Abstract
The production of artificial planted forage is important for the development of animal husbandry in the Qinghai–-Tibet Plateau, and oat forage is one of the main artificial planted forages in the area. However, the suitable oat varieties for harvesting and preparing silage feed in this region are still unclear. To investigate suitable oat forage varieties for silage production and the potential feeding value of different oat forage varieties, 16 oat forage varieties planted in Qinghai Province were selected in this experiment. These oat forages were subjected to two treatments: a group with no inoculants (CK) and a group with self-selected lactic acid bacteria (LAB) inoculants (IN). After 90 days of ensiling, silage quality and in vitro digestibility of the 16 oat forage varieties were determined. The results showed that all oat forage varieties ferment well after ensiling (pH < 4), the CK group had a silage pH range of 3.62–3.95, and the IN group had a silage pH range of 3.68–3.83. Tianyan No.1, Qingtian No.2, and Tianyan No.3 were in the top three in RFV and RFQ rankings in the CK group, while Qinghai 444, Tianyan No.1, and Tianyan No.3 were in the lead in GI rankings. Tianyan No.1, Qingtian No.2, and Everleaf 126 were in the lead in RFV and RFQ rankings in the IN group, while Qinghai 444, Titan, and Tianyan No.1 were in the top three in GI rankings. The dry matter digestibility and 72 h cumulative gas production of the IN group were higher than that of the CK group (p < 0.05). Based on principal component analysis and membership function comprehensive evaluation, Tianyan No.1, Qinghai 444, and Tianyan No.3 ranked the top three, demonstrating that these three oat forage varieties are suitable for silage processing in the Qinghai–Tibet Plateau region.
1. Introduction
Oats (Avena sativa L.), as an annual forage crop, are characterised by their tender and succulent stems and leaves, rich nutritional content, and remarkable tolerance to drought, cold, and poor soil conditions. They exhibit extensive adaptability and high nutritional value, making them particularly valuable in high-altitude pastoral regions [,]. However, the traditional hay-making method has drawbacks, such as serious nutritional loss and limitation by geographical and climatic factors [,], which makes it difficult to meet the sustained demand for high-quality forage in high-altitude pastoral regions. Silage technology, which preserves fresh forage through microbial fermentation, can prolong storage time, reduce nutrient loss, and provide balanced nutrition and has become an effective way to solve the forage problem in high-altitude pastoral areas []. However, the raw material characteristics of different oat forage varieties vary greatly; we hypothesise that these varietal differences will lead to significant variations in silage quality parameters, including fermentation characteristics, nutrient preservation, and fibre degradation.
It is noteworthy that unfavourable conditions such as low temperature in high-altitude regions often lead to unstable silage fermentation, which affects silage quality. To improve silage quality, we further propose that high-quality lactic acid bacteria (LAB) can be added during the ensiling process to promote the production of organic acids and inhibit the reproduction of harmful microorganisms, thus enhancing the stability and nutritional value of silage []. In assessing silage quality, the in vitro gas production method has unique advantages as a test method that simulates rumen digestion in ruminants []. This method not only accurately evaluates the digestibility of organic matter in the rumen but also determines the effects of feed inoculants, which provides a scientific basis for the comprehensive evaluation of silage quality []. The in vitro gas production method allows for the assessment of the nutritional value and digestibility of silage from different oat forage varieties, and the effect of inoculants such as LAB on silage fermentation and digestibility is also investigated.
In this study, 16 oat forage varieties from the Qinghai–Tibet Plateau region were selected to evaluate their silage conventional nutrients and in vitro digestibility of their silage. By combining the in vitro gas production method with traditional evaluation methods, we comprehensively assessed silage quality and identified oat forage varieties suitable for the Qinghai–Tibet Plateau region. These findings provide important theoretical support and practical guidance for the development of forage resources, optimisation of silage preparation, and sustainable development of animal husbandry in high-altitude pastoral regions.
2. Materials and Methods
2.1. Materials
The 16 oat forage varieties selected for the experiment were Qingtian No.2, Baiyan No.7, Baler, Haymaker, Hanma, Bayan No.6, Everleaf 126, Titan, Youmu No.1, Sweet oat, Magnum, Forage plus, Qinghai 444, Tianyan No.3, Monica, and Tianyan No.1, which were all provided by Qinghai Academy of Animal Science and Veterinary Medicine. The experiment was arranged in a completely randomised block design with three replicates per variety. Each plot measured 15 m2 (3 m × 5 m) and consisted of 16 rows, with 0.5 m spacing between adjacent plots. The trial was conducted in Taxiu Township, Guinan County, Qinghai Province (100.75 °E, 35.57 °N), at an altitude of 3266 m, with sowing performed on 21 May 2021. All varieties were harvested at the milk stage with a stubble height of 10 cm. The silage was prepared using self-developed LAB inoculants named “Silage Mate” by the research group, including Lactobacillus plantarum 160, Pediococcus pentosaceus 260, and Lactobacillus buchneri 225 (viable bacterial count ≥ 2.0 × 1010 CFU/g). After harvesting, the oat forages were immediately chopped to 1–2 cm pieces using a forage cutter and thoroughly mixed. A portion of the mixed material was compacted and vacuum-sealed in polyethylene bags (260 mm × 350 mm) using a vacuum sealer (DZQ-390, Anshengke, Fujian, China), with each weighing about 1 kg. The remaining portion was uniformly sprayed with the inoculant, packed into polyethylene bags, each weighing about 1 kg, and vacuum-sealed using the same equipment. Three replicates were prepared for each treatment, and they were left to ferment in the local outdoor environment where the daily temperature ranged between 25 °C and 32 °C. After 90 days of ensiling, the bags were opened, and about 1000 g of silage samples were dried in an oven until constant weight. The dried samples were then crushed in a shredder (2500C, Hongtaiyang, Yongkang, China) and used for the determination of nutritional quality and in vitro fermentation parameters.
2.2. Determination of Conventional Nutrients
The silage samples of each variety were dried in an oven at 65 °C for 48 h to constant weight to determine the dry matter (DM) content []. Crude protein (CP) was determined using the Kjeldahl method []. Water-soluble carbohydrate (WSC) was determined using the anthrone-sulfuric acid colourimetric method, with a plant water-soluble carbohydrate assay kit produced by Suzhou Keming Biotechnology Co., Ltd.(Suzhou, China) Neutral detergent fibre (NDF) and acid detergent fibre (ADF) were determined by the Van Soest detergent fibre method []. Crude ash (Ash) was determined by burning in a muffle furnace.
2.3. Determination of Silage Quality
For each raw material and treated silage sample, 20 g was weighed and mixed with 180 mL of distilled water. The mixtures were sealed and stored 4 °C overnight to allow for aqueous extraction. The next day, the mixtures were filtered through 4 layers of gauze, and a portion of the filtrate was used to determine the pH using a pH meter (pHS-10). The filtrate was further filtered through a 0.22 μm membrane into a sample vial. The concentrations of lactic acid (LA), acetic acid (AA), propionic acid (PA), and butyric acid (BA) were determined using an ultra-performance liquid chromatography system (Thermo Scientific UltiMate 3000, Thermo Fisher Scientific, MA, USA) [], with the chromatography conditions of an RSpak KC-811 column, mobile phase 0.1% H3PO4, flow rate 0.5 mL·min−1, and column temperature 55 °C.
2.4. Feed Quality Rating Index
The feed quality rating index includes relative feeding value (RFV), relative forage quality (RFQ), and forage grading index (GI).
2.4.1. Relative Feeding Value
Relative feeding value (RFV) is calculated using the formula as follows []:
where DMI is dry matter intake (%BW, body weight); NDF is neutral detergent fibre content of forage (g/kgDM); DDM is digestible dry matter (g/kgDM); and ADF is acid detergent fibre content of forage (g/kgDM).
2.4.2. Relative Forage Quality
The formula for relative forage quality (RFQ) is as follows []:
2.4.3. Forage Grading Index
The forage grading index (GI) formula is calculated as follows []:
In calculating GI, NDF was used to predict VDMI, and ADF was used to predict NEL for forage:
where NEL is net energy for lactation (MJ/kg); VDMI is voluntary dry matter intake (kg/d); CP is crude protein content of forage (g/kgDM); NDF is neutral detergent fibre content of forage (g/kgDM); ADF is the acid detergent fibre content of forage (g/kgDM); and BW is the body weight of the cow (set at 600 kg).
2.5. Determination of In Vitro Digestibility
2.5.1. Collection of Rumen Fluid
The rumen fluid was obtained from four grazing male yaks (average body weight: 600 kg) of close weight from Qingbaijiang slaughterhouse in Chengdu, Sichuan Province, China. After extracting the rumen contents from the animals, they were immediately placed into pre-warmed thermos bottles pre-filled with CO2 gas and quickly transported back to the laboratory for freezing and storage at −20 °C. At the time of use, they were thawed in a water bath at 39 °C, filtered through four layers of cheesecloth into a receiving bottle, and water-bathed at 39 °C with continuous ventilation of CO2 to ensure anaerobic conditions.
2.5.2. Preparation of Artificial Buffer Solution
The artificial rumen buffer was prepared according to the method of Goering et al. []; 520.2 mL distilled water + 0.1 mL of solution A (13.2 g CaCl2·2H2O, 10.0 g MnCl2·4H2O, 1.0 g CoCl2·6H2O, 8.0 g FeCl3·6H2O to 100 mL with distilled water) + 208.1 mL of solution B (4 g NH4HCO3, 35 g NaHCO3 to 1000 mL with distilled water) + 208.1 mL of solution C (5.7 g Na2HPO4, 6.2 g KH2PO4, 0.6 g MgSO4·7H2O to 1000 mL with distilled water) + 1.0 mL of solution D (100 mg of resazurin dissolved in 100 mL distilled water) was mixed in a 2000 mL conical flask. Before use, 62.5 mL of solution E (625 mg cysteine hydrochloride dissolved in 95 mL distilled water, followed by 4 mL of 1 mol/L NaOH and 625 mg Na2S·9H2O) was added and mixed well, and the reaction system was placed in a 39 °C constant temperature water bath. During this process, CO2 was continuously injected into the system until the buffer was nearly colourless.
2.5.3. Determination of Gas Production In Vitro
The ANKOM RFS in vitro gas production system and a computer equipped with the corresponding software were used for the measurements. An amount of 1 g of fermentation substrate was accurately weighed in a 250 mL gas-producing bottle. The bottle was preheated in a constant-temperature air-bath shaker for 60 min at 39 °C. Filtered rumen fluid was mixed well with pre-prepared artificial rumen buffer solution at a 1:4 volume ratio, ensuring that CO2 was continuously passed through to maintain an anaerobic environment. A 39 °C water bath was continuously maintained during this process. An amount of 150 mL of well-mixed rumen fluid were added to the gas-producing bottle, and the bottle was tightly sealed. The gas-producing bottle with the cap screwed on was placed in constant-temperature air-bath shaker and incubated at 39 °C for 72 h [].
The software automatically recorded the cumulative and absolute pressures in the gas-producing bottle at each time point on the computer connected to the in vitro gas-producing system. The time points for this experiment were 2 h, 4 h, 8 h, 12 h, 24 h, 36 h, 48 h, and 72 h.
According to the ANKOM RFS system manual, the formula for converting gas production pressure (psi) to gas production volume (mL) at 39 °C is as follows:
where Vx is the volume of gas production at 39 °C (mL); Vj is the headspace volume of the gas production bottle (mL); and Ppsi is the cumulative pressure at a point in time recorded by the software (psi).
2.5.4. Determination of In Vitro Fermentation Parameters
At the end of incubation, the fermentation was terminated in an ice-water bath, and the pH was determined using a pH meter (pHS-10). Ammonia nitrogen (NH3-N) was determined by the improved colorimetric method described by Feng Zongci et al. [].
Nylon cloths were prepared, numbered, dried, and weighed. The corresponding culture solution was filtered through the nylon cloth, and the filtrate was dispensed into 15 mL tubes and stored at −20 °C for the determination of NH3-N. We rinsed the nylon cloth and sample residue with distilled water, placed the washed nylon cloth and residue together into an oven at 65 °C to dry for 48 h, and then transferred them into a desiccator to cool down and weighed them.
The dry matter degradation rate was calculated using the following formula:
where DMD is the dry matter degradability; M is the weight of dry matter of the sample (g); M1 is the weight of the nylon cloth (g); and M2 is the total weight of the dry matter of the fermentation residue and the nylon cloth (g).
2.6. Statistical Analysis
The data were initially collated using Excel 2019.0, followed by statistical analysis using SPSS 25.0 software. A one-way ANOVA model was employed to investigate the measured indicators of each oat forage variety. The general linear model was applied to analyse the effects of variety, treatment, and their interactions on silage evaluation indicators, with multiple comparisons conducted using Duncan’s method. Correlation analysis and principal component analysis were performed on the measured relevant indicators using SPSS to extract the principal components. The membership function was used to calculate the membership values of the principal component scores for each variety, and comprehensive scores were computed based on their respective weights [,].
The membership function was calculated as follows:
where μin is the membership value of the ith principal component score of the nth variety; Xin is the score of the ith principal component of the nth variety; Ximin is the minimum score of the ith principal component of all varieties; and Ximax is the maximum score of the ith principal component of all varieties.
The weights of the membership values were calculated using the following formula:
where wi is the weight of the ith principal component; λi is the contribution rate of the ith principal component; and p is the number of extracted principal components.
The composite score was calculated using the following formula:
where Dn is the composite score of the nth variety; wi is the weight of the ith principal component; μin is the membership value of the ith principal component score of the nth variety; and p is the number of extracted principal components.
3. Results
3.1. Silage Nutrient Composition of Different Oat Forage Varieties
By analysing the silage nutrient composition of different oat forage varieties, we observed significant variations in silage nutrient composition among different oat forage silage; the results are shown in Table 1. It was found that both the variety and the LAB inoculants had highly significant effects on the CP, Ash, WSC, NDF, and ADF contents (p < 0.01); the interaction between varieties and LAB inoculants had a highly significant effect on the contents of NDF and ADF (p < 0.01). Compared with the control group CK, the addition of LAB in the IN group resulted in varying degrees of increase in the CP, Ash, and WSC contents across different oat forage varieties, while the contents of NDF and ADF decreased to varying extents.

Table 1.
Effect of lactic acid bacterial inoculants on silage nutritional quality of 16 oat forage varieties.
In the CK group, the CP content of Qinghai 444 was significantly higher than that of other varieties except Titan (p < 0.01), and the CP content of Tianyan No.1 was significantly lower than that of other varieties (p < 0.01); the Ash content of Qinghai 444 was highly significantly higher than that of other varieties (p < 0.01), and that of Tianyan No.1 was significantly lower than that of other varieties (p < 0.01). The WSC content of Qingtian No.2 was significantly higher than other varieties (p < 0.01), and the WSC content of Everleaf 126 was significantly lower than other varieties (p < 0.01). The NDF content of Bayan No.6 was significantly higher than other varieties (p < 0.01), and the NDF content of Tianyan No.1 was significantly lower than other varieties (p < 0.01). The ADF content of Bayan No.6 was significantly higher than other varieties other than Titan, Monica, and Youmu No.1 (p < 0.01), and the ADF content of Qingtian No.2 was significantly lower than other varieties other than Qinghai 444, Forage plus, Hanma, Tianyan No.3, and Tianyan No.1 (p < 0.01).
In IN group, the CP content of Qinghai 444 was significantly higher than that of other varieties except Titan (p < 0.01), and the CP content of Tianyan No.1 was significantly lower than that of other varieties except Qingtian No.2 and Everleaf 126 (p < 0.01); the Ash content of Qinghai 444 was significantly higher than that of other varieties (p < 0.01), and that of Titan was significantly lower than that of Tianyan No.1 (p < 0.01). The WSC content of Qingtian No.2 was significantly higher than that of other varieties (p < 0.01), and that of Everleaf 126 was significantly lower than that of other varieties (p < 0.01). The NDF content of Magnum was significantly higher than that of other varieties (p < 0.01), and that of Tianyan No.1 was significantly lower than that of other varieties (p < 0.01); the ADF content of Monica was significantly higher than that of other varieties except Youmu No.1 (p < 0.01), and the ADF content of Qingtian 2 was significantly lower than that of other varieties (p < 0.01).
3.2. Silage Quality of Different Oat Forage Varieties
By analysing the silage quality of different oat forage varieties, as shown in Table 2, variety and LAB inoculant had highly significant effects on pH, LA, and AA (p < 0.01); the interaction of both variety and LAB inoculant had highly significant effects on pH (p < 0.01), and no significant effects on LA and AA contents (p < 0.05). PA and BA contents were not detected in all varieties of oat forage silage. Regarding an IN comparison with CK, there was an overall increasing trend in the LA content, while there was an overall decreasing trend in the pH and AA content.

Table 2.
Effect of lactic acid bacterial inoculants on silage quality of 16 oat forage varieties.
The pH of Qinghai 444 in the CK group was significantly higher than other varieties (p < 0.01), and the pH of Tianyan No.3 was significantly lower than other varieties other than Everleaf 126, Hanma, Qingtian No.2, and Tianyan No.1 (p < 0.01). The LA content ranged from 26.87 g/mg to 48.21 g/mg, and the AA content ranged from 1.78 g/mg to 3.27 g/mg. The pH was significantly higher in the IN group Titan than other varieties (p < 0.01) and significantly lower in Tianyan No.1 than other varieties (p < 0.01). The LA content ranged from 38.06 g/mg to 51.53 g/mg, and the AA content ranged from 1.25 g/mg to 2.66 g/mg.
3.3. Feed Quality Rating Index of Different Oat Forage Varieties
Through the analysis of the feed quality evaluation indices of various oat forage varieties, the results showed that the differences between RFQ and RFV was basically similar among the different varieties, and the specific data are shown in Table 3. The variety and LAB inoculant had a highly significant effect on RFV, RFQ, and GI (p < 0.01); the interaction between the variety and LAB inoculant had a significant effect on RFV and RFQ (p < 0.01), and the difference on GI was not significant (p > 0.05). Compared to the CK group, the IN group showed varying degrees of increase in RFV, RFQ, and GI across different oat forage varieties.

Table 3.
Effect of lactic acid bacterial inoculants on silage RFV, RFQ, and GI of 16 oat forage varieties.
The three varieties with the highest RFV and RFQ in the CK group were Tianyan No.1, Qingtian No.2, and Tianyan No.3, while the three varieties with the lowest RFV and RFQ were Bayan No.6, Youmu No.1, and Titan; the three varieties with the highest GI were Qinghai 444, Tianyan No.1, and Tianyan No.3, while the three varieties with the lowest GI were Magnum, Bayan No.6, and Youmu No.1.
The three varieties with the highest RFV and RFQ in the IN group were Tianyan No.1, Qingtian No.2, and Everleaf 126, while the three varieties with the lowest RFV and RFQ were Monica, Youmu No.1, and Magnum; the three varieties with the highest GI were Qinghai 444, Titan, and Tianyan No.1, while the three varieties with the lowest GI were Hanma, Baler, and Youmu No.1.
3.4. In Vitro Digestibility of Silage from Different Oat Forage Varieties
By analysing the in vitro digestibility of silage from different oat forage varieties, the results are shown in Table 4. The LAB inoculant had a significant effect on DMD and NH3-N (p < 0.01), and the difference was not significant for pH (p > 0.05); the variety had a highly significant effect on DMD, pH, and NH3-N (p < 0.01). In addition, the two of the variety and LAB inoculant interaction pH and NH3-N had highly significant effects (p < 0.01), and the difference on DMD was not significant (p > 0.05).

Table 4.
Effect of lactic acid bacterial inoculants on in vitro fermentation parameters of silage from 16 oat forage varieties.
The DMD content of the CK group Haymaker was significantly higher than that of other varieties except Baler and Tianyan No.1 (p < 0.01), and that of Qinghai 444 was significantly lower than that of other varieties except Everleaf 126, Sweet oat, Forage plus, Monica, Bayan No.6, Magnum, and Titan (p < 0.01); the pH of Bayan No.6 was significantly higher than that of other varieties (p < 0.01), and pH of Tianyan No.1 was significantly lower than that of other varieties (p < 0.01). NH3-N of Qinghai 444 was significantly higher than that of other varieties except Magnum and Monica (p < 0.01), and that of Haymaker was significantly lower than that of other varieties (p < 0.01).
The DMD content of Tianyan No.1 in the IN group was significantly higher than that of other varieties except for Haymaker, Baler, Tianyan No.3, Youmu No.1, and Sweet oat (p < 0.01), while the DMD content of Baiyan No.7 was significantly lower than that of other varieties except for Everleaf 126, Magnum, Monica, and Titan (p < 0.01). The pH of Monica was significantly higher than that of other varieties except for Bayan No.6, Qinghai 444, and Titan (p < 0.01). The pH of Tianyan No.1 was significantly lower than that of other varieties except for Baler, Sweet oat, Everleaf 126, Hanma, Tianyan No.3, Haymaker, and Youmu No.1 (p < 0.01), and NH3-N of Qinghai 444 was significantly higher than that of other varieties except for Haymaker, Monica, Titan, Baiyan No.7, and Tianyan No.1 (p < 0.01). The NH3-N of Everleaf 126 was significantly lower than that of other varieties except for Sweet oat, Qingtian No.2, Tianyan No.3, Forage, Bayan No.6, Youmu No.1, Baler, Hanma, and Haymaker (p < 0.01).
From Figure 1 and Figure 2, it can be seen that the gas production of each variety increased with the increase in fermentation time. In the CK group, the three varieties with the highest total gas production were Haymaker, Tianyan No.1, and Youmu No.1, and the three varieties with the lowest total gas production were Qingtian No.2, Monica, and Magnum. In the IN group, the three varieties with the highest total gas production were Haymaker, Tianyan No.3, and Baiyan No.7, and the three varieties with the lowest total gas production were Titan, Qingtian No.2, and Magnum. All varieties had the highest gas production rate between 2 h and 12 h, and the trend of gas production increased slowly after 48 h.
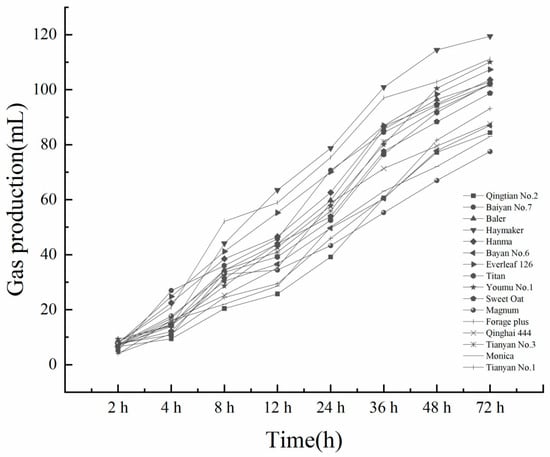
Figure 1.
The 72 h cumulative gas production in the silage CK group of 16 oat forage varieties.
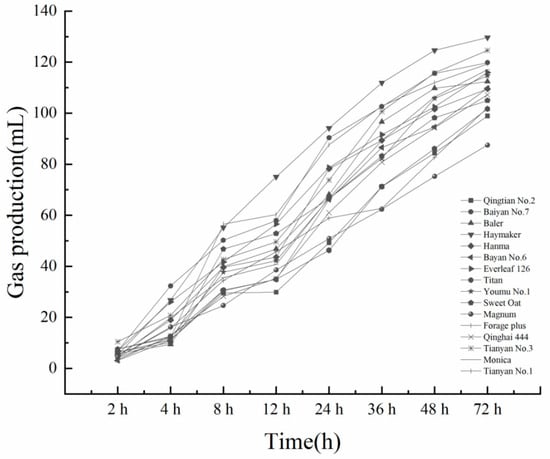
Figure 2.
The 72 h cumulative gas production in the silage IN group of 16 oat forage varieties.
3.5. Comprehensive Evaluation
A principal component analysis was conducted on the standardised nutritional quality data (excluding silage pH and rumen pH) after normalisation, and the standardised data are shown in Table 5. The principal components were extracted according to the principle of eigenvalue > 1. The results are shown in Table 6: principal component 1, with an eigenvalue of 4.951 and a contribution rate of 38.083%; principal component 2, with an eigenvalue of 2.664 and a contribution rate of 20.491%; principal component 3, with an eigenvalue of 1.714 and a contribution rate of 13.182%; and principal component 4, with an eigenvalue of 1.323 and a contribution rate of 10.173%.The cumulative contribution rate of the four principal components was 81.929%, containing 81.929% of the information of the measured indicators.

Table 5.
Standardised data of oat forage silage indicators.

Table 6.
Loading factor, eigenvalue, and contribution rate of principal component.
As shown in Table 6, the 1st principal component includes RFV, RFQ, NDF, and ADF, which are related to the fibre content; the 2nd principal component includes NH3-N, CP, AA, and GP, which are related to the protein content; the 3rd principal component is mainly Ash; and the 4th principal component is mainly LA and WSC.
The formula for the score value of each principal component is as follows:
where F1, F2, F3, and F4 represent the 1st, 2nd, 3rd, and 4th principal component scores, respectively; Y1 to Y13 represent the standardised values of CP to AA in that order.
The results are shown in Table 7.

Table 7.
Principal component scores of silage of 16 oat forage varieties.
The membership function values (μ) for the principal component scores in Table 7 were calculated, and the weights (w) for each principal component were determined based on their contribution rates in Table 6. The integrated score D was calculated and ranked according to w and μ. The higher the value D represented, the higher the nutritive value of the variety was, and the results are shown in Table 8.

Table 8.
Membership function values, comprehensive scores, and rankings of principal component scores of oat forage silage.
From the rankings in Table 7, it can be seen that the three varieties with the best silage quality in the IN group were Tianyan No.1, Qinghai 444, and Tianyan No.3, and the three varieties with the lowest silage quality were Sweet oat, Forage plus, and Magnum; the three varieties with the best silage quality in the CK group were Tianyan No.1, Tianyan No.3, and Qinghai 444, and the three varieties with the lowest silage quality were Bayan No.6, Everleaf 126, and Magnum.
4. Discussion
4.1. Effect of Lactic Acid Bacterial Inoculants on Silage Nutrient Composition of Different Oat Forage Varieties
The nutrient content of silage are crucial indicators for evaluating the nutritional value of oat forage silage. CP content is one of the key parameters for assessing forage nutritional quality, while Ash content reflects the inorganic matter content in silage. NDF content and ADF content influence both the palatability and digestibility of silage. Generally, higher contents of CP, Ash, and WSC, along with lower levels of NDF and ADF, indicate superior silage quality. After ensiling fermentation, the nutrient content of oat forage silage varied among different varieties. The CP content ranged from 4.3% to 6.2% DM in the CK treatment and from 4.39% to 6.28% DM in the IN treatment. Compared with the CK treatment, the CP content of each oat forage variety in the IN treatment showed varying degrees of increase, indicating that the addition of LAB inoculant reduced the amount of nitrogen loss during the ensiling process. The Ash content ranged from 4.2% to 6.46% DM in the CK group and from 4.49% to 6.5% DM in the IN group. The ash content in the IN group was higher than that in the CK group, which was consistent with the findings of Wei et al. []. The WSC content ranged from 3.1% to 12.15% DM in the CK treatment and from 3.22% to 12.41% DM in the IN treatment. Among the 16 oat forage silage varieties tested, the WSC content was all above 3%, which ensured good preservation of the silage []. The WSC content in the IN group was higher than that in the CK group, which might be attributed to the reduction in pH by LAB during the fermentation process, inhibiting the growth of harmful microorganisms and thereby reducing the consumption of WSC. However, during the fermentation process, WSC will continue to be converted into LA, and there was a secondary fermentation conversion, so the content increase was not obvious []. The NDF content in the CK treatment ranged from 44.31% to 59.12% DM, and the ADF content ranged from 22.29% ~31.03% DM. The NDF content ranged from 43.55% to 56.76% DM, and the ADF content ranged from 19.37% to 28.81% DM in the IN treatment. The IN treatment showed a decreasing trend of NDF and ADF contents compared to the CK treatment, which was consistent with the findings of Xu et al. [].
4.2. Effect of Lactic Acid Bacterial Inoculants on Silage Quality of Different Oat Forage Varieties
pH is one of the critical indicators for evaluating the quality of silage. A lower pH can ensure good preservation of silage, and the pH is directly related to the number of LAB in silage []. The fermentation of LAB can rapidly reduce pH, inhibit protein hydrolysis by other harmful microorganisms in a high acid concentration condition, and improve the quality and palatability of the silage []. In this study, the silage pH content ranged from 3.6 to 3.95 in the CK treatment and from 3.68 to 3.83 in the IN treatment, both of which were acidic and favourable for silage fermentation. Shao et al. [] concluded that a pH lower than 4.2 meets the criteria for high-quality silage. Compared to the CK treatment, the pH of Qingtian No.2, Baiyan No.7, Baler, Haymaker, Hanma, Bayan No.6, Titan, Youmu No.1, and Sweet oat in the IN treatment showed varying degrees of reduction, indicating that inoculation with LAB significantly reduced the pH of oat forage silage.
Organic acid is one of the key indicators for evaluating the fermentation quality of silage oat forages. A higher LA content indicates better inhibition of the growth and reproduction of harmful microorganisms and effectively prevents the spoilage of silage oat forages []. The content of AA in silage mainly reflects the aerobic stability and preservation performance of silage []. The PA and BA content should be as little as possible in silage oat forages, especially BA produced by harmful microorganisms such as Clostridium, which not only leads to the decomposition of proteins in silage but also produces a bad odour, which seriously affects the fermentation quality of silage oat forages []. In this study, the WSC content of the raw materials of various oat forage varieties ranged from 3.49% to 12.61% DM. The decrease in the WSC content after ensiling indicates that a significant amount of WSC was consumed as a fermentation substrate, indicating successful silage []. LAB under anaerobic conditions utilised WSC as the main fermentation substrate to produce large amounts of LA, which contributed to the decrease in pH. The lowest pH and higher LA content were obtained after the addition of LAB inoculants, which resulted in the improvement of the fermentation quality of silage. The range of the LA content in the CK group ranged from 28.97 mg/g to 48.21 mg/g and that in the IN group ranged from 38.06 mg/g to 51.53 mg/g. The LA in all the oat forage varieties under the IN treatment was higher than that of the CK treatment, which indicated that the addition of LAB favoured fermentation and promoted LA production. The range of the AA content was 1.78–3.27 mg/g in the CK group and 1.25–2.66 mg/g in the IN group. BA and PA was not detected in all the samples, which indicated that the fermentation was good, likely due to the reduction in pH and the accumulation of acidic substances, which inhibited harmful microorganisms []. This is in agreement with the study of Li et al. []. Therefore, while the nutritional composition of silage varies among different oat forage varieties, the addition of LAB inoculants enables the preparation of high-quality silage across all varieties.
4.3. Effect of Lactic Acid Bacterial Inoculants on Feed Quality Rating Index of Different Oat Forage Varieties
In the experiment, the RFV ranged from 101.87 to 149.77, RFQ ranged from 131.59 to 224.99, and GI ranged from 6.32 MJ/d to 12.65 MJ/d in the CK treatment, while the RFV ranged from 115.97 to 153.53, RFQ ranged from 159.09 to 232.33, and GI ranged from 8.55 MJ/d to 13.89 MJ/d in the IN treatment. Compared to the CK treatment RFV, RFQ, and GI were significantly higher in the IN treatment (p < 0.01). The overall trend of RFQ was the same as that of RFV. The RFQ range of the 16 oat forage varieties after ensiling with LAB inoculants was overall higher than that reported in the central and southern regions of Heilongjiang (128.28~208.78) [], the northwestern region of Hebei (111.00–181.50) [], and the Shigatse region of Tibet (118.03–174.76) [], indicating that the quality of oat forage silage was significantly improved with the addition of LAB. In this experiment, the GI of 16 oat forage varieties ranged from 6.34 MJ/d to 11.91 MJ/d. Qinghai 444 had the highest GI, followed by Tianyan No.1 and Tianyan No.3, while Everleaf 126 had the lowest GI, followed by Bayan No.6. According to the GI classification standard [], eight oat forage varieties belonged to Grade 1 forage, namely Qinghai 444, Tianyan No.1, Tianyan No.3, Sweet oat, Qingtian No.2, Monica, Haymaker, and Magnum, and the remaining eight oat forage varieties belonged to Grade 2 forage. Except for the 4 oat forage varieties in the CK treatment, namely Everleaf 126, Youmu No.1, Bayan No.6, and Magnum, which belonged to Grade 2 forage, all the forages in the IN treatment and the 12 varieties in the CK treatment, namely Qingtian No.2, Baiyan No.7, Baler, Haymaker, Hanma, Titan, Sweet oat, Forage plus, Qinghai 444, Tianyan No.3, Monica, and Tianyan No.1, belonged to Grade 1 forage. Considering the comprehensive performance of RFV, RFQ, and GI, Tianyan No.1 exhibited outstanding results, indicating its suitability as a silage forage variety for local silage processing. Furthermore, the addition of LAB inoculants significantly improved the silage quality of oat forage.
4.4. Effect of Lactic Acid Bacterial Inoculants on In Vitro Digestibility of Silage from Different Oat Forage Varieties
LAB inoculants improved silage quality and digestibility by rapidly lowering pH through LA production, optimising the acid profile, and ultimately increasing DMD []. In the CK treatment, the rumen pH ranged from 6.75 to 7, and the rumen NH3-N concentration ranged from 11.1 mg/dL to 16.51 mg/dL. In the IN treatment, the rumen pH ranged from 6.82 to 7.09, and the rumen NH3-N concentration ranged from 12.85 mg/dL to 17.63 mg/dL. The rumen pH and NH3-N concentration in all groups of this study were within the normal range, indicating that LAB and silage of different oat forage varieties had no adverse effects on the rumen internal environment, which is consistent with the study of Zhang et al. [].
In this experiment, DMD was significantly improved in the IN treatment compared to the CK treatment (p < 0.01), and the DMD of oat forage silage with LAB inoculants basically reached more than 67.13%, which was similar to the results reported by Liu et al. []. Among the CK and IN treatments, the three varieties with the highest dry matter degradation rates were Haymaker, Baler, and Tianyan No.1, which may be associated with the lower average contents of NDF and ADF.
Gas production volume and gas production rate can reflect the degradation rate and rumen microbial activity []. In this experiment, there were significant differences in the gas production characteristics of different varieties of oat forages after in vitro fermentation. The study found that the gas production rates of different oat forage varieties varied significantly between 2 h and 8 h. The gas production rates of Tianyan No.1 and Haymaker increased faster than those of other varieties. The gas production rates of all varieties increased again from 24 h to 36 h, and the rest of the time period was relatively slow. The total gas production of all IN treatments in this experiment was higher than that of the CK treatment, suggesting that the addition of LAB inoculants may increase the activity of rumen microorganisms and promote the degradation of oat forage silage in the rumen [,,]. In the CK treatment, the three varieties with the highest cumulative gas production at 72 h were Haymaker, Tianyan No.1, and Youmu No.1, while the three varieties with the highest cumulative gas production at 72 h in the IN treatment were Haymaker, Tianyan No.3, and Baiyan No.7.
4.5. Integrated Evaluation of Different Oat Forage Varieties for Silage
In this experiment, the 13 indicators determined by the two treatments were combined into four independent factors by principal component analysis and membership function methods. These factors reflected 81.929% of the original variable information. Based on the principal component scores, the comprehensive scores of different treatment qualities were calculated after processing with the membership function. The results of the study showed that the IN treatment was superior to the CK treatment. Specifically, the Tianyan No.1 variety in the IN group obtained the highest comprehensive score of 0.703, followed by Qinghai 444 and Tianyan No.3 in the IN group, with comprehensive scores of 0.688 and 0.677, respectively. These data indicate that the addition of LAB inoculants significantly enhances the quality of silage.
5. Conclusions
Our results confirmed that silage from different oat forage varieties showed variations in nutrient composition, fermentation quality, and in vitro digestibility. Ensiling improved the nutritional value of oat forage. Adding LAB inoculants enhanced the nutritional quality, fermentation, and digestibility of silage. Among the 16 oat forage varieties tested, Tianyan No.1, Qinghai 444, and Tianyan No.3 not only exhibited high comprehensive nutritional value but also demonstrated excellent potential feeding value, making them suitable varieties for cultivation and processing in the Qinghai–Tibet Plateau.
Author Contributions
Conceptualization, H.G.; methodology, H.L. and X.H.; validation, D.Q. and X.L. (Xiangba Lamu); formal analysis, X.L. (Xinyang Li) and D.Q.; investigation, X.Z. and X.L. (Xinyang Li); resources, Z.J., Y.J. and Y.H.; writing—original draft preparation, X.Z.; writing—review and editing, Z.J., Y.J., Y.H., Q.Z. and H.G.; visualization, X.L. (Xiangba Lamu); supervision, Q.Z.; project administration, H.L. and X.H.; funding acquisition, H.L. and H.G. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the National Natural Science Foundation of China (NSFC) Youth Science Fund Project (32301507); Sichuan Provincial Natural Science Foundation (2024NSFSC1198); the National Subsidy Project for Post-Support of Central Universities’ Research Programs (ZYN2025002); the Research, Development, and Integrated Application of Beef Cattle (Yak) Industry Technology in Tibetan Regions (SCCXTD-2024-13); the Integration and Demonstration of Key Technologies for High-Quality Forage Cultivation and Processing in Zêkog County (2024-NK-135); and the Key Laboratory of Superior Forage Germplasm in the Qinghai Tibetan Plateau.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| LAB | Lactic acid bacteria |
| CP | Crude protein |
| Ash | Crude ash |
| WSC | Water-soluble carbohydrate |
| NDF | Neutral detergent fibre |
| ADF | Acid detergent fibre |
| LA | Lactic acid |
| AA | Acetic acid |
| PA | Propionic acid |
| BA | Butyric acid |
| RFV | Relative feeding value |
| RFQ | Relative forage quality |
| GI | Forage grading index |
| DMD | Dry matter degradability |
| DM | Dry matter |
| GP | Gas production |
References
- Ren, C.Z.; Hu, Y.G. Oatology in China; China Agricultural Press: Beijing, China, 2013. [Google Scholar]
- Zhao, G.Q.; Mu, P.; Wei, L.M. Research progress in Avena sativa. Acta Pratac. Sin. 2007, 04, 116–125. [Google Scholar]
- Liu, W.; Jia, Y.S.; Ge, G.T.; Wang, Z.J.; Liu, M.J.; Si, Q.; Bao, J.; Liu, Y.C.; Sun, P.B. Research advance of oat silage. Acta Agrestia. Sin. 2022, 30, 3175–3183. Available online: https://link.cnki.net/urlid/11.3362.S.20221027.1003.008 (accessed on 23 March 2025).
- Kumar, B.; Brar, N.S.; Kumar, A. Nutritious feed for farm animals during lean period: Silage and hay—A review. Forage Res. 2019, 45, 10–22. [Google Scholar]
- Chavan, M.; Jain, S.K.; Pawar, A.B. A review: Silage preparation and silo management techniques. Pharma Innov. J. 2022, 11, 6–11. [Google Scholar]
- Chen, L.J.; Wang, Y.L.; Li, X.; MacAdam, J.W.; Zhang, Y.H. Interaction between plants and epiphytic lactic acid bacteria that affect plant silage fermentation. Front. Microbiol. 2023, 14, 1164904. [Google Scholar] [CrossRef]
- Raghavendra, B.; Tajima, K.; Takusari, N.; Higuchi, K.; Enishi, O.; Kurihara, M. Comparison of in vivo and in vitro techniques for methane production from ruminant diets. Asian-Australas. J. Anim. Sci. 2007, 20, 1049–1056. [Google Scholar]
- Zhai, J.T. Evaluation methods for feed characteristics and nutritional value in ruminants. Chin. Livest. Poult. Breed. 2022, 18, 33–34. [Google Scholar]
- Yang, S. Feed Analysis and Feed Quality Testing Techniques; Beijing Agricultural University Press: Beijing, China, 1993. [Google Scholar]
- Official methods of analysis of AOAC International, 16th ed. Choice Rev. Online 1997, 35, 35–0912. [CrossRef]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fibre, neutral detergent fibre, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Guan, H.; Yan, Y.H.; Li, X.L.; Shuai, Y.; Feng, G.Y.; Ran, Q.F.; Cai, Y.M.; Li, Y.; Zhang, X.Q. Microbial communities and natural fermentation of corn silages prepared with farm bunker-silo in Southwest China. Bioresour. Technol. 2018, 265, 282–290. [Google Scholar] [CrossRef]
- Jia, C.H.; Qian, W.X.; Saimaiti, T.; Ao, W.P.; Abudukelimu, G. Roughage nutritional value evaluation indices and research methods. Pratac. Sci. 2017, 34, 415–427. [Google Scholar]
- Zhang, S.X.; Wang, X.; Ma, L.; Wu, X.M.; Shi, Y.H.; Wang, X.M.; Zhao, W. Comprehensive evaluation of performance and forage quality of silage maize in the farming-pastoral ecotone. Acta. Agrestia. Sin. 2022, 30, 1517–1524. Available online: https://link.cnki.net/urlid/11.3362.S.20220330.1841.032 (accessed on 14 March 2025).
- Goering, H.K.; Van Soest, P.J. Forage Fiber Analyses (Apparatus, Reagents, Prcedures, and Some Applications); Agricultural Research Service: Washington, DC, USA, 1970. [Google Scholar]
- Jiang, F.; Lai, Q.; Gao, Y.H.; Peng, Z.L.; Dong, L.F.; Zhang, J.W.; Li, X.; Liao, Y.P. Effects of supplementing isobutyric acid, 2-methylbutyric acid and valeric acid on in vitro rumen fermentation parameters and nutrient degradation rate in yaks. Chin. J. Anim. Nutr. 2022, 34, 5915–5930. Available online: https://link.cnki.net/urlid/11.5461.S.20220615.1442.026 (accessed on 26 April 2025).
- Feng, Z.C.; Gao, M. Improvement of the clorimetric method for determining ammonia nitrogen content in rumen fluid. Anim. Husb. Feed Sci. 2010, 31, 37. [Google Scholar] [CrossRef]
- Tian, H.; Xiong, H.Q.; Xiong, J.B.; Zhang, H.S.; Cai, H.; Liu, Y. Comprehensive evaluation of the production performance of 14 Silage maize varieties by principal component analysis and subordinate function method. Acta Agric. Univ. Jiangxiensis. 2015, 37, 249–259. [Google Scholar] [CrossRef]
- Li, S.Q.; Tian, S.L.; Li, M.; Ge, X.; Tian, J.C.; Cheng, J.X. Comprehensive evaluation of the nutrition quality of 15 varieties of potatoes by principal component analysis and subordinate function method. Sci. Technol. Food. Ind. 2020, 41, 272–276. [Google Scholar] [CrossRef]
- Wei, J. Effects of different additives on the quality and in vitro digestibility of oat grass silage. Feed Res. 2023, 46, 113–117. [Google Scholar] [CrossRef]
- Muck, R.E. A Lactic Acid Bacteria Strain to Improve Aerobic Stability of Silages; Dairy Forage Research Center: Madison, WI, USA, 1996; pp. 46–47. [Google Scholar]
- Cao, X.J.; Wu, J.Y.; Li, W.X.; Hu, R.R.; Wu, X.L.; Hu, W. Effects of different additives on fermentation quality and nutritional components of oat silage. China Feed. 2022, 21, 60–65. [Google Scholar] [CrossRef]
- Xu, J.Y.; Zhang, K.Y.; Lin, Y.F.; Li, M.X.; Wang, X.K.; Yu, Q.; Sun, H.; Cheng, Q.M.; Xie, Y.X.; Wang, C.M.; et al. Effect of cellulase and lactic acid bacteria on the fermentation quality, carbohydrate conversion, and microbial community of ensiling oat with different moisture contents. Front. Microbiol. 2022, 13, 1013258. [Google Scholar] [CrossRef]
- Gan, L. Effects of Mixed Sowing Ratio and Lactic Acid Bacteria on the Quality of Oats/Common Vetch Mixed Silage with Different Harvest Time in Sichuan Agricultural Areas. Master’s Thesis, Southwest Minzu University, Chengdu, China, 2023. [Google Scholar] [CrossRef]
- Li, H.P.; Guan, H.; Jia, Z.F.; Liu, W.H.; Ma, X.; Liu, Y.; Wang, H.; Ma, L.; Zhou, Q.P. Screening of antifreeze-thawed lactic acid bacteria and their effects on oat silage fermentation quality and aerobic stability. Acta Pratac. Sin. 2022, 31, 158–170. Available online: https://link.cnki.net/urlid/62.1105.S.20220929.1201.011 (accessed on 14 March 2025).
- Shao, T.; Zhang, Z.X.; Shimojo, M.; Wang, T.; Masuda, Y. Comparison of fermentation characteristics of Italian ryegrass (Lolium multiflorum Lam.) and guineagrass (Panicum maximum Jacq.) during the early stage of ensiling. Asian-Australas. J. Anim. Sci. 2005, 18, 1727–1734. [Google Scholar] [CrossRef]
- Danner, H.; Holzer, M.; Mayrhuber, E.; Braun, R. Acetic acid increases stability of silage under aerobic conditions. Appl. Environ. Microbiol. 2003, 69, 562–567. [Google Scholar] [CrossRef]
- Wei, X.Q.; Luo, S.W.; Ha, Z.G.; Mao, H.C.; Wan, Y.; Wu, B.; Kou, Y.J. Effects of adding different additives on the quality, microbial quantity and aerobic stability of whole-crop corn silage. China Dairy Cattle 2018, 12, 8–12. [Google Scholar] [CrossRef]
- Reich, L.J.; Kung, L.M. Effects of combining Lactobacillus buchneri 40788 with various lactic acid bacteria on the fermentation and aerobic stability of corn silage. Anim. Feed Sci. Technol. 2010, 159, 105–109. [Google Scholar] [CrossRef]
- Jia, T.T.; Wu, Z.; Yu, Z. Effect of different lactic acid bacteria additives on the fermentation quality and aerobic stability of oat silage. Pratac Sci. 2018, 35, 1266–1272. [Google Scholar]
- Li, H.P.; Guan, H.; Jia, Z.F.; Liu, W.H.; Chen, S.Y.; Xu, M.L.; Chen, S.C.; Yan, D.H.; Jiang, Y.M.; Gan, L.; et al. Effects of wheat bran and lactic acid bacteria on bale oat silage quality and aerobic stability in the alpine region of northwest Sichuan. Acta Agrestia. Sin. 2023, 31, 302–310. Available online: https://link.cnki.net/urlid/11.3362.S.20221111.1516.004 (accessed on 26 April 2025).
- Li, F.; Li, W.; Li, X. A multi-trait evaluation of the performance of 16 forage oat varieties in central and southern Heilongjiang province. Acta Pratac. Sin. 2023, 32, 82. Available online: https://link.cnki.net/urlid/62.1105.S.20230714.0916.004 (accessed on 14 March 2025).
- Wang, Y.T.; Yang, Z.M.; Liu, J.C.; Li, F.; Yu, L.Q.; Yuan, T.; Liang, X.; Zhou, W.X. Comprehensive evaluation of production performance and nutritional quality of 21 oat varieties in northwest Hebei province. Acta Agrestia. Sin. 2020, 28, 1311–1318. [Google Scholar]
- Wu, H.Y.; Qu, N.; Qu, Z.; Tongsangcuomu; Dawazhuoga; Deyang; Nimazhuoga; Liu, Z.M.; Ma, Y.S. Comparison of crop yield and forage quality of six oat varieties in Angren County, Shigatse. Acta Pratac. Sin. 2022, 31, 72–80. [Google Scholar]
- Zhang, J.K.; Lu, D.X. Recent advance in the quality evaluation indices of roughage. Contemporary Anim. Husb. 2005, 1, 24–26. [Google Scholar]
- Dong, D.; Zhang, L.; Zhao, J.; Dong, Z.; Li, J.; Shao, T. Synergistic Effects of Exogenous Lactobacillus plantarum and Fibrolytic Enzymes on Fermentation Quality, Fiber Degradation, and In Vitro Digestibility of Napiergrass (Pennisetum purpureum) Silage. Agronomy 2025, 15, 340. [Google Scholar] [CrossRef]
- Zhang, J.M.; Guan, H.; Li, H.P.; Jia, Z.F.; Ma, X.; Liu, W.H.; Chen, Y.J.; Chen, S.Y.; Jiang, Y.M.; Gan, L.; et al. Effects of oat: Feed pea sowing ratio and lactic acid bacteria addition on crop silage fermentation and ruminal degradation characteristics of the resulting total mixed ration. Acta Pratac. Sin. 2024, 33, 169–181. Available online: https://link.cnki.net/urlid/62.1105.S.20231110.1004.020 (accessed on 26 April 2025).
- Liu, T.T.; Yang, Y.Y.; Li, Q.F.; Cao, Y.F.; Wang, K.; Shen, Y.Z.; Wang, S.W.; Li, J.G. Effects of silage treatment on three kinds of oat grass nutrients and rumen degradation performance of dairy cows. Feed Ind. 2022, 43, 41–48. [Google Scholar] [CrossRef]
- Pérez-Márquez, S.; Ovani, V.S.; Lima, P.d.M.T.; Lana, Â.M.Q.; Louvandini, H.; Abdalla, A.L.; Maurício, R.M. Tithonia diversifolia Improves In Vitro Rumen Microbial Synthesis of Sheep Diets without Changes in Total Gas and Methane Production. Agronomy 2023, 13, 2768. [Google Scholar] [CrossRef]
- Pan, H.M.; Huang, X.C.; Tian, Y.H.; Sun, Z.W.; Li, C.Y. Effect of silage of oak leaves treated with different additives on rumen degradation rate and microbial community of Yanbian cattle in vitro fermentation. Pratac. Sci. 2023, 40, 2384–2400. [Google Scholar]
- Guo, J.S.; Zhao, G.Y.; Li, S.L.; Feng, Y.L. Effect of adding lactic acid bacteria on quality of barley silage and the rumen degradation rates of neutral detergent fiber and acid detergent fiber. Chin. J. Anim. Sci. 1999, 35, 27–28. [Google Scholar]
- Ellis, J.L.; Bannink, A.; Hindrichsen, I.K. The effect of lactic acid bacteria included as a probiotic or silage inoculant on in vitro rumen digestibility, total gas and methane production. Anim. Feed. Sci. Technol. 2016, 211, 61–74. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).