Abstract
As a product and reproductive organ of ginger (Zingiber officinale Roscoe), the degree of rhizome bulking is a key factor in determining the yield and economic value of ginger. There are few studies on the regulatory mechanism of rhizome bulking in ginger. This study aims to identify the key hormone that regulates ginger rhizome bulking and to screen for critical hormone-associated genes. As research subjects, two ginger accessions—large (L) with a thickened rhizome and small (S) with a slender rhizome—were derived from the same parent plant. The ploidy differences between the two determine variations in gene dosage as well as differential expression patterns. The levels of eight hormones in the rhizome of L and S during different growth stages were analyzed. Differentially expressed genes (DEGs) were identified by combining third-generation transcriptome sequencing technology (PacBio SMART) with quantitative real time PCR (qRT-PCR). Through screening methods such as Orthogonal Partial Least Squares Discriminant Analysis (OPLS-DA), auxin, cytokinin, and salicylic acid were identified as the key differential hormones across various growth periods. Among these, changes in IAA level showed a positive correlation with rhizome bulking. Among them, change in IAA levels was positively correlated with the degree of rhizome bulking. Transcriptome analysis combined with qRT-PCR revealed that the auxin response factor genes ZoARF7 and ZoARF23 are likely to act as positive regulators of rhizome bulking. This study provides a theoretical foundation for elucidating the molecular mechanisms underlying hormone-mediated rhizome bulking in ginger.
1. Introduction
Ginger (Zingiber officinale Roscoe) is an annual crop in northern China, and its use as an economical crop has important applications in the food, medical, and chemical industries [1,2,3]. The rhizome, serving as both the productive and reproductive organ of ginger, directly impacts ginger yield and economic value through its growth and development, making it the subject of significant research [4]. The rhizome of ginger, along with the tubers of potatoes (Solanum tuberosum L.) and the storage roots of sweet potatoes (Ipomoea batatas L.), belong to the category of underground storage organs. Their bulking process is co-regulated by both environmental factors and genetic components [5]. Current research on the bulking of ginger rhizomes has primarily focused on physiological aspects, specifically investigating the effects of external factors such as light and mineral elements on rhizome bulking [6,7]. In contrast, studies on the genetic and molecular regulatory mechanisms underlying rhizome bulking remain limited. In crops such as potatoes and sweet potatoes, plant hormones, as critical regulators of underground storage organ development, have been extensively studied [8]. These findings provide valuable references for investigating the mechanisms underlying rhizome bulking in ginger.
Endogenous hormones play a key role in regulating the bulking of plant product organs, and the development of plant organs is regulated by the biosynthesis and signaling processes of endogenous hormones [9,10]. The CCAAT box-binding factor IbNF-YA1 targets binding to the promoter of IbYUCCA4, a key gene for auxin synthesis, and activates its transcription to increase the level of indole-3-acetic acid (IAA) in sweet potato tubers, promoting the growth of tubers at the early stage but inhibiting their bulking at the later stage [11]. SRD1 (MADS-box protein) activates the proliferation of the formation layer and secondary xylem to achieve the initial bulking of sweet potato tuberous roots, a process that also depends on the regulation of auxin [12]. Gibberellins (GAs) often play a negative role in the formation of product organs [13,14,15]. GA degradation gene StGA2ox1 overexpression lines had higher gibberellin levels than silenced lines, and higher gibberellin levels inhibited the bulking of potato tubers [16]. Brassinolide (BR) functions to improve crop yield and stress tolerance, and the expression of BvBZR, key factor genes in the BR signaling pathway, were positively correlated with the bulking of sugar beet (Beta vulgaris L.) primary roots and sugar accumulation [17]. In potatoes, BR promotes cell bulking and tuber development by activating the receptor StBRI1, which upregulates the activity of plasma membrane proton ATPase 2 (PHA2) [18]. The abscisic acid (ABA) signaling transcription factor StABL1 regulates StGA2o1 to affect potato tuber bulking [19]. Expression levels of the ABA response factor BjABR1 are positively correlated with the degree of mustard (Brassica juncea var. tumida Tsen et Lee) stem bulking [20]. Other hormones have also been reported, such as the silencing of the Carotenoid Cleavage Dioxygenase 8 (CCD8) gene, key in the SL biosynthetic pathway, which results in the dwarfing of the potato plant, increased branching, and the inhibition of tuber development [21].
In ginger, the roles of different plant hormone in rhizome bulking remain controversial, with inconsistent findings reported across studies. Previous studies have demonstrated that the levels of GA, IAA, zeatin (ZT), and jasmonic acid (JA) are positively correlated with rhizome bulking [22]. Some studies have indicated that the levels of IAA, ABA, and JA are positively correlated with rhizome bulking in ginger, whereas ZT and GA exhibit negative correlations with this process [23]. On the other hand, studies on the genetic and molecular regulatory mechanisms underlying rhizome bulking in ginger remain limited. Cao et al. and Ren et al. identified 10 and 11 candidate genes potentially involved in ginger rhizome bulking, respectively, by performing transcriptome sequencing on ginger rhizomes at different growth stages and validating the results through quantitative real-time PCR (qRT-PCR) [4,22]. Among the identified genes, in addition to auxin response factor (ARF), these candidates encompass multiple gene families such as basic helix–loop–helix (bHLH), WRKY transcription factor (WRKY), NAM/ATAF/CUC (NAC), and MYB (myeloblastosis), with diverse functional potentials implicated in rhizome bulking. Elucidating the roles of plant hormones in ginger rhizome bulking and identifying key genes regulating this process remain central objectives in current ginger research. Given the consensus and controversies surrounding the roles of plant hormones, we hypothesize that one or two key regulatory hormones govern the bulking process of ginger rhizomes. By prioritizing these hormones, we aim to refine the candidate pool of hormone-associated genes for targeted screening. To streamline the identification of key differential hormones and associated genes, this study selected two distinct ginger varieties with markedly divergent rhizome bulking capacities as experimental materials. Following preliminary phenotyping to confirm their morphological divergence, hormonal profiling (targeting eight major classes of plant hormones) was conducted on rhizomes at different developmental periods. Transcriptome sequencing was conducted on rhizome samples collected during the peak bulking stage (the rhizome bulking stage) of ginger, followed by the screening of differentially expressed genes (DEGs) and validation through qRT-PCR. The elucidation of key hormones and associated genes governing ginger rhizome bulking will significantly advance our understanding of the hormonal regulatory mechanisms underlying this process, providing critical theoretical foundations for future research.
2. Materials and Methods
2.1. Plant Materials
The experiment was carried out in the Horticultural Experiment Station of Shandong Agricultural University in 2023. The ginger used in the experiment was all planted in cultivation bags with a diameter of 40 cm and a height of 25 cm. There was one ginger plant in each bag, and the cultivation substrate was a mixture of coconut coir and peat (1:1.5, v/v). This study selected two ginger varieties, large ginger material (L) and small ginger material (S) (Figure 1), as research subjects. The VM0-generation L and S were derived from the same ginger plant (Figure 1A), which originated from a natural variation of the ‘Shannong No. 1’ ginger cultivar. The right side of the rhizome is designated as L, which maintains the characteristic hypertrophy of the ‘Shannong No. 1’ cultivar rhizome. The left side of the rhizome is designated as S, characterized by stunted rhizome growth and a reduced bulking level compared to L. The experimental populations of both the L and S cultivars consisted of 90 test plants each. Sampling was conducted at four distinct growth phases of ginger: the seedling stage, the tillering stage, the rhizome bulking stage, and the harvest stage. The phenological phases of ginger growth and corresponding sampling time points were defined as follows: the seedling stage (6–15), the tillering stage (8–15), the rhizome bulking stage (9–15), and the harvest stage (10–15). For sampling, three plants were randomly selected from each cultivar to serve as three biological replicates.
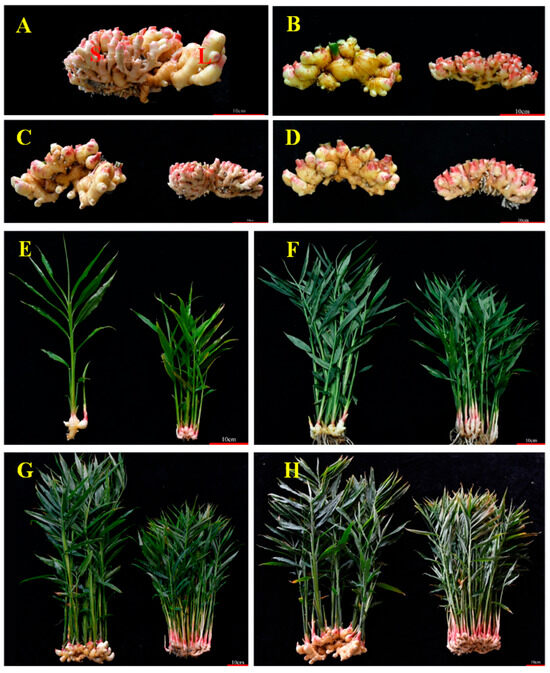
Figure 1.
Phenotype of large ginger and small ginger. (A) VM0 generation of large ginger and small ginger, (B) VM1 generation of large ginger and small ginger, (C) VM2 generation of large ginger and small ginger, (D) VM3 generation of large ginger and small ginger, (E) seedling stage (6–15), (F) tillering stage (8–15), (G) rhizome bulking stage (9–15), and (H) harvest stage (10–15).
2.2. Phenotypic Identification
The growth parameters at harvest maturity were quantified in three successive generations of the variant materials, with five plants per cultivar randomly selected for the systematic measurement of morpho-physiological traits. Ploidy determination in ginger was conducted using apical unexpanded leaves collected during the seedling stage (6–15), with the diploid cultivar ‘Laiwu small ginger’ serving as the control [24]. Ploidy analysis maintained methodological fidelity to the protocol developed by Liu et al. [25], with the core experimental workflow summarized below. Leaf tissues were rapidly fragmented using double-edged razor blades in a lysis buffer, followed by filtration through a 300-mesh nylon mesh. The nuclei suspension was subsequently stained with propidium iodide (Solarbio, Beijing, China). Following processing, ploidy determination was performed using a BD FACSAria III flow cytometer (BD Biosciences, San Jose, CA, USA). Chromosomal observation in cytogenetic variant materials was performed using the root tip squashing technique, with ginger root tips collected during the seedling stage (6–15). Following tissue maceration, chromosome-specific staining, and microscopic preparation, chromosomal visualization was conducted under a Nikon Eclipse Ni-U research microscope (Nikon, Tokyo, Japan) equipped with a DS-Ri2 digital camera system, enabling high-resolution karyotypic documentation for chromosomal enumeration and structural aberration analysis. Ultimately, karyotype analysis of ginger chromosomes was performed using the ImageJ software (version 1.8.0), with the entire experimental and karyotyping procedures aligning with the methodology described by Xin [26].
2.3. Metabolome Analysis of Ginger Rhizome Hormones
Rhizomes from four periods of L and S were used as the test materials, and the four periods were as follows: the seedling stage (6–15), the tillering stage (8–15), the rhizome bulking stage (9–15), and the harvest stage (10–15). Quantitative analysis of eight classes of phytohormones—auxins, cytokinins, gibberellins, abscisic acid, ethylene, jasmonic acid, salicylic acid, and strigolactones—encompassing 88 hormonal metabolites was performed. The procedures for hormone extraction, Liquid Chromatography–Tandem Mass Spectrometry (LC-MS/MS) operation, and data analysis were conducted following the method established by Shen et al. [27].
2.4. Transcriptome Analysis and qRT-PCR Validation
In order to explore the molecular mechanism of the difference in rhizome bulking degrees between the ginger variant materials L and S at the transcriptome level, transcriptome sequencing was performed on the rhizomes of the variant materials during the rhizome bulking stage (9–15). The transcriptome sequencing work was completed by Novogene Company (Beijing, China). Primer design for the target genes was accomplished using Primer Premier 5.0. RNA extraction and cDNA synthesis were performed using an RNA Isolation Kit (Vazyme, Nanjing, China) and a cDNA Synthesis Kit (Vazyme, Nanjing, China), respectively. qRT-PCR was conducted using the ABI Q6 RealTime PCR system. ZoRPII was used as an internal reference gene [28]. The relative expression levels of the target genes were calculated using the 2−ΔΔCT method. The relevant primer information can be found in Table A1.
2.5. Statistical Analysis
The data of different parameters were analyzed using an independent samples t-test in the SPSS statistical software (version 27.0). The homogeneity of variances was first verified using Levene’s test. Significant differences between the two experimental groups (expressed as the mean value ± standard deviation) were determined based on t-test results at p < 0.05. Asterisks denote statistically significant differences between the groups determined by the independent samples t-test (one asterisk denotes p < 0.05 and two asterisks denote p < 0.01). As key parameters of Orthogonal Partial Least Squares Discriminant Analysis (OPLS-DA), the Coefficient of Determination X matrix (R2X) and the Coefficient of Determination Y matrix (R2Y) reflect the model’s ability to fit the original data and classification results, respectively. The Predictive Coefficient of Determination (Q2) reflects the model’s predictive ability, and the model is considered valid when Q2 > 0.5. Plotting was performed using Excel 2016 and heatmaps were constructed using Heml 1.0 software.
3. Results
3.1. Analysis of Growth Parameters and Ploidy Determination in Ginger
Growth indicators and cell ploidy, as critical plant phenotypic traits, show significant differences between groups L and S, as demonstrated in Table 1 and Figure 2. The number of branches in S was 2.40~3.01 times that of L, whereas the fresh weight of the rhizome was only 48.19%~53.60% times that of L. In addition to this, L and S showed significant differences in plant height, stem diameter, and the fresh weight of rhizomes, leaves, and roots (Table 1, p < 0.01). L had both diploid and tetraploid cells belonging to the mixoploid (Figure 2B), while S was diploid (Figure 2C). The integrated analysis of growth indicators and ploidy levels revealed that S exhibited significant variations in both morphological phenotypes and genetic architecture compared to L.

Table 1.
Analysis of growth indicators during harvest stage of large ginger and small ginger.
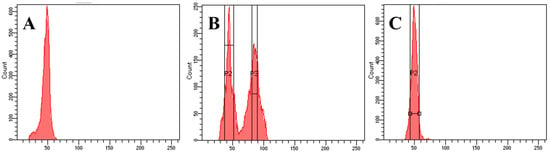
Figure 2.
Identification of ploidy of large ginger and small ginger. (A) Diploid control material, (B) mixoploid large ginger (L), and (C) diploid small ginger (S).
3.2. Karyotype Analysis of Ginger
To validate the flow cytometric results and elucidate karyotypic differences between L and S, chromosome counting and karyotype analysis were systematically conducted on both experimental groups. There were two chromosome types, 2n = 2x = 22 and 2n = 4x = 44, in L (Figure 3A,B), which belonged to the mixoploid group. The chromosome number of S was 2n = 2x = 22 (Figure 3C), which belonged to the diploid group. As shown in Table 2, the relative length of the chromosomes in L diploid and tetraploid cells ranged from 6.41% to 11.74% and 6.32% to 11.59%. The arm ratios ranged from 1.37 to 2.11 and 1.30 to 2.46, with the average arm ratios of 1.75 and 1.71 and the mitotic indices ranged from 32.14% to 42.28% and 31.95% to 43.48%. The ratio of the longest chromosome to the shortest chromosome was 1.83, and the karyotype asymmetry index was 63.56% and 62.90%. The relative length of chromosomes in S somatic cells ranged from 6.45% to 11.85%, the arm ratio ranged from 1.36 to 2.59, with an average arm ratio of 1.84, the mitotic index ranged from 31.06% to 42.28%, the ratio of the longest chromosome to the shortest chromosome was 1.84, and the karyotype asymmetry index was 64.45%. The range of L and S mitotic indices was close, both located in the 31~44% interval, and the average arm ratio and karyotype asymmetry coefficient were, in descending order, S cells > L diploid cells > L tetraploid cells. S somatic and L diploid cells have the same karyotype formula of K(2n) = 8 m + 14 sm, while L tetraploid cells have the karyotype formula K(2n) = 24 m + 20 sm. The karyotype classification of chromosomes in both L and S was type 2A. The chromosome counts between L and S validated the flow cytometry results. Comprehensive karyotype analysis integrating multiple parameters demonstrated the identical karyotypic formulas and classifications in the diploid cells of both groups, while tetraploid cells exhibited distinct differences.
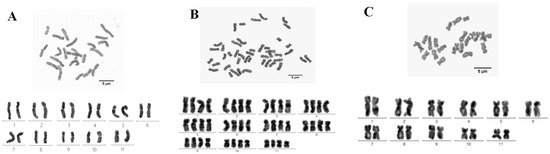
Figure 3.
Chromosome karyotype of large ginger and small ginger. (A) Karyotype of large ginger (L) diploid cells, (B) karyotype of large ginger (L) tetraploid cells, and (C) karyotype of small ginger (S).

Table 2.
Analysis of karyotype parameters of large ginger and small ginger.
3.3. Analysis of Hormone Levels in Ginger Rhizomes
On the basis of clarifying the phenotypic differences between L and S, the study further explores the impact of endogenous hormones on the differences in rhizome bulking. Hormone levels were measured at the seedling stage (6–15), the tillering stage (8–15), the rhizome bulking stage (9–15), and the harvest stage (10–15) using the LC-MS/MS technique, and 32 out of 88 metabolites detected were under the detection limit. Firstly, changes the levels of the five common classes of hormone metabolites were analyzed over four growth periods (Figure 4). During the tillering stage (8–15), ginger is dominated by above-ground growth, rhizome development is relatively slow, and the difference in rhizome size between L and S begins to become apparent (Figure 1F). The levels of indole-3-acetic acid (IAA), 1-aminocyclopropane-1-carboxylic acid (ACC), and abscisic acid (ABA) in L were 53.21%, 42.20%, and 87.67% higher than those in S, respectively, with significant differences between L and S (p < 0.01). At this time, IAA, ACC, and ABA levels were positively correlated with the degree of rhizome bulking. During the rhizome bulking stage (9–15), the above-ground growth of ginger basically ceased, and the rhizome entered the rapid bulking period. The difference in rhizome bulking between rhizomes of L and S increased further (Figure 1G). The IAA level in the rhizome of L was 201.5% higher than in the rhizome of S, with a significant difference (p < 0.01). The levels of other hormones in the rhizome of S were significantly higher than those in L, and only the IAA levels were positively correlated with the size of the rhizome. During the harvest stage (10–15), the growth of both the above-ground and underground parts of ginger essentially ceased (Figure 1H). At this time, S showed a non-significant difference in IAA levels compared to L, while all other hormone levels showed significant differences. Among the five common hormone classes, the IAA levels were consistently correlated with the rhizome bulking trend. The remaining 51 classes of hormone metabolites are presented in the form of a heat map (Figure 5). Endogenous hormone metabolism was more active during the seedling stage (6–15) as the initial period of rhizome development. At this time, almost all metabolites showed significant difference. During the rhizome bulking stage, metabolites that were significantly higher in S than in L included cytokinins (Kinetin-9glucoside (K9G), 6-Benzyladenine (BAP)) and strigolactone (5-Deoxystrigol). Metabolites significantly elevated in the rhizomes of L compared to S included auxin [3-indoleacetonitrile (IAN) and indole-3-butyric acid (IBA)] and jasmonic acid analogs (3-oxo-2-(2-(Z)-Pentenyl) cyclopentane-1-hexanoic acid, OPC-6) cyclopentane-1-hexanoic acid (OPC-6)]. However, no significant differences in their levels were observed between the tillering stage (8–15) and the harvest stage (10–15). The results of the heatmap show that both auxin and jasmonic acid were positively correlated with the trend of rhizome bulking, while cytokinin and strigolactone were negatively correlated. However, merely relying on the comparison of the hormone level of L and S during different growth periods and the results of the significance analysis of the differences are not sufficient to directly determine that the above-mentioned hormones play a key role in the process of rhizome bulking.
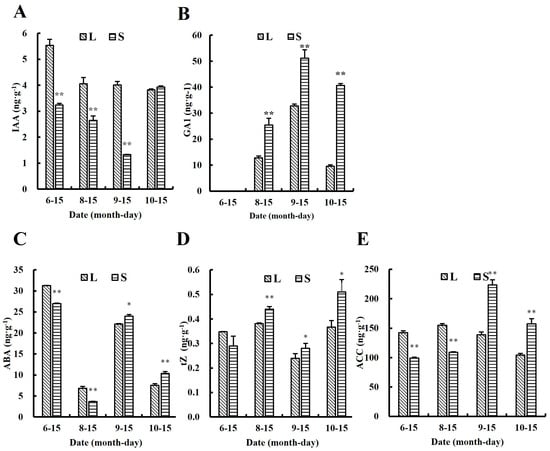
Figure 4.
The levels of common hormone metabolites in large ginger and small ginger at different growth periods. (A) Indole-3-acetic acid (IAA) levels in rhizomes at different growth periods, (B) gibberellic acid 1 (GA1) levels in rhizomes at different growth periods, (C) abscisic acid (ABA) levels in rhizomes at different growth periods, (D) trans zein (tZ) levels in rhizomes at different growth periods, (E) 1-Aminocyclopropanecarboxylic acid (ACC) in rhizomes at different growth periods. Note: In the figure, asterisks denote statistically significant differences between groups determined by independent samples t-tests (one asterisk denotes p < 0.05, and two asterisks denote p < 0.01). Error bars represent the standard deviation (n = 3). The label ‘Date (month-day)’ indicates the sampling time, with the four sampling time points corresponding to the seedling stage, the tillering stage, the rhizome bulking stage, and the harvest stage of ginger, respectively.
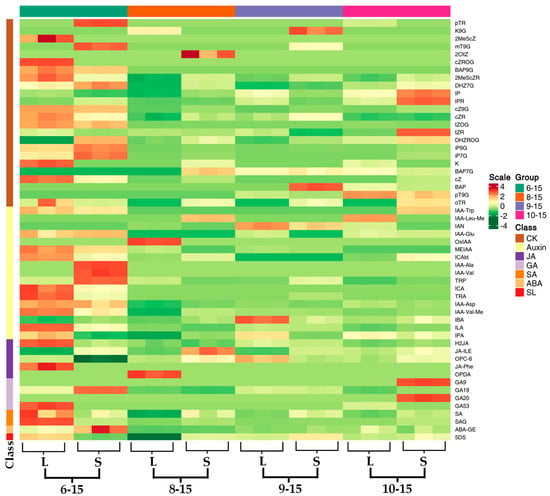
Figure 5.
The levels of hormone metabolites in rhizomes at different growth periods. Note: In the figure, the label ‘month-day’ indicates the sampling time, with the four sampling time points corresponding to the seedling stage, the tillering stage, the rhizome bulking stage, and the harvest stage of ginger, respectively.
3.4. KEGG Functional Classification and OPLS-DA Analysis of Hormones in Ginger Rhizomes
Based on the differential metabolite results, KEGG pathway enrichment was performed to further identify the hormone metabolic pathways that significantly changed during rhizome bulking in L and S (Figure 6A). During the rhizome bulking stage, there was a higher enrichment of significantly different hormone metabolites for tryptophan metabolism and indole alkaloid biosynthesis, and the hormone most closely linked to this pathway was auxin, which uses tryptophan as a synthetic precursor. The data were analyzed using Orthogonal Partial Least Squares Discriminant Analysis (OPLS-DA) for the tillering stage (8–15), the rhizome bulking stage (9–15), and the harvest stage (10–15), and the samples were efficiently divided into two groups (Figure 6B). At this stage, with R2X = 0.539, R2Y = 0.995, and Q2 = 0.988 (Figure A1), the model demonstrated excellent performance in classification tasks, strong predictive capability, and reliable results. VIP scores provided by OPLS-DA yielded the contribution of metabolites to the classification of samples and also provided a visual representation of the role of metabolites in classification.
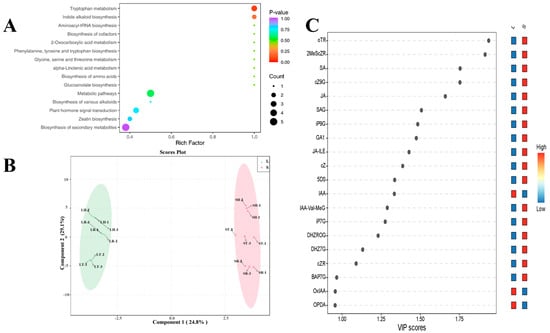
Figure 6.
KEGG functional classification and OPLS-DA analysis of hormone metabolites in ginger rhizomes. (A) KEGG pathways enrichment results of differential hormone during rhizome bulking stage, (B) OPLS-DA analysis of hormone in large ginger and small ginger, and (C) VIP scores of hormone in large ginger and small ginger samples based on OPLS-DA analysis. Note: In Figure 6B, LT and ST represent the tillering stage of Large ginger and Small ginger, respectively; LR and SR denote the rhizome bulking stage of Large ginger and Small ginger, respectively; LH and SH indicate the harvest stage of Large ginger and Small ginger, respectively.
The metabolites with a VIP score > 1.75 were ortho-topolin riboside (oTR), 2-Methylthio-cis-zeatin riboside (2MeScZR), cis-Zeatin-9-glucoside (cZ9G), and salicylic acid (SA) (Figure 6C). The only hormone with a VIP score > 1.25 and significantly higher levels in the rhizomes of L than S was IAA. The three categories of plant hormones exhibited significant differences between L and S across three growth periods. Specifically, cytokinin (oTR, 2-MeScZR, and cZ9G) and salicylic acid (SA) showed an inverse correlation with the rhizome bulking trend (Figure 5). By comprehensively analyzing the comparison of hormone levels during different growth periods, the significance analysis of differences, and the KEGG functional classification, and then combining with the OPLS-DA analysis, it is concluded that auxin, cytokinin, and salicylic acid are the key hormones that lead to the differences in rhizome bulking between L and S. This is generally consistent with our initial hypothesis.
3.5. Transcriptome Analysis of Ginger Rhizomes
Building on the identified key differential hormones between L and S, transcriptome-based screening was carried out to pinpoint the differentially expressed genes associated with these hormones. The rhizomes of L and S during the rhizome bulking stage were selected as test materials for transcriptome analysis. A total of 56,610 shared differentially expressed genes (DEGs) were identified between L and S through transcriptome sequencing (Figure 7B). The differential gene screening criteria were based on |log2 (Fold Change)| > 1 and p-value < 0.05. Figure 7A shows that there were 618 heterogeneous genes in L vs. S during the root and rhizome bulking stages, of which 359 were down-regulated and 259 were up-regulated. To further explore the hormone-related differentially expressed genes, statistics were conducted for auxin, strigolactone, salicylic acid, and cytokinin-related genes (Table 3). There were eight genes related to auxin, four genes related to cytokinin, and three genes related to salicylic acid. Combined with the annotation of transcriptome data and comparison with the whole genome sequence of ginger, the results were as follows after eliminating duplicated genes: five auxin-related genes, including four auxin signaling genes (ZoARF7/23/17, ZoGH3.1) and one transporter protein gene (ZoPIN1b), one cytokinin-responsive regulator gene (ZoARR9) and three salicylate signaling genes (ZoPR1, ZoNPR1, and ZoSABP2).
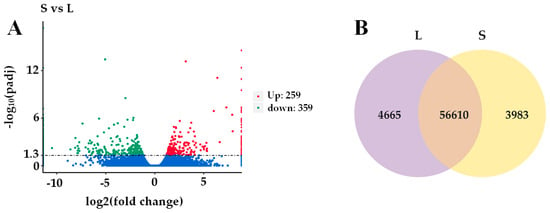
Figure 7.
Volcano plot and Venn diagram of DEGs during rhizome bulking stage. (A) Volcano plot, (B) Venn diagram of DEGs. Note: Blue dots represent genes with non-significant differential expression (adjusted p-value, padj > 0.05).

Table 3.
Differential expression genes (DEGs) related to auxin, cytokinin, and salicylic acid.
3.6. Quantitative Real-Time PCR of DEGs
QRT-PCR was used to validate the transcriptome results. The results (Figure 8) show a consistent trend with the RNA-seq data, indicating a high accuracy of the transcriptome data. The expression levels of ZoARF7 and ZoARF23 in the rhizome of L were significantly higher than those in S, showing a positive correlation with rhizome bulking at this stage. Conversely, ZoARF17 and ZoGH3.1 exhibited the opposite pattern. To further clarify the change in the expression of ZoARF7/23 at different growth periods, the expression levels of both at four growth periods were examined using qRT-PCR. As shown in Figure 9, the expression level of ZoARF7/23 reached its peak during the rhizome bulking stage (9–15), with significantly higher levels observed in L than in S (p < 0.01) The overall expression showed a firstly increasing and then decreasing trend, which was positively correlated with the trend of the degree of rhizome bulking. The qRT-PCR results revealed that ZoARF7/23, an auxin-related gene, not only exhibited differential expression between L and S but also displayed expression level changes that paralleled the trend of rhizome bulking.
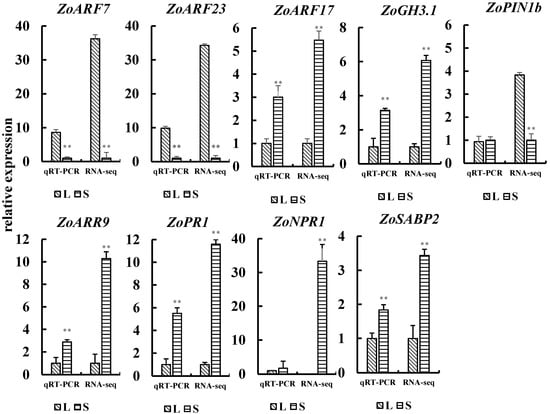
Figure 8.
Analysis of gene expression level related to auxin, cytokinin, and salicylic acid. Note: In the figure, asterisks denote statistically significant differences between groups determined by independent samples t-tests (two asterisks denote p < 0.01). Error bars represent the standard deviation (n = 3).
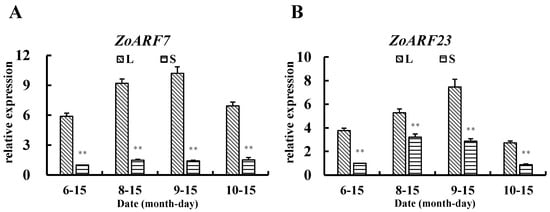
Figure 9.
Analysis of ZoARF7 and ZoARF23 expression at different growth periods. (A) ZoARF7 expression at different growth periods and (B) ZoARF23 expression at different growth periods. Note: In the figure, the label ‘Date (month-day)’ indicates the sampling time, with the four sampling time points corresponding to the seedling stage, the tillering stage, the rhizome bulking stage, and the harvest stage of ginger, respectively. In the figure, asterisks denote statistically significant differences between groups determined by independent samples t-tests (two asterisks denote p < 0.01). Error bars represent the standard deviation (n = 3).
4. Discussion
4.1. The Influence of Chromosomal Ploidy Variation on Rhizome Bulking
The significant and genetically stable difference in rhizome bulking between L and S provides a foundation for analyzing the regulatory mechanism underlying this phenotypic variation. Karyotype analysis revealed that both L and S share identical karyotype classifications, likely attributed to their shared origin from the same parent plant (Figure 1A). Flow cytometry and karyotype analysis further demonstrated that, unlike the mixoploid L (2n = 2x = 22, 2n = 4x = 44), S is a diploid (2n = 2x = 22) with no tetraploid cells. In potatoes, polyploid potatoes exhibit higher gene content compared to diploid potatoes [29], and the weight and size of individual tubers in tetraploid potatoes are greater than those in diploid potatoes [30], which is similar to the situation observed in L and S. Meanwhile, plants of the same species with different ploidy levels exhibit significant differences in endogenous hormone levels [31] and the expression of hormone-related genes [32]. Therefore, variations in rhizome ploidy may affect endogenous hormone levels and the expression of related genes, thereby influencing the degree of rhizome bulking.
4.2. Role of Auxin, Cytokinin, and Salicylic Acid in Rhizome Bulking
Based on hormone level dynamics across growth stages, KEGG pathway enrichment, and OPLS-DA analysis, cytokinins, salicylic acid, and auxin were identified as the primary differential hormones between L and S. As a crucial auxin in plants, the variation in IAA levels has been consistently positively correlated with the degree of rhizome bulking. This observation not only aligns with previous studies on auxin dynamics during ginger rhizome development but is also supported by similar findings in other crops with underground storage organs, such as potato, sugar beet, sweet potato, and cassava (Manihot esculenta) [22,33,34,35,36].
During the seedling stage, both auxin (IAA) and cytokinins (e.g., tZ, oTR) exhibit elevated levels in the rhizomes of L in ginger. This pattern parallels the role of auxin and cytokinin in initiating tuber formation in potatoes [37,38]. However, during the rhizome bulking stage, cytokinin levels exhibit a negative correlation with the degree of rhizome bulking. This contrasts with reports on potatoes and sweet potatoes, where cytokinins are described to play a positive regulatory role in tuber or root bulking [39,40]. The possible reason is that excessively high cytokinin levels inhibit the bulking of rhizomes. As demonstrated in the study by Tao et al., the expression of PCHS-ipt (a cytokinin biosynthesis gene) elevated the cytokinin level in potato stems and leaves but resulted in decreased tuber weight [41]. Auxin may also influence cytokinin levels. Bishopp et al. demonstrated that elevated auxin levels promote the transcription of AHP6, a gene encoding a negative regulator of cytokinin signaling, thereby maintaining cytokinin homeostasis in Arabidopsis roots. Their findings were supported by hormone quantification and transcriptomic analysis [42]. Research on SA has predominantly focused on plant disease resistance and stress tolerance [43,44,45], while its role in regulating storage organ development remains scarcely reported [46]. Auxin and cytokinin may serve as the key hormones modulating the differential regulation of ginger rhizome bulking.
4.3. Potential Influence of ARF Genes on the Bulking of Ginger Rhizomes
Integrated hormone metabolome and transcriptome analysis identified critical differential hormones and their associated differentially expressed genes. The expression levels of ZoARF7 and ZoARF23 not only showed a positive correlation with the rhizome bulking stage but were also consistent with the bulking trend observed across different developmental stages of the rhizomes. ZoARF7 and ZoARF23 are likely to be key genes that positively regulate ginger rhizome bulking. Transcriptome analysis and qRT-PCR validation of rhizomes at different growth stages by Ren et al. and Zhou et al. revealed that ARF is likely involved in regulating rhizome formation [22,47]. Phylogenetic analysis of ARF protein in rice (Oryza sativa L.), Arabidopsis (Arabidopsis thaliana (L.) Heynh.), and potato revealed that ZoARF7 shares high sequence similarity with OsARF7, AtARF1, and StAR7, while ZoARF23 clusters closely with OsARF23, AtARF2, and StARF6 [48,49]. Functional inference of ZoARF7/23 can be deduced through advances in research on their orthologous genes, with these ARFs characterized as putative transcriptional repressors [50]. In rice, the overexpression of OsARF7 confirmed its function in positively regulating root elongation [51]. Through qRT-PCR analysis of potato tuber at different growth periods, it was found that the expression level of StARF6 in stolons and at the initial stage of tuber formation was higher than that in other periods [49]. Previous researchers utilized techniques such as constructing mutants, microscopic observation, and fluorescent probes to discover that a reduction in the expression of OsARF23/24 inhibits the expression of the Rice Morphology Determinant (RMD). This, in turn, suppresses the formation of actin filaments and cell elongation, thereby affecting the distribution gradient of auxin [52]. The aforementioned orthologous genes function as positive regulators in root or stem development and morphogenesis, which is similar to our prediction of ZoARF7/23 function.
However, conflicting findings have been reported. AtARF1/2 exhibits functional redundancy in Arabidopsis, primarily expressed in leaves and floral organs where they promote leaf senescence and floral organ abscission [53]. The heterologous expression of HbARF2 (an ortholog of AtARF2) suppresses pith cell expansion and negatively regulates plant height and stem diameter in tobacco (Nicotiana tabacum L.) [54]. These findings collectively underscore the functional diversity and mechanistic complexity inherent to the ARF gene family. However, the functional characterization of ZoARF7/23 using gain- and/or loss-of-function approaches has been hampered due to the considerable challenges in generating ginger transgenic plants. Therefore, subsequent work will focus on optimizing Agrobacterium infection conditions and selectable markers, as well as refining the ginger regeneration system, to enable the functional validation of ZoARF7 and ZoARF23.
5. Conclusions
This study identified auxin, cytokinin, and salicylic acid as key hormones that regulate ginger rhizome bulking, with IAA levels showing a positive correlation with rhizome bulking. Integrated analysis of transcriptomic profiling and qRT-PCR validation demonstrated that the expression dynamics of auxin response factor genes ZoARF7 and ZoARF23 were aligned with rhizome bulking patterns, suggesting their potential roles as positive regulators of ginger rhizome bulking. However, due to the current technical limitations of the ginger genetic transformation system, the functional validation of ZoARF7 and ZoARF23 could not be conclusively performed. This will become the primary focus of subsequent research efforts. The identification of key hormones and associated genes governing ginger rhizome bulking has not only established a theoretical foundation for elucidating the hormonal regulatory mechanisms underlying rhizome bulking but also provides theoretical support for future molecular breeding strategies in ginger.
Author Contributions
K.W.: Writing—original draft, Validation, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Y.L.: Data curation, Software. S.G.: Methodology, Software. Y.K. and M.L.: Investigation, Methodology, Validation. Z.C.: Resources, Software, Supervision, Validation, Writing—review and editing. K.X.: Funding acquisition, Project administration, Writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Key Research and Development Program of China (grant No. 2023YFD1600202), the National Natural Science Foundation of China (Grant No. 32302530), and the Agricultural Fine Variety Project in Shandong Province of China (Grant No. 2023LZGCQY003).
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A
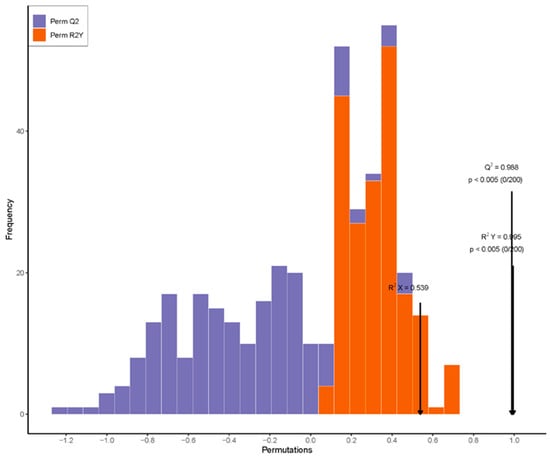
Figure A1.
Orthogonal projections to latent structures discriminant analysis (OPLS-DA) permutation test histogram. Note: In the chart, the black arrow points to the positions of the values of R2X, R2Y, and Q2 on the X-axis.

Table A1.
Information on primers related to quantitative real-time PCR.
Table A1.
Information on primers related to quantitative real-time PCR.
| Gene | Primer Sequence (5′ → 3′) | Tm (°C) |
|---|---|---|
| ZoRPII | F: CTGCTGATGGATACGAATG | 60.0 |
| R: CTGCCCAAGAGAATGAAAG | ||
| ZoARF7 | F: AACTGCGTGTTGGTGTCAGAGG | 61.0 |
| R: CCGTAGTGATTGCGTGGGATGC | ||
| ZoARF23 | F: CAGCGGAAATGCGAGGACAGAG | 60.9 |
| R: GATCGGAGAGAGCAGCGGAAATG | ||
| ZoARF17 | F: GTGGTGGAGGCATTCGGATTGG | 61.5 |
| R: ACTCTCGCTCCTACGGTCCAAC | ||
| ZoGH3.1 | F: TGTTCGTGAAGTCGGAGGTTTGC | 61.4 |
| R: TCGTCTGGGCGGTTGAGGAAG | ||
| ZoPIN1b | F: CCATCAAGAAGGTCGGGCAGAAC | 61.8 |
| R: CACTCGCAGGCGGCATCAAG | ||
| ZoARR9 | F: TCGGACATGACAAGGCTCAGACC | 61.4 |
| R: TTGCTATTGCTGCTGCCACTGG | ||
| ZoPR1 | F: GGATCGGGCGACTGCCAAC | 63.2 |
| R: TCCCACCCACAAGCCCACAG | ||
| ZoNPR1 | F: GAGCATCTCGCCTCCCTCCTC | 62.5 |
| R: ACATCGGTGGACACGGACAGAG | ||
| ZoSABP2 | F: CGCCGCTTCGTTGACATCCG | 62.8 |
| R: AGGCTGTGACCGACGAGGAC |
References
- Varakumar, S.; Umesh, K.V.; Singhal, R.S. Enhanced extraction of oleoresin from ginger (Zingiber officinale) rhizome powder using enzyme-assisted three phase partitioning. Food Chem. 2017, 216, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Fang, H.; Zhang, X.; Yan, Y.-M.; Liu, Y.; Miao, J.; Niu, H.; Feng, W.; Cheng, Y.-X.; Wang, Y. Renoprotective Glycoside Derivatives from Zingiber officinale (Ginger) Peels. J. Agric. Food Chem. 2023, 71, 15170–15185. [Google Scholar] [CrossRef] [PubMed]
- Paula, S.; Linn, B.; Kristina, H.; Jonathan, R.; Johan, J.; Hazem, k.; Cristiane, S.F.; Kristiina, O. Multifunctional Ginger Nanofiber Hydrogels with Tunable Absorption: The Potential for Advanced Wound Dressing Applications. Biomacromolecules 2021, 22, 3202–3215. [Google Scholar] [CrossRef]
- Cao, Z.; Tang, N.; Chen, Z.; Wu, P.; Zheng, J.; Ye, J.; Liu, Y.; Hu, Y.; Zhang, L.; Sun, X.; et al. Transcriptomic analysis reveals transcription factors involved in vascular bundle development and tissue maturation in ginger rhizomes (Zingiber officinale Roscoe). Not. Bot. Horti Agrobot. Cluj-Napoca 2023, 51, 13131. [Google Scholar] [CrossRef]
- Lehretz, G.G.; Sonnewald, S.; Hornyik, C.; Corral, J.M.; Sonnewald, U. Post-transcriptional Regulation of FLOWERING LOCUS T Modulates Heat-Dependent Source-Sink Development in Potato. Curr. Biol. 2019, 29, 1614–1624.e3. [Google Scholar] [CrossRef]
- Jabborova, D.; Choudhary, R.; Azimov, A.; Jabbarov, Z.; Selim, S.; Abu-Elghait, M.; Desouky, S.E.; Azab, I.H.E.; Alsuhaibani, A.M.; Khattab, A.; et al. Composition of Zingiber officinale Roscoe (Ginger), Soil Properties and Soil Enzyme Activities Grown in Different Concentration of Mineral Fertilizers. Horticulturae 2022, 8, 43. [Google Scholar] [CrossRef]
- Lv, X.; Gao, S.; Li, N.; Lv, Y.; Chen, Z.; Cao, B.; Xu, K. Comprehensive insights into the influence of supplemental green light on the photosynthesis of ginger (Zingiber officinale Roscoe). Protoplasma 2022, 259, 1477–1491. [Google Scholar] [CrossRef]
- Zierer, W.; Rüscher, D.; Sonnewald, U.; Sonnewald, S. Tuber and Tuberous Root Development. Annu. Rev. Plant Biol. 2021, 72, 551–580. [Google Scholar] [CrossRef]
- Huang, H.; Wei, Y.; Huang, S.; Lu, S.; Su, H.; Ma, L.; Huang, W. Integrated metabolomic and transcriptomic analyses provide insights into regulation mechanisms during bulbous stem development in the Chinese medicinal herb plant, Stephania kwangsiensis. BMC Plant Biol. 2024, 24, 276. [Google Scholar] [CrossRef]
- Yao, S.; Lan, Z.; Huang, R.; Tan, Y.; Huang, D.; Gu, J.; Pan, C. Hormonal and transcriptional analyses provides new insights into the molecular mechanisms underlying root thickening and isoflavonoid biosynthesis in Callerya speciosa (Champ. ex Benth.) Schot. Sci. Rep. 2021, 11, 9. [Google Scholar] [CrossRef]
- Xue, L.; Wang, Y.; Fan, Y.; Jiang, Z.; Wei, Z.; Zhai, H.; He, S.; Zhang, H.; Yang, Y.; Zhao, N.; et al. IbNF-YA1 is a key factor in the storage root development of sweet potato. Plant J. 2024, 118, 1991–2002. [Google Scholar] [CrossRef] [PubMed]
- Noh, S.A.; Lee, H.-S.; Huh, E.J.; Huh, G.H.; Paek, K.-H.; Shin, J.S.; Bae, J.M. SRD1 is involved in the auxin-mediated initial thickening growth of storage root by enhancing proliferation of metaxylem and cambium cells in sweetpotato (Ipomoea batatas). J. Exp. Bot. 2010, 61, 1337–1349. [Google Scholar] [CrossRef] [PubMed]
- Kondhare, K.R.; Kumar, A.; Patil, N.S.; Malankar, N.N.; Saha, K.; Banerjee, A.K. Development of aerial and belowground tubers in potato is governed by photoperiod and epigenetic mechanism. Plant Physiol. 2021, 187, 1071–1086. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wen, J.; Ke, X.; Zhang, J.; Sun, X.; Wang, C.; Yang, Y. Gibberellin inhibition of taproot formation by modulation of DELLA-NAC complex activity in turnip (Brassica rapa var. rapa). Protoplasma 2021, 258, 925–934. [Google Scholar] [CrossRef]
- Ren, X.; Ma, W.; Xuan, S.; Li, D.; Wang, Y.; Xu, Y.; Feng, D.; Zhao, J.; Chen, X.; Luo, S.; et al. Hormones and carbohydrates synergistically regulate the formation of swollen roots in a Chinese cabbage translocation line. Hortic. Res. 2023, 10, uhad121. [Google Scholar] [CrossRef]
- Kloosterman, B.; Navarro, C.; Bijsterbosch, G.; Lange, T.; Prat, S.; Visser, R.G.F.; Bachem, C.W.B. StGA2ox1 is induced prior to stolon swelling and controls GA levels during potato tuber development. Plant J. 2007, 52, 362–373. [Google Scholar] [CrossRef]
- Wang, W.; Sun, Y.-Q.; Li, G.-L.; Zhang, S.-Y. Genome-wide identification, characterization, and expression patterns of the BZR transcription factor family in sugar beet (Beta vulgaris L.). BMC Plant Biol. 2019, 19, 191. [Google Scholar] [CrossRef]
- Deng, R.; Huang, S.; Du, J.; Luo, D.; Liu, J.; Zhao, Y.; Zheng, C.; Lei, T.; Li, Q.; Zhang, S.; et al. The brassinosteroid receptor StBRI1 promotes tuber development by enhancing plasma membrane H+-ATPase activity in potato. Plant Cell 2024, 36, 3498–3520. [Google Scholar] [CrossRef]
- Jing, S.; Sun, X.; Yu, L.; Wang, E.; Cheng, Z.; Liu, H.; Jiang, P.; Qin, J.; Begum, S.; Song, B. Transcription factor StABI5-like 1 binding to the FLOWERING LOCUS T homologs promotes early maturity in potato. Plant Physiol. 2022, 189, 1677–1693. [Google Scholar] [CrossRef]
- Sun, Q.; Zhou, G.F.; Cai, Y.F.; Fan, Y.H.; Zhu, X.Y.; Liu, Y.H.; He, X.H.; Shen, J.J.; Jiang, H.Z.; Hu, D.W.; et al. Transcriptome analysis of stem development in the tumourous stem mustard Brassica juncea var. tumida Tsen et Lee by RNA sequencing. BMC Plant Biol. 2012, 12, 53. [Google Scholar] [CrossRef]
- Pasare, S.A.; Ducreux, L.J.M.; Morris, W.L.; Campbell, R.; Sharma, S.K.; Roumeliotis, E.; Kohlen, W.; van der Krol, S.; Bramley, P.M.; Roberts, A.G.; et al. The role of the potato (Solanum tuberosum) CCD8 gene in stolon and tuber development. New Phytol. 2013, 198, 1108–1120. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Li, W.B.; Li, Z.X.; Zhang, W.L.; Jue, D.W.; Xing, H.T.; Li, H.L.; Li, Q. Dynamic transcriptome profiling provides insights into rhizome enlargement in ginger (Zingiber officinale Rosc.). PLoS ONE 2023, 18, e0287969. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y. The Mechanism of Ginger Rhizome Formationand Enlargement. Doctor’s Dissertation, Shandong Agricultural University, Tai’an, China, 2021. [Google Scholar]
- Wang, L.; Gao, F.S.; Xu, K.; Li, X. Natural occurrence of mixploid ginger (Zingiber officinale Rosc.) in China and its morphological variations. Sci. Hortic. 2014, 172, 54–60. [Google Scholar] [CrossRef]
- Liu, M.; Wang, G.; Liu, G.; Ma, F.; Bao, Z. Biostimulants Promote the Sedimentation of Salts to Restore Tomato Plant Growth Under Salt Stress. J. Soil Sci. Plant Nutr. 2023, 23, 1830–1844. [Google Scholar] [CrossRef]
- Xin, R. Research on Crossing Compatibility and Karyotype Analysis in Paeonia. Master’s Dissertation, Shenyang Agricultural University, Shenyang, China, 2020. [Google Scholar]
- Sheng, C.; Song, S.; Zhou, W.; Dossou, S.S.K.; Zhou, R.; Zhang, Y.; Li, D.; You, J.; Wang, L. Integrating transcriptome and phytohormones analysis provided insights into plant height development in sesame. Plant Physiol. Biochem. 2023, 198, 107695. [Google Scholar] [CrossRef]
- Lv, Y.; Li, Y.; Liu, X.; Xu, K. Identification of Ginger (Zingiber officinale Roscoe) Reference Genes for Gene Expression Analysis. Front. Genet. 2020, 11, 586098. [Google Scholar] [CrossRef]
- Bozan, I.; Achakkagari, S.R.; Anglin, N.L.; Ellis, D.; Tai, H.H.; Strömvik, M.V. Pangenome analyses reveal impact of transposable elements and ploidy on the evolution of potato species. Proc. Natl. Acad. Sci. USA 2023, 120, 11. [Google Scholar] [CrossRef]
- Guo, H.; Zhou, M.; Zhang, G.; He, L.; Yan, C.; Wan, M.; Hu, J.; He, W.; Zeng, D.; Zhu, B.; et al. Development of homozygous tetraploid potato and whole genome doubling-induced the enrichment of H3K27ac and potentially enhanced resistance to cold-induced sweetening in tubers. Hortic. Res. 2023, 10, uhad017. [Google Scholar] [CrossRef]
- Mahmud, E.; Zhu, H.J.; Kaseb, M.O.; Sajjad, M.Z.; He, N.; Lu, X.Q.; Liu, W.G. Polyploidization Impact on Plant Architecture of Watermelon (Citrullus lanatus). Horticulturae 2024, 10, 24. [Google Scholar] [CrossRef]
- Pu, T.; Wang, Y.; Han, W.; Li, H.; Sun, P.; Suo, Y.; Fu, J. Physiological Characteristics and Transcriptional Differences of Growth Traits of Persimmon with Different Ploidy. Horticulturae 2024, 10, 207. [Google Scholar] [CrossRef]
- Kondhare, K.R.; Patil, A.B.; Giri, A.P. Auxin: An emerging regulator of tuber and storage root development. Plant Sci. 2021, 306, 110854. [Google Scholar] [CrossRef] [PubMed]
- Utsumi, Y.; Tanaka, M.; Utsumi, C.; Takahashi, S.; Matsui, A.; Fukushima, A.; Kobayashi, M.; Sasaki, R.; Oikawa, A.; Kusano, M.; et al. Integrative omics approaches revealed a crosstalk among phytohormones during tuberous root development in cassava. Plant Mol. Biol. 2022, 109, 249–269. [Google Scholar] [CrossRef] [PubMed]
- Ravi, V.; Chakrabarti, S.K.; Makeshkumar, T.; Saravanan, R. Molecular Regulation of Storage Root Formation and Development in Sweet Potato. In Horticultural Reviews; John Wiley & Sons: Hoboken, NJ, USA, 2014; Volume 42. [Google Scholar]
- Abts, W.; Vandenbussche, B.; De Proft, M.P.; Van de Poel, B. The Role of Auxin-Ethylene Crosstalk in Orchestrating Primary Root Elongation in Sugar Beet. Front. Plant Sci. 2017, 8, 444. [Google Scholar] [CrossRef]
- Roumeliotis, E.; Visser, R.G.F.; Bachem, C.W.B. A crosstalk of auxin and GA during tuber development. Plant Signal. Behav. 2014, 7, 1360–1363. [Google Scholar] [CrossRef]
- Hartmann, A.; Senning, M.; Hedden, P.; Sonnewald, U.; Sonnewald, S. Reactivation of Meristem Activity and Sprout Growth in Potato Tubers Require Both Cytokinin and Gibberellin. Plant Physiol. 2011, 155, 776–796. [Google Scholar] [CrossRef]
- Borzenkova, R.A.; Borovkova, M.P. Developmental Patterns of Phytohormone Content in the Cortex and Pith of Potato Tubers as Related to Their Growth and Starch Content. Russ. J. Plant Physiol. 2003, 50, 119–124. [Google Scholar]
- Dong, T.; Zhu, M.; Yu, J.; Han, R.; Tang, C.; Xu, T.; Liu, J.; Li, Z. RNA-Seq and iTRAQ reveal multiple pathways involved in storage root formation and development in sweet potato (Ipomoea batatas L.). BMC Plant Biol. 2019, 19, 136. [Google Scholar] [CrossRef]
- Tao, G.Q.; Stuart, D.; Yong, J.W.H.; Zhang, K.; Farquhar, G.D. Promotion of shoot development and tuberisation in potato by expression of a chimaeric cytokinin synthesis gene at normal and elevated CO2 levels. Funct. Plant Biol. 2010, 37, 43–54. [Google Scholar]
- Bishopp, A.; Help, H.; El-Showk, S.; Weijers, D.; Scheres, B.; Friml, J.; Benková, E.; Mähönen, A.P.; Helariutta, Y. A Mutually Inhibitory Interaction between Auxin and Cytokinin Specifies Vascular Pattern in Roots. Curr. Biol. 2011, 21, 917–926. [Google Scholar] [CrossRef]
- Xu, L.; Zhao, H.; Ruan, W.; Deng, M.; Wang, F.; Peng, J.; Luo, J.; Chen, Z.; Yi, K. ABNORMAL INFLORESCENCE MERISTEM1 Functions in Salicylic Acid Biosynthesis to Maintain Proper Reactive Oxygen Species Levels for Root Meristem Activity in Rice. Plant Cell 2017, 29, 560–574. [Google Scholar] [CrossRef]
- Ali, I.; Wang, X.; Tareen, M.J.; Wattoo, F.M.; Qayyum, A.; Hassan, M.U.; Shafique, M.; Liaquat, M.; Asghar, S.; Hussain, T.; et al. Foliar Application of Salicylic Acid at Different Phenological Stages of Peach Fruit CV. ‘Flordaking’ Improves Harvest Quality and Reduces Chilling Injury during Low Temperature Storage. Plants 2021, 10, 1981. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Liu, Y.; Yuan, D.; Duan, M.; Liu, Y.; Shen, Z.; Yang, C.; Qiu, Z.; Liu, D.; Wen, P.; et al. An R2R3 MYB transcription factor confers brown planthopper resistance by regulating the phenylalanine ammonia-lyase pathway in rice. Proc. Natl. Acad. Sci. USA 2019, 117, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Zhang, S.; Li, F.; Zhang, H.; Sun, R.; Li, G.; Zhang, S. Cloning and Functional Analysis of BraTSD2 Associated with Root Swelling in Turnip (Brassica rapa L.). Horticulturae 2025, 11, 33. [Google Scholar] [CrossRef]
- Zhou, Y.; Yao, L.; Xie, Y.; Huang, B.; Li, Y.; Huang, X.; Yu, L.; Pan, C. Metabolic and transcriptional analysis of tuber expansion in Curcuma kwangsiensis. Sci. Rep. 2025, 15, 1588. [Google Scholar] [CrossRef]
- Wang, D.; Pei, K.; Fu, Y.; Sun, Z.; Li, S.; Liu, H.; Tang, K.; Han, B.; Tao, Y. Genome-wide analysis of the auxin response factors (ARF) gene family in rice (Oryza sativa). Gene 2007, 394, 13–24. [Google Scholar] [CrossRef]
- Yang, C. Functional Analysis of Auxin-responsive Genes StARF2 and StARF5 in Potato Tuber Development. Doctor’s Dissertation, Northwest A&F University, Xianyang, China, 2022. [Google Scholar]
- Shen, C.; Wang, S.; Bai, Y.; Wu, Y.; Zhang, S.; Chen, M.; Guilfoyle, T.J.; Wu, P.; Qi, Y. Functional analysis of the structural domain of ARF proteins in rice (Oryza sativa L.). J. Exp. Bot. 2010, 61, 3971–3981. [Google Scholar] [CrossRef]
- Sun, C.Y.; Fan, K.; Wang, X.; Liu, H.H.; Guo, N.P.; Liu, W.Y.; Ye, G.X.; Lin, W.W.; Lin, W.X.; Li, Z.W. The involvement of auxin response factor OsARF7 in positively regulating root development by mediating the expression of OsCRL1 in rice (Oryza sativa L.). Plant Mol. Biol. 2025, 115, 38. [Google Scholar] [CrossRef]
- Li, G.; Liang, W.; Zhang, X.; Ren, H.; Hu, J.; Bennett, M.J.; Zhang, D. Rice actin-binding protein RMD is a key link in the auxin–actin regulatory loop that controls cell growth. Proc. Natl. Acad. Sci. USA 2014, 111, 10377–10382. [Google Scholar] [CrossRef]
- Ellis, C.M.; Nagpal, P.; Young, J.C.; Hagen, G.; Guilfoyle, T.J.; Reed, J.W. AUXIN RESPONSE FACTOR1andAUXIN RESPONSE FACTOR2 regulate senescence and floral organ abscission in Arabidopsis thaliana. Development 2005, 132, 4563–4574. [Google Scholar] [CrossRef]
- Li, Y.; Yang, S.; Shi, M.; Zhang, S.; Wu, S.; Chen, Y.; Li, W.; Tian, W.-M. HbARF2 and HbARF16.3 function as negative regulators for the radial trunk growth of rubber tree. Ind. Crops Prod. 2020, 158, 112978. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).