Impact of Preharvest Bagging on the Volatile Profile of Vinalopó Table Grapes
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Sample Processing
2.2. Extraction Procedure of Volatile Aroma Compounds and Chromatographic Analyses
2.3. Statistical Analysis
3. Results and Discussion
- Aldehydes (n = 14): Hexanal, (E)-2-hexenal, benzaldehyde, octanal, benzeneacetaldehyde, nonanal, (E)-2-nonenal, decanal, β-citral, α-citral, undecanal, dodecanal, tetradecanal (2 isomers).
- Terpenes (n = 7): o-Cymene, D-limonene, γ -terpinene, terpinolene, caryophyllene, α-bergamotene, β-bisabolene.
- Alcohols (n = 6): 1-hexanol, fenchol, 1-terpinenol, β-terpineol, terpinen-4-ol, L-α-terpineol.
- Esters (n = 4): acetic acid hexyl ester, octanoic acid methyl ester, citronellol acetate, methyl laurate.
- Ketones (n = 2): 6-methyl-5-hepten-2-one, 2,6-di-tert-butyl-p-benzoquinone.
- Terpenoids (n = 1): linalool.
- Carboxilic acids (n = 1): hexanoic acid.
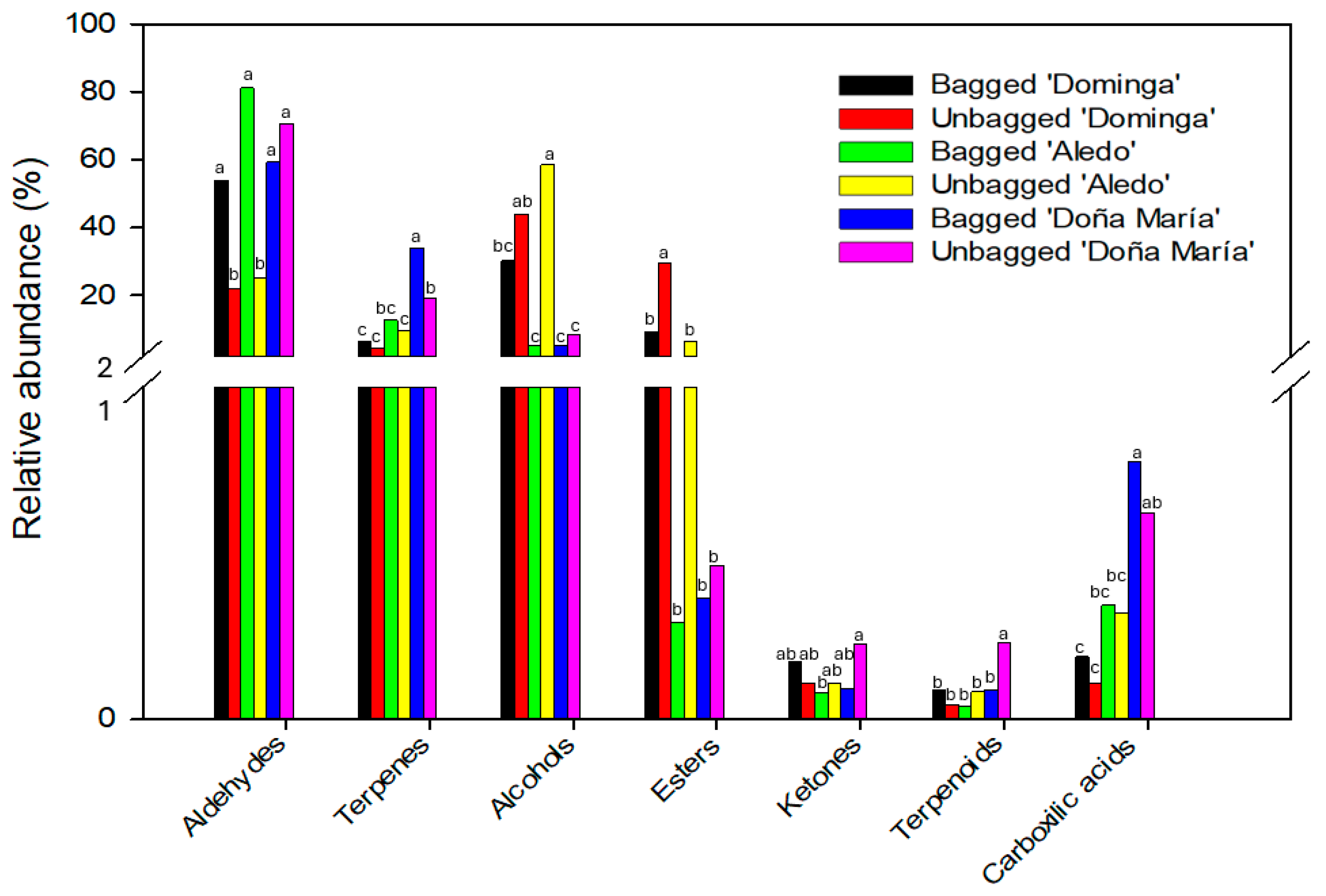
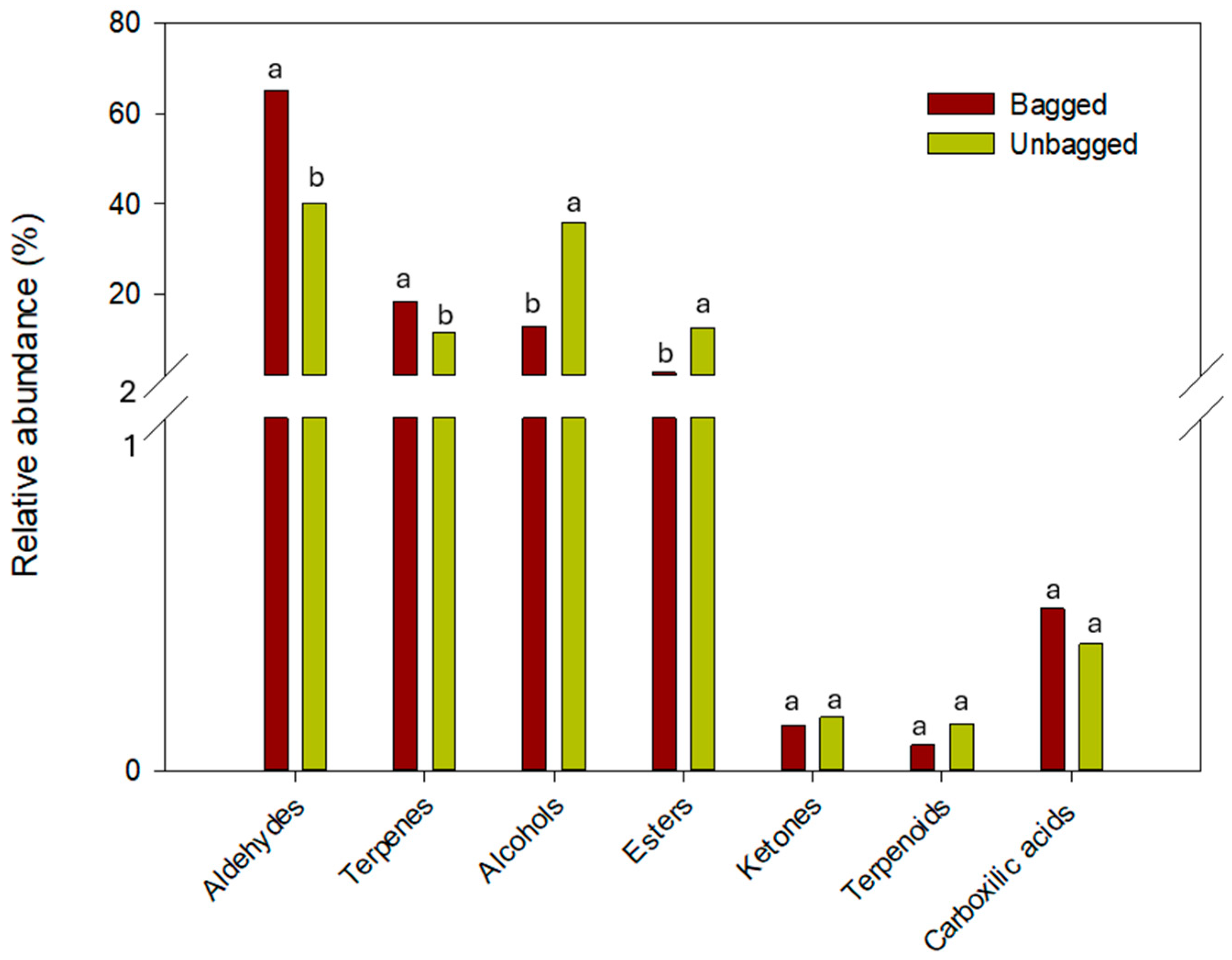

4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variance |
| GC–MS | Gas chromatography–mass spectrometry |
| HS-SPME | Headspace solid-phase microextraction |
| KI | Kovat Index |
| NS | Not significant |
| PCA | Principal component analyses |
| PDO | Protected Designation of Origin |
| RT | Retention time |
References
- Denominación de Origen Protegida Uva de Mesa Embolsada del Vinalopó. Pliego de Condiciones de la Denominación de Origen Protegida Uva de Mesa Embolsada del Vinalopó; Ministerio de Agricultura, Pesca y Alimentación de España: Alicante, Spain, 2015; Available online: https://www.mapa.gob.es/images/es/uva_vinalopo_2015_05_29_tcm30-210665.pdf (accessed on 15 October 2024).
- He, L.; Xu, X.Q.; Wang, Y.; Vanderweide, J.; Sun, R.Z.; Cheng, G.; Chen, W.; Li, S.D.; Li, S.P.; Duan, C.Q.; et al. Differential influence of timing and duration of bunch bagging on volatile organic compounds in Cabernet Sauvignon berries (Vitis vinifera L.). Aust. J. Grape Wine Res. 2022, 28, 75–85. [Google Scholar] [CrossRef]
- Li, Y.; He, L.; Song, Y.; Zhang, P.; Chen, D.; Guan, L.; Liu, S. Comprehensive study of volatile compounds and transcriptome data providing genes for grape aroma. BMC Plant Biol. 2023, 23, 171. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.M.; Anwar, R.; Yousef, A.F.; Li, B.; Luvisi, A.; De Bellis, L.; Aprile, A.; Chen, F. Influence of Bagging on the Development and Quality of Fruits. Plants 2021, 10, 358. [Google Scholar] [CrossRef]
- Sharma, R.R.; Reddy, S.V.R.; Jhalegar, M.J. Pre-harvest Fruit Bagging: A Useful Approach for Plant Protection and Improved Post-harvest Fruit Quality—A Review. J. Hortic. Sci. Biotechnol. 2014, 89, 101–113. [Google Scholar] [CrossRef]
- Lin, J.; Chang, Y.; Yan, Z.; Li, X. Effects of bagging on the quality of pear fruit and pesticide residues. Acta Hortic. 2008, 772, 315–318. [Google Scholar]
- Jia, H.; Araki, A.; Okamoto, G. Influence of fruit bagging on aroma volatiles and skin coloration of ‘Hakuho’ peach (Prunus persica batsch). Postharvest Biol. Technol. 2005, 35, 61–68. [Google Scholar] [CrossRef]
- Wang, X.Y.; Wang, H.L.; Zhang, G.J.; Yan, A.L.; Ren, J.C.; Liu, Z.H.; Zhao, H.B.; Wang, X.T.; Sun, L. Effects of fruit bagging treatment with different types of bags on the contents of phenolics and monoterpenes in muscat-flavored table grapes. Horticulturae 2022, 8, 411. [Google Scholar] [CrossRef]
- Teruel-Andreu, C.; Issa-Issa, H.; Noguera-Artiaga, L.; Sendra, E.; Hernández, F.; Cano-Lamadrid, M. Volatile profile of breba and fig fruits (peel and pulp) from different Ficus carica L. varieties. Sci. Hortic. 2024, 328, 112892. [Google Scholar] [CrossRef]
- Oliveira, A.P.; Silva, L.R.; de Pinho, P.G.; Gil-Izquierdo, A.; Valentão, P.; Silva, B.M.; Ferreres, F.; Guedes de Pinho, P.; Andrade, P.B. Volatile profiling of Ficus carica varieties by HS-SPME and GC–IT-MS. Food Chem. 2010, 123, 548–557. [Google Scholar] [CrossRef]
- NIST. National Institute of Standards and Technology. Available online: https://webbook.nist.gov/chemistry/ (accessed on 19 June 2024).
- Addinsoft. XLSTAT Software, version 9; Addinsoft: Barcelona, Spain, 2010.
- Systat Software. SigmaPlot for Windows, version 12.5; Systat Software Inc.: San Jose, CA, USA, 2013.
- FEMA. Flavors Extracts Manufacturers Association. Available online: https://www.femaflavor.org (accessed on 19 June 2024).
- SAFC. Flavors and Fragrances; SAFC Specialities: Madrid, Spain, 2011. [Google Scholar]
- Schirack, A.V.; Drake, M.A.; Sanders, T.H.; Sandeep, K.P. Characterization of aroma-active compounds in microwave blanched peanuts. J. Food Sci. 2006, 71, C513–C520. [Google Scholar] [CrossRef]
- Canturk, S.; Tangolar, S.; Tangolar, S.; Ada, M. Volatile composition of seven new hybrid table grape cultivars (Vitis vinifera L.). Appl. Fruit Sci. 2025, 67, 1–14. [Google Scholar] [CrossRef]
- Ubeda, C.; Gil i Cortiella, M.; Villalobos-González, L.; Gómez, C.; Pastenes, C.; Peña-Neira, Á. Ripening and storage time effects on the aromatic profile of new table grape cultivars in Chile. Molecules 2020, 25, 5790. [Google Scholar] [CrossRef] [PubMed]
- Blanch, G.P.; Suarez-Coca, D.; de Pascual-Teresa, S.; del Castillo, M.L.R. Antioxidant content and volatile composition of seedless table grape (Vitis vinifera L.) varieties. Eur. Food Res. Technol. 2023, 249, 985–991. [Google Scholar] [CrossRef]
- Kaya, O.; Incesu, M.; Ates, F.; Keskin, N.; Verdugo-Vásquez, N.; Gutiérrez-Gamboa, G. Study of volatile organic compounds of two table grapes (Cv. Italia and Bronx Seedless) along ripening in vines established in the Aegean region (Turkey). Plants 2022, 11, 1935. [Google Scholar] [CrossRef]
- Belitz, H.D.; Grosch, W.; Schieberle, P. Aroma Compounds. In Food Chemistry; Springer: Berlin/Heidelberg, Germany, 2004; pp. 342–408. [Google Scholar]
- Van Gemert, L.J. Odour Thresholds: Compilations of Odour Threshold Values in Air, Water and Other Media, 2nd ed.; Oliemans Punter & Partners BV: Utrecht, The Netherlands, 2011. [Google Scholar]
- Gasiński, A.; Noguera-Artiaga, L.; Kawa-Rygielska, J. Influence of Malted Chickpea on the Composition of Volatiles in Hummus. Molecules 2025, 30, 1231. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information (NCBI). PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov (accessed on 15 October 2024).
- Oliveira, I.; de Pinho, P.G.; Malheiro, R.; Baptista, P.; Pereira, J.A. Volatile profile of Arbutus unedo L. fruits through ripening stage. Food Chem. 2011, 128, 667–673. [Google Scholar] [CrossRef]
- The Good Scents Company. Flavor and Fragrance Information Catalog. Available online: http://www.thegoodscentscompany.com (accessed on 15 October 2024).
- Zhang, L.; Mi, S.; Liu, R.B.; Sang, Y.X.; Wang, X.H. Evaluation of volatile compounds during the fermentation process of yogurts by Streptococcus thermophilus based on odor activity value and heat map analysis. Int. J. Anal. Chem. 2020, 2020, 3242854. [Google Scholar] [CrossRef]
- Wang, W.-N.; Qian, Y.-H.; Liu, R.-H.; Liang, T.; Ding, Y.-T.; Xu, X.-L.; Huang, S.; Fang, Y.-L.; Ju, Y.-L. Effects of table grape cultivars on fruit quality and aroma components. Foods 2023, 12, 3371. [Google Scholar] [CrossRef]
- Ferenczi, A.; Sugimoto, N.; Beaudry, R.M. Emission patterns of esters and their precursors throughout ripening and senescence in ‘Redchief Delicious’ apple fruit and implications regarding biosynthesis and aroma perception. J. Am. Soc. Hortic. Sci. 2021, 146, 297–328. [Google Scholar] [CrossRef]
- Sancho-Galán, P.; Amores-Arrocha, A.; Palacios, V.; Jiménez-Cantizano, A. Volatile composition and sensory characterization of dry white wines made with overripe grapes by means of two different techniques. Foods 2022, 11, 509. [Google Scholar] [CrossRef]
- Yue, X.; Ju, Y.; Cui, Y.; Wei, S.; Xu, H.; Zhang, Z. Evolution of green leaf volatile profile and aroma potential during the berry development in five Vitis vinifera L. cultivars. Food Chem. X 2023, 18, 100676. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.H.; Wang, B.L.; Wang, X.D.; Shi, X.B.; Liu, P.P.; Liu, F.Z.; Wang, H.B. Effects of different color paper bags on aroma development of Kyoho grape berries. J. Integr. Agric. 2019, 18, 70–82. [Google Scholar] [CrossRef]
- Guo, S.H.; Xu, T.F.; Shi, T.C.; Jin, X.Q.; Feng, M.X.; Zhao, X.H.; Duan, C.Q.; Meng, J.F. Cluster bagging promotes melatonin biosynthesis in the berry skins of Vitis vinifera cv. Cabernet Sauvignon and Carignan during development and ripening. Food Chem. 2020, 305, 125502. [Google Scholar] [CrossRef] [PubMed]
| Variety × Treatment | SST (°Brix) | Titratable Acidity (g Tartaric Acid L−1) | MI |
|---|---|---|---|
| Bagged ‘Dominga’ | 17.1 f 2 | 2.55 d | 67.0 a |
| Unbagged ‘Dominga’ | 22.0 c | 3.50 bc | 63.0 b |
| Bagged ‘Aledo’ | 19.0 e | 3.34 c | 57.0 c |
| Unbagged ‘Aledo’ | 19.9 d | 3.80 b | 52.5 d |
| Bagged ‘Doña María’ | 23.2 b | 3.29 a | 70.5 a |
| Unbagged ‘Doña María’ | 25.4 a | 4.58 a | 55.6 cd |
| ANOVA | *** 1 | *** | *** |
| Code | Volatile Compound | Chemical Family | RT 1 | Kovat Index (KI) 2 | Sensory Descriptors | |
|---|---|---|---|---|---|---|
| (min) | Exp. | Lit. | ||||
| V1 | Hexanal | Aldehydes | 4.69 | 823 | 819 | Apple, fatty, green, fresh 3,4 |
| V2 | (E)-2-Hexenal | Aldehydes | 6.13 | 862 | 855 | Almond, apple, fruity, vegetable 4 |
| V3 | 1-Hexanol | Alcohols | 6.49 | 870 | 870 | Green, woody, sweet 4 |
| V4 | Benzaldehyde | Aldehydes | 10.6 | 953 | 955 | Almond, cherry, sweet 4 |
| V5 | Hexanoic acid | Carboxilic acids | 11.41 | 969 | 977 | Cheesy, fatty, sour, pungent 3,4 |
| V6 | 6-methyl-5-Hepten-2-one | Ketones | 11.84 | 978 | 986 | Citrus, mushroom, oily, green 3,4 |
| V7 | Octanal | Aldehydes | 12.86 | 998 | 1001 | Fat, green, oil, pungent, fruity 3,4 |
| V8 | Acetic acid hexyl ester | Esters | 13.40 | 1007 | 1010 | Floral, green, apple, cherry 3,4 |
| V9 | o-Cymene | Terpenes | 14.07 | 1018 | 1018 | Citrus, fresh, solvent 3,4 |
| V10 | D-Limonene | Terpenes | 14.33 | 1022 | 1028 | Citrus, mint 3 |
| V11 | Benzeneacetaldehyde | Aldehydes | 15.06 | 1033 | 1043 | Cocoa, coffee, wine-line 3,4 |
| V12 | γ-Terpinene | Terpenes | 16.09 | 1050 | 1055 | Bitter, citrus, herbaceous 3,4 |
| V13 | Terpinolene | Terpenes | 17.78 | 1077 | 1084 | Pine 3,4 |
| V14 | Linalool | Terpenoids | 18.78 | 1092 | 1098 | Floral, citrus, sweet 3,4 |
| V15 | Nonanal | Aldehydes | 19.09 | 1097 | 1101 | Fat, grape, floral, citrus, melon 3,4 |
| V16 | Fenchol | Alcohols | 19.86 | 1109 | 1110 | Camphor, lemon 3 |
| V17 | Octanoic acid methyl ester | Esters | 20.38 | 1116 | 1120 | Oily, cheese 4 |
| V18 | 1-Terpinenol | Alcohols | 21.05 | 1126 | 1120 | Grapefruit, anise, citrus, fruity 3,4 |
| V19 | β-Terpineol | Alcohols | 21.90 | 1139 | 1144 | Anise, fresh, mint, oil, lilac 3,4 |
| V20 | (E)-2-Nonenal | Aldehydes | 22.71 | 1151 | 1156 | Paper, waxy, fatty 3,4 |
| V21 | Terpinen-4-ol | Alcohols | 23.95 | 1169 | 1162 | Grapefruit, citrus, anise, fresh 3,4 |
| V22 | L-α-Terpineol | Alcohols | 24.94 | 1183 | 1192 | Anise, fresh, mint, oil, lilac 3,4 |
| V23 | Decanal | Aldehydes | 25.85 | 1197 | 1203 | Floral, green, apple, cherry, 3,4 |
| V24 | β-Citral | Aldehydes | 27.81 | 1229 | 1238 | Lemon 3,4 |
| V25 | α-Citral | Aldehydes | 29.64 | 1259 | 1267 | Lemon 3,4 |
| V26 | Undecanal | Aldehydes | 31.97 | 1297 | 1306 | Floral, orange, fatty, rose 3,4 |
| V27 | Citronellol acetate | Esters | 34.21 | 1341 | 1348 | Floral, geranium, rose 3,4 |
| V28 | Dodecanal | Aldehydes | 37.16 | 1399 | 1409 | Green, waxy, floral, sweet 3,4 |
| V29 | Caryophyllene | Terpenes | 37.46 | 1406 | 1414 | Fried, spicy, woody 3,4 |
| V30 | α-Bergamotene | Terpenes | 38.21 | 1422 | 1414 | Fruity, sweet 3,4 |
| V31 | 2,6-di-tert-butyl-p-Benzoquinone | Ketones | 39.38 | 1448 | 1458 | Fennel, fatty 4 |
| V32 | β-Bisabolene | Terpenes | 41.58 | 1498 | 1506 | Floral 3 |
| V33 | Tetradecanal | Aldehydes | 41.76 | 1502 | 1503 | Honey, hay 5 |
| V34 | Methyl laurate | Esters | 42.34 | 1516 | 1524 | Coconut, creamy, fatty, soapy 3,4 |
| V35 | Tetradecanal | Aldehydes | 45.98 | 1605 | 1615 | Honey, hay 5 |
| Volatile Compound | Concentration (μg 100 g−1) | ||
|---|---|---|---|
| Student’s t-Test | Bagged | Unbagged | |
| Aldehydes | |||
| Hexanal | 0.004 1 | 822 | 370 |
| (E)-2-Hexenal | 0.04 | 1024 | 631 |
| Benzaldehyde | 0.13 | 1.94 | 3.68 |
| Octanal | 0.04 | 7.23 | 22.3 |
| Benzeneacetaldehyde | 0.08 | 2.43 | 7.75 |
| Nonanal | 0.63 | 55.9 | 60.8 |
| (E)-2-Nonenal | 0.04 | 10.9 | 3.40 |
| Decanal | 0.32 | 12.3 | 14.6 |
| β-Citral | 0.50 | 1.14 | 1.26 |
| α-Citral | 0.48 | 0.95 | 1.12 |
| Undecanal | 0.11 | 1.37 | 2.06 |
| Dodecanal | 0.10 | 3.91 | 4.93 |
| Tetradecanal | 0.99 | 0.97 | 0.97 |
| Tetradecanal | 0.58 | 0.87 | 0.99 |
| Terpenes | |||
| o-Cymene | 0.85 | 13.9 | 14.4 |
| D-Limonene | 0.24 | 364 | 260 |
| γ-Terpinene | 0.34 | 37.8 | 29.4 |
| Terpinolene | 0.93 | 3.94 | 4.03 |
| Caryophyllene | 0.56 | 2.37 | 2.74 |
| α-Bergamotene | 0.94 | 3.27 | 3.34 |
| β-Bisabolene | 0.85 | 2.42 | 2.53 |
| Alcohols | |||
| 1-Hexanol | 0.003 | 450 | 1710 |
| Fenchol | 0.73 | 1.12 | 1.20 |
| 1-Terpinenol | 0.18 | 1.77 | 2.27 |
| β-Terpineol | 0.77 | 1.82 | 1.71 |
| Terpinen-4-ol | 0.47 | 2.58 | 2.98 |
| L-α-Terpineol | 0.56 | 5.44 | 6.04 |
| Esters | |||
| Acetic acid, hexyl ester | 0.04 | 117 | 656 |
| Octanoic acid methyl ester | 0.11 | 0.432 | 0.682 |
| Citronellol acetate | 0.54 | 0.33 | 0.42 |
| Methyl laurate | 0.95 | 1.54 | 1.56 |
| Ketones | |||
| 6-methyl-5-Hepten-2-one | 0.16 | 1.65 | 2.86 |
| 2,6-di-tert-butyl-p-Benzoquinone | 0.95 | 1.94 | 1.92 |
| Carboxilic acids | |||
| Hexanoic acid | 0.70 | 10.9 | 9.76 |
| Total (mg kg−1) | 0.36 | 29.72 | 38.42 |
| Volatile Compound | Concentration (μg 100 g−1) | |||
|---|---|---|---|---|
| ANOVA | ‘Dominga’ | ‘Aledo’ | ‘Doña María’ | |
| Aldehydes | ||||
| Hexanal | *** 1 | 411 b 2 | 1052 a | 327 b |
| (E)-2-Hexenal | NS | 1078 a | 775 a | 628 a |
| Benzaldehyde | NS | 2.70 a | 3.01 a | 2.73 a |
| Octanal | ** | 28.3 a | 8.13 a | 7.87 a |
| Benzeneacetaldehyde | NS | 3.30 a | 7.10 a | 4.86 a |
| Nonanal | NS | 48.8 a | 68.5 a | 57.7 a |
| (E)-2-Nonenal | *** | 14.4 a | 4.10 ab | 2.94 b |
| Decanal | NS | 11.9 a | 14.1 a | 14.3 a |
| β-Citral | NS | 1.20 a | 1.06 a | 1.33 a |
| α-Citral | NS | 1.17 a | 1.03 a | 0.91 a |
| Undecanal | NS | 1.89 a | 1.73 a | 1.54 a |
| Dodecanal | NS | 5.31 a | 4.18 a | 3.77 a |
| Tetradecanal | NS | 1.14 a | 0.96 a | 0.81 a |
| Tetradecanal | NS | 0.83 a | 0.88 a | 1.08 a |
| Terpenes | ||||
| o-Cymene | NS | 10.6 a | 14.8 a | 16.9 a |
| D-Limonene | NS | 196 a | 362 a | 378 a |
| γ-Terpinene | NS | 19.9 a | 38.2 a | 42.7 a |
| Terpinolene | NS | 2.74 a | 3.68 a | 5.53 a |
| Caryophyllene | NS | 3.51 a | 2.20 a | 1.96 a |
| α-Bergamotene | NS | 4.88 a | 2.60 b | 2.45 b |
| β-Bisabolene | NS | 3.33 a | 1.822 a | 2.271 a |
| Alcohols | ||||
| 1-Hexanol | ** | 1793 a | 1348 ab | 97 b |
| Fenchol | NS | 1.31 a | 1.28 a | 0.90 a |
| 1-Terpinenol | NS | 2.09 a | 1.99 a | 1.99 a |
| β-Terpineol | * | 2.27 a | 1.82 ab | 1.21 b |
| Terpinen-4-ol | NS | 3.62 a | 2.52 a | 2.20 a |
| L-α-Terpineol | NS | 5.94 a | 6.05 a | 5.24 a |
| Esters | ||||
| Acetic acid, hexyl ester | ** | 1013 a | 141 ab | 4.36 b |
| Octanoic acid methyl ester | NS | 0.36 a | 0.57 a | 0.75 a |
| Citronellol acetate | NS | 0.57 a | 0.22 a | 0.34 a |
| Methyl laurate | NS | 1.91 a | 1.51 a | 1.24 a |
| Ketones | ||||
| 6-methyl-5-Hepten-2-one | ** | 4.12 a | 1.78 b | 0.87 b |
| 2,6-di-tert-butyl-p-Benzoquinone | NS | 2.22 a | 1.92 a | 1.65 a |
| Carboxilic acids | ||||
| Hexanoic acid | NS | 6.61 a | 12.9 a | 11.6 a |
| Total (mg kg−1) | * | 46.92 a | 38.92 ab | 16.36 b |
| Volatile Compound | Concentration (μg 100 g−1) | ||||||
|---|---|---|---|---|---|---|---|
| ANOVA | Bagged ‘Dominga’ | Unbagged ‘Dominga’ | Bagged ‘Aledo’ | Unbagged ‘Aledo’ | Bagged ‘Doña María’ | Unbagged ‘Doña María’ | |
| Aldehydes | |||||||
| Hexanal | *** 1 | 538 b 2 | 284 b | 1579 a | 524 b | 350 b | 304 b |
| (E)-2-Hexenal | NS | 1317 a | 840 a | 1110 a | 441 a | 644 a | 611 a |
| Benzaldehyde | NS | 3.23 a | 2.16 a | 1.33 a | 4.70 a | 1.26 a | 4.19 a |
| Octanal | ** | 8.23 b | 48.4 a | 7.58 b | 8.68 b | 5.88 b | 9.85 b |
| Benzeneacetaldehyde | NS | 3.80 a | 2.81 a | 2.11 a | 12.1 a | 1.37 a | 8.34 a |
| Nonanal | NS | 54.5 a | 43.1 a | 66.6 a | 70.4 a | 46.6 a | 68.9 a |
| (E)-2-Nonenal | *** | 25.2 a | 3.55 b | 4.93 b | 3.28 b | 2.52 b | 3.37 b |
| Decanal | NS | 12.8 a | 11.0 a | 13.3 a | 14.9 a | 10.7 a | 17.9 a |
| β-Citral | NS | 1.11 a | 1.28 a | 1.19 a | 0.927 a | 1.11 a | 1.56 a |
| α-Citral | NS | 1.19 a | 1.15 a | 0.99 a | 1.06 a | 0.67 a | 1.14 a |
| Undecanal | NS | 1.39 a | 2.38 a | 1.44 a | 2.01 a | 1.28 a | 1.80 a |
| Dodecanal | NS | 4.28 a | 6.34 a | 3.88 a | 4.47 a | 3.56 a | 3.99 a |
| Tetradecanal | NS | 1.06 a | 1.23 a | 1.09 a | 0.82 a | 0.76 a | 0.86 a |
| Tetradecanal | NS | 0.71 a | 0.94 a | 1.11 a | 0.65 a | 0.78 a | 1.37 a |
| Terpenes | |||||||
| o-Cymene | NS | 11.0 a | 10.2 a | 13.9 a | 15.8 a | 16.7 a | 17.1 a |
| D-Limonene | NS | 186 a | 206 a | 373 a | 351 a | 533 a | 223 a |
| γ-Terpinene | NS | 19.1 a | 20.6 a | 39.6 a | 36.7 a | 54.6 a | 30.7 a |
| Terpinolene | NS | 2.88 a | 2.61 a | 4.04 a | 3.32 a | 4.89 a | 6.18 a |
| Caryophyllene | NS | 2.83 a | 4.18 a | 2.46 a | 1.929 a | 1.81 a | 2.12 a |
| α-Bergamotene | NS | 4.77 a | 4.99 a | 2.68 a | 2.52 a | 2.37 a | 2.52 a |
| β-Bisabolene | NS | 3.56 a | 3.09 a | 1.95 a | 1.69 a | 1.73 a | 2.81 a |
| Alcohols | |||||||
| 1-Hexanol | ** | 1090 ab | 2497 a | 175 b | 2523 a | 85.4 b | 109 b |
| Fenchol | NS | 1.43 a | 1.19 a | 1.12 a | 1.44 a | 0.81 a | 0.98 a |
| 1-Terpinenol | NS | 2.09 a | 2.09 a | 1.64 a | 2.35 a | 1.58 a | 2.39 a |
| β-Terpineol | * | 2.87 a | 1.67 ab | 1.52 ab | 2.12 ab | 1.08 b | 1.34 ab |
| Terpinen-4-ol | NS | 3.89 a | 3.36 a | 1.76 a | 3.28 a | 2.10 a | 2.30 a |
| L-α-Terpineol | NS | 6.74 a | 5.13 a | 5.03 a | 7.06 a | 4.56 a | 5.92 a |
| Esters | |||||||
| Acetic acid, hexyl ester | ** | 338 b | 1689 a | 7.96 b | 275 b | 4.20 b | 4.52 b |
| Octanoic acid methyl ester | NS | 0.09 b | 0.63 ab | 0.59 ab | 0.55 ab | 0.62 ab | 0.874 a |
| Citronellol acetate | NS | 0.65 a | 0.49 a | 0.13 a | 0.32 a | 0.22 a | 0.46 a |
| Methyl laurate | NS | 1.71 a | 2.11 a | 1.36 a | 1.65 a | 1.56 a | 0.93 a |
| Ketones | |||||||
| 6-methyl-5-Hepten-2-one | ** | 3.96 ab | 4.28 a | 0.602 c | 2.95 abc | 0.398 c | 1.34 bc |
| 2,6-di-tert-butyl-p-Benzoquinone | NS | 2.43 a | 2.00 a | 2.06 a | 1.77 a | 1.34 a | 1.97 a |
| Carboxilic acids | |||||||
| Hexanoic acid | NS | 6.93 a | 6.29 a | 11.8 a | 13.9 a | 14.0 a | 9.07 a |
| Total (mg kg−1) | * | 36.66 ab | 57.17 a | 34.44 ab | 43.40 ab | 18.05 ab | 14.67 b |
| Volatile Compound | Odor Threshold (μg 100 g−1) | Bagged ‘Dominga’ | Unbagged ‘Dominga’ | Bagged ‘Aledo’ | Unbagged ‘Aledo’ | Bagged ‘Doña María’ | Unbagged ‘Doña María’ | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OAV | ROC (%) | OAV | ROC (%) | OAV | ROC (%) | OAV | ROC (%) | OAV | ROC (%) | OAV | ROC (%) | ||
| Hexanal 1 | 0.45 2 | 1196 | 39.0 3 | 631 | 43.2 | 3510 | 78.6 | 1164 | 58.9 | 777 | 46.1 | 675 | 49.2 |
| (E)-2-Hexenal | 8.20 | 161 | 5.23 | 102 | 7.02 | 135 | 3.03 | 53.7 | 2.72 | 78.6 | 4.66 | 74.5 | 5.44 |
| 1-Hexanol | 250.00 | 4.36 | 0.14 | 9.99 | 0.68 | 0.70 | 0.02 | 10.1 | 0.51 | 0.34 | 0.02 | 0.44 | 0.03 |
| Octanal | 0.40 | 20.6 | 0.67 | 121 | 8.28 | 18.9 | 0.42 | 21.7 | 1.10 | 14.7 | 0.87 | 24.6 | 1.80 |
| o-Cymene | 0.50 | 22.0 | 0.72 | 20.4 | 1.40 | 27.7 | 0.62 | 31.6 | 1.60 | 33.4 | 1.98 | 34.2 | 2.50 |
| D-Limonene | 1.00 | 186 | 6.05 | 206 | 14.1 | 373. | 8.35 | 351 | 17.7 | 533 | 31.6 | 222 | 16.3 |
| Benzeneacetaldehyde | 0.63 | 6.02 | 0.20 | 4.46 | 0.31 | 3.35 | 0.08 | 19.2 | 0.97 | 2.18 | 0.13 | 13.2 | 0.97 |
| Linalool | 0.60 | 5.37 | 0.18 | 4.32 | 0.30 | 2.40 | 0.05 | 5.98 | 0.30 | 2.68 | 0.16 | 5.73 | 0.42 |
| Nonanal | 2.80 | 19.5 | 0.63 | 15.4 | 1.05 | 23.8 | 0.53 | 25.1 | 1.27 | 16.6 | 0.99 | 24.6 | 1.80 |
| (E)-2-Nonenal | 0.02 | 1325 | 43.2 | 187 | 12.8 | 259 | 5.81 | 172 | 8.72 | 132. | 7.85 | 177.6 | 13.0 |
| Decanal | 0.93 | 13.8 | 0.45 | 11.8 | 0.81 | 14.2 | 0.32 | 16.0 | 0.81 | 11.5 | 0.68 | 19.3 | 1.41 |
| Undecanal | 0.50 | 2.79 | 0.09 | 4.77 | 0.33 | 2.89 | 0.06 | 4.02 | 0.20 | 2.56 | 0.15 | 3.6 | 0.26 |
| Dodecanal | 0.05 | 80.7 | 2.63 | 120 | 8.19 | 73.2 | 1.64 | 84.4 | 4.27 | 67.2 | 3.98 | 75.2 | 5.49 |
| Caryophyllene | 1.00 | 2.83 | 0.09 | 4.18 | 0.29 | 2.46 | 0.06 | 1.93 | 0.10 | 1.81 | 0.11 | 2.12 | 0.15 |
| 2,6-di-tert-butyl-p-Benzoquinone | 0.11 | 22.1 | 0.72 | 18.2 | 1.25 | 18.7 | 0.42 | 16.1 | 0.81 | 12.1 | 0.72 | 17.9 | 1.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andreu-Coll, L.; Noguera-Artiaga, L.; Sendra, E.; Hernández, F. Impact of Preharvest Bagging on the Volatile Profile of Vinalopó Table Grapes. Agronomy 2025, 15, 1066. https://doi.org/10.3390/agronomy15051066
Andreu-Coll L, Noguera-Artiaga L, Sendra E, Hernández F. Impact of Preharvest Bagging on the Volatile Profile of Vinalopó Table Grapes. Agronomy. 2025; 15(5):1066. https://doi.org/10.3390/agronomy15051066
Chicago/Turabian StyleAndreu-Coll, Lucía, Luis Noguera-Artiaga, Esther Sendra, and Francisca Hernández. 2025. "Impact of Preharvest Bagging on the Volatile Profile of Vinalopó Table Grapes" Agronomy 15, no. 5: 1066. https://doi.org/10.3390/agronomy15051066
APA StyleAndreu-Coll, L., Noguera-Artiaga, L., Sendra, E., & Hernández, F. (2025). Impact of Preharvest Bagging on the Volatile Profile of Vinalopó Table Grapes. Agronomy, 15(5), 1066. https://doi.org/10.3390/agronomy15051066










