Abstract
Selenium (Se) is an important micronutrient for the maintenance of human health. In China, however, the population is more severely deficient in Se. Soybean is an important grain and oil crop in the world and serves as a major dietary source. The development of Se biofortification of soybeans may be an effective measure to address human Se deficiency. Arbuscular mycorrhizal fungi (AMF) are ubiquitous soil microorganisms that can enhance nutrient uptake in host plants. So, it is necessary to investigate whether soybean inoculated with AMF can biofortificate Se. In this experiment, we studied the impact of the exogenous application of three Se species (selenite, selenate, and selenomethionine) and two AMF species (Funneliformis mosseae and Glomus versiforme) on Se uptake in soybean seedlings. The results showed that the inoculation of AMF significantly (p < 0.05) improved biomass and P concentration in soybeans. Regardless of exogenous Se addition, the inoculation of AMF improved the Se transfer factor and significantly (p < 0.05) increased Se translocation to the soybean shoot. The inoculation of AMF also significantly (p < 0.05) increased the percentage of available Se in soil with selenite addition. Based on these findings, the combined application of exogenous Se and AMF inoculation represents a viable strategy for the Se biofortification of soybeans.
1. Introduction
Selenium (Se) is an important micronutrient necessary for sustaining human life. Se has antioxidant and regulating immune responses and other various biological functions []. Se deficiency leads to dysfunction in various organs, resulting in conditions such as Creutzfeldt–Jakob disease, macroglossia, and leukodystrophy [,,]. Unfortunately, inadequate Se intake is a widespread global issue, with approximately 15% of the world’s population affected by Se deficiency []. China is facing a more critical Se deficiency challenge, with roughly 51% of its territory lacking sufficient Se. Moreover, the typical daily Se consumption among Chinese individuals, about 40 μg/day, remains substantially lower than the WHO’s advised threshold of 55 μg/day [,]. Se biofortification is a crucial strategy for enhancing Se concentration in crops through agronomic practices. It is regarded as a sustainable approach to improve human Se intake [,]. Soybean is not only an important high-protein crop but also exhibits strong bioconcentration capacity for Se. Through its intrinsic uptake transporter mechanisms, soybean can convert up to 80% of inorganic Se into organic Se species, which demonstrates lower toxicity and higher bioavailability [,]. Therefore, increasing Se concentration in soybean represents an important strategy for improving human health.
Adding Se into soil is an easy and reliable way to increase Se levels in crops. The addition of selenite (Se(IV)) and selenate (Se(VI)) to the soil is a common practice for enhancing Se concentration in crops [], but crops exhibit varying uptake efficiencies for different Se species. The inorganic species of Se(IV) (Na2SO3) and Se(VI) (Na2SO4) represent the primary species available for crop uptake and utilization in soil. Se(VI) is taken up by plant roots via sulfate transporter proteins and is subsequently transported to the leaf chloroplasts, where it is metabolized into organic Se via sulfur assimilation. In contrast, Se(IV) is primarily assimilated into organic Se through S assimilation within the root system. Se(IV) may be transported by phosphate (Pi) transporters [,,]. SeMet is usually the predominant organic form of Se in crops []. The distribution of Se fractions in soil serves as a crucial indicator for assessing Se bioavailability. These fractions include soluble Se (SOL-Se), exchangeable and carbonate Se (EXC-Se), Fe/Mn oxide Se (FMO-Se), organic-matter Se (OM-Se), and residual Se (RES-Se). Generally, SOL-Se and EXC-Se are significant indicators of Se bioavailability, with their concentration directly correlating with Se availability. In contrast, FMO-Se, OM-Se, and RES-Se exhibit very low potency. Although the addition of exogenous Se can directly increase soil Se concentration, the crop utilization efficiency of applied Se remains very low, with typically less than 5% being absorbed by plants [,,]. Consequently, improving the utilization efficiency of Se under conditions of soil application of exogenous Se has become an urgent challenge.
Arbuscular mycorrhizal fungi (AMF) are microorganisms commonly found in soil that can colonize with plant roots, thereby increasing the contact area between the root system and the soil. This interaction enhances the uptake of nutrients by the host plant and increases plant biomass [,,]. Under soil phosphorus (P) deficiency, AMF can significantly enhance P acquisition in host plant, which generally occurs through two pathways: direct uptake through root hairs and root epidermal cells [,], and indirect uptake by AMF mycelium, followed by transfer to root cortical cells []. In addition, AMF can alter soil speciation of metals through the exudation of host plants, thus increasing or altering their access to the plant [,]. In some other studies, inoculation of AMF significantly increased the Se content of garlic (Allium sativum L.) and onion (Allium cepa L.) []. AMF enhanced Se bioavailability in the rhizosphere and promoted Se uptake in winter wheat []. These findings suggested that AMF plays a significant role in regulating Se uptake in crops. However, research on the impact of AMF on Se uptake in soybeans remains limited. Furthermore, the species of applied Se, its concentration, and the species of AMF all influence the Se uptake by crops [,]. Therefore, the mechanisms by which the inoculation of AMF (Funneliformis mosseae and Glomus versiforme) affects Se uptake in soybean, particularly under varying species and concentrations of exogenously applied Se, require further investigation.
In this experiment, we explored the effects of exogenous Se species and inoculation of AMF on Se uptake in soybeans. The purpose of this experiment was to (1) examine the mechanisms of Se uptake and translocation in soybean inoculated with AMF under different Se species, and (2) evaluate the influence of AMF on Se fractions in soil. This study’s results will provide a theoretical foundation for understanding how AMF regulate Se uptake in soybeans and a scientific reference for enhancing crop Se concentration and promoting the sustainable development of Se-enriched agriculture.
2. Materials and Methods
2.1. Pot Experiments
Topsoil (0–20 cm depth) was acquired from uncontaminated farmland in Yangling, Shaanxi Province of China, naturally air-dried, and passed through a 2 mm sieve. The following were basic soil physical and chemical properties according to Bao []: pH 7.67 (water–soil ratio = 1:2.5, pH meter method), and total Se (atomic fluorescence spectrometry), total P (molybdenum blue method), and total potassium (K, flame atomic absorption spectrometry) were 0.19 mg/kg, 1.34 g/kg, and 1.12 g/kg, respectively. The soil was sterilized with autoclaving (121 °C, 2 h). Both sodium selenate and sodium selenite were obtained from Shandong West Asia Chemical Industry Co., Ltd. (Linyi, China), while DL-selenomethionine was obtained from Tokyo Chemical Industry Co., Ltd. (Chuo-ku, Japan). Soybean seeds (Zhonghuang 13 cultivar) were purchased from the Yangling District Seed Station (Xianyang, China). Funneliformis mosseae (Fm) and Glomus versiforme (Gv) were obtained from the Beijing Academy of Agriculture and Forestry (Beijing, China). Fm and Gv are widely found in natural ecosystems, and the two species of AMF can show strong adaptability in different types of soils, with a high affinity for a variety of crops. The results of the study are more generalizable and can be generalized to practical agricultural production or ecological restoration scenarios. The two species of AMF were propagated in soil–sand mixtures (soil: sand = 1:3, v/v) that were autoclaved (121 °C, 2 h), utilizing maize and alfalfa as host plants. Soil moisture content was maintained at 60–70% field holding capacity, avoiding waterlogging leading to anaerobic conditions. After 3 months, the substrate, which included AMF spores, hyphae, and colonized root fragments, was air-dried and stored for the following pot experiment.
Pot experiments were conducted in the greenhouse of Northwest A&F University (temperature range: 18–30 °C, photoperiod: 12 h light/day). A completely randomized three-factor design (3 × 3 × 10) was implemented, with the factors being (1) AMF inoculation treatments, (2) Se species treatments, and (3) Se concentration treatments. The three inoculation treatments included CK (no inoculation control), Fm, and Gv. The three Se species treatments included Se(Ⅳ), Se(Ⅵ), and SeMet. The ten Se treatments included 0 mg/kg (control); Se(Ⅳ) at 0.2, 0.5, 1.0, 5.0, and 10.0 mg/kg; Se(Ⅵ) at 0.5 and 1.0 mg/kg; and SeMet at 0.5 and 1.0 mg/kg. The Se concentration was set with reference to the effect of AMF on selenium uptake in wheat studied by Luo et al. []. The pot experiment consisted of 30 treatments, each of which was replicated three times. The Se solution was sprayed into the soil, mixed well with the soil, and equilibrated for 30 days. Each pot received 4 kg of air-dried soil, with inoculated treatments receiving 500 g of AMF inoculum (mixture of inoculated root fragments, spores, mycelium, and substrates), while uninoculated control was amended with 500 g of sterilized inoculum. The soybean seeds underwent surface sterilization using 5% (v/v) NaOCl for 30 min, and then were thoroughly washed five times with sterile deionized water. A total of 20–25 seeds were sowed per pot and the seedlings were thinned to 8 plants per pot at the cotyledon expansion (VC growth stage). Soil water content was regulated at 60–80% of field holding capacity. After 12 weeks, the seedlings were harvested and separated into shoots and roots. At the same time, soil samples were obtained following a standardized five-point sampling protocol. The shoots and roots were washed three times with tap water and once with deionized water before drying to constant weight at 60 °C and the dry weight (DW) of shoots and roots were measured. The dried plant samples were pulverized and then stored for the subsequent chemical analysis. The soil samples were naturally dried in a cool place, sieved (20 mesh and 100 mesh), and stored for chemical analysis.
2.2. Determination of Mycorrhizal Colonization Rate in Roots of Soybean
The root mycorrhizal colonization rate was determined using the slide length method described in our previous study []. Fresh root samples were collected, cut into 1 cm segments, washed, and stained before microscope observation. The mycorrhizal colonization rate was measured by examining fifty 1 cm root segments per sample under a microscope at 100× magnification. The percentage of root length containing fungal structures was calculated for each segment and averaged across three replicates per treatment.
2.3. Chemical Analysis
2.3.1. Determination of P Concentration in Soybean
Approximately 0.2 g of the plant samples was digested with H2SO4 and H2O2, and the P concentration was determined using the vanadium–molybdate yellow colorimetric method [].
2.3.2. Determination of Se Concentration in Soil and Plant
Plant Se concentration was determined using Wang et al.’s method []. Briefly, about 0.2 g dried plant sample was weighed into a digestion tube, and a mixed acid solution (HNO3:HClO4 = 4:1, v/v) was added to each sample. The mixture was pre-digested in an electrically heated digestion furnace at 90 °C for 30 min, followed by heating to 165 °C until the mixture became clear. After cooling, the selenate was reduced to selenite by adding concentrated HCl and heating at 90 °C for an additional 30 min. The Se concentration was quantified with atomic fluorescence spectrometry (LC-AFS-8530, Beijing Haiguang Instrument Co., Ltd., Beijing, China). Total soil Se concentration was digested with a mixed acid solution (HNO3:HClO4 = 3:2, v/v) in an electrothermal digestion furnace at 165 °C until the soil turned white.
2.3.3. Analysis of Se Fractions in Soil
Soil Se fractions were fractionated through a sequential extraction procedure modified from Wang et al.’s five-step protocol []. The procedure is summarized as follows: (1) SOL-Se: A total of 1.000 g of soil was placed in a polyethylene centrifuge tube (100 mL) and mixed with 10 mL of solution of 0.25 M KCl. The suspension was agitated (25 °C, 1 h) and then centrifuged (4000 rpm, 15 min), and the supernatant liquid was stored at 4 °C for analysis. (2) EXC-Se: The solid residue from the SOL-Se extraction was mixed with 10 mL of buffer (pH 5.0) of 0.7 mol/L KH2PO4 and extracted (25 °C, 4 h). The supernatant was subsequently separated for analysis. (3) FMO-Se: The solid residue from the EXC-Se extraction was mixed with 10 mL of 2.5 M HCl and subjected to intermittent oscillatory extraction (90 °C, 1 h) to isolate Fe/Mn oxide-bound Se (FMO-Se). The supernatant was subsequently separated for analysis. (4) OM-Se: The solid residue from the FMO-Se extraction was mixed with 8 mL 5% K2S2O2 and 2 mL of concentrated HNO3 (1:1), followed by intermittent shaking extraction (90 °C, 3 h). The supernatant was subsequently separated for analysis. (5) RES-Se: HClO4:HNO3 = 4:1 (v/v) mixture at 160–170 °C was used to complete the sequential extraction procedure. The Se concentrations in the extracts were determined with inductively coupled plasma mass spectrometry (ICP-MS) following established protocols.
2.4. Quality Control
Quality control was conducted by regulating the test conditions, establishing replicates, and utilizing standard reference materials (laver: GBW10023; soil: GBW07404). Environment and sample consistency were strictly maintained to minimize systematic errors. The coefficient of variation between replicates was less than 10%, and Se recoveries in the standard reference materials (purslane and soil) ranged from 97.9% to 98%.
2.5. Statistical Analysis
Microsoft Excel 2010 was utilized for the initial data processing. IBM SPSS Statistics 23 was utilized for analysis of variance (ANOVA) and correlation analysis amongst treatments, and Origin 2016 for equation fitting and graphing []. The transport factor (TF) of Se reflects the ability of Se to be transferred from the soybean root system to the shoot, and the bioconcentration factor (BCF) indicates the ability of Se uptake. By calculating the BCF of Se in the shoot parts (BCFshoot/soil) and root system (BCFroot/soil) of soybean, as well as the Se TF (root-to-shoot), the effects of AMF on the Se uptake capacity of soybean plants were further studied.
where Cshoot, Croot, and Csoil represent the Se concentration (mg/kg) in shoot, root, and soil, respectively.
BCFshoot/soil = Cshoot/Csoil
BCFroot/soil = Croot/Csoil
TF = Cshoot/Croot
Se content(Shoot/Root) = Cshoot/Croot × biomass(Shoot/Root)
3. Results and Discussion
3.1. Effects of AMF on P Uptake and Growth of Soybean Grown in Soils Amended with Different Se Species
The high rate of mycorrhizal colonization indicated the strength of the symbiotic relationship between the soybean and AMF. The inoculation of AMF significantly (p < 0.05) increased mycorrhizal colonization rate in roots compared to CK. The colonization rate in soybean roots exceeded 68%. Under low Se conditions (Se(IV) ≤ 0.2 mg/kg, Se(VI) ≤ 0.5 mg/kg, SeMet ≤ 0.5 mg/kg), soybean roots inoculated with Gv exhibited a significantly (p < 0.05) higher colonization rate compared to that inoculated with Fm (Figure 1a–c). The colonization rate of Gv was higher than that of Fm largely due to the different dependence of soybean on the two fungi. Sugars, organic acids, and phenolics secreted by the soybean root system are more conducive to promoting the colonization of Gv []. Different Se species and concentrations exhibited certain effects on the root colonization rate. The colonization rate in soybean root inoculated with Fm under 1 mg/kg Se(VI) addition was significantly (p < 0.05) higher than that under the 0.5 mg/kg Se(VI) treatment. When inoculated with Gv, the colonization rate under Se(VI) treatment was significantly (p < 0.05) higher than that under Se(IV) at the same exogenous Se concentration (0.5 or 1 mg/kg) (Figure 1).
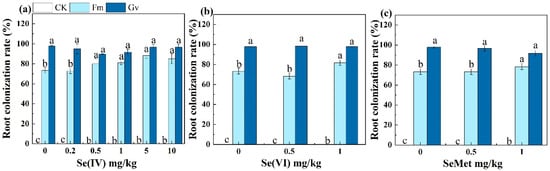
Figure 1.
Effects of Funneliformis mosseae (Fm) and Glomus versiforme (Gv) on root colonization rate in soybean grown in soils amended with different levels of selenite (a), selenate (b), and selenomethionine (c). Notes: selenite—Se(IV); selenate—Se(VI); selenomethionine—SeMet. Values are means ± SE. Different letters indicate significant (p < 0.05) differences among different AMF treatments under the same Se concentration treatment.
The inoculation of AMF significantly (p < 0.05) increased the shoot and root biomass of soybean under Se(Ⅳ) treatment compared to the CK. Specifically, the shoot and root biomass of soybean inoculated with AMF increased by 60.3–94.9% and 73.3–105.3%, respectively, under the 1.0 mg/kg Se(IV) treatment. Under the Se(VI) and SeMet treatments, only the inoculation of Gv significantly (p < 0.05) increased the biomass of the soybean shoots (Table 1). The inoculation of AMF has been shown to increase the biomass of potatoes, leeks, corn, sorghum, and celery [,,]. The increase in soybean biomass may be closely related to the inoculation of AMF, which significantly enhances the uptake efficiency of essential nutrients such as N, P, and potassium (K). This improved nutrient absorption provides better support for soybean growth, thereby promoting biomass growth [].

Table 1.
Effects of Funneliformis mosseae (Fm) and Glomus versiforme (Gv) on shoot and root biomass of soybean grown in soils amended with different levels of selenite, selenate, and selenomethionine (mean ± SE, n = 3).
The inoculation of AMF significantly (p < 0.05) increased P concentration in the shoot and root of soybean (Table 2). With exogenously added Se(IV), Se(VI), and SeMet, along with AMF inoculation, the P concentration in soybean shoot and root was 1.36–2.21, 1.72–2.15, and 1.96–2.35 times and 1.39–3.19, 2.16–3.13, and 1.98–2.29 times, respectively, compared to the CK. This is because the formation of the mycelial network of mycorrhizal roots promotes P uptake in the host plant []. Meanwhile, AMF induces the expression of P transporter protein genes in the plant root system and enhances P uptake by the plant [,]. Additionally, exogenous Se application to soils influenced P uptake in soybeans. The application of 5 mg/kg Se(IV) along with AMF significantly (p < 0.05) increased P uptake in the shoots of soybean. Conversely, the addition of 10 mg/kg Se(IV) in conjunction with the inoculation of AMF significantly (p < 0.05) decreased shoot P uptake in soybeans. This reduction may result from competitive uptake between P and Se(IV) under high Se concentration treatment, which limits P uptake by the soybean plants []. Furthermore, it has been shown that AMF exhibited a synergistic effect under low P concentration, enhancing Se uptake by crops []. Thus, a low concentration of Se in soil promoted P uptake, whereas a high concentration inhibited P uptake.

Table 2.
Effects of Funneliformis mosseae (Fm) and Glomus versiforme (Gv) on shoot and root phosphorus (P) concentration in soybean grown in soils amended with different levels of selenite (0, 0.2, 0.5, 1.0, 5.0, and 10.0 mg/kg), selenate (0, 0.5, and 1.0 mg/kg) and selenomethionine (0, 0.5, and 1.0 mg/kg) (mean ± SE, n = 3).
3.2. Effect of AMF on Se Uptake in Soybean Grown in Soils Amend with Different Se Species
Regardless of whether Se(IV), Se(VI), or SeMet was applied, the inoculation of AMF significantly (p < 0.05) increased shoot Se uptake in soybean under the different Se species and their respective concentrations, more distinct in shoots, with an almost exponential trend. Specifically, under the treatment of 5 mg/kg Se(IV), the shoot Se concentration in soybean inoculated with Fm (7.73 mg/kg) and Gv (10.24 mg/kg) was 1.5 and 1.9 times higher than the CK (uninoculated treatment) (5.18 mg/kg) (Figure 2a). The inoculation of Fm significantly enhanced Se uptake by soybean root only when Se(IV) was added. In contrast, both Fm and Gv significantly (p < 0.05) reduced Se concentration in soybean root when Se(VI) or SeMet was added (Figure 2b). Some studies have shown that AMF inoculation significantly increased the uptake of Se(IV) and Se(VI) in winter wheat []. Luo et al. reported that AMF did not significantly enhance the uptake of SeMet in wheat under hydroponic experiments []. In contrast, the present study found that AMF significantly promoted the uptake of SeMet in soybeans. In soybeans, Se(IV) is predominantly found in the root system whereas Se(VI) is primarily concentrated in the shoot. This distribution occurs because selenite is highly susceptible to transformation into other forms (such as seleno-substituted amino acid oxides and SOL-Se) and tends to be immobilized in the root system. In contrast, selenate can be transported directly through the xylem to the aboveground tissues []. SeMet can enter the plant root system via amino acid permease [], and amorphous transformations can be rapidly transferred to the aboveground parts of the plant. The Se concentration in soybean increased with a higher concentration of exogenous Se (Figure 2). This result is consistent with previous studies []. However, when Se concentration is excessively high, it induces the accumulation of reactive oxygen species, damages cell membranes and proteins, and disrupts cellular function. This, in turn, affects nutrient uptake and ultimately reduces both crop yield and quality []. In this experiment, the effects of AMF on Se uptake in soybeans were found to vary with Se species. This variation reflects different translocation efficiencies of Se species in soybeans. Se accumulation in roots and shoots is a direct source of Se in the seed, and differences at the seedling stage already predict a great likelihood of good results in the seed. We are in the process of following up the work at soybean maturity.
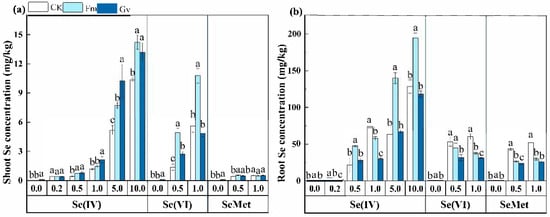
Figure 2.
Effects of Funneliformis mosseae (Fm) and Glomus versiforme (Gv) on Se concentration in shoot (a) and root (b) of soybean grown in soils amended with different levels of selenite, selenate, and selenomethionine. Notes: selenite—Se(IV); selenate—Se(VI); selenomethionine—SeMet. Values are means ± SE. Different letters indicate significant (p < 0.05) differences among different AMF treatments under the same Se concentration treatment.
The transfer factor (TF) of Se reflects the capacity of Se to be transferred from the root to the shoot. The inoculation of AMF significantly (p < 0.05) increased the TF of Se in soybean, both without Se addition and with the exogenous addition of Se(Ⅵ) and SeMet. The TF of Se in AMF-inoculated soybean was 1.8–11.4, 1.7–4.3, and 1.7–3.3 times higher than the control without Se or with exogenously added Se(Ⅵ) and SeMet. The exogenous addition of Se(IV) significantly (p < 0.05) increased the TF of Se in soybean only when inoculated with AMF under the 1 mg/kg Se treatment (Table 3). In addition, both AMF and Se significantly (p < 0.01) influence Se transport in soybeans. It has been demonstrated that soybean absorbs and recycles selenate more efficiently than selenite []. The present experiments indicated that the transport capacity of soybean for selenate was significantly (p < 0.05) higher than that for selenite, which aligned with the findings of the previous studies. The bioconcentration factor (BCF) reflects the capacity of soybeans to absorb and accumulate Se. The shoot Se enrichment factor in AMF-inoculated soybean treated with 1 mg/kg Se(IV) significantly (p < 0.05) increased. Furthermore, the shoot Se BCF of AMF-inoculated soybean was 2.5–4.3 times higher than non-inoculated control under 0.5 mg/kg Se(VI) treatment. It has also been demonstrated that inoculation of AMF significantly increased the enrichment factor of wheat for selenate and selenite []. The inoculation of AMF under SeMet treatment did not significantly affect BCF in soybean shoots.

Table 3.
Effects of Funneliformis mosseae (Fm) and Glomus versiforme (Gv) on bioconcentration factor (BCF) and transport factor (TF) of selenium (Se) in soybean grown in soils amended with different levels of selenite (0, 0.5, and 1.0 mg/kg), selenate (0, 0.5, and 1.0 mg/kg) and selenomethionine (0, 0.5, and 1.0 mg/kg) (n = 3).
3.3. Effects of Different Se Species and AMF on Se Fractions in Rhizosphere Soil of Soybean
Se fractions in soil are crucial factors influencing Se uptake in soybeans. Different species of exogenous Se undergo a series of physical, chemical, and biological reactions after being applied to the soil, resulting in changes to their existing chemical fractions. Regardless of the Se species added, there is a highly significant (p < 0.01; linear regression) positive correlation between Se content in soybean shoot/root and the available Se (SOL-Se + EXC-Se) in the soil (Figure 3). This indicated that the levels of available Se in the soil were key determinants of Se bioavailability. It has been shown that the Se content in both cabbage shoots and roots correlates significantly (p < 0.05) with the SOL-Se concentration in soil []. The effects of different Se fractions in soil on Se uptake in soybeans were further analyzed using stepwise regression equations. This involved inputting independent variables, eliminating those with p > 0.05, obtaining regression equations, and introducing new variables until no additional variables could be reintroduced. The results indicated that SOL-Se in the soil had a significant (p < 0.001) positive correlation with the shoot Se concentration in soybean. In contrast, other Se fractions did not significantly (p < 0.001) affect the shoot Se content in soybeans and were subsequently eliminated from consideration (Table 4). This may be attributed to the fact that SOL-Se, as the primary fraction of Se uptake in soybean, is highly susceptible to translocation to the aboveground parts []. Furthermore, the Se content in soybean root was highly significant (p < 0.001) and positively correlated with soil SOL-Se, EXC-Se, and RES-Se, while it exhibited a highly significant (p < 0.001) negative correlation with OM-Se. The inoculation of AMF may enhance soybean root secretion of organic matter, which can rapidly bind exogenous Se into stable compounds, reducing root uptake [].
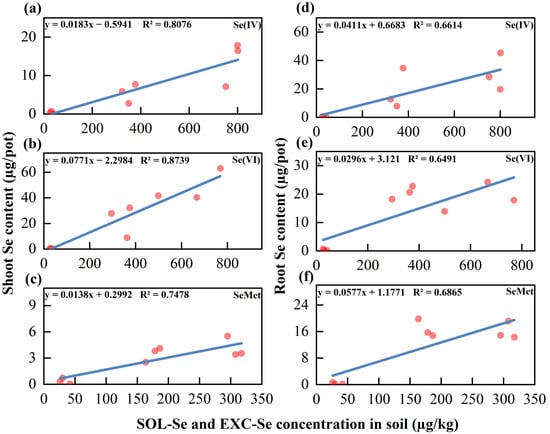
Figure 3.
Correlation between soil available selenium (Se) and Se content in soybean shoot (a–c) and root (d–f) grown in soils amended with selenite (Se(IV)), selenate (Se(VI)) and selenomethionine (SeMet).

Table 4.
Stepwise regression analysis of plant Se concentration and soil Se fractions.
In the absence of exogenous Se addition, the percentage of available Se in AMF-inoculated soil significantly (p < 0.05) increased in comparison with the CK. However, when exogenous Se was added, the percentage and concentration of available Se in AMF-inoculated soil significantly (p < 0.05) increased only under 1 mg/kg Se(IV) treatment. In contrast, the exogenous addition of Se(VI) and SeMet did not significantly (p < 0.05) affect the percentage or concentration of available Se in the soil inoculated with AMF (Figure 4). Li et al. investigated rhizosphere soil and found that the inoculation of AMF significantly increased the proportion of Se bioavailable in the soil, even in the absence of Se addition or exogenous Se(IV) and Se(VI) []. These findings contrast with the results of the present study, which is possibly related to differences in soil sampling methodologies. Additionally, rhizosphere soils are more influenced by plant root activities, resulting in greater differences in their physicochemical and biological properties compared to non-rhizosphere soils []. This variation, in turn, affected the presence of Se in its bioavailable fraction. However, without the supplementation of exogenous Se, the bioavailable Se concentration in AMF-inoculated soil decreased significantly (p < 0.05) by 28–38% in comparison with the CK. This reduction may be closely related to the ability of AMF to enhance the uptake of bioavailable Se by soybean plants. This finding provided a significant foundation for Se biofortification through the use of AMF.
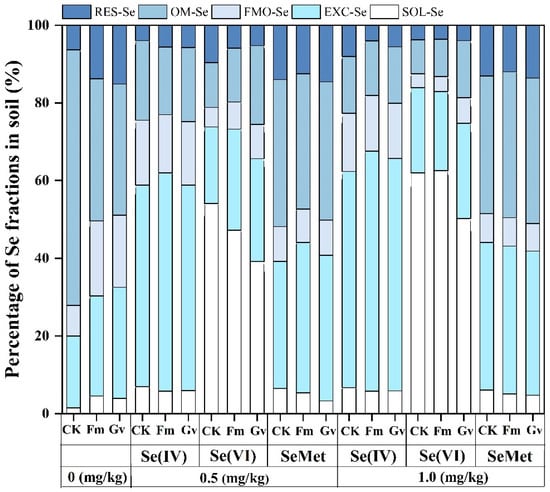
Figure 4.
Effects of Funneliformis mosseae (Fm) and Glomus versiforme (Gv) on the percentage of Se fractions in rhizosphere soils added with different levels of selenite (0, 0.5, and 1.0 mg/kg), selenate (0, 0.5, and 1.0 mg/kg) and selenomethionine (0, 0.5, and 1.0 mg/kg). Notes: selenite—Se(IV); selenate—Se(VI); selenomethionine—SeMet. CK—no inoculation treatment. SOL-Se—soluble Se, EXC-Se—exchangeable and carbonate Se; FMO-Se—Fe/Mn oxide Se; OM-Se—organic-matter Se; RES-Se—residual Se.
4. Conclusions
Both exogenous Se application and the inoculation of AMF increased biomass and P uptake in soybeans. The inoculation of AMF significantly (p < 0.05) increased the transfer factors of Se in soybeans and promoted Se transfer to the shoot. Se(IV) was predominantly retained in the root, while Se(VI) was primarily translocated to the shoot. Furthermore, inoculation of AMF significantly (p < 0.05) improved the percentage of available Se (SOL-Se + EXC-Se) in the rhizosphere soil. Soil available Se was the dominant factor affecting Se uptake in soybeans, while the inoculation of AMF enhanced Se accumulation in soybeans by altering soil Se bioavailability. Both the shoot and root Se contents of soybean showed a significant (p < 0.01) positive correlation with the available Se concentration in soil. The inoculation of AMF can regulate the transport and distribution of Se in soybeans, enhancing the uptake and utilization of Se by altering Se availability in the soil. The present study provided a crucial foundation for the Se biofortification of soybeans through the inoculation of AMF combined with Se application in soil.
Author Contributions
Conceptualization, F.W. and W.X.; formal analysis, F.W. and Y.M.; investigation, F.W.; methodology, W.X. and Y.M.; resources, W.X. and J.Y.; software, Q.C. and J.Y.; supervision, S.H. and Q.C.; validation, S.H. and Y.M.; visualization, Y.M. and H.Z.; writing—original draft, H.Z. and F.W.; writing—review and editing, H.Z. and F.W. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financially supported by the National Natural Science Foundation of China (42077325; 41571456) and the Natural Science Basic Research Program of Shaanxi (Program No. 2023-JC-ZD-17).
Data Availability Statement
All the data generated or analyzed during this study are included in the article.
Acknowledgments
We are very grateful to every scientific researcher who supports and helps us in our work.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Schomburg, L. Dietary selenium and human health. Nutrients 2017, 9, 22. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Zhang, M.; Tang, S.; Li, M.; Wu, R.; Wan, S.; Chen, L.; Wei, X.; Feng, S. Effects and Impact of Selenium on Human Health, a review. Molecules 2024, 30, 50. [Google Scholar] [CrossRef] [PubMed]
- Altekin, E.; Çoker, C.; Şişman, A.R.; Önvural, B.; Kuralay, F.; Kırımlı, Ö. The relationship between trace elements and cardiac markers in acute coronary syndromes. J. Trace Elem. Med. Biol. 2005, 18, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P. The importance of selenium to human health. Lancet 2000, 356, 233–241. [Google Scholar] [CrossRef]
- Tan, L.C.; Nancharaiah, Y.V.; van Hullebusch, E.D.; Lens, P.N. Selenium: Environmental significance, pollution, and biological treatment technologies. In Anaerobic Treatment of Mine Wastewater for the Removal of Selenate and Its Co-Contaminants; CRC Press: Boca Raton, FL, USA, 2018; pp. 9–71. [Google Scholar]
- Dinh, Q.T.; Cui, Z.; Huang, J.; Tran, T.A.T.; Wang, D.; Yang, W.; Zhou, F.; Wang, M.; Yu, D.; Liang, D. Selenium distribution in the Chinese environment and its relationship with human health: A review. Environ. Int. 2018, 112, 294–309. [Google Scholar] [CrossRef]
- Wu, Z.; Bañuelos, G.S.; Lin, Z.-Q.; Liu, Y.; Yuan, L.; Yin, X.; Li, M. Biofortification and phytoremediation of selenium in China. Front. Plant Sci. 2015, 6, 136. [Google Scholar] [CrossRef]
- Bodnar, M.; Marzena, S.; Piotr, K.; Namiesnik, J. Methods of selenium supplementation: Bioavailability and determination of selenium compounds. Crit. Rev. Food Sci. Nutr. 2016, 56, 36–55. [Google Scholar] [CrossRef]
- Welch, R.M.; Graham, R.D. Agriculture: The real nexus for enhancing bioavailable micronutrients in food crops. J. Trace Elem. Med. Biol. 2005, 18, 299–307. [Google Scholar] [CrossRef]
- Wei, H. Comparison of the bioavailability of soybean selenoprotein and sodium selenite. Soybean Sci. 2010, 29, 534–536. [Google Scholar]
- Dai, H.; Wei, S.; Twardowska, I. Biofortification of soybean (Glycine max L.) with Se and Zn, and enhancing its physiological functions by spiking these elements to soil during flowering phase. Sci. Total Environ. 2020, 740, 139648. [Google Scholar] [CrossRef]
- Ros, G.; Van Rotterdam, A.; Bussink, D.; Bindraban, P.S. Selenium fertilization strategies for bio-fortification of food: An agro-ecosystem approach. Plant Soil 2016, 404, 99–112. [Google Scholar] [CrossRef]
- Deng, X.; Liu, K.; Li, M.; Zhang, W.; Zhao, X.; Zhao, Z.; Liu, X. Difference of selenium uptake and distribution in the plant and selenium form in the grains of rice with foliar spray of selenite or selenate at different stages. Field Crops Res. 2017, 211, 165–171. [Google Scholar] [CrossRef]
- Terry, N.; Zayed, A.; De Souza, M.; Tarun, A. Selenium in higher plants. Annu. Rev. Plant Biol. 2000, 51, 401–432. [Google Scholar] [CrossRef] [PubMed]
- Ikram, S.; Li, Y.; Lin, C.; Yi, D.; Heng, W.; Li, Q.; Tao, L.; Hongjun, Y.; Weijie, J. Selenium in plants: A nexus of growth, antioxidants, and phytohormones. J. Plant Physiol. 2024, 296, 154237. [Google Scholar] [CrossRef]
- Poblaciones, M.J.; Rodrigo, S.; Santamaría, O.; Chen, Y.; McGrath, S. Agronomic selenium biofortification in Triticum durum under Mediterranean conditions: From grain to cooked pasta. Food Chem. 2014, 146, 378–384. [Google Scholar] [CrossRef]
- Dai, Z.; Imtiaz, M.; Rizwan, M.; Yuan, Y.; Huang, H.; Tu, S. Dynamics of selenium uptake, speciation, and antioxidant response in rice at different panicle initiation stages. Sci. Total Environ. 2019, 691, 827–834. [Google Scholar] [CrossRef]
- Qi, M.; Wang, D.; Zhai, H.; Zhou, F.; Wu, H.; Zhao, W.; Ren, R.; Shi, J.; Liang, D. Effects of straw amendment on the bioavailability of selenite in soil and its mechanisms. Ecotoxicol. Environ. Saf. 2025, 290, 117578. [Google Scholar] [CrossRef]
- Haug, A.; Graham, R.D.; Christophersen, O.A.; Lyons, G.H. How to use the world’s scarce selenium resources efficiently to increase the selenium concentration in food. Microb. Ecol. Health Dis. 2007, 19, 209–228. [Google Scholar]
- Zhang, F.; Liu, M.; Li, Y.; Che, Y.; Xiao, Y. Effects of arbuscular mycorrhizal fungi, biochar and cadmium on the yield and element uptake of Medicago sativa. Sci. Total Environ. 2019, 655, 1150–1158. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, L.; Meng, S.; Song, X.; Long, R.; Huang, H.; Tang, Y.; Zhou, X.; Li, M.; Sun, B.; et al. The physiological responses of celery (Apium graveolens L.) and its ability to accumulate selenium when inoculated with Funneliformis mosseae. Sci. Hortic. 2024, 326, 112752. [Google Scholar] [CrossRef]
- Wang, J.; Pi, Y.; Li, Y.; Wang, H.; Huang, K.; Wang, X.; Xia, H.; Zhang, X.; Liang, D.; Lv, X.; et al. Transcriptome and metabolome analyses reveal the promoting effects of arbuscular mycorrhizal fungi on selenium uptake in grapevines. Plant Physiol. Biochem. 2025, 219, 109456. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.; Sun, J.; Liu, D.; Li, B.; Zhang, A.; Li, Z.; Tong, Y. Characterization of the promoter of phosphate transporter TaPHT1. 2 differentially expressed in wheat varieties. J. Genet. Genom. 2009, 36, 455–466. [Google Scholar] [CrossRef] [PubMed]
- Nussaume, L.; Kanno, S.; Javot, H.; Marin, E.; Pochon, N.; Ayadi, A.; Nakanishi, T.M.; Thibaud, M.-C. Phosphate import in plants: Focus on the PHT1 transporters. Front. Plant Sci. 2011, 2, 83. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zhao, B.; Zheng, S.; Zhang, X.; Wang, X.; Dong, W.; Xie, Q.; Wang, G.; Xiao, Y.; Chen, F. A phosphate starvation response-centered network regulates mycorrhizal symbiosis. Cell 2021, 184, 5527–5540. [Google Scholar] [CrossRef]
- Klugh-Stewart, K.; Cumming, J.R. Organic acid exudation by mycorrhizal Andropogon virginicus L. (broomsedge) roots in response to aluminum. Soil Biol. Biochem. 2009, 41, 367–373. [Google Scholar] [CrossRef]
- Lindblom, S.D.; Valdez-Barillas, J.R.; Fakra, S.C.; Marcus, M.A.; Wangeline, A.L.; Pilon-Smits, E.A.H. Influence of microbial associations on selenium localization and speciation in roots of Astragalus and Stanleya hyperaccumulators. Environ. Exp. Bot. 2013, 88, 33–42. [Google Scholar] [CrossRef]
- Golubkina, N.; Amagova, Z.; Matsadze, V.; Zamana, S.; Tallarita, A.; Caruso, G. Effects of arbuscular mycorrhizal fungi on yield, biochemical characteristics, and elemental composition of garlic and onion under selenium supply. Plants 2020, 9, 84. [Google Scholar] [CrossRef]
- Li, J.; Awasthi, M.K.; Xing, W.; Liu, R.; Bao, H.; Wang, X.; Wang, J.; Wu, F. Arbuscular mycorrhizal fungi increase the bioavailability and wheat (Triticum aestivum L.) uptake of selenium in soil. Ind. Crops Prod. 2020, 150, 112383. [Google Scholar] [CrossRef]
- Zhou, F.; Yang, W.; Wang, M.; Miao, Y.; Cui, Z.; Li, Z.; Liang, D. Effects of selenium application on Se content and speciation in Lentinula edodes. Food Chem. 2018, 265, 182–188. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Z.; Gu, M.; Li, H.; Shohag, M.J.I.; Shen, F.; Wang, X.; Wei, Y. Combined use of arbuscular mycorrhizal fungus and selenium fertilizer shapes microbial community structure and enhances organic selenium accumulation in rice grain. Sci. Total Environ. 2020, 748, 141166. [Google Scholar] [CrossRef]
- Bao, S. Analytical methods of soil agrochemistry. In Soil Science Society of China Beijing, 3rd ed.; China Agricultural Science and Technology Press: Beijing, China, 2000. [Google Scholar]
- Luo, W.; Li, J.; Ma, X.; Niu, H.; Hou, S.; Wu, F. Effect of arbuscular mycorrhizal fungi on uptake of selenate, selenite, and selenomethionine by roots of winter wheat. Plant Soil 2019, 438, 71–83. [Google Scholar] [CrossRef]
- Wu, F.; Ye, Z.; Wong, M.H. Intraspecific differences of arbuscular mycorrhizal fungi in their impacts on arsenic accumulation by Pteris vittata L. Chemosphere 2009, 76, 1258–1264. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Ali, F.; Wang, M.; Dinh, Q.T.; Zhou, F.; Bañuelos, G.S.; Liang, D. Understanding boosting selenium accumulation in Wheat (Triticum aestivum L.) following foliar selenium application at different stages, forms, and doses. Environ. Sci. Pollut. Res. 2020, 27, 717–728. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Cui, Z.; Xue, M.; Peng, Q.; Zhou, F.; Wang, D.; Dinh, Q.T.; Liu, Y.; Liang, D. Assessing the uptake of selenium from naturally enriched soils by maize (Zea mays L.) using diffusive gradients in thin-films technique (DGT) and traditional extractions. Sci. Total Environ. 2019, 689, 1–9. [Google Scholar] [CrossRef]
- Öpik, M.; Zobel, M.; Cantero, J.J.; Davison, J.; Facelli, J.M.; Hiiesalu, I.; Jairus, T.; Kalwij, J.M.; Koorem, K.; Leal, M.E.; et al. Global sampling of plant roots expands the described molecular diversity of arbuscular mycorrhizal fungi. Mycorrhiza 2013, 23, 411–430. [Google Scholar] [CrossRef]
- Yang, Q.; Ravnskov, S.; Neumann Andersen, M. Nutrient uptake and growth of potato: Arbuscular mycorrhiza symbiosis interacts with quality and quantity of amended biochars. J. Plant Nutr. Soil Sci. 2020, 183, 220–232. [Google Scholar] [CrossRef]
- Tran, B.T.T.; Watts-Williams, S.J.; Cavagnaro, T.R. Impact of an arbuscular mycorrhizal fungus on the growth and nutrition of fifteen crop and pasture plant species. Funct. Plant Biol. 2019, 46, 732–742. [Google Scholar] [CrossRef]
- Chandrasekaran, M. Arbuscular mycorrhizal fungi mediated enhanced biomass, root morphological traits and nutrient uptake under drought stress: A meta-analysis. J. Fungi 2022, 8, 660. [Google Scholar] [CrossRef]
- Hammer, E.C.; Balogh-Brunstad, Z.; Jakobsen, I.; Olsson, P.A.; Stipp, S.L.; Rillig, M.C. A mycorrhizal fungus grows on biochar and captures phosphorus from its surfaces. Soil Biol. Biochem. 2014, 77, 252–260. [Google Scholar] [CrossRef]
- Li, S.; Chi, S.; Lin, C.; Cai, C.; Yang, L.; Peng, K.; Huang, X.; Liu, J. Combination of biochar and AMF promotes phosphorus utilization by stimulating rhizosphere microbial co-occurrence networks and lipid metabolites of Phragmites. Sci. Total Environ. 2022, 845, 157339. [Google Scholar] [CrossRef]
- Paszkowski, U.; Kroken, S.; Roux, C.; Briggs, S.P. Rice phosphate transporters include an evolutionarily divergent gene specifically activated in arbuscular mycorrhizal symbiosis. Proc. Natl. Acad. Sci. USA 2002, 99, 13324–13329. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Yu, T.; Qi, H.; Li, F.; Yang, Z. Analysis of phosphorus and sulfur effect on soil selenium bioavailability based on diffusive gradients in thin films technique and sequential extraction. Chemosphere 2022, 302, 134831. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Wu, M.; Zhang, Z.; Su, R.; He, H.; Zhang, X. The interaction of arbuscular mycorrhizal fungi and phosphorus inputs on selenium uptake by alfalfa (Medicago sativa L.) and selenium fraction transformation in soil. Front. Plant Sci. 2020, 11, 966. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhu, N.; Liang, X.; Zheng, L.; Zhang, C.; Li, Y.-F.; Zhang, Z.; Gao, Y.; Zhao, J. A comparative study on the accumulation, translocation and transformation of selenite, selenate, and SeNPs in a hydroponic-plant system. Ecotoxicol. Environ. Saf. 2020, 189, 109955. [Google Scholar] [CrossRef]
- White, P.J.; Broadley, M.R. Biofortification of crops with seven mineral elements often lacking in human diets–iron, zinc, copper, calcium, magnesium, selenium and iodine. New Phytol. 2009, 182, 49–84. [Google Scholar] [CrossRef]
- Radawiec, A.; Rutkowska, B.; Tidaback, J.A.; Gozdowski, D.; Knapowski, T.; Szulc, W. The impact of selenium fertilization on the quality characteristics of spring wheat grain. Agronomy 2021, 11, 2100. [Google Scholar] [CrossRef]
- Gupta, M.; Gupta, S. An overview of selenium uptake, metabolism, and toxicity in plants. Front. Plant Sci. 2017, 7, 2074. [Google Scholar] [CrossRef]
- Deng, X.; Liao, J.; Zhao, Z.; Qin, Y.; Liu, X. Distribution and speciation of selenium in soybean proteins and its effect on protein structure and functionality. Food Chem. 2022, 370, 130982. [Google Scholar] [CrossRef]
- Peng, Q.; Guo, L.; Ali, F.; Li, J.; Qin, S.; Feng, P.; Liang, D. Influence of pak choi plant cultivation on Se distribution, speciation and bioavailability in soil. Plant Soil 2016, 403, 331–342. [Google Scholar] [CrossRef]
- Lyu, C.; Chen, J.; Li, L.; Zhao, Z.; Liu, X. Characteristics of Se in water-soil-plant system and threshold of soil Se in seleniferous areas in Enshi, China. Sci. Total Environ. 2022, 827, 154372. [Google Scholar] [CrossRef]
- Li, Z.; Liang, D.; Peng, Q.; Cui, Z.; Huang, J.; Lin, Z. Interaction between selenium and soil organic matter and its impact on soil selenium bioavailability: A review. Geoderma 2017, 295, 69–79. [Google Scholar] [CrossRef]
- Guo, H.; Chen, J.; Zhong, B.; Liu, C.; Wu, J.; He, L.; Ye, Z.; Liu, D. Heavy metal concentration, enzyme activity, and physical and chemical properties of rhizosphere and non-rhizosphere soils containing Moso bamboo. Acta Ecol. Sin. 2017, 27, 6149–6156. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).