A Combined Strategy Using Funneliformis mosseae and Phosphorus Addition for Enhancing Oat Drought Tolerance
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site and Design
2.2. Experimental Materials
2.3. Sample Collection and Analyses
2.3.1. Sample Collection
2.3.2. Laboratory Analysis
2.4. Statistical Analysis
3. Results and Analysis
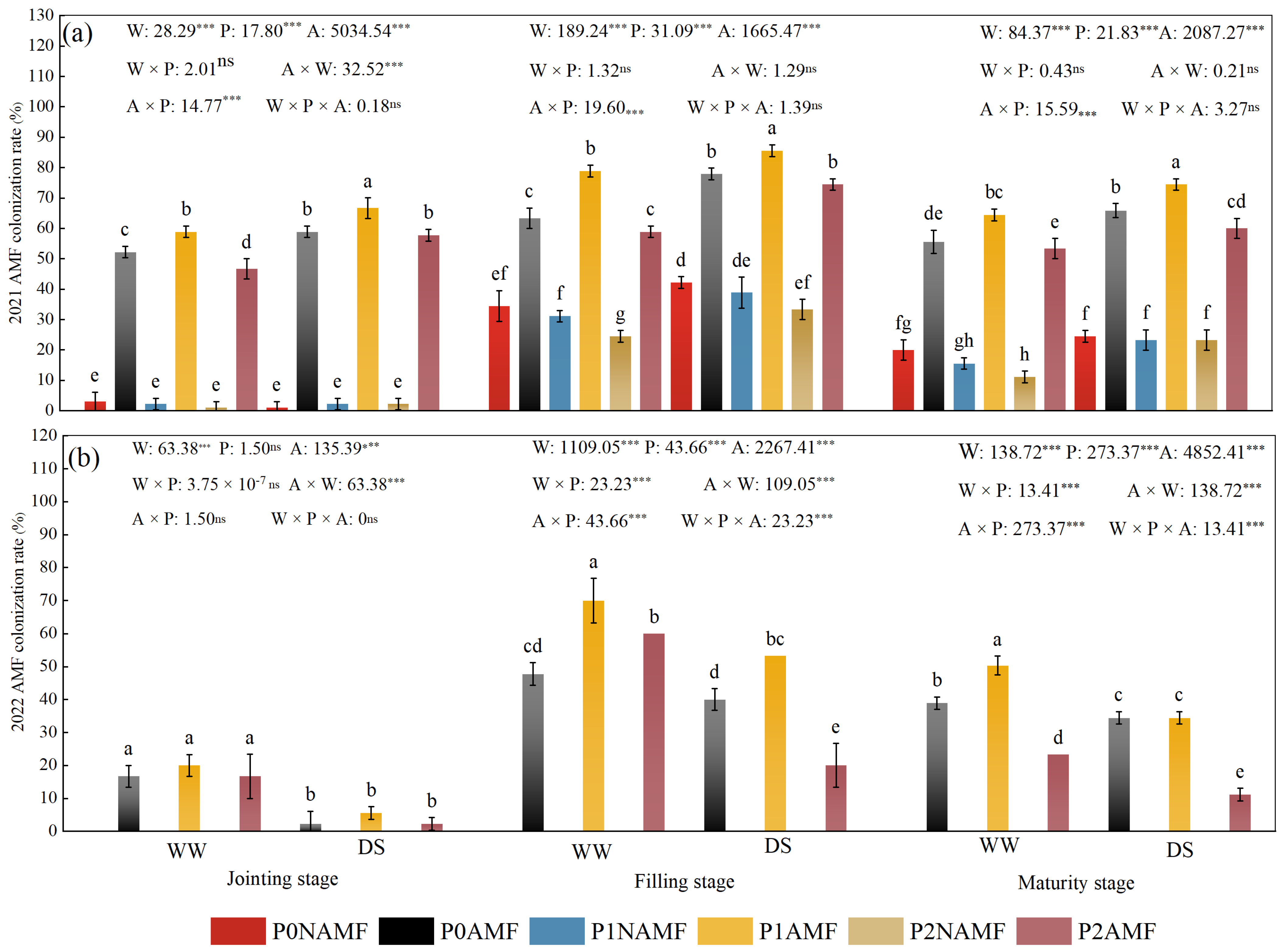
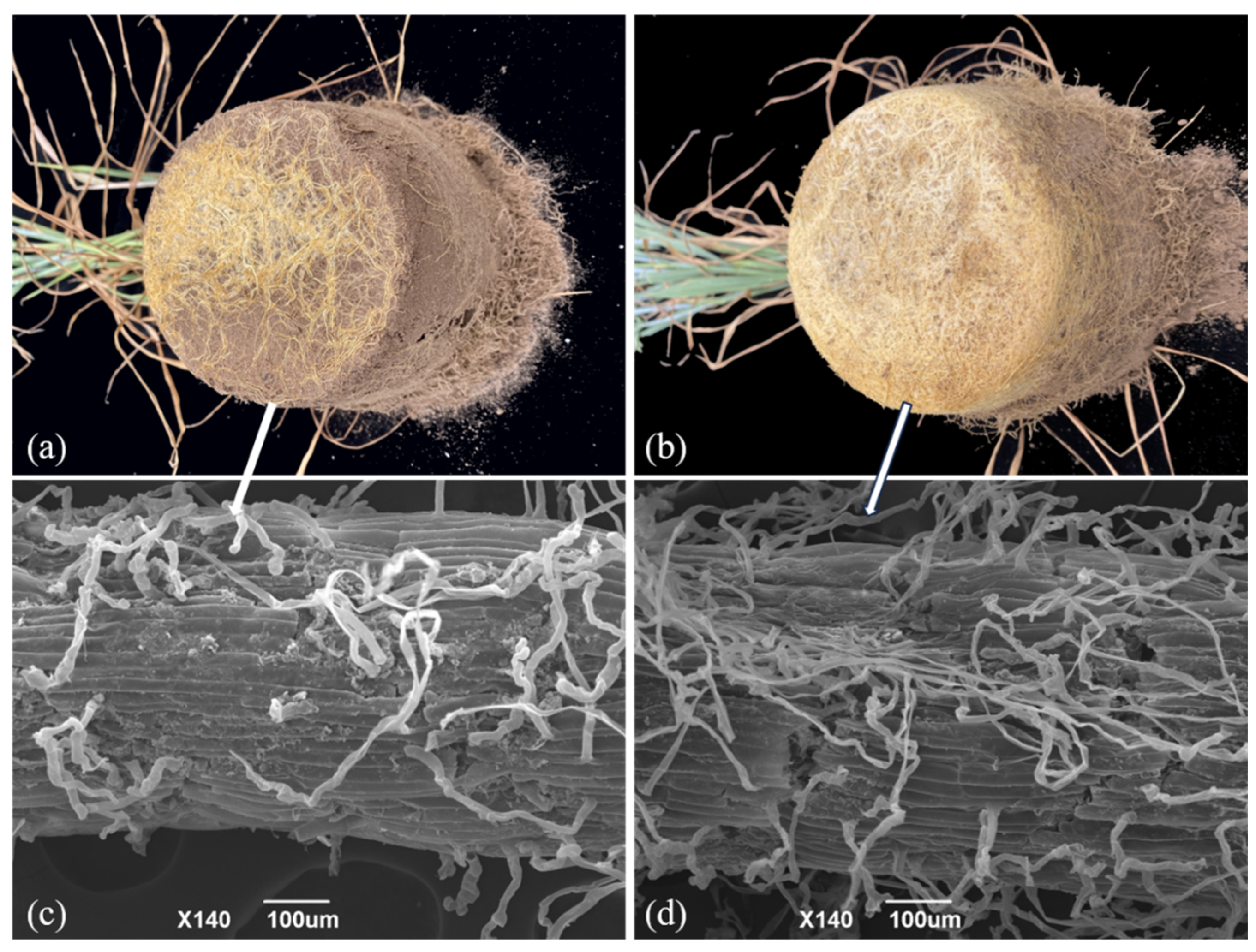
3.1. Colonization of Oat Roots by AMF
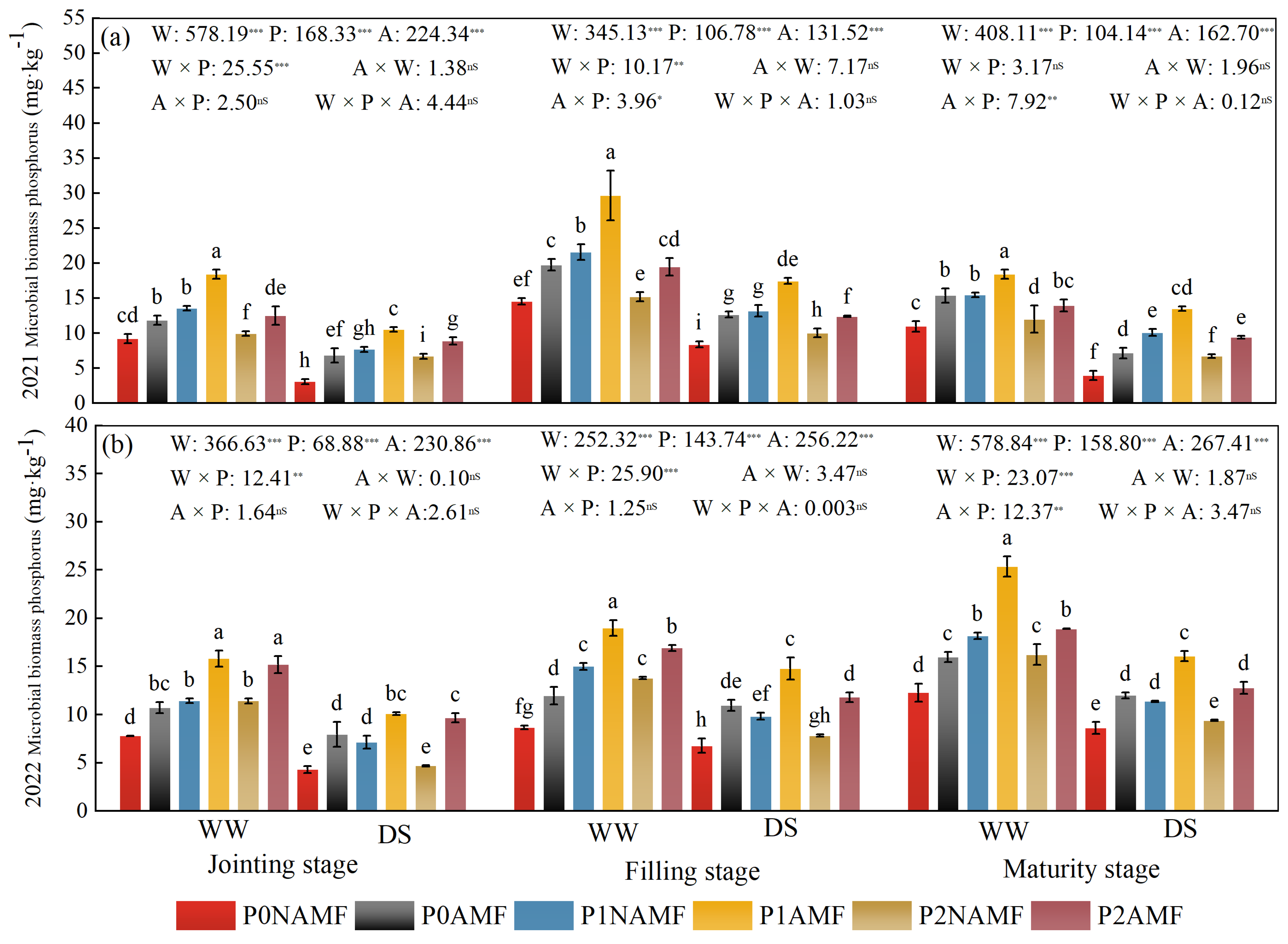
3.2. MBP in Oat Soil
3.3. Ammonium N and Nitrate N Contents in Oat Soil
3.4. Total P and Available P Contents in Oat Soil
3.5. Total N and Total P Content in Oat Plants
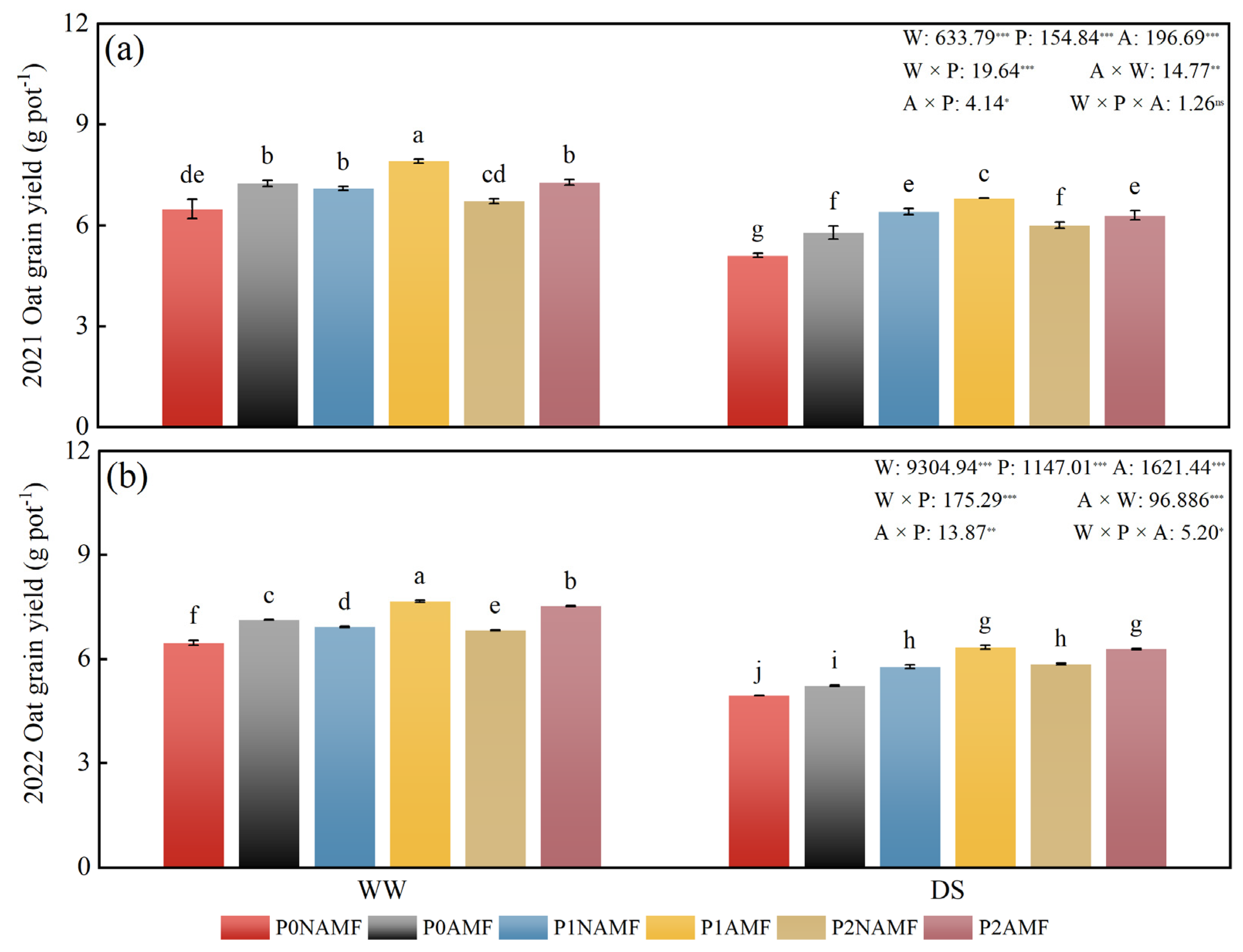
3.6. Oat Grain Yield
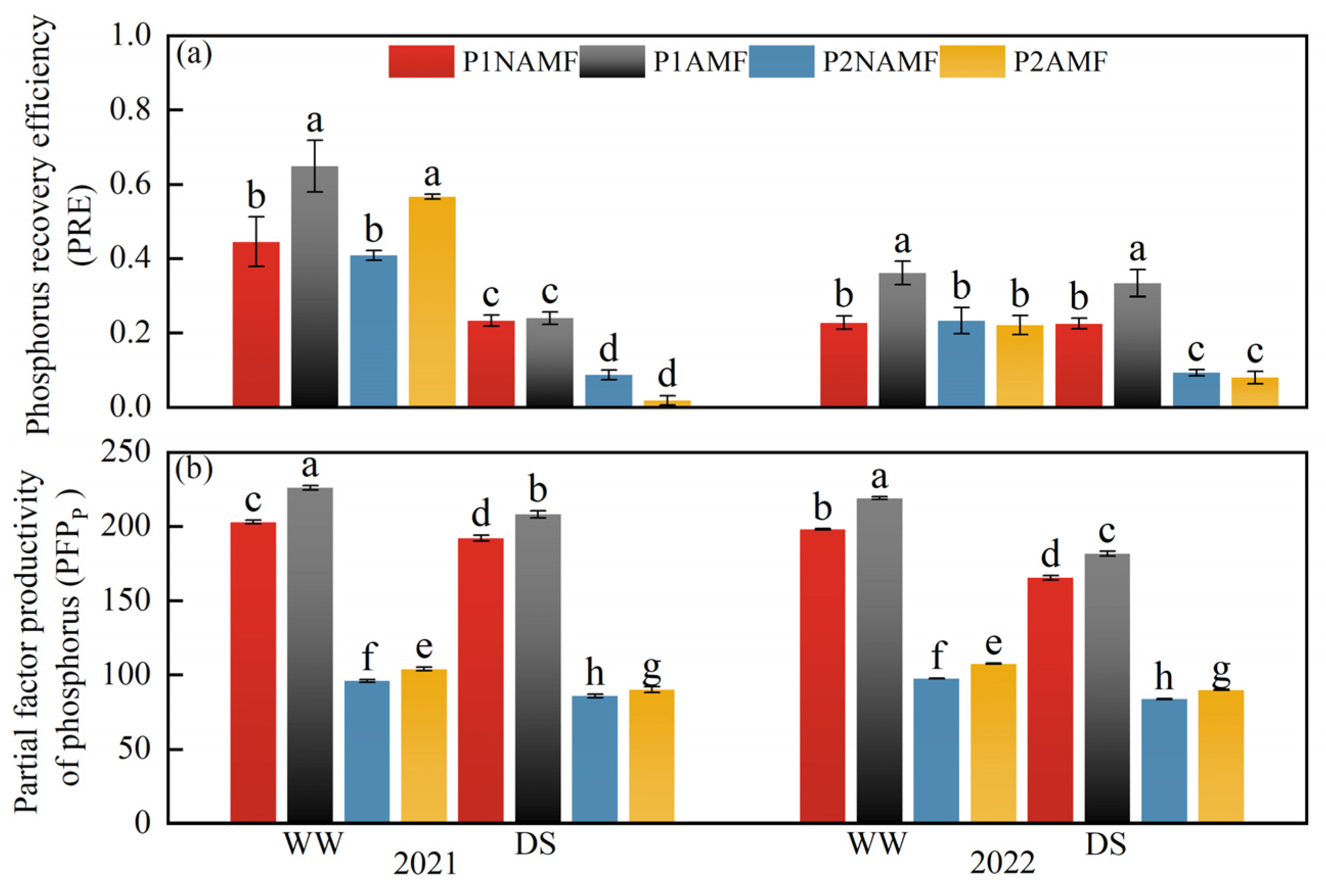
3.7. P Productivity and Uptake Efficiency
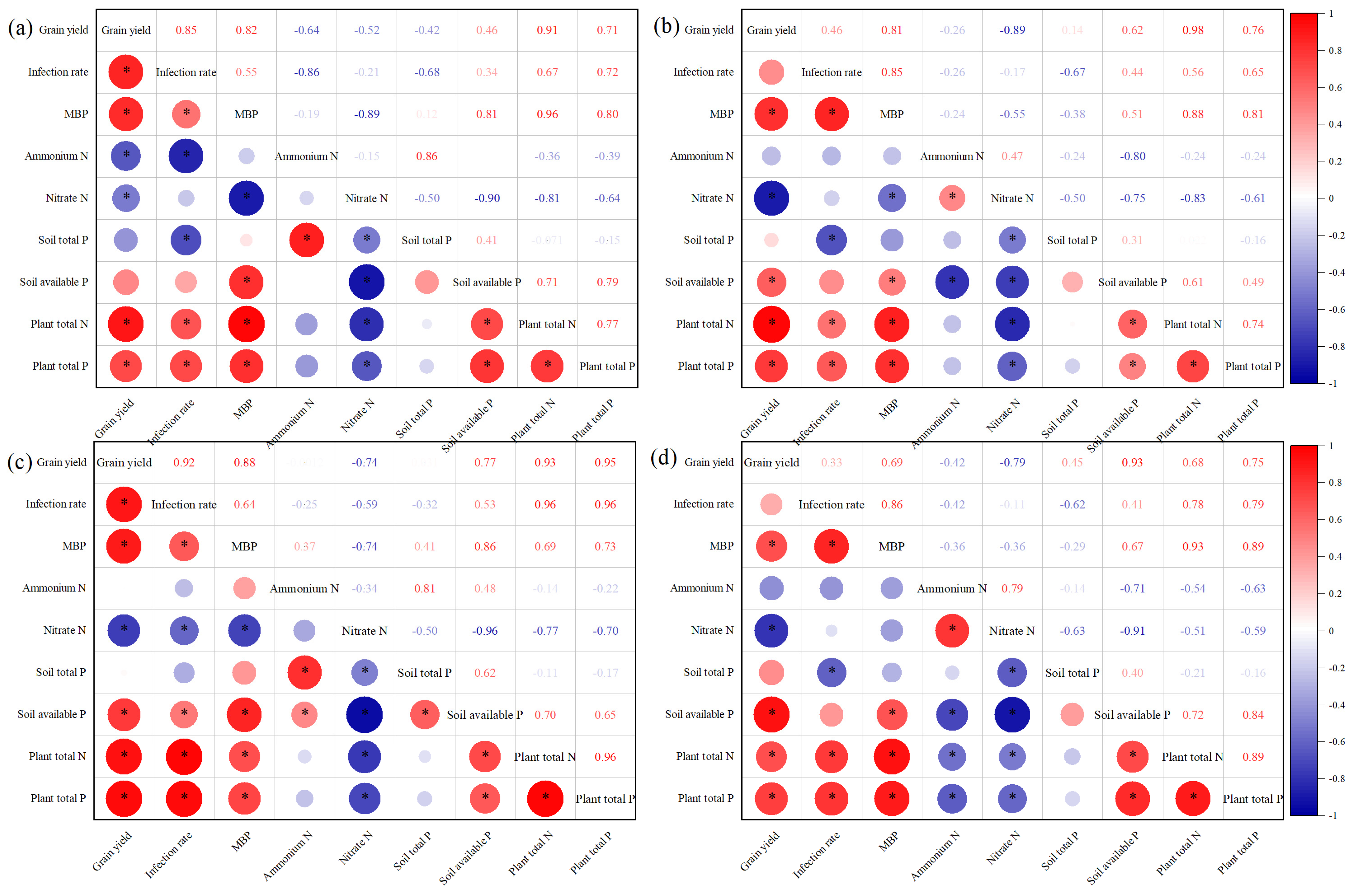
3.8. Relationship Between Oat Grain Yield and Each Index
4. Discussion
4.1. AMF Colonization of Oat Roots with P Addition Under Drought Stress
4.2. AMF and P Addition Enhance N and P Uptake in Oat Plants Under Drought Stress
4.3. AMF and P Addition Mitigate Drought-Induced Oat Yield Loss
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A


References
- Ahmad, M.; Gul-Zaffar; Dar, Z.A.; Habib, M. A review on oat (Avena sativa L.) as a dual-purpose crop. Sci. Res. Essays 2014, 9, 52–59. [Google Scholar]
- Sikora, P.; Tosh, S.M.; Brummer, Y.; Olsson, O. Identification of high β-glucan oat lines and localization and chemical characterization of their seed kernel β-glucans. Food Chem. 2013, 137, 83–91. [Google Scholar] [CrossRef]
- Zhou, Q.P.; Yan, H.B.; Liang, G.L.; Jia, Z.F.; Liu, W.H.; Tian, L.H.; Chen, Y.J.; Chen, S.Y. Analysis of the forage and grain productivity of oat cultivars. Acta Prataculturae Sin. 2015, 24, 120–130. [Google Scholar]
- Ning, T.; Sang, M.J.; Guo, X.Y.; Li, C.; Du, S.X. Spatial and temporal distribution characteristics of rainfall erosivity during 2000–2016 in Shanxi province. Sci. Soil Water Conserv. 2020, 18, 1–7. [Google Scholar]
- Lambers, H. Phosphorus acquisition and utilization in plants. Annu. Rev. Plant Biol. 2022, 73, 17–42. [Google Scholar] [CrossRef]
- Lambers, H.; Chapin, F.S.; Pons, T.L. Plant Physiological Ecology, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 187–263. [Google Scholar]
- McLaughlin, M.J.; McBeath, T.M.; Smernik, R.; Stacey, S.P.; Ajiboye, B.; Guppy, C. The chemical nature of P accumulation in agricultural soils—Implications for fertiliser management and design: An Australian perspective. Plant Soil 2011, 349, 69–87. [Google Scholar] [CrossRef]
- Ashwin, R.; Bagyaraj, D.J.; Mohan Raju, B. Ameliorating the drought stress tolerance of a susceptible soybean cultivar, MAUS 2 through dual inoculation with selected rhizobia and AM fungus. Fungal Biol. Biotechnol. 2023, 10, 10. [Google Scholar] [CrossRef]
- Ray, J.G.; Nadesan, S.T. Arbuscular Mycorrhiza as an Essential Ecotechnological Tool: A Critical Review of Literature on the Role of AMF in the Sustainability of Cultivation and Conservation of Palms. Alger. J. Biosci. 2024, 5, 67–103. [Google Scholar]
- John, S.A.; Ray, J.G. Optimization of environmental and the other variables in the application of arbuscular mycorrhizal fungi as an ecotechnological tool for sustainable paddy cultivation: A critical review. J. Appl. Microbiol. 2023, 134, lxad111. [Google Scholar] [CrossRef]
- Ray, J.G. Ecology of arbuscular mycorrhizal association in coconut (Cocos nucifera L.) palms: Analysis of factors influencing AMF in fields. Rhizosphere 2024, 32, 100961. [Google Scholar] [CrossRef]
- Grümberg, B.C.; Urcelay, C.; Shroeder, M.A.; Vargas-Gil, S.; Luna, C.M. The role of inoculum identity in drought stress mitigation by arbuscular mycorrhizal fungi in soybean. Biol. Fertil. Soils 2015, 51, 1–10. [Google Scholar] [CrossRef]
- Genre, A.; Lanfranco, L.; Perotto, S.; Bonfante, P. Unique and common traits in mycorrhizal symbioses. Nat. Rev. Microbiol. 2020, 18, 649–660. [Google Scholar] [CrossRef]
- Sun, J.; Jia, Q.; Li, Y.; Zhang, T.; Chen, J.; Ren, Y.; Dong, K.; Xu, S.; Shi, N.-N.; Fu, S. Effects of arbuscular mycorrhizal fungi and biochar on growth, nutrient absorption, and physiological properties of maize (Zea mays L.). J. Fungi 2022, 8, 1275. [Google Scholar] [CrossRef]
- Paravar, A.; Maleki Farahani, S.; Rezazadeh, A.; Keshavarz Afshar, R. How nano-iron chelate and arbuscular mycorrhizal fungi mitigate water stress in Lallemantia species: A growth and physio-biochemical properties. J. Plant Nutr. Soil Sci. 2024, 187, 621–638. [Google Scholar] [CrossRef]
- Birhane, E.; Bongers, F.; Damtew, A.; Tesfay, A.; Norgrove, L.; Kuyper, T.W. Arbuscular mycorrhizal fungi improve nutrient status of Commiphora myrrha seedlings under drought. J. Arid Environ. 2023, 209, 104877. [Google Scholar] [CrossRef]
- Tanaka, Y.; Yano, K. Nitrogen delivery to maize via mycorrhizal hyphae depends on the form of N supplied. Plant Cell Environ. 2005, 28, 1247–1254. [Google Scholar] [CrossRef]
- Attarzadeh, M.; Balouchi, H.; Rajaie, M.; Dehnavi, M.M.; Salehi, A. Improvement of Echinacea purpurea performance by integration of phosphorus with soil microorganisms under different irrigation regimes. Agric. Water Manag. 2019, 221, 238–247. [Google Scholar] [CrossRef]
- Liao, X.; Zhao, J.; Xu, L.; Tang, L.; Li, J.; Zhang, W.; Xiao, J.; Xiao, D.; Hu, P.; Nie, Y. Arbuscular mycorrhizal fungi increase the interspecific competition between two forage plant species and stabilize the soil microbial network during a drought event: Evidence from the field. Appl. Soil Ecol. 2023, 185, 104805. [Google Scholar] [CrossRef]
- Ryan, M.H.; Graham, J.H. Little evidence that farmers should consider abundance or diversity of arbuscular mycorrhizal fungi when managing crops. New Phytol. 2018, 220, 1092–1107. [Google Scholar] [CrossRef]
- Zhang, L.; Shi, N.; Fan, J.; Wang, F.; George, T.S.; Feng, G. Arbuscular mycorrhizal fungi stimulate organic phosphate mobilization associated with changing bacterial community structure under field conditions. Environ. Microbiol. 2018, 20, 2639–2651. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, J.; George, T.S.; Limpens, E.; Feng, G. Arbuscular mycorrhizal fungi conducting the hyphosphere bacterial orchestra. Trends Plant Sci. 2022, 27, 402–411. [Google Scholar] [CrossRef]
- Wang, G.; Jin, Z.; George, T.S.; Feng, G.; Zhang, L. Arbuscular mycorrhizal fungi enhance plant phosphorus uptake through stimulating hyphosphere soil microbiome functional profiles for phosphorus turnover. New Phytol. 2023, 238, 2578–2593. [Google Scholar] [CrossRef]
- Augé, R.M.; Toler, H.D.; Saxton, A.M. Arbuscular mycorrhizal symbiosis alters stomatal conductance of host plants more under drought than under amply watered conditions: A meta-analysis. Mycorrhiza 2015, 25, 13–24. [Google Scholar] [CrossRef]
- Zhang, B.; Lv, Y.F.; Li, Y.; Li, L.; Jia, J.Q.; Feng, M.C.; Wang, C.; Song, X.Y.; Yang, W.D.; Shafiq, F. Inoculation with Rhizophagus intraradices Confers Drought Stress Tolerance in Oat by Improving Nitrogen and Phosphorus Nutrition. J. Soil Sci. Plant Nutr. 2023, 23, 2039–2052. [Google Scholar] [CrossRef]
- Cornejo, P.; Rubio, R.; Borie, F. Mycorrhizal propagule persistence in a succession of cereals in a disturbed and undisturbed andisol fertilized with two nitrogen sources. Chil. J. Agric. Res. 2010, 68, 426–434. [Google Scholar] [CrossRef]
- Lee, A.; Neuberger, P.; Omokanye, A.; Hernandez-Ramirez, G.; Kim, K.; Gorzelak, M.A. Arbuscular mycorrhizal fungi in oat-pea intercropping. Sci. Rep. 2023, 13, 390. [Google Scholar] [CrossRef]
- Tian, H.; Jia, Z.; Liu, W.; Wei, X.; Wang, H.; Bao, G.; Li, J.; Zhou, Q. Effects of arbuscular mycorrhizal fungi on growth and nutrient accumulation of oat under drought conditions. Agronomy 2023, 13, 2580. [Google Scholar] [CrossRef]
- Wang, J.Y.; Turner, N.C.; Liu, Y.X.; Siddique, K.H.; Xiong, Y.C. Effects of drought stress on morphological, physiological and biochemical characteristics of wheat species differing in ploidy level. Funct. Plant Biol. 2016, 44, 219–234. [Google Scholar] [CrossRef]
- Lendzemo, V.W.; Kuyper, T.W.; Matusova, R.; Bouwmeester, H.J.; Ast, A.V. Colonization by arbuscular mycorrhizal fungi of sorghum leads to reduced germination and subsequent attachment and emergence of Striga hermonthica. Plant Signal. Behav. 2007, 2, 58–62. [Google Scholar] [CrossRef]
- Vierheilig, H.; Coughlan, A.P.; Wyss, U.; Piché, Y. Ink and Vinegar, a Simple Staining Technique for Arbuscular-Mycorrhizal Fungi. Appl. Environ. Microbiol. 1998, 64, 5004–5007. [Google Scholar] [CrossRef]
- Trouvelot, A.; Kough, J.; Gianinazzi-Pearson, V. Mesure du taux de mycorhization VA d’un systeme radiculaire. Recherche de méthodes d’estimation ayant une signification fonctionnelle. In Physiological and Genetical Aspects of Mycorrhizae; INRA: Paris, France, 1986; pp. 217–221. [Google Scholar]
- Bao, S.D. Soil Agrochemical Analysis, 3rd ed.; China Agriculture Press: Beijing, China, 2000; pp. 242–282. [Google Scholar]
- Brookes, P.; Powlson, D.; Jenkinson, D. Measurement of microbial biomass phosphorus in soil. Soil Biol. Biochem. 1982, 14, 319–329. [Google Scholar] [CrossRef]
- Thioub, M.; Ewusi-Mensah, N.; Sarkodie-Addo, J.; Adjei-Gyapong, T. Arbuscular mycorrhizal fungi inoculation enhances phosphorus use efficiency and soybean productivity on a Haplic Acrisol. Soil Tillage Res. 2019, 192, 174–186. [Google Scholar] [CrossRef]
- Syers, J.K.; Johnston, A.E.; Curtin, D. Efficiency of Soil and Fertilizer Phosphorus Use: Reconciling Changing Concepts of Soil Phosphorus Behaviour with Agronomic Information; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2008; Chapter 4; pp. 27–44. [Google Scholar]
- Cassman, K.G.; Peng, S.; Olk, D.; Ladha, J.; Reichardt, W.; Dobermann, A.; Singh, U. Opportunities for increased nitrogen-use efficiency from improved resource management in irrigated rice systems. Field Crops Res. 1998, 56, 7–39. [Google Scholar] [CrossRef]
- Qi, C.X.; Yi, G.T.; Fu, X.X.; Wang, H.L.; Wang, J.L.; Wu, J.H. Effects of arbuscular mycorrhizal fungi on physiological indexes of Potentilla anserina under drought stress. Acta Agrestia Sin. 2021, 29, 2407–2412. [Google Scholar]
- Li, F.; Deng, J.; Nzabanita, C.; Li, Y.; Duan, T. Growth and physiological responses of perennial ryegrass to an AMF and an Epichloë endophyte under different soil water contents. Symbiosis 2019, 79, 151–161. [Google Scholar] [CrossRef]
- Zhang, Z.F.; Zhang, J.C.; Huang, Y.Q.; Yang, H.; Luo, Y.J.; Luo, A.Y. Effects of arbuscular mycorrhizal fungi on plant drought tolerance: Research progress. Chin. J. Ecol. 2013, 32, 1607–1612. [Google Scholar]
- Rillig, M.C.; Mardatin, N.F.; Leifheit, E.F.; Antunes, P.M. Mycelium of arbuscular mycorrhizal fungi increases soil water repellency and is sufficient to maintain water-stable soil aggregates. Soil Biol. Biochem. 2010, 42, 1189–1191. [Google Scholar] [CrossRef]
- Huang, H.; Wang, X.; Li, J.; Gao, Y.; Yang, Y.; Wang, R.; Zhou, Z.; Wang, P.; Zhang, Y. Trends and directions in oats research under drought and salt stresses: A bibliometric analysis (1993–2023). Plants 2024, 13, 1902. [Google Scholar] [CrossRef]
- Wang, J.G.; Zheng, R.; Bai, S.L.; Liu, S.; Yan, W. Responses of ectomycorrhiza to drought stress:A review. Chin. J. Ecol. 2012, 31, 1571–1576. [Google Scholar]
- Lee, K.J.; Park, H.; Lee, I.S. Morphology of arbuscular mycorrhizal roots and effects of root age and soil texture on the mycorrhizal infection in Panax ginseng CA Meyer. J. Ginseng Res. 2004, 28, 149–156. [Google Scholar] [CrossRef]
- Bao, X.Z.; Wang, Y.T.; Olsson, P.A. Arbuscular mycorrhiza under water-Carbon-phosphorus exchange between rice and arbuscular mycorrhizal fungi under different flooding regimes. Soil Biol. Biochem. 2019, 129, 169–177. [Google Scholar] [CrossRef]
- Zhang, S.B.; Wang, Y.S.; Yin, X.F.; Liu, J.B.; Wu, F.X. Development of arbuscular mycorrhizal (AM) fungi and their influences on the absorption of N and P of maize at different soil phosphorus application levels. J. Plant Nutr. Fert. 2017, 23, 649–657. [Google Scholar]
- Guo, Y.E.; Li, Y.D.; Gao, P.; Wang, Z.G.; Duan, Y.Y. Effects of Claroideoglomus etunicatum and grass endophyte on the growth of Lolium perenne under different phosphorus levels. Acta Agrestia Sin. 2018, 26, 1458–1466. [Google Scholar]
- Konvalinková, T.; Püschel, D.; Řezáčová, V.; Gryndlerová, H.; Jansa, J. Carbon flow from plant to arbuscular mycorrhizal fungi is reduced under phosphorus fertilization. Plant Soil 2017, 419, 319–333. [Google Scholar] [CrossRef]
- Salmeron-Santiago, I.A.; Martínez-Trujillo, M.; Valdez-Alarcón, J.J.; Pedraza-Santos, M.E.; Santoyo, G.; Lopez, P.A.; Larsen, J.; Pozo, M.J.; Chávez-Bárcenas, A.T. Carbohydrate and lipid balances in the positive plant phenotypic response to arbuscular mycorrhiza: Increase in sink strength. Physiol. Plant. 2023, 175, e13857. [Google Scholar] [CrossRef]
- Balzergue, C.; Puech-Pagès, V.; Bécard, G.; Rochange, S.F. The regulation of arbuscular mycorrhizal symbiosis by phosphate in pea involves early and systemic signalling events. J. Exp. Bot. 2011, 62, 1049–1060. [Google Scholar] [CrossRef]
- Qin, Z.X.; Zhu, M.; Guo, T. Influence of mycorrhizal inoculation on physiological and biochemical characteristics of maize (Zea mays) under water stress. Plant Nutr. Fert. Sci. 2013, 19, 510–516. [Google Scholar]
- Ryan, M.H.; Graham, J.H. Is there a role for arbuscular mycorrhizal fungi in production agriculture? Plant soil 2002, 244, 263–271. [Google Scholar] [CrossRef]
- Cavagnaro, T.R.; Barrios-Masias, F.H.; Jackson, L.E. Arbuscular mycorrhizas and their role in plant growth, nitrogen interception and soil gas efflux in an organic production system. Plant Soil 2012, 353, 181–194. [Google Scholar] [CrossRef]
- Welc, M.; Ravnskov, S.; Kieliszewska-Rokicka, B.; Larsen, J. Suppression of other soil microorganisms by mycelium of arbuscular mycorrhizal fungi in root-free soil. Soil Biol. Biochem. 2010, 42, 1534–1540. [Google Scholar] [CrossRef]
- Shen, Y.; Zhang, F.; Yang, Y.; Lu, G.; Yang, X. Arbuscular mycorrhizal fungi alter plant N and P resorption of dominant species in a degraded grassland of northern China. Ecol. Indic. 2023, 150, 110195. [Google Scholar] [CrossRef]
- Schmied, G.; Hilmers, T.; Mellert, K.H.; Uhl, E.; Buness, V.; Ambs, D.; Steckel, M.; Biber, P.; Šeho, M.; Hoffmann, Y.D. Nutrient regime modulates drought response patterns of three temperate tree species. Sci. Total Environ. 2023, 868, 161601. [Google Scholar] [CrossRef]
- Deng, L.; Peng, C.; Kim, D.G.; Li, J.; Liu, Y.; Hai, X.; Liu, Q.; Huang, C.; Shangguan, Z.; Kuzyakov, Y. Drought effects on soil carbon and nitrogen dynamics in global natural ecosystems. Earth-Sci. Rev. 2021, 214, 103501. [Google Scholar] [CrossRef]
- Yu, H.Y.; He, W.X.; Zou, Y.N.; Alqahtani, M.D.; Wu, Q.S. Arbuscular mycorrhizal fungi and rhizobia accelerate plant growth and N accumulation and contribution to soil total N in white clover by difficultly extractable glomalin-related soil protein. Appl. Soil Ecol. 2024, 197, 105348. [Google Scholar] [CrossRef]
- Chen, Y.X.; Wen, Z.H.; Meng, J.; Liu, Z.Q.; Wei, J.L.; Liu, X.Y.; Ge, Z.Y.; Dai, W.N.; Lin, L.; Chen, W.F. Positive effects of biochar application and Rhizophagus irregularis inoculation on mycorrhizal colonization, rice seedlings and phosphorus cycling in paddy soils. Pedosphere 2024, 34, 361–373. [Google Scholar] [CrossRef]
- Ma, K.; Yang, J.J.; Li, L.; Wang, Y.Q.; Wang, Y.; Ma, H.C. Drought stress effects of nutrients of the Bombax ceiba at the root zone soil and plant’s body after inoculation of AMF. J. Cent. South Univ. For. Technol. 2017, 37, 90–95+102. [Google Scholar]
- Bender, S.F.; Conen, F.; Van der Heijden, M.G. Mycorrhizal effects on nutrient cycling, nutrient leaching and N2O production in experimental grassland. Soil Biol. Biochem. 2015, 80, 283–292. [Google Scholar] [CrossRef]
- Suriyagoda, L.D.; Ryan, M.H.; Renton, M.; Lambers, H. Above-and below-ground interactions of grass and pasture legume species when grown together under drought and low phosphorus availability. Plant Soil 2011, 348, 281–297. [Google Scholar] [CrossRef]
- Fall, A.F.; Nakabonge, G.; Ssekandi, J.; Founoune-Mboup, H.; Badji, A.; Ndiaye, A.; Ndiaye, M.; Kyakuwa, P.; Anyoni, O.G.; Kabaseke, C. Combined effects of indigenous arbuscular mycorrhizal fungi (AMF) and NPK fertilizer on growth and yields of maize and soil nutrient availability. Sustainability 2023, 15, 2243. [Google Scholar] [CrossRef]
- Ul Haq, J.; Sharif, M.; Akbar, W.A.; Ur Rahim, H.; Ahmad Mian, I.; Ahmad, S.; Alatalo, J.M.; Khan, Z.; Mudassir, M. Arbuscular mycorrhiza fungi integrated with single super phosphate improve wheat-nitrogen-phosphorus acquisition, yield, root infection activity, and spore density in alkaline-calcareous soil. Gesunde Pflanz. 2023, 75, 539–548. [Google Scholar] [CrossRef]
- Smith, S.E.; Jakobsen, I.; Grønlund, M.; Smith, F.A. Roles of arbuscular mycorrhizas in plant phosphorus nutrition: Interactions between pathways of phosphorus uptake in arbuscular mycorrhizal roots have important implications for understanding and manipulating plant phosphorus acquisition. Plant Physiol. 2011, 156, 1050–1057. [Google Scholar] [CrossRef]
- Li, C.J.; Li, H.G.; Hoffland, E.; Zhang, F.S.; Zhang, J.L.; Kuyper, T.W. Common mycorrhizal networks asymmetrically improve chickpea N and P acquisition and cause overyielding by a millet/chickpea mixture. Plant Soil 2022, 472, 279–293. [Google Scholar] [CrossRef]
- Catalin, S.I.; Ionut, M. Drought phenomena and groundwater scarcity in eastern Romania (Siret-Prut region). EGU Gen. Assem. 2013, 15, 6997. [Google Scholar]
- Luo, W.; Zuo, X.; Ma, W.; Xu, C.; Li, A.; Yu, Q.; Knapp, A.K.; Tognetti, R.; Dijkstra, F.A.; Li, M.H. Differential responses of canopy nutrients to experimental drought along a natural aridity gradient. Ecology 2018, 99, 2230–2239. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Z.; Guo, M.; Qu, L.; Biere, A. Effects of arbuscular mycorrhizal fungi on plant growth and herbivore infestation depend on availability of soil water and nutrients. Front. Plant Sci. 2023, 14, 1101932. [Google Scholar] [CrossRef]
- Oliveira, H.; Pereira, S.; Santos, M.G. Cenostigma microphyllum seedlings in semiarid region grow faster under arbuscular mycorrhizal symbiosis, regardless of water availability. J. Arid Environ. 2023, 212, 104962. [Google Scholar] [CrossRef]
- Li, X.; Zhang, J.L. Uptake of ammonium and nitrate by external hypaae of arbuscular mycorrhizal fungi. J. Plant Nutr. Fert. 2009, 15, 683–689. [Google Scholar]
- Zhang, B.B.; Zhang, H.; Wang, H.; Wang, P.; Wu, Y.X.; Wang, M.M. Effect of phosphorus additions and arbuscular mycorrhizal fungal inoculation on the growth, physiology, and phosphorus uptake of wheat under two water regimes. Soil Sci. Plant Anal. 2018, 49, 862–874. [Google Scholar] [CrossRef]
- Wang, W.; Vinocur, B.; Altman, A. Plant responses to drought, salinity and extreme temperatures: Towards genetic engineering for stress tolerance. Planta 2003, 218, 1–14. [Google Scholar] [CrossRef]
- Li, Y.; Li, L.; Zhang, B.; Lv, Y.F.; Feng, M.C.; Wang, C.; Song, X.Y.; Yang, W.D.; Zhang, M.J. AMF inoculation positively regulates soil microbial activity and drought tolerance of oat. J. Plant Nutr. Fert. 2023, 29, 1135–1149. [Google Scholar]
- Das, D.; Ullah, H.; Himanshu, S.K.; Tisarum, R.; Cha-Um, S.; Datta, A. Arbuscular mycorrhizal fungi inoculation and phosphorus application improve growth, physiological traits, and grain yield of rice under alternate wetting and drying irrigation. J. Plant Physiol. 2022, 278, 153829. [Google Scholar] [CrossRef]
- Cui, X.; Wang, B.; Chen, Z.; Guo, J.; Zhang, T.; Zhang, W.; Shi, L. Comprehensive physiological, transcriptomic, and metabolomic analysis of the key metabolic pathways in millet seedling adaptation to drought stress. Physiol. Plant. 2023, 175, e14122. [Google Scholar] [CrossRef]
- Al-Karaki, G.; McMichael, B.; Zak, J. Field response of wheat to arbuscular mycorrhizal fungi and drought stress. Mycorrhiza 2004, 14, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, C.N.; Li, H.B.; Wang, H.; Wang, X.X. Research progress of arbuscular mycorrhizal fungi in vegetable production. Microbiol. China 2021, 48, 4282–4295. [Google Scholar]
- Smith, S. Structural diversity in (vesicular)-arbuscular mycorrhizal symbiosis. New Phytol. 1997, 114, 373–388. [Google Scholar] [CrossRef] [PubMed]

| Year | Organic Carbon (g kg−1) | Total Phosphorus (mg kg−1) | Available Phosphorus (mg kg−1) | Alkali-Hydrolyzable Nitrogen (mg kg−1) | pH |
|---|---|---|---|---|---|
| 2021 | 10.1 | 73.5 | 3.5 | 49.4 | 8.1 |
| 2022 | 8.9 | 64.2 | 5.7 | 21.4 | 8.2 |
| Year | Treatment | Ammonium N (mg kg−1) | Nitrate N (mg kg−1) | |||||
|---|---|---|---|---|---|---|---|---|
| Jointing Stage | Filling Stage | Maturity Stage | Jointing Stage | Filling Stage | Maturity Stage | |||
| 2021 | Water treatment | WW | 7.77 b | 6.84 b | 6.21 b | 7.01 b | 10.47 a | 7.00 b |
| DS | 8.92 a | 8.31 a | 6.97 a | 9.18 a | 11.65 a | 7.87 a | ||
| p-value | 0.002 | 0.001 | 0.007 | <0.001 | 0.278 | 0.033 | ||
| F | 12.42 ** | 15.87 ** | 8.86 ** | 26.51 *** | ns | 5.18 * | ||
| P treatment | P0 | 8.90 a | 7.70 a | 6.95 a | 8.66 a | 14.34 a | 7.59 a | |
| P1 | 8.28 a | 7.91 a | 6.55 a | 8.20 a | 9.56 b | 8.09 a | ||
| P2 | 7.86 a | 7.11 a | 6.28 a | 7.42 a | 9.27 b | 6.62 b | ||
| p-value | 0.092 | 0.382 | 0.183 | 0.255 | <0.001 | 0.007 | ||
| F | ns | ns | ns | ns | 52.39 *** | 6.42 ** | ||
| AMF treatment | NAMF | 8.90 a | 7.99 a | 6.94 a | 8.74 a | 11.68 a | 7.60 a | |
| AMF | 7.79 b | 7.15 a | 6.24 b | 7.44 b | 10.44 a | 7.27 a | ||
| p-value | 0.003 | 0.079 | 0.014 * | 0.030 | 0.253 | 0.446 | ||
| F | 11.09 ** | ns | 7.78 | 5.35 * | ns | ns | ||
| 2022 | Water treatment | WW | 7.76 b | 6.92 b | 6.06 b | 12.28 b | 7.03 b | 6.38 b |
| DS | 12.45 a | 10.02 a | 9.12 a | 21.30 a | 27.65 a | 18.25 a | ||
| p-value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||
| F | 145.92 *** | 65.36 *** | 99.88 *** | 79.46 *** | 158.25 *** | 478.73 *** | ||
| P treatment | P0 | 9.57 a | 8.27 a | 7.23 a | 18.36 a | 20.58 a | 13.60 a | |
| P1 | 10.39 a | 8.90 a | 7.72 a | 16.67 a | 17.82 a | 12.04 a | ||
| P2 | 10.36 a | 8.23 a | 7.82 a | 15.35 a | 13.62 a | 11.31 a | ||
| p-value | 0.782 | 0.737 | 0.783 | 0.532 | 0.48 | 0.768 | ||
| F | ns | ns | ns | ns | ns | ns | ||
| AMF treatment | NAMF | 10.82 a | 8.95 a | 7.88 a | 18.61 a | 18.55 a | 12.57 a | |
| AMF | 9.39 a | 7.99 a | 7.29 a | 14.98 a | 16.13 a | 12.06 a | ||
| p-value | 0.178 | 0.21 | 0.415 | 0.088 | 0.609 | 0.845 | ||
| F | ns | ns | ns | ns | ns | ns | ||
| Year | Water Treatment | P Treatment | AMF Treatment | Ammonium N (mg kg−1) | Nitrate N (mg kg−1) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Jointing Stage | Filling Stage | Maturity Stage | Jointing Stage | Filling Stage | Maturity Stage | ||||
| 2021 | WW | P0 | NAMF | 8.76 cd | 7.02 de | 6.81 cd | 7.57 e | 14.80 b | 6.99 f |
| AMF | 7.64 fg | 6.22 e | 6.10 fg | 6.45 f | 12.29 d | 7.59 de | |||
| P1 | NAMF | 8.13 e | 7.17 de | 6.32 ef | 7.39 e | 9.30 h | 7.81 cd | ||
| AMF | 6.90 h | 6.48 e | 5.31 h | 7.16 e | 8.86 j | 7.43 ef | |||
| P2 | NAMF | 8.00 ef | 7.39 de | 6.66 de | 7.59 e | 9.04 i | 7.07 ef | ||
| AMF | 7.21 gh | 6.77 de | 6.09 fg | 5.91 f | 8.55 k | 5.15 h | |||
| DS | P0 | NAMF | 10.09 a | 9.55 a | 7.90 a | 11.48 a | 16.73 a | 7.54 de | |
| AMF | 9.13 bc | 8.01 bc | 6.98 cd | 9.14 c | 13.55 c | 8.26 b | |||
| P1 | NAMF | 9.46 b | 9.09 ab | 7.39 b | 9.71 b | 10.27 e | 8.08b c | ||
| AMF | 8.62 d | 8.89 ab | 7.21 bc | 8.56 d | 9.83 f | 9.04 a | |||
| P2 | NAMF | 8.96 cd | 7.71 cd | 6.61 de | 8.73 cd | 9.94 f | 8.10 bc | ||
| AMF | 7.26 gh | 6.60 de | 5.76 gh | 7.45 e | 9.57 g | 6.18 g | |||
| ANOVA | W | p-value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| P | <0.001 | 0.052 | <0.001 | <0.001 | <0.001 | <0.001 | |||
| A | <0.001 | 0.005 | <0.001 | <0.001 | <0.001 | 0.003 | |||
| W × P | <0.001 | 0.006 | <0.001 | <0.001 | <0.001 | 0.164 | |||
| A × W | 0.429 | 0.623 | 0.483 | 0.003 | 0.002 | 0.018 | |||
| A × P | 0.458 | 0.489 | 0.534 | <0.001 | <0.001 | 0.164 | |||
| W × A ×P | 0.009 | 0.564 | 0.022 | 0.003 | <0.001 | 0.015 | |||
| 2022 | WW | P0 | NAMF | 7.53 g | 6.91 e | 5.20 g | 15.10 e | 8.46 e | 7.84 d |
| AMF | 6.63 h | 5.53 f | 5.48 fg | 11.17 h | 7.54 ef | 7.08 d | |||
| P1 | NAMF | 8.49 f | 7.00 e | 5.93 ef | 13.67 f | 7.76 ef | 6.84 de | ||
| AMF | 7.34 g | 7.25 de | 5.84 fg | 11.88 gh | 6.54 fg | 5.48 e | |||
| P2 | NAMF | 9.00 e | 7.73 d | 7.51 d | 12.46f g | 6.59 fg | 5.45 e | ||
| AMF | 7.58 g | 7.10 e | 6.39 e | 9.43 i | 5.29 g | 5.59 e | |||
| DS | P0 | NAMF | 12.32 b | 11.47 a | 9.89 a | 26.60 a | 36.29 a | 18.67 b | |
| AMF | 11.79 c | 9.19 c | 8.36 c | 20.58 c | 30.03 b | 20.81 a | |||
| P1 | NAMF | 13.88 a | 11.48 a | 10.04 a | 22.39 b | 30.28 b | 18.88 b | ||
| AMF | 11.87 b | 9.89 b | 9.06 b | 18.75 c | 26.70 c | 16.96 c | |||
| P2 | NAMF | 13.72 c | 9.11 c | 8.74 bc | 21.43 d | 21.94 d | 17.75b c | ||
| AMF | 11.15 c | 9.02 c | 8.64 bc | 18.09 d | 20.68 d | 16.45 c | |||
| ANOVA | W | p-value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| P | <0.001 | <0.001 | 0.002 | <0.001 | <0.001 | <0.001 | |||
| A | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.051 | |||
| W × P | 0.001 | <0.001 | <0.001 | 0.001 | <0.001 | 0.471 | |||
| A × W | 0.003 | 0.001 | 0.026 | 0.005 | <0.001 | 0.539 | |||
| A × P | <0.001 | <0.001 | 0.937 | 0.002 | 0.004 | 0.006 | |||
| W × A ×P | 0.003 | <0.001 | 0.001 | 0.200 | 0.001 | 0.007 | |||
| Year | Treatments | Total P (mg kg−1) | Available P (mg kg−1) | |||||
|---|---|---|---|---|---|---|---|---|
| Jointing Stage | Filling Stage | Maturity Stage | Jointing Stage | Filling Stage | Maturity Stage | |||
| 2021 | Water treatment | WW | 70.34 a | 61.79 b | 51.84 b | 8.18 a | 5.66 b | 3.77 b |
| DS | 77.79 a | 68.38 a | 58.53 a | 6.53 b | 4.60 a | 2.82 a | ||
| p-value | 0.057 | 0.044 | 0.043 | <0.001 | 0.005 | 0.007 | ||
| F | ns | 4.38 * | 4.42 * | 18.03 *** | 9.85 ** | 8.78 ** | ||
| P treatment | P0 | 62.21 c | 56.87c | 47.27 b | 6.62 b | 4.34 b | 2.61 b | |
| P1 | 73.78 b | 64.37 b | 53.25 b | 7.45 ab | 5.08 b | 3.12 b | ||
| P2 | 86.21 a | 74.01 a | 65.03 a | 8.20 a | 5.97 a | 4.16 a | ||
| p-value | <0.001 | <0.001 | <0.001 | 0.010 | 0.001 | <0.001 | ||
| F | 40.03 *** | 17.69 *** | 21.05 *** | 5.76 * | 10.41 ** | 11.16 *** | ||
| AMF treatment | NAMF | 78.85 a | 70.53 a | 60.29 a | 6.89 a | 4.73 b | 2.95 a | |
| AMF | 69.28 b | 59.64 b | 50.08 b | 7.83 a | 5.53 a | 3.64 a | ||
| p-value | 0.013 | <0.001 | 0.001 | 0.065 | 0.040 | 0.062 | ||
| F | 6.90 * | 15.42 *** | 12.45 ** | ns | 4.77 * | ns | ||
| 2022 | Water treatment | WW | 64.86 a | 56.33 a | 45.19 b | 5.40 a | 5.10 a | 2.11 a |
| DS | 72.23 a | 61.76 a | 51.93 a | 4.81 a | 3.76 a | 1.69 a | ||
| p-value | 0.091 | 0.152 | 0.044 | 0.157 | 0.139 | 0.087 | ||
| F | ns | ns | 4.59 * | ns | ns | ns | ||
| P treatment | P0 | 57.82c | 49.49c | 41.20 b | 4.15 b | 2.36 b | 1.45 b | |
| P1 | 69.02 b | 59.63 b | 49.13 a | 5.20 a | 3.63 a | 1.96 ab | ||
| P2 | 78.78 a | 68.02 a | 55.34 a | 5.96 a | 4.30 a | 2.29 a | ||
| p-value | <0.001 | <0.001 | 0.001 | <0.001 | <0.001 | 0.01 | ||
| F | 21.51 *** | 25.59 *** | 10.90 ** | 13.15 *** | 19.70 *** | 5.74 * | ||
| AMF treatment | NAMF | 72.80 a | 62.85 a | 52.72 a | 4.55 b | 2.95 b | 1.50 b | |
| AMF | 64.29 b | 55.25 b | 44.40 b | 5.65 a | 3.93 a | 2.30 a | ||
| p-value | 0.048 | 0.04 | 0.01 | 0.005 | 0.017 | <0.001 | ||
| F | 4.37 * | 4.78 * | 7.85 * | 9.57 ** | 6.61 * | 19.38 *** | ||
| Year | Water Treatment | P Treatment | AMF Treatment | Total P (mg kg−1) | Available P (mg kg−1) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Jointing Stage | Filling Stage | Maturity Stage | Jointing Stage | Filling Stage | Maturity Stage | ||||
| 2021 | WW | P0 | NAMF | 60.89 g | 57.15 f | 47.02 f | 6.79 f | 4.30 j | 2.69 g |
| AMF | 53.47 h | 50.31 g | 43.29 g | 7.70 d | 5.21 f | 3.81 d | |||
| P1 | NAMF | 75.78 d | 65.52 e | 52.99 de | 8.10 c | 5.43 e | 3.47 e | ||
| AMF | 65.79 f | 55.71 f | 45.42 fg | 9.09 a | 6.02 c | 3.76 d | |||
| P2 | NAMF | 88.48 b | 78.91 b | 68.51 b | 8.28 b | 6.17 b | 4.13 c | ||
| AMF | 77.64 d | 63.14 e | 53.81 d | 9.11 a | 6.82 a | 4.79 a | |||
| DS | P0 | NAMF | 70.66 e | 64.07 e | 54.13 d | 5.07 i | 3.46 i | 1.66 i | |
| AMF | 63.83 f | 55.96 f | 44.63 fg | 6.12 g | 4.37 g | 2.29 h | |||
| P1 | NAMF | 82.52 c | 71.90 c | 63.63 c | 5.84 h | 3.97 h | 2.38 h | ||
| AMF | 71.02 e | 64.35 e | 50.96 e | 6.76 f | 4.91 f | 2.86 f | |||
| P2 | NAMF | 94.77 a | 85.64 a | 75.44 a | 7.24 e | 5.05 e | 3.36 e | ||
| AMF | 83.95 c | 68.35 d | 62.37 c | 8.17 c | 5.85 c | 4.35 b | |||
| ANOVA | W | p-value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| P | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |||
| A | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |||
| W × P | <0.001 | <0.001 | 0.164 | <0.001 | <0.001 | <0.001 | |||
| A × W | 0.003 | 0.002 | 0.018 | 0.104 | <0.001 | 0.866 | |||
| A × P | <0.001 | <0.001 | <0.001 | 0.041 | <0.001 | <0.001 | |||
| W × A ×P | 0.003 | <0.001 | 0.015 | 0.045 | <0.001 | <0.001 | |||
| 2022 | WW | P0 | NAMF | 56.01 h | 48.39 h | 39.53 h | 4.07 g | 2.16 h | 1.32 f |
| AMF | 52.60 i | 43.34 i | 34.17 i | 5.38 de | 2.96 f | 2.03 d | |||
| P1 | NAMF | 69.26 e | 61.20 d | 52.04 d | 4.84 f | 3.45 e | 1.61 e | ||
| AMF | 60.22 g | 53.94 f | 40.43 h | 5.74 c | 4.45 b | 2.51 b | |||
| P2 | NAMF | 81.14 b | 70.10 b | 56.13 c | 5.59 cd | 4.26 c | 2.03 d | ||
| AMF | 69.93 e | 61.05 d | 48.84 e | 6.79 a | 5.27 a | 3.15 a | |||
| DS | P0 | NAMF | 63.55 f | 55.77 ef | 47.12 ef | 3.01 h | 1.69 i | 0.94 g | |
| AMF | 59.11 g | 50.48 g | 43.98 g | 4.12 g | 2.61 g | 1.52 e | |||
| P1 | NAMF | 77.64 c | 66.89 c | 58.37 b | 4.60 f | 2.61 g | 1.52 e | ||
| AMF | 68.99 e | 56.48 e | 45.69 fg | 5.63 c | 4.00 d | 2.22 c | |||
| P2 | NAMF | 89.20 a | 74.75 a | 63.13 a | 5.21 e | 3.54 e | 1.61 e | ||
| AMF | 74.88 d | 66.20 c | 53.28 b | 6.26 b | 4.30b c | 2.35 bc | |||
| ANOVA | W | p-value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| P | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |||
| A | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |||
| W × P | 0.121 | 0.006 | 0.003 | <0.001 | <0.001 | <0.001 | |||
| A × W | 0.137 | 0.177 | 0.457 | 0.37 | 0.114 | 0.001 | |||
| A × P | <0.001 | 0.001 | <0.001 | 0.078 | <0.001 | 0.003 | |||
| W × A ×P | 0.225 | 0.104 | 0.022 | 0.226 | 0.001 | 0.18 | |||
| Year | Treatments | Total N (g kg−1) | Total P (g kg−1) | |||||
|---|---|---|---|---|---|---|---|---|
| Jointing Stage | Filling Stage | Maturity Stage | Jointing Stage | Filling Stage | Maturity Stage | |||
| 2021 | Water treatment | WW | 12.86 a | 8.57 a | 5.39 a | 4.46 a | 3.58 a | 2.81 a |
| DS | 12.41 a | 8.20 a | 5.17 a | 2.73 b | 2.36 b | 1.99 b | ||
| p-value | 0.685 | 0.651 | 0.707 | <0.001 | <0.001 | <0.001 | ||
| F | ns | ns | ns | 75.77 *** | 107.62 *** | 42.81 *** | ||
| P treatment | P0 | 10.41 b | 6.42 c | 4.11 b | 3.28 a | 2.80 a | 2.19 a | |
| P1 | 14.61 a | 10.16 a | 6.78 a | 3.76 a | 3.05 a | 2.46 a | ||
| P2 | 12.89 a | 5.56 b | 4.96 b | 3.74 a | 3.06 a | 2.54 a | ||
| p-value | 0.002 | <0.001 | <0.001 | 0.571 | 0.688 | 0.370 | ||
| F | 8.86 ** | 18.83 *** | 22.89 *** | ns | ns | ns | ||
| AMF treatment | NAMF | 10.80 b | 7.27 b | 4.61 b | 3.31 a | 2.78 a | 2.22 b | |
| AMF | 14.47 a | 9.50 a | 5.95 a | 3.88 a | 3.15 a | 2.58 a | ||
| p-value | <0.001 | 0.003 | 0.013 | 0.168 | 0.193 | 0.084 | ||
| F | 24.02 *** | 11.35 ** | 7.25 * | ns | ns | ns | ||
| 2022 | Water treatment | WW | 20.48 a | 15.99 a | 9.48 a | 4.12 a | 3.02 a | 2.18 a |
| DS | 18.54 b | 15.36 a | 9.62 a | 2.59 b | 2.22 b | 1.71 b | ||
| p-value | 0.013 | 0.466 | 0.838 | <0.001 | <0.001 | <0.001 | ||
| F | 7.27 * | ns | ns | 74.05 *** | 31.89 *** | 35.91 *** | ||
| P treatment | P0 | 17.90 b | 14.80 a | 8.40 b | 3.09 a | 2.47 a | 1.80 a | |
| P1 | 20.22 a | 16.09 a | 10.36 a | 3.48 a | 2.71 a | 2.03 a | ||
| P2 | 20.41 a | 16.14 a | 9.88 ab | 3.49 a | 2.68 a | 2.00 a | ||
| p-value | 0.012 | 0.358 | 0.043 | 0.610 | 0.621 | 0.273 | ||
| F | 5.49 * | ns | 3.67 * | ns | ns | ns | ||
| AMF treatment | NAMF | 18.62 b | 13.84 b | 8.30 b | 3.01 b | 2.32 b | 1.81 b | |
| AMF | 20.40 a | 17.52 a | 10.79 a | 3.70 a | 2.92 a | 2.08 a | ||
| p-value | 0.025 | <0.001 | <0.001 | 0.056 | 0.003 | 0.030 | ||
| F | 5.75 * | 99.82 *** | 29.43 *** | ns | 10.82 ** | 5.38 * | ||
| Year | Water Treatment | P Treatment | AMF Treatment | Total N (g kg−1) | Total P (g kg−1) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Jointing Stage | Filling Stage | Maturity Stage | Jointing Stage | Filling Stage | Maturity Stage | ||||
| 2021 | WW | P0 | NAMF | 8.59g | 5.39 h | 3.63 f | 3.67 d | 3.18 d | 2.28 d |
| AMF | 12.45 d | 8.06 e | 4.71 e | 4.11 c | 3.43 c | 2.62 c | |||
| P1 | NAMF | 12.83 d | 9.14 cd | 5.80 b | 4.19 c | 3.42 c | 2.68 c | ||
| AMF | 16.79 a | 11.41 a | 7.95 a | 5.14 b | 3.89 b | 3.06 b | |||
| P2 | NAMF | 11.55 ef | 7.67 f | 4.73 e | 4.13 c | 3.34 c | 2.69 c | ||
| AMF | 14.94 b | 9.47 b | 5.52 c | 5.48 a | 4.19 a | 3.51 a | |||
| DS | P0 | NAMF | 8.40 g | 5.06 h | 3.46 f | 2.49 h | 2.08 h | 1.71 g | |
| AMF | 12.20 de | 7.17 g | 4.61 e | 2.830 f | 2.49 ef | 2.14 e | |||
| P1 | NAMF | 12.39 d | 8.98 d | 5.55 c | 2.71g | 2.33 g | 1.95 f | ||
| AMF | 16.41 a | 11.11 a | 7.81 a | 3.01 e | 2.58 e | 2.15 e | |||
| P2 | NAMF | 11.02 f | 7.36 fg | 4.51 e | 2.65 g | 2.37 fg | 1.98 f | ||
| AMF | 14.06 c | 9.49 bc | 5.10 d | 2.69 g | 2.34 g | 1.99 f | |||
| ANOVA | W | p-value | 0.003 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| P | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |||
| A | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |||
| W × P | 0.278 | 0.063 | 0.18 | <0.001 | <0.001 | <0.001 | |||
| A × W | 0.626 | 0.121 | 0.94 | <0.001 | <0.001 | <0.001 | |||
| A × P | 0.047 | 0.206 | <0.001 | <0.001 | 0.261 | 0.05 | |||
| W × A ×P | 0.771 | 0.159 | 0.246 | <0.001 | <0.001 | <0.001 | |||
| 2022 | WW | P0 | NAMF | 17.11 f | 13.36 f | 6.97 h | 3.54 d | 2.50 d | 2.04 cd |
| AMF | 20.38 c | 16.94 cd | 9.86 e | 4.08 b | 3.23 b | 2.18 b | |||
| P1 | NAMF | 19.39 de | 13.69 f | 8.19 g | 3.77 cd | 2.79 c | 2.15 b | ||
| AMF | 22.61 b | 18.39 b | 10.96 bc | 4.77 a | 3.47 a | 2.32 a | |||
| P2 | NAMF | 19.80 cd | 14.60 e | 8.84 f | 3.78 c | 2.68 c | 2.09 bc | ||
| AMF | 23.58 a | 18.98 a | 12.04 a | 4.78 a | 3.47 a | 2.31 a | |||
| DS | P0 | NAMF | 16.58 f | 12.52 g | 6.94 h | 2.20 g | 1.89 g | 1.30 f | |
| AMF | 17.24 f | 16.38 d | 9.81 e | 2.54 f | 2.25 e | 1.68 e | |||
| P1 | NAMF | 19.47 de | 14.98 e | 10.69 cd | 2.36f g | 1.98 fg | 1.62 e | ||
| AMF | 19.40 de | 17.29 c | 11.60 b | 3.02 e | 2.62 cd | 2.04 cd | |||
| P2 | NAMF | 19.08 e | 13.87 f | 8.19 g | 2.39 fg | 2.10 ef | 1.67 e | ||
| AMF | 19.18 e | 17.12 c | 10.49 d | 2.99 e | 2.50 d | 1.94 d | |||
| ANOVA | W | p-value | <0.001 | <0.001 | 0.018 | <0.001 | <0.001 | <0.001 | |
| P | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |||
| A | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |||
| W × P | 0.003 | <0.001 | <0.001 | 0.29 | 0.761 | <0.001 | |||
| A × W | <0.001 | <0.001 | <0.001 | 0.003 | 0.001 | <0.001 | |||
| A × P | 0.34 | 0.44 | <0.001 | 0.004 | 0.307 | 0.563 | |||
| W × A ×P | 0.293 | <0.001 | <0.001 | 0.61 | 0.049 | 0.067 | |||
| Year | Soil Index | WW | DS | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Importance Ranking | Relative Contribution Rate (%) | F | p-Value | Importance Ranking | Relative Contribution Rate (%) | F | p-Value | ||
| 2021 | MBP | 1 | 88.7 | 114 | 0.002 | 1 | 77.5 | 49.9 | 0.002 |
| Ammonium N | 2 | 4.2 | 7.6 | 0.002 | 3 | 1.5 | 7.4 | 0.01 | |
| Nitrate N | 4 | 1.5 | 3.2 | 0.046 | 2 | 20.5 | 70.3 | 0.002 | |
| Total P | 5 | 1.4 | 15.0 | 0.002 | 4 | 0.4 | 2.4 | 0.106 | |
| Available P | 3 | 4.1 | 20.7 | 0.002 | 5 | <0.1 | 0.3 | 0.764 | |
| 2022 | MBP | 1 | 56.2 | 19.9 | 0.002 | 1 | 77.4 | 46.7 | 0.002 |
| Ammonium N | 5 | 0.8 | 6.6 | 0.004 | 3 | 5.0 | 9.6 | 0.004 | |
| Nitrate N | 4 | 1.0 | 6.0 | 0.006 | 4 | 2.4 | 6.5 | 0.008 | |
| Total P | 2 | 32.1 | 36.7 | 0.002 | 5 | 1.0 | 2.9 | 0.104 | |
| Available P | 3 | 10.0 | 44.5 | 0.002 | 2 | 14.3 | 17.4 | 0.002 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, B.; Li, X.; Bao, J.; Tian, Z.; Zhang, F.; Zhang, M. A Combined Strategy Using Funneliformis mosseae and Phosphorus Addition for Enhancing Oat Drought Tolerance. Agronomy 2025, 15, 2033. https://doi.org/10.3390/agronomy15092033
Zhang B, Li X, Bao J, Tian Z, Zhang F, Zhang M. A Combined Strategy Using Funneliformis mosseae and Phosphorus Addition for Enhancing Oat Drought Tolerance. Agronomy. 2025; 15(9):2033. https://doi.org/10.3390/agronomy15092033
Chicago/Turabian StyleZhang, Bin, Xueqin Li, Jieyu Bao, Ziming Tian, Fusuo Zhang, and Meijun Zhang. 2025. "A Combined Strategy Using Funneliformis mosseae and Phosphorus Addition for Enhancing Oat Drought Tolerance" Agronomy 15, no. 9: 2033. https://doi.org/10.3390/agronomy15092033
APA StyleZhang, B., Li, X., Bao, J., Tian, Z., Zhang, F., & Zhang, M. (2025). A Combined Strategy Using Funneliformis mosseae and Phosphorus Addition for Enhancing Oat Drought Tolerance. Agronomy, 15(9), 2033. https://doi.org/10.3390/agronomy15092033






