Abstract
Biochar has garnered considerable attention as a soil amendment due to its unique physicochemical properties. Its application not only enhances soil carbon sequestration but also improves nutrient availability. Incorporating biochar into soil is regarded as a promising strategy for mitigating global climate change while delivering substantial environmental and agricultural benefits. In this study, biochar was extracted from Siraitia grosvenorii and subsequently modified through alkali treatment. A laboratory incubation experiment was conducted to assess the effects of unmodified (JMC) and modified (GXC) biochar, applied at different rates (1%, 2%, and 4%), on organic carbon mineralization and soil nutrient dynamics. Results indicated that, at equivalent application rates, JMC-treated soils exhibited lower CO2 emissions than those treated with GXC, with emissions increasing alongside biochar dosage. After the incubation, the 1% JMC treatment exhibited a mineralization rate of 17.3 mg·kg−1·d−1, which was lower than that of the control (CK, 18.8 mg·kg−1·d−1), suggesting that JMC effectively inhibited organic carbon mineralization and reduced CO2 emissions, thereby contributing positively to carbon sequestration in Siraitia grosvenorii farmland. In contrast, GXC application significantly enhanced soil nutrient levels, particularly increasing available phosphorus (AP) by 14.33% to 157.99%. Furthermore, partial least squares structural equation modeling (PLS-SEM) identified application rate and pH as the key direct factors influencing soil nutrient availability.
1. Introduction
Biochar is a carbon-rich material produced through pyrolysis of biomass under anaerobic or oxygen-limited conditions. It is characterized by high porosity and strong adsorption capacity. When applied to soil, biochar can enhance carbon sequestration and reduce CO2 emissions [1]. For instance, Ojeda et al. [2] reported that two types of biochar derived from pine exhibited significant CO2 adsorption capacity and promoted soil carbon storage. Similarly, Sultan et al. [3] found that wheat straw biochar was more effective in enhancing soil carbon storage. Converting biomass waste into biochar has been recognized as a strategy to reduce greenhouse gas emissions caused by biomass combustion and decomposition. It is estimated that large-scale biochar production could reduce current global greenhouse gas emissions by up to 12% [4].
In addition to carbon sequestration, biochar contains a variety of oxygen-containing functional groups, cations, and essential nutrients that contribute to improved soil fertility. For example, Bolan et al. [5] demonstrated that biochar significantly enhanced carbon storage and improved soil quality and fertility across different soil types and climates when used as a soil amendment. Chen et al. [6] also reported that biochar was more effective than traditional NPK fertilizers in increasing several soil quality parameters, including soil organic carbon, total nitrogen, available phosphorus, and pH.
In recent years, increasing attention has been paid to modified biochar due to its enhanced effects on soil properties and carbon sequestration compared to raw biochar. Biochar modification can be achieved through physical methods such as ball milling or chemical treatments involving acid or alkali impregnation [7]. Ghorbani et al. [8] reported that both acidic and alkaline modifiers used in biochar modification reduce the polarization of its structure. Moreover, biochar can enhance its effectiveness in carbon sequestration and soil fertility, indicating a potentially positive role in long-term carbon storage. Wang et al. [9] found that KOH-modified biochar performed significantly better than raw biochar in improving soil biochemical properties and reducing greenhouse gas emissions. Similarly, Liu et al. [10] prepared raw, acid-modified, and alkali-modified biochar from rice straw, and found that the alkali-modified biochar exhibited superior effects on increasing soil pH and organic carbon content. Kazem [11] also found that the application of chemically modified biochar (KOH-treated) significantly improved soil water-holding capacity and plant-available water content. Similarly, Hua et al. [12] reported that modified biochar markedly increased soil moisture content, microbial biomass carbon (MBC), microbial biomass nitrogen (MBN), and the MBC/MBN ratio, while also altering the relative abundance of dominant bacterial communities in the soil. These findings collectively provide theoretical support for the targeted regulation of soil health using modified biochar in agricultural production.
Senna obtusifolia is a widely cultivated medicinal plant in China, with Guangxi Province being one of the major production areas. However, its straw is often discarded directly, posing both environmental and resource-utilization challenges. Meanwhile, soils in Siraitia grosvenorii farmlands in Guangxi frequently suffer from continuous declines in organic carbon content, acidification, and poor soil fertility. Previous studies have shown that alkaline modification can increase the surface oxygen content and pH of biochar, dissolve ash components, and concentrate organic matter. Modified biochar generally exhibits greater adsorption capacity and more functional adsorption sites than unmodified biochar [13].
In this study, Senna obtusifolia was used as feedstock to produce raw and alkali-modified biochar. A 60-day soil incubation experiment was conducted to evaluate the effects of modified Senna obtusifolia biochar on soil organic carbon mineralization and carbon fractions. We hypothesize the following: (1) biochar application will inhibit CO2 emissions, and modified biochar will exhibit a stronger inhibitory effect than raw biochar; and (2) biochar will improve soil nutrient availability, with modified biochar showing more pronounced effect. The objectives of this study are as follows: (1) to investigate the effects of modified Senna obtusifolia biochar on soil nutrients, enzyme activities, and organic carbon fractions; and (2) to examine the interrelationships among soil nutrients, soil organic carbon mineralization, organic carbon fractions, and enzyme activities under the application of modified Senna obtusifolia biochar, with the aim of providing theoretical support for the dual role of enhancing soil organic carbon sequestration and improving nutrient availability in agricultural soils.
2. Materials and Methods
2.1. Experimental Materials
Soil samples were collected from the Siraitia grosvenorii high-yield cultivation demonstration site in Shengli Village, Baoli Town, Yongfu County, Guilin City, Guangxi Zhuang Autonomous Region, China (25°4′43″ N, 110°0′54″ E). Stones and visible plant and animal residues were removed from the soil, which was then air-dried, ground with a mortar and pestle, passed through 10-mesh and 60-mesh nylon sieves, and stored in sealed bags for subsequent analyses of physicochemical properties (Table 1).

Table 1.
Basic properties of the test soil.
Senna obtusifolia used in this study was collected from Yongfu County, Guilin City, Guangxi Zhuang Autonomous Region, China. The straw was air-dried, ground, and passed through a 0.25 mm sieve, then stored in sealed bags for further use. The preparation of Senna obtusifolia biochar (JMC) involved placing the straw powder into a crucible and pyrolyzing it under limited oxygen conditions at 500 °C for 2 h in a muffle furnace. For the preparation of modified Senna obtusifolia biochar (GXC), sodium carbonate (Na2CO3) and straw powder were mixed at a mass ratio of 1:1 in a beaker, followed by the addition of 100 mL deionized water. The mixture was stirred for 2 h, then filtered and dried in an oven at 60 °C for 24 h. Subsequently, the dried material was pyrolyzed under limited oxygen conditions at 500 °C for 2 h [14]. Both types of biochar were passed through a 0.25 mm sieve and stored in sealed bags for later use.
2.2. Experimental Design
The experiment was initiated in 2023 at Guangxi Normal University. A total of seven treatments were established, each with three replicates. Three biochar application rates (1%, 2%, and 4% by weight) were applied. Treatments were labeled based on biochar type and application rate: JMC1, JMC2, JMC4, GXC1, GXC2, and GXC4.
Air-dried soil (1.5 kg) was placed into 2 L polyethylene containers, and appropriate amounts of JMC or GXC were mixed thoroughly with the soil according to the treatment design. Deionized water was added to adjust and maintain soil moisture at 40% of field capacity using the gravimetric method. Samples were incubated at 25 °C for 60 days. Soil samples were collected on days 1, 3, 8, 15, 25, 40, and 60 for analysis. Soil samples were collected using a randomized sampling method and subsequently divided into two portions, each placed in a sealed plastic bag. One portion was stored at 4 °C for the analysis of fresh sample parameters, while the other portion was air-dried at room temperature for the determination of dry sample parameters.
For soil mineralization measurements, 50 g of soil was placed into a polyethylene container and mixed with biochar at the corresponding rate. The mixture was pre-incubated for one week under the same conditions. A small beaker containing 10 mL of 0.1 mol·L−1 NaOH solution was placed inside each container and sealed with a lid. CO2 evolution was measured at the same intervals to determine soil carbon mineralization rates.
2.3. Measurement Methods
Biochar characterization: The surface morphology of the biochar was characterized using a Hitachi Regulus 8100 field emission scanning electron microscope (SEM) under magnifications ranging from 500× to 40,000× and an accelerating voltage of 20 kV. The surface functional groups of the biochar were analyzed using a Fourier-transform infrared (FTIR) spectrometer (Spectrum Two, PerkinElmer, Waltham, MA, USA). Samples were prepared using the KBr pellet method, and spectra were recorded over the wavenumber range of 4000–400 cm−1.
Soil analysis: Soil pH was determined using a pH meter at a soil-to-water ratio of 2.5:1. Cation exchange capacity (CEC) was determined by the barium chloride–sulfate forced-exchange method. Available phosphorus (AP) was measured using the sodium carbonate-molybdenum-antimony colorimetric method. Available potassium (AK) was extracted with ammonium acetate and measured by flame photometry. Soil organic carbon (SOC) was determined by potassium dichromate oxidation and spectrophotometry.
Soil microbial biomass carbon (MBC) was determined using the chloroform fumigation-extraction method. Soils were fumigated for 24 h, followed by extraction with 0.5 mol/L K2SO4 and shaking for 30 min. The extract was analyzed using a total organic carbon (TOC) analyzer (Multi N/C 3100, Analytik Jena, Jena, Germany). Soil dissolved organic carbon (DOC) was also measured using the same TOC analyzer [15]. Readily oxidizable carbon (ROC) was extracted using 333 mmol/L KMnO4 and quantified with a spectrophotometer (UV-1200, Mapada, Shanghai, China) [16]. Soil catalase activity was measured by potassium permanganate titration. Urease activity was determined using urea as the substrate, with incubation at 37 °C for 24 h, followed by colorimetric detection using the phenol–sodium hypochlorite method and UV–visible spectrophotometry [17]. Sucrase activity was assessed using sucrose as the substrate, incubated at 37 °C for 24 h, and measured via the 3,5-dinitrosalicylic acid (DNS) colorimetric method [18]. The soil organic carbon mineralization rate was assessed using the alkali absorption method [19].
2.4. Statistical Analysis
All data were processed using Microsoft Excel 2022 and SPSS 26.0. One-way analysis of variance (ANOVA) was conducted to assess differences among sampling times for each treatment. The least significant difference (LSD) test was used for post hoc comparisons at a significance level of p < 0.05. Graphs were generated using Origin 2022. Correlation analysis between soil organic carbon fractions and other physicochemical parameters was visualized using correlation plots. Partial least squares structural equation modeling (PLS-SEM) was conducted using the “plspm” package in R (version 4.3.3) to identify potential pathways directly influencing soil CO2 emissions.
3. Results
3.1. Characterization and Elemental Composition of Different Types of Biochar
As shown in Figure 1, significant structural differences were observed between JMC and GXC. The surface of JMC exhibited a relatively intact, lamellar structure with a smooth texture. In contrast, GXC showed a rougher and more fragmented surface morphology, characterized by irregular flocculent particles and a higher degree of porosity.

Figure 1.
Scanning electron microscopy (SEM) images and FTIR spectra of Senna obtusifolia biochar before and after modification.
Fourier-transform infrared spectroscopy (FTIR) analysis provided evidence of the functional groups present on the biochar surfaces. A broad absorption peak around 3400 cm−1 corresponds to the stretching vibration of hydroxyl groups (–OH) [20]. The intensity of the –OH peak was notably enhanced in the modified biochar (GXC), suggesting an increase in the number of hydroxyl groups, which can serve as binding sites for metal ions and improve the adsorption capacity.
In addition, absorption bands near 1450 cm−1 and 880 cm−1 were primarily associated with the out-of-plane bending vibrations of carbonate groups (CO32−) [21]. These bands were markedly stronger in the modified biochar, indicating a higher carbonate content, which may also contribute to improved sorption properties and increased alkalinity.
3.2. Effects of Different Types of Biochar on Soil pH and Cation Exchange Capacity (CEC)
The application of both JMC and GXC significantly increased the soil pH throughout the incubation period (Figure 2a,b). In all treatments, the soil pH remained consistently higher than that of the control group (CK), indicating the alkalizing effect of biochar amendments. Two-way analysis of variance (ANOVA) revealed that both biochar type and application rate had highly significant effects on soil pH (p < 0.01) (Table 2).
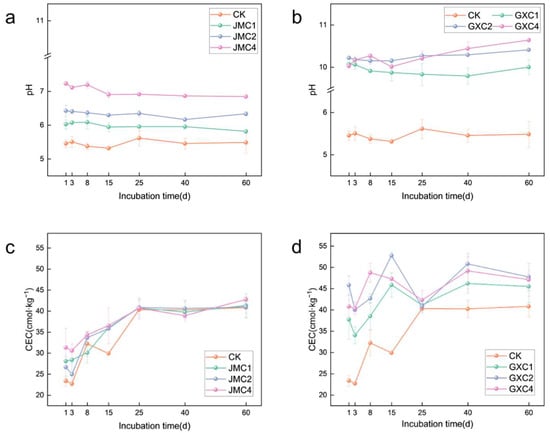
Figure 2.
Effect of different biochars on soil pH (a,b) and CEC (c,d). Significance analysis is shown in Tables S1 and S2.

Table 2.
Two-way ANOVA analysis of the effect of different types of biochar application on soil pH and CEC.
Following the application of JMC, the soil cation exchange capacity (CEC) exhibited an initial increase, followed by a stabilization phase (Figure 2c). An increasing trend was observed from day 1 to day 25 of incubation, followed by a stabilization period from day 25 to day 60.
In the GXC-treated soils, CEC values were consistently higher than those of the CK throughout the experiment (Figure 2d). A notable drop in CEC was observed on day 25, followed by a subsequent increase, suggesting a temporary disturbance possibly due to microbial or chemical dynamics, which later recovered as the soil system reached a new equilibrium. Two-way analysis of variance (ANOVA) indicated that only the type of biochar had a significant effect on cation exchange capacity (CEC) (p < 0.01) (Table 2).
3.3. Effects of Different Types of Biochar on Available Potassium (AK), Available Phosphorus (AP), and Alkali-Hydrolyzable Nitrogen (AN)
The application of JMC significantly increased the content of available potassium in the soil (Figure 3a). Throughout the incubation period, soils treated with JMC exhibited consistently higher AK levels than the control (CK). On day 60, the application of 1%, 2%, and 4% JMC resulted in increases of 2.15-, 3.69-, and 5.05-fold, respectively, compared with CK. Similarly, the application of GXC also enhanced the soil AK content, but unlike JMC, the GXC-treated soils showed a clear trend of initial increase followed by a decline and eventual stabilization. On day 8, AK content in soils treated with 1%, 2%, and 4% GXC was 2.62, 4.25, and 4.96 times higher than in CK, respectively (Figure 3b).
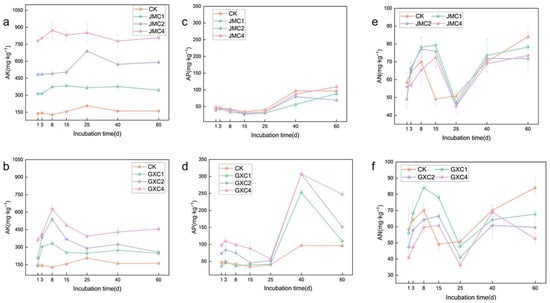
Figure 3.
Effect of different biochars on AK (a,b), AP (c,d), and AN (e,f). Significance analyses are shown in Tables S3 through S5.
The application of JMC resulted in a decrease in available phosphorus content in the initial stage, followed by an increase over time (Figure 3c). During the incubation period, the AP content in CK remained higher than in soils treated with 1% and 2% JMC. However, by day 60, the AP content in the soil treated with 4% JMC exceeded that in the CK. A similar trend was observed in the early stages (first 40 days) for GXC-treated soils, followed by a decline in AP content in the later stage (Figure 3d). On day 60, the application of 1%, 2%, and 4% GXC increased soil AP content by 14.33%, 57.84%, and 157.99%, respectively, compared to CK.
Both JMC and GXC treatments resulted in a general trend of initial increase, subsequent decline, and then a secondary rise in alkali-hydrolyzable nitrogen content (Figure 3e,f). Notably, on day 25, AN content in all JMC- and GXC-treated soils was lower than that in CK. Even on the final day of incubation (day 60), AN levels in all treated soils remained below those of the control.
3.4. Effects of Different Types of Biochar on Soil Carbon Mineralization
The application of JMC and GXC significantly influenced the rate of soil organic carbon (SOC) mineralization (Figure 4). On day 1 of the incubation, the organic carbon mineralization rates in soils treated with 1%, 2%, and 4% JMC were 1.17, 1.20, and 1.32 times those of the control (CK), respectively. Similarly, the mineralization rates in soils treated with the same proportions of GXC were 1.52, 1.70, and 1.72 times those of CK. From days 1 to 25, the SOC mineralization rates in both JMC- and GXC-treated soils rapidly declined, and from days 25 to 60, the rates leveled off. On day 60, the SOC mineralization rates in soils treated with 2% and 4% JMC remained higher than those of CK, at 1.36 and 1.38 times, respectively. The mineralization rates for soils treated with 1%, 2%, and 4% GXC were 1.61, 1.45, and 1.84 times those of CK. Over the entire incubation period, the SOC mineralization rate was highest in the early stages and decreased over time, eventually stabilizing towards the end of the experiment.
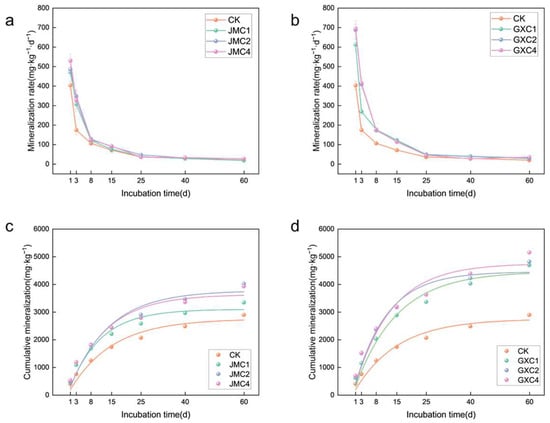
Figure 4.
Rate of organic carbon mineralization (a,b) and cumulative mineralization (c,d) of different types of biochar.
The application of GXC also significantly affected the cumulative mineralization of soil organic carbon (Figure 4). The first-order kinetic equation C = C0 (1 − e−kt) was used to fit the dynamic changes in cumulative mineralization and incubation time in soils treated with JMC and GXC. The fitting coefficients (R2 > 0.930) indicate a good fit.
3.5. Impact of Different Types of Biochar on Organic Carbon Components Before and After Modification
The application of JMC to the soil resulted in an increase in the soil’s organic carbon (SOC) content (Figure 5a). Throughout the 60-day incubation period, SOC contents under all application rates of JMC were higher than those of the control (CK). Notably, the application of 4% JMC showed a significant difference from CK during the entire incubation period (p < 0.05). Initially, the addition of GXC to the soil led to a noticeable change in SOC content; however, as the cultivation time increased, the effect of GXC on SOC decreased. By day 60, the SOC content in the GXC-treated soil was nearly identical to that in CK (Figure 5b).
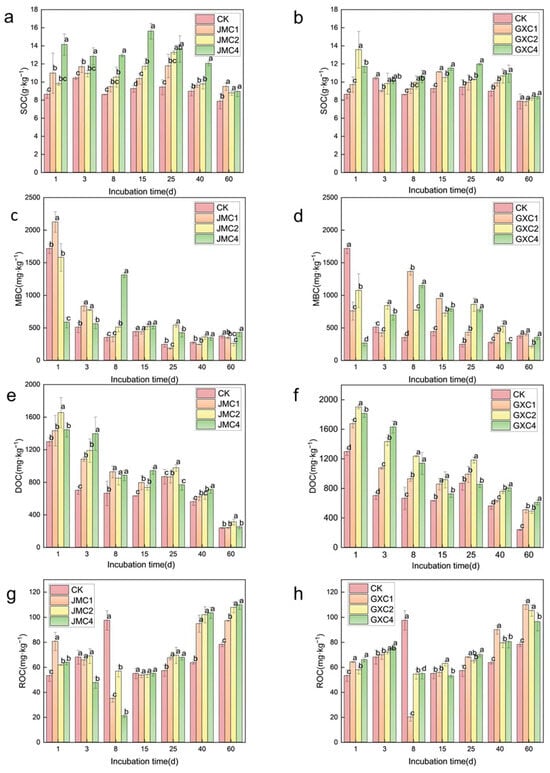
Figure 5.
Effects of different types of biochar on organic carbon (a,b), microbial biomass carbon (c,d), dissolved organic carbon (e,f), and reactive organic carbon (g,h). Different lowercase letters represent significant differences (p < 0.05).
After applying JMC to the soil, the microbial biomass carbon (MBC) content showed a general decreasing trend throughout the experimental period (Figure 5c). Application of 1% and 2% JMC increased soil MBC during days 1–3 of incubation, whereas the application of 4% JMC did not enhance MBC compared to the control (CK) during the same period. However, MBC under 4% JMC exceeded that of CK starting from day 8. The trend of MBC in the GXC-treated soil was similar to that of JMC, showing a general decrease (Figure 5d). On day 1, the MBC content in GXC-treated soil was lower than that in CK.
The content of dissolved organic carbon (DOC) in soils treated with both JMC and GXC generally showed a decreasing trend (Figure 5e,f). On day 60, the application of 2% JMC resulted in a 1.31-fold increase in MBC compared to the control (CK) (p < 0.05). The DOC content in the 1%, 2%, and 4% GXC treatments was 2.14, 2.04, and 2.56 times higher than that of CK, respectively.
After applying JMC to the soil, the content of reactive organic carbon (ROC) exhibited a trend of initial decrease followed by an increase (Figure 5g). By day 60, the ROC content in the 1%, 2%, and 4% JMC treatments was 1.24, 1.38, and 1.40 times higher than that of CK, respectively. Similarly, the ROC content in soils treated with GXC followed a comparable trend of initial decrease and subsequent increase (Figure 5h). By day 60, the ROC content in the 1%, 2%, and 4% GXC treatments was 1.40, 1.35, and 1.23 times higher than that of CK, respectively.
3.6. Effect of Different Types of Biochar on Soil Enzyme Activities
The application of JMC to the soil resulted in significant fluctuations in the soil urease activity (Figure 6a). During the early stage of cultivation (days 1–8), the urease activity increased. However, by day 15, there was a sudden decline, with the urease activity of the JMC1 treatment being lower than that of the control group (CK). During the later stage of cultivation (days 25–60), the urease activity showed a trend of initial increase followed by a decrease. On day 25, the urease activity in the 1%, 2%, and 4% JMC treatments was 1.07, 1.12, and 1.27 times higher than that of CK, respectively.
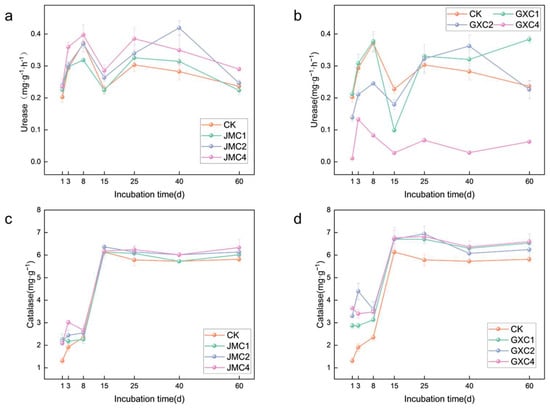
Figure 6.
Effect of different types of biochar on urease activity (a,b) and catalase activity (c,d). Significance analyses are shown in Tables S6 and S7.
After applying GXC to the soil, the urease activity followed a similar trend to that observed with JMC treatment (Figure 6b). On day 15, the urease activity in the 1%, 2%, and 4% GXC treatments was lower than that of CK. By day 60, only the GXC1 treatment showed a higher urease activity compared to the control group (CK).
After applying both JMC and GXC to the soil, the overall hydrogen peroxide (H2O2) activity followed a trend of initial increase followed by stabilization (Figure 6c,d). On day 60, the hydrogen peroxide activity in the 1%, 2%, and 4% JMC treatments was 1.03, 1.06, and 1.09 times higher than that of CK, respectively. The hydrogen peroxide activity in the 1%, 2%, and 4% GXC treatments was 1.12, 1.07, and 1.13 times higher than that of CK, respectively.
3.7. Correlation and Structural Equation Modeling Analysis
Pearson correlation analysis was performed to evaluate the influence of soil environmental factors on CO2 emissions. After the application of JMC, CO2 emissions showed a significant negative correlation with DOC and MBC (p < 0.01), and a significant positive correlation with ROC (p < 0.05) (Figure 7a). After the application of GXC, CO2 emissions were significantly negatively correlated with DOC (p < 0.01) and positively correlated with ROC (p < 0.05). Additionally, CO2 emissions also showed a positive correlation with CEC and AP (Figure 7b).
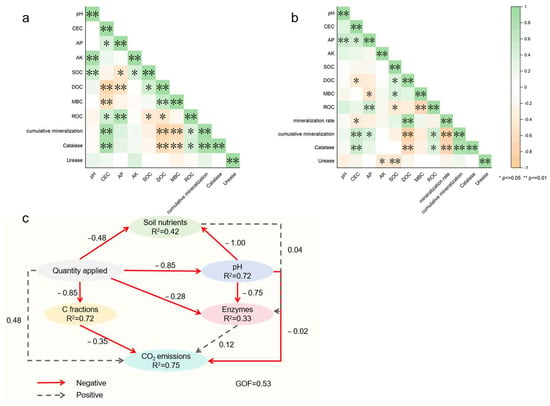
Figure 7.
Correlation analysis between application of different biochar to soil environmental factors and CO2 emissions, (a) JMC; (b) GXC; (c) partial least squares structural equation modeling (PLS-SEM) analysis results. * represents significant differences (p < 0.05); ** represents significant differences (p < 0.01).
Partial least squares structural equation modeling (PLS-SEM) was used to explain the variation in soil CO2 emissions (Figure 7c). The application rate of biochar and soil carbon components directly influenced CO2 emissions. Furthermore, the application rate and pH had a direct effect on soil nutrients.
4. Discussion
4.1. Effects of Different Types of Biochar on Soil CO2 Emissions
Our study found that, compared to the control (CK), GXC significantly promoted CO2 emissions (Figure 4d), and there was a positive correlation with the application rate of biochar. This can primarily be attributed to the substantial proportion of unstable organic carbon remaining in the GXC material, which stimulates microbial activity in the soil [3]. Additionally, previous studies have shown that biochar can either promote or inhibit soil CO2 emissions, depending on the type of biochar used [22]. The lower carbon content (19.65%) of GXC may also contribute to the increased CO2 emissions.
Our study also found that the application of JMC1 reduced the organic carbon mineralization rate in loquat soil (Figure 4a). Similarly, Hu et al. [19] observed that applying different proportions of calcium/magnesium-modified biochar to citrus orchard soils showed that a 1% application rate had greater carbon sequestration potential. Biochar exhibits a strong adsorption effect on native organic carbon, which can explain its greater inhibitory effect on organic carbon mineralization [23,24]. Furthermore, native organic carbon in the soil, when adsorbed by the biochar applied to the soil, is sequestered in the pores of the biochar [25].
The effects of applying the same proportions of JMC and GXC to loquat farmland soil on CO2 emissions were different, with JMC resulting in lower CO2 emissions (Figure 4c,d). This is because JMC has a higher C/N ratio (33.76), which enhances its ability to sequester soil organic carbon [26]. Similarly, Zhang et al. [27] found that biochars with a C/N ratio between 20 and 100 can reduce greenhouse gas emissions, as an increase in the C/N ratio leads to reduced mineralization of nitrogen in the soil.
Soil CO2 emissions are also related to the soil’s physical and chemical properties. Pearson correlation analysis showed that the cation exchange capacity (CEC) had a significant positive correlation with cumulative CO2 emissions (p < 0.01) (Figure 7). Similarly, Wang et al. [9] applied KOH-modified biochar and unmodified biochar to soil and found a positive correlation between CO2 emissions and CEC. It is noteworthy that JMC and GXC applied to loquat farmland soils showed different trends, with GXC significantly increasing soil CEC (Figure 2d), which could also be an important reason for the differences in CO2 emissions. The structural equation model (PLS-SEM) revealed that carbon components are the direct drivers of CO2 emissions (Figure 7c). Pearson correlation analysis also found a negative correlation between DOC and CO2 emissions (p < 0.01) and a positive correlation between ROC and CO2 emissions (p < 0.05). Jiang et al. [28] observed that after the application of biochar, the increased DOC was not further mineralized by microorganisms into CO2 but remained in the soil environment. DOC may promote the movement of carbon deeper into the soil, where it can be fixed. However, if DOC is mineralized by microorganisms or filtered out, it could lead to greater carbon losses. Wang et al. [29] found that soil DOC and ROC contents were closely related to CO2 emissions, but ROC explained CO2 emissions more effectively than DOC. Our results also confirm this finding.
4.2. Effects of Different Types of Biochar on Soil Organic Carbon and Its Carbon Component Composition
The application of JMC and GXC increased the soil’s SOC content (Figure 5a,b). As a factor that directly influences soil organic carbon mineralization rate and carbon sequestration capacity, the increase in SOC can be attributed to biochar being a carbon-rich material containing a significant amount of aromatic compounds [30]. In this study, the SOC content in the soil treated with JMC showed a significant difference compared to the control (CK) (p < 0.05) (Figure 5a). Li et al. [31] found that a high C/N ratio can reduce the decomposition of organic matter by soil microorganisms. The C/N ratio of JMC is higher than that of GXC (Table 3). Throughout the entire incubation period, the application of 4% JMC significantly increased SOC levels (p < 0.05), which may be attributed to the higher biochar addition enhancing the stable carbon content in the soil.

Table 3.
Basic physicochemical properties of Senna obtusifolia biochar before and after modification.
DOC and ROC, as the active carbon components in the soil, are highly sensitive to changes in the soil environment [32]. Both JMC and GXC increased the content of DOC and ROC (Figure 5c–f). This could be because the addition of an external carbon source increased the amount of unstable carbon in the soil. This carbon is mainly utilized by microorganisms and, compared to other carbon components, is more easily accessible, leading to higher microbial activity [33]. Similarly, Zhang et al. [34] found that biochar application significantly increased the contents of DOC and ROC. Comparable findings have also been reported by other scholars [31]. Jiang et al. [30] pointed out that changes in DOC and ROC are primarily influenced by soil properties, biochar types, and environmental conditions. It is noteworthy that the application of GXC did not result in a significant difference in soil MBC content (p < 0.05) (Figure 5d), which contrasts with the findings of some previous studies [35,36]. We speculate that the decrease in CO2 emissions with increasing MBC may be attributed to the enhancement of stable carbon in the soil due to biochar application, which is preferentially decomposed by Gram-negative bacteria. This is further supported by the observed positive correlation with ROC. Additionally, some studies have reported that biochar application does not significantly affect soil MBC, which may be related to factors such as pH and CEC [9].
4.3. Effects of Different Types of Biochar on Soil Nutrient Transformation
The application of biochar has been shown to positively influence the chemical properties of soil and significantly affect soil nutrient dynamics. In this study, the application of JMC and GXC biochars led to an increase in soil pH, which was positively correlated with the application rate (Figure 2a,b). This finding is consistent with the results of previous studies [37,38,39]. Biochar contains various mineral elements, which often form carbonates, contributing to the alkalinity of the biochar and, consequently, increasing soil pH upon application [40]. Partial least squares structural equation modeling (PLS-SEM) further revealed that both pH and biochar application rate were direct factors influencing soil nutrient availability (Figure 7). Similar conclusions were reported by [41], who found that increasing the pH of acidic soils can enhance microbial activity, accelerate the mineralization of soil organic matter, and improve plant nutrient uptake.
The application of JMC and GXC also increased the soil cation exchange capacity (CEC) (Figure 2c,d). Binh Thanh et al. [42] similarly reported that biochar application could enhance soil CEC through mechanisms such as increased surface negative charge density due to biochar oxidation. Chen et al. [43] found that available phosphorus (AP) is a key factor influencing bacterial community composition, thereby affecting soil nutrient dynamics and plant growth. In this study, GXC application significantly increased soil AP levels (Figure 3d). Pearson correlation analysis further indicated a significant positive correlation between CEC and AP (p < 0.05) (Figure 7). We hypothesize that the stronger effect of GXC on increasing soil CEC may account for the more pronounced increase in AP.
Soil enzyme activity reflects the metabolic capacity of the soil and is an important indicator of soil fertility, biological activity, and overall soil quality [44]. Catalase activity, in particular, facilitates the decomposition of hydrogen peroxide, thereby reducing its toxic effects on microorganisms and providing nutrients to support microbial growth. In this study, both JMC and GXC enhanced catalase activity, with GXC showing slightly higher effectiveness (Figure 6c,d). This may be attributed to the surface structure of GXC, which possesses strong adsorption capacity, allowing it to adsorb both soil enzymes and the substrates required for enzymatic reactions, thus creating a more favorable environment for microbial activity [44]. On the 15th day of incubation, urease activity in the soil declined significantly (Figure 6a,b), while catalase activity showed a marked increase (Figure 6c,d). We speculate that the availability of soil nutrients may have triggered the microbial production of specific enzymes, leading to an adjustment in the relative proportions of various soil enzymes [45].
5. Conclusions
Through a 60-day soil incubation experiment, we found that both Jujube straw biochar (JMC) and modified Jujube straw biochar (GXC) increased soil CO2 emissions. However, the application of 1% JMC significantly reduced the organic carbon mineralization rate on the final day of the experiment, with the rate being lower than that of the control (CK). In the long term, the 1% JMC treatment could have a positive effect on reducing CO2 emissions, which contradicts our initial hypothesis. Additionally, we observed that the GXC treatment notably increased soil pH, CEC, and AP. The application of low doses (1%, 2%) of GXC also significantly enhanced urease activity, suggesting a positive effect of GXC on certain soil nutrients. This finding is consistent with our second hypothesis. In summary, these findings provide a valuable reference for research on carbon sequestration and nutrient enhancement in agricultural soils through the application of biochar and modified biochar. It should be noted that, in this study, biochar was applied once to simulate the use of basal fertilizer under field conditions; thus, the potential adverse effects of repeated biochar applications on soil and plants were not addressed. Furthermore, the scope of this research was limited to farmland soil used for Siraitia grosvenorii cultivation. Further investigations are planned to explore these aspects in greater depth.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy15081877/s1, Tables S1–S7 show the effect of different biochar on pH, CEC, AP, AK, AN, catalase, and urease, respectively.
Author Contributions
Data curation, Y.B.; funding acquisition, Y.H.; investigation, G.L. and J.L.; project administration, L.H.; software, Y.B.; supervision, H.D. and L.P.; validation, S.L.; visualization, A.L. and L.L.; writing—original draft, Y.B.; writing—review and editing, L.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Guangxi Key R&D Program (Guike AB25069119).
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Acknowledgments
Thanks to all of the editors and reviewers for their critical review of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bolan, S.; Sharma, S.; Mukherjee, S.; Kumar, M.; Rao, C.S.; Nataraj, K.C.; Singh, G.; Vinu, A.; Bhowmik, A.; Sharma, H.; et al. Biochar modulating soil biological health: A review. Sci. Total Environ. 2024, 914, 169585. [Google Scholar] [CrossRef]
- Ojeda, G.; Gil, J.M.; Mattana, S.; Bachmann, J.; Quenea, K.; Sobral, A.J.F.N. Biochar ageing effects on soil respiration, biochar wettability and gaseous CO2 adsorption. Mitig. Adapt. Strateg. Glob. Chang. 2024, 29, 11. [Google Scholar] [CrossRef]
- Sultan, S.; Khan, K.S.; Akmal, M.; Ahmed, Z.I.; Hussain, Q.; Khosa, S.A. Carbon mineralization in subtropical dryland soil amended with different biochar sources. Arab. J. Geosci. 2019, 12, 451. [Google Scholar] [CrossRef]
- Song, B.; Almatrafi, E.; Tan, X.; Luo, S.; Xiong, W.; Zhou, C.; Qin, M.; Liu, Y.; Cheng, M.; Zeng, G.; et al. Biochar-based agricultural soil management: An application-dependent strategy for contributing to carbon neutrality. Renew. Sustain. Energy Rev. 2022, 164, 112529. [Google Scholar] [CrossRef]
- Bolan, N.; Hoang, S.A.; Beiyuan, J.; Gupta, S.; Hou, D.; Karakoti, A.; Joseph, S.; Jung, S.; Kim, K.-H.; Kirkham, M.B.; et al. Multifunctional applications of biochar beyond carbon storage. Int. Mater. Rev. 2022, 67, 150–200. [Google Scholar] [CrossRef]
- Chen, S.; Liu, G.; Hong, Y.; Ma, Y.; Guo, S.; Yan, P.; Mi, W. Biochar Amendment was Less Effective for Rice Yield Improvement but More Effective for Soil Quality Relative to Inorganic Fertilization in a Low Fertility Paddy Soil. J. Soil Sci. Plant Nutr. 2024, 24, 4918–4928. [Google Scholar] [CrossRef]
- Kumar, A.; Bhattacharya, T.; Shaikh, W.A.; Chakraborty, S.; Sarkar, D.; Biswas, J.K. Biochar Modification Methods for Augmenting Sorption of Contaminants. Curr. Pollut. Rep. 2022, 8, 519–555. [Google Scholar] [CrossRef]
- Ghorbani, M.; Amirahmadi, E.; Cornelis, W.; Zoroufchi Benis, K. Understanding the physicochemical structure of biochar affected by feedstock, pyrolysis conditions, and post-pyrolysis modification methods—A meta-analysis. J. Environ. Chem. Eng. 2024, 12, 114885. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, C.; Wang, W. Response mechanisms of agricultural soil biochemical properties and CO2 emissions to field application of modified biochar. J. Soils Sediments 2024, 24, 1194–1205. [Google Scholar] [CrossRef]
- Liu, S.; Xie, Z.; Zhu, Y.; Zhu, Y.; Jiang, Y.; Wang, Y.; Gao, H. Adsorption characteristics of modified rice straw biochar for Zn and in-situ remediation of Zn contaminated soil. Environ. Technol. Innov. 2021, 22, 101388. [Google Scholar] [CrossRef]
- Ghassemi-Golezani, K.; Farhangi-Abriz, S. Improving plant available water holding capacity of soil by solid and chemically modified biochars. Rhizosphere 2022, 21, 100469. [Google Scholar] [CrossRef]
- Hua, B.; Li, Z.; Gao, W.; Feng, H.; Chen, N.; Li, J.; Ji, X.; Zhang, L.; Wu, Z.; Yan, S.; et al. Soil amendment in plastic greenhouse using modified biochar: Soil bacterial diversity responses and microbial biomass carbon and nitrogen. Biotechnol. Lett. 2021, 43, 655–666. [Google Scholar] [CrossRef]
- Bao, Z.; Shi, C.; Tu, W.; Li, L.; Li, Q. Recent developments in modification of biochar and its application in soil pollution control and ecoregulation. Environ. Pollut. 2022, 313, 120184. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, Y.; Zong, Y.; Yu, J.; Ding, H.; Kong, Y.; Ma, J.; Ding, L. Efficient removal of cadmium by salts modified-biochar: Performance assessment, theoretical calculation, and quantitative mechanism analysis. Bioresour. Technol. 2022, 361, 127717. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Liu, Q.; Chen, Z.; Wen, Z.; Liu, Y.; Huang, L.; Yu, C.; Feng, Y. Organic amendments perform better than inorganic amendments in reducing the absorption and accumulation of cadmium in lettuce. Environ. Sci. Pollut. Res. 2023, 30, 117277–117287. [Google Scholar] [CrossRef]
- Sun, T.; Gao, G.; Yang, W.; Sun, Y.; Huang, Q.; Wang, L.; Liang, X. High-efficiency remediation of Hg and Cd co-contaminated paddy soils by Fe-Mn oxide modified biochar and its microbial community responses. Biochar 2024, 6, 57. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, K.; Liu, J.; Chen, Y.; Han, L. Changes in soil properties and CO2 emissions after biochar addition: Role of pyrolysis temperature and aging. Sci. Total Environ. 2022, 839, 156333. [Google Scholar] [CrossRef]
- Bashir, S.; Hussain, Q.; Akmal, M.; Riaz, M.; Hu, H.; Ijaz, S.S.; Iqbal, M.; Abro, S.; Mehmood, S.; Ahmad, M. Sugarcane bagasse-derived biochar reduces the cadmium and chromium bioavailability to mash bean and enhances the microbial activity in contaminated soil. J. Soils Sediments 2018, 18, 874–886. [Google Scholar] [CrossRef]
- Hu, L.; Huang, R.; Zhou, L.; Qin, R.; He, X.; Deng, H.; Li, K. Effects of magnesium-modified biochar on soil organic carbon mineralization in citrus orchard. Front. Microbiol. 2023, 14, 1109272. [Google Scholar] [CrossRef]
- Zhao, L.; Cao, X.; Mašek, O.; Zimmerman, A. Heterogeneity of biochar properties as a function of feedstock sources and production temperatures. J. Hazard. Mater. 2013, 256–257, 1–9. [Google Scholar] [CrossRef]
- Xu, Y.; Qu, W.; Sun, B.; Peng, K.; Zhang, X.; Xu, J.; Gao, F.; Yan, Y.; Bai, T. Effects of added calcium-based additives on swine manure derived biochar characteristics and heavy metals immobilization. Waste Manag. 2021, 123, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Purakayastha, T.J.; Das, K.C.; Gaskin, J.; Harris, K.; Smith, J.L.; Kumari, S. Effect of pyrolysis temperatures on stability and priming effects of C3 and C4 biochars applied to two different soils. Soil Tillage Res. 2016, 155, 107–115. [Google Scholar] [CrossRef]
- Kasozi, G.N.; Zimmerman, A.R.; Nkedi-Kizza, P.; Gao, B. Catechol and Humic Acid Sorption onto a Range of Laboratory-Produced Black Carbons (Biochars). Environ. Sci. Technol. 2010, 44, 6189–6195. [Google Scholar] [CrossRef]
- Sobek, A.; Stamm, N.; Bucheli, T.D. Sorption of Phenyl Urea Herbicides to Black Carbon. Environ. Sci. Technol. 2009, 43, 8147–8152. [Google Scholar] [CrossRef]
- Zimmerman, A.R.; Gao, B.; Ahn, M.-Y. Positive and negative carbon mineralization priming effects among a variety of biochar-amended soils. Soil Biol. Biochem. 2011, 43, 1169–1179. [Google Scholar] [CrossRef]
- Li, B.; Guo, Y.; Liang, F.; Liu, W.; Wang, Y.; Cao, W.; Song, H.; Chen, J.; Guo, J. Global integrative meta-analysis of the responses in soil organic carbon stock to biochar amendment. J. Environ. Manag. 2024, 351, 119745. [Google Scholar] [CrossRef]
- Zhang, Q.; Xiao, J.; Xue, J.; Zhang, L. Quantifying the Effects of Biochar Application on Greenhouse Gas Emissions from Agricultural Soils: A Global Meta-Analysis. Sustainability 2020, 12, 3436. [Google Scholar] [CrossRef]
- Jiang, X.; Haddix, M.L.; Cotrufo, M.F. Interactions between biochar and soil organic carbon decomposition: Effects of nitrogen and low molecular weight carbon compound addition. Soil Biol. Biochem. 2016, 100, 92–101. [Google Scholar] [CrossRef]
- Wang, W.J.; Dalal, R.C.; Moody, P.W.; Smith, C.J. Relationships of soil respiration to microbial biomass, substrate availability and clay content. Soil Biol. Biochem. 2003, 35, 273–284. [Google Scholar] [CrossRef]
- Jiang, M.; Li, C.; Gao, W.; Cai, K.; Tang, Y.; Cheng, J. Comparison of long-term effects of biochar application on soil organic carbon and its fractions in two ecological sites in karst regions. Geoderma Reg. 2022, 28, e00477. [Google Scholar] [CrossRef]
- Li, S.; Wei, W.; Liu, S. Long-Term Organic Amendments Combined with Nitrogen Fertilization Regulates Soil Organic Carbon Sequestration in Calcareous Soil. Agronomy 2023, 13, 291. [Google Scholar] [CrossRef]
- Chen, W.; Liao, X.; Wu, Y.; Liang, J.B.; Mi, J.; Huang, J.; Zhang, H.; Wu, Y.; Qiao, Z.; Li, X.; et al. Effects of different types of biochar on methane and ammonia mitigation during layer manure composting. Waste Manag. 2017, 61, 506–515. [Google Scholar] [CrossRef]
- Virk, A.L.; Kan, Z.-R.; Liu, B.-Y.; Qi, J.-Y.; He, C.; Liu, Q.-Y.; Zhao, X.; Zhang, H.-L. Impact of biochar water extract addition on soil organic carbon mineralization and C fractions in different tillage systems. Environ. Technol. Innov. 2021, 21, 101193. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, W.; Sun, X.; Jiang, J.; Li, D.; Tang, G.; Xu, W.; Jia, H. Biochar Aged for Five Years Altered Carbon Fractions and Enzyme Activities of Sandy Soil. Land 2023, 12, 1645. [Google Scholar] [CrossRef]
- Khan, N.; Clark, I.; Sanchez-Monedero, M.A.; Shea, S.; Meier, S.; Bolan, N. Maturity indices in co-composting of chicken manure and sawdust with biochar. Bioresour. Technol. 2014, 168, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Li, L.-J.; Han, X.-Z. Changes of soil properties and carbon fractions after long-term application of organic amendments in Mollisols. Catena 2016, 143, 140–144. [Google Scholar] [CrossRef]
- Shwe, K.K.; Chaopayao, N.; Yampracha, S. Mitigation of soil salinity by addition of different rice straw biochar doses in salt-affected acid soil. Environ. Res. Commun. 2024, 6, 095028. [Google Scholar] [CrossRef]
- Yang, L.; Liao, F.; Huang, M.; Yang, L.; Li, Y. Biochar Improves Sugarcane Seedling Root and Soil Properties under a Pot Experiment. Sugar Tech 2015, 17, 36–40. [Google Scholar] [CrossRef]
- Zha, Y.; Zhao, L.; Wei, J.; Niu, T.; Yue, E.; Wang, X.; Chen, Y.; Shi, J.; Zhou, T. Effect of the application of peanut shell, bamboo, and maize straw biochars on the bioavailability of Cd and growth of maize in Cd-contaminated soil. Front. Environ. Sci. 2023, 11, 1240633. [Google Scholar] [CrossRef]
- Tang, C.; Yang, J.; Xie, W.; Yao, R.; Wang, X. Effect of Biochar Application on Soil Fertility, Nitrogen Use Efficiency and Balance in Coastal Salt-Affected Soil under Barley-Maize Rotation. Sustainability 2023, 15, 2893. [Google Scholar] [CrossRef]
- Windeatt, J.H.; Ross, A.B.; Williams, P.T.; Forster, P.M.; Nahil, M.A.; Singh, S. Characteristics of biochars from crop residues: Potential for carbon sequestration and soil amendment. J. Environ. Manag. 2014, 146, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Binh Thanh, N.; Nam Ngoc, T.; Chau Minh Thi, L.; Trang Thuy, N.; Thanh Van, T.; Binh Vu, T.; Tan Van, L. The interactive effects of biochar and cow manure on rice growth and selected properties of salt-affected soil. Arch. Agron. Soil Sci. 2018, 64, 1744–1758. [Google Scholar] [CrossRef]
- Chen, Y.; Qiu, Y.; Hao, X.; Tong, L.; Li, S.; Kang, S. Does biochar addition improve soil physicochemical properties, bacterial community and alfalfa growth for saline soils? Land Degrad. Dev. 2023, 34, 3314–3328. [Google Scholar] [CrossRef]
- Jin, X.X.; Zhang, T.X.; Hou, Y.T.; Bol, R.; Zhang, X.J.; Zhang, M.; Yu, N.; Meng, J.; Zou, H.T.; Wang, J.K. Review on the effects of biochar amendment on soil microorganisms and enzyme activity. J. Soils Sediments 2024, 24, 2599–2612. [Google Scholar] [CrossRef]
- Deng, L.; Peng, C.; Huang, C.; Wang, K.; Liu, Q.; Liu, Y.; Hai, X.; Shangguan, Z. Drivers of soil microbial metabolic limitation changes along a vegetation restoration gradient on the Loess Plateau, China. Geoderma 2019, 353, 188–200. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).