Abstract
Microplastics (MPs), as emerging pollutants, have elicited global concerns. However, few studies have evaluated MPs with varying sizes and their adverse effects on plant growth in farmland soils. In this study, a greenhouse pot experiment was conducted to evaluate the effects of polyethylene (PE) and polybutylene adipate terephthalate (PBAT) with two particle sizes (100 μm and 1000 μm) on the growth, photosynthetic properties, and antioxidant enzyme activity of buckwheat (Fagopyrum esculentum Moench), as well as soil properties. Overall, the results showed that MPs had a certain inhibitory effect on buckwheat growth, especially with the PE treatment of 100 μm particle size. The addition of PE and PBAT inhibited photosynthesis, induced oxidative stress, and decreased soil nutrient availability (specifically ammonium nitrogen, nitrate nitrogen, available potassium, and available phosphorus content), reducing above and belowground biomass. In addition, we observed that the type and size of MPs had a significant effect on buckwheat growth parameters. Degradable MPs (PBAT) showed less toxicity than non-degradable MPs (PE), and MPs with a smaller microplastic particle size (100 μm) displayed a greater inhibitory effect than larger ones (1000 μm). In conclusion, MPs showed significant inhibitory effects on the growth of buckwheat plants, highlighting the necessity for further research in this area.
1. Introduction
Microplastics (MPs, particles less than 5 mm) are emerging contaminants globally that have attracted researchers’ attention due to their potential threat to the terrestrial ecosystem, particularly in agricultural soils [1]. In agriculture, film mulching, drip irrigation tape, organic fertilizers, greenhouse materials, wastewater irrigation, and atmospheric deposition are identified as the main sources of MPs that enter the soil [2]. MPs can affect soil ecosystem functioning by changing soil structure and properties [3], microbial community structure, and diversity [4]; can be easily taken up or adsorbed by plant roots [5]; and can cause human health risks [6].
In 2019, around 370 million tons of plastic were produced globally, and approximately 28% of plastic waste was directly disseminated into the environment [7]. Plastic products can break down into plastic debris and continually accumulate in soil, which can then affect crop growth. This has already become a potential environmental issue, highlighting the need for further exploration of the mechanisms of microplastics’ effects on plant growth and soil properties [8].
Polyethylene (PE) is one of the most used plastics in agriculture, accounting for about 40% of the polymer composition [9]. Polyethylene microplastics are mainly derived from the degradation of plastic film mulching. PE plastics are characterized as having slow degradation and low recycling efficiency, which also has negative effects on soil properties and terrestrial animals [10]. Due to the deep concern regarding potential environmental pollution, biodegradable plastics have been developed to replace traditional plastic, such as polylactic acid (PLA), polybutylene succinate (PBS), and polybutylene adipate terephthalate (PBAT) [11,12,13]. These biodegradable plastics are promising alternatives to traditional plastic and can lead to a reduction in plastic residues in agriculture soils. However, they have also sparked debate regarding their potential environmental problems [14]. MPs in soil can affect plant growth via (1) affecting the soil nutrient availability by altering the soil’s physical, chemical, and biological properties [15] and (2) being adsorbed by the seed epidermis and root cell wall pores, thereby blocking pores in the seed capsule, and accumulating on root hairs in a later growth stage, disrupting the absorption and transportation of water and nutrients in the plant [16]. However, there is still a lack of studies on plant growth and soil properties using MPs of different degradability, especially considering different particle sizes.
The northeast region of Inner Mongolia serves as a crucial region for production, playing a vital role in ensuring a stable supply of essential food resources and contributing significantly to national food security [17]. Buckwheat (Fagopyrum esculentum Moench) is a minor food crop globally, yet it is a popular traditional food in Asian countries such as China, Japan, and Korea. In recent years, it has been widely grown and consumed in Europe and North America [18]. Due to its high content of flavonoids and dietary fiber, buckwheat is considered a nutritional food that is gaining global attention for its potential to lower blood pressure, blood sugar, and blood lipids [19]. It has also been demonstrated to inhibit diabetes and possesses antioxidative, anti-inflammatory, and antitumor properties. Common buckwheat seeds are enriched in Zn, Cu, and Mn, which is an important dietary source of essential trace elements for the human body [20]. Buckwheat is also used as a cover crop in agriculture due to its ability to enhance soil health, suppress weeds, and attract pollinators [21]. In some regions, particularly in parts of Europe and North America, buckwheat honey is also renowned for its rich flavor, distinct aroma, and numerous health benefits, such as its antioxidant properties and high mineral content [22].
In this semiarid region of China where buckwheat is produced, drip irrigation is one of the most water-efficient techniques for farmland irrigation [23]. Drip irrigation is the predominant irrigation method in this region for achieving higher buckwheat yields. Due to the frequent use of drip tape, buckwheat production is exposed to environments with agricultural mulch. Thus, the accumulation of MPs in buckwheat may pose significant risks to human health. Hence, it is thus imperative to evaluate the effects of MPs on the soil–plant system. Such research will help in understanding the full scope of MPs’ impacts and in developing mitigation strategies to protect both environmental and human health.
Some studies have evaluated the impact of MPs on plant growth and soil properties, such as those conducted by Ren et al. [4], Lian et al. [11], and Li et al. [24]. However, these studies have primarily focused on the effect of MP concentrations on the soil–plant system. Consequently, there is a lack of research specifically on how MPs with varying sizes and degradability affect plant growth, soil properties, and specifically plant photosynthesis and antioxidant enzyme activity. Therefore, the aim of this study was to address the gaps in understanding the adverse effects of MPs of various sizes on plant growth, soil properties, and particularly plant photosynthesis and antioxidant enzyme activity. A greenhouse pot experiment was designed to test two different sizes (100 μm and 1000 μm) of PE and PBAT on common buckwheat growth, soil chemical properties, photosynthesis characteristics, and physiological indicators. We hypothesized that (1) PBAT MPs and PE MPs have varying impacts on plant growth and soil properties due to their different biodegradability, and (2) non-degradable MPs (PE) have a greater adverse effect than degradable MPs (PBAT), and a smaller microplastic particle size (100 μm) leads to a greater inhibitory effect than larger particles (1000 μm).
2. Materials and Methods
2.1. Microplastics
PE and PBAT powder were purchased from Dongguan City Jiecheng Plastic Chemical Co., Ltd., Guangdong, China. Each type of MP has two different particle sizes: 100 μm and 1000 μm. The particle sizes of four types of MPs were determined using scanning electron microscopy (SEM, Hitachi S-3400 N, SEM, Hitachi S-3400N, Hitachi High-Technologies Corporation, Tokyo, Japan) (Figure 1).

Figure 1.
Scanning electron microscopy images of the PBAT (polybutylene adipate terephthalate) of 100 μm (a) and 1000 μm (b) particle sizes; PE (polyethylene) of 100 μm (c) and 1000 μm (d) particle sizes.
2.2. Tested Plant and Soil
In the present study, the crop plant used was common buckwheat. The seed cultivar of buckwheat was Tongqiao No.5, which was purchased from Tongliao Agriculture and Animal Husbandry Science Research Institute, Tongliao, Inner Mongolia, China. Prior to use, the seeds were sterilized with a 3% H2O2 solution for 12 h and then rinsed repeatedly with distilled water. This procedure was performed to eliminate potential surface contaminants, such as fungi and bacteria, without damaging the seeds. Seeds of uniform size were then selected for further use.
The tested surface soil (0–15 cm) was collected from a field in Xiliaohe Village, Tongliao, Inner Mongolia, China (43°76′ N, 122°42′ E). This field had no history of using plastic materials. The collected soil was air-dried at room temperature for two weeks then sieved through a 2 mm sieve and stored at room temperature for further use. The basic physicochemical properties of the tested soil are presented in Table 1.

Table 1.
Basic physicochemical properties of the experimental soil.
2.3. Experiment Design and Setup
This experiment was conducted from June to August 2022. Two types of MPs were used in this experiment: (1) polyethylene (PE) and (2) polybutylene adipate terephthalate (PBAT). The two particle sizes of PE and PBAT were 100 μm and 1000 μm. The microplastics were mixed into the soil at a weight ratio of 0.1% (w/w), which was selected based on previous studies [3]. Five treatments were established: CK (control, clean soil without PE and PBAT), PE1 (soil amended with 100 μm PE), PE2 (soil amended with 1000 μm PE), PBAT1 (soil amended with 100 μm PBAT), and PBAT2 (soil amended with 1000 μm PBAT). Each treatment was replicated in triplicate.
The pots used in this experiment were 18.5 cm high with a square side length of 10.5 cm at the bottom and 16 cm at the top, resulting in a volume of 3.3 L. Two kilograms of pre-prepared soil were mixed with microplastics and homogenized and then transferred into each plastic pot. Deionized water was added to all pots, and the soil moisture was adjusted to 75% of the field maximum moisture capacity. Subsequently, the pots were placed in a dark room and pre-incubated for 10 days. Fifteen buckwheat seeds were grown in each pot at 1 cm depth, and after one week of growth, nine seedlings per pot were selected and retained for the experiment. The soil moisture was maintained at 75% of the field’s maximum moisture capacity by regularly adding deionized water and weighing the pots every other day throughout the experiment. At weeks 3 and 5, 100 mL Hoagland nutritive solution was added to each pot to ensure optimal development. Detailed information on the Hoagland nutritive solution is presented in Table S1. All pots were placed randomly in a greenhouse (natural light, temperature: 22–30 °C and humidity: 75–85%), and their positions were shifted every other day. At 20, 40, and 60 days after sowing (DAS), plant growth parameters, photosynthesis indicators, and antioxidant enzyme activities were measured. On the 60th day of the experiment, soil samples were collected for the determination of chemical properties.
2.4. Measurement of Analytical Indicators
2.4.1. Buckwheat Growth Parameters
The plant height was measured using steel tape. Three plants were randomly selected from each plot, rinsed with distilled water to remove any soil particles, and then separated into roots and shoots. Above- and belowground buckwheat biomass was dried in an oven at 80 °C until a constant weight was achieved.
2.4.2. Chlorophyll and Photosynthetic Parameters
The relative chlorophyll content and photosynthetic parameters were measured. The three highest fully expanded leaves on each plant were selected based on their size and developmental stage for consistency across measurements and were measured with a portable plant SPAD-502Plus relative chlorophyll meter (Konica Minolta Inc., Tokyo, Japan). The average of SPAD values from three leaves was represented as the relative chlorophyll content.
The photosynthetic parameters, including the net photosynthesis rate (Pn), transpiration rate (Tr), intercellular CO2 concentration (Ci), and stomatal conductance (Gs), were measured. The most fully expanded fresh leaves were measured and recorded using an LI-Cor LI-6400XT Portable Photosynthesis System (LI-6400, LI-COR Inc., Lincoln, NE, USA). Measurements were taken in the noon hours (9:00–11:00) to avoid potential stomatal closure during the middle of the day. The CO2 concentration and air flow were set to 400 μmol (CO2) mol–1 and 500 μmol s–1, respectively.
2.4.3. Leaf Antioxidant Activity
After the determination of chlorophyll content and photosynthetic parameters, the upper-third, fully expanded leaves of buckwheat seedlings were harvested, immediately frozen in liquid nitrogen, and subsequently stored at −20 °C. This procedure was adopted to preserve the enzymatic activity and ensure the accurate measurement of leaf physiological parameters, including antioxidant enzyme activities.
Fresh leaves (approximately 0.20 g) were ground with 5 mL phosphate buffer solution (pH = 7.8) containing 0.05 M Na2HPO4 and 0.05 M NaH2PO4, followed by centrifugation at 12,000 r min−1 for 15 min at 4 °C. The supernatant was then collected for enzyme activity measurement. The superoxide dismutase (SOD, EC 1.15.1.1), peroxidase (POD, EC 1.11.1.7), and catalase (CAT, EC 1.11.1.6) activities, as well as malonic dialdehyde (MDA) content were determined. The above parameters were measured following the instructions of the assay kit purchased from Coming Biotechnology Co., Ltd. (Suzhou, China).
2.4.4. Soil Chemical Properties
After all plants were harvested, soil samples were collected from each plot and then combined to achieve a composite sample. The representative samples were put into a plastic bag and brought to the laboratory for further analysis. Any visible plant roots, stones, litter, and debris were removed by hand from all of the collected soil samples; then, all of the soil samples were air-dried in the shade for two weeks for chemical analysis. The potassium dichromate oxidation method (K2Cr2O7) was employed to quantify the soil organic carbon (SOC), followed by applying the van Bemmelen conversion factor of 1.724 to calculate the soil organic matter (SOM) [25]. Total nitrogen content (TN) was determined using the Kjeldahl method [26]. Available nitrogen (NH4+-N and NO3−-N) content was extracted from fresh soil using 2 mol L−1 KCl and quantified using a continuous flow analyzer. Soil available phosphorus (AP) content was determined using the Olsen method [27]. Available potassium (AK) content was measured using a flame photometer after extraction with ammonium acetate [28].
2.5. Data Analysis
All of the data are expressed as mean values and standard deviation (SD). A one-way analysis of variance (ANOVA) was performed using R software (Version 4.2.2) to test the significance of MP addition in soil to chemical properties and plant growth parameters at three sampling times. The effects of all three factors (the type and size of MPs and sampling times) and their interactive effects were assessed using a three-way ANOVA. A correlation analysis was conducted using Pearson’s coefficient analysis in R software. Figures were prepared using Origin Pro 2021.
3. Results
3.1. Buckwheat Growth
The microplastics showed an inhibitory effect on plant growth, with variations observed across different sampling times (Figure 2). Both the MP type and size had a significant effect on height (p < 0.05) (Table S2). The difference in plant height among the treatments was minimal at 20 DAS. However, treatments with smaller MP particle sizes exhibited significantly reduced plant height compared to CK. At 40 DAS, only PE2 showed a 16.7% decrease in plant height relative to CK. At 60 DAS, except for PBAT2, the presence of PE and PBAT significantly inhibited plant growth compared to the control (Figure 2a). An inhibitory effect was observed on aboveground biomass accumulation, especially at 40 and 60 DAS, and a size-dependent effect was observed both in PE and PBAT treatments. However, a minor inhibitory effect on underground biomass was noted at 40 and 60 DAS, with some significant differences observed. Among these, PE1 exhibited the strongest inhibition effect on root growth (Figure 2b).
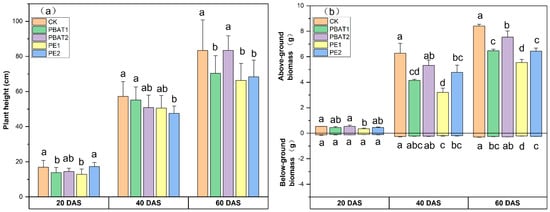
Figure 2.
Effects of different types and sizes of MPs on plant height (a) and aboveground and belowground biomass (b) at 20, 40, and 60 days after sowing. Different lowercase letters indicate significant differences between treatments (p < 0.05). CK: Control, plants treated with clean soil without PE and PBAT. PBAT1 and PBAT2: plants treated with poly (butylene adipate-co-terephthalate) microplastics at sizes of 100 μm and 1000 μm, respectively. PE1 and PE2: plants treated with polyethylene microplastics at sizes of 100 μm and 1000 μm, respectively.
3.2. Chlorophyll Content and Photosynthetic Parameters
The type of MP significantly affected Pn (p < 0.05), while the size of MPs significantly affected Gs (p < 0.01). Both the MP type and size had a significant effect on Tr (p < 0.05) (Table S2). Compared to CK, the addition of PE and PBAT with different sizes showed varying effects on photosynthesis parameters at different cultivation times (Figure 3). At 20 and 40 DAS, PE treatments exhibited a lower Pn value, while PBAT treatments showed a minor inhibitory effect, especially with larger sizes (Figure 3a). Regarding Gs, no significant difference was found at 20 or 40 DAS compared to CK. However, the lowest Gs value was observed in PE1 at 60 DAS, and smaller sizes of PE had lower Gs values than larger sizes at 40 and 60 DAS (Figure 3b). PE1 had the lowest Tr value at 20 and 40 DAS, up to 34.32% lower than that of CK (Figure 3c). The addition of PE and PBAT resulted in an increasing trend of Ci at 40 and 60 DAS, but no significant difference was observed in Ci among different sizes within the same type of MP (Figure 3d).
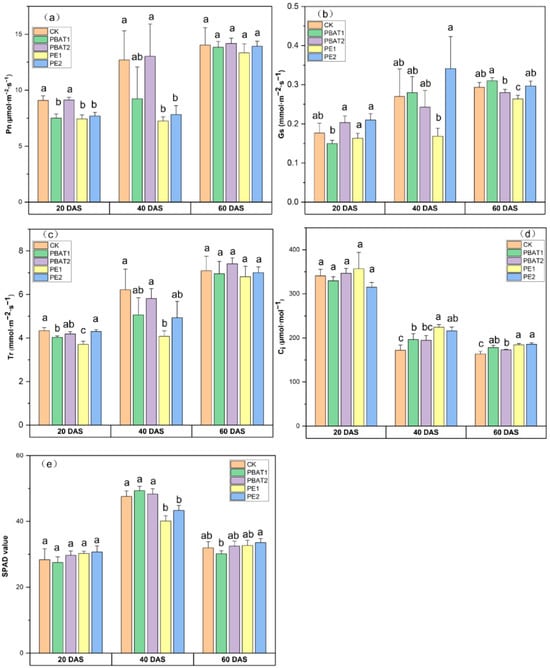
Figure 3.
Effects of different types and sizes of MPs on Pn (a), Gs (b), Tr (c), Ci (d), and SPAD (e) values at 20, 40, and 60 days after sowing. Different lowercase letters indicate significant differences between treatments (p < 0.05). CK: Control, plants treated with clean soil without PE and PBAT. PBAT1 and PBAT2: plants treated with poly (butylene adipate-co-terephthalate) microplastics at sizes of 100 μm and 1000 μm, respectively. PE1 and PE2: plants treated with polyethylene microplastics at sizes of 100 μm and 1000 μm, respectively.
3.3. Leaf Antioxidant Activity
The buckwheat plant showed a strong oxidative-stress response to MP addition (Figure 4). Only the MP type and DAS showed a significant effect on the activity of SOD and POD and MDA content (Table S2). SOD activity was significantly enhanced in the presence of MPs, and a type-dependent effect was observed. Regardless of size, PE treatments showed a higher SOD value than others, with CK having the lowest value. PBAT1 showed a higher SOD value than PBAT2 at 20 and 40 DAS, while PE1 and PE2 mostly did not show a significant difference except at 40 DAS (Figure 4a). POD activity in all treatments remained relatively stable at 20 and 40 DAS. However, an obvious significant difference was observed at 60 DAS, with the POD activity in MP treatment increasing by up to 61.03% compared to CK. Additionally, there was no significant difference in POD activity between different sizes of MPs of the same type (Figure 4b). The addition of MPs increased CAT activity, and the changes in CAT activity followed the same trend at 20 and 60 DAS, with all MP treatments showing increased CAT activity compared to CK, except for PBAT1. Among the treatments, PE2 exhibited the highest CAT activity. At 40 DAS, only PBAT2 showed an increase in CAT activity by 8.30% compared to CK (Figure 4c). An increasing trend was observed in MDA contents throughout the entire buckwheat cultural period. No significant difference was observed in MDA contents among the same type of MP, regardless of size. The MDA contents increased by up to 52.43% in PE and 64.91% in PBAT treatments (Figure 4d).
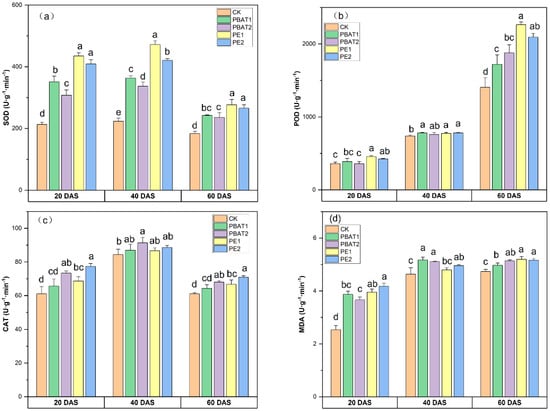
Figure 4.
Effects of different types and sizes of MPs on the activities of SOD (a), POD (b), CAT (c), and the contents of MDA (d) at 20, 40, and 60 days after sowing. Different lowercase letters indicate significant differences between treatments (p < 0.05). CK: Control, plants treated with clean soil without PE and PBAT. PBAT1 and PBAT2: plants treated with poly (butylene adipate-co-terephthalate) microplastics at sizes of 100 μm and 1000 μm, respectively. PE1 and PE2: plants treated with polyethylene microplastics at sizes of 100 μm and 1000 μm, respectively.
3.4. Soil Chemical Properties
All PBAT and PE treatments showed no significant change in soil organic matter (SOM) content (Table 2). The soil total nitrogen content in PBAT2 treatment decreased by 0.12 g kg−1 compared to CK. The addition of PBAT and PE significantly reduced soil NH4+-N and AP content, while no significant effect-size differences were observed between the same types of plastic additions. PE2 had the lowest value for both parameters, 17.00% and 11.07% lower than CK in NH4+-N and AP content, respectively. Regarding soil NO3−-N and AK content, only PE treatments induced a significant decrease compared to CK. Among the PE treatments, PE2 showed the largest reduction, with a decrease of 6.39% in NO3−-N and 19.87% in AK compared to CK.

Table 2.
Effects of MP addition on soil chemical properties.
3.5. Relationship Between Soil Chemical Properties and Buckwheat Growth Parameters
The findings from the correlation analysis revealed a positive relationship between buckwheat plant (above- and belowground) biomass and soil chemical properties such as NH4+-N, AK, and AP while indicating a negative correlation with Ci, SOD, POD, and CAT activity. Conversely, a distinct negative correlation was observed between soil chemical properties (NH4+-N, NO3−-N, AK, and AP) and Ci, SOD, POD, and CAT activity (Figure 5).
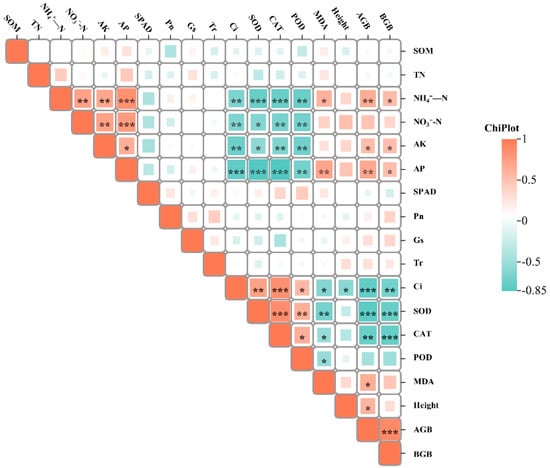
Figure 5.
Pearson correlation between buckwheat growth parameters and soil chemical properties. SOD, superoxide dismutase; POD, peroxidase; CAT, catalase; MDA, malonic dialdehyde; Pn, net photosynthesis rate; Gs, stomatal conductance; Tr, transpiration rate; Ci, intercellular CO2 concentration; SPAD, SPAD value; SOM, soil organic matter; TN, total nitrogen content; NH4+-N, ammonium nitrogen; NO3−-N, nitrate nitrogen; AP, available phosphorus; AK, available potassium; Height, plant height; AGB, aboveground plant biomass; and BGB, belowground plant biomass. *, **, and *** indicate significant correlations at the 0.5, 0.1, and 0.001 levels, respectively.
4. Discussion
4.1. Effects of MPs on Buckwheat Growth
Microplastics, as emerging agricultural pollutants, have demonstrated an inhibitory effect on plant growth [29]. In this study, the addition of PBAT and PE resulted in a significant decrease in both the aboveground and belowground biomass of buckwheat. The potential inhibitory mechanism in plant growth is the presence of microplastics, which may alter the key soil properties such as bulk density, porosity, aggregates, and water-holding capacity [30]. This can change the water storage and further affect the crop’s uptake process, ultimately resulting in decreased biomass accumulation. In the present study, aboveground and belowground buckwheat biomass was found to be positively correlated with soil nutrients (Figure 5). However, the presence of PBAT and PE led to a decrease in soil NH4+-N, AP, and AK content. This reduction in available nutrients limited the absorption capacity of buckwheat roots, thereby restricting buckwheat growth. It was also supported by the increase in photosynthesis inhibition and antioxidant enzyme activities, which indicated that buckwheat growth suffered an inhibitory effect, resulting in the reduction in plant biomass accumulation (Figure 2 and Figure 3).
In this study, the varying adverse effects of two types of MPs on buckwheat performance were investigated. The results revealed the distinct impacts of PE and PBAT on buckwheat growth. Overall, PE induced a greater inhibitory effect on plant growth than PBAT did, which is supported by other studies. For instance, Yang and Gao [31] found that the negative effects from PE microplastics on rice plant growth were stronger than those of PBAT. Koskei et al. [32] reported that exposure to non-biodegradable plastic showed more negative effects on maize growth and production. However, Lian et al. [11] argued that the biodegradability is an important factor affecting plant growth and reported that biodegradable MPs (such as polylactic acid, PLA) exhibited an inhibitory effect on root length and root fresh weight compared to unbiodegradable MPs (such as polyethylene and PE). One potential reason for this finding is the stress induced by the degradation of byproducts [33].
Our results found that smaller MPs showed a greater inhibitory effect on plant growth. This may be because they can easily enter the plant root and clog pores, reducing the uptake of water and nutrients by plants, thereby reducing biomass accumulation [34]. Similar studies also confirmed that small MPs (<2 μm) can penetrate the root stele by entering cracks, particularly at sites of lateral roots and root tips [35].
4.2. Effects of MPs on Buckwheat Photosynthesis
Photosynthesis is an important process in oxygen and energy supply. Recent studies have confirmed that MPs exhibit negative effects on SPAD values and photosynthetic indices, such as Pn, Gs, Ci, and Tr [4]. In the present study, we also observed that the addition of PE and PBAT reduced SPAD values as well as Gs, Tr, and Pn values. This may be due to the severe environmental pollution caused by MPs, leading to significant alterations in both soil physicochemical properties and crop physiology [36]. However, photosynthetic parameters in both PBAT and PE treatments presented various changes during the buckwheat growth period. These results may indicate that MPs have a complex effect on the soil–plant system. Therefore, more in-depth studies on the application of MPs in soil properties and plant physiological indices are needed to explore the potential mechanism.
Both biodegradability and size are crucial factors that influence plant growth. Compared to biodegradable microplastics, non-biodegradable microplastics show more harmful effects on plant growth. In this study, smaller non-biodegradable microplastics (PE, 100 μm) showed more negative effects on buckwheat photosynthesis. It is well known that the transport of MPs in soils is closely related to their particle size. Theoretically, smaller MPs are more likely to enter plant roots and have toxic effects on plants via possible mechanisms such as physical blockage and mechanical damage [37].
4.3. Effects of MPs on Buckwheat Leaf Antioxidant Enzyme Activity
Antioxidant enzymes, such as SOD, POD, and CAT, play a crucial role in scavenging oxygen species (ROS) that are produced during normal plant metabolism, but excess ROS are generated when plants suffer xenobiotic stress [38]. Higher levels of ROS can cause damage to cells and tissues and disrupt normal metabolic processes [39]. In many cases, the activities of SOD, POD, and CAT increase to defend against the toxicity induced by the addition of MPs [37].
In the present study, regardless of their particle size, SOD, POD, and CAT activities generally increased across the three sampling times. This indicated that both PE and PBAT had a toxic effect on buckwheat growth and induced oxidative stress, as confirmed by numerous other studies [37]. In most cases, treatments with the same type of MP but different particle sizes led to a significant difference in SOD, POD, and CAT activities, suggesting that MP particle size is an important factor affecting the plant defense system. PE treatments generally exhibited higher antioxidant enzyme activity than PBAT treatments. Typically, when environmental stress exceeds the regulating ability of the antioxidant defense system, the defense ability may be lost [38]. Considering the reduction in biomass accumulation, it was indicated that the increased activities of POD, SOD, and CAT exceeded the buckwheat’s defense ability.
Some reports have found that exposure to MPs can cause severe oxidative damage during the early growth stage, resulting in higher activities of CAT, POD, and SOD both aboveground and belowground at 50 days after transplanting (DAT) compared to those at 80 DAT [40]. However, our findings revealed that CAT and SOD activities were higher at 20 and 40 days after sowing, while POD activity was higher at 60 days after sowing. This suggested that the plant defense system exhibited a complex response to the toxic effects of MPs, which may also be influenced by other factors such as plant species, culture duration, and the types and size of MPs [41,42].
MDA, a decomposition product of polyunsaturated fatty acid peroxidation in the cells, has been used as an indicator to evaluate the extent of membrane lipid peroxidation damage [38]. MDA is typically positively correlated with ROS levels [37]. In this study, soil treatment with PE and PBAT resulted in a decrease in MDA content in buckwheat leaves, while no significant effect was observed based on MP particle size. This indicated that there was an excessive accumulation of ROS that was not quickly scavenged by antioxidant enzymes, leading to increased lipid peroxidation and affecting the normal metabolism of buckwheat.
4.4. Effects of MPs on Soil Chemical Indicators
Numerous studies in microplastic ecology have demonstrated that exposure to microplastics can lead to diverse alterations in soil properties, including changes in pH levels, soil bulk density, water-holding capacity, and soil nutrient dynamics [43,44]. In the present study, soil chemical properties, especially soil available nutrients, were clearly influenced by PBAT and PE addition (Table 1). We did not find a significant influence of PBAT and PE addition on soil organic matter (Table 2). Some studies suggested that biodegradable plastics can be easily decomposed and utilized by microorganisms, leading to an increase in soil organic carbon. However, PE microplastics may disrupt microbial community composition and activity, thus potentially slowing down the decomposition of soil organic matter [45,46]. Qian et al. [47] reported that the accumulation of plastic led to a decrease in soil organic matter and total nitrogen content. These inconsistencies highlight the multifaceted impact of microplastics on specific soil properties, underscoring the need for further investigation into their varied effects. Our results revealed that both PE and PBAT MPs significantly decreased soil NO3−-N, NH4+-N, AP, and AK content. This finding was supported by Wang et al. [48], who revealed that PE and PBAT addition significantly decreased soil NO3−-N, NH4+-N, and AK content but increased AP content [49]. We also observed that the inhibitory effects on the soil properties mentioned above were more pronounced in the PE treatment than the PBAT treatment. However, some studies have argued that biodegradable MPs can be degraded easily by microorganisms compared to non-biodegradable MPs. The degradation process is influenced by various factors, including temperature, humidity, incubation time, polymer size, and types of microorganisms [50,51]. Nevertheless, some studies found that both biodegradable and non-biodegradable MPs have no significant effect on some soil physiochemical properties, such as pH [49], electrical conductivity [52], and total sulfur content [52]. The effects of biodegradable and non-biodegradable microplastics on soil properties vary depending on experimental conditions, application rates, plant species, soil type, and incubation time. Therefore, the long-term effects of conventional and biodegradable microplastics on soil–plant systems should be investigated across various soil and plant types.
5. Conclusions
Our results indicated that the addition of PE and PBAT inhibited buckwheat above- and belowground biomass and photosynthesis, decreased soil nutrient content, and increased antioxidant enzyme activities. Buckwheat showed type- and particle size-dependent responses to the two types of microplastics. Compared to CK, the addition of MPs reduced buckwheat plant height and decreased aboveground biomass by up to 20.4% and 34.07%, respectively, in the PE1 treatment. Additionally, soil TN content decreased by 11.65% in PBAT2, while NH4+-N, NO3−-N, AP, and AK were reduced by up to 17.00%, 6.39%, 11.07%, and 19.87%, respectively, in PE2. Regardless of MP particle size, PBAT showed a lower inhibitory effect than PE treatments. Compared to larger MPs, small MPs tended to cause more severe adverse impacts. Overall, our results showed various inhibitions of buckwheat growth in the presence of PE and PBAT throughout the entire cultivation period. While PBAT MPs show promise as a less harmful alternative to conventional plastics, a thorough understanding of their effects on the soil–plant system is essential.
This study should be considered a preliminary exploration of the effects of microplastics on soil and plant parameters. Further research is needed to assess their broader ecological impacts, including effects on soil microbial communities and additional soil properties. Studies incorporating varying application rates, multiple plant species, and long-term field trials will be essential to fully understand these interactions. A comprehensive investigation will help determine whether the benefits of using biodegradable plastics such as PBAT in agriculture outweigh potential drawbacks, thereby guiding future practices toward environmental sustainability.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy15051064/s1, Table S1: Detailed information about Hoagland nutritive solution in this study, Table S2: ANOVA of effect of MPs types(T), sizes (S) and days after sowing (DAS) on above- and below-ground biomass, antioxidants activity and photosynthetic parameters.
Author Contributions
L.Z.: Conceptualization, Methodology, Formal analysis, Investigation, Writing—original draft preparation, Writing—review and editing. J.C.: Methodology, Investigation, Formal analysis. Y.Z. (Yufen Zhang): Methodology, Investigation, Formal analysis. Y.Z. (Yi Zhou): Investigation, Resources, Methodology. Q.W.: Investigation, Resources, Methodology. B.Z.: Conceptualization, Writing—review and editing, Funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research is supported by the China Agricultural Research System of Oat and Buckwheat (CARS-07).
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare that they have no competing interests.
References
- Meng, F.; Yang, X.; Riksen, M.; Geissen, V. Effect of different polymers of microplastics on soil organic carbon and nitrogen—A mesocosm experiment. Environ. Res. 2022, 204, 111938. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, J.; Liu, Y.; Chen, L.; Tao, S.; Liu, W. Distribution characteristics of microplastics in agricultural soils from the largest vegetable production base in China. Sci. Total Environ. 2021, 756, 143860. [Google Scholar] [CrossRef]
- Qi, Y.; Beriot, N.; Gort, G.; Lwanga, E.; Gooren, H.; Yang, X.; Geissen, V. Impact of plastic mulch film debris on soil physicochemical and hydrological properties. Environ. Pollut. 2020, 266, 115097. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Tang, J.; Wang, L.; Liu, Q. Microplastics in soil-plant system: Effects of nano/microplastics on plant photosynthesis, rhizosphere microbes and soil properties in soil with different residues. Plant Soil. 2021, 462, 561–576. [Google Scholar] [CrossRef]
- Li, R.; Tu, C.; Li, L.; Wang, X.; Yang, J.; Feng, Y.; Zhu, X.; Fan, Q.; Luo, Y. Visual tracking of label-free microplastics in wheat seedlings and their effects on crop growth and physiology. Hazard. Mater. 2023, 456, 131675. [Google Scholar] [CrossRef]
- Sun, X.; Yuan, X.; Jia, Y.; Feng, L.; Zhu, F.; Dong, S.; Liu, J.; Kong, X.; Tian, H.; Duan, J.; et al. Differentially charged nanoplastics demonstrate distinct accumulation in Arabidopsis thaliana. Nat. Nanotechnol. 2020, 15, 755–760. [Google Scholar] [CrossRef] [PubMed]
- An Analysis of European Plastics Production, Demand and Waste Data, in Plastics—The Facts 2020; Plastics Europe: 2020. Available online: https://plasticseurope.org/knowledge-hub/plastics-the-facts-2020/ (accessed on 3 March 2024).
- Zhang, K.; Hamidian, A.; Tubić, A.; Zhang, Y.; Fang, J.; Wu, C.; Lam, P. Understanding plastic degradation and microplastic formation in the environment: A review. Environ. Pollut. 2021, 274, 116554. [Google Scholar] [CrossRef]
- Liu, M.; Lu, S.; Song, Y.; Lei, L.; Hu, J.; Lv, W.; Zhou, W.; Cao, C.; Shi, H.; Yang, X.; et al. Microplastic and mesoplastic pollution in farmland soils in suburbs of Shanghai, China. Environ. Pollut. 2018, 242, 855–862. [Google Scholar] [CrossRef]
- Silva, C.J.M.; Silva, A.; Campos, D.; Soares, A.; Pestana, J.; Gravato, C. Lumbriculus variegatus (oligochaeta) exposed to polyethylene microplastics: Biochemical, physiological and reproductive responses. Ecotoxicol. Environ. Saf. 2021, 207, 111375. [Google Scholar] [CrossRef]
- Lian, Y.; Liv, W.; Shi, R.; Zeb, A.; Wang, Q.; Li, J.; Zheng, Z.; Tang, J. Effects of polyethylene and polylactic acid microplastics on plant growth and bacterial community in the soil. Hazard. Mater. 2022, 435, 129057. [Google Scholar] [CrossRef]
- Rafiqah, S.A.; Khalina, A.; Harmaen, A.; Tawakkal, I.; Zaman, K.; Asim, M.; Nurrazi, M.; Lee, C. A review on properties and application of bio-based poly (butylene succinate). Polymer 2021, 13, 1436. [Google Scholar] [CrossRef] [PubMed]
- Zumstein, M.T.; Schintlmeister, A.; Nelson, T.; Wagner, R.; Kohler, H.; Mcneill, K.; Sander, M. Biodegradation of synthetic polymers in soils: Tracking carbon into CO2 and microbial biomass. Sci. Adv. 2018, 4, eaas9024. [Google Scholar] [CrossRef]
- Moreno, M.M.; González-Mora, S.; Villena, J.; Campos, J.A.; Moreno, C. Deterioration pattern of six biodegradable, potentially low-environmental impact mulches in field conditions. Environ. Manage. 2017, 200, 490–501. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, S.; Xu, J.; Allen, S.D.; Khan, S.; Nadir, S.; Arif, M.S.; Yasmeen, T. Unraveling consequences of soil micro- and nano-plastic pollution on soil-plant system: Implications for nitrogen (N) cycling and soil microbial activity. Chemosphere 2020, 260, 127578. [Google Scholar] [CrossRef]
- Bosker, T.; Bouwman, L.J.; Brun, N.R.; Behrens, P.; Vijver, M.G. Microplastics accumulate on pores in seed capsule and delay germination and root growth of the terrestrial vascular plant Lepidium sativum. Chemosphere 2019, 226, 774–781. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Pang, Z.; Lv, J.; Ju, H.; Li, L.; Fu, J. Satellite observations reveal decreasing soil erosion in Northeast Inner Mongolia, China, over the past four decades. Front. Earth Sci. 2022, 10, 988521. [Google Scholar]
- Tohgi, K.; Kohno, K.; Takahashi, H.; Matsuo, H.; Nakayama, S.; Morita, E. Usability of Fag e 2 ImmunoCAP in the diagnosis of buckwheat allergy. Arch. Dermatol. Res. 2011, 303, 635–642. [Google Scholar] [CrossRef]
- Domingos, I.F.N.; Bilsborrow, P.E. The effect of variety and sowing date on the growth, development, yield and quality of common buckwheat (Fagopyrum esculentum Moench). Eur. J. Agron. 2021, 126, 126264. [Google Scholar] [CrossRef]
- Bonafaccia, G.; Marocchini, M.; Kreft, I. Composition and technological properties of the flour and bran from common and tartary buckwheat. Food Chem. 2003, 80, 9–15. [Google Scholar] [CrossRef]
- Boglaienko, D.; Soti, P.; Shetty, K.G.; Jayachandran, K. Buckwheat as a cover crop in Florida: Mycorrhizal status and soil analysis. Agroecol. Sustain. Food Syst. 2014, 38, 1033–1046. [Google Scholar] [CrossRef]
- Ongalbek, D.; Tokul-Ölmez, Ö.; Şahin, B.; Küçükaydın, S.; Aydoğmuş-Öztürk, F.; Sıcak, Y.; Yeskaliyeva, B.; Öztürk, M. Classification of buckwheat honey produced in Kazakhstan according to their biochemical ingredients and bioactivities by chemometric approach. Food Chem. 2024, 451, 139409. [Google Scholar] [CrossRef] [PubMed]
- Sa, R.; Yang, H.; Zhang, R.; Li, Y. Shallow-buried drip irrigation promoted the enrichment of beneficial microorganisms in surface soil. Rhizosphere 2023, 28, 100776. [Google Scholar]
- Li, W.; Wang, Z.; Li, W.; Li, Z. Impacts of microplastics addition on sediment environmental properties, enzymatic activities and bacterial diversity. Chemosphere 2022, 307, 135836. [Google Scholar] [CrossRef] [PubMed]
- Mebius, L.J. A rapid method for the determination of organic carbon in soil. Anal. Chim. Acta. 1960, 22, 120–124. [Google Scholar] [CrossRef]
- Barbano, D.M.; Clark, J.L.; Dunham, C.E.; Flemin, R.J. Kjeldahl method for determination of total nitrogen content of milk: Collaborative study. Assoc. Off. Agric. Chem. 1990, 73, 849–859. [Google Scholar] [CrossRef]
- Olsen, S.; Sommers, L.; Page, A. Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties of Phosphorus; ASA Monograph 9; Wiley: New York, NY, USA, 1982; pp. 403–430. [Google Scholar]
- Helmke, P.A.; Sparks, D.L. Lithium, Sodium, Potassium, Rubidium, and Cesium. In Methods of Soil Analysis: Part 3 Book Series No.5. Soil Science Society of America; Madison, Ed.; Wiley: New York, NY, USA, 1996; pp. 551–573. [Google Scholar]
- Qi, Y.; Yang, X.; Mejia Pelaez, A.; Huerta Lwanga, E.; Beriot, N.; Gertsen, H.; Garbeva, P.; Geissen, V. Macro- and micro- plastics in soil-plant system: Effects of plastic mulch film residues on wheat (Triticum aestivum) growth. Sci. Total Environ. 2018, 645, 1048–1056. [Google Scholar] [CrossRef]
- Zhang, Z.; Cui, Q.; Chen, L.; Zhu, X.; Zhao, S.; Duan, C.; Zhang, X.; Song, D.; Fang, L. A critical review of microplastics in the soil-plant system: Distribution, uptake, phytotoxicity and prevention. J. Hazard. Mater. 2022, 424, 127750. [Google Scholar] [CrossRef]
- Yang, C.; Gao, X. Impact of microplastics from polyethylene and biodegradable mulch films on rice (Oryza sativa L.). Sci. Total Environ. 2022, 828, 154579. [Google Scholar] [CrossRef]
- Koskei, K.; Munyasya, A.N.; Wang, Y.-B.; Zhao, Z.-Y.; Zhou, R.; Indoshi, S.N.; Wang, W.; Cheruiyot, W.K.; Mburu, D.M.; Nyende, A.B.; et al. Effects of increased plastic film residues on soil properties and crop productivity in agro-ecosystem. Hazard. Mater. 2021, 414, 125521. [Google Scholar] [CrossRef]
- Boots, B.; Russell, C.W.; Green, D.S. Effects of microplastics in soil ecosystems: Above and below ground. Environ. Sci. Technol. 2019, 53, 11496–11506. [Google Scholar] [CrossRef]
- Yu, Z.; Song, S.; Xu, X.; Ma, Q.; Lu, Y. Sources, migration, accumulation and influence of microplastics in terrestrial plant communities. Environ. Exp. Bot. 2021, 192, 104635. [Google Scholar] [CrossRef]
- Li, L.; Luo, Y.; Li, R.; Zhou, Q.; Peijnenburg, W.J.G.M.; Yin, N.; Yang, J.; Tu, C.; Zhang, Y. Effective uptake of submicrometre plastics by crop plants via a crack-entry mode. Nat. Sustain. 2020, 3, 929–937. [Google Scholar] [CrossRef]
- Zang, H.; Zhou, J.; Marshall, M.R.; Chadwick, D.R.; Wen, Y.; Jones, D.L. Microplastics in the agroecosystem: Are they an emerging threat to the plant-soil system? Soil Biol. Biochem. 2020, 148, 107926.29. [Google Scholar] [CrossRef]
- Yu, Y.; Li, J.; Song, Y.; Zhang, Z.; Yu, S.; Xu, M.; Zhao, Y. Stimulation versus inhibition: The effect of microplastics on pak choi growth. Appl. Soil. Ecol. 2022, 177, 104505. [Google Scholar] [CrossRef]
- Gao, M.; Liu, Y.; Song, Z. Effects of polyethylene microplastic on the phytotoxicity of di-n-butyl phthalate in lettuce (Lactuca sativa L. var. ramosa Hort). Chemosphere 2019, 237, 124482. [Google Scholar] [CrossRef]
- Wiegand, C.; Pflugmacher, S. Ecotoxicological effects of selected cyanobacterial secondary metabolites a short review. Toxicol. Appl. Pharmacol. 2005, 203, 201–218. [Google Scholar] [CrossRef]
- Zhao, X.; Xie, H.; Zhao, X.; Zhang, J.; Li, Z.; Yin, W.; Yuan, A.; Zhou, H.; Manan, S.; Nazar, M.; et al. Combined Inhibitory Effect of Canada Goldenrod Invasion and Soil Microplastics on Rice Growth. Environ. Res. Public. Health 2022, 19, 11947. [Google Scholar] [CrossRef]
- Ge, J.; Li, H.; Liu, P.; Zhang, Z.; Ouyang, Z.; Guo, X. Review of the toxic effect of microplastics on terrestrial and aquatic plants. Sci. Total Environ. 2021, 791, 148333. [Google Scholar] [CrossRef]
- Yu, H.; Qi, W.; Cao, X.; Wang, Y.; Li, Y.; Xu, Y.; Zhang, X.; Peng, J.; Qu, J. Impact of microplastics on the foraging, photosynthesis and digestive systems of submerged carnivorous macrophytes under low and high nutrient concentrations. Environ. Pollut. 2022, 292, 118220. [Google Scholar] [CrossRef]
- Rillig, M.C.; Lehmann, A.; Machado, A.; Yang, G. Microplastic effects on plants. New Phytol. 2019, 223, 1066–1070. [Google Scholar] [CrossRef]
- Machado, A.A.; Lau, C.W.; Kloas, W.; Bergmann, J.; Bachelier, J.B.; Faltin, E.; Becker, R.; Görlich, A.S.; Rillig, M.C. Microplastics can change soil properties and affect plant performance. Environ. Sci. Technol. 2019, 53, 6044–6052. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Wang, J.; Lv, J.; Wang, Z.; Peng, Y.; Shang, J.; Wang, X. Microplastic additions alter soil organic matter stability and bacterial community under varying temperature in two contrasting soils. Sci. Total Environ. 2022, 838, 156471. [Google Scholar] [CrossRef]
- Shi, R.; Liu, W.; Lian, Y.; Zeb, A.; Wang, Q. Type-dependent effects of microplastics on tomato (Lycopersicon esculentum L.): Focus on root exudates and metabolic reprogramming. Sci. Total Environ 2023, 859, 160025. [Google Scholar] [CrossRef]
- Qian, H.; Zhang, M.; Liu, G.; Lu, T.; Qu, Q.; Du, B.; Pan, X. Effects of soil residual plastic film on soil microbial community structure and fertility. Water Air And. Soil. Pollution. 2018, 229, 261. [Google Scholar] [CrossRef]
- Wang, W.; Xie, Y.; Li, H.; Dong, H.; Li, B.; Guo, Y.; Wang, Y.; Guo, X.; Yin, T.; Liu, X.; et al. Responses of lettuce (Lactuca sativa L.) growth and soil properties to conventional non-biodegradable and new biodegradable microplastics. Environ. Pollut. 2023, 341, 122897. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Cui, Q.; Li, Y.; Zhang, K.; Lu, X.; Zhang, Y. Effect of LDPE and biodegradable PBAT primary microplastics on bacterial community after four months of soil incubation. Hazard. Mater. 2022, 429, 128353. [Google Scholar] [CrossRef]
- Agarwal, S. Biodegradable polymers: Present opportunities and challenges in providing a microplastic-free environment. Macromol. Chem. Phys. 2020, 221, 2000017. [Google Scholar] [CrossRef]
- Yuan, J.; Ma, J.; Sun, Y.; Zhou, T.; Zhao, Y.; Yu, F. Microbial degradation and other environmental aspects of microplastics/plastics. Sci. Total Environ. 2020, 715, 136968. [Google Scholar] [CrossRef]
- Han, Y.; Teng, Y.; Wang, X.; Wen, D.; Gao, P.; Yan, D.; Yang, N. Biodegradable PBAT microplastics adversely affect pakchoi (Brassica chinensis L.) growth and the rhizosphere ecology: Focusing on rhizosphere microbial community composition, element metabolic potential, and root exudates. Sci. Total Environ. 2024, 912, 169048. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).