Adaptive Plasticity of Phragmites australis in Aboveground and Belowground Productivity Under Salinization and Nitrogen Enrichment
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Mesocosm Experiment
2.3. Data Analysis
3. Results
3.1. Effects of Salinity and Nitrogen Enrichment Level on Height of P. australis
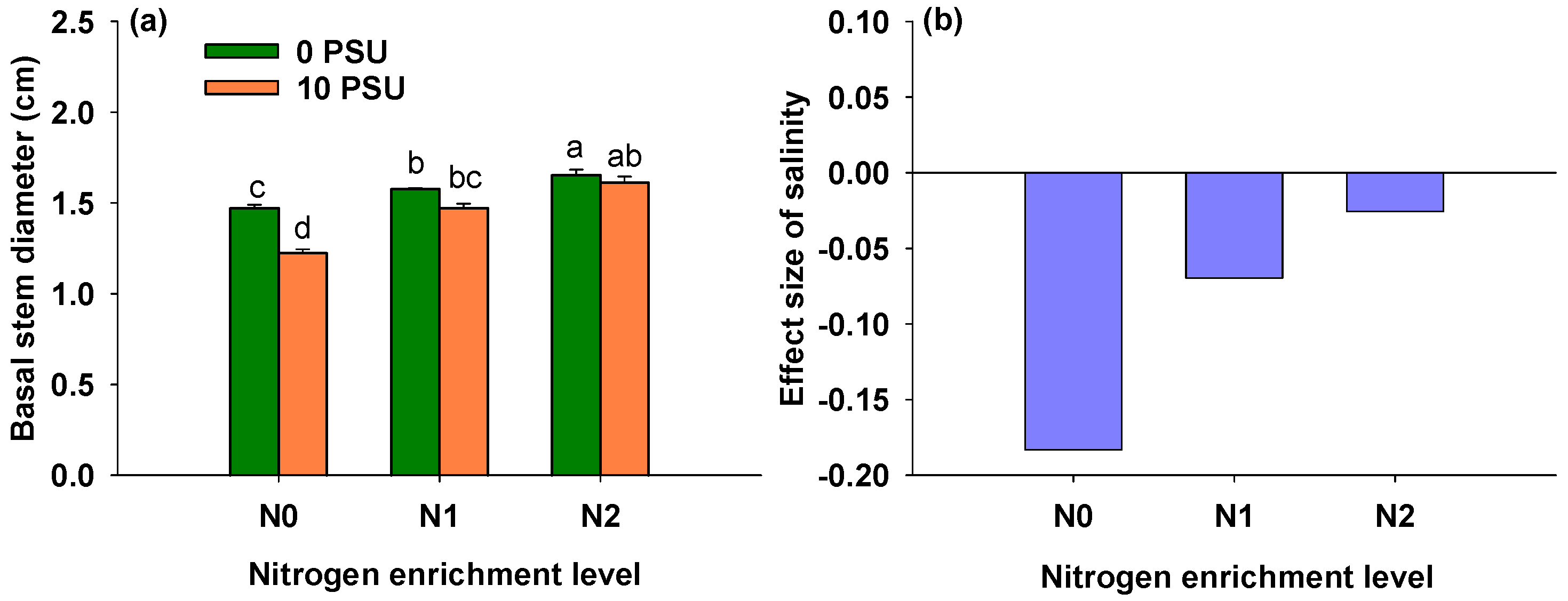
3.2. Effects of Salinity and Nitrogen Enrichment Level on Basal Stem Diameter of P. australis
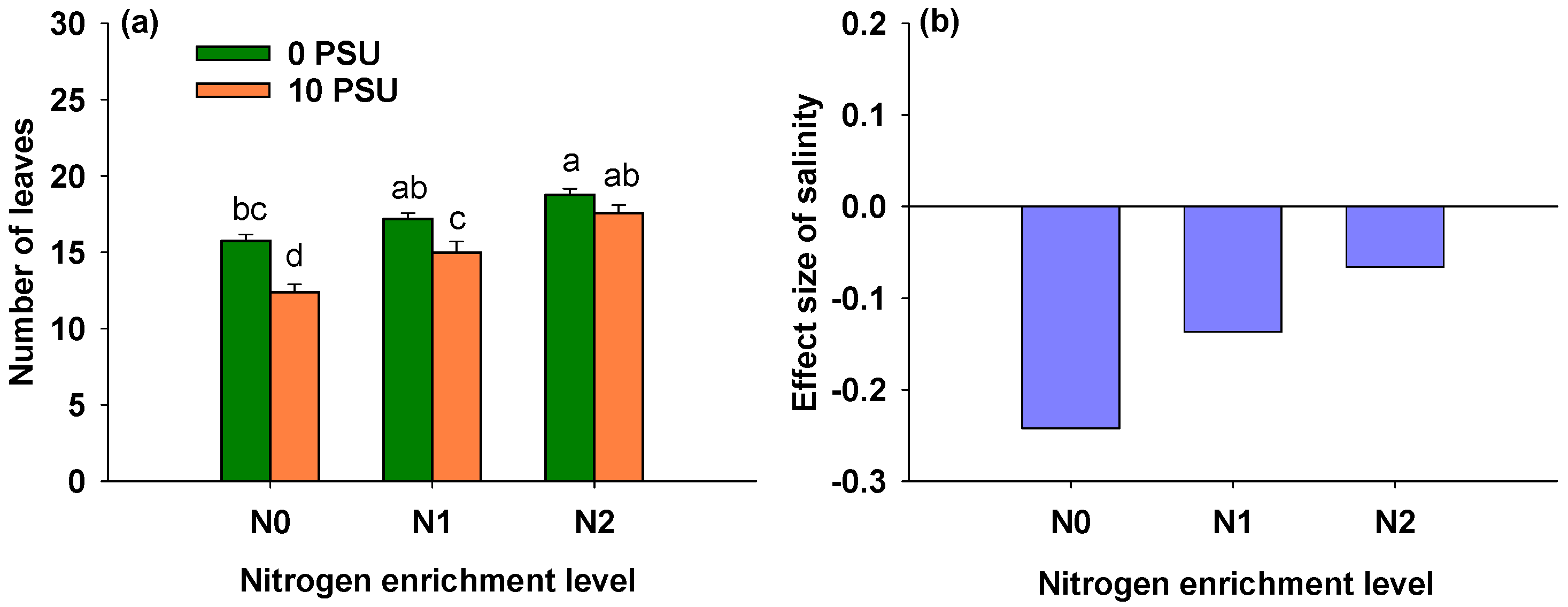
3.3. Effects of Salinity and Nitrogen Enrichment Level on Number of Leaves of P. australis
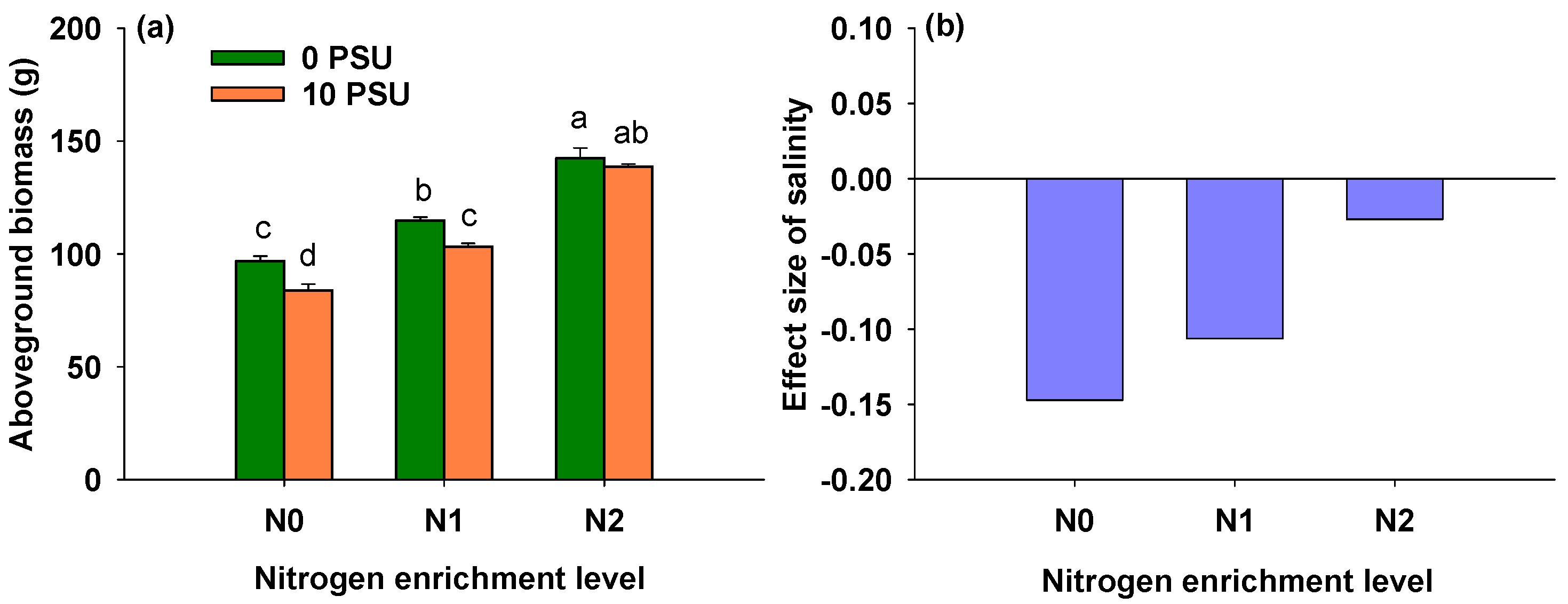
3.4. Effects of Salinity and Nitrogen Enrichment Level on Aboveground Biomass of P. australis
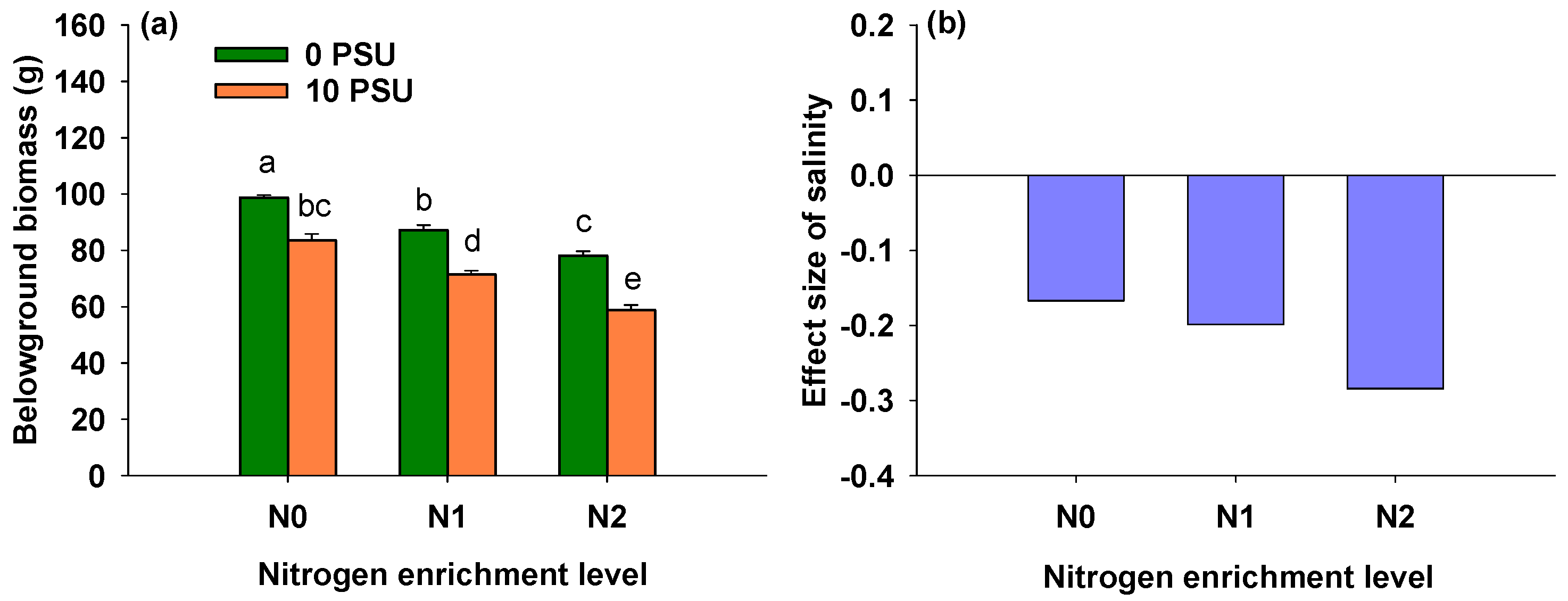
3.5. Effects of Salinity and Nitrogen Enrichment Level on Belowground Biomass of P. australis
3.6. Effects of Salinity and Nitrogen Enrichment Level on Ratio of Belowground to Aboveground Biomass of P. australis
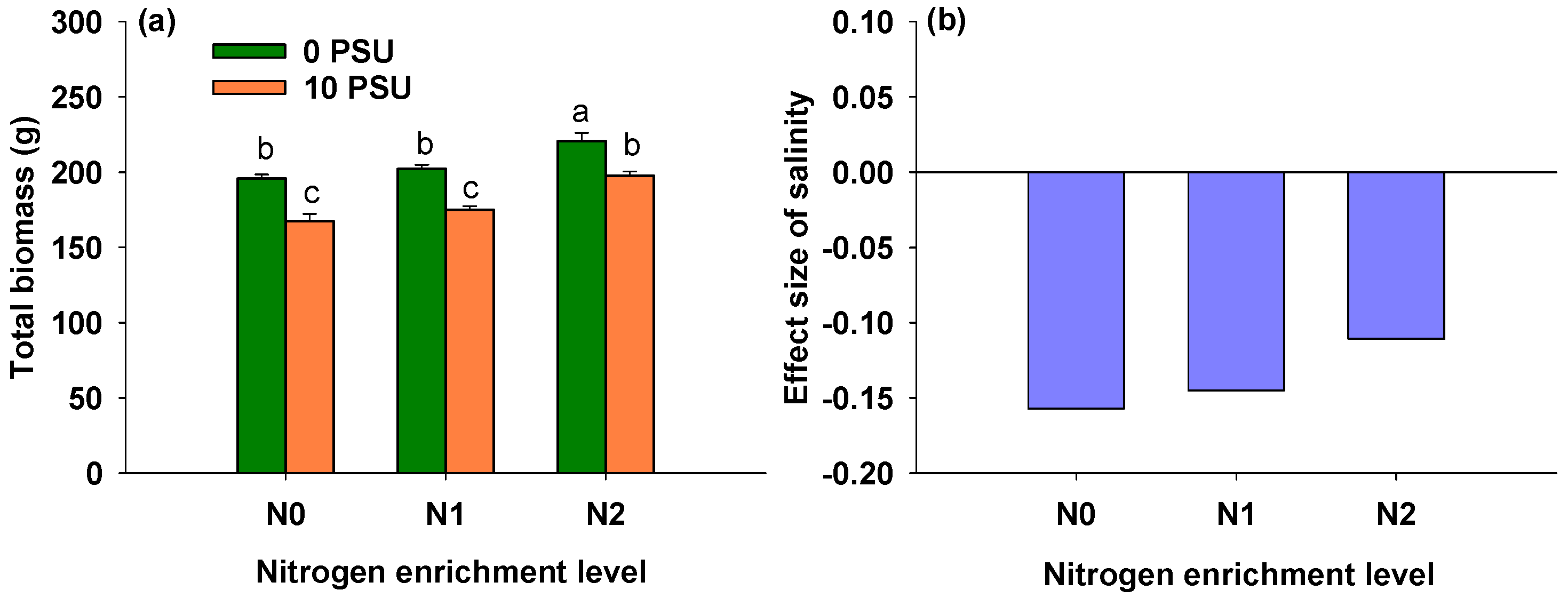
3.7. Effects of Salinity and Nitrogen Enrichment Level on Total Biomass of P. australis
4. Discussion
4.1. Aboveground Growth of P. australis Under Different Salinity and Nitrogen Enrichment Conditions
4.2. Belowground Biomass of P. australis Under Different Salinity and Nitrogen Enrichment Conditions
4.3. Ratio of Belowground to Aboveground Biomass of P. australis Under Different Salinity and Nitrogen Enrichment Conditions
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bloomfield, J.A.; Rose, T.J.; King, G.J. Sustainable harvest: Managing plasticity for resilient crops. Plant Biotechnol. J. 2014, 12, 517–533. [Google Scholar] [CrossRef]
- Milke, J.; Galczynska, M.; Wróbel, J. The importance of biological and ecological properties of Phragmites australis (cav.) Trin. ex Steud., in phytoremendiation of aquatic ecosystems-the review. Water 2020, 12, 1770. [Google Scholar] [CrossRef]
- Cízková, H.; Kucera, T.; Poulin, B.; Kvet, J. Ecological basis of ecosystem services and management of wetlands dominated by common reed (Phragmites australis): European perspective. Diversity 2023, 15, 629. [Google Scholar] [CrossRef]
- Jakubaszek, A. Nitrogen and phosphorus accumulation in horizontal subsurface flow constructed wetland. Agronomy 2021, 11, 1317. [Google Scholar] [CrossRef]
- Currin, C.A.; Wainright, S.C.; Able, K.W.; Weinstein, M.P.; Fuller, C.M. Determination of food web support and trophic position of the mummichog, Fundulus heteroclitus, in New Jersey smooth cordgrass (Spartina alterniflora), common reed (Phragmites australis), and restored salt marshes. Estuaries 2003, 26, 495–510. [Google Scholar] [CrossRef]
- Li, X.Y.; Jin, K.; Qin, P.; Liu, C.X.; Zhu, X.Z.; Zhang, Y.Y.; Zong, Q.L. Enhancement effect of Phragmites australis roots on soil shear strength in the Yellow River Delta. Sustainability 2024, 16, 10657. [Google Scholar] [CrossRef]
- Zhang, W.; Ge, Z.M.; Li, S.H.; Tan, L.S.; Zhou, K.; Li, Y.L.; Xie, L.N.; Dai, Z.J. The role of seasonal vegetation properties in determining the wave attenuation capacity of coastal marshes: Implications for building natural defenses. Ecol. Eng. 2022, 175, 106494. [Google Scholar] [CrossRef]
- Gao, X.Q.; Bi, Y.X.; Su, L.; Lei, Y.; Gong, L.; Dong, X.H.; Li, X.Z.; Yan, Z.Z. Unveiling the nitrogen and phosphorus removal potential: Comparative analysis of three coastal wetland plant species in lab-scale constructed wetlands. J. Environ. Manag. 2024, 351, 119864. [Google Scholar] [CrossRef]
- Whitaker, K.; Rogers, K.; Saintilan, N.; Mazumder, D.; Wen, L.; Morrison, R.J. Vegetation persistence and carbon storage: Implications for environmental water management for Phragmites australis. Water Resour. Res. 2015, 51, 5284–5300. [Google Scholar] [CrossRef]
- Silan, G.; Buosi, A.; Bertolini, C.; Sfriso, A. Dynamics and drivers of carbon sequestration and storage capacity in Phragmites australis-dominated wetlands. Estuar. Coast. Shelf Sci. 2024, 298, 108640. [Google Scholar] [CrossRef]
- Wang, X.H.; Yu, J.; Zhou, D.; Dong, H.F.; Li, Y.Z.; Lin, Q.X.; Guan, B.; Wang, Y.L. Vegetative ecological characteristics of restored reed (Phragmites australis) wetlands in the Yellow River Delta, China. Environ. Manag. 2012, 49, 325–333. [Google Scholar] [CrossRef]
- Chen, G.Z.; Bai, J.H.; Yu, L.; Wang, W.; Wang, Y.Q.; Qiu, J.C.; Cui, B.S. Phragmites australis straw and biochar additives regulate soil organic carbon fractions in a degraded coastal salt marsh. Ecol. Eng. 2024, 206, 107328. [Google Scholar] [CrossRef]
- Shi, C.Q.; Li, Y.; Yu, S.P.; Hu, B.Z.; Jin, L. Fluorescence spectral characteristics of soil dissolved organic matter in different plant formations after reverting farmland to wetland. Spectrosc. Spectr. Anal. 2020, 40, 3472–3476. [Google Scholar]
- Kankiliç, G.B.; Metin, A. Phragmites australis as a new cellulose source: Extraction, characterization and adsorption of methylene blue. J. Mol. Liq. 2020, 312, 113313. [Google Scholar] [CrossRef]
- Köbbing, J.F.; Beckmann, V.; Thevs, N.; Peng, H.; Zerbe, S. Investigation of a traditional reed economy (Phragmites australis) under threat: Pulp and paper market, values and netchain at Wuliangsuhai Lake, Inner Mongolia, China. Wetl. Ecol. Manag. 2016, 24, 357–371. [Google Scholar] [CrossRef]
- Machaka, M.; Khatib, J.; Baydoun, S.; Elkordi, A.; Assaad, J.J. The effect of adding Phragmites australis fibers on the properties of concrete. Buildings 2022, 12, 278. [Google Scholar] [CrossRef]
- Baran, M.; Váradyová, Z.; Krácmar, S.; Hedbávny, J. The common reed (Phragmites australis) as a source of roughage in ruminant nutrition. Acta Vet. Brno 2002, 71, 445–449. [Google Scholar] [CrossRef]
- Vazic, T.; Svircek, Z.; Dulic, T.; Krstic, K.; Obreht, I. Potential for energy production from reed biomass in the Vojvodina region (north Serbia). Renew. Sustain. Energy Rev. 2015, 48, 670–680. [Google Scholar] [CrossRef]
- Patuzzi, F.; Mimmo, T.; Cesco, S.; Gasparella, A.; Baratieri, M. Common reeds (Phragmites australis) as sustainable energy source: Experimental and modelling analysis of torrefaction and pyrolysis processes. Glob. Change Biol. Bioenergy 2013, 5, 367–374. [Google Scholar] [CrossRef]
- Hay, C.C.; Morrow, E.; Kopp, R.E.; Mitrovica, J.X. Probabilistic reanalysis of twentieth-century sea-level rise. Nature 2015, 517, 481–484. [Google Scholar] [CrossRef]
- Nevermann, H.; AghaKouchak, A.; Shokri, N. Sea level rise implications on future inland migration of coastal wetlands. Glob. Ecol. Conserv. 2023, 43, e02421. [Google Scholar] [CrossRef]
- IPCC. Summary for policymakers. In Climate Change 2023; IPCC: Geneva, Switzerland, 2023. [Google Scholar]
- Talke, S.A.; Jay, D.A. Changing tides: The role of natural and anthropogenic factors. Annu. Rev. Mar. Sci. 2020, 12, 121–151. [Google Scholar] [CrossRef] [PubMed]
- Solohin, E.; Widney, S.E.; Craft, C.B. Declines in plant productivity drive loss of soil elevation in a tidal freshwater marsh exposed to saltwater intrusion. Ecology 2020, 101, e03148. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Biemond, B.; van Keulen, D.; Huismans, Y.; van Westen, R.M.; de Swart, H.E.; Dijkstra, H.A.; Kranenburg, W.M. Global increases of salt intrusion in estuaries under future environmental conditions. Nat. Commun. 2025, 16, 3444. [Google Scholar] [CrossRef]
- Li, F.; Pennings, S.C. Response and recovery of low-salinity marsh plant communities to presses and pulses of elevated salinity. Estuaries Coasts 2019, 42, 708–718. [Google Scholar] [CrossRef]
- Jesus, J.; Castro, F.; Niemelä, A.; Borges, M.T.; Danko, A.S. Evaluation of the impact of different soil salinization processes on organic and mineral soils. Water Air Soil Pollut. 2015, 226, 102. [Google Scholar] [CrossRef]
- Shokri, N.; Hassani, A.; Sahimi, M. Multi-scale soil salinization dynamics from global to pore scale: A review. Rev. Geophys. 2024, 62, e2023RG000804. [Google Scholar] [CrossRef]
- Stevens, C.J. Nitrogen in the environment. Science 2019, 363, 578–580. [Google Scholar] [CrossRef]
- Gruber, N.; Galloway, J.N. An Earth-system perspective of the global nitrogen cycle. Nature 2008, 451, 293–296. [Google Scholar] [CrossRef]
- Sun, Q.H.; Li, J.N.; Zhou, C.H.; Lei, K.; Jiang, W.J. Source analysis of nitrogen pollution in basin by export coefficient modeling and microbial source tracking with mutual verification. Front. Mar. Sci. 2025, 12, 1527098. [Google Scholar] [CrossRef]
- Deng, O.P.; Huang, S.; Wang, C.; Wei, Y.C.; Xia, Y.Q.; Liu, Z.H.; Zhang, X.M.; Xiao, W.; He, T.T.; Wu, X.B.; et al. Atmospheric nitrogen pollution control benefits the coastal environment. Environ. Sci. Technol. 2023, 58, 449–458. [Google Scholar] [CrossRef]
- Duarte, C.M.; Krause-Jensen, D. Intervention options to accelerate ecosystem recovery from coastal eutrophication. Front. Mar. Sci. 2018, 5, 470. [Google Scholar] [CrossRef]
- Rabalais, N.N.; Turner, R.E.; Díaz, R.J.; Justic, D. Global change and eutrophication of coastal waters. Ices J. Mar. Sci. 2009, 66, 1528–1537. [Google Scholar] [CrossRef]
- Johnson, D.S.; Warren, R.S.; Deegan, L.A.; Mozdzer, T.J. Saltmarsh plant responses to eutrophication. Ecol. Appl. 2016, 26, 2647–2659. [Google Scholar] [CrossRef]
- White, J.R.; DeLaune, R.D.; Justic, D.; Day, J.W.; Pahl, J.; Lane, R.R.; Boynton, W.R.; Twilley, R.R. Consequences of Mississippi River diversions on nutrient dynamics of coastal wetland soils and estuarine sediments: A review. Estuar. Coast. Shelf Sci. 2019, 224, 209–216. [Google Scholar] [CrossRef]
- Goodwillie, C.; McCoy, M.W.; Peralta, A.L. Long-term nutrient enrichment, mowing, and ditch drainage interact in the dynamics of a wetland plant community. Ecosphere 2020, 11, e03252. [Google Scholar] [CrossRef]
- Smith, V.H. Eutrophication of freshwater and coastal marine ecosystems—A global problem. Environ. Sci. Pollut. Res. 2003, 10, 126–139. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.T.; Khan, I.; Chattha, M.U.; Maqbool, R.; Ziaulhaq, M.; Lihong, W.; Usman, S.; Rasheed, A.; Hassan, M.U.; Hashem, M.; et al. The critical role of nitrogen in plants facing the salinity stress: Review and future prospective. Not. Bot. Horti Agrobot. Cluj-Napoca 2023, 51, 13347. [Google Scholar] [CrossRef]
- Maatallah, M.; Zribi, O.T.; Salhi, M.; Abdelly, C.; Barhoumi, Z. Combined effects of salinity and nitrogen levels on some physiological and biochemical aspects at the halophytic forage legume Sulla carnosa. Arch. Agron. Soil Sci. 2023, 69, 119–134. [Google Scholar] [CrossRef]
- Guo, H.; Gao, F.; Pang, J.; Wang, H.; Wang, H.; Wang, Y.; Whitt, A.A.; Ma, C. Plant-plant interactions of Phragmites australis and Suaeda salsa as mediated by combined influences of salinity and tidal level changes. Plant Soil 2022, 474, 141–161. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, L.; Yi, H.; Lan, S.; Chen, L.; Han, G. Nitrogen addition alters plant growth in China’s Yellow River Delta coastal wetland through direct and indirect effects. Front. Plant Sci. 2022, 13, 1016949. [Google Scholar] [CrossRef]
- Fu, H.Q.; Yang, Y.Q. How plants tolerate salt stress. Curr. Issues Mol. Biol. 2023, 45, 5914–5934. [Google Scholar] [CrossRef]
- Negrao, S.; Schmöckel, S.M.; Tester, M. Evaluating physiological responses of plants to salinity stress. Ann. Bot. 2017, 119, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.J.; Ma, X.L.; Wan, P.; Liu, L.Y. Plant salt-tolerance mechanism: A review. Biochem. Biophys. Res. Commun. 2018, 495, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Katuwal, K.B.; Xiao, B.; Jespersen, D. Physiological responses and tolerance mechanisms of seashore paspalum and centipedegrass exposed to osmotic and iso-osmotic salt stresses. J. Plant Physiol. 2020, 248, 153154. [Google Scholar] [CrossRef] [PubMed]
- Kesawat, M.S.; Satheesh, N.; Kherawat, B.S.; Kumar, A.; Kim, H.U.; Chung, S.M.; Kumar, M. Regulation of reactive oxygen species during salt stress in plants and their crosstalk with other signaling molecules-current perspectives and future directions. Plants 2023, 12, 864. [Google Scholar] [CrossRef]
- Muchate, N.S.; Nikalje, G.C.; Rajurkar, N.S.; Suprasanna, P.; Nikam, T.D. Plant salt stress: Adaptive responses, tolerance mechanism and bioengineering for salt tolerance. Bot. Rev. 2016, 82, 371–406. [Google Scholar] [CrossRef]
- Wang, Q.; Li, S.S.; Li, J.R.; Huang, D. The utilization and roles of nitrogen in plants. Forests 2024, 15, 1191. [Google Scholar] [CrossRef]
- Farhan, M.; Sathish, M.; Kiran, R.; Mushtaq, A.; Baazeem, A.; Hasnain, A.; Hakim, F.; Naqvi, S.A.H.; Mubeen, M.; Iftikhar, Y.; et al. Plant nitrogen metabolism: Balancing resilience to nutritional stress and abiotic challenges. Phyton-Int. J. Exp. Bot. 2024, 93, 581–609. [Google Scholar] [CrossRef]
- Iqbal, N.; Umar, S.; Khan, N.A. Nitrogen availability regulates proline and ethylene production and alleviates salinity stress in mustard (Brassica juncea). J. Plant Physiol. 2015, 178, 84–91. [Google Scholar] [CrossRef]
- Tomar, R.S.; Kataria, S.; Jajoo, A. Behind the scene: Critical role of reactive oxygen species and reactive nitrogen species in salt stress tolerance. J. Agron. Crop Sci. 2021, 207, 577–588. [Google Scholar] [CrossRef]
- Hao, S.H.; Wang, Y.R.; Yan, Y.X.; Liu, Y.H.; Wang, J.Y.; Chen, S. A review on plant responses to salt stress and their mechanisms of salt resistance. Horticulturae 2021, 7, 132. [Google Scholar] [CrossRef]
- Wang, T.S.; Liu, L.N.; Zuo, Q.; Wu, X.; Xu, Y.Q.; Shi, J.C.; Sheng, J.D.; Jiang, P.G.; Ben-Gal, A. Characterizing the hysteretic effects of water and salinity stresses on root-water-uptake. Agric. Water Manag. 2024, 305, 109121. [Google Scholar] [CrossRef]
- Hu, X.F.; Wang, D.; Ren, S.; Feng, S.; Zhang, H.Z.; Zhang, J.Z.; Qiao, K.; Zhou, A.M. Inhibition of root growth by alkaline salts due to disturbed ion transport and accumulation in Leymus chinensis. Environ. Exp. Bot. 2022, 200, 104907. [Google Scholar] [CrossRef]
- Karahara, I.; Horie, T. Functions and structure of roots and their contributions to salinity tolerance in plants. Breed. Sci. 2021, 71, 89–108. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Zhang, X.L.; Liu, J.M.; Gao, X.D.; Bai, J.; Hao, Y.L.; Cui, H.C. A mechanism coordinating root elongation, endodermal differentiation, redox homeostasis and stress response. Plant J. 2021, 107, 1029–1039. [Google Scholar] [CrossRef]
- Ahmad, N.; Jiang, Z.J.; Zhang, L.J.; Hussain, I.; Yang, X.P. Insights on phytohormonal crosstalk in plant response to nitrogen stress: A focus on plant root growth and development. Int. J. Mol. Sci. 2023, 24, 3631. [Google Scholar] [CrossRef]
- Newsholme, P.; Rebelato, E.; Abdulkader, F.; Krause, M.; Carpinelli, A.; Curi, R. Reactive oxygen and nitrogen species generation, antioxidant defenses, and β-cell function: A critical role for amino acids. J. Endocrinol. 2012, 214, 11–20. [Google Scholar] [CrossRef]
- Tsukagoshi, H. Defective root growth triggered by oxidative stress is controlled through the expression of cell cycle-related genes. Plant Sci. 2012, 197, 30–39. [Google Scholar] [CrossRef]
- Walch-Liu, P.; Ivanov, I.I.; Filleur, S.; Gan, Y.B.; Remans, T.; Forde, B.G. Nitrogen regulation of root branching. Ann. Bot. 2006, 97, 875–881. [Google Scholar] [CrossRef]
- Huang, F.Q.; Peñuelas, J.; Sardans, J.; Collins, S.L.; Yu, K.L.; Liu, M.Q.; Pei, J.Y.; Ke, W.B.; Ye, J.S. Plant use of water across soil depths regulates species dominance under nitrogen addition. Plant Divers. 2025, 47, 479–488. [Google Scholar] [CrossRef]
- Mazhar, S.; Pellegrini, E.; Contin, M.; Bravo, C.; De Nobili, M. Impacts of salinization caused by sea level rise on the biological processes of coastal soils—A review. Front. Environ. Sci. 2022, 10, 909415. [Google Scholar] [CrossRef]
- Howarth, R.W. Coastal nitrogen pollution: A review of sources and trends globally and regionally. Harmful Algae 2008, 8, 14–20. [Google Scholar] [CrossRef]


| Source of Variance | df | F | p |
|---|---|---|---|
| Height of P. australis | |||
| Salinity | 1, 24 | 68.842 | <0.001 *** |
| Nitrogen enrichment level | 2, 24 | 87.094 | <0.001 *** |
| Salinity × Nitrogen enrichment level | 2, 24 | 6.073 | 0.007 ** |
| Basal stem diameter of P. australis | |||
| Salinity | 1, 24 | 48.276 | <0.001 *** |
| Nitrogen enrichment level | 2, 24 | 73.835 | <0.001 *** |
| Salinity × Nitrogen enrichment level | 2, 24 | 11.707 | <0.001 *** |
| Number of leaves of P. australis | |||
| Salinity | 1, 24 | 33.266 | <0.001 *** |
| Nitrogen enrichment level | 2, 24 | 33.510 | <0.001 *** |
| Salinity × Nitrogen enrichment level | 2, 24 | 3.882 | 0.035 * |
| Aboveground biomass of P. australis | |||
| Salinity | 1, 24 | 24.685 | <0.001 *** |
| Nitrogen enrichment level | 2, 24 | 188.146 | <0.001 *** |
| Salinity × Nitrogen enrichment level | 2, 24 | 3.629 | 0.042 * |
| Belowground biomass of P. australis | |||
| Salinity | 1, 24 | 150.181 | <0.001 *** |
| Nitrogen enrichment level | 2, 24 | 90.532 | <0.001 *** |
| Salinity × Nitrogen enrichment level | 2, 24 | 3.923 | 0.034 * |
| Ratio of belowground to aboveground biomass | |||
| Salinity | 1, 24 | 58.096 | <0.001 *** |
| Nitrogen enrichment level | 2, 24 | 686.846 | <0.001 *** |
| Salinity × Nitrogen enrichment level | 2, 24 | 19.290 | <0.001 *** |
| Total biomass of P. australis | |||
| Salinity | 1, 24 | 73.858 | <0.001 *** |
| Nitrogen enrichment level | 2, 24 | 27.634 | <0.001 *** |
| Salinity × Nitrogen enrichment level | 2, 24 | 0.787 | 0.467 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Tian, X.; Yang, C.; Guo, C.; Li, Y.; Lyu, X.; Li, N.; Guo, H. Adaptive Plasticity of Phragmites australis in Aboveground and Belowground Productivity Under Salinization and Nitrogen Enrichment. Agronomy 2025, 15, 2031. https://doi.org/10.3390/agronomy15092031
Wang Y, Tian X, Yang C, Guo C, Li Y, Lyu X, Li N, Guo H. Adaptive Plasticity of Phragmites australis in Aboveground and Belowground Productivity Under Salinization and Nitrogen Enrichment. Agronomy. 2025; 15(9):2031. https://doi.org/10.3390/agronomy15092031
Chicago/Turabian StyleWang, Yinhua, Xinyi Tian, Chen Yang, Changcheng Guo, Yifan Li, Xin Lyu, Ningning Li, and Hongyu Guo. 2025. "Adaptive Plasticity of Phragmites australis in Aboveground and Belowground Productivity Under Salinization and Nitrogen Enrichment" Agronomy 15, no. 9: 2031. https://doi.org/10.3390/agronomy15092031
APA StyleWang, Y., Tian, X., Yang, C., Guo, C., Li, Y., Lyu, X., Li, N., & Guo, H. (2025). Adaptive Plasticity of Phragmites australis in Aboveground and Belowground Productivity Under Salinization and Nitrogen Enrichment. Agronomy, 15(9), 2031. https://doi.org/10.3390/agronomy15092031






