Abstract
Strong UV-B radiation is one of the main climatic characteristics of the Qinghai–Tibet Plateau. Plants grown on the Tibetan Plateau are exposed to high-intensity UV radiation and therefore require effective mechanisms to adapt to these stresses. However, little attention has been paid to the response of grass–endophytic fungi symbiosis to UV-B radiation in this area. In this study, we investigated the relationship between Epichloë gansuensis and the growth and antioxidant responses of Achnatherum inebrians seedlings exposed to different UV-B doses, aiming to evaluate the growth and antioxidant capacity of A. inebrians seedlings under UV-B stress. The plant height, tillers, biomass, electrical conductivity, soluble sugars, malondialdehyde (MDA), proline, hydrogen peroxide (H2O2), superoxide dismutase (SOD), polyphenol oxidase (POD), and catalase (CAT) of A. inebrians seedlings were determined under different intensities of UV-B radiation treatments. The results showed that, with the increase in UV-B radiation intensity, the plant height, tiller and biomass of A. inebrians seedlings showed a decreasing trend, the electrical conductivity increased, malondialdehyde content increased, soluble sugar and proline content decreased, SOD, POD, and CAT activities showed a decreasing trend, and the content of H2O2 increased, which means that the UV-B radiation was able to inhibit the morphogenesis and aggravate the membrane lipid peroxidation of A. inebrians seedlings. The tolerance of the A. inebrians–E. gansuensis symbiont to UV-B may enable it to have a high infection rate on the Qinghai–Tibet Plateau.
1. Introduction
Since the 1980s, human activities, particularly the emission of chlorofluorocarbons (CFCs) have significantly depleted the stratospheric ozone. According to 2024 data released by the National Aeronautics and Space Administration (NASA), the Antarctic ozone hole reached an area of 23.1 million km2 in 2023. While this was smaller than the peak levels recorded in the early 2000s, it exceeded the minimal extent observed in 2019, with a renewed downward trend emerging in 2024 [1]. Additionally, Arctic ozone depletion exhibits high interannual variability; for instance, unique meteorological conditions during the winter and spring of 2020 triggered an unprecedented ozone loss event [2]. These depletion dynamics have led to elevated levels of UV-B radiation reaching the Earth’s surface. UV-B radiation is an abiotic stress factor that impacts plant growth, development, and environmental adaptation [3]. Numerous studies have shown that increased UV-B has adverse effects on both aquatic and terrestrial organisms and affects ecosystem interactions [4,5,6,7,8,9]. Significant impacts on ecosystems could result from indirect effects of increased UV-B through influences on the magnitude and direction of interactions between organisms [10]. Caldwell et al. proposed that the most critical effects of UV-B on plants are mediated through alterations in regulatory processes that modify plant morphology and secondary metabolism [11]. High-intensity, continuous full-wavelength UV-B induces DNA damage, oxidative stress, and lipid/protein oxidation in plants, ultimately disrupting normal growth and development. This phenomenon is collectively termed UV-B stress [12,13]. Therefore, the ability of plants to repair damage and protect themselves is very important.
High-altitude ecosystems, such as mountain ranges and plateaus, are exposed to significantly higher levels of UV-B radiation compared to low-elevation regions at the same latitudes, primarily due to reduced atmospheric attenuation [14]. The Qinghai–Tibet Plateau in the western part of China is a relatively harsh environment for living organisms [15]. At the same time, the Qinghai–Tibet Plateau is characterized by high altitude, a thin atmosphere, and a low concentration of suspended particles in the air, resulting in relatively high intensity of solar UV-B radiation reaching the near-surface layer [16]. As the third pole of the world and one of the significant centers of ozone depletion [17], the Qinghai–Tibet Plateau provides a unique environment for studying the physiological and ecological response mechanisms of plants to UV-B radiation through investigations of organisms that inhabit and are distributed across this region.
Endophytic fungi of grasses have co-evolved with their host plants over an extended period. They spend most or all of their life cycle within the host without causing external symptoms, forming a mutualistic symbiotic relationship with grasses [18]. Through long-term co-evolution, grass endophytic fungi have developed a mutualistic symbiosis with their host plants. This symbiotic relationship has conferred many unique ecological advantages to the host plants. Studies have shown a mutualistic symbiosis between endophytic fungi and grasses. The endophytes acquire essential nutrients (e.g., sugars, amino acids) and propagation vectors (seeds) from their host grasses. In return, the endophytic fungi enhance the tolerance of grasses to both biotic (insects, diseases, herbivores, etc.) [19,20,21] and abiotic stress (drought, salt, temperature, heavy metals, etc.) [22,23,24]. These protective effects are achieved through modifications by endophytic fungi in host morphology, physiology, and biochemical pathways, ultimately promoting the growth and adaptability of the host grasses to the environment [25]. However, studies on the effects of grass endophytic fungi on their hosts under UV-B radiation are limited. More studies are needed to elucidate the effects and impacts of grass endophytic fungi on host plants under this adverse condition.
Achnatherum inebrians (drunken horse grass) is a perennial grass species widely distributed across alpine and subalpine grasslands in some provinces of China, including Gansu, Xinjiang, Qinghai, Inner Mongolia, and Tibet [26,27]. Currently, two endophytic species have been identified in association with A. inebrians: Epichloë gansuensis isolated from the Sunan region of Gansu [28,29] and E. inebrians isolated from the Tianshan region of Xinjiang [30,31]. Both species form a close symbiotic relationship with A. inebrians. Enhanced UV-B radiation has the most direct and significant impact on plants, which in turn has an indirect impact on ecosystems [32]. Thus, it is important to understand the effect of endophytic fungi on UV-B radiation response of the A. inebrians in the Qinghai–Tibet Plateau. The average infection rate of endophytic fungi in A. inebrians on the Qinghai–Tibet Plateau is about 90%, and the area has strong UV-B radiation [33]. It is possible that the A. inebrians–endophytic fungi symbiont may have a unique adaptation to strong UV-B radiation. The growth of plants and their antioxidant capacity are the fundamental manifestations of plants’ responses to environmental stress [34]. In order to study the responses of the biomass and antioxidant capacity of E. gansuensis-infected (E+) plants and non-E. gansuensis-infected (E−) plants under UV-B stress, we hypothesized that under UV-B stress E+ A. inebrians plants would grow better than E− plants and possess a stronger antioxidant capacity. In order to test this hypothesis, we used E+ and E− A. inebrians seedlings as experimental materials and compared and analyzed their growth, membrane lipid peroxidation, and antioxidant enzyme activities under different UV-B treatments.
2. Materials and Methods
2.1. Collection of Experimental Materials
In October 2023, the mature individual plants of wild A. inebrians with fully ripened seedheads were collected on the wild permanent grassland of Qinghai–Tibet Plateau. The sampling point is located at N38°50′51.6″ E99°39′57.2″, with an altitude of 2231 m in Sunan County, Gansu Province, on the Qinghai–Tibet Plateau (Figure 1).
Figure 1.
The map of the sampling point. Note: The light blue area indicates the location of the Qinghai–Tibet Plateau in China, and the red points represent the sample points where we collected seeds for this experiment.
The E+ A. inebrians was identified using the aniline blue staining method of Li et al. [35]. If smooth, continuous blue hyphae appeared along the interstitial space of the cells, and it was determined that the plant was infected with E. gansuensis.
To obtain E− seeds, half of the seeds collected from the same infected mother plant were treated with systemic fungicides to eliminate the fungus. According to the method of Li et al. (2016) [36], we treated seeds with thiophanate methyl (70% effective component, NIPPON SODA CO., LTD., Tokyo, Japan; repackaged by Zouping Dexin Biotechnology Co., Ltd., Zouping, Shandong, China) at a 100-times dilution for 2 h. After immersion, the surface was washed with distilled water to remove residual fungicide. Establish populations of asexual reproduction lines with (E+) and without (E−) E. gansuensis in greenhouse of the State Key Laboratory of plateau ecology and agriculture, and offspring seeds were collected for future pot experiments.
2.2. Experimental Design
Healthy offspring seeds of A. inebrians were selected and divided into two groups: E+ and E−. The seeds were sterilized with 2% NaClO solution for 0.4 h, and then rinsed no less than five times with distilled water [37]. After the surface moisture was absorbed with filter paper, the seeds were evenly placed in Petri dishes (9 cm) covered with double-layer moist filter paper. Fifty seeds with uniform diameter and full individuals were placed in each dish. They were incubated in an artificial climatic chamber (PRX-350B Ningbo Plantronics Instrument Co., Ltd., luminous flux density 200 μmol·m−2·s−1, light period 16 h, temperature 25 °C, humidity 70%; dark period 8 h, temperature 20 °C, humidity 60%). The number of sprouted seeds was recorded and rehydrated daily. After 7 d, the neatly grown seedlings were selected and transferred into pots (diameter: 24 cm; height: 15 cm) filled with 3:1 mix of sterilized soil and vermiculite, five plants per pot and transplanting depth 2 cm. Subsequently, the transplanted seedlings pots were assigned at random to a position within a constant-temperature greenhouse (temperature: (26 ± 2) °C, moisture: (42 ± 2)%, light intensity was 20,000–40,000 lux) and watered sufficiently to keep the surface of the soil moist. Following the appearance of the second fully expanded leaf [38], A. inebrians seedlings were subjected to UV-B radiation treatments and natural light treatments as controls. Hoagland’s solution was used to quantitatively water the pots every 7 days [39]. Three UV-B radiation levels (CK, UV-B+, UV-B++) were set up in the experiment, CK was exposed to natural light at 20,000–40,000 lux. UV-B+ is an increase of 8–10 μW/cm2/h in UV-B radiation on the basis of natural light. UV-B++ is an increase of 14–16 μW/cm2/h in UV-B radiation on the basis of natural light. All treatments were repeated three times independently. Five pots were planted for each treatment, for a total of 90 pots. UV-B lamps were hung in parallel above the plants to simulate UV-B radiation treatment. Fluorescent tubes were covered with a cellulose bis-acetate film (0.13 nm thick) to isolate small amounts of ecologically irrelevant inactivating radiation UV-C (<280 nm). The film of cellulose bisacetate was changed every 10 d to ensure the stability of fluorescent lamp radiation after filtration. The treatments were irradiated daily with UV-B fluorescent lamps from 10:00 am until 16:00 pm [40]. After 30 d, the relevant indexes of seedlings were measured.
2.3. Measurements
Ten representative plants were randomly selected from each treatment to investigate the number of tillers, the height of the plants was measured with a tape measure. Whole plants were then carefully removed from pots, washed with distilled water, and dried on filter paper. After that, the plants were dried at 75 °C to a constant weight to determine the biomass.
About 4.0 g of leaf tissue per plant was harvested for malondialdehyde (MDA), electrical conductivity, proline content, soluble sugar content, antioxidant enzyme analysis following 30 days of UV-B treatment. Each treatment measured three plants, with each plant serving as one replicate.
The electrical conductivity was determined by the method of Bao (2016) [41]. Weigh 0.1 g of A. inebrians leaves and rinse well with distilled water. Put them into test tubes containing 8 mL of distilled water and soak them at room temperature for 5 h. Use distilled water as blank control. Measure the electrical conductivity of the solution in each test tube using an electrical conductivity meter. After the measurement, cap the test tubes and place them in a boiling water bath until the leaves turn yellow and sink, indicating that the cell tissues are killed and the membranes are fully permeable. The electrical conductivity of the solution in each test tube was again determined.
The soluble sugar content was determined using the anthrone method of Wang et al. (2015) [42]. The anthrone spectrophotometer method was applied to measure total soluble sugar content. Leaf samples (0.2 g) were homogenized with 5 mL deionized water in a tube and heated for 30 min in a boiling water bath. The supernatant was filtered and then adjusted to 25 mL water in flask as the original extraction. The 0.5 mL of extract was mixed with deionized water (1.5 mL), anthrone ethyl acetate (0.5 mL), and concentrated sulfuric acid (5 mL) and seated for 1 min in a boiling water bath. The absorbance at a wavelength of 630 nm was measured after the sample was cooled down for the measurement of total soluble sugar content.
MDA content refers to the method of Liu (2013) [43]. Weigh 0.7 g of leaf tissue, add 1 mL of 10% TCA (trichloroacetic acid) and a small amount of quartz sand, grind until homogenized, add 6 mL of TCA for further grinding, centrifuge the homogenate at 4000 r/min for 17 min; the supernatant is the sample extract. Next, 2 mL of the centrifuged supernatant was aspirated, 2 mL of 0.6% TBA (thiobarbituric acid) solution was added, and the homogenate was reacted in a boiling water bath for 15 min. After rapid cooling, the supernatant was centrifuged at 4000 r/min for 15 min. The absorbance at wavelengths of 532 nm, 600 nm, and 450 nm was measured.
where m is the fresh weight of the sample.
MDA (μmol/g FW) = (6.45 × (D532 − D600) − 0.56 × D450) × 5/m
Proline content was determined using the method from Shi et al. (2024) [44]. Leaves were freeze-dried, 0.5 g was finely powdered, and 5 mL of sulfosalicylic acid (3%) was added and maintained at 100 °C for 10 min. A total of 2 mL of supernatant was combined with 2 mL each of glacial acetic acid and acidic ninhydrin solution (2.5% w/v) and heated for 25 min at 100 °C. A total of 4 mL of toluene was added to the cooled mixture, and the absorbance was measured at a wavelength of 520 nm to obtain the μg/g dry matter concentration.
Superoxide dismutase (SOD) activity was determined using the nitrogen blue tetrazolium method [45]. Next, 0.1 g of leaf tissue was accurately weighed into a pre-cooled mortar, and 1 mL of phosphate buffer (50 mM, pH 7.8) was added to the mortar for thorough and uniform grinding. The mixture was then transferred into a dry and sterile 2 mL centrifuge tube, and an additional 1 mL of phosphate buffer used to rinse the mortar was also transferred into the 2 mL centrifuge tube. The supernatant was then centrifuged at 12,000 rpm for 15 min at 4 °C, and the enzyme extract was taken and stored in a refrigerator at 4 °C. Three dry and transparent sterile test tubes were selected, one of which was used as the measurement tube, and the other two were used as control tubes. Next, 50 μL of SOD extract and 5 mL of 4-nitro blue tetrazolium chloride (NBT) reaction solution were added to the measurement tubes, and the reaction was performed at 25 °C for 20 min under fluorescent lamps with the light intensity set at 4000 Lux. One control tube was used as the control without SOD enzyme solution and reacted for 20 min under fluorescent lamps with an intensity of 4000 Lux, while the other one reacted without SOD enzyme solution in a dark environment for 20 min. The other control tube without SOD enzyme solution that reacted in a dark environment for 20 min was used as the reference. The absorbance at 560 nm was measured spectrophotometrically and the SOD activity was calculated as follows:
ACK: absorbance value of the light-contrast tube; AE: absorbance value of the sample tube; V: total volume of enzyme extract; VT: volume of enzyme solution used in the assay; m: fresh weight of the sample.
Polyphenol oxidase (POD) activity was measured by the guaiacol method [46]. A total of 0.5 g of leaf tissue was weighed into a mortar and pestle, and 1 mL of extract solution (0.05 mol/L pH 5.5 phosphate buffer) was added for grinding. Then, 9 mL of extract solution was added, and all the homogenate was transferred into a centrifuge tube, and centrifuged at 4 °C for 10 min at 4000 r/min; the supernatant was the crude extract of the enzyme. To determine the enzyme activity, 4 mL of reaction mixture (2 mL of phosphate buffer, 1 mL of enzyme solution, and 1 mL of 0.05 mol/L guaiacol) and 1 mL of 2% H2O2 were added to the test tube, which was immediately shaken and poured into the cuvette. The absorbance value was recorded at 470 nm using a spectrophotometer; this value was recorded immediately and then again every 30 s for a total duration of 4 min. The buffer was used as a control instead of the enzyme solution.
ΔOD is the difference in absorbance at the initial velocity stage of the reaction, and the colorimetric wavelength is 470 nm; Δt is the time corresponding to ΔOD (min); ΔOD/Δt is the slope of the straight line portion, in which the absorbance is proportional to the reaction time; V1 is the total volume of enzyme extracted (mL); V2 is the volume of enzyme extracted (mL) taken for the determination; W is the fresh weight of the plant samples (g); and 0.01 was taken as a change in absorbance value (ΔOD) of 0.01 for 1 unit of enzyme activity (u).
Catalase (CAT) activity was measured with reference to the method of Li et al. (2013) [47]. An amount of 1 g of plant leaf material was ground with 1 mL of chilled NaH2PO4/Na2HPO4 buffer (PBS, 50 mM, pH 7.8) containing 0.1 mM ethylenediaminetetraacetic acid (EDTA) and 1% polyvinylpyrrolidone (PVP). The homogenate was centrifuged at 12,000× g for 30 min, and the supernatant (enzyme extraction) was collected. The enzyme extraction was added to 3 mL 50 mM PBS buffer (pH 7.0) and incubated at 25 °C for 5 min; then the reaction was started by the addition of 6 μM H2O2, and the absorbance changes were recorded at 240 nm for 2 min. An absorbance change of 0.1 unit min−1 was defined as one unit of CAT activity.
Hydrogen peroxide (H2O2) content was determined according to the method of Velikova et al. (2000) [48]. Leaf tissues (0.5 g) were homogenized in ice bath with 5 mL 0.1% (w:v) TCA. The homogenate was centrifuged at 12,000× g for 15 min and 0.5 mL of the supernatant was added to 0.5 mL 10 mM potassium phosphate buffer (pH 7.0) and 1 mL 1 M KI. The absorbency of the supernatant was read at 390 nm. The content of H2O2 was given on a standard curve.
2.4. Data Analysis
The data were the mean ± standard error of the three replications. The experimental data were statistically analyzed with the SPSS statistical software program (Version 27.0, Chicago, IL, USA), and plotted using the Origin software platform (Version 2019, OriginLab Corporation, Northampton, MA, USA). The data on growth, membrane lipid peroxidation, and antioxidant properties of A. inebrians seedlings were tested for normality and our data conformed to a normal distribution. Then, the data were subjected to one-way ANOVA using SPSS 27.0 software, and the significance of the differences between different treatments was determined using the new compound extreme deviation (Duncan’s) test at the 0.05 level. An RDA (redundancy analysis) was conducted using the Canoco software program (Version 5.02 trial, Windows release) to analyze the relationship among plant growth, antioxidant capacity, UV-B radiation, and E. gansuensis.
3. Results
3.1. Growth Parameters of E+ and E− A. inebrians Seedlings Under UV-B Radiation
The plant height, tillers, and biomass of A. inebrians seedlings showed a decreasing trend with increasing radiation intensity under different UV-B radiation treatments, which was significantly lower than that of the blank control (p < 0.05). This indicated that high UV-B radiation had an inhibitory effect on the morphogenesis of A. inebrians seedlings. In the blank control, E. gansuensis significantly increased plant height and tiller number of A. inebrians seedlings (p < 0.05) (Figure 2), except for the insignificant difference (p > 0.05) in biomass. With an increasing intensity of UV-B radiation, E. gansuensis infection significantly increased the plant height, tillers, and biomass of host A. inebrians seedlings (p < 0.05). This indicated that the E. gansuensis infection could alleviate the inhibitory effect of UV-B radiation on plant morphogenesis to a certain extent.
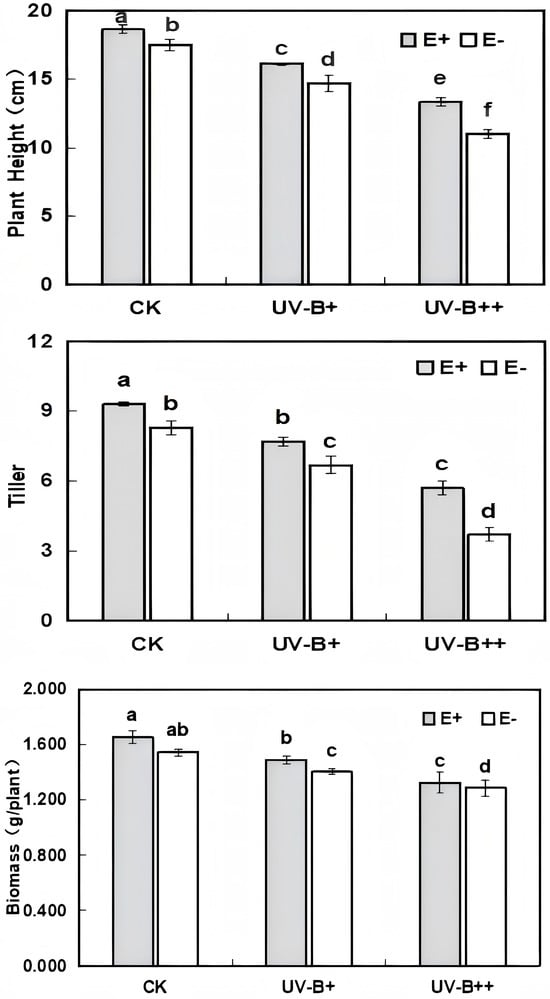
Figure 2.
Effect of different UV-B radiation treatments on the plant height, tillers, and biomass of A. inebrians seedlings. Different lowercase letters in the figure indicate the variability of each indicator at the 0.05 level under different treatments (ANOVA).
3.2. Membrane Lipid Peroxidation Levels in E+ and E− A. inebrians Seedlings Under UV-B RADIATION
Significant differences (p < 0.05) in electrical conductivity, soluble sugars, and MDA content were observed between E+ and E− A. inebrians seedlings under CK conditions. With the increase in UV-B treatment, electrical conductivity and MDA tended to increase, while soluble sugar and proline tended to decrease. E. gansuensis significantly reduced the electrical conductivity and MDA content under UV-B+ and UV-B++ treatments (Figure 3). Soluble sugar content was significantly affected under UV-B+ treatment but not under UV-B++ treatment. Interestingly, although proline decreased significantly under UV-B treatment, there was no significant difference between E+ and E− seedlings under the same treatment conditions.
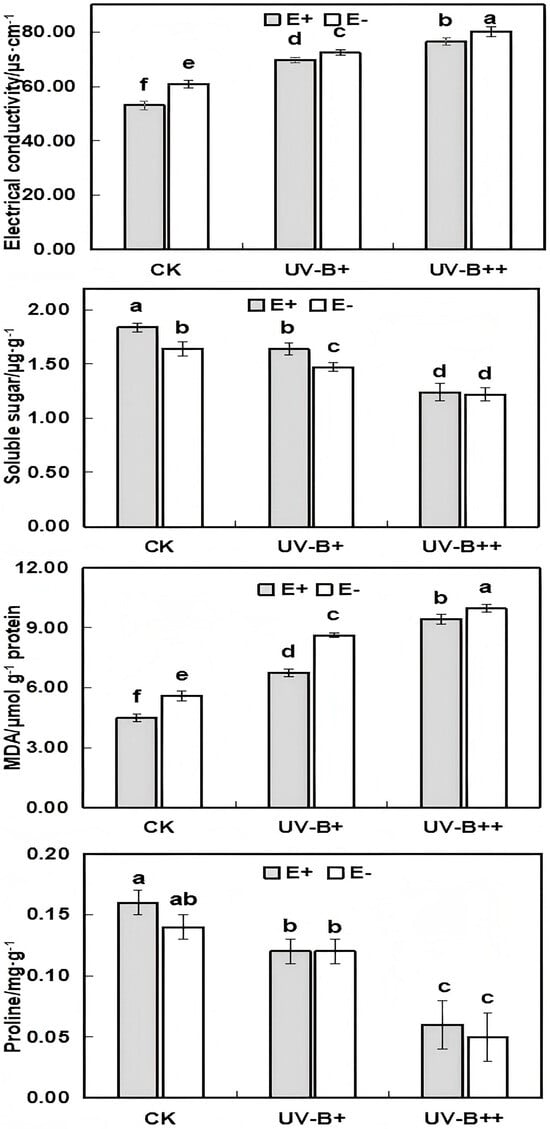
Figure 3.
Effect of different UV-B radiation treatments on electrical conductivity, soluble sugar, malondialdehyde (MDA), and proline in A. inebrians seedlings. Different lowercase letters in the figure indicate the variability of each indicator at the 0.05 level under different treatments (ANOVA).
3.3. Activities of Antioxidant Enzymes in E+ and E− A. inebrians Seedlings Under UV-B Radiation
The activities of SOD, POD, and CAT in A. inebrians seedlings showed a decreasing trend, while the content of H2O2 showed an increasing trend with the enhancement of UV-B radiation intensity. The E. gansuensis significantly reduced the accumulation of H2O2 (p < 0.05) and significantly enhanced the activities of SOD, POD, and CAT (p < 0.05) (Figure 4), which effectively mitigated membrane lipid peroxidation in the host A. inebrians seedlings and improved their resistance to strong UV-B radiation. Interestingly, E. gansuensis significantly decreased H2O2 under UV-B+ treatment, but increased H2O2 under UV-B++ treatment.
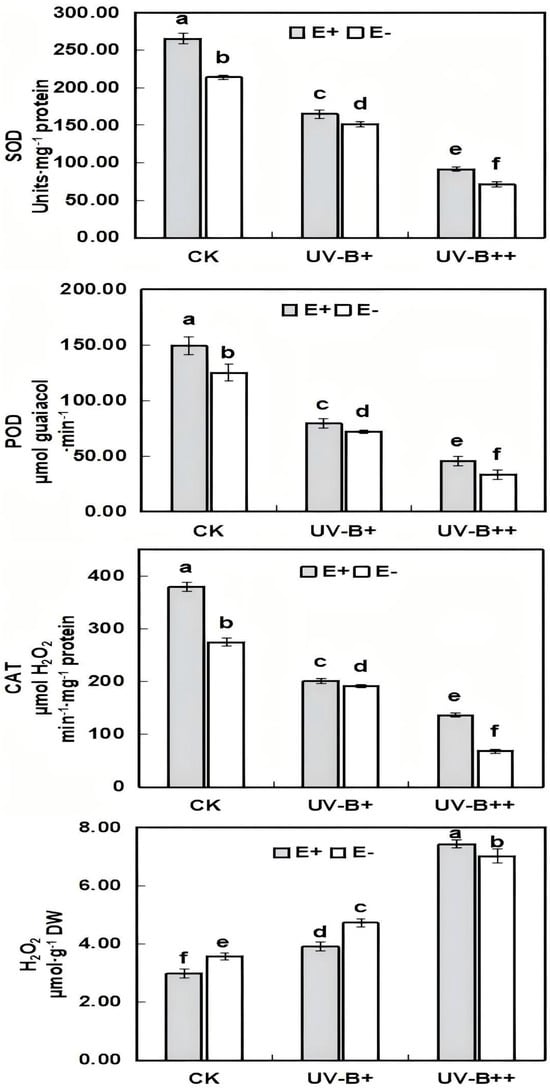
Figure 4.
Effects of different UV-B radiation treatments on superoxide dismutase (SOD), polyphenol oxidase (POD), catalase (CAT), and hydrogen peroxide (H2O2) in A. inebrians seedlings. Different lowercase letters in the figure indicate the variability of each indicator at the 0.05 level under different treatments (ANOVA).
3.4. RDA Analysis Among E. gansuensis Treatment, UV-B Treatment, Plant Growth, Lipid Peroxidation, and Antioxidant Capacity
The first and second axis are 96.08% and 0.3%, respectively (Figure 5). The UV-B treatment was negatively associated with plant growth and antioxidant enzymes, and positively associated with H2O2, electrical conductivity, and MDA. E. gansuensis was positively associated with plant growth and antioxidant enzymes, and negatively associated with H2O2, electrical conductivity, and MDA. The effect of E. gansuensis on antioxidant enzymes was weaker than biomass. UV-B had a stronger effect on the growth, lipid peroxidation, and antioxidant parameters of A. inebrians than E. gansuensis.
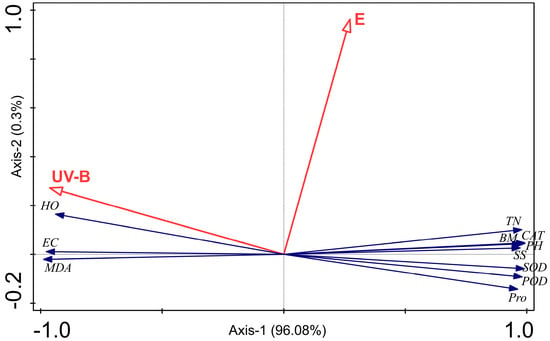
Figure 5.
The result of RDA analysis among endophytic fungi treatment; UV-B treatment, plant growth, lipid peroxidation, and antioxidant capacity. Note: HO indicates H2O2; EC indicates electrical conductivity; TN indicates tiller number; BM indicates biomass; PH indicates plant height; SS indicates soluble sugar; Pro indicates proline; E indicates E. gansuensis treatment; UV-B indicates UV-B treatment.
4. Discussion
To date, the response mechanisms of more than 200 plant species to enhanced UV-B radiation have been extensively studied both domestically and internationally, and the results indicate that UV-B causes various degrees of damage to more than two-thirds of plants [49]. This is reflected in preventing plant growth and development, reducing plant height, tiller number, biomass, and increasing the activity of antioxidant enzymes [50,51,52,53,54]. The studies on the effect of Epichloë endophytic fungi on cool season grasses under UV-B stress are limited. Our study confirmed the hypothesis and explored the effects of E. gansuensis on the morphology, membrane lipid peroxidation, and activities of major antioxidant enzymes of A. inebrians seedlings under different UV-B treatments. This study is useful for understanding the mechanism by which E. gansuensis enhances the tolerance of host A. inebrians to UV-B radiation in the Qinghai–Tibet Plateau region.
4.1. Effect of UV-B Radiation on the Growth of E+ and E− A. inebrians Seedlings
In this study, high-intensity UV-B radiation inhibited the morphogenesis of A. inebrians seedlings. This phenomenon was also observed in the response of other Poaceae to UV-B stress. Zhang et al. (2011) studied found that enhanced UV-B radiation significantly reduced the biomass accumulation in various organs of golden buckwheat (Fagopyrum dibotrys) [55]. Liu et al. (2010) showed that higher doses of UV-B radiation significantly inhibited the growth of winter wheat (Triticum aestivum), as evidenced by a reduced leaf area, plant dwarfing, reduced branching, and decreased biomass [56]. In Bacelar et al. (2015), enhanced UV-B caused reductions in plant height and total biomass in triticale (× Triticosecale Wittm.) crops [57].
Under UV-B radiation, growth parameter analyses of A. inebrians seedlings revealed that E+ plants exhibited superior adaptability. With increasing UV-B intensity, E+ individuals demonstrated significant advantages in plant height, tiller number, and biomass accumulation compared to E− seedlings. These findings indicate that E. gansuensis enhances host growth under UV-B stress. Previous studies confirmed that under abiotic stress (low temperature, heavy metals, salt, drought, etc.), Epichloë endophytic fungi can generally increase the biomass of host grasses [58,59,60,61]. The results of our study are similar with this conclusion.
Previous studies have shown that there is an absorption peak of IAA (Indole-3-Acetic acid) within the UV-B wavelength range, which means that under strong UV-B radiation, some IAA undergoes photo-oxidation and cannot provide sufficient IAA to the plants, thereby causing the plants to fail to grow tall [62,63]. However, the infection of Epichloë endophytic fungi can induce systemic hormones and defense responses in grasses [64]. For instance, one of the main components of the systemic response of ryegrass (Lolium perenne) to E. festucae infection is increased gene expression related to the synthesis, transportation, and response to plant hormones. These plant hormones include salicylic acid, jasmonic acid, gibberellin, ethylene, abscisic acid, cytokinin, and auxin. Therefore, the infection of endophytic fungi may have enhanced the hormone levels within the ryegrass host, resulting in a higher biomass of the host ryegrass. Therefore, E. gansuensis may have increased the hormone content within A. inebrians, thereby leading to an increase in biomass of A. inebrians.
4.2. Effect of UV-B Radiation on Membrane Lipid Peroxidation in E+ and E− A. inebrians Seedlings
Lipid peroxidation is a marker of oxidative stress [65]. Adverse stress often forces plants to produce excessive reactive oxygen species, impairing their growth and development [66,67,68]. MDA, a reference substance for the strength of membrane lipid peroxidation and the degree of plasma membrane damage in plant cells [69], has a content that is directly proportional to the damage to plasma membranes [70]. In addition, the accumulation of proline is one of the effective ways for plants to cope with multiple threats [71,72,73]. Soluble sugar is an important osmoregulatory substance that plays a significant role in the regulation of plant growth under conditions of adversity and stress [74]. When the cell membrane is damaged, the membrane permeability increases, which leads to the extravasation of electrolytes from the cell, resulting in an increase in the electrical conductivity of the plant cell extract. Therefore, the magnitude of the electrical conductivity reflects the extent of biofilm damage. The results of this study showed that with increasing UV-B radiation, the electrical conductivity and MDA content of A. inebrians seedlings significantly increased, and the soluble sugar and proline content decreased. Similar results were found in He et al. (2004), where UV-B radiation treatment resulted in decreased proline content and increased MDA content in wheat seedlings [75]. However, studies by Kirova et al. (2024) found different results: that UV-B treatment led to an increase in both MDA and proline content in wheat [76]. In these studies, we note that not only are the materials studied different but also that the intensity of UV-B treatment varies. Therefore, these factors may be responsible for the differing results.
Previous studies have shown that Epichloë endophytic fungi effectively reduce oxidative damage and increase host membrane lipid peroxidation under abiotic stress (drought, cold, salt, heavy metals, etc.) [44,77,78]. Both Zhao (2023) and Nie et al. (2024) demonstrated that Epichloë endophytic fungi enhance proline accumulation and reduce MDA content in A. inebrians leaves under drought stress [78,79]. Gou (2007) found that the MDA content and relative electrical conductivity of E− A. inebrians were higher than those of E+ A. inebrians; furthermore, Epichloë endophytic fungi can lead to an increase in the content of proline and soluble sugar in the leaves of A. inebrians under salt stress treatment [80]. In this study, E. gansuensis significantly reduced electrical conductivity and MDA of A. inebrians seedlings under different UV-B radiation intensity treatments. However, proline and soluble sugar content decreased significantly with increasing UV-B intensity, while the difference between E+ and E− was not significant under the same treatment. This result suggests that E. gansuensis reduces the membrane lipid peroxidation of A. inebrians but has a more significant effect on inhibiting MDA levels and electrical conductivity. Similarly, in Xu et al. (2017), lower levels of MDA content were observed in E+ tall fescue (Festuca arundinacea) exposed to drought and heat treatment [81]. However, a study on tall fescue showed that endophytic fungi infection did not change MDA content under salinity stress compared to uninfected plants [82]. Differences in these results may be related to specific metabolic mechanisms of grass or grass–endophytic fungi interactions, which need to be further investigated.
4.3. Effect of UV-B Radiation on Antioxidant Enzyme Systems of E+ and E− A. inebrians Seedlings
Plants have important antioxidant enzymes, such as POD, SOD, and CAT, which can convert excessive intracellular reactive oxygen species (H2O2) into harmless substances in order to protect plant growth from the severe attack of adversity [83,84]. Different abiotic stress conditions often induce an increase in the levels of key antioxidant enzymes such as SOD, POD, and CAT in plants, with SOD converting superoxide radicals to H2O2, which is then further converted by POD and CAT to eliminate the oxidative damage effects [85,86,87]. The results of this study showed that with increasing UV-B radiation, SOD, POD, and CAT activities in A. inebrians seedlings showed a decreasing trend, while H2O2 content showed an increasing trend. The findings are consistent with the results of Amirikhah et al. (2021), who claimed that high doses of gamma ray radiation decreased CAT and SOD activities and increased H2O2 contents in tall fescue seedlings; under low dose gamma radiation, CAT activity in E+ plants showed a significant increase [88]. Both Chen et al. (2019) and Barabás et al. (1998) found that the activity of SOD, POD, and CAT in wheat seedlings decreased with increasing intensity of UV-B radiation [89,90].
It has been confirmed in many studies that the enhanced production of antioxidant enzymes by Epichloë endophytic fungi is one of the most important mechanisms by which they increase the resistance of host grasses to abiotic stresses (drought, cold, salt, etc.) [91]. Chen (2022) and Zhang (2012) demonstrated that E+ A. inebrians exhibited significantly elevated activities of POD, SOD, and CAT compared to E− plants when subjected to saline-alkali stress and cadmium stress [92,93]. In this study, E. gansuensis significantly reduced the accumulation of H2O2 and significantly enhanced the activities of SOD, POD, and CAT in A. inebrians seedlings, which was consistent with this conclusion. With the enhancement of UV-B radiation, the antioxidant enzyme system of A. inebrians was activated. Compared with E− plant seedlings, E+ could more effectively increase the antioxidant enzyme activities, thereby providing more effective protection against UV-B stress in A. inebrians and alleviating UV-B induced damage. Interestingly, we found that E. gansuensis infection reduced H2O2 under UV-B+ but increased it under UV-B++. Under UV-B+ treatment, E. gansuensis enhanced the H2O2 scavenging capacity of A. inebrians. UV-B++ stimulated the production of H2O2 in A. inebrians. However, the protective effect of E. gansuensis on the host has decreased. Under different doses of UV-B radiation, the scavenging characteristics and mechanisms of H2O2 by E. gansuensis need to be studied. Different plants exhibit varying growth and antioxidant characteristics under different environmental stresses [34]. This might be related to the antioxidant metabolic pathway of the E. gansuensis-A. inebrians symbiosis under UV-B stress.
4.4. Relationship Among E. gansuensis Treatment, UV-B Treatment, Plant Growth, Lipid Peroxidation, and Antioxidant Capacity
Over the past decades, studies on the effects of Epichloë endophytic fungi symbiosis on host grasses under abiotic stresses have focused on stresses such as drought, salt, temperature, and heavy metals, while very little research has been conducted on UV-B radiation. The results in our study showed that E. gansuensis was positively correlated with plant height, tiller number, biomass, CAT, soluble sugar, SOD, POD, and proline under UV-B stress. The effect of E. gansuensis on the biomass of A. inebrians was stronger than its effect on antioxidant enzyme activity. This result may indicate that under UV-B stress, E. gansuensis is more focused on positive effects on biomass. Endophytic fungi can enhance the tolerance of A. inebrians to UV-B radiation. This seems to provide a plausible explanation for the observed phenomenon that the infection rate of Epichloë endophytic fungi remains about 90% on the Qinghai–Tibet Plateau. In other studies on the enhancement of abiotic stress tolerance in host grasses by endophytic fungi, although the intensity of the influence of endophytic fungi and abiotic stress factors was not compared, the growth of endophytic fungal symbionts and their antioxidant capacity gradually weakened as abiotic stress increased [20,94,95]. These findings also support our conclusion. However, the effect of endophytic fungi on A. inebrians is weaker than that of UV-B radiation. Similarly, in Shi et al. (2024), the authors found that the effects of cadmium stress on the growth and antioxidant capacity of Elymus dahucirus collected from the Qinghai–Tibet Plateau were stronger than those of the Epichloë endophytic fungi; although the Epichloë endophytic fungi mitigated the damage caused by cadmium stress [44].
Our results confirmed the hypothesis we proposed. The presence of E. gansuensis was positive for the growth and antioxidant capacity of A. inebrians. During long-term adaptive evolution, plants can continuously regulate their protective mechanisms to adapt to the increasing UV-B radiation by increasing the synthesis of flavonoids and alkaloidal UV-absorbing substances themselves [96,97]. Previous studies have confirmed that E. gansuensis possesses genes associated with the production of synthesis of terpenoid indole alkaloids [30]. These terpenoid indole alkaloids can absorb UV-B radiation and are believed to protect plants from UV-B radiation [98]. This can explain why E+ A. inebrians has better tolerance to UV-B stress.
The Qinghai–Tibet Plateau serves as a critical ecological security barrier and the primary pastoral production base in China [99]. Its unique topography and the complex and variable environment caused by high altitude have profoundly shaped the ecosystem structure and function in this region. The Qinghai–Tibet Plateau encompasses diverse vegetation types, including subtropical evergreen broad-leaved forests, temperate grasslands, Afro-Asian deserts, and alpine meadows. In this unique environment, Poaceae species are widely distributed, forming the dominant or associated species that characterize the alpine steppe and meadow communities with distinct high-altitude features in this region [100,101]. Previous investigations have shown that more than 12 genera of grasses can be symbiotic with Epichloë endophytic fungi in the Qinghai–Tibet Plateau [33]. Stronger UV-B radiation is one of the main climatic features of this area. Therefore, investigating the response mechanisms of grass–endophytic symbioses to UV-B radiation is crucial for understanding how the symbiotic relationship between grasses and endophytic fungi adapts to this region under the backdrop of global climate change.
5. Conclusions
At present, studies on the response of Epichloë endophytic fungi to UV-B radiation are relatively limited. Our study mainly investigates the effects of E. gansuensis on the growth and antioxidant capacity of A. inebrians seedlings under UV-B stress. The results showed that E. gansuensis enhances the growth and antioxidant capacity of A. inebrians seedlings under UV-B stress. This is consistent with the hypothesis we proposed. Our study indicates that E. gansuensis can enhance the tolerance of A. inebrians to UV-B radiation on the Tibetan Plateau. The tolerance of the A. inebrians–endophytic fungi symbiont to UV-B may enable it to have a higher infection rate under the strong UV-B conditions of the Qinghai–Tibet Plateau.
Author Contributions
Conceptualization, C.W. and Q.S.; methodology, X.L.; software, C.W. and Q.S.; validation, C.W., X.L., and Q.S.; formal analysis, X.L.; investigation, X.L.; resources, X.L.; data curation, X.L.; writing—original draft preparation, C.W.; writing—review and editing, C.W. and Q.S.; visualization, C.W. and Q.S.; supervision, X.L. and Q.S.; project administration, X.L. and Q.S.; funding acquisition, Q.S. and X.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by The National Natural Science Foundation of China (32360349).
Data Availability Statement
The datasets presented in this article are not readily available because the data are part of an ongoing study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- National Aeronautics and Space Administration. NASA Ozone Watch: Annual Records. Available online: https://ozonewatch.gsfc.nasa.gov/statistics/annual_data.html (accessed on 3 June 2025).
- Grooss, J.U.; Müller, R. Simulation of record arctic stratospheric ozone depletion in 2020. J. Geophys. Res.-Atmos. 2021, 126, e2020JD033339. [Google Scholar] [CrossRef]
- Zhang, F.; Sun, M.; Li, D.X.; You, M.H.; Yan, J.J.; Bai, S.Q. Metabolomic analysis of Elymus sibiricus exposed to UV-B radiation stress. Pratac. Sci. 2024, 29, 5133. [Google Scholar] [CrossRef] [PubMed]
- Bin, L.; Qing, Z. Effect of enhanced UV-B radiation on plant flavonoids. Chin. J. Eco-Agric. 2007, 15, 191–194. [Google Scholar]
- Caldwell, M.M.; Bornman, J.F.; Ballaré, C.L.; Flint, S.D.; Kulandaivelu, G. Terrestrial ecosystems, increased solar ultraviolet radiation, and interactions with bother climate change factors. Photochem. Photobiol. Sci. 2007, 6, 252–266. [Google Scholar] [CrossRef]
- Dai, Q.J.; Yan, B.; Huang, S.B.; Liu, X.Z.; Peng, S.B.; Miranda, M.L.L.; Chavez, A.Q.; Vergara, B.S.; Olszyk, D.M. Response of oxidative stress defense systems in rice (Oryza sativa) leaves with supplemental UV-B radiation. Physiol. Plant. 1997, 101, 301–308. [Google Scholar] [CrossRef]
- Kakani, V.G.; Reddy, K.R.; Zhao, D.; Gao, W. Senescence and hyperspectral reflectance of cotton leaves exposed to ultraviolet-B radiation and carbon dioxide. Physiol. Plant. 2004, 121, 250–257. [Google Scholar] [CrossRef]
- Li, Y.S.; Jin, J.; Wang, G.H.; Liu, X.B. Preventive mechanisms to UV- B radiation damages in Crops. Soils Crops 2016, 5, 223–233. [Google Scholar]
- Rastogi, R.P.; Singh, S.P.; Incharoensakdi, A.; Häder, D.-P.; Sinha, R.P. Ultraviolet radiation-induced generation of reactive oxygen species, DNA damage and induction of UV-absorbing compounds in the cyanobacterium Rivularia sp. HKAR-4. S. Afr. J. Bot. 2014, 90, 163–169. [Google Scholar] [CrossRef]
- McLeod, A.R.; Rey, A.; Newsham, K.K.; Lewis, G.C.; Wolferstan, P. Effects of elevated ultraviolet radiation and endophytic fungi on plant growth and insect feeding in Lolium perenne, Festuca rubra, F. arundinacea and F. pratensis. J. Photochem. Photobiol. B. 2001, 62, 97–107. [Google Scholar] [CrossRef]
- Caldwell, M.; Teramura, A.H.; Tevini, M.; Bornman, J.F.; Bjorn, L.O.; Kulandaivelu, G. Effects of increased solar ultraviolet-radiation on terrestrial plants. Ambio 1995, 24, 166–173. [Google Scholar]
- Liu, M.Y.; Sun, W.J.; Ma, Z.T.; Guo, C.C.; Chen, J.H.; Wu, Q.; Wang, X.Y.; Chen, H. Integrated network analyses identify MYB4R1 neofunctionalization in the UV-B adaptation of Tartary buckwheat. Plant Commun. 2022, 3, 100414. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Liu, H.T. How plants protect themselves from ultraviolet-B radiation stress. Plant Physiol. 2021, 187, 1096–1103. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Jia, Z.P.; Wang, J.; Du, M.N.; Su, X. Response of antioxidant enzyme system and total flavonoid of woody plant Hippophae neurocarpa seedlings to enhanced UV-B radiation. Guihaia 2020, 40, 1595–1601. [Google Scholar]
- Shi, S.B.; Shang, Y.X.; Zhu, P.J.; Yang, L. Effects of short term enhanced UV-B radiation on the PS II photochemical efficiency of alpine plant Saussurea superba. Chin. J. Appl. Ecol. 2011, 22, 1147–1154. [Google Scholar] [CrossRef]
- Zhou, X.J.; Luo, C.; Li, W.L.; Shi, J.E. Changes of total ozone in whole China and its low contents center in Qing-Zang Plateau regions. Chin. Sci. Bull. 1995, 1396–1398. [Google Scholar]
- Liu, Y.; Li, W.L. Deepening of ozone valley over Tibetan Plateau and its possible influences. Acta Meteorol. Sin. 2001, 97–106. [Google Scholar]
- Zhang, P.P. Characteristic of Endophytic Fungi Isolated from Elymus and Their Effect on Host Resistances. Master’s Thesis, Xinjiang Agricultural University, Urumqi, China, 2013. [Google Scholar]
- Pennell, C.G.L.; Popay, A.J.; Rolston, M.P.; Townsend, R.J.; Lloyd-West, C.M.; Card, S.D. Avanex unique endophyte technology: Reduced insect food source at airports. Environ. Entomol. 2016, 45, 101–108. [Google Scholar] [CrossRef]
- Song, M.L.; Chai, Q.; Li, X.Z.; Yao, X.; Li, C.J.; Christensen, M.J.; Nan, Z.B. An asexual Epichloë endophyte modifies the nutrient stoichiometry of wild barley (Hordeum brevisubulatum) under salt stress. Plant Soil 2015, 387, 153–165. [Google Scholar] [CrossRef]
- Tian, P.; Nan, Z.B.; Li, C.J.; Spangenberg, G. Effect of the endophyte Neotyphodium lolii on susceptibility and host physiological response of perennial ryegrass to fungal pathogens. Eur. J. Plant Pathol. 2008, 122, 593–602. [Google Scholar] [CrossRef]
- Kannadan, S.; Rudgers, J.A. Endophyte symbiosis benefits a rare grass under low water availability. Funct. Ecol. 2008, 22, 706–713. [Google Scholar] [CrossRef]
- Song, M.L.; Li, X.Z.; Saikkonen, K.; Li, C.J.; Nan, Z.B. An asexual Epichloë endophyte enhances waterlogging tolerance of Hordeum brevisubulatum. Fungal Ecol. 2015, 13, 44–52. [Google Scholar] [CrossRef]
- Zhou, L.Y.; Li, C.J.; Zhang, X.X.; Johnson, R.; Bao, G.S.; Yao, X.; Chai, Q. Effects of cold shocked Epichloë infected Festuca sinensis on ergot alkaloid accumulation. Fungal Ecol. 2015, 14, 99–104. [Google Scholar] [CrossRef]
- Jin, Y.Y.; SAMAN, B.; Tian, P.; Peng, Z.C.; Hou, F.J.; Chun, J.L. Research developments on the effects of grass—endophyte fungi symbiosis onsoil physical and chemical properties and microbes. Pratac. Sci. 2019, 36, 1292–1307. [Google Scholar]
- Ma, Y.L.; Li, C.J.; White, J.F. Effects of aqueous extracts of endophyte-infected grass Achnatherum inebrians on growth and development of pea aphid Acyrthosiphon pisum. Insects 2021, 12, 944. [Google Scholar] [CrossRef]
- Zhang, X.X.; Li, C.J.; Zhi, B.N. Neotyphodium endophyte increases Achnatherum inebrians (drunken horse grass) resistance to herbivores and seed predators. Weed Res. 2012, 52, 70–78. [Google Scholar] [CrossRef]
- Leuchtmann, A.; Bacon, C.W.; Schardl, C.L.; White, J.F.; Tadych, M. Nomenclatural realignment of Neotyphodium species with genus Epichloë. Mycologia 2014, 106, 202–215. [Google Scholar] [CrossRef]
- Li, C.J.; Nan, Z.B.; Li, F. Biological and physiological characteristics of Neotyphodium gansuense symbiotic with Achnatherum inebrians. Microbiol. Res. 2008, 163, 431–440. [Google Scholar] [CrossRef]
- Chen, L.; Li, X.Z.; Li, C.J.; Swoboda, G.A.; Young, C.A.; Sugawara, K.; Leuchtmann, A.; Schardl, C.L. Two distinct Epichloë species symbiotic with Achnatherum inebrians, drunken horse grass. Mycologia 2015, 107, 863–873. [Google Scholar] [CrossRef]
- Li, X.L.; Zheng, R.; Deng, M.H.; Wang, B.C.; Liu, R.G.; Wang, J.F. Effects of endophytic fungi on growth and nitrogen metabolism of Achnatherum inebrians under different nitrogen concentrations. Pratac. Sci. 2024, 66, 1–24. [Google Scholar]
- He, L. Effect of Inhanced UV-B Radiation on the Diversity of Arbuscular Mycorrhizal Fungi in Alpine Meadow Soil Ecosystem. Master’s Thesis, Lanzhou University, Lanzhou, China, 2009. [Google Scholar]
- Bao, G.S.; Zhang, X.X.; Li, X.Z.; Wei, X.X.; Li, C.J. Incidence and isolation of endophyte in native grasses of Qinghai Plateau. Pratac. Sci. 2015, 32, 1997–2007. [Google Scholar]
- Shabala, S. Plant Stress Physiology, 2nd ed.; Cabi: Wallingford, UK, 2017. [Google Scholar]
- Li, C.J.; Nan, Z.B.; Liu, Y.; Paul, V.H.; Peter, D. Studies on the detection of endophytes in drunken horse grass (Achnatherum inebrians). In Proceedings of the Annual Meeting of Chinese Society for Plant Pathology, Guangzhou, China, 18–20 July 2008; p. 4. [Google Scholar]
- Li, N.N.; Zhao, Y.F.; Xia, C.; Zhong, R.; Zhang, X.X. Effects of thiophanate methyl on seed borne Epichloë fungal endophyte of Achnatherum inebrians. Pratac. Sci. 2016, 33, 1306–1314. [Google Scholar]
- Lan, Y. Physiological Responses of Three Echinochloa Forages to Saline-Alkaline Stress and Comprehensive Evaluation for Their Saline-Alkaline Tolerance. Master’s Thesis, Ningxia University, Yinchuan, China, 2022. [Google Scholar]
- Huang, X.W.; Zhang, Y.J.; Feng, Q.; Chen, W.X.; Zhang, W.H.; Li, B.Y. Analysis of wheat seeding leaf proteome after UV-B radiation. J. China Agric. Univ. 2012, 17, 31–36. [Google Scholar]
- Zhao, Z.R.; Kou, M.Z.; Zhong, R.; Xia, C.; Christensen, M.J.; Zhang, X.X. Transcriptome analysis revealed plant hormone biosynthesis and response pathway modification by Epichloë gansuensis in Achnatherum inebrians under different soil moisture availability. J. Fungi 2021, 7, 640. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Han, F.; Yue, X.G.; Shi, S.B.; Wang, X.Y. Effects of long-term intensified UV-B radiation on the photosynthetic rates and antioxidative systems of three plants in alpine meadows. Acta Bot. Boreali-Occident. Sin. 2005, 67, 2010–2016. [Google Scholar]
- Bao, L.L. Comparative Analyses of the Physiological Responses of Different Rice Cultivarsto Cold Stress. Master’s Thesis, Chongqing Normal University, Chongqing, China, 2016. [Google Scholar]
- Wang, L.X.; Fang, C.; Wang, K. Physiological responses of Leymus Chinensis to long-term salt, alkali and mixed salt-alkali stresses. J. Plant Nutr. 2015, 38, 526–540. [Google Scholar] [CrossRef]
- Liu, Y. Study on the Effect of Salt Stress on the Seed Germination and Seedling Growth of Qinyin No3 Naked Oats. Master’s Thesis, Qinghai University, Xining, China, 2013. [Google Scholar]
- Shi, Q.; Simpson, W.R.; Li, Y.L.; Xu, C.T.; De, K.; Li, X.Z. Epichloë bromicola enhances Elymus dahucirus plant growth and antioxidant capacity under cadmium stress. Agronomy 2024, 14, 365. [Google Scholar] [CrossRef]
- Li, Z.Q. Physiological Responses of Cotton to Salt Stress After Adaptation Under Low Salinity in Seeding Stage. Master’s Thesis, Chinese Academy of Agricultural Sciences, Beijing, China, 2017. [Google Scholar]
- Wang, W.L.; Wang, Z.; Wang, J.Y. Optimization of determination method of peroxidase activity in plant. Res. Explor. Lab. 2010, 29, 21–23. [Google Scholar]
- Li, X.N.; Yang, Y.L.; Jia, L.Y.; Chen, H.J.; Wei, X. Zinc-induced oxidative damage, antioxidant enzyme response and proline metabolism in roots and leaves of wheat plants. Ecotoxicol. Environ. Saf. 2013, 89, 150–157. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants—Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Lou, Y.S.; Zhang, Z.; Wu, J. Crop growth, yield and quality as affected by ultraviolet-B (UV-B) radiation elevating. J. Agro-Environ. Sci. 2020, 39, 812–821. [Google Scholar]
- Badridze, G.; Kacharava, N.; Chkhubianishvili, E.; Rapava, L.; Kikvidze, M.; Chanishvili, S.; Chigladze, L. Influence of ultraviolet irradiation and acid precipitations on the content of antioxidants in wheat leaves. Appl. Ecol. Env. Res. 2015, 13, 993–1013. [Google Scholar] [CrossRef]
- Lou, Y.S.; Wu, L.; Ren, L.X.; Yan, M.; Zhao, S.D.; Zhu, H.W.; Zhang, Y.W. Effects of silicon application on diurnal variations of physiological properties of rice leaves of plants at the heading stage under elevated UV-B radiation. Int. J. Biometeorol. 2016, 60, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Rudnoy, S.; Majlath, I.; Pal, M.; Paldi, K.; Racz, I.; Janda, T. Interactions of S-methylmethionine and UV-B can modify the defence mechanisms induced in maize. Acta Physiol. Plant. 2015, 37, 148. [Google Scholar] [CrossRef]
- Xu, K. Studies on the Growth and Photosynthetic Responses of Two Hybid Rice Cultivars to Enhanced U-VB Radiation. Master’s Thesis, Central China Normal University, Wuhan, China, 2006. [Google Scholar]
- Xu, K.; Qiu, B.S. Responses of superhigh-yield hybrid rice Liangyoupeijiu to enhancement of ultraviolet-B radiation. Plant Sci. 2006, 172, 139–149. [Google Scholar] [CrossRef]
- Zhang, Y.F.; He, P.; Zhang, C.P. Influences of enhanced UV-B radiation and drought stress on biomass accumulation and allocation of Fagopyrum dibotrys. China J. Chin. Mater. Med. 2011, 36, 2032–2037. [Google Scholar]
- Liu, L.L.; Zhang, W.H.; Fan, Y.L.; Lu, Y.W.; Miao, X.L. Effects of different dose UV-B radiation on the morphological and physiological indices of winter wheat seedlings. Chin. J. Ecol. 2010, 29, 314–318. [Google Scholar] [CrossRef]
- Bacelar, E.; Moutinho-Pereira, J.; Ferreira, H.; Correia, C. Enhanced ultraviolet-B radiation affect growth, yield and physiological processes on triticale plants. Procedia Environ. Sci. 2015, 29, 219–220. [Google Scholar] [CrossRef]
- Decunta, F.A.; Perez, L.I.; Malinowski, D.P.; Molina-Montenegro, M.A.; Gundel, P.E. A systematic review on the effects of Epichloë fungal endophytes on drought tolerance in cool-season grasses. Front. Plant Sci. 2021, 12, 644731. [Google Scholar] [CrossRef]
- Wang, Z.F.; Li, C.J.; White, J. Effects of Epichloë endophyte infection on growth, physiological properties and seed germination of wild barley under saline conditions. J. Agron. Crop Sci. 2020, 206, 43–51. [Google Scholar] [CrossRef]
- Yang, Y. Neotyphodium Endophyte in festuca sinensis and Effect on Cold Tolerance to Host. Master’s Thesis, Lanzhou University, Lanzhou, China, 2010. [Google Scholar]
- Zhang, X.X.; Fan, X.M.; Li, C.J.; Nan, Z.B. Effects of cadmium stress on seed germination, seedling growth and antioxidative enzymes in Achnatherum inebrians plants infected with a Neotyphodium endophyte. Plant Growth Regul. 2010, 60, 91–97. [Google Scholar] [CrossRef]
- Li, Y.S.; Liu, X.B.; Henson, J.F. Advances in crop responses to enhanced UV-B radiation. Appl. Ecol. Environ. Res. 2016, 14, 339–367. [Google Scholar] [CrossRef]
- Ros, J.; Tevini, M. Interaction of UV-radiation and IAA during growth of seedlings and hypocotyl segments of sunflower. J. Plant Physiol. 1995, 146, 295–302. [Google Scholar] [CrossRef]
- Schmid, J.; Day, R.; Zhang, N.X.; Dupont, P.Y.; Cox, M.P.; Schardl, C.L.; Minards, N.; Truglio, M.; Moore, N.; Harris, D.R.; et al. Host tissue environment directs activities of an Epichloë Endophyte, While it induces systemic hormone and defense responses in its native Perennial Ryegrass host. Mol. Plant-Microbe Interact. 2017, 30, 138–149. [Google Scholar] [CrossRef]
- Hamilton, C.E.; Gundel, P.E.; Helander, M.; Saikkonen, K. Endophytic mediation of reactive oxygen species and antioxidant activity in plants: A review. Fungal Divers. 2012, 54, 1–10. [Google Scholar] [CrossRef]
- Malencic, D.; Kiprovski, B.; Popovic, M.; Prvulovic, D.; Miladinovic, J.; Djordjevic, V. Changes in antioxidant systems in soybean as affected by Sclerotinia sclerotiorum (Lib.) de Bary. Plant Physiol. Biochem. 2010, 48, 903–908. [Google Scholar] [CrossRef]
- Wang, Q.Z.; Wu, C.H.; Xie, B.; Liu, Y.; Cui, J.; Chen, G.; Zhang, Y.W. Model analysing the antioxidant responses of leaves and roots of switchgrass to NaCl-salinity stress. Plant Physiol. Biochem. 2012, 58, 288–296. [Google Scholar] [CrossRef]
- Yildiztugay, E.; Sekmen, A.H.; Turkan, I.; Kucukoduk, M. Elucidation of physiological and biochemical mechanisms of an endemic halophyte Centaurea tuzgoluensis under salt stress. Plant Physiol. Biochem. 2011, 49, 816–824. [Google Scholar] [CrossRef]
- Huff, A. Peroxidase-catalysed oxidation of chlorophyll by hydrogen peroxide. Phytochemistry 1982, 21, 261–265. [Google Scholar] [CrossRef]
- Catala, A. Lipid peroxidation of membrane phospholipids generates hydroxy-alkenals and oxidized phospholipids active in physiological and/or pathological conditions. Chem. Phys. Lipids 2009, 157, 1–11. [Google Scholar] [CrossRef]
- Delauney, A.J.; Verma, D.P.S. Proline biosynthesis and osmoregulation in plants. Plant J. 1993, 4, 215–223. [Google Scholar] [CrossRef]
- Hsu, S.Y.; Hsu, Y.T.; Kao, C.H. The effect of polyethylene glycol on proline accumulation in rice leaves. Biol. Plant. 2003, 46, 73–78. [Google Scholar] [CrossRef]
- Kishor, P.B.K.; Sangam, S.; Amrutha, R.N.; Laxmi, P.S.; Naidu, K.R.; Rao, K.; Rao, S.; Reddy, K.J.; Theriappan, P.; Sreenivasulu, N. Regulation of proline biosynthesis, degradation, uptake and transport in higher plants: Its implications in plant growth and abiotic stress tolerance. Curr. Sci. 2005, 88, 424–438. [Google Scholar]
- Wang, C.; Chen, J.B.; Pang, Z.L.; Li, N.N.; Dong, H.H.; Li, D.D. The response and screening of germplasm tolerant to mixed saline-alkali stress in sweet sorghum. Crops 2016, 56–61. [Google Scholar] [CrossRef]
- He, J.M.; She, X.P.; Wang, R.B.; Liu, C. Physiological and ecological effects of enhanced UV-B radiation on wheat seedling under NaCl stress condition. Acta Bot. Boreali-Occident. Sin. 2004, 24, 1810–1815. [Google Scholar]
- Kirova, E.; Moskova, I.; Manova, V.; Koycheva, Y.; Tsekova, Z.; Borisova, D.; Nikolov, H.; Dimitrov, V.; Sergiev, I.; Kocheva, K. Exogenous cytokinin 4PU-30 modulates the response of wheat and einkorn seedlings to ultraviolet B radiation. Plants 2024, 13, 1401. [Google Scholar] [CrossRef]
- Lee, K.; Missaoui, A.; Mahmud, K.; Presley, H.; Lonnee, M. Interaction between grasses and Epichloë endophytes and its significance to biotic and abiotic stress tolerance and the rhizosphere. Microorganisms 2021, 9, 2186. [Google Scholar] [CrossRef]
- Nie, X.M.; Zhao, Z.R.; Zhang, X.X.; Bastias, D.A.; Nan, Z.B.; Li, C.J. Endophytes alleviate drought-derived oxidative damage in Achnatherum inebrians plants through increasing antioxidants and regulating host stress responses. Microb. Ecol. 2024, 87, 73. [Google Scholar] [CrossRef]
- Zhao, Z.R. The Mechanism of Response of Achnatherum inebrians-Epichloë Endophyte Symbiont to Drought Stress. Master’s Thesis, Lanzhou University, Lanzhou, China, 2023. [Google Scholar]
- Gou, X.Y. Effects of Neotyphodium Endophyte on Salt Tolerance to Drunken Horse Grass (Achnatherum inebrians). Master’s Thesis, Lanzhou University, Lanzhou, China, 2007. [Google Scholar]
- Xu, L.X.; Li, X.S.; Han, L.B.; Li, D.Y.; Song, G.L. Epichloë endophyte infection improved drought and heat tolerance of tall fescue through altered antioxidant enzyme activity. Eur. J. Hortic. Sci. 2017, 82, 90–97. [Google Scholar] [CrossRef]
- Xie, Y.; Sun, X.Y.; Feng, Q.J.; Luo, H.J.; Wassie, M.; Amee, M.; Amombo, E.; Chen, L. Comparative physiological and metabolomic analyses reveal mechanisms of Aspergillus aculeatus-mediated abiotic stress tolerance in tall fescue. Plant Physiol. Biochem. 2019, 142, 342–350. [Google Scholar] [CrossRef]
- Chandra, A.; Dubey, A. Effect of ploidy levels on the activities of Δ1-pyrroline-5-carboxylate synthetase, superoxide dismutase and peroxidase in Cenchrus species grown under water stress. Plant Physiol. Biochem. 2010, 48, 27–34. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Srivastava, S.; Penna, S.; D’Souza, S.F. Thiourea orchestrates regulation of redox state and antioxidant responses to reduce the NaCl-induced oxidative damage in Indian mustard (Brassica juncea (L.) Czern.). Plant Physiol. Biochem. 2011, 49, 676–686. [Google Scholar] [CrossRef] [PubMed]
- Kumari, G.J.; Reddy, A.M.; Naik, S.T.; Kumar, S.G.; Prasanthi, J.; Sriranganayakulu, G.; Reddy, P.C.; Sudhakar, C. Jasmonic acid induced changes in protein pattern, antioxidative enzyme activities and peroxidase isozymes in peanut seedlings. Biol. Plant. 2006, 50, 219–226. [Google Scholar] [CrossRef]
- Singh, R.; Singh, S.; Tripathi, R.; Agrawal, S.B. Supplemental UV-B radiation induced changes in growth, pigments and antioxidant pool of bean (Dolichos lablab) under field conditions. J. Environ. Biol. 2011, 32, 139–145. [Google Scholar] [PubMed]
- Yang, S.H.; Wang, L.J.; Li, S.H.; Duan, W.; Loescher, W.; Liang, Z.C. The effects of UV-B radiation on photosynthesis in relation to Photosystem II photochemistry, thermal dissipation and antioxidant defenses in winter wheat (Triticum aestivum L.) seedlings at different growth temperatures. Funct. Plant Biol. 2007, 34, 907–917. [Google Scholar] [CrossRef]
- Amirikhah, R.; Etemadi, N.; Sabzalian, M.R.; Nikbakht, A.; Eskandari, A. Gamma radiation negatively impacted seed germination, seedling growth and antioxidant enzymes activities in tall fescue infected with Epichloë endophyte. Ecotoxicol. Environ. Saf. 2021, 216, 112169. [Google Scholar] [CrossRef]
- Barabás, K.N.; Szegletes, Z.; Pestenácz, A.; Fülöp, K.; Erdei, L. Effects of excess UV-B irradiation on the antioxidant defence mechanisms in wheat (Triticum aestivum L.) seedlings. J. Plant Physiol. 1998, 153, 146–153. [Google Scholar] [CrossRef]
- Chen, Z.J.; Ma, Y.; Weng, Y.; Yang, R.Q.; Gu, Z.X.; Wang, P. Effects of UV-B radiation on phenolic accumulation, antioxidant activity and physiological changes in wheat (Triticum aestivum L.) seedlings. Food Biosci. 2019, 30, 100409. [Google Scholar] [CrossRef]
- Hamilton, C.E.; Bauerle, T.L. A new currency for mutualism? Fungal endophytes alter antioxidant activity in hosts responding to drought. Fungal Divers. 2012, 54, 39–49. [Google Scholar] [CrossRef]
- Chen, Y.Q. The Saline-Alkali Tolerance of Seed of Epichloë Endophytic Fungi-Achnatherum inebrians Symbiont and Effects of Nitrogen Applicationon Seed Yield. Master’s Thesis, Lanzhou University, Lanzhou, China, 2022. [Google Scholar]
- Zhang, X.X. Response of Achnatherum inebrians/Neotyphodium gansuense Symbiont to Stresses and Secondary Metabolites Activities. Ph.D. Thesis, Lanzhou University, Lanzhou, China, 2012. [Google Scholar]
- Oberhofer, M.; Güsewell, S.; Leuchtmann, A. Effects of natural hybrid and non-hybrid Epichloë endophytes on the response of Hordelymus europaeus to drought stress. New Phytol. 2014, 201, 242–253. [Google Scholar] [CrossRef]
- Soleimani, M.; Hajabbasi, M.A.; Afyuni, M.; Mirlohi, A.; Borggaard, O.K.; Holm, P.E. Effect of endophytic fungi on cadmium tolerance and bioaccumulation by Festuca Arundinacea and Festuca Pratensis. Int. J. Phytorem. 2010, 12, 535–549. [Google Scholar] [CrossRef]
- Hideg, É.; Jansen, M.A.K.; Strid, Å. UV-B exposure, ROS, and stress: Inseparable companions or loosely linked associates? Trends Plant Sci. 2013, 18, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Suesslin, C.; Frohnmeyer, H. An Arabidopsis mutant defective in UV-B light-mediated responses. Plant J. 2003, 33, 591–601. [Google Scholar] [CrossRef] [PubMed]
- Binder, B.Y.K.; Peebles, C.A.M.; Shanks, J.V.; San, K.Y. The effects of UV-B Stress on the production of terpenoid Indole alkaloids in Catharanthus roseus hairy roots. Biotechnol. Prog. 2009, 25, 861–865. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.W.; Zhao, X.Q.; Zhang, X.L.; Wang, X.G.; Geng, Y.Y.; Hu, L.Y.; Zhao, N.; Mao, S.J.; Liu, H.J.; Kang, S.P.; et al. Sustainable development of ecological grass-based livestock husbandry in Qinghai-Tibet Plateau alpine area: Principle, technology and practice. Acta Ecol. Sin. 2020, 40, 6324–6337. [Google Scholar]
- Wu, G.L.; Du, G.Z. Germination is related to seed mass in grasses (Poaceae) of the eastern Qinghai-Tibetan Plateau, China. Nord. J. Bot. 2007, 25, 361–365. [Google Scholar] [CrossRef]
- Zhou, Q.P.; Ji, Y.J.; Bruijn, K.D.; Liang, G.L.; Yan, H.B. Preliminary evaluation of native grasses collected from alpine rangelands in Qinghai Province, China, as materials for breeding grazing-tolerant fine herbage. Grassl. Sci. 2009, 55, 41–45. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).