Abstract
The growing demand for sustainable phytosanitary products has renewed interest in botanical insecticides as viable pest control tools. Amid rising demand for sustainable crop protection, this study screens Cerrado plants traditionally used in medicine to pinpoint bioactive compounds that could replace synthetic pesticides. These products have complex chemical compositions, with compounds acting synergistically through multiple mechanisms, including oral (ingestion of allelochemicals) and topical (contact of allelochemicals on epidermis) toxicity. This study evaluated the oral and topical toxicity of aqueous leaf extracts from Anemopaegma arvense (AEAa), Coussarea hydrangeifolia (AECh), Tapirira guianensis (AETg), and Duguetia furfuracea (AEDf) on Plutella xylostella. In the oral toxicity test, first-instar larvae were fed treated diets until pupation, with biological parameters monitored until adulthood. The extracts caused an average of 45% larval mortality, reduced pupal duration, and lowered egg production. In the topical toxicity test, only the extract from T. guianensis showed significant effect (p = 0.0171), causing 30% mortality in third-instar larvae. The other extracts showed no significant topical toxicity, and AECh showed no lethal or sublethal effects at all. Phytochemical screening was assessed by quantitative spectrophotometric assays, and semi-quantitative classical colorimetric tests. Major compound classes identified were tannins, flavonoids, triterpenoids, coumarins, and alkaloids. These findings highlight the potential of the evaluated plant extracts for pest control, particularly via ingestion, while also underscoring the need for further studies to better understand their efficacy and mechanisms of action.
1. Introduction
Integrated pest management (IPM) is a holistic strategy that seeks to keep pest populations below their economic injury levels while minimizing the use of synthetic pesticides [1,2,3]. Achieving this balance necessitates an understanding of ecosystem dynamics, crop-specific tolerance thresholds, and cost–benefit ratios [4,5]. Among the many strategies available, those rooted in biotechnology, insect nutrition, and host–plant resistance play a prominent role [6]. Plants that have developed a resistance to insects through chemical attributes and can alter their biology (antibiosis) or preference (antixenosis) for a certain insect species are examples that fit within the IPM toolkit [6,7]. Whether attractive or repellent, these plants have great ecological and nutritional appeal, but many of them have not yet been fully explored and are not completely understood [6,8]. Since IPM emerged to meet the global market’s demands for safer tools for the ecosystem and human health, botanical insecticides have been regarded as a safe way to control pests [9,10].
Botanical insecticides typically contain complex blends of secondary metabolites that act via multiple routes: penetration through the cuticle (contact), entry into the tracheal system (fumigant), or ingestion after systemic translocation [11,12,13,14]. This chemical diversity allows simultaneous behavioral and physiological disruptions in target pests, underscoring the need to evaluate diverse application methods for a single product [12].
In recent years, the scientific community has intensified its research, seeking to elucidate the insecticidal potential within these plants [15,16]. Recent studies show that a wide spectrum of plant-based products can suppress agricultural pests via multiple modes of action. Cerrado natives such as Miconia albicans (sw.) Triana and Ludwigia spp. produce oviposition inhibition and oral toxicity, revealing an under-explored resource [13,17,18,19,20]. These results reveal how the understanding of already known botanical species can still be exploited for agricultural use.
The diamondback moth, Plutella xylostella (Linnaeus, 1758) (Lepidoptera: Plutellidae), is one of the most destructive pests of cruciferous crops worldwide [21,22,23,24]. In tropical and subtropical regions, it can complete more than 20 generations per year, causing substantial economic losses, especially in Southeast Asia, where cruciferous vegetables are dietary staples [23]. Heavy reliance on a limited set of chemical classes—most notably pyrethroids—has driven resistance to over 100 active ingredients [25,26]. P. xylostella’s economic importance, rapid generation cycle, well-studied physiology, and extensive record of insecticide resistance, therefore, render it an ideal biological model for assessing the efficacy of new botanical insecticides.
Once chemical control happens mainly in undeveloped countries [23], the study of the effects of botanical insecticides on this pest becomes particularly relevant, as these products can be created at home and at a reduced cost using local resources [8]. Thus, such products can be a viable solution for agricultural families, smallholder farmers, and communities that seek to reduce their dependence on synthetic chemical products, which may sometimes be inaccessible or inadequate for their circumstances, favoring the use of diverse tools for the ecologically correct handling of pests [27].
In this context, the present study analyzed the insecticidal potential and chemical composition of four botanical extracts from species present in the Brazilian Cerrado relative to P. xylostella: Anemopaegma arvense (Vell.) Stellf. ex de Souza (Bignoniaceae) (AEAa), Coussarea hydrangeifolia (Benth.) Benth. & Hook.f. ex Müll.Arg. (AECh), Tapirira guianensis Aubl. (Anarcadiaceae) (AETg), and Duguetia furfuracea A. St.-Hill (Annonaceae) (AEDf). These species were chosen based on the popular knowledge of small producers in the region of Dourados (Mato Grosso do Sul), Brazil, who use these species for medicinal and agricultural purposes.
2. Materials and Methods
The assays were performed at the Insect–Plant Interaction Laboratory (LIIP), located in the LAPACA building, unit 2 of the Federal University of Grande Dourados, Mato Grosso do Sul, Brazil. The rearing and experiments were established in a controlled environment at a temperature of 25 ± 1 °C (77 °F), relative humidity of 70 ± 5%, and a photo period of 12 h. The collection of botanical material was authorized by the Brazilian National Research Council (CNPq)/Council for Genetic Heritage Management (CGEN/MMA) under number AD06DBA.
2.1. Stock Rearing of Plutella xylostella
P. xylostella larvae were collected from vegetable gardens in Dourados, Mato Grosso do Sul, Brazil, in 2024 and transferred to plastic pots (30 × 15 × 12 cm), which contained a sheet of paper towel and two collard green leaves (Brassica oleraceae var. acephala) serving as a food source. The collard greens used were previously sanitized with sodium hypochlorite (5%) and sterilized with UV light for 15 min (wavelength 253.7 nm and l55 μW/cm2 of radiation levels). The larvae remained in the pots until pupation, when they were removed and stored in adult cages (9 × 19 × 19 cm).
The collard green disks were placed in adult cages, arranged on filter paper disks (both 4 cm Ø), and used as substrates for oviposition. Cotton soaked in a 10 mg/mL honey solution was used as a food source for the adults. After oviposition, the collard green leaves and filter paper disks were placed in the plastic pots (30 × 15 × 12 cm), where they remained until the larvae emerged.
Rearing maintenance was carried out daily, when the collard green leaves were replaced with new leaves. All individuals used in the experiments were removed from the stock rearing process. The methodology was adapted from [13,17,18,19].
2.2. Preparation of Aqueous Botanical Extracts
The collection took place in August 2023, during the morning period between 7:00 am and 11:00 am at the Lagoa Grande settlement in Itahum, Mato Grosso do Sul (MS) (Table 1). The leaves of each plant were detached and cleaned with running water. Subsequently, the material was dried in a forced-air-circulation oven for three days at 40 °C (100 °F) and ground in a knife mill to obtain a fine powder, which was stored in plastic jars. Each plant species was identified, and its exsiccate was deposited in the Herbarium of the Federal University of Grande Dourados (DDMS-UFGD Herbarium).

Table 1.
Botanical species, family, and collection location of the botanical species used in the experiments.
The aqueous extracts were prepared using the maceration method. In total, 3 g of plant powder was mixed in 30 mL of distilled water (10% concentration). The mixture was homogenized with a glass rod and then left to rest for 24 h in a refrigerated environment (2 °C). Before use, it was filtered with filter paper. The extracts were prepared daily during the period of the experiments. The extracts obtained were from T. guianensis (AETg), C. hydrangeifolia (AECh), D. furfuracea (AEDf), and A. arvense (AEAa). The methodology was adapted from [13,17,18,19].
2.3. Plutella xylostella Oral Toxicity
The methodology used was adapted from [13,19]. We isolated 48 h old larvae, one per Petri plate (5 cm Ø). In each plate, we placed a filter paper disk (5 cm Ø) that was moistened in distilled water and two collard green leaf (Brassica oleraceae var. acephala) disks (4 cm Ø). The disks were previously submerged in their respective treatment for 30 s and left to dry naturally. We changed the disks daily throughout the larval stage of P. xylostella. Subsequently, we collected the pupae, and after 24 h, we weighed them using a Bel Mark analytical balance with 0.001 g precision. They were then transferred to test tubes covered with cotton until emergence.
After the emergence of the adults, 24 h old larvae couples were identified by observing the ventral side of the last segment of the abdomen (two round spots in females and a longitudinal cleft in males). The couples were placed in cages (10.5 cm Ø × 9 cm) containing cotton moistened in a 10% honey solution, a filter paper disk, and a collard green disk (both 9 cm Ø). We inspected the cages daily, and the eggs laid on the disks were stored in Petri dishes (9 cm Ø) and later covered with filter paper. This procedure lasted until the death of the adults. At 4 and 5 days after oviposition, the caterpillars were counted from the stored eggs (Figure 1).
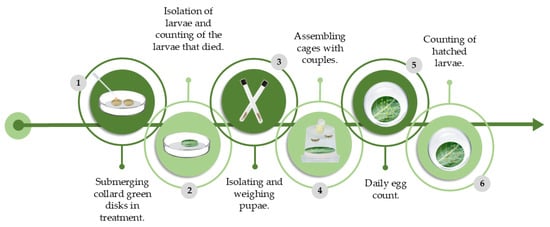
Figure 1.
Schematic and chronological representation of the methodology used to evaluate lethal and sublethal effects of AETg, AEDf, AECh, and AEAa on Plutella xylostella through oral toxicity experiment. Adapted from [13].
The experiment was set up in a completely randomized manner, with 50 individuals for each treatment. The control used was distilled water. The parameters evaluated in the larval stage were as follows: larval duration and viability; pupal duration, viability, and biomass; number of eggs, egg viability, longevity of males and females, and period and oviposition of adults. In addition, the mortality speed index (MSI) was calculated using the formula adapted from [28] (Equation (1)). MSI indicates the mortality rate (larvae/day), and t1 denotes the duration of the experiment (days).
2.4. Topical Toxicity on P. xylostella Larvae
The methodology used was adapted from [29]. We isolated third-instar larvae in Petri dishes (5 cm Ø) (one per plate) containing paper filter disks, (5 cm Ø) and using a Biopet 8 Channel BSN023A Micropipette, we topically applied 0.05 mL of the respective treatments (AETg, AECh, AEDf, and AEAa) on the dorsal part of the larvae.
After 5 min, the larvae were transferred to another Petri dish (5 cm Ø) containing a collard green disk (Brassica oleraceae var. acephala) (5 cm Ø). The collard green disks were exchanged daily. The larvae were monitored for four days, and the mortality rate was determined by a lack of movement visible to the naked eye, even under mechanical stimuli. Each treatment consisted of 30 replicates, and each contained one P. xylostella larva (Figure 2). The control used was distilled water.
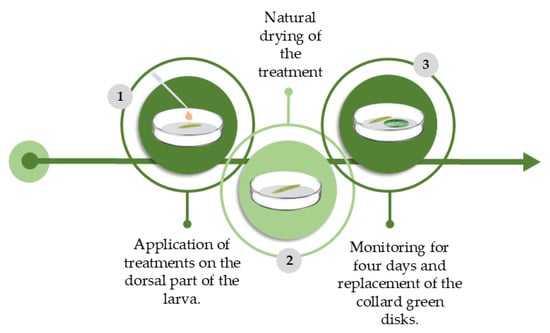
Figure 2.
Schematic and chronological representation of the methodology used to evaluate lethal and sublethal effects of AETg, AEDf, AECh, and AEAa on Plutella xylostella through larval topic toxicity experiment. Image made by the authors.
2.5. Topical Toxicity on Plutella xylostella Pupae
The methodology for the experiment was adapted from [29]. Pupae up to three days old were lined up on a sheet of filter paper, and using a Biopet 8-Channel BSN023A Micropipette, 0.05 mL of their respective treatments was topically applied. After 5 min, the pupae were isolated in Petri dishes (5 cm Ø), one per dish. We monitored the pupae for seven days, and the mortality rate was recorded based on whether the adult emerged. Each treatment consisted of 30 replicates, and each contained one P. xylostella pupa (Figure 3). The control used was distilled water.
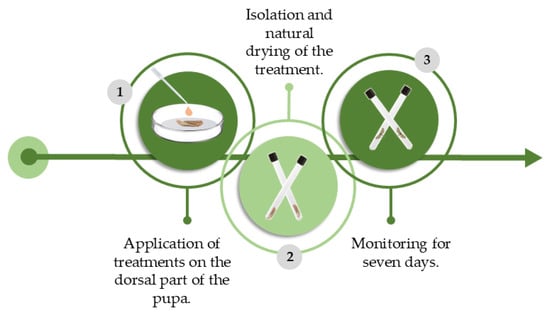
Figure 3.
Schematic and chronological representation of the methodology used to evaluate lethal and sublethal effects of AETg, AEDf, AECh, and AEAa on Plutella xylostella through pupa topic toxicity experiment. Image made by the authors.
2.6. Topical Toxicity on Plutella xylostella Eggs
The methodology used during the experiment was adapted from [30]. P. xylostella eggs up to 24 h old were removed from the stock and subsequently organized into groups of 10 on filter paper sheets. We submerged the paper fragments in their respective treatments for 5 s and left them to dry at room temperature for 20 min before isolating them in closed Petri dishes (5 cm in Ø), with one per dish. The dishes were monitored every 24 h for 5 days, and the number of hatched larvae was counted (egg survival). Each treatment consisted of 10 replicates, and each contained 10 P. xylostella eggs (Figure 4). The control used was distilled water.
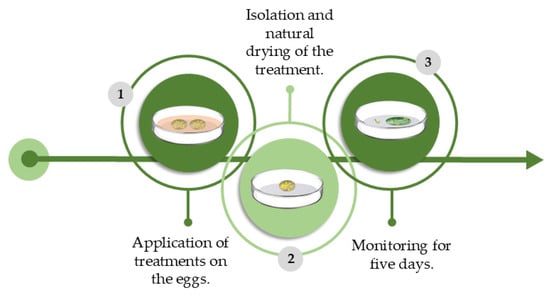
Figure 4.
Schematic and chronological representation of the methodology used to evaluate lethal and sublethal effects of AETg, AEDf, AECh, and AEAa on P. xylostella through eggs topic toxicity experiment. Image made by the authors.
2.7. Chemical Analysis of the Extract
2.7.1. Determination of Phenolic Commixture Contents
To quantify the content of phenolic compounds, 0.1 mL of the sample was added to 0.5 mL of the Folin–Ciocalteu reagent and 1 mL of 70% ethanol. After 1 min of incubation, 1.5 mL of 20% sodium carbonate was added, and the analysis was performed on a spectrophotometer (Global Trade Technology, Jaboticabal, Brazil) at a wavelength of 760 nm [31]. Quantification was carried out by examining the analytical curve of gallic acid, and the result was expressed in µg gallic acid equivalent (GAE) per mL of the extract.
2.7.2. Determination of Flavonoid Contents
To quantify the flavonoids, 1 mL of 2% aluminum chloride in methanol was added to 1 mL of the sample, and after 15 min of reaction, readings were performed on a spectrophotometer at a wavelength of 430 nm. The results were expressed in µg rutin equivalent (RE) per mL of the extract [31].
2.7.3. Determination of Tannin Levels
The tannin content was determined via the Folin–Denis spectrophotometric method [32], with adaptations in the volumes of the reagents and without changing the proportions. In total, 0.5 mL of the Foli–Denis reagent was added to 0.5 mL of the sample (1 mg mL−1), followed by stirring and allowing the mixture to stand for 3 min. Sequentially, 0.5 mL of sodium carbonate at 0.75 mol L−1 was added, the mixture was homogenized, and the reaction was allowed to proceed for 2 h in the dark. Absorbance was measured at a wavelength of 725 nm, and the result was expressed in mg of tannic acid equivalent (TAE) per mL of the extract.
2.7.4. Determination of Alkaloid Contents
The methodology of [33] was applied with some adaptations. For each extract sample, a 4 mL aliquot was removed and acidified to a pH value between 2 and 2.5 with 1 mol L−1 HCl. Then, 4 mL of the acidified solution from each replicate was transferred to test tubes, and 0.2 mL of the Dragendorff reagent was added to each tube and centrifuged at 2400 RPM for 30 min. After centrifugation, the supernatant was discarded, and the residue was treated with 0.1 mL of absolute ethyl alcohol. Subsequently, 0.2 mL of 1% sodium sulfite was added and centrifuged again at 2400 RPM for 30 min. Then, the supernatant was discarded, and the residue was treated with 0.2 mL of concentrated nitric acid.
After the procedures described above, the resulting content of each repetition was transferred to 5 mL volumetric flasks, and the volume was completed with distilled water. Then, 0.5 mL of this solution was removed, and 2.5 mL of 3% (m:V) thiourea was added. Each sample was examined using a spectrophotometer at a wavelength of 435 nm. The blank was obtained by mixing nitric acid and thiourea. The alkaloid concentration was calculated by preparing an analytical curve using berberine as a standard at concentrations of 1.40 to 4.10 µg mL−1. Using the data obtained from the standard, the absorbance curve versus berberine concentration was plotted, and a linear regression was fitted.
2.7.5. Semi-quantitative Phytochemical Prospecting
Phytochemical prospecting was carried out for some cases in which quantitative tests could not be properly performed, and this was carried out through semi-quantitative tests, following the methodology of [34]. The analyzed classes were as follows: coumarins, saponins, anthraquinones, steroids and triterpenoids, proteins, amino acids, and anthocyanidins. A cross test was used, for which the following definitions are provided: (−) negative, (+) weak positive, (++) moderate positive, and (+++) strong positive (++++).
2.8. Statistical Analysis
The data adjustments were tested for the Gaussian, gamma (inverse link, identity, log, and quadratic functions), and binomial and quasi-binomial models. All data that did not fit one of these models were analyzed using the nonparametric Kruskal–Wallis ranking at a significance level of 5%. The goodness of fit of the above models was assessed with a half-normal plot. All models were analyzed using the R 4.2.0 program [35].
The models that best fitted the data distribution during the oral toxicity experiment were the binomial model (larval and pupal viability), Gaussian model (number of eggs laid), and nonparametric Kruskal–Wallis ranking (larval and pupal duration; pupal biomass; egg viability; male and female longevity; and oviposition period). The model that best fitted the data distribution during the topical toxicity experiment was the binomial model.
3. Results
3.1. Oral Toxicity
Oral toxicity experiments were carried out to evaluate the effects caused by the ingestion of treatments during the larval stage of individuals. Larval durations differed from the control for the extracts evaluated, except for AECh (DF = 4, p < 0.0001). None of the treatments caused the extension of larval duration, and the greatest reduction was caused by AEDf (DF = 4, p < 0.0001). Likewise, the larval viability of P. xylostella showed a reduction with respect to AETg, AEDf, and AEAa but not relative to AECh (DF = 4, p = 0.0002). Furthermore, AEDf presented the highest mortality rate index (MSI) (larvae/day) (Table 2).

Table 2.
Parametric ranking using a binomial model for larval viability (mean ± SE *) and nonparametric Kruskal–Wallis ranking (rank KW) for larval duration (mean ± SE), and mortality rate index (MSI) (larvae/day) of P. xylostella, after ingestion of botanical extracts in the larval stage.
AEDf promoted a 47.41% reduction in the duration of the larval stage compared to the control, while the percentage was 24.30% for AETg and AEAa, which did not differ from AECh. The extracts that induced mortality recorded an average of 44.67% lethality, with no statistical differences among them, although the average viability index associated with AEDf was approximately 40% higher than that observed in the treatments with AETg and AEAa.
Regarding pupal biomass, the only treatment that exhibited a reduction compared to the control was AECh, with a decrease of 12.19% (DF = 4, p < 0.0001). Pupal viability was also affected by the treatments, and AETg and AEAa resulted in mortality values of 18.53% and 24.62%, respectively (DF = 4, p = 0.0018). AECh did not present pupal mortality; however, it prolonged the duration of this stage by 28.63%, while AETg, AEDf, and AEAa decreased their number of days by approximately 29% (Table 3).

Table 3.
Parametric ranking using binomial model for pupal viability (mean ± SE *) and nonparametric Kruskal–Wallis ranking (rank KW) for pupal duration and pupal biomass (mean ± SE) of Plutella xylostella, after ingestion of botanical extracts in larval stage.
Female fertility decreased after the ingestion of the extract containing all treatments, indicating that exposure to the treatments caused a reduction in the number of eggs laid. AEDf, AEAa, and AETg were those that exhibited the most reduced oviposition by 62.06%, 59.99%, and 52.26%, respectively, while AECh promoted a decrease of 29.56%. Egg viability did not differ significantly when compared in relation to the control. The oviposition period was affected differently. For AETg and AEDf, the number of days of the female fertile period decreased by 43.39% and 66.98%, respectively. For AECh, there was an increase of 30.47% in the number of oviposition days (Table 4).

Table 4.
Parametric ranking, using Gaussian model, for the number of eggs (mean ± SE *) and nonparametric Kruskal–Wallis ranking (rank KW) for egg viability and oviposition period (mean ± SE) of Plutella xylostella, using different botanical extracts.
The longevity of adult specimens did not differ significantly from the control for any of the extracts, although there was a decrease in the number of days of the adult phase for both males and females. Only AECh showed the opposite effect, in which the number of days was extended by 30.76% and 19.78% for males and females, respectively (Table 5).

Table 5.
Kruskal–Wallis nonparametric ranking (rank KW) for male and female longevity (mean ± SE *) of Plutella xylostella after ingestion of botanical extracts when in the larval stage.
3.2. Topic Toxicity
The topical toxicity experiments were carried out to evaluate the effects caused by the topical application of treatments on the individuals during their egg, larval, and pupal stages (Table 6).

Table 6.
Parametric ranking, using binomial model for egg, larval, and pupal viability (mean ± SE *), of P. xylostella, after topical application in their respective life stages.
The topical application on the eggs and the larvae revealed that only one treatment was significantly different (p = 0.0171) from the control—AETg—with mortality of 26.67% and 30%, respectively. For pupal viability, AEDf caused the highest mortality (44%); however, no treatment differed significantly.
3.3. Chemical Analysis
Phenolic compounds, flavonoids, tannins, coumarins, saponins, anthraquinones, steroids, and triterpenoids were found in all plant extracts. AETg presented the highest amount of phenolic compounds (approximately 51% higher than the average), flavonoids (approximately 30% higher than the average), alkaloids (together with AECh, approximately 47% higher than the average), and tannins (together with AECh, approximately 50% higher than the average). AEAa contained the lowest amount of these substances (Table 7).

Table 7.
Determination of the levels (mean ± SE) of phenolic compounds, flavonoids, tannins, and alkaloids of aqueous extracts (10%) of leaves of C. hydrangeifolia, T. guianensis, D. furfuracea, and A. arvense.
For coumarins, saponins, and anthraquinones, the cross test revealed weak positives for all extracts, with the exception of AECh. AEDf and AECh were classified as moderately positive for triterpenoids, while the others were only weakly positive. Anthocyanidins were the substances present in the smallest quantities, and only AEDf was classified as a false positive (Table 8).

Table 8.
Determination of the presence of coumarins, saponins, anthraquinones, steroids, triterpenoids, and anthocyanidins in aqueous extracts (10%) of the leaves of C. hydrangeifolia, T. guianensis, D. furfuracea, and A. arvense.
4. Discussion
The nutritional composition and presence of allelochemicals in food directly impact the development, behavior, and reproduction of insects, especially during the larval stage, when feeding is crucial for the stocking of nutrients [6]. In this sense, secondary metabolites can induce physiological stress, reduce growth, and increase mortality rates [13,17,18,19,36,37,38,39]. Insects could develop tolerance mechanisms, such as taste adaptation, the consumption of carbohydrates that mask unpleasant flavors, and the activation of detoxifying enzymes, such as P450 [40,41,42,43], even though such adaptations can generate metabolic consequences, which could result in sublethal effects.
Thus, some of the secondary metabolites found in the botanical extracts of this study are compounds such as alkaloids, flavonoids, and triterpenoids, which are commonly found in botanical extracts; they are known for their deterrent, antifeedant, and toxic properties, acting on the metabolism, nervous system, and growth of different species of pests [44,45,46,47,48,49]. In bioassays with P. xylostella, the AETg, AEDf, and AEAa extracts caused the highest larval mortality rate (on average 48%). In addition, a reduction in the larval-phase period during these treatments was observed; this indicates the species’ adaptive strategy in the face of physiological stress, aiming to complete the life cycle more quickly [17,19]. The shortening of the larval stage is beneficial from an agronomic point of view, since it is at this stage that the greatest damage to crops occurs [50,51].
The ingestion of botanical extracts also negatively affected the adult phase, with a decrease of over 50% in oviposition in the AETg, AEDf, and AEAa treatments, in addition to a significant reduction (p < 0.0021) in the reproductive period of females. These sublethal effects may also be associated with the ingestion of commixtures during the larval phase because the nutritional quality of larval food directly interferes with the formation of ovarioles in adults and, therefore, interferes with their reproductive capacity [52,53,54,55]. For example, protein HSC70-3 assists P. xylostella larvae with respect to tolerating host allelochemicals, likewise producing smaller pupae and fewer eggs, reinforcing the link between larval stress and adult fertility [56]. Despite the reduction in the total number of eggs, viability was not compromised, reinforcing the idea that the number of female reproductive structures (ovarioles) may have been affected, but not their viability.
Regarding topical application, only AETg exhibited ovicidal and larvicidal action, while no extract caused significant mortality in pupae, although the survival of pupae in AEDf was reduced (56.66%). These results may be related to the morphological characteristics of the different stages of the insect’s development. The rough and microporous chorion of P. xylostella eggs facilitates the adhesion and penetration of topically applied chemical compounds [57,58], while the thin cuticle present on larvae allows greater substance permeability [11]. For example, ref. [59] showed that Cordyceps fumosorosea conidia attached most readily to the acanthoid and spinous micro-projections on P. xylostella larval cuticles, but they scarcely attached to the smooth, highly sclerotized surfaces, such as the head capsule. Moreover, stronger attachment correlated with higher pathogenicity, and the same acanthoid structures could have likely increased the residence time and wettability of AETg, facilitating the diffusion of allelochemicals across the still-thin larval cuticle. In contrast, the pupae’s rigid cuticle renders the absorption of the compounds difficult, offering greater protection [60].
Thus, the results indicate that botanical extracts acted mainly through ingestion, causing mortality and important sublethal effects, such as increased larvae mortality and a reduction in reproductive potential. The observed mode of action is strongly associated with the presence of secondary metabolites, which were identified through chemical analysis. The T. guianensis extract (AETg) contained the highest levels of phenolic compounds, and it was precisely this treatment that produced the greatest larval mortality and topical ovicidal/larvicidal activity. Phenolics and tannins can form complexes with digestive enzymes, reduce nutrient assimilation, and thereby hasten death or shorten the larval period (as observed for AETg, AEDf, and AEAa) [44,45,61]. Flavonoids such as quercetin modulate cytochrome P450s, impairing detoxification and affecting reproductive behavior—an effect observed here with up to a 62% decrease in egg numbers for AEDf [46,62]. Simultaneously, the alkaloids present in the extracts could interfere with cholinergic transmission, inducing paralysis or antifeedant behavior [45,47], which is reflected in high mortality speed index (MSI) values. The shortened pupal phase and reduced fertility could also be attributed to triterpenoids, which are known to mimic ecdysone hormones, disrupt molting, and yield less-fertile adults [48,63,64].
While AETg and AEDf contained several classes of secondary metabolites, AEAa exhibited low chemical diversity, suggesting that not only the quantity but also the type and combination of commixtures influence toxicity [61]. Thus, the presence of these chemical classes and their biological effects on P. xylostella pinpoint the synergistic role of secondary metabolites and highlight T. guianensis as the most promising source for future Cerrado-derived bio-insecticidal formulations. The presence of these compounds in botanical insecticides is a valuable tool for integrated pest management, offering a sustainable alternative to synthetic insecticides. Their diverse modes of action, including deterrence, toxicity, and growth inhibition, contributes to reducing pest resistance and minimizing environmental impacts. In addition, their natural origin helps preserve ecological balance. Thus, the strategic use of botanical extracts, together with other control techniques, can improve pest control programs, making them more effective and environmentally responsible.
The mortality observed was not the only relevant result; the decrease in adult fertility also has the potential to reduce the number of offspring produced by the pest and control its population level in over the medium to long term, without needing to kill the insect immediately after ingestion. In addition, oviposition repellency and food preference effects should also be investigated in order to better understand the extract’s mechanisms of action. Finally, it is important that new solvents are tested, as the most economically valuable active ingredients may not have been extracted using only water alone as a solvent.
5. Conclusions
The results of the present study demonstrate that the aqueous botanical extracts tested have insecticidal potential against P. xylostella, acting mainly through ingestion. The AETg, AEDf, and AEAa extracts exhibited larval toxicity (average of 48%) and sublethal effects, such as reduced larval durations, reduced oviposition rates, and shortened oviposition period, directly affecting the development and fecundity of the species. These effects are associated with the presence of secondary composites such as alkaloids, flavonoids, and triterpenoids. To develop commercially viable products from these extracts, further assays using alternative solvents (e.g., hydro-alcoholic systems) are recommended to enhance larvicidal activity and sublethal impacts. Equally important, future studies should include ecotoxicological tests on key non-target organisms (parasitoids, pollinators, and aquatic invertebrates) to ensure that new formulations deliver effective control without compromising environmental safety.
Author Contributions
Conceptualization, I.M.P.M.P., S.A.d.S., and R.M.M.; methodology, I.M.P.M.P., S.A.d.S., C.A.L.C., and R.M.M.; formal analysis, I.M.P.M.P. and A.S.N.F.; investigation, I.M.P.M.P. and S.A.d.S.; writing—original draft preparation, I.M.P.M.P.; writing—review and editing, I.M.P.M.P., S.A.d.S., J.R.C.M., and R.M.M.; supervision, S.A.d.S. and R.M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Development of Education, Science, and Technology of Mato Grosso do Sul (FUNDECT) for the resources provided under No. 83/029.649/2024; National Council for Scientific and Technological Development, Brazil (CNPq), to the last author, in process 316993/2023-9.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We would like to thank the support from the Federal University of Grande Dourados, Dourados, MS, the Postgraduate Program in Entomology and Biodiversity Conservation, and Itaipu Parquetec.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Stern, V.M.; Smith, R.F.; Van den Bosch, R.; Hagen, K.S. The integration of chemical and biological control of the spotted alfalfa aphid: The integrated control concept. Hilgardia 1959, 29, 81–101. [Google Scholar] [CrossRef]
- Smith, R.F.; Van den Bosch, R. Integrated control. In Pest Control: Biological, Physical and Selected Chemical Methods; Kilgore, W.W., Doutt, R.L., Eds.; Academic Press: Cambridge, MA, USA, 1967; pp. 295–340. [Google Scholar]
- Dent, D. Insect Pest Management, 2nd ed.; CABI: Wallingford, UK, 2000. [Google Scholar]
- Luckmann, W.H.; Metcalf, R.L. Introduction to Insect Pest Management, 2nd ed.; John Wiley & Sons: New York, NY, USA, 1982. [Google Scholar]
- Kogan, M.; Jenson, P. Perspectives in Ecological Theory and Integrated Pest Management; Oxford University Press: Oxford, UK; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Panizzi, A.R.; Parra, J.R.P. Insect Bioecology and Nutrition for Integrated Pest Management, 1st ed.; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar] [CrossRef]
- Baldin, E.L.L. Resistência de Plantas a Insetos: Fundamentos e Aplicações; FEALQ: Piracicaba, Brazil, 2019. [Google Scholar]
- Ribeiro, L.P.; Vendramim, J.D.; Baldin, E.L. Inseticidas Botânicos no Brasil: Aplicações, Potencialidades e Perspectivas; FEALQ: Piracicaba, Brazil, 2023. [Google Scholar]
- Vendramim, J.D.; Castiglioni, E. Aleloquímicos, resistência e plantas inseticidas. In Bases e Técnicas do Manejo de Insetos; UFSM: Santa Maria, CA, USA, 2000. [Google Scholar]
- Embrapa Milho e Sorgo. Área de Refúgio: Recomendações de Uso Para o Plantio do Milho Transgênico Bt; Embrapa: Sete Lagoas, Brazil, 2014. [Google Scholar]
- Gullan, P.J.; Cranston, P.S. Insetos: Fundamentos da Entomologia, 5th ed.; Roca: Rio de Janeiro, Brazil, 2017. [Google Scholar]
- Lengai, G.M.W.; Muthomi, J.W.; Mbega, E.R. Phytochemical activity and role of botanical pesticides in pest management for sustainable agricultural crop production. Sci. Afr. 2020, 7, e00239. [Google Scholar] [CrossRef]
- Padial, I.M.P.M.; de Souza, S.A.; Malaquias, J.B.; Cardoso, C.A.L.; Pachú, J.K.S.; Fioratti, C.A.G.; Mussury, R.M. Leaf Extracts of Miconia albicans (Sw.) Triana (Melastomataceae) Prevent the Feeding and Oviposition of Plutella xylostella (Linnaeus, 1758) (Lepidoptera: Plutellidae). Agronomy 2023, 13, 890. [Google Scholar] [CrossRef]
- Naimi, I.; Bouamama, H.; Ba M’hamed, T. Chemical Composition, Repellency, and Insecticidal Activity of Pinus halepensis Leaf Essential Oil from Morocco on Adults of Rhyzopertha dominica (Fabricius) (Coleoptera: Bostrichidae) and Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae). Plants 2025, 14, 407. [Google Scholar] [CrossRef]
- Isman, M.B.; Grieneisen, M.L. Botanical insecticide research: Many publications, limited useful data. Trends Plant Sci. 2014, 19, 140–145. [Google Scholar] [CrossRef]
- Popescu, I.E.; Gostin, I.N.; Blidar, C.F. An Overview of the Mechanisms of Action and Administration Technologies of Essential Oils Used as Green Insecticides. AgriEngineering 2024, 6, 1195–1217. [Google Scholar] [CrossRef]
- Ferreira, E.A.; de Souza, S.A.; Domingues, A.; da Silva, M.M.M.; Padial, I.M.P.M.; de Carvalho, E.M.; Cardoso, C.A.L.; da Silva, S.V.; Mussury, R.M. Phytochemical Screening and Bioactivity of Ludwigia spp. in the Control of Plutella xylostella (Lepidoptera: Plutellidae). Insects 2020, 11, 596. [Google Scholar] [CrossRef]
- De Souza, S.A.; Padial, I.M.P.M.; de Souza, T.S.; Domingues, A.; Ferreira, E.A.; Mauad, M.; Cardoso, C.A.L.; Malaquias, J.B.; Oliveira, L.V.Q.; Formagio, A.S.N.; et al. Evaluation of Bioinseticide in the Control of Plutella xylostella (Linnaeus, 1758): A Laboratory Study for Large-Scale Implementation. Sustainability 2025, 17, 1626. [Google Scholar] [CrossRef]
- De Souza, S.A.; Padial, I.M.P.M.; Domingues, A.; Mauad, J.R.C.; Formagio, A.S.N.; Campos, J.F.; Malaquias, J.B.; Mussury, R.M. An Interesting Relationship between the Insecticidal Potential of Simarouba sp. in the Biology of Diamondback Moth. Sustainability 2023, 15, 7759. [Google Scholar] [CrossRef]
- Rocha, J.D.; Carneiro, F.M.; Fernandes, A.S.; Morais, J.M.; Borges, L.L.; Chen-Chen, L.; de Almeida, L.M.; Bailão, E.F.L.C. Toxic Potential of Cerrado Plants on Different Organisms. Int. J. Mol. Sci. 2022, 23, 3413. [Google Scholar] [CrossRef]
- Furlong, M.J.; Wright, D.J.; Dosdall, L.M. Diamondback moth ecology and management: Problems, progress and prospects. Annu. Rev. Entomol. 2013, 58, 517–541. [Google Scholar] [CrossRef] [PubMed]
- Agriculture Victoria. Diamondback Moth. Available online: https://agriculture.vic.gov.au/biosecurity/pest-insects-and-mites/priority-pest-insects-and-mites/diamondback-moth (accessed on 10 January 2025).
- Mason, P. Plutella xylostella (diamondback moth). CABI Compend. 2022. Available online: https://www.cabi.org/cpc/datasheet/42318 (accessed on 10 January 2025). [CrossRef]
- Muthomi, P.K.; Seal, D.; Liburd, O.E. Diamondback Moth Management in Cole Crops: ENY2119/IN1443, 3/2025; EDIS, University of Florida IFAS Extension: Gainesville, FL, USA, 2025; Available online: https://edis.ifas.ufl.edu/publication/IN1443 (accessed on 6 June 2025).
- Capinera, J.L. Diamondback Moth, Plutella xylostella (Linnaeus) (Insecta: Lepidoptera: Plutellidae): EENY-119/IN276, rev. 5/2000. EDIS 2000, 2002. [Google Scholar] [CrossRef]
- APRD (Arthropod Pesticide Resistance Database). Plutella xylostella. 2025. Available online: https://www.pesticideresistance.org/display.php?page=speciesearId=571 (accessed on 10 May 2025).
- Dougoud, J.; Toepfer, S.; Bateman, M.L.; Jenner, W.H. Efficacy of Homemade Botanical Insecticides Based on Traditional Knowledge: A Review. Agron. Sustain. Dev. 2019, 39, 37. [Google Scholar] [CrossRef]
- Maguire, J.D. Speed of germination-aid in selection and evaluation for seedling emergence and vigor. Crop Sci. 1962, 2, 176–177. [Google Scholar] [CrossRef]
- Santos, M.S.; Zanardi, O.Z.; Pauli, K.S.; Forim, M.R.; Yamamoto, P.T.; Vendramim, J.D. Toxicity of an azadirachtin-based biopesticide on Diaphorina citri kuwayama (Hemiptera: Liviidae) and its ectoparasitoid Tamarixia radiata (Waterston) (Hymenoptera: Eulophidae). Crop Prot. 2015, 74, 116–123. [Google Scholar] [CrossRef]
- Liu, J.; Tian, Z.; Li, R.; Ni, S.; Sun, H.; Yin, F.; Li, Z.; Zhang, Y.; Li, Y. Key Contributions of the Overexpressed Plutella xylostella Sigma Glutathione S-Transferase 1 Gene (PxGSTs1) in the Resistance Evolution to Multiple Insecticides. J. Agric. Food Chem. 2024, 72, 2560–2572. [Google Scholar] [CrossRef]
- Djeridane, A.; Yousfi, M.; Nadjemi, B.; Boutassouna, D.; Stocker, P.; Vidal, N. Antioxidant activity of some Algerian medicinal plants extracts containing phenolic compounds. Food Chem. 2006, 97, 654–660. [Google Scholar] [CrossRef]
- Pansera, M.R.; Santos, A.C.A.; Paese, K.; Wasum, R.; Rossato, M.; Rota, L.D.; Pauletti, G.F.; Serafini, L. Análise de taninos totais em plantas aromáticas e medicinais cultivadas no Rio Grande do Sul. Rev. Bras. Farmacogn. 2003, 13, 17–22. [Google Scholar] [CrossRef]
- Oliveira, M.A.C.; Albuquerque, M.M.; Xavier, H.S.; Strattmann, R.R.; Grangeiro Júnior, S.; Queiroz, A.T. Desenvolvimento e validação de metodologia para quantificação de alcalóides totais como berberina em fitoterápico contendo Berberis vulgaris L. Rev. Bras. Farmacogn. 2006, 16, 357–364. [Google Scholar] [CrossRef]
- Filho, A.C.P.M.; Filho, J.G.O.; Castro, C.F.S. Estudo físico-químico, fitoquímico e atividades biológicas do extrato do fruto maduro de Brosimum gaudichaudii Tréc. (Moraceae). Sci. Electron. Arch. 2021, 14, 1309. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 10 January 2025).
- Zhou, J.; Zhang, Z.; Liu, H.; Guo, M.; Deng, J. Inhibition Effect of Non-Host Plant Volatile Extracts on Reproductive Behaviors in the Diamondback Moth Plutella xylostella (Linnaeus). Insects 2024, 15, 227. [Google Scholar] [CrossRef] [PubMed]
- Rocha, A.d.N.; Souza, S.A.d.; Fioratti, C.A.G.; Mauad, J.R.C.; Mauad, M.; Mussury, R.M. Tradescantia pallida (Commelinaceae) Promotes Reductions in Plutella xylostella (Lepidoptera: Plutellidae) Populations. Agronomy 2022, 12, 2646. [Google Scholar] [CrossRef]
- Peres, L.L.S.; Sobreiro, A.I.; Couto, I.F.S.; Silva, R.M.; Pereira, F.F.; Heredia-Vieira, S.C.; Cardoso, C.A.L.; Mauad, M.; Scalon, S.P.Q.; Verza, S.S.; et al. Chemical Compounds and Bioactivity of Aqueous Extracts of Alibertia spp. in the Control of Plutella xylostella L. (Lepidoptera: Plutellidae). Insects 2017, 8, 125. [Google Scholar] [CrossRef] [PubMed]
- Faca, E.C.; Ferreira, E.A.; Pereira, H.C.; Rodrigues, A.; da Silva, R.M.; Fioratti, C.A.G.; Pereira, F.F.; Mussury, R.M. Selectivity of Water-Based Extracts of Serjania spp. on Tetrastichus howardi (Hymenoptera: Eulophidae), an Endoparasitoid of Plutella xylostella (Lepidoptera: Plutellidae). Braz. J. Biol. 2025, 85, e284440. [Google Scholar] [CrossRef]
- Glendinning, J.I.; Nelson, N.; Bernays, E.A. How do inositol and glucose modulate feeding in Manduca sexta caterpillars? J. Exp. Biol. 2000, 203, 1299–1315. [Google Scholar] [CrossRef]
- Schoonhoven, L.M. Long-term sensitivity changes in some insect taste receptors. Drug Res. 1978, 28, 23–77. [Google Scholar]
- Usher, B.F.; Bernays, E.A.; Barbehenn, R.V. Antifeedant tests with larvae of Pseudaletia unipuncta—Variability of behavioral response. Entomol. Exp. Appl. 1988, 48, 203–212. [Google Scholar] [CrossRef]
- Brattsten, L.B.; Wilkinson, C.F.; Eisner, T. Herbivore–plant interactions: Mixed function oxidases and secondary plant substances. Science 1977, 196, 1349–1352. [Google Scholar] [CrossRef]
- Bhambhani, S.; Kondhare, K.R.; Giri, A.P. Diversity in Chemical Structures and Biological Properties of Plant Alkaloids. Molecules 2021, 26, 3374. [Google Scholar] [CrossRef]
- Yang, Y.; Saand, M.A.; Huang, L.; Abdelaal, W.B.; Zhang, J.; Wu, Y.; Li, J.; Sirohi, M.H.; Wang, F. Applications of Multi-Omics Technologies for Crop Improvement. Front. Plant Sci. 2021, 12, 563953. [Google Scholar] [CrossRef]
- Simmonds, M.S. Importance of Flavonoids in Insect-Plant Interactions: Feeding and Oviposition. Phytochemistry 2001, 56, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Cui, B.; Huang, X.; Li, S.; Hao, K.; Chang, B.H.; Tu, X.; Pang, B.; Zhang, Z. Quercetin Affects the Growth and Development of the Grasshopper Oedaleus asiaticus (Orthoptera: Acrididae). J. Econ. Entomol. 2019, 112, 1175–1182. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Yang, S.; Huang, J.; Zhou, L. Insecticidal Triterpenes in Meliaceae: Plant Species, Molecules and Activities: Part (Aphanamixis-Chukrasia). Int. J. Mol. Sci. 2021, 22, 13262. [Google Scholar] [CrossRef] [PubMed]
- Pereira, V.; Figueira, O.; Castilho, P.C. Flavonoids as Insecticides in Crop Protection—A Review of Current Research and Future Prospects. Plants 2024, 13, 776. [Google Scholar] [CrossRef]
- Chafino, S.; Ureña, E.; Casanova, J.; Casacuberta, E.; Franch-Marro, X.; Martín, D. Upregulation of E93 Gene Expression Acts as the Trigger for Metamorphosis Independently of the Threshold Size in the Beetle Tribolium castaneum. Cell Rep. 2019, 27, 1039–1049. [Google Scholar] [CrossRef]
- Truman, J.W. The Evolution of Insect Metamorphosis. Curr. Biol. 2019, 29, 1252–1268. [Google Scholar] [CrossRef]
- Niitepõld, K.; Boggs, C. Carry-Over Effects of Larval Food Stress on Adult Energetics and Life History in a Nectar-Feeding Butterfly. Ecol. Entomol. 2022, 47, 391–399. [Google Scholar] [CrossRef]
- Salinas, P.J. Studies on the Ecology and Behavior of the Larvae Plutella xylostella (Linnaeus) (Lepidoptera: Plutellidae) III. Effects of Size and Shape of the Host Plant Leaves. Turrialba 1990, 40, 40–43. [Google Scholar]
- Moller, J. Investigations on a Laboratory Culture of the Diamond-Back Moth, Plutella maculipennis (Curt.) (Lep., Tineidae). J. Appl. Entomol. 1988, 105, 5. [Google Scholar] [CrossRef]
- Costa, D.C.M. Toxicidade de Extratos Botânicos de Fabácea e Rubiácea para Spodoptera frugiperda (J.E. Smith, 1797) (Lepidoptera: Noctuidae). Master’s Thesis, Universidade Federal de Lavras, Lavras, Brazil, 2015. [Google Scholar]
- Qiao, Q.; Zheng, C.; Feng, H.; Huang, S.; Wang, B.; Zaheer, U.; He, W. HSC70-3 in the Gut Regurgitant of Diamondback Moth, Plutella xylostella: A Candidate Effector for Host Plant Adaptation. Insects 2025, 16, 489. [Google Scholar] [CrossRef]
- Gallo, D.; Nakano, O.; Silveira, N.S.; Carvalho, R.P.L.; Baptista, G.C.; Berti Filho, E.; Parra, J.R.P.; Zucchi, R.A.; Alves, S.B.; Vendramim, J.D.; et al. Entomologia Agrícola; FEALQ: Piracicaba, Brazil, 2002. [Google Scholar]
- Torres, A.L.; Júnior, A.L.B.; Medeiros, C.A.M.; Barros, R. Efeito de Extratos Aquosos de Azadirachta indica, Melia azedarach e Aspidosperma pyrifolium no Desenvolvimento e Oviposição de Plutella xylostella. Fitossanidade 2006, 65, 447–457. [Google Scholar] [CrossRef]
- Lei, Y.; Hussain, A.; Guan, Z.; Wang, D.; Jaleel, W.; Lyu, L.; He, Y. Unraveling the Mode of Action of Cordyceps fumosorosea: Potential Biocontrol Agent against Plutella xylostella (Lepidoptera: Plutellidae). Insects 2021, 12, 179. [Google Scholar] [CrossRef] [PubMed]
- Rafael, J.A.; Melo, G.A.R.d.; Carvalho, C.J.B.d.; Casari, S.A.; Constantino, R. Insetos do Brasil: Diversidade e Taxonomia, 2nd ed.; INPA: Manaus, Brazil, 2024. [Google Scholar] [CrossRef]
- Gajger, I.; Dar, S. Plant Allelochemicals as Sources of Insecticides. Insects 2021, 12, 189. [Google Scholar] [CrossRef] [PubMed]
- Alvarenga, N.; Ferro, E.A. Bioactive Triterpenes and Related Compounds from Celastraceae. Stud. Nat. Prod. Chem. 2005, 30, 635–702. [Google Scholar] [CrossRef]
- González-Coloma, A.; López-Balboa, C.; Santana, O.; Reina, M.; Fraga, B.M. Triterpene-Based Plant Defenses. Phytochem. Rev. 2011, 10, 245–260. [Google Scholar] [CrossRef]
- Christenhusz, M.J.M.; Byng, J.W. The Number of Known Plants Species in the World and Its Annual Increase. Phytotaxa 2016, 261, 201–217. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).