Abstract
This study investigated the effects of organic soil amendments derived from agricultural byproducts—specifically cow manure (CM) at 0% and 1% w/w, and rice husk biochar (RHB) at 0%, 1%, 3%, and 5% w/w—on soil health, plant growth, and the accumulation of bioactive compounds in sunflower sprouts. The application of 1% CM significantly improved the soil properties—enhancing macroaggregates (MaAs) by 54.5%, mesoaggregates (MeAs) by 16.7%, and soil organic carbon (SOC) by 27.2%. It also increased the shoot and root biomass by 22.3% and 25.8%, respectively, and boosted soil respiration by 67.0%, while reducing the nitrate (NO3−) content by 33.7%. However, the CM also decreased the total phenolic content (TPC) by 21% and chlorophyll by 44.7%. The RHB, particularly at rates of 1–3% w/w, increased the MaAs by 62%, microaggregates (MiAs) by 3%, leaf area by up to 43.9%, root-to-shoot ratio by 26.5%, SOC by 13.1%, and DPPH antioxidant activity by 42.8%, while lowering the MeAs by 9% and NO3− content by up to 56.1%. In contrast, excessive RHB application (5% w/w) negatively impacted root development. The interaction effects revealed that the combination of 1% w/w CM with 1% w/w RHB maximized the MaAs by 12%, increased the root dry biomass by 101.9%, and also increased the TPC by 40.1% compared to the manure-only treatment. The Principal Component Analysis (PCA) indicated that CM primarily promoted plant growth and respiration, while RHB contributed to organic matter retention and nutrient availability. Applying 1% w/w CM and 1% w/w RHB showed promising effects and is recommended for short-cycle crop production.
1. Introduction
Sandy loam soils are widely distributed across Thailand, particularly in the central, northeastern, and parts of the northern regions, where they are extensively used for cultivating crops, such as rice, cassava, sugarcane, maize, and other field crops [1]. Their texture ranges from sandy and sandy loam to sandy clay loam, with fertility levels varying from low to medium. Due to land availability pressures, these soils have been continuously used for agriculture in both upland and lowland areas. However, their inherent limitations, including low water retention, poor nutrient-holding capacity, and susceptibility to erosion, present significant challenges for sustainable crop production [2].
One promising approach is the application of soil organic amendments (SOAs) derived from agricultural residues and locally available organic materials, which offer dual benefits: improving soil health while promoting sustainable nutrient recycling. These amendments—produced through composting, pyrolysis (e.g., biochar), anaerobic digestion (e.g., digestate), and other biomass conversion processes—transform underutilized organic materials into valuable, multifunctional products. When responsibly applied, SOAs enhance the soil structure, boost microbial diversity, improve nutrient cycling, and contribute to long-term soil fertility [3,4,5,6]. Their use also aligns with cleaner production and circular economy principles, reducing environmental burdens while supporting resource-efficient agriculture.
Among SOAs, cow manure (CM) and biochar have received growing attention for their synergistic potential. CM, a nutrient-rich organic input generated through livestock operations, contributes significantly to soil fertility, aggregation, and microbial activity by increasing soil organic carbon (SOC) [7,8]. It also serves as a slow-release source of macro- and micronutrients, supporting plant growth and reducing dependency on synthetic fertilizers [9,10]. However, when mismanaged, manure application can pose risks, such as nutrient leaching, salinity buildup, and greenhouse gas (GHG) emissions [11,12].
In contrast, biochar—produced via the pyrolysis of biomass, like rice husks biochar (RHB)—acts as a stable carbon input that enhances soil aeration, water retention, and nutrient availability, while also aiding in long-term carbon sequestration [13,14]. Biochar also contributes to climate change mitigation by reducing methane and nitrous oxide emissions, immobilizing pollutants, and stabilizing organic matter during decomposition [15,16]. However, the effectiveness of both CM and biochar depends on the feedstock type, processing conditions, and environmental factors [17,18,19,20]. The co-application of CM and biochar has shown synergistic effects for improving soil fertility, aggregation, microbial function, and crop yield [21,22,23].
Beyond the agronomic benefits, the application of SOAs has been linked to enhanced climate resilience. By improving the soil structure and water-holding capacity, these amendments can buffer crops against drought and environmental stress [24]. Additionally, SOAs support microbial resistance and resilience under extreme conditions, thus preserving essential ecosystem functions [25,26]. Importantly, the reuse of underutilized organic materials—such as crop residues and animal-derived byproducts—for agricultural purposes contributes to GHG emission reduction and positions SOAs as key components of carbon-smart agriculture and sustainable land use strategies.
Despite the numerous studies highlighting the agronomic benefits of SOAs, few have investigated their combined effects on soil physical structure, nutrient dynamics, and plant secondary metabolites—particularly in short-cycle cropping systems. These secondary metabolites, including phenolic compounds, antioxidants, and chlorophylls, are essential for plant defense, stress adaptation, and human health benefits, yet their responses to organic amendments have not been comprehensively characterized. Likewise, short-cycle crops, such as sunflower sprouts, provide a practical and sensitive model for evaluating the early-stage effects of soil amendments. Their rapid growth, high responsiveness to soil conditions, and growing significance in functional food production enable the detection of the short-term impacts of amendments on biomass accumulation and biochemical composition. In contrast to long-cycle crops that require extended observation periods, sunflower sprouts allow for the timely assessment of soil–plant interactions, making them particularly relevant for rapid-cycle cultivation systems and urban agriculture, where soil reuse and efficient input management are essential.
Therefore, this study aims to (i) assess the co-application effects of CM and RHB on soil aggregation, SOC stabilization, and nutrient availability in sandy loam soil; (ii) evaluate their influence on shoot and root biomass and the accumulation of bioactive compounds—including the total phenolic content (TPC), DPPH antioxidant activity, and total chlorophyll—in sunflower sprouts; and (iii) determine the optimal amendment combinations for enhancing agronomic performance and functional crop traits. It is hypothesized that the co-application of CM and RHB in short-cycle systems improves soil structural integrity, enhances SOC retention, and increases nutrient availability, resulting in greater plant growth and bioactive compound accumulation. Additionally, biochar is expected to exert a maximal benefit at moderate application rates, beyond which diminishing returns may occur due to nutrient imbalance or suboptimal physical conditions.
2. Materials and Methods
2.1. Soil, Manure, and Rice Husk Biochar Preparation
The soil used in this study was collected from San Pa Tong, Chiang Mai, Thailand (18°38′21.5″ N; 98°50′09.6″ E). It belongs to the Sanpatong series (Sp), classified as Typic Haplustalfs according to the United States Department of Agriculture (USDA) Soil Taxonomy. The soil is classified as sandy loam, comprising 73.8% sand and 16.4% loam, according to USDA texture classification. This soil type is well drained and derived from semi-recent alluvial deposits. Surface samples (0–15 cm depth) were collected, air-dried for 2–3 days, and sieved through a 2 mm mesh (Sieve No. 10, ASTM E11, Humboldt Mfg. Co., Elgin, IL, USA) to remove coarse particles.
The rice husk used for biochar production was sourced from a local rice mill owned by farmers in the study area, where rice husks are typically regarded as agricultural byproducts with limited reuse on-farm. Prior to pyrolysis, rice husks were oven-dried at 65 °C for 8 h. RHB was then produced under controlled pyrolysis conditions using an automated biomass pyrolyzing furnace. The process involved a heating rate of 10 °C/min, maintained for 1 h at a peak temperature of 500 °C to ensure uniform thermal treatment and carbonization. Similarly, the CM used in this study was raw (non-composted), collected fresh from livestock farmers in the same community, where it is routinely generated as part of standard animal husbandry. The manure was air-dried under shade for five days, then ground and sieved through a 2 mm mesh before application. Laboratory analysis indicated a carbon-to-nitrogen (C/N) ratio of 20.2.
The initial physicochemical properties of the soil, CM, and RHB were analyzed prior to application and are presented in Table 1.

Table 1.
Initial properties of soil, cow manure (CM), and rice husk biochar (RHB) prior to use for the experiment.
2.2. Experimental Setup
The experiment was conducted in February 2025 and was designed to include two CM application rates (0% and 1% w/w) and four RHB application rates (0%, 1%, 3%, and 5% w/w), based on the dry weight of the soil. Each treatment was replicated three times, resulting in a total of 24 experimental units. CM was applied at 1% w/w due to its high nutrient content, which at higher concentrations could cause phytotoxicity or nutrient imbalance. In contrast, RHB was tested at higher rates (1–5% w/w) to explore its structural benefits on soil aggregation and carbon retention, as biochar functions primarily as a physical and chemical amendment rather than as a nutrient input. The selected application rates were informed by previous research and align with locally recommended agronomic practices for organic amendments. Based on its incorporation into the top 15 cm of soil, with a bulk density of 1.3 g/cm3, the 1%, 3%, and 5% w/w rates correspond to approximately 19.5, 58.5, and 97.5 t/ha, respectively.
Approximately 400 g of soil was placed into square plastic pots with dimensions of 10.5 × 16.3 × 5.6 cm (width × length × height), and the respective amounts of CM and RHB were added to each pot. The use of pots for sunflower sprout (Helianthus annuus L.) cultivation followed the traditional small-scale planting method commonly practiced by Thai farmers. Moreover, a pot experiment was chosen to ensure precise control over amendment application and soil conditions, which is especially important given the variability in composition and nutrient content of organic amendments derived from agricultural residues [24]. Soil moisture was initially adjusted to 60 ± 10% water-filled pore space (WFPS) (Equation (1)) by adding deionized (DI) water to the pots using a graduated cylinder, followed by thoroughly mixing the moist soil with a spatula.
where WFPS represents water-filled pore space (%), SWC denotes volumetric soil water content (vol. %), BD refers to soil bulk density (g/cm), and PD is particle density (2.65 g/cm) [27].
Sunflower sprout seeds (Chia Tai Produce Co., Ltd., Bangkok, Thailand) were surface-sterilized with 2% (v/v) sodium hypochlorite, thoroughly rinsed, and soaked in water at room temperature for 24 h. Healthy, sprouted seeds were selected and transplanted into square plastic pots, with each pot containing 45 seeds. The pots were arranged in a randomized complete block design within an incubator set at 25 °C. Soil moisture was maintained at 60 ± 10% WFPS, with DI water added when moisture levels dropped by more than 10%. Light-emitting diode (LED) lights (Slim Set T5 Plant Grow 9W, Lamptan, Bangkok, Thailand) provided a consistent light spectrum (400–780 nm) across all pots, ensuring uniform illumination. Germination occurred within 10 days, after which both soil and sunflower sprouts were collected for further analysis. All treatments were conducted in triplicate.
2.3. Soil Sampling and Analyses
After harvesting, soil samples were divided into two portions: one air-dried for seven days, sieved through a 2 mm sieve, and stored at room temperature for physicochemical analysis, while the other was wet-sieved and immediately frozen at −20 °C to preserve integrity for soil respiration measurements. All analyses were performed in triplicate to ensure data reliability.
2.3.1. Soil Texture
The initial soil texture was analyzed using a modified pipette method [28]. Briefly, organic matter (OM) was removed from a 10 g soil sample with H2O2 at 90 °C for 45 min, followed by salt removal through repeated DI water washes and centrifugation at 3577× g (Varispin 4, Novapro Co., Ltd., Seoul, Republic of Korea). Soil dispersion was achieved by shaking with sodium metaphosphate overnight. Sand was separated using a 50 µm sieve, oven-dried at 105 °C, and weighed. The remaining suspension was diluted to 1000 mL, stirred, and left to settle for 5 h before extracting 25 mL of the clay fraction, which was also oven-dried and weighed. Soil texture was classified based on the recorded proportions of sand, silt, and clay using the USDA soil texture classification system.
2.3.2. pH and Electrical Conductivity
The soil pH and electrical conductivity (EC) were measured in DI water using a 1:2 soil-to-solution ratio [29] with a multifunction TDS/EC/pH/salinity meter (Ex–9909, Chachoengsao, Thailand).
2.3.3. Water-Holding Capacity
The water-holding capacity (WHC) was determined following the method described by Motsara and Roy [30]. The soil and amendments were oven-dried at 48 °C for two days to standardize moisture content. Five grams of each material was placed on a filter paper-lined funnel over a graduated cylinder, gradually saturated with DI water over seven hours, and then left to drain for 24 h. The weight of the saturated samples was recorded, and WHC was calculated as the percentage of water retained relative to the dry weight.
2.3.4. Organic Matter and Organic Carbon
Loss on ignition (LOI) was used to determine soil organic matter content, which is commonly used to estimate organic carbon (OC), assuming OM contains 58% OC [30]. For LOI analysis, 2 g of air-dried soil (<2 mm) (W2) was placed in a pre-weighed porcelain crucible (W1) and combusted in a muffle furnace (LT 3/11/B170, Nabertherm, Lilienthal/Bremen, Germany) at 550 °C for 4 h. After cooling in a desiccator, the crucible with the remaining ash was weighed (W3). OM and OC content were then calculated using Equations (2) and (3).
2.3.5. Water-Stable Aggregates
Water-stable aggregates (WSAs) were assessed using a modified wet-sieving method [31,32]. Approximately 50 g of air-dried soil was placed on a 2 mm sieve, submerged in DI water, and soaked for 5 min. The sieve was manually shaken 50 times over 2 min in an up-and-down motion. The remaining aggregates were collected, dried at 50 °C, and weighed to determine water-stable macroaggregates (>2 mm, MaAs). The soil fraction passing through the 2 mm sieve was transferred onto a 250 μm sieve, subjected to the same procedure, and weighed to calculate mesoaggregates (250 μm—2 mm, MeAs). Finally, the soil fraction passing through the 250 μm sieve was dried and weighed to determine microaggregates (<250 μm, MiAs), with all fractions calculated using Equation (4).
where WSAi represents the water-stable aggregation for each size class i, Wa denotes the weight of aggregates retained on the sieve after wet sieving for size i, and W0 refers to the initial weight of aggregates placed on the sieve before wet sieving.
2.3.6. Soil Respiration
Soil respiration was assessed using a modified method from Page [33]. Frozen soil samples were thawed for 1 h, and 25 g of soil was placed in aluminum cups inside airtight containers. A glass bottle with 20 mL of 1 N NaOH was added to each container to absorb carbon dioxide (CO2) from microbial activity, ensuring no direct soil contact. The containers were sealed and incubated at 25 °C in the dark for 24 h, with a control setup containing NaOH without soil. After incubation, the NaOH solution was treated with 8 mL of 3 N BaCl2 to precipitate BaCO3. Two drops of phenolphthalein were added as the indicator, and unreacted NaOH was titrated with HCl. The CO2 evolution rate, indicating microbial activity, was calculated using Equation (5).
where CO2–C is expressed in mg/kg/hour and is calculated using the following parameters, B represents the volume (mL) of acid required to titrate the NaOH in the control bottles, and V is the volume (mL) of acid needed to titrate the NaOH in the bottles exposed to soil respiration. N denotes the normality of the acid, and E is the equivalent weight used to express the data in terms of CO2 (E = 22). T refers to the incubation time (h), and Ws represents the weight of the incubated soil (kg).
2.3.7. Nitrate, Available Phosphorus, and Available Potassium
Briefly, nitrate (NO3−) was extracted from soil by shaking 10 g of soil with 20 mL of 0.5 M K2SO4 for 30 min [30]. The extract was then centrifuged at 3577× g for 5 min and filtered through Whatman No. 5 filter paper (Cat No.1005–110, Cytiva, Shanghai, China). The NO3− concentration was determined by reacting the extract with 5% salicylic acid, forming a colored complex, and measuring the absorbance at 410 nm using a UV/Vis double-beam spectrophotometer (Lambda 365, PerkinElmer Ltd., Bangkok, Thailand).
For available phosphorus (P), Olsen’s method was followed as described by Motsara and Roy [30]. Using this method, 1.0 g of soil was extracted with 20 mL of 0.5 M NaHCO3 by shaking for 30 min on an orbital shaker (MS–NOR–30, Major Science, Saratoga, CA, USA). The extract was then centrifuged at 3577× g for 5 min, filtered through Whatman No. 5 filter paper, and analyzed for P concentration at 882 nm using a UV/V is double-beam spectrophotometer.
For available potassium (K), 2.5 g of soil was extracted with 25 mL of 1 M NH4C2H3O2 by shaking for 30 min [30]. The extract was then filtered and analyzed for available K using a flame photometer (AE–FP8201, A&E Lab (UK) Co., Ltd., London, UK) at 383 nm.
2.4. Plant Sampling and Measures
After ten days of germination, the number of germinated sunflower sprouts was recorded. The sprouts and roots were carefully removed, rinsed with tap water to remove soil particles, and then washed with DI water. After air-drying at room temperature for 5 min, the samples were placed in Ziplock bags and stored at 4 °C for further analysis. All analyses were performed in triplicate.
2.4.1. Germination Rate
The germination rate was determined based on the number of germinated sunflower sprouts and was calculated using the following equation:
2.4.2. Leaf Area Measurement
The width and the length of sunflower leaves were measured using a ruler. These data were used to calculate average total leaf area per sprout using the equation below [34]:
where 0.73 is the coefficient, W1 and L1 represent the width and length of the first leaf, and W2 and L2 correspond to the width and length of the second leaf. All measurements were recorded in centimeters (cm), and the average total leaf area per sprout was expressed in square centimeters (cm2).
2.4.3. Root-to-Shoot Ratio
The shoot and root lengths of 24 sprouts per pot were measured using a ruler and recorded in centimeters (cm). These measurements were then used to calculate the root-to-shoot ratio using the following equation:
The roots and shoots were carefully separated using scissors and weighed to determine fresh biomass. The roots were then oven-dried at 60 °C for 72 h before measuring dry biomass. Shoots were analyzed for TPC, DPPH antioxidant capacity, and total chlorophyll.
2.4.4. Total Phenolic Content
After cleaning, sunflower sprout shoots were finely ground using a specialized grinder. Hydrophilic and lipophilic compounds were extracted following Deng et al. [35], with slight modifications. For lipophilic extraction, 0.5 g of the sample was extracted twice with 5 mL of tetrahydrofuran in a shaking water bath at 37 °C for 30 min, followed by centrifugation at 3944× g for 30 min. Supernatants were pooled to obtain the lipophilic extract. Hydrophilic extraction was performed on the residue using a methanol–acetic acid–water solution (50:3.7:46.3, v/v/v) under the same conditions. Extracts were stored at −20 °C and analyzed within 24 h.
TPC was measured using the Folin–Ciocalteu method. Briefly, 0.50 mL of extract was mixed with 2.5 mL of diluted Folin–Ciocalteu reagent, incubated for 4 min, then combined with 2 mL of saturated sodium carbonate solution and left in the dark for 2 h. Absorbance was recorded at 760 nm using a UV–VIS spectrophotometer (Shimadzu, Kyoto, Japan). Gallic acid was used as the standard, and TPC was expressed as mg gallic acid equivalents (mg GAEs) per 100 g fresh weight (FW), calculated from both lipophilic and hydrophilic extracts.
2.4.5. Antioxidant Activity
The antioxidant capacity of the extracts was assessed using a 1,1–diphenyl–2–picrylhydrazyl (DPPH) assay, following Ghafoor et al. [36] with slight modifications. A total of 1 mL of extract was mixed with 2 mL of DPPH solution, vortexed, and incubated at room temperature for 30 min. Absorbance was then measured at 517 nm using a Shimadzu UV–VIS spectrophotometer. The DPPH radical scavenging activity was calculated accordingly.
where CTrolox is trolox equivalent concentration from the standard curve (µmol/mL), Vextract is volume of extract used (mL), msample is mass of the sample used for extraction (g), and the factor 10 adjusts the result to per 10 g of sample. The total DPPH scavenging activity was determined by summing the antioxidant activities derived from the lipophilic and hydrophilic extraction fractions.
2.4.6. Total Chlorophyll Content
The total chlorophyll content was determined following a modified method from Barua et al. [37]. Fresh shoot (1 g) was finely chopped, weighed, and ground with 20 mL of 80% acetone using a mortar and pestle. The homogenate was centrifuged at 5589× g for 5 min, and the supernatant was filtered into a 100 mL volumetric flask. The extraction was repeated with additional 80% acetone until the residue became colorless. The final volume was adjusted to 100 mL, and absorbance was measured at 645 nm and 663 nm using a Shimadzu UV–VIS spectrophotometer. Total chlorophyll content was calculated and expressed as mg per 100 g of fresh tissue.
where OD is optical density, V is final volume of chlorophyll extract in 80% acetone (mL), W is fresh weight of tissue extracted (g), and the factor 100 adjusts the result to per 100 g of sample.
2.5. Statistical Analyses
Statistical analyses were performed using R (RStudio v2022.02.1). Residual normality was assessed using the Shapiro–Wilk test, and non-normal data were transformed as appropriate: log transformation was applied to NO3− concentration, germination rate, and total WSAs, while square root transformation was used for average total leaf area. Levene’s test evaluated variance homogeneity. Outliers were assessed through Q-Q plots and by calculating studentized residuals. Influential outliers (|residual| > 3) were inspected and removed if determined to have resulted from data entry errors or experimental anomalies. Data were analyzed via analysis of variance (ANOVA), including interaction effects, with Tukey’s post hoc test for multiple comparisons (α = 0.05). Principal Component Analysis (PCA) was conducted using FactoMineR to assess treatment effects on soil and plant variables. Biplots were generated with factoextra, incorporating ellipses and color coding to visualize amendment rate effects.
3. Results and Discussion
Due to the limited availability of published studies on sunflower sprouts under comparable soil amendment conditions, some of the nutrient-related findings in this study are discussed in relation to the results for other crops. While we acknowledge the species-specific nature of nutrient uptake and metabolism, these comparisons are included to provide contextual insights and to highlight plausible mechanisms that may be relevant to early-stage plant development under organic amendment treatments.
3.1. Soil Aggregation and Physicochemical Properties
3.1.1. Effects on Soil Aggregation
The distribution of three sizes of water-stable aggregates—MaAs (>2 mm), MeAs (250 μm—2 mm), and MiAs (<250 μm)—was significantly influenced by the application of CM, RHB, and their interaction (Table 2). The addition of 1% w/w CM resulted in an increase in the MaA proportion from 0.011 to 0.017 and the MeA from 0.330 to 0.385, while the proportion of MiAs decreased from 0.659 to 0.599 (Figure 1a). This shift toward larger aggregates suggests that CM may improve soil structure by enhancing aggregate stability, likely due to the increased organic matter input. Organic materials, such as manure, are known to contribute binding agents—including polysaccharides and decomposed organic residues—that support macroaggregate formation [38,39,40]. Previous studies have demonstrated that manure enhances MaA formation more effectively than inorganic fertilizers [41,42], with similar trends showing increased MaAs and decreased MiAs following organic amendment application [43,44,45]. Although the microbial activity was not assessed in this study, previous research has shown that organic inputs can stimulate microbial processes [46], which contribute to MaA formation through the production of extracellular binding agents and the physical entanglement of soil particles [47].

Table 2.
Summary of analysis of variance (ANOVA) for the effects of cow manure (CM), rice husk biochar (RHB), and their interaction.
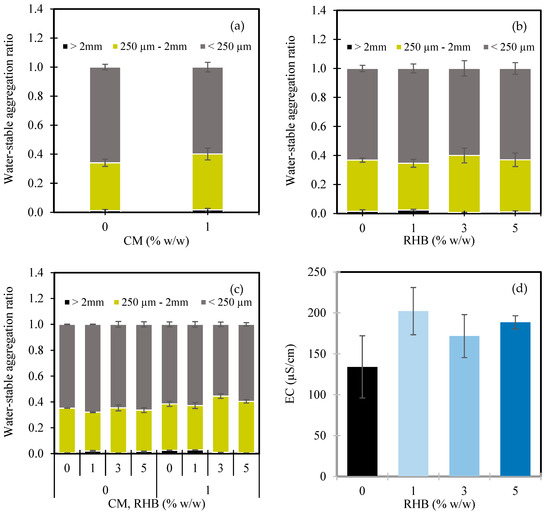
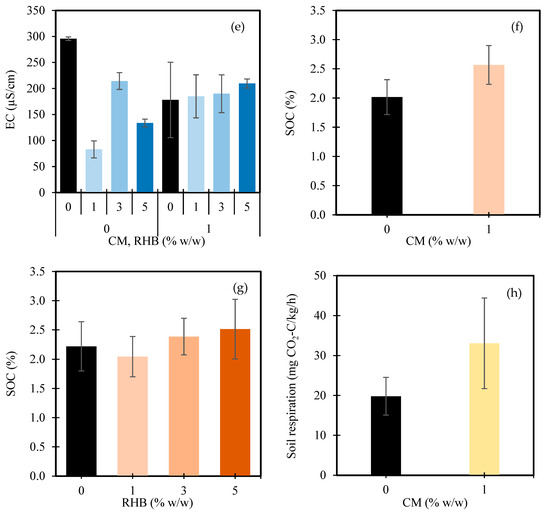
Figure 1.
Water-stable aggregates greater than 2 mm, between 250 µm and 2 mm, and less than 250 µm, as influenced by cow manure (CM) (a), rice husk biochar (RHB) (b), and the interaction between CM and RHB (c); electrical conductivity of soil as influenced by RHB (d) and the interaction between CM and RHB (e); soil organic carbon (SOC) as influenced by CM (f) and RHB (g); and soil respiration as influenced by CM (h). In Figure panels (c,e), the X-axes are grouped by CM application level: the left four bars represent CM at 0% w/w, and the right four bars represent CM at 1% w/w. Within each group, the bars correspond to increasing RHB application rates of 0%, 1%, 3%, and 5% w/w, respectively. Values represent means ± standard deviation.
The RHB application influenced the distribution of the soil aggregate sizes in a variable manner (Figure 1b). The highest MaA proportion (0.024) was observed at 1% w/w RHB, while 3% w/w RHB led to a notable reduction to 0.007. The MeA content peaked at 3% w/w RHB (0.393) and remained elevated at 5% w/w (0.360), compared to the control (0.354). Meanwhile, the MiA proportions increased at 1% w/w RHB (0.654) compared to 0% RHB (0.632), but generally declined at higher RHB rates, indicating improved soil aggregation at low-to-moderate biochar levels. These findings suggest that moderate applications of RHB enhance soil aggregate stability [14,48], whereas higher doses may disrupt particle bonding—possibly due to an excess of fine particles or reduced microbial cohesion. Similar effects have been observed in rice cultivation with wheat straw biochar [49]. However, some studies have reported no significant impact of herbaceous-derived biochar on aggregation in sandy soils [50], and Blanco-Canqui [48] noted that the effects of biochar on aggregation can vary widely depending on the soil type.
The interaction between the CM and RHB further affected the aggregate distribution (Figure 1c). In the absence of CM, increasing the RHB from 0% to 3% w/w raised the MeA proportions and reduced the MiA, reflecting the binding of MiAs into MaAs and improved structure. Under the CM-amended conditions, 3% w/w RHB yielded the highest MeA content (0.436) and the lowest MiA proportion (0.555), suggesting the synergistic effect of both amendments on soil aggregation. The 1% w/w RHB also had the highest MaA proportion in the presence of CM, suggesting that the combination of RHB (at 1–3% w/w) and CM effectively enhances soil structure. The improved soil aggregation likely resulted from the interaction between the biochar and organic matter, which promotes the binding of soil particles and stabilizes larger aggregates, contributing to better aeration, water infiltration, and long-term soil health.
3.1.2. Effects on pH and Electrical Conductivity
The soil pH was not significantly affected by CM, RHB, or their interaction (Table 2), indicating that none of the amendments, whether applied individually or in combination, had a statistically measurable impact on soil pH under the conditions of this experiment. This stability may be attributed to the soil’s buffering capacity and the balanced composition of CM, which contains both acidic components (e.g., NH4+) and basic cations (e.g., Ca2+ and Mg2+) that help maintain pH equilibrium [51]. Consistent with this, Ozlu and Kumar [42] reported that long-term CM application helped maintain neutral soil pH in field conditions. Other studies have also shown that CM can increase pH in acidic-to-neutral soils, suggesting a buffering effect that brings the soil closer to neutrality [52,53,54,55].
While RHB has often been reported to raise soil pH in acidic soils due to its alkaline nature [56,57,58,59], its effect largely depends on the relative pH of the amendment and the initial soil conditions. In this study, the initial soil pH was 6.7, which was comparable to the RHB pH of 6.8, whereas the manure exhibited a substantially higher pH of 8.9. Consequently, no liming effect was observed following the RHB application. The mineral composition of the RHB used in this study was determined using X-ray fluorescence (XRF) spectroscopy and compared with biochars derived from other agricultural byproducts, such as longan wood and corn cobs (Appendix A). The RHB exhibited a markedly high silica (Si) content (35.9%), while its calcium (Ca) and magnesium (Mg) concentrations were relatively low, at 0.6% and 0.4%, respectively. These differences suggest that RHB, being rich in silica but deficient in basic cations, such as Ca and Mg, may have a weaker liming effect and lower buffering capacity than woody or manure-derived biochars. This mineral profile helps explain the minimal pH change observed in the soils treated with RHB in this study. The full XRF elemental profiles and comparative biochar data are provided in Appendix A. These trends are also consistent with earlier studies that have reported the limited liming potential of silica-rich biochars [60]. Moreover, the short duration of the 10-day experiment may have allowed for short-term acidifying processes—such as the release of acidic functional groups, the microbial production of organic acids, and H+ exchange reactions—to predominate. A longer incubation period may be required for the gradual release of the alkaline ash and carbonate compounds in the biochar to exert a more pronounced pH-increasing effect.
The EC, a key indicator of soil salinity and ion availability, was significantly influenced by the RHB and its interaction with the CM (Table 2). In the single-factor analysis, where the EC values were averaged across all CM levels, the EC increased from 134.0 µS/cm in the control (0% w/w RHB) to 202.2 µS/cm at 1% w/w RHB (+50.8%) (Figure 1d). However, it decreased slightly at 3% and 5% w/w RHB, to 171.7 and 188.4 µS/cm, respectively, suggesting a nonlinear response. Interestingly, the interaction analysis revealed more nuanced trends (Figure 1e). The highest EC value (295.7 µS/cm) occurred in the control group with 0% w/w CM and 0% w/w RHB—suggesting an accumulation of salts in the unamended soil. However, when either CM, RHB, or both were applied, the EC values declined. For example, applying 1% w/w RHB alone reduced the EC to 83.0 µS/cm, while for combinations of CM and RHB, it ranged between 178.0 and 209.7 µS/cm. Chen et al. [61] also found a decrease in the EC after aged biochar application. The extent of EC reduction may also be linked to the characteristics of the RHB, as its influence on the EC depends on the feedstock type and pyrolysis conditions [62].
3.1.3. Effects on Soil Organic Carbon
The SOC content was significantly influenced by both the CM and RHB applications, while the interaction between them was not statistically significant (Table 2). The application of 1% w/w CM increased the SOC from 2.0% in the control (0% w/w CM) to 2.6%, representing a 27.2% improvement (Figure 1f). This rise in the SOC is attributed to the direct carbon input from the manure [7,63] and the enhancement of microbial activity that contributes to OM decomposition and stabilization [39,64].
Regarding RHB, a gradual increase in the SOC was observed with higher application rates (Figure 1g). Compared to the control (0% w/w RHB, 2.2%), 1% w/w RHB slightly decreased the SOC to 2.1% (−7.7%), but the SOC content subsequently increased to 2.4% at 3% w/w RHB (+7.7%) and further to 2.5% at 5% w/w RHB (+13.1%). These results suggest that higher doses of RHB are more effective for enhancing the SOC due to the biochar’s high carbon content and its resistance to microbial decomposition, which promotes carbon sequestration in the soil over time [65,66,67].
3.1.4. Effects on Soil Respiration
The soil respiration, as indicated by CO2-C release, was significantly affected by the CM application only (Table 2). The application of 1% w/w CM increased the soil respiration from 19.8 mg CO2-C/kg/h in the control (0% w/w CM) to 33.1 mg CO2-C/kg/h—representing a 67.0% increase (Figure 1h). This substantial rise reflects the enhanced microbial activity driven by the addition of organic substrates in the CM, which serve as a carbon and energy source for soil microorganisms [68]. The moderate C/N ratio (20.2; Table 1) of the raw manure may have contributed to this response by promoting microbial decomposition without causing strong nitrogen immobilization, thereby facilitating both mineralization and CO₂ release. Additional support for this comes from previous findings linking manure application to the activation of hydrolytic enzymes, such as cellobiohydrolase and acetylglucosaminidase, and increased expression of carbon cycle-related functional genes [69]. Furthermore, soil respiration is closely associated with inorganic nitrogen availability, which is also affected by manure amendments [70]. Numerous studies have shown that manure additions result in elevated CO₂ emissions [71] and stimulate microbial respiration, particularly among bacteria and actinomycetes [72].
Although the RHB and the CM × RHB interaction did not significantly affect soil respiration in this study (Table 2), a slight increase was observed with the 1% and 3% w/w RHB applications. This lack of statistical significance may be attributed to the relatively inert nature of biochar [73], which contains less labile carbon compared to fresh organic amendments like cow manure. The observed trends are consistent with the findings of Fatima et al. [74], who reported dynamic changes in soil respiration depending on the biochar application rate and incubation time. In their study, respiration was not significantly affected by 1% and 2% biochar applications during the first 12 days of incubation. Conversely, neutral or negative effects of biochar on soil respiration have also been reported. For example, Han et al. [69] reported no significant effect of applying 20 t/ha biochar combined with mineral fertilizer on soil respiration in a subtropical tea plantation, while Hammerschmiedt et al. [75] similarly found no impact from cattle manure combined with biochar. In addition, Wei et al. [76] reported reduced respiration in biochar-amended soils treated with manure during hormone remediation efforts.
3.1.5. Effects on Soil Nitrate, Phosphorus, and Potassium
The nitrate content in the soils was significantly influenced by both the CM and RHB, although their interaction effect was not significant (Table 2). The CM application at 1% w/w reduced the NO3− content from 18.5 mg/kg in the control (0% w/w CM) to 12.3 mg/kg—a 33.7% decrease (Figure 2a). This reduction aligns with the increase in the shoot and root biomass, suggesting enhanced nitrogen assimilation and utilization efficiency under the nutrient-rich conditions provided by the manure [77]. Similarly, Zhihui et al. [78] observed a reduction in nitrate accumulation in soil with manure application, which concurrently improved the N uptake and crop yield. Similarly, the RHB application showed a dose-dependent impact on the NO3− levels. The highest NO3− content was observed in the control group (0% w/w RHB), at 23.5 mg/kg. Increasing the RHB to 1% w/w led to a marked reduction in the NO3− to 10.3 mg/kg (−56.1%). However, at 3% w/w RHB, the NO3− levels increased slightly to 15.5 mg/kg, before dropping again to 12.2 mg/kg at 5% w/w RHB (−47.9% relative to the control) (Figure 2b). These results suggest that biochar application can reduce the NO3− accumulation in soils, possibly by improving the nitrogen uptake efficiency and reducing NO3− leaching or conversion into less available forms in the soil [79,80].
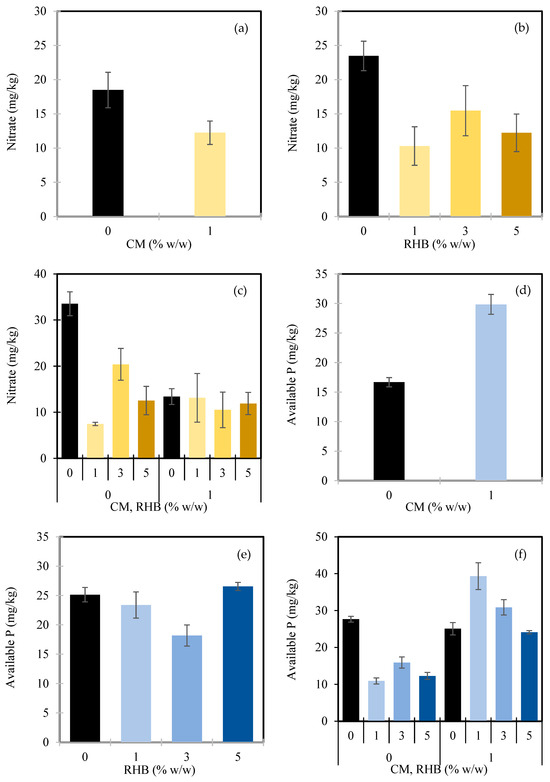
Figure 2.
Nitrate (NO3−) concentrations in soils as influenced by manure (CM) (a), rice husk biochar (RHB) (b), and the interaction between CM and RHB (c). Available phosphorus (P) concentrations as affected by CM (d), RHB (e), and the interaction between CM and RHB (f). In Figure panels (c,f), the X-axes are grouped by CM application level: the left four bars represent CM at 0% w/w, and the right four bars represent CM at 1% w/w. Within each group, the bars correspond to increasing RHB application rates of 0%, 1%, 3%, and 5% w/w, respectively. The values represent the means ± standard deviation.
Regarding the interaction effect, when 1% w/w CM was applied, the NO3− levels were already substantially reduced across all the RHB rates compared to the untreated control (Figure 2c). At 0% w/w RHB, the NO3− content was 13.4 mg/kg, representing a 60.0% reduction from the 0% w/w CM + 0% w/w RHB control. Increasing the RHB to 1%, 3%, and 5% w/w further adjusted the NO3− levels to 13.1 mg/kg, 10.5 mg/kg, and 11.9 mg/kg, respectively. These reductions were relatively modest—ranging from −2.0% to −21.6%—compared to the CM-only treatment, suggesting that the CM alone was primarily responsible for lowering the NO3− accumulation, while the additional RHB provided only minor incremental effects. This finding supports studies that have highlighted the dominant role of organic amendments, like manure, in nitrogen dynamics. Although microbial processes were not directly measured, the significant increase in soil respiration under the CM application (Section 3.1.4) suggests enhanced microbial activity. This may indicate active nitrogen transformations, such as immobilization and denitrification, which are consistent with the findings of Rufino et al. [81], who reported that manure amendments influence nitrogen dynamics via microbial pathways in integrated crop–livestock systems. In contrast, biochar, while improving soil structure and nutrient retention, has a more limited effect on nitrate reduction. RHB likely affects soil properties, such as the cation exchange capacity (CEC) and moisture retention, rather than directly influencing nitrogen cycling [82]. Previous research suggests that biochar can enhance nutrient retention when combined with organic amendments, but the limited impact of RHB on nitrate reduction in this study may depend on the biochar properties or application rates [83].
The available P in the soil was significantly influenced by both the CM and RHB, including their interaction (Table 2). The application of 1% w/w CM markedly increased the available P from 16.7 mg/kg in the control (0% w/w CM) to 29.9 mg/kg—an increase of 78.9% (Figure 2d). This substantial improvement is likely attributed to the phosphorus content in the CM and its stimulation of microbial activity that enhanced phosphorus mineralization [84,85,86]. In contrast, the response to the RHB was nonlinear. Compared to the control (0% w/w RHB), which had an available P content of 25.1 mg/kg, the application of 1% and 3% w/w RHB reduced the available P to 23.4 mg/kg (−7.0%) and 18.2 mg/kg (−27.7%), respectively. However, increasing the RHB to 5% w/w led to a recovery in the available P levels, reaching 26.6 mg/kg—5.6% higher than the control (Figure 2e). These fluctuations suggest that low-to-moderate levels of RHB may temporarily immobilize P due to its high surface area and adsorption properties [87]. At higher rates, however, RHB may improve soil structure and microbial activity, enhancing P availability [88]. The effect of biochar on P retention varies depending on the feedstock types, application rate, and duration [89]. While short-term applications have often shown reduced P availability, longer-term studies (≥12 months) have tended to report neutral or positive effects [90,91,92].
With 1% w/w CM applied, the available P substantially increased across all RHB levels (Figure 2f). At 0% w/w RHB, the P content was 25.1 mg/kg. Adding 1% w/w RHB boosted this to 39.4 mg/kg (+56.9%), while 3% and 5% w/w RHB maintained elevated levels of 30.9 mg/kg (+23.1%) and 24.1 mg/kg (−3.8%), respectively, relative to the 0% w/w RHB + CM treatment. These results indicate that biochar’s impact on P availability is strongly dependent on CM’s presence. In CM-amended soils, biochar appears to enhance the P availability—likely because of the P content in CM and its role in improving soil structure, microbial activity, and nutrient cycling. This is consistent with the findings of Arif et al. [93], who reported an increase in soil P with the application of biochar and organic fertilizer. However, in unamended CM soils, biochar may immobilize phosphorus due to its high adsorption capacity, reducing its availability [94]. Thus, the synergistic effect of CM and moderate levels of RHB is more beneficial for increasing soil’s available P.
The available K content in the soil was not significantly affected by any of the factors (Table 2). This suggests that, under the conditions of this study, neither the CM nor RHB application had a measurable impact on altering the soil’s available K levels. This lack of a significant effect, despite the higher available K content in the CM and RHB, could be attributed to the K uptake by the sunflower sprouts. Young plants may require high amounts of K during the early growth stages [95], which could temporarily reduce soil K levels and mask the effect of added K. Additionally, K in the soil can become fixed to soil particles and soil organic matter, thereby reducing its availability [96].
3.2. Growth Performance of and Biomass Allocation in Sunflower Sprouts
3.2.1. Changes in Germination Rate and Leaf Area
The germination rate of the sunflower sprouts was not significantly affected by the CM, RHB, or their interaction (Table 2). This suggests that the presence or absence of these amendments did not influence the percentage of seeds that successfully germinated under the conditions of this study.
In contrast, the average total leaf area per sprout was significantly influenced by the RHB application (p < 0.05), while the CM and the CM × RHB interaction had no significant effects (Table 2). The leaf area increased progressively with higher RHB application rates (Figure 3a). Compared to the control (0% w/w RHB), which had an average leaf area of 3.30 cm2, the application of 1% w/w RHB increased the leaf area by 20.6%, the 3% w/w RHB led to an 11.8% increase, and the 5% w/w RHB resulted in the highest increase of 43.9%. This trend suggests that RHB can enhance early vegetative growth in sunflower sprouts and nutrient uptake by improving the soil’s physical and chemical properties, such as water retention, aeration, and nutrient availability [97]. These improvements likely support more efficient photosynthesis and cell expansion, resulting in larger leaf development [98]. Similar trends were observed in soybeans [99] and tomatoes and peppers [100] with biochar application. Interestingly, the lower effect observed at 3% w/w RHB compared to 1% w/w was unexpected, and may reflect nonlinear plant responses to the amendment levels or microenvironmental variability during the experiment. Although the interaction between the CM and RHB was not significant in this study, other research has shown that combining organic manure with biochar can increase the leaf area of crops, such as sweet potato [101] and cashew seedlings [102].
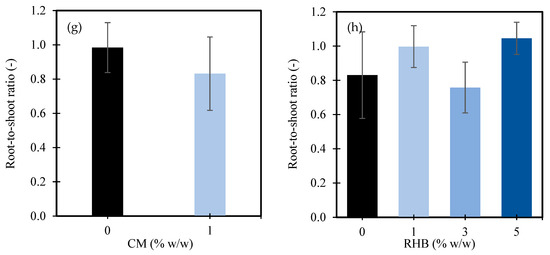
Figure 3.
Average total leaf area per sprout as affected by rice husk biochar (RHB) (a). Shoot fresh biomass as influenced by manure (CM) (b) and the interaction between CM and RHB (c). Root biomass as influenced by CM (d), RHB (e), and the interaction between CM and RHB (f). Root-to-shoot ratio as influenced by CM (g) and RHB (h). In Figure panels (c,f), the X-axes are grouped by CM application level: the left four bars represent CM at 0% w/w, and the right four bars represent CM at 1% w/w. Within each group, the bars correspond to increasing RHB application rates of 0%, 1%, 3%, and 5% w/w, respectively. Values represent means ± standard deviation.
3.2.2. Changes in Plant Biomass and Root-to-Shoot Ratio
The application of the CM had a significant effect on all the biomass-related traits of the sunflower sprouts, including the shoot fresh biomass (p < 0.001), root fresh and dry biomass (p < 0.001), and the root-to-shoot ratio (p < 0.05) (Table 2). In contrast, the RHB significantly influenced only the root dry biomass (p < 0.01) and the root-to-shoot ratio (p < 0.01), while its effects on the shoot and root fresh biomass were not statistically significant. Notably, a significant interaction between the CM and RHB was observed for both the shoot and root fresh biomass (p < 0.05) and root dry biomass (p < 0.001), suggesting that the combined application of these amendments influenced biomass accumulation more than their individual effects.
In terms of the shoot biomass, the application of 1% w/w CM increased the shoot fresh biomass from 27.0 g to 33.0 g, representing a 22.3% improvement over the control (Figure 3b). The interaction between the CM and RHB further highlighted these nuanced effects (Figure 3c and Figure 4). Without CM, increasing the RHB from 0% to 1% w/w resulted in a substantial rise in the shoot biomass from 22.7 g to 29.9 g—a 31.9% increase. However, higher RHB rates of 3% and 5% w/w led to lower shoot biomass values of 27.2 g and 28.1 g, respectively, indicating diminishing returns beyond 1% w/w RHB. Conversely, under 1% w/w CM, the highest shoot biomass was observed with 0% w/w RHB (36.4 g). Adding 1% w/w RHB reduced the biomass to 31.5 g (−13.5%), while 3% and 5% w/w RHB resulted in values of 31.1 g and 32.9 g, respectively. These results suggest that while both the CM and RHB individually enhance shoot growth, their combined effects are not strictly additive. Moderate RHB levels appear to be more beneficial in the absence of CM, whereas CM alone may already provide optimal conditions, with additional RHB contributing limited or slightly negative effects at higher application rates.

Figure 4.
Representative shoot and root growth of sunflower sprouts under 0% and 1% w/w cow manure (CM) combined with 0%, 1%, 3%, and 5% w/w rice husk biochar (RHB) treatments.
Similarly, the application of 1% w/w CM significantly increased the root biomass, with the root fresh biomass rising from 7.0 g to 8.8 g (+25.8%) and the root dry biomass increasing from 1.6 g to 2.2 g (+32.5%) compared to the control (0% w/w CM) (Figure 3d). The observed increases in both the shoot and root biomass with CM application are likely attributed to improvements in the soil structure (Section 3.1.1) and nutrient availability [103], which together enhanced root development and nutrient uptake. Although microbial activity was not directly measured, the significant increase in the soil respiration observed under the CM treatment (Section 3.1.4) suggests elevated biological activity, likely driven by the microbial decomposition of organic substrates. Supporting evidence for this comes from Zhang et al. [104], who showed that organic manure enhanced cotton root growth by stimulating soil enzyme activity and improving nutrient content. Likewise, Ibrahim et al. [105] reported increased root length density in wheat and rice following farm manure application, while Yang et al. [106] found that combining farmyard manure with chemical fertilizer improved the root weight and length density in rice.
The RHB also had a significant effect on the root dry biomass, although the fresh biomass responses were more variable. Increasing the RHB from 0% to 1% w/w improved the root fresh biomass from 7.5 g to 9.1 g (+20.4%) and the dry biomass from 1.8 g to 2.3 g (+24.2%) (Figure 3e), aligning with previous reports of biochar enhancing root growth [107,108]. However, at the higher RHB rates (3% and 5% w/w), the root biomass declined—particularly at 3% w/w RHB, where the root fresh biomass dropped to 3.82 g (−49.2%) and the dry biomass to 1.62 g (−11%). This pattern suggests the presence of a threshold beyond which excessive biochar application may reduce its effectiveness, potentially due to initial nutrient immobilization or changes in soil moisture dynamics [109].
The interaction between the CM and RHB (Figure 3f and Figure 4) revealed more nuanced responses. Without CM, increasing the RHB from 0% to 1% w/w boosted the root fresh biomass from 5.39 g to 8.68 g (+61%) and the dry biomass from 1.08 g to 2.18 g (+101.9%). However, further increases to 3% and 5% w/w led to reductions in the root fresh biomass to 6.95 g (−20%) and 6.94 g (−20%), respectively. In contrast, under 1% w/w CM, the root fresh biomass peaked at 9.65 g with 1% w/w RHB, followed by a slight drop to 9.42 g at 3% RHB, and a further drop to 8.10 g at 5%. The root dry biomass showed a similar trend; it was highest at 2.56 g with 1% w/w RHB, slightly lower at 2.34 g with 3% w/w RHB, and lowest at 2.07 g with 5% w/w RHB. This interaction suggests that combining 1% w/w CM with 1% w/w RHB is optimal for root growth, while higher RHB rates may offset the positive effect of manure. Similar findings were reported by Adekiya et al. [110], who observed increased radish root weight with biochar and poultry manure, and Zhang et al. [104], who found that biochar combined with organic manure improved the root length and area of cotton, though the responses varied by crop stage.
For the root-to-shoot ratio, the application of 1% w/w CM led to a reduction in the root-to-shoot ratio from 0.98 in the control (0% w/w CM) to 0.83—a 15.3% decrease (Figure 3g). This decline indicates that the CM promoted shoot growth more than root development, likely due to its ability to supply readily available nutrients, enhancing the aboveground biomass accumulation and shifting resource allocation toward shoot expansion [84]. In contrast, the RHB showed a nonlinear effect on the root-to-shoot ratio (Figure 3h). At 1% w/w RHB, the ratio increased from 0.83 to 1.00, representing a 20.5% improvement. However, at 3% w/w RHB, the ratio declined to 0.76, a decrease of 8.4% compared to the control. Interestingly, the application of 5% w/w RHB resulted in the highest root-to-shoot ratio of 1.05, marking a 26.5% increase over the control. These results suggest that while low and high RHB levels may enhance root development relative to the shoot biomass—indicating that plants prioritize root development to maximize resources uptake [111]—the allocation of more resources to shoot growth at the intermediate level of 3% is often observed in environments with abundant resources [112], where plants invest in shoot growth, as evidenced by the higher nutrient availability and increased plant shoot growth, but reduced root development in this study.
3.3. Bioactive Compounds in Sunflower Sprouts
3.3.1. Changes in Total Phenolic Content
In the bioactive compound analysis, the 5% w/w RHB treatment was excluded to prioritize the analysis of the lower application rates, allowing us to allocate resources more effectively while still capturing the key trends in the bioactive compound production. The ANOVA results demonstrate that the CM, RHB, and their interaction had a highly significant effect on the TPC of the sunflower sprouts (p < 0.001 for all factors, Table 2). These findings indicate that both the CM and RHB independently influenced the TPC, while their combination produced interdependent effects, suggesting a more complex interaction than simple additive contributions.
When examining the effect of the CM alone, a clear reduction in the TPC was observed with manure application (Figure 5a). At 0% w/w CM, the TPC reached 59.7 mg/100 g FW, while at 1% w/w CM it decreased to 47.2 mg/100 g FW, representing a reduction of approximately 21%. This decline implies that the nutrient-rich conditions provided by the manure may have shifted the plant’s metabolic focus from secondary metabolite production, such as phenolics, toward primary growth processes, suggesting that manure’s role in enhancing phenolic accumulation may be more long-term—improving the soil microbial activity and nutrient cycling rather than directly stimulating biosynthesis during a short cropping cycle. Similar trends were reported in white mugwort, where chicken manure increased the TPC only after multiple harvests [113].
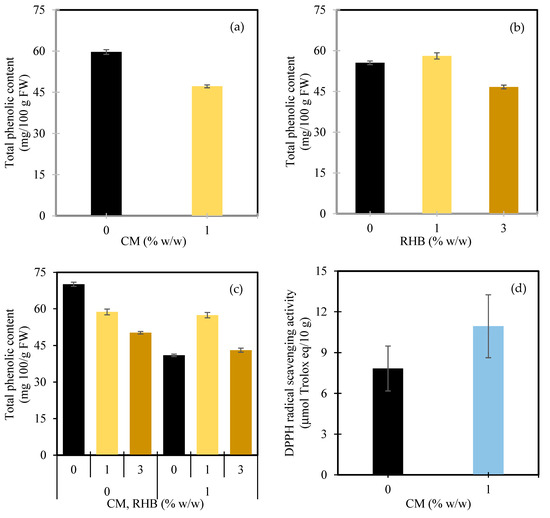
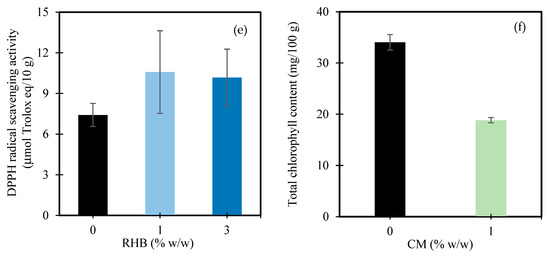
Figure 5.
Total phenolic content (TPC) of sunflower sprouts as affected by cow manure (CM) (a), rice husk biochar (RHB) (b), and the interaction between CM and RHB (c); DPPH radical scavenging activity as influenced by CM (d) and RHB (e); and total chlorophyll content as influenced by CM (f). In Figure panel (c), the X-axes are grouped by CM application level: the left three bars represent CM at 0% w/w, and the right three bars represent CM at 1% w/w. Within each group, the bars correspond to increasing RHB application rates of 0%, 1%, and 3% w/w, respectively. Values represent means ± standard deviation.
Although several studies have reported increased TPC in crops, such as white mugwort [113], lettuce [114], sesame [115], and wild passion fruit [116], following manure application, these findings must be interpreted with caution, as phenolic responses are often species-specific. The observed increases are frequently attributed to an enhanced availability of phenylalanine, a key precursor in the phenylpropanoid pathway [117]. However, when nitrogen levels are high, phenylalanine is directed toward protein synthesis instead of secondary metabolite production, potentially explaining the reduced TPC observed in some manure-treated plants [118]. Despite this, the TPC responses to organic fertilization remain inconsistent. Some studies have found no clear trend [119,120], while others have reported no significant effect [121], underscoring the complexity of phenolic biosynthesis, which is influenced by nutrient availability, microbial interactions, and environmental conditions.
In contrast, the response to the RHB followed a nonlinear trend (Figure 5b). At 0% w/w RHB, the TPC was 55.5 mg/100 g FW. A moderate increase was observed at 1% w/w RHB to 58.1 mg/100 g FW, marking a 4.6% improvement. However, at 3% w/w RHB, the TPC dropped to 46.7 mg/100 g FW, reflecting a 16% decrease compared to the 0% w/w RHB treatment. This response may be attributed to nutrient imbalances, microbial shifts, or altered plant metabolism associated with excess biochar inputs [122]. Such trends have also been reported in other species; for example, Regmi et al. [123] observed reduced polyphenol and flavonoid levels in Viola cornuta with 10–25% biochar, while Yang et al. [106] reported growth benefits at moderate biochar doses (600–900 kg/ha) in tobacco, but adverse effects at higher rates.
While the main effect of RHB suggests a slight increase in the TPC at the 1% w/w application, it is important to note that this reflects an average across both CM levels. The interaction analysis reveals opposing trends depending on manure’s presence—indicating that RHB’s effect on phenolic accumulation is strongly influenced by whether CM is applied. This highlights the need to interpret the main effects cautiously when significant interactions are present.
The interaction between the CM and RHB revealed even more nuanced effects (Figure 5c). Without CM, the TPC decreased steadily with increasing RHB—from 70.1 mg/100 g FW at 0% w/w RHB, to 58.7 mg/100 g at 1% w/w RHB (−16.2%), and to 50.2 mg/100 g at 3% w/w RHB (−28.4%). However, under 1% w/w CM, the trend was reversed. The lowest TPC was recorded with 0% w/w RHB (41.0 mg/100 g), but adding 1% w/w RHB significantly increased the TPC to 57.4 mg/100 g FW—an improvement of approximately 40.1%. Even at 3% w/w RHB, the TPC slightly increased to 43.1 mg/100 g FW (+5.2%) compared to the manure-only treatment. Although CM alone tends to reduce the TPC, its combination with a moderate rate of RHB—particularly at 1% w/w—can counteract this decline and significantly enhance phenolic accumulation. This suggests that manure may alter the plant’s response to biochar, possibly by influencing nutrient dynamics, microbial interactions, or physiological stress responses. While the organic inputs used in previous studies differ from those in the present experiment, the observed synergy between CM and RHB is consistent with the broader findings in the literature. For instance, Sharma et al. [124] reported that combining biochar with vermicompost and jaggery enhanced the TPC in guava, likely by stimulating microbial activity and improving nutrient availability. Similarly, Antonious [125] found that CM improved the nutrient profile of sweet potato, including the total phenolics, whereas biochar alone had minimal effect. These studies support the general concept that biochar, when co-applied with biologically active organic inputs, can enhance plants’ secondary metabolite production. However, amendment-specific responses and crop types must be carefully considered when interpreting such outcomes.
3.3.2. Changes in Antioxidant Activity
The DPPH radical scavenging activity of the sunflower sprouts was significantly influenced by both the CM (p < 0.001) and RHB (p < 0.01), while their interaction was not statistically significant (Table 2). Specifically, the application of 1% w/w CM resulted in a marked increase in antioxidant activity, rising from 7.83 μmol Trolox eq/10 g in the control to 10.94 μmol Trolox eq/10 g—an improvement of approximately 39.7% (Figure 5d). This observation aligns with broader evidence suggesting that organic amendments, such as cow, cattle, and quail manure, can enhance the antioxidant capacity in plants, as reflected by the increased DPPH and oxygen radical absorbance capacity (ORAC) values [126,127]. While these previous studies were conducted on different crop species, they support the general mechanism by which nutrient-rich organic inputs improve soil fertility, enhance nutrient availability, and potentially stimulate microbial activity—factors known to contribute to antioxidant biosynthesis and plant resilience [128]. Additionally, it has been proposed that such amendments may alter the soil nutrient dynamics or microbial communities in ways that induce mild physiological stress, thereby triggering antioxidant defense responses [129]. In the current study, the observed DPPH increase under the manure and biochar treatments in the sunflower sprouts may reflect similar processes, although species-specific responses and environmental conditions must be considered.
Interestingly, while the CM significantly increased the DPPH radical scavenging activity, it concurrently reduced the TPC. This apparent discrepancy may be explained by the distinct metabolic pathways involved. Manure creates nutrient-rich, low-stress soil conditions that tend to favor the production of non-phenolic antioxidants, such as ascorbic acid and glutathione, which are highly effective in DPPH assays [130,131]. As a result, the overall antioxidant activity may increase even when the phenolic accumulation remains limited. This observation aligns with findings by Ibrahim and Jaafar [132], who reported that DPPH activity in Labisia pumila was not solely dependent on the phenolic content, suggesting that the TPC alone may not be a comprehensive indicator of antioxidant capacity.
Without the RHB, the value was 7.41 μmol Trolox eq/10 g. With 1% w/w RHB, the activity increased to 10.58 μmol Trolox eq/10 g, representing a 42.8% increase (Figure 5e). At 3% w/w RHB, the value slightly declined to 10.17 μmol Trolox eq/10 g, though it still reflected a 37.3% improvement over the control. These results suggest that biochar application, particularly at moderate rates, may enhance antioxidant potential in sunflower sprouts. While species-specific responses can vary, previous studies of other crops under stress conditions have shown that biochar can enhance antioxidant responses by improving soil health, reducing oxidative stress, and supporting plant metabolism. For example, Hasnain et al. [133] reported improved antioxidant enzyme activity in plants under abiotic stress following biochar application. Similarly, Jiang et al. [134] and Ghassemi-Golezani and Rahimzadeh [135] observed enhanced antioxidant performance in Mentha and dill, respectively, in response to biochar. Though these examples involve different species, they illustrate the potential underlying mechanisms—such as improved nutrient status or reduced environmental stress—that may also have contributed to the observed effects in the sunflower sprouts. Further studies focusing on species-specific mechanisms are warranted to confirm these interactions.
3.3.3. Changes in Total Chlorophyll Content
The total chlorophyll content of the sunflower sprouts was significantly influenced by the CM application, while the RHB and its interaction with CM showed no significant effects (Table 2). Specifically, the chlorophyll content decreased from 34.0 mg/100 g FW in the control (0% w/w CM) to 18.8 mg/100 g FW under 1% w/w CM—representing a 44.7% reduction (Figure 5f). Although similar reductions in chlorophyll content have been reported in other plant species, these findings are consistent with those of Sharma et al. [136], who observed reduced chlorophyll levels in kiwifruit vines treated with 100% poultry manure—suggesting a shift in plant metabolism under nutrient-rich conditions, where resources are redirected toward growth and biomass accumulation rather than pigment synthesis. In contrast, Thepsilvisut et al. [113] reported increased chlorophyll a + b content in white mugwort under higher chicken manure applications from the third harvest onward, attributing the improvement to enhanced photosynthesis and cumulative yield benefits over time.
Although the RHB and its interaction with CM had no significant effect on the chlorophyll content in this study, previous research has reported mixed results regarding the impact of biochar. Liu et al. [137] found that combining biochar with fertilizers increased the chlorophyll content in soybeans by 16.6%. Similarly, Yang and Zhang [138] observed a 26.4% increase in cornflower chlorophyll content when 15% biochar and 10% CM were added to compost. In contrast, Ghaedi et al. [139] reported a decline in chlorophyll content in safflower under water stress when biochar was applied alone. Cong et al. [140] also noted that while a moderate level of biochar improved chlorophyll levels in maize, excessive application rates (>12 t/ha) led to declines in both chlorophyll content and overall plant growth. These findings suggest that biochar’s influence on chlorophyll is largely indirect—improving soil conditions and microbial activity rather than directly supplying nutrients for chlorophyll synthesis.
3.4. Principal Component Analysis (PCA) of Soil and Plant Responses to Amendments
A PCA was conducted to evaluate the contributions of various plant and soil indices to the variability among the treatments involving the CM and RHB applications (Figure 6a,b). The first two principal components, PC1 (48.4%) and PC2 (21.3%), accounted for a cumulative variance of 69.7%. The PCA biplot for CM (Figure 6a) clearly differentiates between the manure-free (CM 0% w/w) and manure-amended (CM 1% w/w) treatments. The treatments without manure are clustered predominantly on the left side of the plot, and are strongly associated with a higher total chlorophyll content, TPC, root-to-shoot ratio, and MiAs. This indicates that the plants grown without manure allocated resources more toward stress adaptation mechanisms and the production of secondary metabolites [113,136].
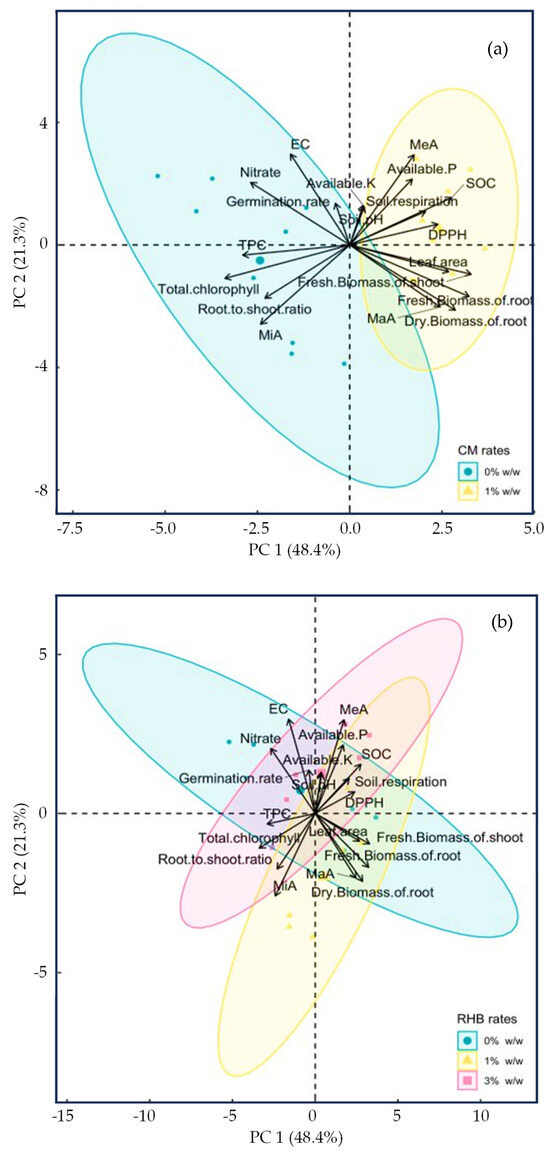
Figure 6.
Principal Component Analysis (PCA) biplot showing the effect of cow manure (CM) (a) and rice husk biochar (RHB) (b) applications on soil and plant parameters. The variation in point sizes is a rendering artifact and does not represent any variable or data value.
Conversely, the manure-amended treatments form a distinct cluster on the right side of the plot, showing strong positive correlations with the SOC, available P, soil respiration, pH, leaf area, DPPH activity, and biomass parameters, including the fresh shoot biomass and fresh and dry root biomass. This pattern supports the notion that manure application enhances soil fertility, OM accumulation, microbial activity, and overall plant growth [126,127,141]. Parameters, such as EC, NO3− content, available K, and germination rate, are positioned centrally on the PCA plot, indicating limited differentiation between the manure treatments. Additionally, a clear trade-off is evident between the growth-related parameters and stress–response indicators, as the manure-amended treatments exhibited reduced total chlorophyll content and TPC. This emphasizes that under the nutrient-rich conditions provided by manure amendments, plants prioritize biomass accumulation at the expense of stress-induced metabolite production [128,142]. These insights also highlight the potential of livestock-derived organic materials—when applied appropriately—to serve not only as a nutrient source but also as a sustainable soil conditioner that supports circular economy models in agriculture.
The PCA biplot for RHB (Figure 6b) illustrates distinct shifts in the plant and soil characteristics associated with varying biochar application rates (0%, 1%, and 3% w/w). The treatments without biochar (0% w/w RHB) are clustered distinctly on the left side of the PCA plot and show strong correlations with a higher total chlorophyll content, TPC, root-to-shoot ratio, and MiAs. This suggests that in the absence of RHB, plants rely more on stress adaptation mechanisms, such as chlorophyll retention and antioxidant production, likely due to lower soil fertility and reduced nutrient-buffering capacity [143].
The treatments with biochar at 1% and 3% w/w rates progressively shift toward the right side of the PCA plot, exhibiting stronger correlations with enhanced SOC, available P, soil respiration, leaf area, biomass parameters (fresh shoot biomass, fresh root biomass, and dry root biomass), and DPPH antioxidant activity. However, the broader dispersion of the data points with 3% w/w RHB suggests greater variability in the plant and soil responses, possibly due to complex interactions involving nutrient dynamics or microbial activity [144].
Similar to the CM treatments, parameters such as EC, NO3− content, available K, germination rate, and soil pH, display limited differentiation among the biochar treatments. This suggests that these parameters are relatively insensitive or exhibit variable responses to biochar amendments. Additionally, the PCA results clearly indicate that while RHB application (especially at rates of 1% and 3% w/w) significantly enhances long-term soil health and biomass productivity, its effect on the TPC secondary metabolite accumulation and total chlorophyll content is relatively weak, as these parameters are more closely associated with biochar-free treatments (0% w/w RHB). This implies that biochar’s influence on secondary metabolites and chlorophyll content is likely dependent on factors such as biochar properties, soil conditions, and specific plant species [139,145]. From a resource management perspective, the use of RHB not only valorizes underutilized agricultural byproducts but also improves soil carbon storage and nutrient dynamics—demonstrating how biochar application contributes to low-emission agriculture, reduced material losses, and enhanced resource circularity.
4. Conclusions
This study highlights the potential of CM and RHB—both sourced from agricultural byproducts—as effective soil amendments that improve soil health, support plant growth, and influence bioactive compound accumulation in sunflower sprouts. The application of 1% w/w CM enhanced the SOC, aggregate stability, and soil respiration, which translated into increased shoot and root biomass. However, this nutrient-rich amendment also led to a reduction in chlorophyll and the total phenolic content, reflecting a trade-off between growth and antioxidant accumulation. The RHB, particularly at 1–3%, improved the soil structure, SOC, and antioxidant activity, while it reduced nitrate accumulation. Nonetheless, its excessive application (5% w/w) impaired root development, underscoring the importance of dose optimization. Based on these findings, the co-application of 1% w/w CM and 1% w/w RHB is recommended as an effective and practical strategy for enhancing soil quality and plant bioactive compounds.
Although not consistently synergistic, their combined use highlights the importance of balanced amendment strategies tailored to specific soil and crop requirements. Both CM and RHB are derived from underutilized agricultural residues, making them suitable for integration into circular economy practices that promote resource efficiency, reduce agricultural waste, and minimize dependence on synthetic inputs. In short-cycle crop systems, such as sunflower sprouts, their application offers a practical approach to improving soil structure and nutrient management within a limited timeframe.
Nevertheless, the short duration of this experiment limits the interpretation of the long-term effects, particularly regarding organic matter transformation and sustained nutrient availability. To strengthen the evidence base, future studies should include long-term, field-scale experiments to assess the cumulative impacts of organic amendments over time. Incorporating a life cycle assessment (LCA) will also be essential to evaluate the environmental costs and benefits associated with the production and application of materials, such as biochar and manure. Although microbial processes were not directly measured, the observed increase in soil respiration indicates elevated microbial activity. Future research should include direct assessments of microbial communities and functions to validate the mechanisms inferred from the respiration data and enhance the understanding of soil–plant–microbe interactions. Furthermore, evaluating the economic feasibility and adoption potential will be crucial for supporting the practical implementation of sustainable soil amendment strategies in sustainable agriculture.
Author Contributions
Funding acquisition, P.K.; methodology, T.R. and T.B.; formal analysis, T.R.; investigation, T.R. and P.U.; writing—original draft preparation, T.R. and P.U.; writing—review and editing, T.B. and P.K.; supervision, P.K. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financially supported by the Fundamental Fund and the Targeted Research Fund, with additional support from Chiang Mai University. The postdoctoral fellowships awarded to Thidarat Rupngam and Patchimaporn Udomkun are gratefully acknowledged.
Data Availability Statement
The datasets used and/or analyzed in the current study are available from the corresponding author upon reasonable request.
Acknowledgments
We would like to acknowledge all support from Chaing Mai University.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A
Elemental Composition Analysis
The elemental composition analysis was carried out through X-ray fluorescence (XRF) (Malvern Panalytical Ltd., Malvern, UK).

Table A1.
Chemical composition of rice husk biochar (RHB), longan wood biochar (LWB), and corn-cob biochar (CCB).
Table A1.
Chemical composition of rice husk biochar (RHB), longan wood biochar (LWB), and corn-cob biochar (CCB).
| Element | RHB (%) 1 | LWB (%) | CCB (%) |
|---|---|---|---|
| Oxygen (O) | 48.45 | 39.04 | 37.85 |
| Nitrogen (N) | 4.08 | 28.01 | 31.45 |
| Carbon (C) | 3.50 | 23.02 | 24.15 |
| Calcium (Ca) | 0.58 | 3.34 | 0.04 |
| Hydrogen (H) | 0.29 | 2.17 | 2.2 |
| Potassium (K) | 3.69 | 2.8 | 3.2 |
| Phosphorus (P) | 0.89 | 0.43 | 0.15 |
| Chlorine (Cl) | 1.54 | 0.15 | 0.15 |
| Magnesium (Mg) | 0.37 | 0.87 | 0.11 |
| Sulfur (S) | 0.18 | 0.08 | 0.03 |
| Silicon (Si) | 35.90 | 0.03 | 0.59 |
| Sodium (Na) | 0.03 | 0.03 | 0.01 |
| Iron (Fe) | 0.18 | 0.01 | 0.05 |
| Aluminum (Al) | 0.10 | 0.01 | 0.01 |
| Copper (Cu) | ND 2 | 0.01 | 0.01 |
| Strontium (Sr) | ND | 0 | ND |
| Titanium (Ti) | 0.01 | ND | ND |
| Manganese (Mn) | 0.14 | ND | ND |
| Zinc (Zn) | 0.05 | ND | 0.01 |
| Bromine (Br) | ND | ND | 0.00 |
| Rubidium (Rb) | 0.10 | 0.00 | 0.00 |
| Chromium (Cr) | 0.01 | ND | ND |
1 Mean range from three replications. 2 ND = not detected.
References
- Funakawa, S.; Yanai, J.; Hayashi, Y.; Hayashi, T.; Watanabe, T.; Noichana, C.; Panitkasate, T.; Katawatin, R.; Kosaki, T.; Natawa, E. Soil organic matter dynamics in a sloped sandy cropland of Northeast Thailand with special reference to the spatial distribution of soil properties. Jpn. J. Trop. Agric. 2006, 50, 199–207. [Google Scholar] [CrossRef]
- Šimanský, V.; Juriga, M.; Jonczak, J.; Uzarowicz, L.; Stępień, W. How relationships between soil organic matter parameters and soil structure characteristics are affected by the long-term fertilization of a sandy soil. Geoderma 2019, 342, 75–84. [Google Scholar] [CrossRef]
- Ding, Y.; Gao, X.; Shu, D.; Siddique, K.H.M.; Song, X.; Wu, P.; Li, C.; Zhao, X. Enhancing soil health and nutrient cycling through soil amendments: Improving the synergy of bacteria and fungi. Sci. Total Environ. 2024, 923, 171332. [Google Scholar] [CrossRef]
- Guo, X.X.; Liu, H.T.; Zhang, J. The role of biochar in organic waste composting and soil improvement: A review. Waste Manag. 2020, 102, 884–899. [Google Scholar] [CrossRef] [PubMed]
- Lal, R. Soil carbon sequestration to mitigate climate change. Geoderma 2004, 123, 1–22. [Google Scholar] [CrossRef]
- Akhtar, M.; Malik, A. Roles of organic soil amendments and soil organisms in the biological control of plant-parasitic nematodes: A review. Bioresour. Technol. 2000, 74, 35–47. [Google Scholar] [CrossRef]
- Gross, A.; Glaser, B. Meta-analysis on how manure application changes soil organic carbon storage. Sci. Rep. 2021, 11, 5516. [Google Scholar] [CrossRef]
- Dunjana, N.; Nyamugafata, P.; Shumba, A.; Nyamangara, J.; Zingore, S. Effects of cattle manure on selected soil physical properties of smallholder farms on two soils of Murewa, Zimbabwe. Soil Use Manag. 2012, 28, 221–228. [Google Scholar] [CrossRef]
- Mustafa, A.; Hu, X.; Abrar, M.M.; Shah, S.A.A.; Nan, S.; Saeed, Q.; Kamran, M.; Naveed, M.; Conde-Cid, M.; Hongjun, G.; et al. Long-term fertilization enhanced carbon mineralization and maize biomass through physical protection of organic carbon in fractions under continuous maize cropping. Appl. Soil Ecol. 2021, 165, 103971. [Google Scholar] [CrossRef]
- Hammerschmiedt, T.; Holatko, J.; Pecina, V.; Huska, D.; Latal, O.; Kintl, A.; Radziemska, M.; Muhammad, S.; Gusiatin, Z.M.; Kolackova, M.; et al. Assessing the potential of biochar aged by humic substances to enhance plant growth and soil biological activity. Chem. Biol. Technol. Agric. 2021, 8, 46. [Google Scholar] [CrossRef]
- Rayne, N.; Aula, L. Livestock manure and the impacts on soil health: A review. Soil Syst. 2020, 4, 64. [Google Scholar] [CrossRef]
- Marques-dos-Santos, C.; Serra, J.; Attard, G.; Marchaim, U.; Calvet, S.; Amon, B. Available technical options for manure management in environmentally friendly and circular livestock production. In Technology for Environmentally Friendly Livestock Production; Springer International Publishing: Cham, Switzerland, 2023; pp. 147–176. [Google Scholar] [CrossRef]
- Linam, F.; Limmer, M.A.; Ebling, A.M.; Seyfferth, A.L. Rice husk and husk biochar soil amendments store soil carbon while water management controls dissolved organic matter chemistry in well-weathered soil. J. Environ. Manag. 2023, 339, 117936. [Google Scholar] [CrossRef]
- Lehmann, J.; Rillig, M.C.; Thies, J.; Masiello, C.A.; Hockaday, W.C.; Crowley, D. Biochar effects on soil biota—A review. Soil Biol. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Supriyadi, S.; Widjajani, B.W.; Murniyanto, E. The effect of rice husk biochar and cow manure on some soil characteristics, N and P uptake and plant growth of soybean in Alfisol. J. Trop. Soils 2022, 27, 59–66. [Google Scholar] [CrossRef]
- Oni, B.A.; Oziegbe, O.; Olawole, O.O. Significance of biochar application to the environment and economy. Ann. Agric. Sci. 2019, 64, 222–236. [Google Scholar] [CrossRef]
- Antonious, G.F. Duality of biochar and organic manure co-composting on soil heavy metals and enzymes activity. Appl. Sci. 2025, 15, 3031. [Google Scholar] [CrossRef]
- Tazebew, E.; Addisu, S.; Bekele, E.; Alemu, A.; Belay, B.; Sato, S. Sustainable soil health and agricultural productivity with biochar-based indigenous organic fertilizers in acidic soils: Insights from Northwestern Highlands of Ethiopia. Discov. Sustain. 2024, 5, 205. [Google Scholar] [CrossRef]
- Yao, R.; Li, H.; Zhu, W.; Yang, J.; Wang, X.; Yin, C.; Jing, Y.; Chen, Q.; Xie, W. Biochar and potassium humate shift the migration, transformation and redistribution of urea-N in salt-affected soil under drip fertigation: Soil column and incubation experiments. Irrig. Sci. 2022, 40, 267–282. [Google Scholar] [CrossRef]
- Agegnehu, G.; Srivastava, A.K.; Bird, M.I. The role of biochar and biochar-compost in improving soil quality and crop performance: A review. Appl. Soil Ecol. 2017, 119, 156–170. [Google Scholar] [CrossRef]
- Banik, C.; Koziel, J.A.; De, M.; Bonds, D.; Chen, B.; Singh, A.; Licht, M.A. Biochar-swine manure impact on soil nutrients and carbon under controlled leaching experiment using a Midwestern Mollisols. Front. Environ. Sci. 2021, 9, 609621. [Google Scholar] [CrossRef]
- Brtnicky, M.; Datta, R.; Holatko, J.; Bielska, L.; Gusiatin, Z.M.; Kucerik, J.; Hammerschmiedt, T.; Danish, S.; Radziemska, M.; Mravcova, L.; et al. A critical review of the possible adverse effects of biochar in the soil environment. Sci. Total Environ. 2021, 796, 148756. [Google Scholar] [CrossRef] [PubMed]
- Agyarko-Mintah, E.; Cowie, A.; Singh, B.P.; Joseph, S.; Zwieten, L.V.; Cowie, A.; Harden, S.; Smillie, R. Biochar increases nitrogen retention and lowers greenhouse gas emissions when added to composting poultry litter. Waste Manag. 2017, 61, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Zarezadeh, S.; Zheng, Y.; Jenkins, S.N.; Mercer, G.D.; Moheimani, N.R.; Singh, P.; Mickan, B.S. Sustainable soil management in agriculture under drought stress: Utilising waste-derived organic soil amendments and beneficial impacts on soil bacterial processes. Appl. Soil Ecol. 2025, 206, 105870. [Google Scholar] [CrossRef]
- Bastida, F.; Torres, I.F.; Hernández, T.; García, C. The impacts of organic amendments: Do they confer stability against drought on the soil microbial community? Soil Biol. Biochem. 2017, 113, 173–183. [Google Scholar] [CrossRef]
- Sun, Y.; Tao, C.; Deng, X.; Liu, H.; Shen, Z.; Liu, Y.; Li, R.; Shen, Q.; Geisen, S. Organic fertilization enhances the resistance and resilience of soil microbial communities under extreme drought. J. Adv. Res. 2023, 47, 1–12. [Google Scholar] [CrossRef]
- Fichtner, T.; Goersmeyer, N.; Stefan, C. Influence of soil pore system properties on the degradation rates of organic substances during soil aquifer treatment (SAT). Appl. Sci. 2019, 9, 496. [Google Scholar] [CrossRef]
- Kroetsch, D.; Wang, C. Particle size distribution. Soil Sampl. Methods Anal. 2008, 2, 713–725. [Google Scholar] [CrossRef]
- Hendershot, W.H.; Lalande, H.; Duquette, M. Soil reaction and exchangeable acidity. In Soil Sampling and Methods of Analysis; CRC Press: Boca Raton, FL, USA, 1993. [Google Scholar] [CrossRef]
- Motsara, M.R.; Roy, R.N. Guide to Laboratory Establishment for Plant Nutrient Analysis; FAO Fertilizer and Plant Nutrition Bulletin, 19; FAO: Rome, Italy, 2008; Available online: https://www.fao.org/4/i0131e/i0131e00.htm (accessed on 13 April 2025).
- Smallholder Agriculture. Soil Health Evaluation Manual (Version 6.4). 2020. Available online: https://smallholder-sha.org/wp-content/uploads/2020/08/soiltoolkitmanual_sv6.4_edits_8_2020_pom_rewrite_changeaccepted.pdf (accessed on 27 December 2024).
- USDA Agricultural Research Service. Soil Aggregate Analysis. Available online: https://www.ars.usda.gov/ARSUserFiles/30640500/Glomalin/Soil%20aggregate%20analysis.pdf (accessed on 27 December 2024).
- Page, A.L.; Miller, R.H.; Keeney, D.R. (Eds.) Methods of Soil Analysis. Part 2: Chemical and Microbiological Properties, 2nd ed.; Agronomy Monograph No. 9, Part 2; Front Matter—1982—Agronomy Monographs—Wiley Online Library; American Society of Agronomy: Madison, WI, USA, 1982. [Google Scholar]
- Goyne, P.J.; Woodruff, D.R.; Churchett, J.D. Environmental causes of yield variation in rain grown sunflower in Central Queensland. Aust. J. Exp. Agric. 1978, 18, 129–134. [Google Scholar] [CrossRef]
- Deng, G.F.; Lin, X.; Xu, X.R.; Gao, L.L.; Xie, J.F.; Li, H.B. Antioxidant capacities and total phenolic contents of 56 vegetables. J. Funct. Foods 2013, 5, 260–266. [Google Scholar] [CrossRef]
- Ghafoor, K.; Ozcan, M.M.; AL-Juhaimi, F.; Babiker, E.E.; Fadimu, G.J. Changes in quality, bioactive compounds, fatty acids, tocopherols, and phenolic composition in oven- and microwave-roasted poppy seeds and oil. LWT Food Sci. Technol. 2019, 99, 490–496. [Google Scholar] [CrossRef]
- Barua, U.; Das, R.P.; Gogoi, B. Chlorophyll estimation in some minor fruits of Assam. Ecol. Environ. Conserv. 2016, 22, 1787–1789. [Google Scholar] [CrossRef]
- Upadhyay, S.K.; Srivastava, A.K.; Rajput, V.D.; Chauhan, P.K.; Bhojiya, A.A.; Jain, D.; Chaubey, G.; Dwivedi, P.; Sharma, B.; Minkina, T. Root exudates: Mechanistic insight of plant growth promoting rhizobacteria for sustainable crop production. Front. Microbiol. 2022, 13, 916488. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hu, N.; Ge, T.; Kuzyakov, Y.; Wang, Z.L.; Li, Z.; Tang, Z.; Chen, Y.; Wu, C.; Lou, Y. Soil aggregation regulates distributions of carbon, microbial community and enzyme activities after 23-year manure amendment. Appl. Soil Ecol. 2017, 111, 65–72. [Google Scholar] [CrossRef]
- Lehmann, J.; Kleber, M. The contentious nature of soil organic matter. Nature 2015, 528, 60–68. [Google Scholar] [CrossRef]
- Zhang, J.; Chi, F.; Wei, D.; Zhou, B.; Cai, S.; Li, Y.; Kuang, E.; Sun, L.; Li, L.J. Impacts of long-term fertilization on the molecular structure of humic acid and organic carbon content in soil aggregates in black soil. Sci. Rep. 2019, 9, 11908. [Google Scholar] [CrossRef] [PubMed]
- Ozlu, E.; Kumar, S. Response of soil organic carbon, pH, electrical conductivity, and water stable aggregates to long-term annual manure and inorganic fertilizer. Soil Sci. Soc. Am. J. 2018, 82, 1243–1251. [Google Scholar] [CrossRef]
- Mikha, M.M.; Hergert, G.W.; Benjamin, J.G.; Jabro, J.D.; Nielsen, R.A. Long-term manure impacts on soil aggregates and aggregate-associated carbon and nitrogen. Soil Sci. Soc. Am. J. 2015, 79, 626–636. [Google Scholar] [CrossRef]
- Yu, H.; Ding, W.; Luo, J.; Geng, R.; Cai, Z. Long-term application of organic manure and mineral fertilizers on aggregation and aggregate-associated carbon in a sandy loam soil. Soil Tillage Res. 2012, 124, 170–177. [Google Scholar] [CrossRef]
- Mikha, M.M.; Rice, C.W. Tillage and manure effects on soil and aggregate-associated carbon and nitrogen. Soil Sci. Soc. Am. J. 2004, 68, 809–816. [Google Scholar] [CrossRef]
- Mohammadi, K.; Heidari, G.; Khalesro, S.; Sohrabi, Y. Soil management, microorganisms and organic matter interactions: A review. Afr. J. Biotechnol. 2011, 10, 19840. [Google Scholar] [CrossRef]
- Verchot, L.V.; Dutaur, L.; Shepherd, K.D.; Albrecht, A. Organic matter stabilization in soil aggregates: Understanding the biogeochemical mechanisms that determine the fate of carbon inputs in soils. Geoderma 2011, 161, 182–193. [Google Scholar] [CrossRef]
- Blanco-Canqui, H. Biochar and soil physical properties. Soil Sci. Soc. Am. J. 2017, 81, 687–711. [Google Scholar] [CrossRef]
- Abulaiti, A.; She, D.; Liu, Z.; Sun, X.; Wang, H. Application of biochar and polyacrylamide to revitalize coastal saline soil quality to improve rice growth. Environ. Sci. Pollut. Res. 2023, 30, 18731–18747. [Google Scholar] [CrossRef]
- Jeffery, S.; Meinders, M.B.; Stoof, C.R.; Bezemer, T.M.; van de Voorde, T.F.; Mommer, L.; van Groenigen, J.W. Biochar application does not improve the soil hydrological function of a sandy soil. Geoderma 2015, 251, 47–54. [Google Scholar] [CrossRef]
- Vanotti, M.B.; García-González, M.C.; Szögi, A.A.; Harrison, J.H.; Smith, W.B.; Moral, R. Removing and recovering nitrogen and phosphorus from animal manure. Anim. Manure Prod. Charact. Environ. Concerns Manag. 2020, 67, 275–321. [Google Scholar] [CrossRef]
- Van Dang, L.; Ngoc, N.P.; Hung, N.N. Soil quality and pomelo productivity as affected by chicken manure and cow dung. Sci. World J. 2021, 2021, 6289695. [Google Scholar] [CrossRef] [PubMed]
- Uzoma, K.C.; Inoue, M.; Andry, H.; Fujimaki, H.; Zahoor, A.; Nishihara, E. Effect of cow manure biochar on maize productivity under sandy soil condition. Soil Use Manag. 2011, 27, 205–212. [Google Scholar] [CrossRef]
- Ewulo, B.S. Effect of poultry dung and cattle manure on chemical properties of clay and sandy clay loam soil. J. Anim. Vet. Adv. 2005, 4, 839–841. [Google Scholar]
- Whalen, J.K.; Chang, C.; Clayton, G.W.; Carefoot, J.P. Cattle manure amendments can increase the pH of acid soils. Soil Sci. Soc. Am. J. 2000, 64, 962–966. [Google Scholar] [CrossRef]
- Da Silva Mendes, J.; Fernandes, J.D.; Chaves, L.H.G.; Guerra, H.O.C.; Tito, G.A.; de Brito Chaves, I. Chemical and physical changes of soil amended with biochar. Water Air Soil Pollut. 2021, 232, 338. [Google Scholar] [CrossRef]
- Ghorbani, M.; Asadi, H.; Abrishamkesh, S. Effects of rice husk biochar on selected soil properties and nitrate leaching in loamy sand and clay soil. Int. Soil Water Conserv. Res. 2019, 7, 258–265. [Google Scholar] [CrossRef]
- Oladele, S.O. Changes in physicochemical properties and quality index of an Alfisol after three years of rice husk biochar amendment in rainfed rice–Maize cropping sequence. Geoderma 2019, 353, 359–371. [Google Scholar] [CrossRef]
- Abrishamkesh, S.; Gorji, M.; Asadi, H.; Bagheri–Marandi, G.H.; Pourbabaee, A.A. Effects of rice husk biochar application on the properties of alkaline soil and lentil growth. Plant Soil Environ. 2015, 61, 475–482. [Google Scholar] [CrossRef]
- Karam, D.S.; Nagabovanalli, P.; Rajoo, K.S.; Ishak, C.F.; Abdu, A.; Rosli, Z.; Muharam, F.M.; Zulperi, D. An overview on the preparation of rice husk biochar, factors affecting its properties, and its agriculture application. J. Saudi Soc. Agric. Sci. 2022, 21, 149–159. [Google Scholar] [CrossRef]
- Chen, X.; Lewis, S.; Heal, K.V.; Lin, Q.; Sohi, S.P. Biochar engineering and ageing influence the spatiotemporal dynamics of soil pH in the charosphere. Geoderma 2021, 386, 114919. [Google Scholar] [CrossRef]
- Asadi, H.; Ghorbani, M.; Rezaei-Rashti, M.; Abrishamkesh, S.; Amirahmadi, E.; Chen, C.; Gorji, M. Application of rice husk biochar for achieving sustainable agriculture and environment. Rice Sci. 2021, 28, 325–343. [Google Scholar] [CrossRef]
- Maillard, É.; Angers, D.A. Animal manure application and soil organic carbon stocks: A meta-analysis. Glob. Change Biol. 2014, 20, 666–679. [Google Scholar] [CrossRef]
- Lin, Y.; Ye, G.; Kuzyakov, Y.; Liu, D.; Fan, J.; Ding, W. Long-term manure application increases soil organic matter and aggregation, and alters microbial community structure and keystone taxa. Soil Biol. Biochem. 2019, 134, 187–196. [Google Scholar] [CrossRef]
- Liu, S.; Kong, F.; Li, Y.; Jiang, Z.; Xi, M.; Wu, J. Mineral-ions modified biochars enhance the stability of soil aggregate and soil carbon sequestration in a coastal wetland soil. Catena 2020, 193, 104618. [Google Scholar] [CrossRef]
- Du, Z.L.; Zhao, J.K.; Wang, Y.D.; Zhang, Q.Z. Biochar addition drives soil aggregation and carbon sequestration in aggregate fractions from an intensive agricultural system. J. Soils Sediments 2017, 17, 581–589. [Google Scholar] [CrossRef]
- Lehmann, J.; Gaunt, J.; Rondon, M. Bio-char sequestration in terrestrial ecosystems—A review. Mitig. Adapt. Strateg. Glob. Change 2006, 11, 403–427. [Google Scholar] [CrossRef]
- Holatko, J.; Bielska, L.; Hammerschmiedt, T.; Kucerik, J.; Mustafa, A.; Radziemska, M.; Kintl, A.; Baltazar, T.; Latal, O.; Brtnicky, M. Cattle manure fermented with biochar and humic substances improve the crop biomass, microbiological properties and nutrient status of soil. Agronomy 2022, 12, 368. [Google Scholar] [CrossRef]
- Han, Z.; Xu, P.; Li, Z.; Guo, S.; Li, S.; Liu, S.; Wu, S.; Wang, J.; Zou, J. Divergent effects of biochar amendment and replacing mineral fertilizer with manure on soil respiration in a subtropical tea plantation. Biochar 2023, 5, 73. [Google Scholar] [CrossRef]
- López-López, G.; Lobo, M.C.; Negre, A.; Colombàs, M.; Rovira, J.M.; Martorell, A.; Reolid, C.; Sastre-Conde, I. Impact of fertilisation practices on soil respiration, as measured by the metabolic index of short-term nitrogen input behaviour. J. Environ. Manag. 2012, 113, 517–526. [Google Scholar] [CrossRef]
- Lai, R.; Arca, P.; Lagomarsino, A.; Cappai, C.; Seddaiu, G.; Demurtas, C.E.; Roggero, P.P. Manure fertilization increases soil respiration and creates a negative carbon budget in a Mediterranean maize (Zea mays L.)-based cropping system. Catena 2017, 151, 202–212. [Google Scholar] [CrossRef]
- Kheyrodin, H.; Ghazvinian, K.; Taherian, M. Tillage and manure effect on soil microbial biomass and respiration, and on enzyme activities. Afr. J. Biotechnol. 2012, 11, 14652–14659. [Google Scholar]
- Sanei, H.; Rudra, A.; Przyswitt, Z.M.M.; Kousted, S.; Sindlev, M.B.; Zheng, X.; Nielsen, S.B.; Petersen, H.I. Assessing biochar’s permanence: An inertinite benchmark. Int. J. Coal Geol. 2024, 281, 104409. [Google Scholar] [CrossRef]
- Fatima, S.; Riaz, M.; Al-Wabel, M.I.; Arif, M.S.; Yasmeen, T.; Hussain, Q.; Roohi, M.; Fahad, S.; Ali, K.; Arif, M. Higher biochar rate strongly reduced decomposition of soil organic matter to enhance C and N sequestration in nutrient-poor alkaline calcareous soil. J. Soils Sediments 2021, 21, 148–162. [Google Scholar] [CrossRef]
- Hammerschmiedt, T.; Holatko, J.; Kucerik, J.; Mustafa, A.; Radziemska, M.; Kintl, A.; Malicek, O.; Baltazar, T.; Latal, O.; Brtnicky, M. Manure maturation with biochar: Effects on plant biomass, manure quality and soil microbiological characteristics. Agriculture 2022, 12, 314. [Google Scholar] [CrossRef]
- Wei, Z.; Wang, J.J.; Fultz, L.M.; White, P.; Jeong, C. Application of biochar in estrogen hormone-contaminated and manure-affected soils: Impact on soil respiration, microbial community and enzyme activity. Chemosphere 2021, 270, 128625. [Google Scholar] [CrossRef]
- Geng, Y.; Cao, G.; Wang, L.; Wang, S. Effects of equal chemical fertilizer substitutions with organic manure on yield, dry matter, and nitrogen uptake of spring maize and soil nitrogen distribution. PLoS ONE 2019, 14, e0219512. [Google Scholar] [CrossRef]
- Wen, Z.; Shen, J.; Blackwell, M.; Li, H.; Zhao, B.; Yuan, H. Combined applications of nitrogen and phosphorus fertilizers with manure increase maize yield and nutrient uptake via stimulating root growth in a long-term experiment. Pedosphere 2016, 26, 62–73. [Google Scholar] [CrossRef]
- Cao, H.; Ning, L.; Xun, M.; Feng, F.; Li, P.; Yue, S.; Song, J.; Zhang, W.; Yang, H. Biochar can increase nitrogen use efficiency of Malus hupehensis by modulating nitrate reduction of soil and root. Appl. Soil Ecol. 2019, 135, 25–32. [Google Scholar] [CrossRef]
- Liu, Z.; He, T.; Cao, T.; Yang, T.; Meng, J.; Chen, W. Effects of biochar application on nitrogen leaching, ammonia volatilization and nitrogen use efficiency in two distinct soils. J. Soil Sci. Plant Nutr. 2017, 17, 515–528. [Google Scholar] [CrossRef]
- Rufino, M.C.; Rowe, E.C.; Delve, R.J.; Giller, K.E. Nitrogen cycling efficiencies through resource-poor African crop–livestock systems. Agric. Ecosyst. Environ. 2006, 112, 261–282. [Google Scholar] [CrossRef]
- Lehmann, J.; Joseph, S. (Eds.) Biochar for Environmental Management: Science, Technology and Implementation, 2nd ed.; Routledge: Abingdon-on-Thames, UK, 2015. [Google Scholar] [CrossRef]
- Fischer, D.; Glaser, B. Synergisms between compost and biochar for sustainable soil amelioration. In Management of Organic Waste; InTech: London, UK, 2012. [Google Scholar] [CrossRef]
- Garg, S.; Bahl, G.S. Phosphorus availability to maize as influenced by organic manures and fertilizer P associated phosphatase activity in soils. Bioresour. Technol. 2008, 99, 5773–5777. [Google Scholar] [CrossRef]
- Sharpley, A.N.; McDowell, R.W.; Kleinman, P.J. Amounts, forms, and solubility of phosphorus in soils receiving manure. Soil Sci. Soc. Am. J. 2004, 68, 2048–2057. [Google Scholar] [CrossRef]
- Griffin, T.S.; Honeycutt, C.W.; He, Z. Changes in soil phosphorus from manure application. Soil Sci. Soc. Am. J. 2003, 67, 645–653. [Google Scholar] [CrossRef]
- Jha, P.; Neenu, S.; Rashmi, I.; Meena, B.P.; Jatav, R.C.; Lakaria, B.L.; Biswas, A.K.; Singh, M.; Patra, A.K. Ameliorating effects of Leucaena biochar on soil acidity and exchangeable ions. Commun. Soil Sci. Plant Anal. 2016, 47, 1252–1262. [Google Scholar] [CrossRef]
- Shi, Y.; Yu, Y.; Chang, E.; Wang, R.; Hong, Z.; Cui, J.; Zhang, F.; Jiang, J.; Xu, R. Effect of biochar incorporation on phosphorus supplementation and availability in soil: A review. J. Soils Sediments 2023, 23, 672–686. [Google Scholar] [CrossRef]
- Rashmi, I.; Jha, P.; Biswas, A.K. Phosphorus sorption and desorption in soils amended with subabul biochar. Agric. Res. 2020, 9, 371–378. [Google Scholar] [CrossRef]
- Gao, S.; DeLuca, T.H.; Cleveland, C.C. Biochar additions alter phosphorus and nitrogen availability in agricultural ecosystems: A meta-analysis. Sci. Total Environ. 2019, 654, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Glaser, B.; Lehr, V.I. Biochar effects on phosphorus availability in agricultural soils: A meta-analysis. Sci. Rep. 2019, 9, 9338. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chen, C.; Gray, E.M.; Boyd, S.E.; Yang, H.; Zhang, D. Roles of biochar in improving phosphorus availability in soils: A phosphate adsorbent and a source of available phosphorus. Geoderma 2016, 276, 1–6. [Google Scholar] [CrossRef]
- Arif, M.; Ali, S.; Ilyas, M.; Riaz, M.; Akhtar, K.; Ali, K.; Adnan, M.; Fahad, S.; Khan, I.; Shah, S.; et al. Enhancing phosphorus availability, soil organic carbon, maize productivity and farm profitability through biochar and organic–inorganic fertilizers in an irrigated maize agroecosystem under semi-arid climate. Soil Use Manag. 2021, 37, 104–119. [Google Scholar] [CrossRef]
- Kizito, S.; Luo, H.; Lu, J.; Bah, H.; Dong, R.; Wu, S. Role of nutrient-enriched biochar as a soil amendment during maize growth: Exploring practical alternatives to recycle agricultural residuals and to reduce chemical fertilizer demand. Sustainability 2019, 11, 3211. [Google Scholar] [CrossRef]
- Römheld, V.; Kirkby, E.A. Research on potassium in agriculture: Needs and prospects. Plant Soil 2010, 335, 155–180. [Google Scholar] [CrossRef]
- Das, I.; Pradhan, M. Potassium-solubilizing microorganisms and their role in enhancing soil fertility and health. In Potassium Solubilizing Microorganisms for Sustainable Agriculture; Meena, V., Maurya, B., Verma, J., Meena, R., Eds.; Springer: New Delhi, India, 2016. [Google Scholar] [CrossRef]
- Wang, J.; Shi, D.; Huang, C.; Zhai, B.; Feng, S. Effects of common biochar and acid-modified biochar on growth and quality of spinach in coastal saline soils. Plants 2023, 12, 3232. [Google Scholar] [CrossRef]
- Yan, X.; Wang, Z.; Zhao, M.; Hao, J.; Liu, J.; Yan, Y.; Sun, P.; Jia, Y.; Ge, G. Hydrothermal biochar enhances the photosynthetic efficiency and yield of alfalfa by optimizing soil chemical properties and stimulating the activity of microbial communities. Sci. Rep. 2024, 14, 31420. [Google Scholar] [CrossRef]
- Zhu, Q.; Kong, L.J.; Shan, Y.Z.; Yao, X.D.; Zhang, H.J.; Xie, F.T.; Xue, A.O. Effect of biochar on grain yield and leaf photosynthetic physiology of soybean cultivars with different phosphorus efficiencies. J. Integr. Agric. 2019, 18, 2242–2254. [Google Scholar] [CrossRef]
- Graber, E.R.; Meller Harel, Y.; Kolton, M.; Cytryn, E.; Silber, A.; Rav David, D.; Tsechansky, L.; Borenshtein, M.; Elad, Y. Biochar impact on development and productivity of pepper and tomato grown in fertigated soilless media. Plant Soil 2010, 337, 481–496. [Google Scholar] [CrossRef]
- Agbede, T.M.; Oyewumi, A. Benefits of biochar, poultry manure and biochar–poultry manure for improvement of soil properties and sweet potato productivity in degraded tropical agricultural soils. Resour. Environ. Sustain. 2022, 7, 100051. [Google Scholar] [CrossRef]
- Nduka, B.A.; Ogunlade, M.O.; Adeniyi, D.O.; Oyewusi, I.K.; Ugioro, O.; Mohammed, I. The influence of organic manure and biochar on cashew seedling performance, soil properties and status. Agric. Sci. 2019, 10, 110–120. [Google Scholar] [CrossRef]
- Agegnehu, G.; Nelson, P.N.; Bird, M.I. Crop yield, plant nutrient uptake and soil physicochemical properties under organic soil amendments and nitrogen fertilization on Nitisols. Soil Tillage Res. 2016, 160, 1–13. [Google Scholar] [CrossRef]
- Zhang, Z.; Dong, X.; Wang, S.; Pu, X. Benefits of organic manure combined with biochar amendments to cotton root growth and yield under continuous cropping systems in Xinjiang, China. Sci. Rep. 2020, 10, 4718. [Google Scholar] [CrossRef]
- Ibrahim, M.; Yamin, M.; Sarwar, G.; Anayat, A.; Habib, F.; Ullah, S. Tillage and farm manure affect root growth and nutrient uptake of wheat and rice under semi-arid conditions. Appl. Geochem. 2011, 26, S194–S197. [Google Scholar] [CrossRef]
- Yang, C.; Yang, L.; Yang, Y.; Ouyang, Z. Rice root growth and nutrient uptake as influenced by organic manure in continuously and alternately flooded paddy soils. Agric. Water Manag. 2004, 70, 67–81. [Google Scholar] [CrossRef]
- Zou, Z.; Fan, L.; Li, X.; Dong, C.; Zhang, L.; Zhang, L.; Fu, J.; Han, W.; Yan, P. Response of plant root growth to biochar amendment: A meta-analysis. Agronomy 2021, 11, 2442. [Google Scholar] [CrossRef]
- Zhu, Q.; Kong, L.; Xie, F.; Zhang, H.; Wang, H.; Ao, X. Effects of biochar on seedling root growth of soybeans. Chil. J. Agric. Res. 2018, 78, 549–558. [Google Scholar] [CrossRef]
- Biederman, L.A.; Harpole, W.S. Biochar and its effects on plant productivity and nutrient cycling: A meta-analysis. GCB Bioenergy 2013, 5, 202–214. [Google Scholar] [CrossRef]
- Adekiya, A.O.; Agbede, T.M.; Aboyeji, C.M.; Dunsin, O.; Simeon, V.T. Effects of biochar and poultry manure on soil characteristics and the yield of radish. Sci. Hortic. 2019, 243, 457–463. [Google Scholar] [CrossRef]
- Comas, L.H.; Becker, S.R.; Cruz, V.M.V.; Byrne, P.F.; Dierig, D.A. Root traits contributing to plant productivity under drought. Front. Plant Sci. 2013, 4, 442. [Google Scholar] [CrossRef]
- Hermans, C.; Hammond, J.P.; White, P.J.; Verbruggen, N. How do plants respond to nutrient shortage by biomass allocation? Trends Plant Sci. 2006, 11, 610–617. [Google Scholar] [CrossRef]
- Thepsilvisut, O.; Chutimanukul, P.; Sae-Tan, S.; Ehara, H. Effect of chicken manure and chemical fertilizer on the yield and qualities of white mugwort at dissimilar harvesting times. PLoS ONE 2022, 17, e0266190. [Google Scholar] [CrossRef] [PubMed]
- Anugoolprasert, O.; Rithichai, P. Effect of high quality organic fertilizer on yield and quality of lettuce. Thai J. Sci. Technol. 2015, 4, 81–94. [Google Scholar] [CrossRef]
- Elhanafi, L.; Houhou, M.; Rais, C.; Mansouri, I.; Elghadraoui, L.; Greche, H. Impact of excessive nitrogen fertilization on the biochemical quality, phenolic compounds, and antioxidant power of Sesamum indicum L. seeds. J. Food Qual. 2019, 2, 9428092. [Google Scholar] [CrossRef]
- Pacheco, A.C.; Feba, L.G.T.; Serra, E.G.; Takata, W.H.S.; Gorni, P.H.; Yoshida, C.H.P. The use of animal manure in the organic cultivation of Passiflora incarnata L. increases the content of phenolic compounds in the leaf and the antioxidant activity of the plant. Org. Agric. 2021, 11, 567–575. [Google Scholar] [CrossRef]
- Misra, D.; Dutta, W.; Jha, G.; Ray, P. Interactions and regulatory functions of phenolics in soil-plant-climate nexus. Agronomy 2023, 13, 280. [Google Scholar] [CrossRef]
- Li, J.; Zhu, Z.; Gerendás, J. Effects of nitrogen and sulfur on total phenolics and antioxidant activity in two genotypes of leaf mustard. J. Plant Nutr. 2008, 31, 1642–1655. [Google Scholar] [CrossRef]
- Łata, B. Variability in enzymatic and non-enzymatic antioxidants in red and green-leafy kale in relation to soil type and N-level. Sci. Hortic. 2014, 168, 38–45. [Google Scholar] [CrossRef]
- Amarowicz, R.; Ambroziak, B.C.; Janiak, M.A. Effect of N fertilization on the content of phenolic compounds in Jerusalem artichoke (Helianthus tuberosus L.) tubers and their antioxidant capacity. Agronomy 2020, 10, 1215. [Google Scholar] [CrossRef]
- Rodnuch, N.; Phongsri, S.; Aninbon, C. Responses of landrace rice to organic fertilizer for physiological traits, grain yield and total phenolic content. Curr. Appl. Sci. Technol. 2019, 19, 297–305. [Google Scholar]
- Knoblauch, C.; Priyadarshani, S.H.R.; Haefele, S.M.; Schröder, N.; Pfeiffer, E.-M. Impact of biochar on nutrient supply, crop yield and microbial respiration on sandy soils of northern Germany. Eur. J. Soil Sci. 2021, 72, 1885–1901. [Google Scholar] [CrossRef]
- Regmi, A.; Poudyal, S.; Singh, S.; Coldren, C.; Moustaid-Moussa, N.; Simpson, C. Biochar influences phytochemical concentrations of Viola cornuta flowers. Sustainability 2023, 15, 3882. [Google Scholar] [CrossRef]
- Sharma, R.; Kumar, R.; Bakshi, P.; Amit Jasrotia Sinha, B.K.; Sharma, N.; Sharma, P.; Kumar, V.; Sood, M.; Dadheechi, M. Synergistic impact of vermicompost, biochar and jaggery on antioxidants, phenols and flavonoids in Guava cv. L-49. BioResources 2024, 19, 8173–8187. [Google Scholar] [CrossRef]
- Antonious, G.F. Impact of biochar and organic fertilizers on sweet potato yield, quality, ascorbic acid, β-carotene, sugars, and phenols contents. Int. J. Environ. Health Res. 2024, 34, 3708–3719. [Google Scholar] [CrossRef]
- Liwanda, N.; Nurinayah, I.; Mubayyinah, H.; Pratiwi, A.R.R.; Wahyuningrum, T.; Ashari, R.Z.; Aisyah, S.I.; Nurcholis, W. Effect of cow manure fertilizer on growth, polyphenol content, and antioxidant activity of purslane plants. Int. J. Chem. Biochem. Sci. 2023, 23, 43–54. [Google Scholar]
- Ribeiro, F.N.S.; Rocha, T.T.; Medeiros, A.P.R.; Germano, C.M.; de Assis, R.M.A.; de Carvalho, A.A.; Bertolucci, S.K.V.; Pinto, J.E.B.P. Manure application enhances the biomass production, phytochemical contents, antioxidant, and essential oil of Lippia dulcis. Rev. Ciência Agronômica 2024, 55, e20238873. [Google Scholar] [CrossRef]
- Rostaei, M.; Fallah, S.; Carrubba, A.; Lorigooini, Z. Organic manures enhance biomass and improve content, chemical compounds of essential oil and antioxidant capacity of medicinal plants: A review. Heliyon 2024, 10, e36693. [Google Scholar] [CrossRef]
- Duri, L.G.; Pannico, A.; Petropoulos, S.A.; Caporale, A.G.; Adamo, P.; Graziani, G.; Ritieni, A.; De Pascale, S.; Rouphael, Y. Bioactive compounds and antioxidant activity of lettuce grown in different mixtures of monogastric-based manure with lunar and Martian soils. Front. Nutr. 2022, 9, 890786. [Google Scholar] [CrossRef]
- Krupodorova, T.; Barshteyn, V.; Dzhagan, V.; Pluzhnyk, A.; Zaichenko, T.; Blume, Y. Enhancement of antioxidant activity and total phenolic content of Fomitopsis pinicola mycelium extract. Fungal Biol. Biotechnol. 2024, 11, 18. [Google Scholar] [CrossRef] [PubMed]
- Gaafar, A.A.; Ali, S.I.; El-Shawadfy, M.A.; Salama, Z.A.; Sękara, A.; Ulrichs, C.; Abdelhamid, M.T. Ascorbic acid induces the increase of secondary metabolites, antioxidant activity, growth, and productivity of the common bean under water stress conditions. Plants 2020, 9, 627. [Google Scholar] [CrossRef]
- Ibrahim, M.H.; Jaafar, H.Z.E. The Relationship of nitrogen and C/N ratio with secondary metabolites levels and antioxidant activities in three varieties of Malaysian Kacip Fatimah (Labisia pumila Blume). Molecules 2011, 16, 5514–5526. [Google Scholar] [CrossRef] [PubMed]
- Hasnain, M.; Munir, N.; Abideen, Z.; Zulfiqar, F.; Koyro, H.W.; El-Naggar, A.; Caçador, I.; Duarte, B.; Rinklebe, J.; Yong, J.W.H. Biochar-plant interaction and detoxification strategies under abiotic stresses for achieving agricultural resilience: A critical review. Ecotoxicol. Environ. Saf. 2023, 249, 114408. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Xu, L.; Liu, Y.; Su, W.; Yan, J.; Xu, D. Effect of biochar on the growth, photosynthesis, antioxidant system and cadmium content of Mentha piperita ‘Chocolate’ and Mentha spicata in cadmium-contaminated soil. Agronomy 2022, 12, 2737. [Google Scholar] [CrossRef]
- Ghassemi-Golezani, K.; Rahimzadeh, S. The biochar-based nanocomposites influence the quantity, quality and antioxidant activity of essential oil in dill seeds under salt stress. Sci. Rep. 2022, 12, 21903. [Google Scholar] [CrossRef]
- Sharma, S.; Rana, V.S.; Rana, N.; Sharma, U.; Gudeta, K.; Alharbi, K.; Ameen, F.; Bhat, S.A. Effect of organic manures on growth, yield, leaf nutrient uptake and soil properties of kiwifruit (Actinidia deliciosa Chev.) cv. Allison. Plants 2022, 11, 3354. [Google Scholar] [CrossRef]
- Liu, M.; Linna, C.; Ma, S.; Ma, Q.; Guo, J.; Wang, F.; Wang, L. Effects of biochar with inorganic and organic fertilizers on agronomic traits and nutrient absorption of soybean and fertility and microbes in purple soil. Front. Plant Sci. 2022, 13, 871021. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, L. Biochar and cow manure organic fertilizer amendments improve the quality of composted green waste as a growth medium for the ornamental plant Centaurea cyanus L. Environ. Sci. Pollut. Res. Int. 2022, 29, 45474–45486. [Google Scholar] [CrossRef]
- Ghaedi, M.; Bijanzadeh, E.; Behpouri, A.; Najafi-Ghiri, M. Biochar application affected biochemical properties, yield and nutrient content of safflower under water stress. Sci. Rep. 2024, 14, 20228. [Google Scholar] [CrossRef]
- Cong, M.; Hu, Y.; Sun, X.; Yan, H.; Yu, G.; Tang, G.; Chen, S.; Xu, W.; Jia, H. Long-term effects of biochar application on the growth and physiological characteristics of maize. Front. Plant Sci. 2023, 14, 1172425. [Google Scholar] [CrossRef] [PubMed]
- AG, B.; Rashwan, E.; El-Sharkawy, T.A. Effect of organic manure, antioxidant and proline on corn (Zea mays L.) grown under saline conditions. Environ. Biodivers. Soil Secur. 2017, 1, 203–217. [Google Scholar] [CrossRef]
- Sugier, D.; Sugier, P.; Jakubowicz-Gil, J.; Gawlik-Dziki, U.; Zając, A.; Król, B.; Chmiel, S.; Kończak, M.; Pięt, M.; Paduch, R. Nitrogen fertilization and solvents as factors modifying the antioxidant and anticancer potential of Arnica montana L. flower head extracts. Plants 2022, 12, 142. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Vancov, T.; Penuelas, J.; Sardans, J.; Singla, A.; Alrefaei, A.F.; Song, X.; Fang, Y.; Wang, W. Optimal biochar application rates for mitigating global warming and increasing rice yield in a subtropical paddy field. Exp. Agric. 2021, 57, 283–299. [Google Scholar] [CrossRef]
- Fan, C.; Cui, Y.; Zhang, Q.; Yin, N.; Cai, X.; Yuan, X.; Senadheera, S.; Cho, Y.; Ok, Y.S. A critical review of the interactions between rhizosphere and biochar during the remediation of metal(loid) contaminated soils. Biochar 2023, 5, 87. [Google Scholar] [CrossRef]
- Duan, S.; AL-Huqail, A.A.; Alsudays, I.M.; Younas, M.; Aslam, A.; Shahzad, A.N.; Qayyum, M.F.; Rizwan, M.; Hamooud, Y.A.; Shaghaleh, H.; et al. Effects of biochar types on seed germination, growth, chlorophyll contents, grain yield, sodium, and potassium uptake by wheat (Triticum aestivum L.) under salt stress. BMC Plant Biol. 2024, 24, 487. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).