Environmental and Health Impacts of Pesticides and Nanotechnology as an Alternative in Agriculture
Abstract
1. Introduction
2. Environmental Impact of Conventional Pesticides
3. Oxidative Stress Risk Associated with Pesticide Exposure
4. Pesticides in Food and Their Effect on Human Health
5. Nanotechnology as an Alternative to Pesticides in Agriculture
6. Regulations
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 2,4-D | 2,4-dichlorophenoxyacetic acid |
| 2,4-DDE | 2,4-dichlorodiphenyldichloroethylene |
| 4,4-DDE | 4,4-dichlorodiphenyldichloroethylene |
| β-BHC | beta-hexachlorocyclohexane |
| CAT | catalase |
| DDT | dichlorodiphenyltrichloroethane |
| DEE | diethyl ether |
| e-nose | electronic nose |
| EPA | Environmental Protection Agency |
| FAO | Food and Agriculture Organization of the United Nations |
| FDA | Food and Drug Administration |
| e-tongue | electronic tongue |
| γ-BHC | gamma-hexachlorocyclohexane (Lindane) |
| GPx | glutathione peroxidase |
| GST | glutathione-S-transferase |
| HCB | hexachlorobenzene |
| LD50 | median lethal dose |
| MRLs | maximum residue limits |
| RNS | reactive nitrogen species |
| ROS | reactive oxygen species |
| SOD | superoxide dismutase |
| TBARSs | thiobarbituric acid reactive substances |
| UPLC-MS/MS | ultra-performance liquid chromatography coupled with tandem mass spectrometry |
| USDA | U.S. Department of Agriculture |
References
- Van Dijk, M.; Morley, T.; Rau, M.L.; Saghai, Y. A meta-analysis of projected global food demand and population at risk of hunger for the period 2010–2050. Nat. Food 2021, 2, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Makame, K.R.; Sherif, M.; Östlundh, L.; Sandor, J.; Adam, B.; Nagy, K. Are encapsulated pesticides less harmful to human health than their conventional alternatives? Protocol for a systematic review of in vitro and in vivo animal model studies. Environ. Int. 2023, 174, 107924. [Google Scholar] [CrossRef]
- Khan, B.A.; Nadeem, M.A.; Nawaz, H.; Amin, M.M.; Abbasi, G.H.; Nadeem, M.; Ali, M.; Ameen, M.; Javaid, M.M.; Maqbool, R. Pesticides: Impacts on agriculture productivity, environment, and management strategies. In Emerging Contaminants and Plants: Interactions, Adaptations and Remediation Technologies; Springer International Publishing: Cham, Switzerland, 2023; pp. 109–134. [Google Scholar]
- Lazarević-Pašti, T.; Milanković, V.; Tasić, T.; Petrović, S.; Leskovac, A. With or Without You?—A Critical Review on Pesticides in Food. Foods 2025, 14, 1128. [Google Scholar] [CrossRef]
- Abdollahdokht, D.; Gao, Y.; Faramarz, S.; Poustforoosh, A.; Abbasi, M.; Asadikaram, G.; Nematollahi, M.H. Conventional agrochemicals towards nano-biopesticides: An overview on recent advances. Chem. Biol. Technol. Agric. 2022, 9, 13. [Google Scholar] [CrossRef]
- Mostafalou, S.; Abdollahi, M. Pesticides: An update of human exposure and toxicity. Arch. Toxicol. 2017, 91, 549–599. [Google Scholar] [CrossRef] [PubMed]
- Tudi, M.; Daniel Ruan, H.; Wang, L.; Lyu, J.; Sadler, R.; Connell, D.; Chu, C.; Phung, D.T. Agriculture development, pesticide application and its impact on the environment. Int. J. Environ. Res. Public Health 2021, 18, 1112. [Google Scholar] [CrossRef]
- Padhiary, M.; Kumar, R. Assessing the environmental impacts of agriculture, industrial operations, and mining on agro-ecosystems. In Smart Internet of Things for Environment and Healthcare; Springer Nature Switzerland: Cham, Switzerland, 2024; pp. 107–126. [Google Scholar]
- Beaumelle, L.; Tison, L.; Eisenhauer, N.; Hines, J.; Malladi, S.; Pelosi, C.; Thouvenot, L.; Phillips, H.R. Pesticide effects on soil fauna communities—A meta-analysis. J. Appl. Ecol. 2023, 60, 1239–1253. [Google Scholar] [CrossRef]
- Rubira, R.J.; Correia, R.R.; Batista, V.R.; Pazin, W.M.; González, F.G.; Otero, J.C.; Teixeira, G.R.; Job, A.E. Assessing the negative impact of chlorantraniliprole, isoxaflutole, and simazine pesticides on phospholipid membrane models and tilapia gill tissues. Environ. Pollut. 2024, 349, 123904. [Google Scholar] [CrossRef]
- Hamed, M.A.; Akhigbe, T.M.; Adeogun, A.E.; Adesoye, O.B.; Akhigbe, R.E. Impact of organophosphate pesticides exposure on human semen parameters and testosterone: A systematic review and meta-analysis. Front. Endocrinol. 2023, 14, 1227836. [Google Scholar] [CrossRef]
- Zhou, W.; Li, M.; Achal, V. A comprehensive review on environmental and human health impacts of chemical pesticide usage. Emerg. Contam. 2024, 11, 100410. [Google Scholar] [CrossRef]
- Desye, B.; Tesfaye, A.H.; Daba, C.; Alemseged, E.A.; Angaw, Y.; Ebrahim, A.M.; Natnael, T.; Hassen, S.; Woretaw, L. Pesticide safe use practice and acute health symptoms, and associated factors among farmers in developing countries: A systematic review and meta-analysis of an epidemiological evidence. BMC Public Health 2024, 24, 3313. [Google Scholar] [CrossRef] [PubMed]
- Radulović, J.; Lučić, M.; Nešić, A.; Onjia, A. Multivariate assessment and risk ranking of pesticide residues in citrus fruits. Foods 2023, 12, 2454. [Google Scholar] [CrossRef]
- Jiang, X.; Yang, F.; Jia, W.; Jiang, Y.; Wu, X.; Song, S.; Shen, H.; Shen, J. Nanomaterials and nanotechnology in agricultural pesticide delivery: A review. Langmuir 2024, 40, 18806–18820. [Google Scholar] [CrossRef]
- Yadav, N.; Garg, V.K.; Chhillar, A.K.; Rana, J.S. Recent advances in nanotechnology for the improvement of conventional agricultural systems: A review. Plant Nano Biol. 2023, 4, 100032. [Google Scholar] [CrossRef]
- Ale, A.; Andrade, V.S.; Gutierrez, M.F.; Bacchetta, C.; Rossi, A.S.; Santo Orihuela, P.; Desimone, M.F.; Cazenave, J. Nanotechnology-based pesticides: Environmental fate and ecotoxicity. Toxicol. Appl. Pharmacol. 2023, 471, 116560. [Google Scholar] [CrossRef]
- Bueno, V.; Wang, P.; Harrisson, O.; Bayen, S.; Ghoshal, S. Impacts of a porous hollow silica nanoparticle-encapsulated pesticide applied to soils on plant growth and soil microbial community. Environ. Sci. Nano 2022, 9, 1476–1488. [Google Scholar] [CrossRef]
- Zia, R.; Taj, A.; Younis, S.; Bukhari, S.Z.; Latif, F.; Feroz, Y.; Fatima, K.; Imran, A.; Bajwa, S.Z. Application of nanosensors for pesticide detection. In Nanosensors for Smart Agriculture; Elsevier: Amsterdam, The Netherlands, 2022; pp. 259–302. [Google Scholar]
- Ahmed, F.; Dwivedi, S.; Shaalan, N.M.; Kumar, S.; Arshi, N.; Alshoaibi, A.; Husain, F.M. Development of selenium nanoparticle based agriculture sensor for heavy metal toxicity detection. Agriculture 2020, 10, 610. [Google Scholar] [CrossRef]
- Guo, M.; Pang, J.; Wang, Y.; Bi, C.; Xu, Z.; Shen, Y.; Yang, J.; Wang, H.; Sun, Y. Nanobodies-based colloidal gold immunochromatographic assay for specific detection of parathion. Anal. Chim. Acta 2024, 1310, 342717. [Google Scholar] [CrossRef]
- Aswani, R.; Radhakrishnan, E.; Visakh, P. Nanoformulations for Agricultural Applications: State-of-the-Art, New Challenges, and Opportunities. In Nanoformulations for Agricultural Applications; Wiley: Hoboken, NJ, USA, 2025; pp. 1–34. [Google Scholar]
- Khan, J.; Kaur, A.; Alam, M.A.; Yadav, A.; Paramanick, D.; Chaudhary, S. Nanopesticides, Nanoherbicides, and Nanofertilizers: Risks and environmental acceptability. In Nanopesticides, Nanoherbicides, and Nanofertilizers; CRC Press: Boca Raton, FL, USA, 2023; pp. 94–113. [Google Scholar]
- Andrade, V.S.; Ale, A.; Antezana, P.E.; Desimone, M.F.; Cazenave, J.; Gutierrez, M.F. Ecotoxicity of nanosilver on cladocerans and the role of algae provision. Environ. Sci. Pollut. Res. 2023, 30, 27137–27149. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Goss, G.G. Nanotechnology in agriculture: Comparison of the toxicity between conventional and nano-based agrochemicals on non-target aquatic species. J. Hazard. Mater. 2022, 439, 129559. [Google Scholar] [CrossRef]
- Xuan, L.; Ju, Z.; Skonieczna, M.; Zhou, P.K.; Huang, R. Nanoparticles-induced potential toxicity on human health: Applications, toxicity mechanisms, and evaluation models. MedComm 2023, 4, e327. [Google Scholar] [CrossRef] [PubMed]
- Santo-Orihuela, P.L.; Foglia, M.L.; Targovnik, A.M.; Miranda, M.V.; Desimone, M.F. Nanotoxicological effects of SiO2 nanoparticles on Spodoptera frugiperda Sf9 cells. Curr. Pharm. Biotechnol. 2016, 17, 465–470. [Google Scholar] [CrossRef]
- Pathak, V.M.; Verma, V.K.; Rawat, B.S.; Kaur, B.; Babu, N.; Sharma, A.; Dewali, S.; Yadav, M.; Kumari, R.; Singh, S. Current status of pesticide effects on environment, human health and it’s eco-friendly management as bioremediation: A comprehensive review. Front. Microbiol. 2022, 13, 962619. [Google Scholar] [CrossRef]
- Gabriel Ortega, J.; Ávila Demera, J.; Ayón Villao, F.; Morán Morán, J.; Álvarez Plúa, A.; Flores Ramírez, H. Utilización de plaguicidas por agricultores en Puerto La Boca, Manabí. Una reflexión sobre sus posibles consecuencias. J. Selva Andin. Biosph. 2023, 11, 47–65. [Google Scholar] [CrossRef]
- Rangel-Ortiz, E.; Landa-Cansigno, O.; Páramo-Vargas, J.; Camarena-Pozos, D.A. Prácticas de manejo de plaguicidas y percepciones de impactos a la salud y al medio ambiente entre usuarios de la cuenca del Río Turbio, Guanajuato, México. Acta Univ. 2023, 33, 1–26. [Google Scholar] [CrossRef]
- Rajmohan, K.; Chandrasekaran, R.; Varjani, S. A review on occurrence of pesticides in environment and current technologies for their remediation and management. Indian J. Microbiol. 2020, 60, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Streletskii, R.; Astaykina, A.; Krasnov, G.; Gorbatov, V. Changes in bacterial and fungal community of soil under treatment of pesticides. Agronomy 2022, 12, 124. [Google Scholar] [CrossRef]
- de Souza, R.M.; Seibert, D.; Quesada, H.B.; de Jesus Bassetti, F.; Fagundes-Klen, M.R.; Bergamasco, R. Occurrence, impacts and general aspects of pesticides in surface water: A review. Process Saf. Environ. Prot. 2020, 135, 22–37. [Google Scholar] [CrossRef]
- Alengebawy, A.; Abdelkhalek, S.T.; Qureshi, S.R.; Wang, M.-Q. Heavy metals and pesticides toxicity in agricultural soil and plants: Ecological risks and human health implications. Toxics 2021, 9, 42. [Google Scholar] [CrossRef]
- Rajan, D.K.; Mohan, K.; Rajarajeswaran, J.; Divya, D.; Thanigaivel, S.; Zhang, S. Toxic effects of organophosphate pesticide monocrotophos in aquatic organisms: A review of challenges, regulations and future perspectives. Environ. Res. 2024, 244, 117947. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, Q.; Chen, B.; Dong, T.; Chen, M. Toxic effects of pesticides on the marine microalga Skeletonema costatum and their biological degradation. Sci. China Earth Sci. 2023, 66, 663–674. [Google Scholar] [CrossRef]
- Syafrudin, M.; Kristanti, R.A.; Yuniarto, A.; Hadibarata, T.; Rhee, J.; Al-Onazi, W.A.; Algarni, T.S.; Almarri, A.H.; Al-Mohaimeed, A.M. Pesticides in drinking water—A review. Int. J. Environ. Res. Public Health 2021, 18, 468. [Google Scholar] [CrossRef]
- Kumar, V.; Sharma, N.; Sharma, P.; Pasrija, R.; Kaur, K.; Umesh, M.; Thazeem, B. Toxicity analysis of endocrine disrupting pesticides on non-target organisms: A critical analysis on toxicity mechanisms. Toxicol. Appl. Pharmacol. 2023, 474, 116623. [Google Scholar] [CrossRef]
- Elhamalawy, O.; Bakr, A.; Eissa, F. Impact of pesticides on non-target invertebrates in agricultural ecosystems. Pestic. Biochem. Physiol. 2024, 202, 105974. [Google Scholar] [CrossRef] [PubMed]
- Nicolopoulou-Stamati, P.; Maipas, S.; Kotampasi, C.; Stamatis, P.; Hens, L. Chemical pesticides and human health: The urgent need for a new concept in agriculture. Front. Public Health 2016, 4, 148. [Google Scholar] [CrossRef] [PubMed]
- Miranda, R.A.; Silva, B.S.; de Moura, E.G.; Lisboa, P.C. Pesticides as endocrine disruptors: Programming for obesity and diabetes. Endocrine 2023, 79, 437–447. [Google Scholar] [CrossRef]
- Gea, M.; Zhang, C.; Tota, R.; Gilardi, G.; Di Nardo, G.; Schilirò, T. Assessment of five pesticides as endocrine-disrupting chemicals: Effects on estrogen receptors and aromatase. Int. J. Environ. Res. Public Health 2022, 19, 1959. [Google Scholar] [CrossRef] [PubMed]
- Cavalier, H.; Trasande, L.; Porta, M. Exposures to pesticides and risk of cancer: Evaluation of recent epidemiological evidence in humans and paths forward. Int. J. Cancer 2023, 152, 879–912. [Google Scholar] [CrossRef]
- Djekkoun, N.; Lalau, J.-D.; Bach, V.; Depeint, F.; Khorsi-Cauet, H. Chronic oral exposure to pesticides and their consequences on metabolic regulation: Role of the microbiota. Eur. J. Nutr. 2021, 60, 4131–4149. [Google Scholar] [CrossRef]
- Silveira-Gramont, M.I.; Aldana-Madrid, M.L.; Piri-Santana, J.; Valenzuela-Quintanar, A.I.; Jasa-Silveira, G.; Rodríguez-Olibarria, G. Plaguicidas agrícolas: Un marco de referencia para evaluar riesgos a la salud en comunidades rurales en el estado de Sonora, México. Rev. Int. De Contam. Ambient. 2018, 34, 7–21. [Google Scholar] [CrossRef]
- Raj, J.; Chandra, M.; Dogra, T.D.; Pahuja, M.; Raina, A. Determination of median lethal dose of combination of endosulfan and cypermethrin in wistar rat. Toxicol. Int. 2013, 20, 1. [Google Scholar] [CrossRef]
- Chen, T.; Chen, H.; Wang, A.; Yao, W.; Xu, Z.; Wang, B.; Wang, J.; Wu, Y. Methyl Parathion Exposure Induces Development Toxicity and Cardiotoxicity in Zebrafish Embryos. Toxics 2023, 11, 84. [Google Scholar] [CrossRef]
- Urióstegui-Acosta, M.; Tello-Mora, P.; de Jesús Solís-Heredia, M.; Ortega-Olvera, J.M.; Piña-Guzmán, B.; Martín-Tapia, D.; González-Mariscal, L.; Quintanilla-Vega, B. Methyl parathion causes genetic damage in sperm and disrupts the permeability of the blood-testis barrier by an oxidant mechanism in mice. Toxicology 2020, 438, 152463. [Google Scholar] [CrossRef] [PubMed]
- Zuo, W.; Zhang, X.; Chang, J.B.; Ma, W.B.; Wei, J.J. Bromadiolone poisoning leading to subarachnoid haemorrhage: A case report and review of the literature. J. Clin. Pharm. Ther. 2019, 44, 958–962. [Google Scholar] [CrossRef]
- Lu, A.; Yuan, F.; Yao, Y.; Wen, W.; Lu, H.; Wu, S.; Wang, L. Reversible leukoencephalopathy caused by 2 rodenticides bromadiolone and fluroacetamide: A case report and literature review. Medicine 2021, 100, e25053. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.F.; Zhang, Z.J.; You, C.J.; Chen, L. A case of bromadiolone poisoning leading to digestive tract, abdominal hemorrhage and secondary paralytic ileus. J. Ind. Hyg. Occup. Dis. 2022, 40, 707–709. [Google Scholar]
- Wu, J.; Chen, J. One case of bromadiolone poisoning leading to intestinal necrosis and severe coagulopathy. J. Ind. Hyg. Occup. Dis. 2023, 41, 863–865. [Google Scholar]
- Hossain, M.; Suchi, T.T.; Samiha, F.; Islam, M.M.; Tully, F.A.; Hasan, J.; Rahman, M.A.; Shill, M.C.; Bepari, A.K.; Rahman, G.S.; et al. Coenzyme Q10 ameliorates carbofuran induced hepatotoxicity and nephrotoxicity in wister rats. Heliyon 2023, 9, e13727. [Google Scholar] [CrossRef]
- Liu, J.; Li, R.-X.; Yang, C.-S.; Zhao, L.-L.; Liu, X.; Zhang, B. Clinical study and observation on the effect of hemoperfusion therapy treatment on central nervous system injury in patients with 2, 4-dichlorophenoxyacetic acid poisoning. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 2403–2408. [Google Scholar]
- Yang, Q.; Guo, Y.; Lian, L.; Li, J.; Blatchley, E., 3rd. CH 3 NCl 2 Formation from Chlorination of Carbamate Insecticides. Environ. Sci. Technol. 2019, 53, 13098–13106. [Google Scholar]
- Freitas, L.M.; Valadares, L.P.d.A.; Camozzi, M.G.M.; de Oliveira, P.G.; Ferreira Machado, M.R.; Lima, F.C. Animal models in the neurotoxicology of 2,4-D. Hum. & Exp. Toxicol. 2019, 38, 1178–1182. [Google Scholar] [CrossRef]
- Chauhan, R.; Rawat, A.; Sama, S.; Goel, D.; Ahmad, S. A case series of 2,4 diethylamine poisoning–what the future holds for us. Trop. Dr. 2023, 53, 389–392. [Google Scholar] [CrossRef]
- Seven, B.; Kültiğin; Çavuşoğlu; Yalçin, E.; Acar, A. Investigation of cypermethrin toxicity in Swiss albino mice with physiological, genetic and biochemical approaches. Sci. Rep. 2022, 12, 11439. [Google Scholar] [CrossRef]
- Xie, Y.; Gong, L.; Liu, S.; Yan, J.; Zhao, S.; Xia, C.; Li, K.; Liu, G.; Mazhar, M.W.; Zhao, J. Antioxidants improve β-cypermethrin degradation by alleviating oxidative damage and increasing bioavailability by Bacillus cereus GW-01. Environ. Res. 2023, 236, 116680. [Google Scholar] [CrossRef] [PubMed]
- Richardson, J.R.; Fitsanakis, V.; Westerink, R.H.; Kanthasamy, A.G. Neurotoxicity of pesticides. Acta Neuropathol. 2019, 138, 343–362. [Google Scholar] [CrossRef] [PubMed]
- Ha, M.; Dong, Z.; Li, L.; Wang, L.; Liu, C. Cypermethrin induced liver oxidative DNA damage via the JNK/c-Jun pathway. J. Hyg. Res. 2023, 52, 497–505. [Google Scholar]
- Bhatta, O.P.; Chand, S.; Chand, H.; Poudel, R.C.; Lamichhane, R.P.; Singh, A.K.; Subedi, N. Imidacloprid poisoning in a young female: A case report. J. Med. Case Rep. 2023, 17, 43. [Google Scholar] [CrossRef] [PubMed]
- Sriapha, C.; Trakulsrichai, S.; Tongpoo, A.; Pradoo, A.; Rittilert, P.; Wananukul, W. Acute imidacloprid poisoning in Thailand. Ther. Clin. Risk Manag. 2020, 16, 1081–1088. [Google Scholar] [CrossRef]
- Sriapha, C.; Trakulsrichai, S.; Intaraprasong, P.; Wongvisawakorn, S.; Tongpoo, A.; Schimmel, J.; Wananukul, W. Imidacloprid poisoning case series: Potential for liver injury. Clin. Toxicol. 2020, 58, 136–138. [Google Scholar] [CrossRef]
- Perananthan, V.; Mohamed, F.; Shahmy, S.; Gawarammana, I.; Dawson, A.; Buckley, N. The clinical toxicity of imidacloprid self-poisoning following the introduction of newer formulations. Clin. Toxicol. 2021, 59, 347–350. [Google Scholar] [CrossRef]
- Mehtap, K.; Ezgi, Ö.; Tugce, B.; Fatma, K.E.; Gul, O. Benomyl induced oxidative stress related DNA damage and apoptosis in H9c2 cardiomyoblast cells. Toxicol. Vitr. 2021, 75, 105180. [Google Scholar] [CrossRef]
- Kumar, S.; Baggi, T.R.; Zughaibi, T. Forensic toxicological and analytical aspects of carbamate poisoning–A review. J. Forensic Leg. Med. 2022, 92, 102450. [Google Scholar] [CrossRef]
- Jiao, H.; Yuan, T.; Wang, X.; Zhou, X.; Ming, R.; Cui, H.; Hu, D.; Lu, P. Biochemical, histopathological and untargeted metabolomic analyses reveal hepatotoxic mechanism of acetamiprid to Xenopus laevis. Environ. Pollut. 2023, 317, 120765. [Google Scholar] [CrossRef]
- Kang, Y.; Wu, T.; Han, B.; Yang, S.; Wang, X.; Wang, Q.; Gao, J.; Dai, P. Interaction of acetamiprid, Varroa destructor, and Nosema ceranae in honey bees. J. Hazard. Mater. 2024, 471, 134380. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.-N.; Song, S.; Huang, Y.; Kannan, K.; Sun, H.; Zhang, T. Insights into free and conjugated forms of neonicotinoid insecticides in human serum and their association with oxidative stress. Environ. Health 2023, 1, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Costas-Ferreira, C.; Durán, R.; Faro, L.R.F. Toxic Effects of Glyphosate on the Nervous System: A Systematic Review. Int. J. Mol. Sci. 2022, 23, 4605. [Google Scholar] [CrossRef] [PubMed]
- Pirollo, K.F.; Moghe, M.; Guan, M.; Rait, A.S.; Wang, A.; Kim, S.-S.; Chang, E.H.; Harford, J.B. A pralidoxime nanocomplex formulation targeting transferrin receptors for reactivation of brain acetylcholinesterase after exposure of mice to an anticholinesterase organophosphate. Int. J. Nanomed. 2024, 19, 307–326. [Google Scholar] [CrossRef]
- Ajiboye, T.O.; Oladoye, P.O.; Olanrewaju, C.A.; Akinsola, G.O. Organophosphorus pesticides: Impacts, detection and removal strategies. Environ. Nanotechnol. Monit. Manag. 2022, 17, 100655. [Google Scholar] [CrossRef]
- Jayaraj, R.; Megha, P.; Sreedev, P. Organochlorine pesticides, their toxic effects on living organisms and their fate in the environment. Interdiscip. Toxicol. 2016, 9, 90. [Google Scholar] [CrossRef]
- Kamalesh, T.; Kumar, P.S.; Rangasamy, G. An insights of organochlorine pesticides categories, properties, eco-toxicity and new developments in bioremediation process. Environ. Pollut. 2023, 333, 122114. [Google Scholar] [CrossRef]
- Mdeni, N.L.; Adeniji, A.O.; Okoh, A.I.; Okoh, O.O. Analytical evaluation of carbamate and organophosphate pesticides in human and environmental matrices: A review. Molecules 2022, 27, 618. [Google Scholar] [CrossRef]
- Rezende-Teixeira, P.; Dusi, R.G.; Jimenez, P.C.; Espindola, L.S.; Costa-Lotufo, L.V. What can we learn from commercial insecticides? Efficacy, toxicity, environmental impacts, and future developments. Environ. Pollut. 2022, 300, 118983. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.R.; Alagawany, M.; Bilal, R.M.; Gewida, A.G.; Dhama, K.; Abdel-Latif, H.M.; Amer, M.S.; Rivero-Perez, N.; Zaragoza-Bastida, A.; Binnaser, Y.S. An overview on the potential hazards of pyrethroid insecticides in fish, with special emphasis on cypermethrin toxicity. Animals 2021, 11, 1880. [Google Scholar] [CrossRef]
- Jacob, M.S.; Iyyadurai, R.; Jose, A.; Fleming, J.J.; Rebekah, G.; Zachariah, A.; Hansdak, S.G.; Alex, R.; Chandiraseharan, V.K.; Lenin, A. Clinical presentation of type 1 and type 2 pyrethroid poisoning in humans. Clin. Toxicol. 2022, 60, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef]
- Al-Shehri, S.S. Reactive oxygen and nitrogen species and innate immune response. Biochimie 2021, 181, 52–64. [Google Scholar] [CrossRef]
- Black, H.S. A synopsis of the associations of oxidative stress, ROS, and antioxidants with diabetes mellitus. Antioxidants 2022, 11, 2003. [Google Scholar] [CrossRef]
- Reddy, V.P. Oxidative stress in health and disease. Biomedicines 2023, 11, 2925. [Google Scholar] [CrossRef]
- Liu, T.; Sun, L.; Zhang, Y.; Wang, Y.; Zheng, J. Imbalanced GSH/ROS and sequential cell death. J. Biochem. Mol. Toxicol. 2022, 36, e22942. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Valbuena, D.S.; Meléndez-Flórez, M.P.; Villegas, V.E.; Sánchez, M.C.; Rondón-Lagos, M. Daño celular y genético como determinantes de la toxicidad de los plaguicidas. Cienc. En Desarro. 2020, 11, 25–42. [Google Scholar] [CrossRef]
- Kiani, Z.; Asadikaram, G.; Faramarz, S.; Salimi, F.; Ebrahimi, H. Pesticide exposure and Alzheimer’s disease: A case-control Study. Curr. Alzheimer Res. 2022, 19, 892–903. [Google Scholar] [CrossRef] [PubMed]
- Mazari, A.M.; Zhang, L.; Ye, Z.-W.; Zhang, J.; Tew, K.D.; Townsend, D.M. The multifaceted role of glutathione S-transferases in health and disease. Biomolecules 2023, 13, 688. [Google Scholar] [CrossRef]
- Georgiou-Siafis, S.K.; Tsiftsoglou, A.S. The key role of GSH in keeping the redox balance in mammalian cells: Mechanisms and significance of GSH in detoxification via formation of conjugates. Antioxidants 2023, 12, 1953. [Google Scholar] [CrossRef]
- Li, X.; Naseem, S.; Hussain, R.; Ghaffar, A.; Li, K.; Khan, A. Evaluation of DNA damage, biomarkers of oxidative stress, and status of antioxidant enzymes in freshwater fish (Labeo rohita) exposed to pyriproxyfen. Oxidative Med. Cell. Longev. 2022, 2022, 5859266. [Google Scholar] [CrossRef]
- Selmi, S.; Rtibi, K.; Grami, D.; Sebai, H.; Marzouki, L. Malathion, an organophosphate insecticide, provokes metabolic, histopathologic and molecular disorders in liver and kidney in prepubertal male mice. Toxicol. Rep. 2018, 5, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Sukkar, D.; Wagner, L.; Bonnefoy, A.; Falla-Angel, J.; Laval-Gilly, P. Imidacloprid and amitraz differentially alter antioxidant enzymes in honeybee (Apis mellifera) hemocytes when exposed to microbial pathogen-associated molecular patterns. Sci. Total Environ. 2025, 969, 178868. [Google Scholar] [CrossRef]
- Hilgert Jacobsen-Pereira, C.; dos Santos, C.R.; Troina Maraslis, F.; Pimentel, L.; Feijó, A.J.L.; Iomara Silva, C.; de Medeiros, G.d.S.; Costa Zeferino, R.; Curi Pedrosa, R.; Weidner Maluf, S. Markers of genotoxicity and oxidative stress in farmers exposed to pesticides. Ecotoxicol. Environ. Saf. 2018, 148, 177–183. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, V.; Thakur, S.; Banerjee, B.D.; Rautela, R.S.; Grover, S.S.; Rawat, D.S.; Pasha, S.T.; Jain, S.K.; Ichhpujani, R.L. Paraoxonase-1 genetic polymorphisms and susceptibility to DNA damage in workers occupationally exposed to organophosphate pesticides. Toxicol. Appl. Pharmacol. 2011, 252, 130–137. [Google Scholar] [CrossRef]
- Andersen, H.R.; Wohlfahrt-Veje, C.; Dalgård, C.; Christiansen, L.; Main, K.M.; Nellemann, C.; Murata, K.; Jensen, T.K.; Skakkebæk, N.E.; Grandjean, P. Paraoxonase 1 polymorphism and prenatal pesticide exposure associated with adverse cardiovascular risk profiles at school age. PLoS ONE 2012, 7, e36830. [Google Scholar] [CrossRef]
- Andersen, H.R.; Tinggaard, J.; Grandjean, P.; Jensen, T.K.; Dalgård, C.; Main, K.M. Prenatal pesticide exposure associated with glycated haemoglobin and markers of metabolic dysfunction in adolescents. Environ. Res. 2018, 166, 71–77. [Google Scholar] [CrossRef]
- Declerck, K.; Remy, S.; Wohlfahrt-Veje, C.; Main, K.M.; Van Camp, G.; Schoeters, G.; Vanden Berghe, W.; Andersen, H.R. Interaction between prenatal pesticide exposure and a common polymorphism in the PON1 gene on DNA methylation in genes associated with cardio-metabolic disease risk—An exploratory study. Clin. Epigenetics 2017, 9, 35. [Google Scholar] [CrossRef]
- Organisation for Economic Co-operation and Development. Test No. 451: Carcinogenicity Studies. Available online: https://www.oecd.org/en/publications/2018/06/test-no-451-carcinogenicity-studies_g1gh2955.html (accessed on 1 June 2025).
- Organisation for Economic Co-operation and Development. Test No. 414: Prenatal Developmental Toxicity Study. Available online: https://www.oecd.org/en/publications/2018/06/test-no-414-prenatal-developmental-toxicity-study_g1gh293d.html (accessed on 1 June 2025).
- Organisation for Economic Co-operation and Development. Test No. 416: Two-Generation Reproduction Toxicity. Available online: https://www.oecd.org/en/publications/test-no-416-two-generation-reproduction-toxicity_9789264070868-en.html (accessed on 1 June 2025).
- Ames, B.N.; McCann, J.; Yamasaki, E. Methods for detecting carcinogens and mutagens with the salmonella/mammalian-microsome mutagenicity test. Mutat. Res./Environ. Mutagen. Relat. Subj. 1975, 31, 347–363. [Google Scholar] [CrossRef] [PubMed]
- Organisation for Economic Co-operation and Development. Test No. 487: In Vitro Mammalian Cell Micronucleus Test. Available online: https://www.oecd.org/content/dam/oecd/en/publications/reports/2023/07/test-no-487-in-vitro-mammalian-cell-micronucleus-test_g1g6fb2a/9789264264861-en.pdf (accessed on 1 June 2025).
- Organisation for Economic Co-operation and Development. Test No. 473: In Vitro Mammalian Chromosomal Aberration Test. Available online: https://www.oecd.org/env/test-no-473-in-vitro-mammalian-chromosomal-aberration-test-9789264264649-en.html (accessed on 1 June 2025).
- Collins, A.R. The Comet Assay for DNA Damage and Repair: Principles, Applications, and Limitations. Mol. Biotechnol. 2004, 26, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Moser, V.C. Functional assays for neurotoxicity testing. Toxicol. Pathol. 2011, 39, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Pope, C.N. Organophosphorus pesticides: Do they all have the same mechanism of toxicity? J. Toxicol. Environ. Health Part B Crit. Rev. 1999, 2, 161–181. [Google Scholar] [CrossRef]
- Organisation for Economic Co-operation and Development. Test No. 424: Neurotoxicity Study in Rodents. Available online: https://www.oecd.org/env/test-no-424-neurotoxicity-study-in-rodents-9789264071025-en.html (accessed on 1 June 2025).
- Nabi, S.U.; Ali, S.I.; Rather, M.A.; Sheikh, W.M.; Altaf, M.; Singh, H.; Mumtaz, P.T.; Mishra, N.C.; Nazir, S.U.; Bashir, S.M. Organoids: A new approach in toxicity testing of nanotherapeutics. J. Appl. Toxicol. 2022, 42, 52–72. [Google Scholar] [CrossRef]
- Song, L.; Zan, C.; Liang, Z.; Chen, X.; Li, J.; Ren, N.; Shi, Y.; Zhang, M.; Lan, L.; Li, H. Potential value of FAPI PET/CT in the detection and treatment of fibrosing mediastinitis: Preclinical and pilot clinical investigation. Mol. Pharm. 2023, 20, 4307–4318. [Google Scholar] [CrossRef]
- Wang, X.; Yan, M.; Zhou, J.; Song, W.; Xiao, Y.; Cui, C.; Gao, W.; Ke, F.; Zhu, J.; Gu, Z. Delivery of acetamiprid to tea leaves enabled by porous silica nanoparticles: Efficiency, distribution and metabolism of acetamiprid in tea plants. BMC Plant Biol. 2021, 21, 337. [Google Scholar] [CrossRef]
- Ireland, D.; Zhang, S.; Bochenek, V.; Hsieh, J.-H.; Rabeler, C.; Meyer, Z.; Collins, E.-M.S. Differences in neurotoxic outcomes of organophosphorus pesticides revealed via multi-dimensional screening in adult and regenerating planarians. Front. Toxicol. 2022, 4, 948455. [Google Scholar] [CrossRef]
- Kasteel, E.E.; Nijmeijer, S.M.; Darney, K.; Lautz, L.S.; Dorne, J.L.C.; Kramer, N.I.; Westerink, R.H. Acetylcholinesterase inhibition in electric eel and human donor blood: An in vitro approach to investigate interspecies differences and human variability in toxicodynamics. Arch. Toxicol. 2020, 94, 4055–4065. [Google Scholar] [CrossRef] [PubMed]
- Keresteš, O.; Pohanka, M. Affordable portable platform for classic photometry and low-cost determination of cholinesterase activity. Biosensors 2023, 13, 599. [Google Scholar] [CrossRef]
- Akiyama, H.; Iwasaki, Y.; Ito, R. Basic Principles for Setting MRLs for Pesticides in Food Commodities in Japan. Food Saf. 2024, 12, 34–51. [Google Scholar] [CrossRef]
- Tanaka, R. Setting of Maximum Residue Limits (MRLs) for Pesticides in Foods. Yakugaku Zasshi J. Pharm. Soc. Jpn. 2025, 145, 95–99. [Google Scholar] [CrossRef]
- Díaz-Vallejo, J.; Barraza-Villarreal, A.; Yañez-Estrada, L.; Hernández-Cadena, L. Plaguicidas en alimentos: Riesgo a la salud y marco regulatorio en Veracruz, México. Salud Pública De México 2021, 63, 486–497. [Google Scholar] [CrossRef] [PubMed]
- Jia, Q.; Liao, G.-Q.; Chen, L.; Qian, Y.-Z.; Yan, X.; Qiu, J. Pesticide residues in animal-derived food: Current state and perspectives. Food Chem. 2024, 438, 137974. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Yu, H.; Guo, Y.; Xie, Y.; Cheng, Y.; Qian, H.; Yao, W. Recent advance in probiotics for the elimination of pesticide residues in food and feed: Mechanisms, product toxicity, and reinforcement strategies. Crit. Rev. Food Sci. Nutr. 2024, 64, 12025–12039. [Google Scholar] [CrossRef] [PubMed]
- Radulović, J.; Lučić, M.; Onjia, A. GC-MS/MS and LC-MS/MS analysis followed by risk ranking of mepiquat and pyrethroids in coffee. J. Food Compos. Anal. 2024, 129, 106100. [Google Scholar] [CrossRef]
- Karimi, P.; Sadeghi, S.; Kariminejad, F.; Sadani, M.; Sheikh Asadi, A.M.; Oghazyan, A.; Bay, A.; Mahmudiono, T.; Fakhri, Y. The concentration of pesticides in tomato: A global systematic review, meta-analysis, and health risk assessment. Environ. Sci. Pollut. Res. 2023, 30, 103390–103404. [Google Scholar] [CrossRef] [PubMed]
- Arbo, M.D.; Garcia, S.C.; Sarpa, M.; Junior, F.M.D.S.; Nascimento, S.N.; Garcia, A.L.H.; Da Silva, J. Brazilian workers occupationally exposed to different toxic agents: A systematic review on DNA damage. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2022, 879, 503519. [Google Scholar] [CrossRef]
- Scorza, F.A.; Finsterer, J.; Beltramim, L.; Bombardi, L.M.; de Almeida, A. Telomere length and pesticide residues in food-A causal link? J. Acad. Nutr. Diet. 2023, S2212–S2672. [Google Scholar] [CrossRef]
- Rodríguez-López, A.; Mejía-Saucedo, R.; Calderón-Hernández, J.; Labrada-Martagón, V.; Yáñez-Estrada, L. Alteraciones del ciclo menstrual de adolescentes expuestas no ocupacionalmente a una mezcla de plaguicidas de una zona agrícola de San Luis Potosí, México. Estudio piloto. Rev. Int. De Contam. Ambient. 2020, 36, 997–1010. [Google Scholar] [CrossRef]
- de Andrade, J.C.; Galvan, D.; Kato, L.S.; Conte-Junior, C.A. Consumption of fruits and vegetables contaminated with pesticide residues in Brazil: A systematic review with health risk assessment. Chemosphere 2023, 322, 138244. [Google Scholar] [CrossRef]
- Park, B.K.; Kwon, S.H.; Yeom, M.S.; Joo, K.S.; Heo, M.J. Detection of pesticide residues and risk assessment from the local fruits and vegetables in Incheon, Korea. Sci. Rep. 2022, 12, 9613. [Google Scholar] [CrossRef]
- Qi, S.-Y.; Xu, X.-L.; Ma, W.-Z.; Deng, S.-L.; Lian, Z.-X.; Yu, K. Effects of organochlorine pesticide residues in maternal body on infants. Front. Endocrinol. 2022, 13, 890307. [Google Scholar] [CrossRef]
- Daisley, B.A.; Chernyshova, A.M.; Thompson, G.J.; Allen-Vercoe, E. Deteriorating microbiomes in agriculture–the unintended effects of pesticides on microbial life. Microbiome Res. Rep. 2022, 1, 6. [Google Scholar] [CrossRef]
- Arora, S.; Murmu, G.; Mukherjee, K.; Saha, S.; Maity, D. A comprehensive overview of nanotechnology in sustainable agriculture. J. Biotechnol. 2022, 355, 21–41. [Google Scholar] [CrossRef] [PubMed]
- Usman, M.; Farooq, M.; Wakeel, A.; Nawaz, A.; Cheema, S.A.; ur Rehman, H.; Ashraf, I.; Sanaullah, M. Nanotechnology in agriculture: Current status, challenges and future opportunities. Sci. Total Environ. 2020, 721, 137778. [Google Scholar] [CrossRef] [PubMed]
- Kah, M.; Kookana, R.S.; Gogos, A.; Bucheli, T.D. A critical evaluation of nanopesticides and nanofertilizers against their conventional analogues. Nat. Nanotechnol. 2018, 13, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Münzel, T.; Kuntic, M.; Lelieveld, J.; Aschner, M.; Nieuwenhuijsen, M.J.; Landrigan, P.J.; Daiber, A. The links between soil and water pollution and cardiovascular disease. Atherosclerosis 2025, 403, 119160. [Google Scholar] [CrossRef]
- Wang, Q.; Shan, C.; Zhang, P.; Zhao, W.; Zhu, G.; Sun, Y.; Wang, Q.; Jiang, Y.; Shakoor, N.; Rui, Y. The combination of nanotechnology and potassium: Applications in agriculture. Environ. Sci. Pollut. Res. 2024, 31, 1890–1906. [Google Scholar] [CrossRef]
- Monteiro, R.A.; Camara, M.C.; de Oliveira, J.L.; Campos, E.V.R.; Carvalho, L.B.; de Freitas Proenca, P.L.; Guilger-Casagrande, M.; Lima, R.; do Nascimento, J.; Gonçalves, K.C. Zein based-nanoparticles loaded botanical pesticides in pest control: An enzyme stimuli-responsive approach aiming sustainable agriculture. J. Hazard. Mater. 2021, 417, 126004. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Li, L.; Zhao, R.; Sun, Z.; Wang, Y.; Yu, M.; Pan, S.; Guo, X.; Xu, Y.; Wang, H. Nanoencapsulation-based fabrication of eco-friendly pH-responsive pyraclostrobin formulations with enhanced photostability and adhesion to leaves. J. Environ. Chem. Eng. 2023, 11, 109688. [Google Scholar] [CrossRef]
- Mishra, A.K.; Pandey, M.; Dewangan, H.K.; Sl, N.; Sahoo, P.K. A comprehensive review on liver targeting: Emphasis on nanotechnology-based molecular targets and receptors mediated approaches. Curr. Drug Targets 2022, 23, 1381–1405. [Google Scholar]
- Li, C.; Han, Y.; Gao, T.; Zhang, J.; Xu, D.-X.; Wāng, Y. Insecticidal activity of metallic nanopesticides synthesized from natural resources: A review. Environ. Chem. Lett. 2023, 21, 1141–1176. [Google Scholar] [CrossRef]
- Agredo-Gomez, A.; Molano-Molano, J.; Portela-Patiño, M.; Rodríguez-Páez, J. Use of ZnO nanoparticles as a pesticide: In vitro evaluation of their effect on the phytophagous Puto barberi (mealybug). Nano-Struct. Nano-Objects 2024, 37, 101095. [Google Scholar] [CrossRef]
- Badawy, A.A.; Abdelfattah, N.A.; Salem, S.S.; Awad, M.F.; Fouda, A. Efficacy assessment of biosynthesized copper oxide nanoparticles (CuO-NPs) on stored grain insects and their impacts on morphological and physiological traits of wheat (Triticum aestivum L.) plant. Biology 2021, 10, 233. [Google Scholar] [CrossRef]
- El-Ansary, M.S.M.; Hamouda, R.A.; Elshamy, M.M. Using biosynthesized zinc oxide nanoparticles as a pesticide to alleviate the toxicity on banana infested with parasitic-nematode. Waste Biomass Valorization 2022, 13, 405–415. [Google Scholar] [CrossRef]
- Gupta, R.; Malik, P.; Rani, R.; Solanki, R.; Ameta, R.K.; Malik, V.; Mukherjee, T.K. Recent progress on nanoemulsions mediated pesticides delivery: Insights for agricultural sustainability. Plant Nano Biol. 2024, 8, 100073. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, Y.; Cao, C.; Zhao, P.; Huang, Q.; Cao, L. Optimization and characterization of pyraclostrobin nanoemulsion for pesticide delivery: Improving activity, reducing toxicity, and protecting ecological environment. Colloids Surf. A Physicochem. Eng. Asp. 2024, 692, 134051. [Google Scholar] [CrossRef]
- Wei, N.; Hou, C.; Liu, Z.; Liang, Q.; Lv, Z.; Meng, X.; Feng, J. Preparation of fenpropathrin nanoemulsions for eco-friendly management of Helicoverpa armigera: Improved insecticidal activity and biocompatibility. Colloids Surf. A Physicochem. Eng. Asp. 2023, 656, 130442. [Google Scholar] [CrossRef]
- Abdelaal, K.; Essawy, M.; Quraytam, A.; Abdallah, F.; Mostafa, H.; Shoueir, K.; Fouad, H.; Hassan, F.A.; Hafez, Y. Toxicity of essential oils nanoemulsion against Aphis craccivora and their inhibitory activity on insect enzymes. Processes 2021, 9, 624. [Google Scholar] [CrossRef]
- Beegum, S.; Das, S. Nanosensors in agriculture. In Agricultural Nanobiotechnology; Elsevier: Amsterdam, The Netherlands, 2022; pp. 465–478. [Google Scholar]
- Wu, J.; Liu, H.; Chen, W.; Ma, B.; Ju, H. Device integration of electrochemical biosensors. Nat. Rev. Bioeng. 2023, 1, 346–360. [Google Scholar] [CrossRef]
- Furizal, F.; Ma’arif, A.; Firdaus, A.A.; Rahmaniar, W. Future potential of E-nose technology: A review. Int. J. Robot. Control Syst. 2023, 3, 449–469. [Google Scholar] [CrossRef]
- Tan, J.; Xu, J. Applications of electronic nose (e-nose) and electronic tongue (e-tongue) in food quality-related properties determination: A review. Artif. Intell. Agric. 2020, 4, 104–115. [Google Scholar] [CrossRef]
- Ali, M.M.; Hashim, N.; Abd Aziz, S.; Lasekan, O. Principles and recent advances in electronic nose for quality inspection of agricultural and food products. Trends Food Sci. Technol. 2020, 99, 1–10. [Google Scholar] [CrossRef]
- Moshayedi, A.J.; Sohail Khan, A.; Hu, J.; Nawaz, A.; Zhu, J. E-nose-driven advancements in ammonia gas detection: A comprehensive review from traditional to cutting-edge systems in indoor to outdoor agriculture. Sustainability 2023, 15, 11601. [Google Scholar] [CrossRef]
- Kah, M.; Walch, H.; Hofmann, T. Environmental fate of nanopesticides: Durability, sorption and photodegradation of nanoformulated clothianidin. Environ. Sci. Nano 2018, 5, 882–889. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, S.; Duan, Z.; Wang, L. Pulmonary toxicology assessment of polyethylene terephthalate nanoplastic particles in vitro. Environ. Int. 2022, 162, 107177. [Google Scholar] [CrossRef] [PubMed]
- Teng, M.; Zhao, X.; Wang, C.; Wang, C.; White, J.C.; Zhao, W.; Zhou, L.; Duan, M.; Wu, F. Polystyrene nanoplastics toxicity to zebrafish: Dysregulation of the brain–intestine–microbiota axis. ACS Nano 2022, 16, 8190–8204. [Google Scholar] [CrossRef]
- Hou, S.; Li, C.; Wang, Y.; Sun, J.; Guo, Y.; Ning, X.; Ma, K.; Li, X.; Shao, H.; Cui, G. Silica nanoparticles cause activation of NLRP3 inflammasome in-vitro model-using microglia. Int. J. Nanomed. 2022, 17, 5247. [Google Scholar] [CrossRef] [PubMed]
- Miao, W.; Zhu, B.; Xiao, X.; Li, Y.; Dirbaba, N.B.; Zhou, B.; Wu, H. Effects of titanium dioxide nanoparticles on lead bioconcentration and toxicity on thyroid endocrine system and neuronal development in zebrafish larvae. Aquat. Toxicol. 2015, 161, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Magaye, R.; Zhao, J. Recent progress in studies of metallic nickel and nickel-based nanoparticles’ genotoxicity and carcinogenicity. Environ. Toxicol. Pharmacol. 2012, 34, 644–650. [Google Scholar] [CrossRef] [PubMed]
- Moo-Muñoz, A.J.; Azorín-Vega, E.P.; Ramírez-Durán, N.; Moreno-Pérez, M.P. Estado de la producción y consumo de plaguicidas en méxico†[state of the production and consumption of pesticides in México]. Trop. Subtrop. Agroecosystems 2020, 23, 43. [Google Scholar] [CrossRef]
- Molteni, R.; Alonso-Prados, J.L. National Institute for Agricultural and Food Research and Technology (INIA). In Study of the Different Evaluation Areas in the Pesticide Risk Assessment Process; EFSA Journal: Parma, Italy, 2020; Volume 18, p. e181113. [Google Scholar]
- Kudsk, P.; Mathiassen, S.K. Pesticide regulation in the European Union and the glyphosate controversy. Weed Sci. 2020, 68, 214–222. [Google Scholar] [CrossRef]
- EPA, United States Environmental Protection Agency. Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA) and Federal Facilities. Available online: https://www.epa.gov/enforcement/federal-insecticide-fungicide-and-rodenticide-act-fifra-and-federal-facilities#Summary (accessed on 1 June 2025).
- Geissen, V.; Silva, V.; Lwanga, E.H.; Beriot, N.; Oostindie, K.; Bin, Z.; Pyne, E.; Busink, S.; Zomer, P.; Mol, H.; et al. Cocktails of pesticide residues in conventional and organic farming systems in Europe–Legacy of the past and turning point for the future. Environ. Pollut. 2021, 278, 116827. [Google Scholar] [CrossRef]
- European Union. Reglamento (ce) n o 1107/2009 del Parlamento Europeo y del Consejo de 21 de Octubre de 2009 Relativo a la Comercialización de Productos Fitosanitarios y por el que se Derogan las Directivas 79/117/CEE y 91/414/CEE del Consejo. Available online: https://eur-lex.europa.eu/legal-content/ES/TXT/?uri=CELEX%3A32009R1107&qid=1739790021373 (accessed on 1 June 2025).
- Bejarano González, F.; AguileraMárquez, D.; Álvarez Solís, J.D.; Arámbula Meraz, E.; Arellano Aguilar, O.; Bastidas Bastidas, P.d.J.; Beltrán Camacho, V.d.l.A.; Bernardino Hernández, H.U.; Betancourt Lozano, M.; Calderón Vázquez, C.L. Los plaguicidas altamente peligrosos en México. Texcoco RAPAM 2015, 263. [Google Scholar]
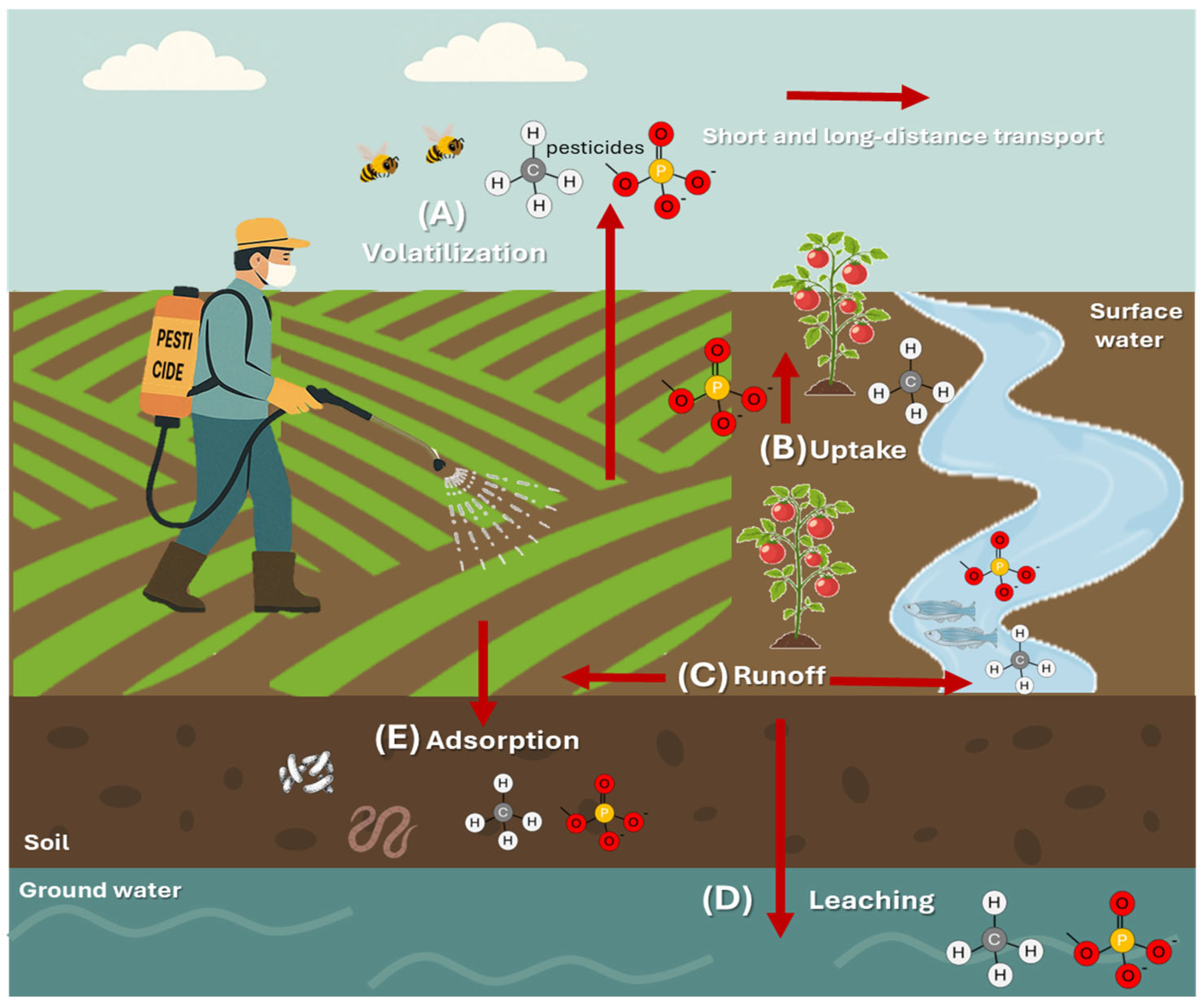
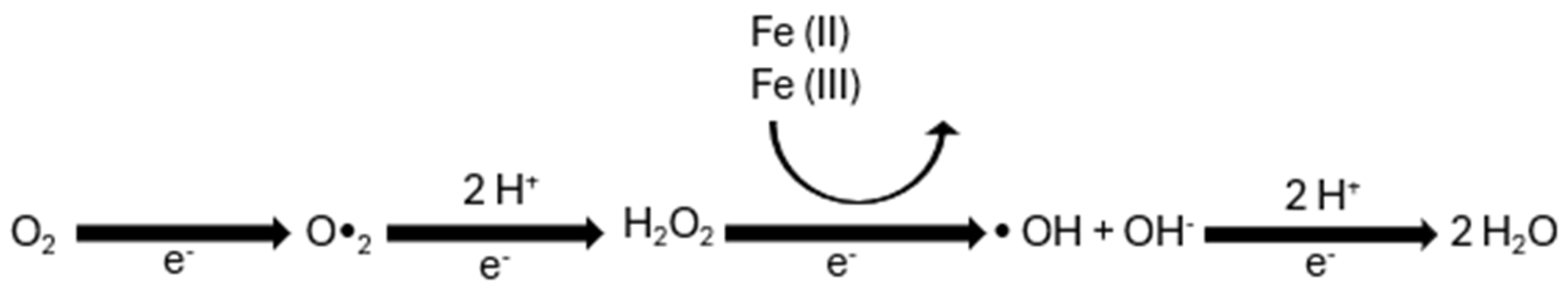

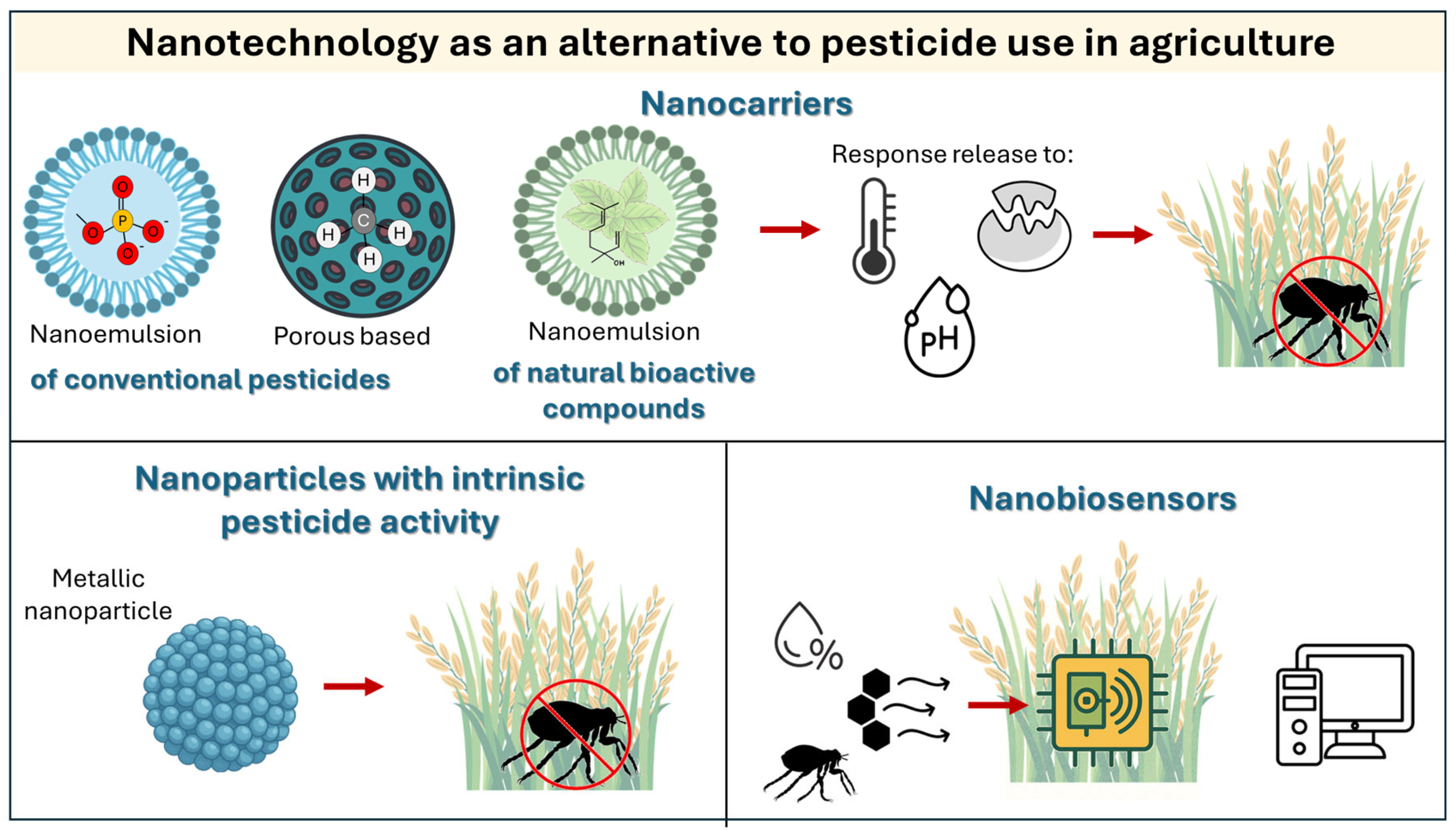
| Pesticide | Type | Target Organism | Associate Damage | DL50 | Reference |
|---|---|---|---|---|---|
| Parathion/Methyl parathion | Organophosphate | Herbicide | In vivo studies have linked its use to the development of heart disease, an increase in CAT, TBARS, and GPx biomarkers, and a decrease in SOD, resulting in an overload of oxidative stress, alterations in acetylcholinesterase levels, and overstimulation of the central nervous system. | 6–14 mg/kg/2–30 mg/kg | [47,48] |
| Rotencidal | Coumarin | Bromadiolone | At low concentrations, it has been linked to the appearance of oxidative stress in short exposures and the destabilization of biomolecules. For acute exposures, bromadiolone has been linked to the inhibition of the carboxylation of vitamin K-dependent coagulation factors (II, VII, IX, and X), producing an anticoagulant effect. It is also widely related to the deterioration of the intestinal mucosa and bleeding in the digestive and urinary tract. There have been cases related to exposure to bromadiolone and the development of diseases of the central nervous system or conditions affecting the brain mass, such as leukoencephalopathy. | 1.125 mg/kg | [49,50,51,52] |
| Carbofuran | Carbamates | Herbicide and insecticide | After exposure to humans, a considerable increase in oxidative stress has been reported in several organs, including the liver, brain, kidney, and heart, which leads to the propagation of necrosis in hepatic and nephrotic cells. | 8–14 mg/kg | [53,54,55] |
| 2,4-D | Phenoxyacetic Acid | Herbicide | It is a widely used compound that causes significant damage to the environment and humans. In addition to the increase in oxidative stress and destabilization of biomolecules, it has been highly related to the inhibition of growth in cells and tissues. Its effects have been studied in different in vivo models, which found a behavioral pattern in terms of neurotoxicity and a decrease in motor skills was observed. Biochemically, it showed a decrease in serotonin levels or a decrease in dopamine levels and its metabolites depending on the brain area analyzed. | 639–764 mg/kg | [56,57] |
| Cypermethrin | Pyrethroid | Acaracide | Often used in mixtures, its acute and subacute exposure causes clinical symptoms, such as pneumonia, acute kidney injury, tearing, acute respiratory failure, and diarrhea. Cypermethrin primarily acts by delaying the closure of voltage-sensitive sodium channels. Most of the effects caused by poisoning with this pesticide are neurotoxic, particularly in the respiratory and gastrointestinal tracts. Cases of cardiotoxic conditions have been reported, but these are insufficient to associate them with cypermethrin poisoning. | 240–4123 mg/kg | [58,59,60,61] |
| Imidacloprid | Neonicotinoid | Insecticide | The most widely used neonicotinoid in the world is known to produce oxidative stress upon exposure. It has also been observed that, in the case of oral ingestion, the main symptoms and associated damage are gastrointestinal without corrosive lesions and neurological effects, such as dyspnea, coma, and diaphoresis. There is a particular relationship between imidacloprid poisoning and the development of various types of liver damage, which sometimes occurs late. | 450–650 mg/kg | [62,63,64,65] |
| Benomyl | Carbamate | Fungicide | Linked to the generation of systemic oxidative stress. In vitro studies in rat cardiomyoblasts (H9c2) demonstrated a 2-fold increase in ROS and glutathione levels measured in cells exposed to benomyl compared to controls. Exposure to benomyl has been shown to induce apoptosis, oxidative stress, and DNA damage. | >10,000 mg/kg | [66,67] |
| Acetamiprid | Neonicotinoid | Insecticide | The severe oxidative stress generated by this pesticide is linked to genotoxic damage and the formation of cleavages in tRNA due to the changes it generates in biomolecules. Isolated cases have been reported where poisoning with acetamiprid triggered lactic acidosis, hyperglycemia, and intestinal obstruction. | 217 mg/kg | [68,69,70] |
| Glyphosate | Organophosphate | Herbicide | Exposure to pesticides during the early stages of development can severely disrupt normal cell growth by interfering with several critical signaling pathways, leading to significant changes in cell differentiation, neuronal development, and myelination. Furthermore, glyphosate appears to have a notable toxic effect on neurotransmission, generating oxidative stress, neuroinflammation, and mitochondrial dysfunction, which can result in neuronal death through mechanisms such as autophagy, necrosis, or apoptosis. These neurotoxic effects are also associated with the development of behavioral disorders and impaired motor skills. | 4320 mg/kg | [71,72] |
| Test Name | Evaluated Focus | Basis | Reference |
|---|---|---|---|
Acute Toxicity Evaluation (Oral, dermal, inhalation)
| Acute toxicity tests. | Designed to assess the immediate effects of exposure to different pesticides. Tests are classified by exposure routes and evaluated within 24 to 96 h. | [98,99,100] |
Chronic Toxicity Evaluation
| Chronic toxicity tests. | Chronic toxicity tests evaluate the effects of prolonged and repeated low-dose exposures. | [98,99,100] |
Genotoxicity Tests
| Toxicological studies based on a pesticide’s ability to damage DNA and cause point mutations. | Due to the high reactivity of pesticides, they can induce mutations, chromosomal aberrations, or DNA strand breaks. These tests encompass the main mechanisms of DNA damage caused by pesticides. | [101,102,103,104] |
Neurotoxicity Studies
| Evaluation of pesticide effects on the central nervous system, especially those caused by organophosphates and carbamates. | By detecting the inhibition of key enzymes in the central nervous system, it is possible to identify motor or behavioral alterations in animal models and relate them to cognitive impairment. | [105,106,107] |
Toxicokinetic Assays
| General evaluation of a pesticide. | Analyzing ADME helps understand how long a pesticide can remain reactive in the body and where it might accumulate. | [108,109,110] |
Biochemical Tests
| Evaluation of alterations in enzymatic systems based on the central nervous system. | These tests assess a pesticide’s effects on specific metabolic and enzymatic systems, usually in the liver or nervous system, depending on the pesticide’s nature. | [111,112,113] |
| Pesticide | Study | Conclusion | Reference |
|---|---|---|---|
| Mixtures of organochlorine and organophosphate pesticides, most notably 2,4-DDE, 4,4-DDE, γ-BHC, and β-BHC | A group of 29 adolescents was studied, with 75% of them belonging to families of agricultural day laborers. Additionally, 43.7% had gardens at home, and 64.28% used pesticides. The study linked interactions with pesticides to menstrual cycle disruption. | In serum levels of sexual hormones, more than 40% of adolescents presented alterations in their hormonal profile, and 96.9% of adolescents had detectable plasma levels of pesticides. However, some indications suggested a relationship between 4,4-DDE in plasma and alterations in the menstrual cycle; no statistically significant differences were found. This may be due to the group chosen and the time designated for the study. | [123] |
| More than 100 pesticides classified as carcinogenic by the EPA | A meta-analysis of the presence of pesticides in different fruits and vegetables. | Within the study, various pesticides found in fruits and vegetables, including grapes, mangoes, tomatoes, strawberries, apples, and peppers, were compiled. These pesticides are widely linked to the development of chronic degenerative diseases, alterations in the endocrine system, and disruptions in reproductive health in both adults and children. | [124] |
| A total of 91 samples were identified as exceeding the permitted MRLs in Korea, including Chlorfenapyr, Procymidone, Etofenprox, Pendimethalin, and Fluopyram | A total of 1146 fruits and vegetables were collected from a Korean market and tested for 15 pesticides of interest. | Although the identified pesticides are related to damage to the central nervous system, endocrine system, and liver conditions, it is necessary to note that they were identified in only 8.9% of the total samples, compared to other countries, where this percentage is lower. | [125] |
| Pesticides such as DDT, dieldrin, and HCB | The factors influencing the presence of organochlorine pesticides in breast milk and the resulting damage to children were addressed. | Organochlorine pesticides can act as endocrine disruptors, and estrogen-inducing pesticides can accumulate with exposure to water, soil, environmental exposure, or food. Levels of HCB or DDT residues have been linked to decreased birth weight and head circumference. The opposite effect can occur with certain OGC pesticides, thanks to lipogenesis. | [126] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñoz-Bautista, J.M.; Bernal-Mercado, A.T.; Martínez-Cruz, O.; Burgos-Hernández, A.; López-Zavala, A.A.; Ruiz-Cruz, S.; Ornelas-Paz, J.d.J.; Borboa-Flores, J.; Ramos-Enríquez, J.R.; Del-Toro-Sánchez, C.L. Environmental and Health Impacts of Pesticides and Nanotechnology as an Alternative in Agriculture. Agronomy 2025, 15, 1878. https://doi.org/10.3390/agronomy15081878
Muñoz-Bautista JM, Bernal-Mercado AT, Martínez-Cruz O, Burgos-Hernández A, López-Zavala AA, Ruiz-Cruz S, Ornelas-Paz JdJ, Borboa-Flores J, Ramos-Enríquez JR, Del-Toro-Sánchez CL. Environmental and Health Impacts of Pesticides and Nanotechnology as an Alternative in Agriculture. Agronomy. 2025; 15(8):1878. https://doi.org/10.3390/agronomy15081878
Chicago/Turabian StyleMuñoz-Bautista, Jesús Martín, Ariadna Thalía Bernal-Mercado, Oliviert Martínez-Cruz, Armando Burgos-Hernández, Alonso Alexis López-Zavala, Saul Ruiz-Cruz, José de Jesús Ornelas-Paz, Jesús Borboa-Flores, José Rogelio Ramos-Enríquez, and Carmen Lizette Del-Toro-Sánchez. 2025. "Environmental and Health Impacts of Pesticides and Nanotechnology as an Alternative in Agriculture" Agronomy 15, no. 8: 1878. https://doi.org/10.3390/agronomy15081878
APA StyleMuñoz-Bautista, J. M., Bernal-Mercado, A. T., Martínez-Cruz, O., Burgos-Hernández, A., López-Zavala, A. A., Ruiz-Cruz, S., Ornelas-Paz, J. d. J., Borboa-Flores, J., Ramos-Enríquez, J. R., & Del-Toro-Sánchez, C. L. (2025). Environmental and Health Impacts of Pesticides and Nanotechnology as an Alternative in Agriculture. Agronomy, 15(8), 1878. https://doi.org/10.3390/agronomy15081878







