Identification, Pathogenicity and Fungicide Sensitivity of Colletotrichum Species Causing Anthracnose on Polygonatum cyrtonema Hua
Abstract
1. Introduction
2. Materials and Methods
2.1. Disease Samples Collection and Colletotrichum Strain Isolation
2.2. Morphological and Cultured Characterization
2.3. DNA Extraction, PCR Amplification, and DNA Sequencing
2.4. Pathogenicity and Aggressiveness Assays of Six Colletotrichum spp.
2.5. Determination of Physiological Characteristics
2.6. Fungicide Assays
3. Results
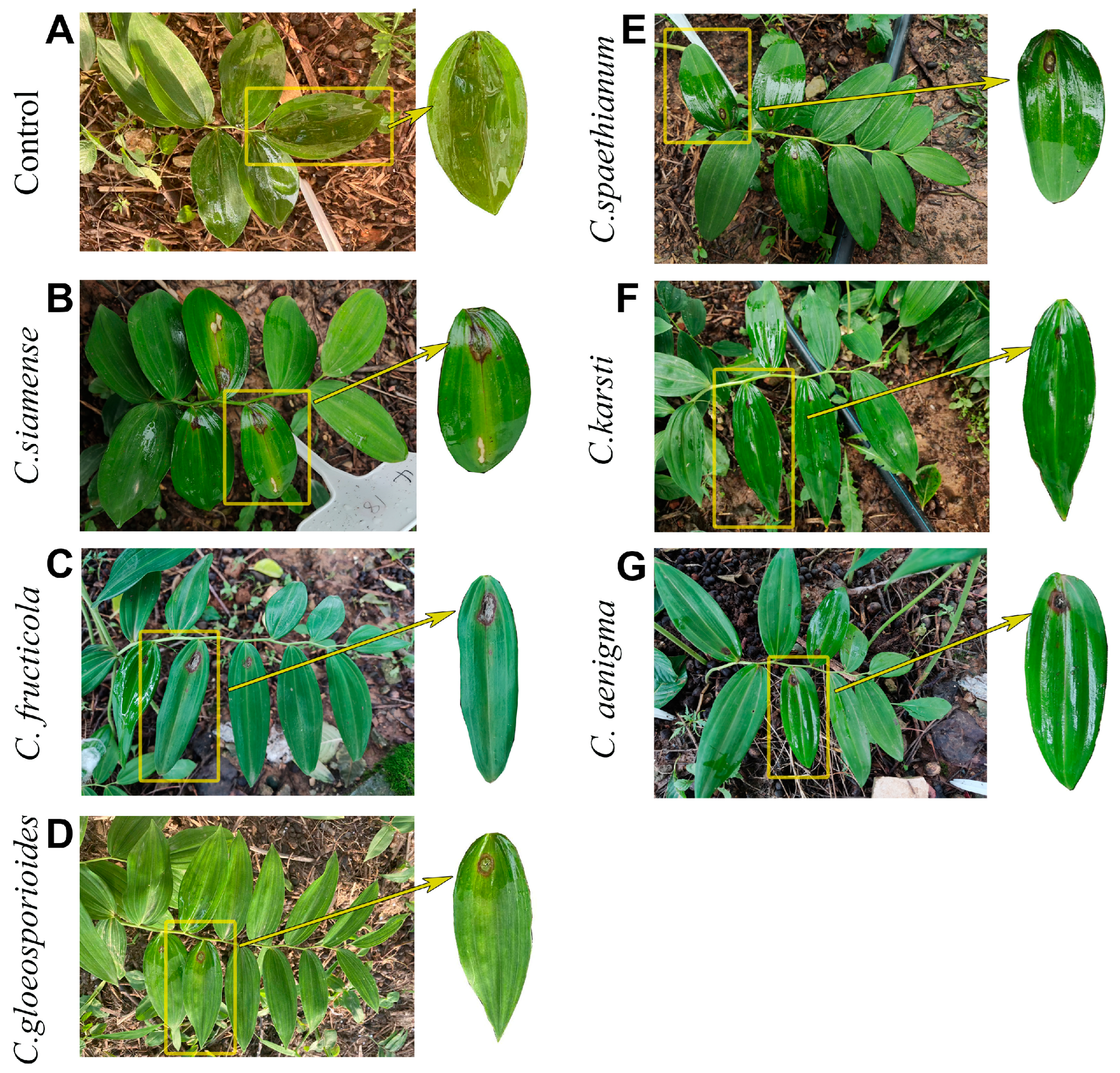
3.1. Disease Symptoms of Anthracnose of P. cyrtonema in Fields and Pathogen Isolation
3.2. Molecular Identification and Phylogenetic Analysis
3.3. Morphological Characteristics of Colletotrichum Pathogens of P. cyrtonema Anthracnose
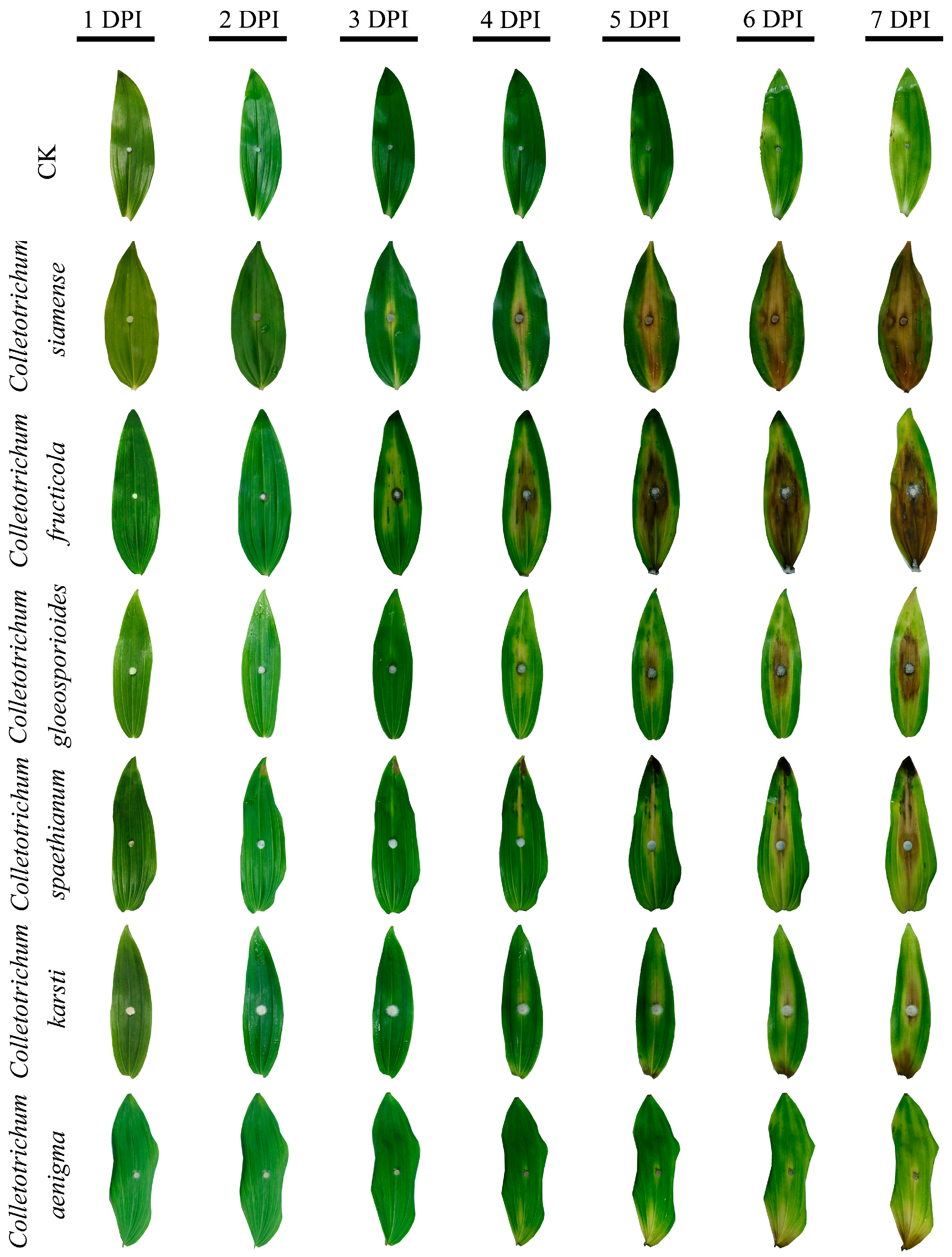
3.4. Pathogenicity Test and Comparison of Six Colletotrichum Species
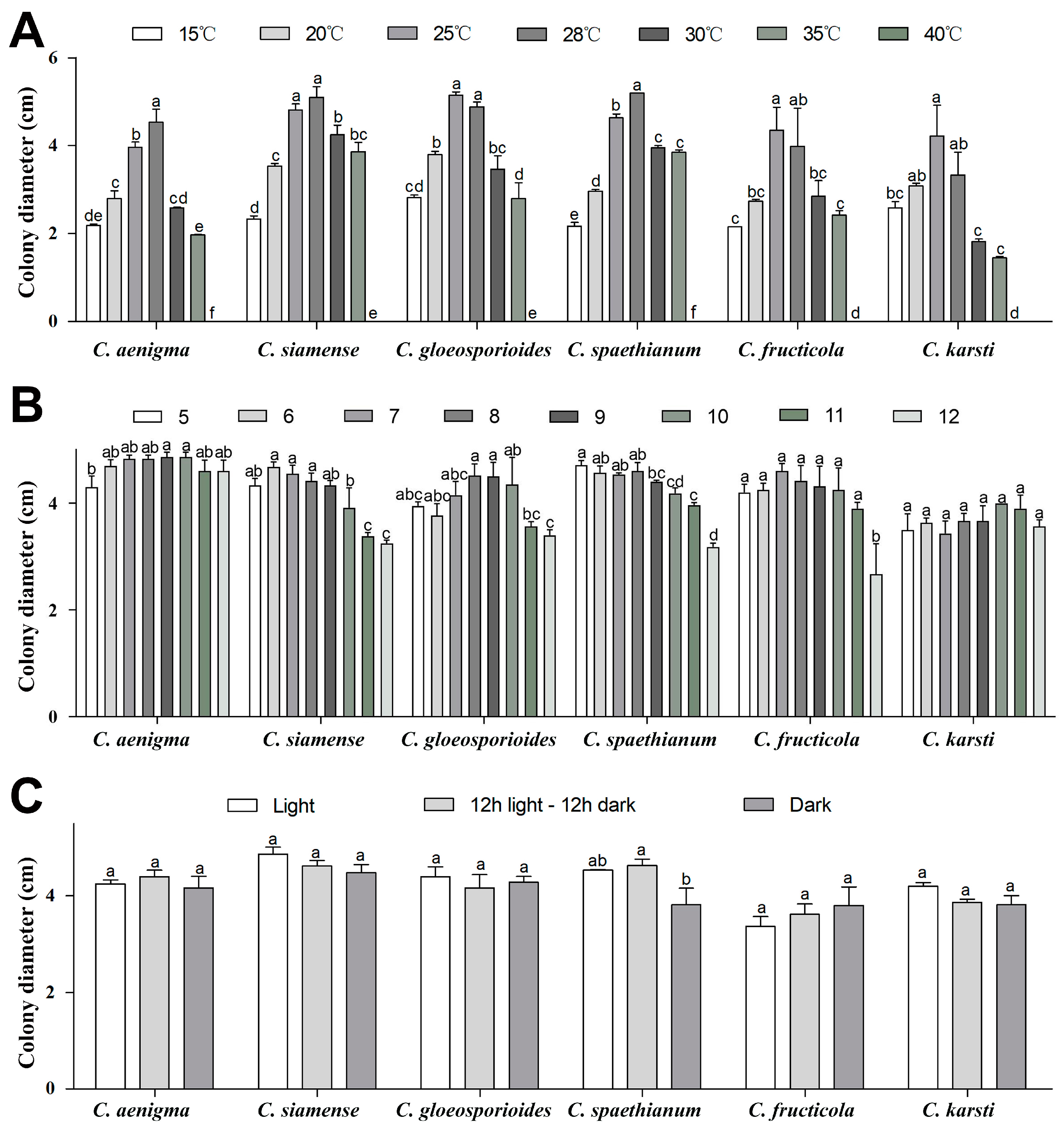
3.5. Cultural Characteristics of the Six Colletotrichum Species
3.6. Fungicide Sensitivity Testing of Colletotrichum Species
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fan, Q.J.; Zhou, Q.Y.; Zhang, S.; Li, Y.; Li, J.; Chen, X.; Sun, L. First report of leaf spot disease caused by Alternaria alternata on Polygonatum cyrtonema Hua in Hunan province of China. Plant Dis. 2024, 108, 530. [Google Scholar] [CrossRef]
- Li, M.; Chen, X.; Li, Y.; Yuan, Y. Research progress on pharmacological effects of Polygonatum polysaccharides. Acta Chin. Med. Pharmacol. 2024, 52, 95–101. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, W.; Shen, B.; Zhao, R.; Xu, L.; Xie, M.; Kang, T. Textual research of Polygonatum sibiricum. Acta Chin. Med. Pharmacol. 2019, 47, 81–86. [Google Scholar] [CrossRef]
- Chen, H.; Feng, S.; Sun, Y.; Hao, Z.; Feng, W.; Zheng, X. Advances in studies on chemical constituents of three medicinal plants from Polygonatum Mill. and their pharmacological activities. Chin. Tradit. Herb. Drugs 2015, 46, 2329–2338. [Google Scholar] [CrossRef]
- Su, W.; Liu, Y.; Jiang, Y.; Xie, J.; Pan, X.; Liu, J.; Si, J. Status of Polygonati rhizome industry and suggestion for its sustainable development. China J. Chin. Mater. Medica 2018, 43, 2831–2835. [Google Scholar] [CrossRef]
- Chen, X.; Ye, W.; Zhong, J. Research on the application and industrial development of the medicinal plant Polygonatum sibiricum Red. Hortic. Seed 2024, 44, 62–63+66. [Google Scholar] [CrossRef]
- Luo, M.; Zhang, W.; Deng, C.; Tan, Q.; Luo, C.; Luo, S. Advances in studies of medicinal crop Polygonatum cyrtonema Hua. Lishizhen Med. Mater. Medica Res. 2016, 27, 1467–1469. [Google Scholar] [CrossRef]
- Li, J.; Cai, H.; Du, Y.; Chen, H.; Qu, K.; Wei, L.; Miao, Y.; Liu, D. Morphological, molecular and pathogenic characterization of Botryosphaeria dothidea causing leaf blight disease of Polygonatum cyrtonema in China. Physiol. Mol. Plant Pathol. 2024, 134, 102424. [Google Scholar] [CrossRef]
- Dan, Y.; Ma, W.; Tang, Z.; Xu, Q.; Song, Z.; Liu, M.; Yin, F. Biological characterization and fungicide screening of Colletotrichum fioriniae causing Polygonatum cyrtonema Hua anthracnose. J. Chin. Med. Mater. 2023, 46, 1876–1881. [Google Scholar] [CrossRef]
- Jeffries, P.; Dodd, J.C.; Jeger, M.J.; Plumbley, R.A. The biology and control of Colletotrichum species on tropical fruit crops. Plant Pathol. 1999, 39, 343–366. [Google Scholar] [CrossRef]
- Wang, Y.; Hao, X.; Wang, L.; Bin, X.; Wang, X.; Yang, Y. Diverse Colletotrichum species cause anthracnose of tea plants (Camellia sinensis (L.) O. Kuntze) in China. Sci. Rep. 2016, 6, 35287. [Google Scholar] [CrossRef] [PubMed]
- Dan, Y.; Liu, M.; Cui, X.; Ma, W.; Tang, Z.; Yin, F. Identification of pathogen causing anthracnose on Polygonatum cyrtonema Hua. Plant Prot. 2022, 49, 288–293. [Google Scholar] [CrossRef]
- Dan, Y.; Tang, Z.; Ma, W.; Cui, X.; Xu, Q.; Song, Z.; Yin, F.; Liu, M. Identification, biological characterization, and fungicide screening of the pathogen causing Polygonatum cyrtonema Hua anthracnose. Acta Phytopathol. Sin. 2023, 53, 796–809. [Google Scholar] [CrossRef]
- Ma, J.; Xiao, X.; Wang, X.; Guo, M. Colletotrichum spaethianum causing anthracnose on Polygonatum cyrtonema Hua in Anhui province, China. Plant Dis. 2020, 105, 509. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Chen, J.; Zhang, Z.; Zhong, J.; Zhu, J. Identification and fungicide screening of pathogen causing anthracnose on Polygonatum cyrtonema (Huangjing) in Hunan province of China. Southwest China J. Agric. Sci. 2023, 36, 91–97. [Google Scholar] [CrossRef]
- Tan, Q.; Li, J.; Xue, Y.; Zhao, D.; Yang, C.; He, H. Identification of the pathogen of anthracnose of Polygonatum cyrtonema from Guizhou. J. Chin. Med. Mater. 2023, 46, 12–16. [Google Scholar] [CrossRef]
- Kim, C.H.; Hassan, O.; Chang, T. Diversity, pathogenicity, and fungicide sensitivity of Colletotrichum species associated withapple anthracnose in South Korea. Plant Dis. 2020, 104, 2866–2874. [Google Scholar] [CrossRef]
- Prasad, M.S.L.; Sujatha, K.; Naresh, N.; Rao, S.C. Variability in Sclerotium rolfsii associated with collar rot of sunflower. Indian Phytopathol. 2012, 65, 161–165. [Google Scholar] [CrossRef]
- Talbot, N.J. Nucleic Acid Isolation and Analysis; Oxford University Press: Oxford, UK, 2001. [Google Scholar]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR protocols: A guide to methods and applications. Mycologia 1990, 18, 315–322. [Google Scholar] [CrossRef]
- Carbone, I.; Kohn, L.M. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 1999, 91, 553–556. [Google Scholar] [CrossRef]
- O’Donnell, K.; Cigelnik, E. Two divergent Intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. Mol. Phylogenetics Evol. 1997, 7, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Templeton, M.D.; Rikkerink, E.H.A.; Solon, S.L.; Crowhurst, R.N. Cloning and molecular characterization of the glyceraldehyde-3-phosphate dehydrogenase-encoding gene and cDNA from the plant pathogenic fungus Glomerella cingulata. Gene 1992, 122, 225–230. [Google Scholar] [CrossRef]
- Zhang, L.; Li, X.; Zhou, Y.; Tan, G.; Zhang, L. Identification and characterization of Colletotrichum species associated with Camellia sinensis anthracnose in Anhui province, China. Plant Dis. 2021, 105, 2649–2657. [Google Scholar] [CrossRef]
- Hou, Y.; Xin, H.; Zhang, X.; Fan, X.; Liu, S.; Xu, J. Sensitivity of Fusarium pseudograminis to epoxiconazole in Henan province. Acta Phytopathol. Sin. 2023, 53, 307–316. [Google Scholar] [CrossRef]
- Machuca, A.; Durán, N. Optimization of some parameters influencing Thermoascus aurantiacus growth: Effects of lignin-related compounds. J. Ind. Microbiol. 1996, 16, 224–229. [Google Scholar] [CrossRef]
- Wang, K.; Jiang, S.; Pu, T.; Fan, L.; Su, F.; Ye, M. Antifungal activity of phenolic monoterpenes and structure-related compounds against plant pathogenic fungi. Nat. Prod. Res. 2019, 33, 1423–1430. [Google Scholar] [CrossRef]
- Deng, H.; Wang, Y.; Chen, S.; Jin, C.; Chen, Y.; Guo, K. Identification and laboratory toxicity test of the pathogen of leaf blight of Polygonatum cyrtonema in Xinhua of Hunan province. Hunan Agric. Sci. 2023, 4, 61–65. [Google Scholar] [CrossRef]
- Feng, W.; Wang, C.; Ju, Y.; Chen, Z.; Wu, X.; Fang, D. Identifying the biological characteristics of anthracnose pathogens of Blueberries (Vaccinium corymbosum L.) in China. Forests 2024, 15, 117. [Google Scholar] [CrossRef]
- Kaur, D.; Kaur, A.; Singh, H.; Arora, A. First report of Colletotrichum siamense causing fruit drop in kinnow mandarin (Citrus nobilis x Citrus deliciosa) in Punjab, India. Crop Prot. 2024, 175, 106464. [Google Scholar] [CrossRef]
- Zhang, A.; Li, L.; Xie, X.; Chai, A.; Shi, Y.; Xing, D.; Yu, Z.; Li, B. Identification and genetic diversity analysis of the pathogen of anthracnose of pepper in Guizhou. Plants 2024, 13, 728. [Google Scholar] [CrossRef]
- Talhinhas, P.; Baroncelli, R. Colletotrichum species and complexes: Geographic distribution, host range and conservation status. Fungal Divers. 2021, 110, 109–198. [Google Scholar] [CrossRef]
- Qian, H.; Xu, P.; Chi, M.; Huang, J. Mixed infection by Fusarium oxysporum and Alternaria tenuissima on sweet potato Fusarium wilt. J. Plant Prot. 2017, 44, 867–868. [Google Scholar] [CrossRef]
- Qin, R.; Zhang, Y.; Li, Q.; Huang, S.; Chen, X.; Guo, T.; Tang, L. Leaf spot caused by Colletotrichum siamense, C. fructicola, and C. aeschynomenes on Ixora chinensis in Guangxi, China. Plant Dis. 2024, 108, 225. [Google Scholar] [CrossRef]
- Chang, J.; Zhai, F.; Zhang, Y.; Wang, D.; Shu, J.; Yao, X. Identification and characterization of Colletotrichum fioriniae and C. fructicola that cause anthracnose in pecan. Front. Plant Sci. 2022, 13, 1043750. [Google Scholar] [CrossRef]
- Muhammad, U.H.; Tan, Q.; Mazharul, K.M.; Muhammad, A.; Yin, W.; Zhu, F.; Luo, C. Sensitivity of C. fructicola and C. siamense of peach in China to multiple classes of fungicides and characterization of pyraclostrobin-resistant isolates. Plant Dis. 2021, 105, 3459–3465. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, T.; Zhang, D.; Wu, H.; Pan, L.; Liao, N.; Liu, W. First report of anthracnose caused by Colletotrichum siamense and C. fructicola of Camellia chrysantha in China. Plant Dis. 2021, 105, 2020. [Google Scholar] [CrossRef] [PubMed]
- Guginski-Piva, C.A.; Bogo, A.; Gomes, B.R.; Menon, J.K.; Nodari, R.O.; Welter, L.J. Morphological and molecular characterization of Colletotrichum nymphaeae and C. fructicola associated with anthracnose symptoms of grape in Santa Catarina State, southern Brazil. J. Plant Dis. Prot. 2018, 125, 405–413. [Google Scholar] [CrossRef]
- Park, M.S.; Kim, B.R.; Park, I.H.; Hahm, S.S. First report of two Colletotrichum Species associated with bitter rot on apple fruit in Korea—C. fructicola and C. siamense. Mycobiology 2018, 46, 154–158. [Google Scholar] [CrossRef]
- Li, H.; Zhou, G.; Liu, J.; Xu, J. Population Genetic Analyses of the Fungal Pathogen Colletotrichum fructicola on Tea-Oil Trees in China. PLoS ONE 2017, 11, e0156841. [Google Scholar] [CrossRef]
- Kang, N.; Xiang, M.; Xiao, L.; Zheng, Z.; Yang, H.; Zeng, J.; Jiang, H.; Chen, J.; Chen, M. First report of Colletotrichum fructicola causing anthracnose on Paeonia lactiflora in China. Plant Dis. 2023, 107, 4022. [Google Scholar] [CrossRef]
- Du, L.; Du, C.; Ding, C. First report of Colletotrichum fructicola causing anthracnose on Rosa chinensis in China. Plant Dis. 2023, 107, 3316. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; Chu, L.; Yang, L.; Dong, N.; Dan, Z.; Zhong, H.; Dong, C. First report of Colletotrichum fructicola causing anthracnose on Epimedium sagittatum in China. Plant Dis. 2024, 108, 813. [Google Scholar] [CrossRef]
- Huang, D.; Li, M.; Qin, Q.P. Identification, pathogenicity, and fungicide sensitivity of Colletotrichum Spaethianum isolates, the causal agents of anthracnose of daylily in Shanghai, China. Res. Sq. 2022. [Google Scholar] [CrossRef]
- Hyde, K.D.; Cai, L.; Cannon, P.F.; Crouch, J.A.; Zhang, J.Z. Colletotrichum—Names in current use. Fungal Divers. 2009, 39, 147–182. [Google Scholar] [CrossRef]
- Bonde, M.R.; Nester, S.E.; Berner, D.K. Effects of daily temperature highs on development of Phakopsora pachyrhizi on soybean. Phytopathology 2012, 102, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Nega, A. Climate change impacts on agriculture: A review of plant diseases and insect pests in Ethiopia and East Africa, with adaptation and mitigation strategies. Adv. Agric. 2025, 2025, 5606701. [Google Scholar] [CrossRef]
- Ciofini, A.; Negrini, F.; Baroncelli, R.; Baraldi, E. Management of Post-Harvest Anthracnose: Current Approaches and Future Perspectives. Plants 2022, 11, 1856. [Google Scholar] [CrossRef]
- Ma, J.; Yuan, Q.; Wang, Y.; Miao, L.; Xin, B. Screening of chemical Fungicides for controlling Alfalfa anthracnose caused by Colletotrichum linicola. Chin. J. Grassl. 2016, 38, 84–90+115. [Google Scholar] [CrossRef]
- Fan, K.; Qi, Y.; Fu, L.; Li, L.; Liu, X.; Qu, J.; Li, D.; Dong, A.; Peng, Y.; Wang, Q. Identification and Fungicide Screening of Fungal Species Associated with Walnut Anthracnose in Shaanxi and Liaoning Provinces, China. Plant Dis. 2024, 108, 599–607. [Google Scholar] [CrossRef]
- Mora-Aguilera, J.A.; Ríos-López, E.G.; Yáñez-Zúñiga, M.; Rebollar-Alviter, A.; Nava-Díaz, C.; Leyva-Mir, S.G.; Sandoval-Islas, J.S.; Tovar-Pedraza, M.J. Sensitivity to MBC fungicides and prochloraz of Colletotrichum gloeosporioides species complex isolates from mango orchards in Mexico. J. Plant Dis. Prot. 2021, 128, 481–491. [Google Scholar] [CrossRef]
- Soe, Z.Z.; Shin, Y.H.; Kang, H.S.; Jeun, Y.C. Chemical Resistance of Diaporthe citri against Systemic Fungicides on Citrus. Plant Pathol. J. 2023, 39, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Vinggaard, A.M.; Hass, U.; Dalgaard, M.; Andersen, H.R.; BonefeldJørgensen, E.; Christiansen, S.; Laier, P.; Poulsen, M.E. Prochloraz: An imidazole fungicide with multiple mechanisms of action. Int. J. Androl. 2006, 29, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Zhang, M.; Tang, S.; Li, M.; Wu, R.; Wan, S.; Chen, L.; Wei, X.; Li, F. Research Progress on Benzimidazole Fungicides: A Review. Molecules 2024, 29, 1218. [Google Scholar] [CrossRef] [PubMed]
- Karadimos, D.A.; Karaoglanidis, G.S.; Tzavella–Klonari, K. Biological activity and physical modes of action of the Qo inhibitor fungicides trifloxystrobin and pyraclostrobin against Cercospora Beticola. Crop Prot. 2005, 24, 23–29. [Google Scholar] [CrossRef]
- Hu, S.; Zhang, S.; Xiao, W.; Liu, Y.; Yu, H.; Zhang, C. Diversity and characterization of resistance to pyraclostrobin in Colletotrichum spp. from Strawberry. Agronomy 2023, 13, 2824. [Google Scholar] [CrossRef]




| Taxon | Strains | GenBank Accession Numbers | |||
|---|---|---|---|---|---|
| ITS | ACT | GAPDH | CHS | ||
| Colletotrichum aenigma | HJ-11 | PP551230 | PP227177 | PP227179 | PP5558343 |
| Colletotrichum siamense | HJ-18 | PP551582 | PP227180 | PP227181 | PP227182 |
| Colletotrichum gloeosporioides | HJ-34 | PP551515 | PP227183 | PP227167 | PP227184 |
| Colletotrichum spaethianum | HJ-41 | PP551521 | PP227168 | PP227170 | PP227169 |
| Colletotrichum fructicola | HJ-43 | PP551516 | PP227171 | PP227173 | PP227172 |
| Colletotrichum karsti | HJ-Y2 | PP551573 | PP227174 | PP227176 | PP227175 |
| Isolates | Appearance | Length Mean (Range, SD) (µm) (n = 50) | Width Mean (Range, SD) (µm) (n = 50) |
|---|---|---|---|
| C. aenigma | cylindrical, aseptate, transparent | 14.9 (10.71~22.16, 2.24) | 6.26 (4.23~9.60, 1.16) |
| C. siamense | cylindrical, aseptate, transparent | 15.01 (10.26~17.71, 1.59) | 5.31 (3.36~6.45, 0.6) |
| C. gloeosporioides | cylindrical, aseptate, transparent | 13.74 (10.77~17.44, 1.55) | 4.52 (3.04~6.34, 0.7) |
| C. spaethianum | acinacifoliate, aseptate, transparent | 15.40 (9.24~19.87, 2.08) | 4.50 (3.37~8.95, 0.81) |
| C. fructicola | monocytes, transparent, oval, or spindle-shaped, slightly curved | 16.93 (9.62~21.07, 2.32) | 5.85 (4.16~7.37, 0.81) |
| C. karsti | cylindrical with both ends rounded, transparent, smooth, aseptate | 12.33 (9.40~15.59, 1.52) | 7.09 (5.87~7.93, 0.57) |
| Isolates | Fungicide Name | Toxic Regression Equation | R | EC50 (μg/mL) |
|---|---|---|---|---|
| C. siamense | 30% Pyraclostrobin | y = 1.0522x + 5.0904 | 0.9417 | 0.825 |
| 450g/L Prochloraz | y = 0.9473x + 5.5869 | 0.9965 | 0.239 | |
| 50% Carbendazim | y = 2.1008x + 5.2753 | 0.9401 | 0.753 | |
| C. gloeosporioides | 30% Pyraclostrobin | y = 0.693x + 4.8046 | 0.8863 | 1.861 |
| 450 g/L Prochloraz | y = 1.2494x + 5.716 | 0.9916 | 0.265 | |
| 50% Carbendazim | y = 3.5325x + 5.6201 | 0.9474 | 0.688 | |
| C. spaethianum | 30% Pyraclostrobin | y = 0.3603x + 5.6388 | 0.8503 | 0.017 |
| 450 g/L Prochloraz | y = 1.4154x + 5.6337 | 0.9869 | 0.363 | |
| 50% Carbendazim | y = 2.0481x + 5.144 | 0.9779 | 0.852 | |
| C. fructicola | 30% Pyraclostrobin | y = 0.2613x + 5.2771 | 0.9688 | 0.087 |
| 450 g/L Prochloraz | y = 0.9313x + 5.6221 | 0.9732 | 0.213 | |
| 50% Carbendazim | y = 2.3866x + 5.7348 | 0.9342 | 0.540 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, H.; Li, J.; Du, Y.; Wu, D.; Chen, J.; Chen, H.; Qu, K.; Miao, Y.; Liu, D. Identification, Pathogenicity and Fungicide Sensitivity of Colletotrichum Species Causing Anthracnose on Polygonatum cyrtonema Hua. Agronomy 2025, 15, 1215. https://doi.org/10.3390/agronomy15051215
Cai H, Li J, Du Y, Wu D, Chen J, Chen H, Qu K, Miao Y, Liu D. Identification, Pathogenicity and Fungicide Sensitivity of Colletotrichum Species Causing Anthracnose on Polygonatum cyrtonema Hua. Agronomy. 2025; 15(5):1215. https://doi.org/10.3390/agronomy15051215
Chicago/Turabian StyleCai, Huixia, Jinxin Li, Yanling Du, Di Wu, Jinyi Chen, Hong Chen, Kaili Qu, Yuhuan Miao, and Dahui Liu. 2025. "Identification, Pathogenicity and Fungicide Sensitivity of Colletotrichum Species Causing Anthracnose on Polygonatum cyrtonema Hua" Agronomy 15, no. 5: 1215. https://doi.org/10.3390/agronomy15051215
APA StyleCai, H., Li, J., Du, Y., Wu, D., Chen, J., Chen, H., Qu, K., Miao, Y., & Liu, D. (2025). Identification, Pathogenicity and Fungicide Sensitivity of Colletotrichum Species Causing Anthracnose on Polygonatum cyrtonema Hua. Agronomy, 15(5), 1215. https://doi.org/10.3390/agronomy15051215





