Abstract
Microplastics and heavy metals (HMs) in soil pose significant environmental and health risks, yet the interactions between mulch film residues and HMs, and their effects on maize productivity, remain poorly understood. This study examined the impacts of long-term traditional polyethylene mulch film (TMF) and biodegradable mulch film (BMF) residues on soil properties, maize root accumulation of HMs, the arbuscular mycorrhizal fungi (AMF) community, and maize productivity under open field conditions. TMF residues significantly increased the soil total carbon (TC), C/N ratio, and bioaccumulation coefficients (BACs) of arsenic (As) and cadmium (Cd) while lowering soil pH and water content. These changes altered AMF colonization and enriched the Paraglomus genus, leading to enhanced maize leaf antioxidant activity and reduced chlorophyll content, although maize growth was not statistically affected. In contrast, they improved soil nutrient availability (e.g., nitrogen and phosphorus), increased TC and the C/N ratio, and reduced soil pH. Notably, BMF residues decreased the BACs of As and Cd, reduced AMF spore density without altering community structure, and ultimately enhanced maize biomass. These effects were associated with BMF’s ability to lower pH and chelate HMs, thereby mitigating their bioavailability and promoting plant growth. Furthermore, the enriched abundance of AMF species, particularly from the Claroideoglomus genus, facilitated heavy metal chelation and reduced HM accumulation in plants. The findings underscore the potential of BMF and AMF for co-remediation of microplastics and HMs, highlighting the importance of mulching strategies for sustainable agriculture.
1. Introduction
Traditional plastic mulch films (TMF) are extensively utilized in agriculture due to their effectiveness in controlling weeds, retaining soil moisture, regulating temperature, and enhancing crop yields [1,2,3]. However, their extensive application has led to persistent environmental issues, as residual TMFs degrade under environmental factors, such as UV radiation and physical disturbances, forming fragments, fibers, and microplastics that accumulate in soils [4,5,6]. These plastic residues significantly alter soil physicochemical properties, degrade soil structure, disrupt microbial communities, and impair plant growth and ecosystem functioning [4,7,8]. Therefore, the accumulation of plastic-derived particles in agricultural soils poses a serious threat to soil health, terrestrial organisms, and the long-term sustainability of ecosystems [9,10,11].
To mitigate plastic mulch pollution associated with TMFs, Biodegradable Mulch Films (BMFs) have emerged as a potential alternative. Although BMFs are designed to decompose into CO2 and water under ideal laboratory conditions, their degradation in field settings is often incomplete and highly variable, influenced by factors such as soil characteristics and climate [12,13]. Studies have shown that BMFs, particularly those made from PLA and PBAT, can degrade slowly, with substantial amounts of microplastics remaining even after 180 days [14]. This incomplete degradation may worsen soil microplastic pollution, disrupt nutrient cycling, and negatively affect soil quality and ecosystem functioning, potentially threatening food security [15,16,17].
Although the environmental risks of plastic mulch residues are increasingly recognized, research on their combined effects with other contaminants, particularly heavy metals, remains scarce. Beyond their direct impact on soil and plants, plastic mulches can adsorb and act as carriers of heavy metals [18,19]. Given that agricultural soils are contaminated with heavy metals, the interaction between plastic residues and heavy metals has become a growing concern [20]. Microplastics from degraded mulch can increase heavy metal pollution risk by forming composite pollution systems [21,22]. Plastics readily adsorb heavy metals through various mechanisms, including hydrophobic partitioning and electrostatic interactions, with the adsorption capacity influenced by factors such as plastic aging and type [23,24]. These adsorbed heavy metals can desorb and alter heavy metal bioavailability and plant uptake [25,26]. For instance, studies have shown that plastics can enhance arsenic (As) bioavailability and increase cadmium (Cd) accumulation in plant roots and even facilitate heavy metal transport to the plant rhizosphere [27,28,29]. Therefore, understanding the interplay between plastic mulch residues, heavy metal concentrations, and heavy metal accumulation in crop roots under long-term film coverage is crucial.
AMFs are key components of soil ecosystems and form symbiotic relationships with the majority of terrestrial plants [30,31]. These fungi enhance nutrient and water uptake in host plants through extensive extraradical hyphae, thereby improving plant resilience and overall health [32,33,34]. AMF can also mitigate heavy metal toxicity in plants through their extensive hyphal networks [35,36,37], which can capture pollutants [38]. AMF can reduce pollutant accumulation in crops through mechanisms like chelation and immobilization [39,40,41]. Plastic residues can negatively affect AMF, potentially through the release of harmful substances and alteration of soil properties during degradation [42]. This interference with plant–fungal symbioses can, in turn, impact heavy metal immobilization and plant uptake [27,38]. Therefore, investigating the effects of plastic mulch residues on both soil heavy metal content and AMF-plant symbiosis, particularly AMF’s role of AMF in modulating heavy metal uptake by plant roots, is critical.
Maize (Zea mays L.) is an essential cereal crop globally, with annual production exceeding 1.2 billion tons and significant nutritional value [43]. Given that 95% of global food production relies on soil-based agriculture, maintaining soil health is paramount [44]. Maize establishes symbiotic relationships with AMF, which enhance nutrient uptake and improve tolerance to heavy metal toxicity [45,46,47]. Therefore, understanding how mulch film residues alter soil nutrient dynamics and subsequently affect maize growth is critical for sustainable agricultural practices. To address knowledge gaps regarding the effects of plastic film residues, a field experiment was conducted in Sichuan Province using maize (Zea mays L.). This study simulated the long-term effects of traditional film (TMF) and short-term effects of biodegradable film (BMF) residues on soil and plant systems under open field conditions. Specifically, we investigate the effects of these treatments on heavy metal accumulation in maize roots, AMF community structure, and maize growth. We hypothesized that (1) TMF residues would increase, while BMF residues would decrease heavy metal concentrations in both soil and roots, and (2) TMF residues would negatively, while BMF residues would positively impact AMF function and soil health, ultimately affecting plant growth. This study aims to elucidate the complex interactions between plastic film residues, heavy metals, and soil AMF, providing crucial insights for sustainable agricultural practices and mitigating the risks of combined soil pollution.
2. Materials and Methods
2.1. Experimental Field and Design
Qiushi Farm, the experimental site at the Chenglong Campus of Sichuan Normal University (30°34′ N, 104°11′ E) in Chengdu, Sichuan Province, China, features a subtropical humid monsoon climate with average temperatures ranging from 14 °C to 22 °C and an annual rainfall of 771.8 mm. The soil, primarily a loam–clay mixture, maintains a pH of 6.5 to 7.0 and contains 2–4% organic matter belonging to Alfisols “(https://www.resdc.cn/ (accessed on 9 March 2025))”, providing suitable conditions for agricultural studies as described by Wang et al. [48].
As the experimental field is totally open under field conditions, a split-plot design was utilized, covering a 40-m by 50-m field that had served as a seedling nursery for over a decade. Six replicated plots, each measuring 10 × 8 m, were established with 5-m buffer zones to reduce edge effects. Each main plot was divided into three subplots assigned to one of three treatments: Control (CK), Traditional Mulch Film (TMF), or Biodegradable Mulch Film (BMF). CK treatment involved no plastic film, whereas TMF used residual polyethylene (PE) film and BMF employed residual biodegradable films made from PBAT and PLA, both 0.01 mm thick. The PE films were sourced from Chengdu Jiuzhou Fengle Agricultural Technology Co., Ltd. (Chengdu, China), and the PBAT+PLA films from Jialemi Horticultural Technology Co., Ltd. (Shaoxing, China).
Building on previously published long-term observation data on film residue, this study focused on the key tillage layer (0–30 cm) to simulate the film residue rate after 32 years of continuous use. To accurately reflect real-world field conditions, we consulted the relevant literature to determine the fragment size distribution of plastic film in mulched areas [49,50]. This resulted in the categorization of fragments into two size classes: microplastic fragments <4 cm2 and those 4–25 cm2. Based on the 32-year film residual rate, we buried two sizes of these film fragments in the soil. Three treatments were established: (1) Traditional Mulch Film (TMF) at 360 kg/ha, (2) Biodegradable Mulch Film (BMF) at 180 kg/ha, and (3) a control (CK) with no added plastic film.
This study focused on maize (Zea mays L.), which was cultivated after the rapeseed harvest in April 2024. The maize planting process began on 4 April 2024 and seed germination was completed by 13 April. Transplantation into the prepared experimental plots was performed on 22 April, maintaining a planting density of approximately 55,560 plants per hectare. Row and plant spacing were set at 35 cm to ensure uniform growth. Prior to maize transplantation, base fertilization was applied to the soil. Fertilizer was added at a rate of 120 kg of nitrogen (N), 60 kg of phosphorus (P), and 90 kg of potassium (K) per hectare, following a 4:2:3 N:P:K ratio. The nitrogen source was a mixture of potassium nitrate and urea. Subsequent fertilization was carried out as necessary during the growing season to meet the requirements of the crop. Maize growth was monitored at three stages: the sixth-leaf stage (30 days post-transplantation), heading stage (60 days), and maturity stage (90 days). The sampling period at the heading stage was emphasized due to its critical impact on yield and biomass production. When maize was transplanted into the plots, the morphology of film residues, including newly introduced films and those aged for 20 days and one year, was examined using scanning electron microscopy (SEM; SU8010, Hitachi, Tokyo, Japan) (Figure A1).
2.2. Plant Leaf and Root Processing
Fresh maize leaves (0.5 g) were homogenized to determine chlorophyll a and b levels, as well as catalase and peroxidase activities, using a colorimetric method [51]. To assess arbuscular mycorrhizal fungi (AMF) infection in roots, the trypan blue staining technique was employed [52]. Fine root segments (~1 cm) were boiled in 5% potassium hydroxide at 90 °C for 60 min, rinsed thoroughly with tap water, acidified in 2% hydrochloric acid (HCl) for 5 min, and stained with 0.05% trypan blue at 90 °C for 10 min. After staining, the roots were decolorized for 2–3 days. For each biological replicate, 30 root segments were randomly selected, with 10 segments mounted per slide for examination under a microscope at 200× magnification. AMF spore density in the soil was determined following the protocol by Brundrett et al. [53]. Briefly, 10 g of soil was homogenized in 100 mL of tap water, soaked for 20 min, and passed through a double-layer sieve (20 and 400 mesh). The retained residues were rinsed with 200 mL of distilled water, centrifuged at 3000 rpm for 3 min, and the supernatant was mixed with a 45–50% sucrose solution. After a second centrifugation for 2 min, the supernatant was filtered through a 400-mesh sieve, and the sucrose solution was subsequently washed away. The remaining material was then transferred to a Petri dish for spore quantification under a stereomicroscope.
2.3. Sampling and Sample Processing
Sampling was performed when over 80% of the plants in each plot had reached the specified growth stage. Plant samples, comprising the aboveground parts and fine roots, were collected by cutting the stems at ground level using a sickle. Aboveground tissues were separated from roots directly in the field. All samples were quickly transported to the laboratory for immediate physiological assessment. The plant materials were then dried at 60 °C until a consistent weight was achieved, after which the dry weight and moisture content were measured.
Soil samples were obtained from the 0–20 cm layer around maize plants using a soil auger and a five-point method described by Wang et al. [48]. Soil attached to fine roots was carefully removed, homogenized, and combined into a composite sample per plot. Six samples were taken per treatment, totaling 18 for all three treatments. Each sample was then passed through a 2 mm sieve and divided into three parts: one portion was air-dried and ground for subsequent analyses, another was stored at 4 °C for detailed physicochemical measurements, and the remainder was placed in 25 mL centrifuge tubes and frozen at −80 °C for microbial studies.
2.4. Soil Physicochemical and Heavy Metal Analysis
Soil physicochemical properties were analyzed using standard methods. Soil moisture content was measured by drying samples at 105 °C. Soil pH was determined in a 1:2.5 soil-to-water suspension. Bulk density was evaluated using the core method based on dry weight. Total carbon (TC) was quantified using the potassium dichromate oxidation method [54] and total nitrogen (TN) was assessed after digestion using a high-temperature strong acid procedure, followed by spectrophotometric analysis [55]. The total inorganic nitrogen (AN) was extracted with 2 M KCl, with NO3−-N concentrations measured by ultraviolet–visible spectrophotometry and NH4⁺-N concentrations determined colorimetrically using the indophenol blue method [56]. The available phosphorus (AP) was extracted using sodium bicarbonate, and the total phosphorus (TP) was quantified using the ammonium molybdate spectrophotometric method [57].
For heavy metal analysis, including copper (Cu), cadmium (Cd), lead (Pb), arsenic (As), zinc (Zn), and chromium (Cr), dried maize root and rhizosphere soil samples (0.5 g each) underwent hot-plate acid digestion following China National Standards [58]. Samples were sequentially digested with 10 mL of hydrochloric acid (HCl), 15 mL of nitric acid (HNO₃), and 5 mL of hydrofluoric acid (HF) at 90–140 °C until the solution became viscous. After cooling, 1 mL of perchloric acid (HClO₄) was added, and the mixture was heated at 160–180 °C until the white fumes nearly dissipated. The residues were dissolved in diluted nitric acid (~1:100), transferred to 50 mL volumetric flasks, and brought to the final volume. The heavy metal concentrations in the digested solutions were measured using an atomic absorption spectrophotometre (AAS) (PinAAcle 900T, PerkinElmer, Shelton, CT, USA).
The heavy metal bioaccumulation coefficient (BAC) was calculated to assess the ability of maize roots to accumulate metals from the soil [59]. The BAC was determined using the following equation:
where C(root) represents the heavy metal concentration in maize roots (mg/kg dry weight) and C(soil) represents the corresponding heavy metal concentration in rhizosphere soil (mg/kg dry weight).
BAC = C(root)/C(soil),
2.5. DNA Extraction, PCR, and Sequencing
To examine arbuscular mycorrhizal fungi (AMF) communities in response to mulch residue treatments, soil samples collected at the maize heading stage (60 days after transplantation) were analyzed. A total of 12 soil samples (4 replicates per treatment) were processed. Genomic DNA was extracted from 0.5–0.7 g of soil using the method described by Liu et al. [60]. DNA quality was evaluated using 0.7% agarose gel electrophoresis, and quantification was performed.
A nested PCR protocol was employed to amplify the AMF-specific 18S rRNA gene region for Illumina MiSeq sequencing (Illumina (China) Scientific Equipment Co., Ltd, Shanghai, China). The first PCR used primers AML1 and AML2 [61], as well as AMV4.5NF and AMDGR [62], to amplify the target region and add Illumina adapter sites. The second PCR incorporated Illumina indexing primers and flow-cell attachment sites. The first PCR thermal cycling conditions were as follows: 94 °C for 1 min (initial denaturation), followed by 12 cycles of 94 °C for 20 s, 65 °C for 45 s, and 72 °C for 45 s, with a final extension at 72 °C for 5 min. The second PCR thermal cycling conditions included 94 °C for 1 min, followed by 18 cycles of 94 °C for 20 s, 50 °C for 30 s, and 72 °C for 30 s, with a final extension at 72 °C for 5 min. PCR products were verified by 2% agarose gel electrophoresis to confirm successful target fragment amplification. Sequencing was performed using the Illumina MiSeq platform. All sequence data were submitted to the NCBI (National Center for Biotechnology Information) sequence read archive under the accession number PRJNA1224526.
2.6. Data Analysis
Raw sequencing data were processed using FLASH for paired-end merging, followed by barcode removal and quality filtering using Trimmomatic (version 0.39). Sequences with an average base quality score below 25, lengths shorter than 200 bp, or ambiguous bases (N) were removed. Amplicon sequence variants (ASVs) were clustered using the UPARSE algorithm in Usearch at a 97% similarity threshold, with the most abundant sequence in each OTU designated as the representative. Taxonomic assignments were performed using the UCLUST classifier against the UNITE database. Representative sequences were aligned using PyNAST (version 1.2.2-4), and phylogenetic trees were constructed using FastTree (version 2.1.11). To standardize sequencing depth, all samples were rarefied to a minimum library size of 83,381 reads per sample.
2.7. Statistical Analysis
Differences in soil physicochemical properties among treatments were analyzed using one-way ANOVA followed by Duncan’s multiple-range test (p < 0.05). Chao1 and Shannon diversity indices were employed to assess α-diversity, while Principal Coordinate Analysis (PCoA) based on Bray–Curtis distances were utilized to assess microbial community composition. These analyses were performed using the Vegan package in R (version 4.3.3). To compare α-diversity across treatments (CK, TMF, and BMF), a nonparametric Wilcoxon test was applied. Visualizations of α-diversity were created with GraphPad Prism (version 10.1.2). Differences in microbial communities were further examined using PCoA to illustrate Bray–Curtis dissimilarities, and a Permutational Multivariate Analysis of Variance (PERMANOVA) was conducted to test the significance of treatment effects on microbial composition. Microbial co-occurrence networks were generated to explore soil microbial dynamics, with key network metrics (e.g., the number of nodes, average path length, average degree, and modularity) calculated using the graph package in R. Positive and negative cohesion values were determined to evaluate the complexity of AMF communities, and network visualizations were produced with Gephi software (version 0.10.1). The relative contributions of AMF community composition and soil properties to Cd and As accumulation were analyzed using the “glmm.hp” package in R (version 4.3.3).
3. Results
3.1. Maize Biomass and Leaf Trait
Both the TMF and BMF residue surfaces exhibited roughening during the maize growing period (Figure A1). These residues and the microplastics derived from them may significantly influence soil properties and maize growth characteristics. The physiological, biochemical, and symbiotic indices under different treatments were analyzed (Figure 1). Compared to the CK group, chlorophyll content was significantly decreased in the TMF treatment (p < 0.01) and increased in the BMF treatment (Figure 1a). Leaf catalase and peroxidase activities were significantly higher in the TMF treatment than in the CK group (p < 0.0001 and p < 0.05, respectively), while no significant difference was observed between the BMF and CK treatments (Figure 1b,c).
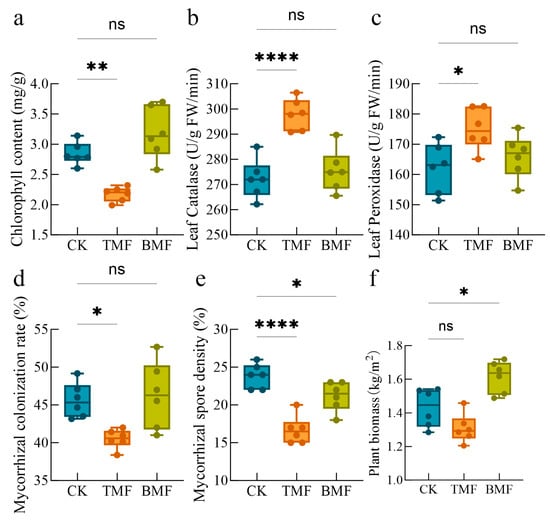
Figure 1.
Comparison of maize leaf chlorophyll content (a), catalase activity (b), peroxidase activity (c), mycorrhizal colonization ratio (d), mycorrhizal spore density (e), and maize biomass (f) across different treatments. Data are expressed as mean ± standard error (n = 6). Significant differences were assessed using the Kruskal–Wallis (KW) test followed by Dunn’s post hoc analysis, with * p < 0.05, ** p < 0.01, **** p < 0.0001, and “ns” indicating no significant difference.
Regarding mycorrhizal symbiosis, the mycorrhizal colonization rate was significantly lower in the TMF treatment (p < 0.05), but no significant difference was observed in the BMF treatment and the CK group (Figure 1d). The mycorrhizal spore density was significantly lower in the TMF (p < 0.0001) and BMF (p < 0.05) treatments than in the CK group (Figure 1e). Plant biomass in the BMF treatment was significantly higher than that in the CK group (p < 0.05), whereas the TMF treatment exhibited a decrease, although this was not statistically significant, compared to the CK group (Figure 1f). These results indicate that the TMF treatment negatively affected chlorophyll content, mycorrhizal colonization, and spore density, potentially due to increased stress. In contrast, the BMF treatment promoted chlorophyll content and plant biomass, suggesting its potential for supporting soil–plant health.
3.2. Soil Physicochemical Properties
The physicochemical properties of the soil under different treatments are summarized in Table 1. Compared with CK, the pH in the TMF treatment was significantly lower (p < 0.05), and the pH in the BMF treatment soil was the lowest among the three treatments (p < 0.05). The SWC in the TMF treatment was significantly reduced compared to that in the CK group (p < 0.05), whereas the SWC decreased in the BMF treatment, although this difference was not statistically significant. The TC content increased significantly under both TMF and BMF treatments relative to CK (p < 0.05 and p < 0.05, respectively), with the BMF treatment showing the highest TC levels. There were no significant differences in soil TN and TP contents among the CK, TMF, and BMF treatments. However, the C/N ratio was significantly higher in the TMF and BMF groups than in the CK group (p < 0.05 and p < 0.05, respectively). Soil AN and AP contents were significantly elevated in the BMF treatment (p < 0.05 and p < 0.05, respectively), with no significant difference between CK and TMF treatments.

Table 1.
Soil physicochemical properties under different treatments.
3.3. Soil Heavy Metal and Root Bioaccumulation Coefficient
The heavy metal contents in soils under different treatments are shown in Figure 2, with the relative changes illustrated in Figure A2. Under TMF treatment, the soil Cu, Cd, Pb, As, Zn, and Cr contents did not differ significantly compared to those in the CK group (Figure 2a–f). In contrast, the BMF treatment significantly increased the soil Cu content relative to CK, while the contents of the soil of Cd, Pb, As, Zn, and Cr did not differ significantly from CK (Figure 2a–f). Regarding relative changes (Figure A3), the TMF treatment resulted in a relative increase in soil Cu, Cd, As, Zn, and Cr contents, whereas soil Pb content showed minor reductions. Conversely, the BMF treatment led to relative increases in soil Cu, Cd, and Zn contents but caused a decrease in soil Pb, As, and Cr contents.
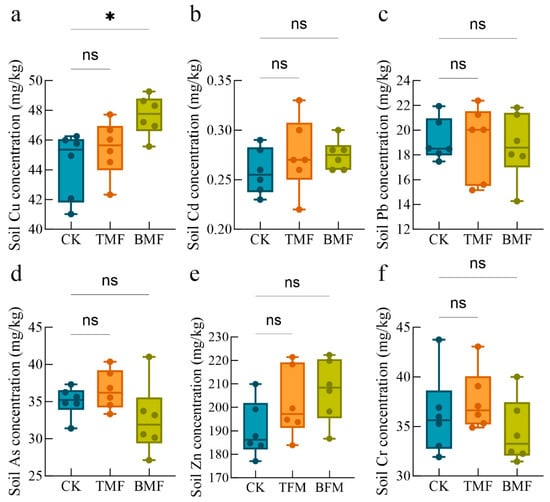
Figure 2.
Soil heavy metal contents, including Cr (a), Cd (b), As (c), Zn (d), Pb (e), and Cu (f), under different treatments are shown. Data are expressed as mean ± standard error (n = 6). Significant differences were determined using the Kruskal–Wallis (KW) test followed by Dunn’s post hoc analysis, where * denotes p < 0.05 and “ns” indicates no significant difference.
The heavy metal BACs in maize fine roots under different treatments are shown in Figure 3. Compared with CK, the BACs of Cd and As were significantly lower in the BMF treatment (p < 0.05 and p < 0.05, respectively), while no significant differences were observed between CK and TMF treatments (Figure 3b,d). In contrast, the BACs of Cu, Pb, Zn, and Cr showed no significant differences among the CK, TMF, and BMF treatments (Figure 3a,c,e,f). Regarding relative changes (Figure A3), under the TMF treatment, root BACs of Cd and As exhibited a relative increase, whereas root Cu, Pb, and Cr showed notable relative reductions. A minor decrease was also observed for Zn content. In contrast, BMF treatment led to relative increases in the BACs of Pb, Zn, and Cr, but caused a decrease in the BACs of root Cu, Cd, and As. Meanwhile, the BAC of soil Zn experienced minimal relative changes. These results suggest that the TMF treatment tends to enhance the enrichment of Cd and As in maize fine roots, whereas the BMF treatment tends to enhance the enrichment of Pb and Cr in maize fine roots. This indicates that BMF may specifically decrease the accumulation of Cd and As in maize roots.
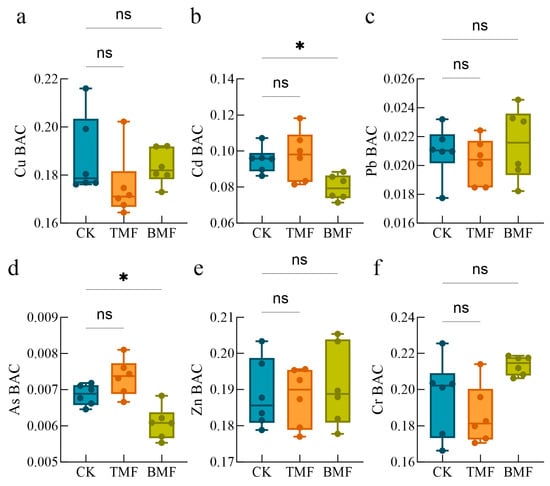
Figure 3.
Heavy metal (HM) enrichment coefficients in maize roots, including Cr (a), Cd (b), As (c), Zn (d), Pb (e), and Cu (f), under different treatments. Data are shown as mean ± standard error (n = 6). Significant differences were determined using the Kruskal–Wallis (KW) test followed by Dunn’s post hoc analysis, where * indicates p < 0.05 and “ns” denotes no significant difference.
3.4. AMF Diversity and Community Composition
The diversity and community structure of AMF under different treatments are shown in Figure 4 and Figure A3. Regarding AMF richness, the Chao1 index (Figure 4a) did not show significant differences among CK, TMF, and BMF treatments. However, for AMF diversity, the Shannon index (Figure 4b) was significantly lower in the BMF treatment compared to CK (p < 0.05), while no significant difference was observed between CK and TMF treatments. PCoA based on Bray–Curtis dissimilarity (Figure 4c) revealed a distinct clustering of AMF communities across treatments. PCo1 and PCo2 explained 22.8% and 12.8% of the variance in AMF community structure, respectively. Pairwise comparisons showed that AMF community composition differed significantly between CK and TMF (R = 0.41; p < 0.05), whereas the difference between CK and BMF was not significant (R = 0.07; p > 0.05). The relative abundances of dominant AMF genera are presented in Figure 4d. Across all treatments, the main genera included Paraglomus, Glomus, and Claroideoglomus, along with other minor genera. According to Figure A4, Paraglomus and Glomus were the most dominant genera in the TMF treatment, and their relative abundances were significantly higher than those in CK (p < 0.05 and p < 0.0001, respectively), whereas no difference was observed between the BMF and CK treatments. In contrast, the relative abundance of Claroideoglomus significantly decreased in TMF and increased in BMF compared to CK (p < 0.0001 and p < 0.05, respectively). Overall, these results suggest that while AMF richness remained unaffected, the BMF treatment significantly reduced AMF diversity and the TMF treatment altered AMF community composition.
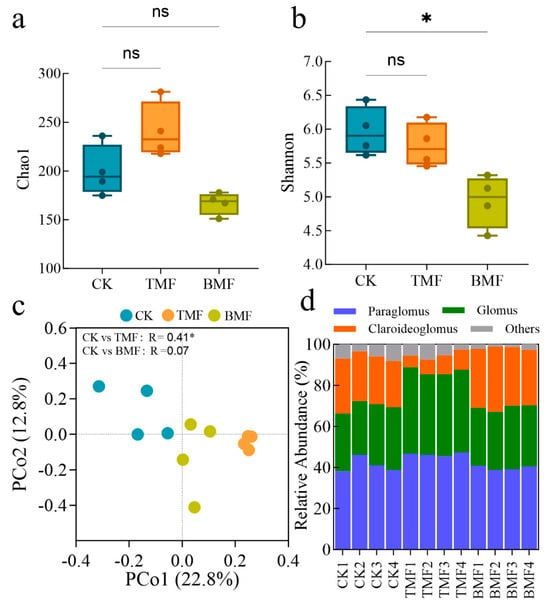
Figure 4.
Chao1 (a) and Shannon (b) diversity indices, Principal Coordinate Analysis (PCoA) with PERMANOVA test based on Bray–Curtis distances (c), and the relative abundance of the top three genera (d) in the AMF community across different treatments. Data are expressed as mean ± standard error (n = 4). Significant differences were assessed using the Kruskal–Wallis (KW) test followed by Dunn’s post hoc analysis, with * p < 0.05 indicating significance and “ns” denoting no significant difference.
3.5. AMF Community Co-Occurrence Network
AMF community co-occurrence network analysis was performed to evaluate the ecological interactions within the CK, TMF, and BMF treatments (Table 2 and Figure 5). Network visualizations (Figure 5a–c) revealed distinct structural differences between the three groups. The TMF network had a higher number of nodes and edges (462, 12,825), while the BMF network contained the smallest number of nodes (244, 4671) compared with the CK network (317, 6215). Similarly, the TMF exhibited a higher average degree (55.52) and modularity (0.71), while the BMF network exhibited a lower average degree (38.29) and modularity (0.48) compared to CK (39.21, 0.55). However, the network density was highest in the BMF treatment (0.16), whereas the CK and TMF networks displayed identical densities (0.12; Table 2). In the CK and TMF networks, nodes corresponding to the main AMF genera, Paraglomus, Glomus, Claroideoglomus, and unclassified Glomeromycota, formed cohesive clusters with abundant connections. In particular, the TMF network showed the highest modularity and extensive cooperation owing to the dense interactions across nodes. In contrast, the BMF network was less connected, with smaller less modular clusters and fewer cohesive patterns, indicating a reduced interaction complexity.

Table 2.
Topological properties of network structure for different treatments.
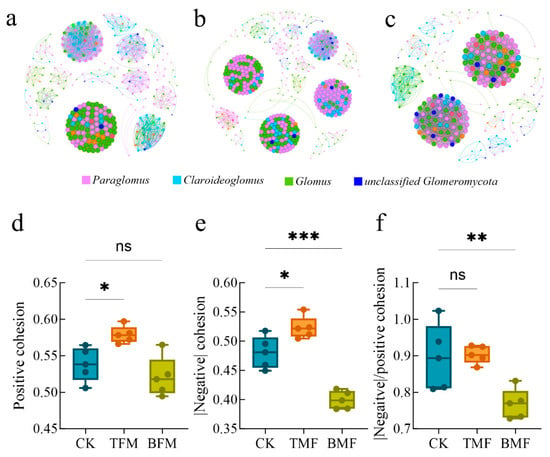
Figure 5.
Co-occurrence networks and topological attributes of soil AMF communities in CK (a), TMF (b), and BMF (c) soils. Microbial network stability indices, including positive cohesion (d), negative cohesion (e), and network complexity (f), across soils under different treatments. Significant differences were evaluated using the Kruskal–Wallis (KW) test followed by Dunn’s post hoc analysis, with * p < 0.05, ** p < 0.01, and *** p < 0.001 indicating significance and “ns” denoting no significant difference.
Network complexity was further evaluated using positive and negative cohesion metrics (Figure 5d–f). Positive cohesion, representing cooperative interactions, was significantly higher in the TMF treatment than in CK (p < 0.05) but did not differ significantly from BMF (Figure 5d). In contrast, negative cohesion, which indicates competitive interactions, was significantly higher in the TMF (p < 0.05) and significantly lower in the BMF (p < 0.001) treatments than in CK (Figure 5e). The ratio of negative to positive cohesion, which reflects the overall interaction balance, was significantly lower in the BMF treatment than in CK but did not differ significantly from TMF, with this simple network suggesting lower complexity in BMF (Figure 5f). Overall, the TMF treatment facilitated the development of a more cooperative and specialized AMF network, while the BMF network exhibited reduced complexity.
3.6. Linking Heavy Metals and Biomass
Regression analysis was used to evaluate the relationships between maize biomass and heavy metal BACs under CK, TMF, and BMF treatments (Figure 6). The Cd and As BACs showed significant negative correlations with maize biomass (R2 = 0.22 and R2 = 0.29, respectively; p < 0.05). Cu, Pb, and Cr exhibited significant positive regressions (R2 = 0.21, 0.27, and 0.27, respectively; p < 0.05), while Zn showed no significant regression (R2 = 0.03, p > 0.05).
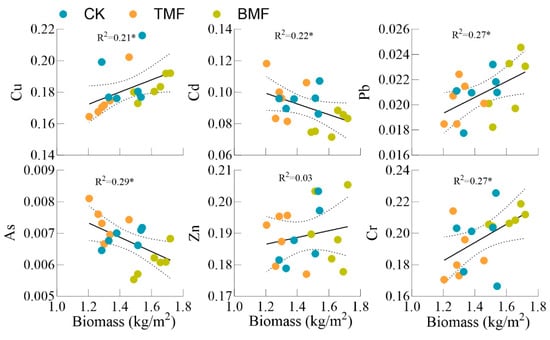
Figure 6.
Linear regression analysis showing the relationship between maize biomass and heavy metal bioaccumulation coefficients. Dashed lines represent the regression fit for the data. Significance levels defined as follows: * p < 0.05.
3.7. Factors Driving Maize Biomass
The factors influencing maize biomass were analyzed using linear mixed models (Figure 7). The model explained 99% of the variation in biomass (adj. R2 = 0.99, p < 0.01), with heavy metal bioaccumulation contributing 51.9%, AMF community characteristics accounting for 30.6%, and soil properties explaining the remaining 17.5%. Among soil factors, soil TC was identified as the dominant predictor, showing a significant positive effect on biomass, while AP had a minimal impact. Within the AMF community, Claroideoglomus abundance and network complexity exhibited strong positive effects on biomass, highlighting their critical role in maintaining functional microbial interactions. These interactions not only support nutrient uptake but also help maize to mitigate environmental stresses. In terms of heavy metals, Cr, Cu, Zn, As, and Cd were significant drivers of biomass variation, demonstrating noticeable impacts on maize growth. In summary, soil carbon, AMF community structure (Claroideoglomus and network complexity), and heavy metals emerged as key drivers of maize growth.
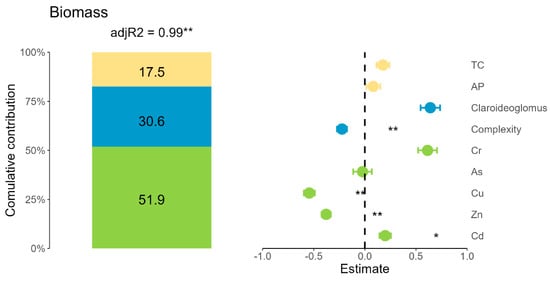
Figure 7.
Individual effect of the model predictors on maize biomass. Parameters were classified into soil factors (TC, total carbon, and AP, available phosphorus), AMF community factors (Claroideoglomus and network complexity), and heavy metals (Cr, As, Cu, Zn, and Cd). Significant differences in the effects of these parameters were indicated by * p < 0.05 and ** p < 0.01. Abbreviations: TC, total carbon; AP, available phosphorus.
4. Discussion
The findings of this study provide nuanced support for the stated hypotheses. TMF treatment increased AMF network complexity while simultaneously inducing a shift in community composition, specifically decreasing the abundance of Claroideoglomus. This, in turn, led to an increase in Cd and As uptake and inhibited the growth of maize. These results substantiate the hypothesis that TMF negatively impacts AMF function and increases heavy metal concentrations in the soil. Conversely, BMF treatment reduced AMF complexity but enriched Claroideoglomus, aiding in Cd and As reduction while increasing nutrient levels, ultimately promoting maize growth. The effect of BMF corroborates the hypothesis that it positively influences special AMF diversity and decreases heavy metal uptake, highlighting the complex interactions within the soil microbiome.
4.1. Heavy Metal Dynamics in Soil and Maize Fine Roots
This study revealed distinct mechanisms through which traditional film (TMF) and biodegradable film (BMF) residues influence the dynamics of heavy metals, particularly As and Cd, in soil (Figure 2) and maize fine roots (Figure 3). The contrasting effects of TMF and BMF on heavy metal behavior underscore the complexity of their interactions with soil properties and plant uptake. These findings contribute important ecological insights into the implications of using different types of plastic films in agricultural practices, offering a deeper understanding of their role in soil–plant systems.
Under TMF treatment, the absolute concentrations of heavy metals in the soil (including Cu, Cd, Pb, As, Zn, and Cr) did not significantly differ from those in the control (CK). However, this study presents new evidence that TMF can increase the relative concentrations of toxic metals, such as Cu, Cd, and As, while slightly reducing Pb and Cr levels (Figure 2 and Figure A2). These changes suggest that TMF modifies the relative proportions of heavy metals in the soil, potentially enhancing the bioavailability of toxic metals such as Cd and As. This observation is further supported by BAC for Cd and As in maize fine roots, as reported in Figure 3 and Figure A3. Several mechanisms may explain these findings. The degradation of TMF residues can alter soil physicochemical properties, such as pH and organic matter content, which are critical factors influencing heavy metal mobility and transformation [63]. For instance, TMF residues can lower soil pH (Table 1), facilitating the desorption of heavy metals through proton competition, thereby increasing their bioavailability [19,64]. TMF also influences soil aggregation and structure, potentially enhancing the mobility of heavy metals. By stabilizing soil aggregates or providing a porous structure, TMF may promote the accumulation of certain metals in plant roots [64,65]. Additionally, microplastics derived from TMF degradation, such as polyethylene (PE), have been shown to facilitate the transformation of metals into more stable organic-bound forms [66]. However, other studies suggest that the impact of TMF on metal mobility and bioavailability varies depending on soil conditions and the specific metals involved [67]. The observed increases in BACs for Cd and As in maize fine roots under TMF treatment align with previous findings, indicating that long-term application of plastic mulch can alter metal concentrations in soil, likely due to changes in soil properties and metal mobility [26]. Critically, we draw attention to the role of TMF-derived microplastics, such as polyethylene (PE), in transforming metals into more bioavailable forms. These results highlight the potential risks associated with TMF use, as the enhanced bioavailability and uptake of toxic metals such as Cd and As could pose significant threats to crop safety and human health.
In contrast, BMF treatment exhibited a distinct and arguably more environmentally favorable pattern in heavy-metal dynamics. While BMF significantly increased the soil Cu content, it simultaneously reduced the relative concentrations of Pb, As, and Cr (Figure 2 and Figure A2). Importantly, our results reveal that the BACs of Cd and As in maize fine roots were significantly lower under BMF treatment, as illustrated in Figure 3 and Figure A3. This suggested that BMF may effectively mitigate the accumulation of these toxic metals in plants. The observed reductions in Cd and As accumulation following BMF treatment can be attributed to several mechanisms. During degradation, BMF releases low-molecular-weight organic compounds, such as lactic acid and hydroxybutyric acid, which can chelate heavy metals such as Cd and As, thereby transforming them into less mobile and bioavailable forms [68]. These organic compounds bind with heavy metals to form stable complexes, which subsequently decrease their accessibility for plant absorption [69]. While prior research has demonstrated the chelation behavior of organic acids, our study adds new evidence linking these processes directly to reduced Cd and As accumulation in maize fine roots under BMF application, reinforcing the functional advantages of BMF. Moreover, the increased surface area and roughness of BMF-derived particles enhance their adsorption capacity for heavy metals, further limiting their mobility [70]. Additionally, BMF degradation alters soil properties such as pH and organic matter content, which can significantly influence heavy metal behavior. Furthermore, our findings emphasize a previously underexplored role of the AMF genus, such as with regard to Claroideoglomus, which appeared more abundant under BMF treatment. This mycorrhizal activity likely contributed to the immobilization of toxic metals, particularly As, providing an additional buffer against bioaccumulation [71]. Together, these mechanisms highlight the soil-improving properties of BMF and underscore its role as a biodegradable alternative with long-term benefits for reducing heavy metal uptake by plants.
The contrasting impacts of TMF and BMF on heavy metal dynamics highlight the crucial need to comprehend the mechanisms underlying plastic mulch degradation and its interactions within soil and plant systems. TMF tends to enhance the bioavailability and root uptake of toxic metals, including Cd and As, potentially through alterations in soil properties, direct metal adsorption, and the impact on soil aggregation. Conversely, BMF’s potential to mitigate these risks is particularly significant, likely owing to the chelating properties of its degradation products and their beneficial influence on soil microbial communities.
4.2. Maize Physiology and Growth Dynamics
In our study, maize biomass was primarily driven by three key factors: heavy metal bioaccumulation (51.9%), the structure of AMF communities (particularly Claroideoglomus abundance and network complexity) (30.6%), and soil total carbon (TC) content (17.5%), which collectively explained the variations in maize biomass (Figure 7). Under TMF treatment, biomass was notably compromised by increased Cd and As bioaccumulation, reduced mycorrhizal colonization, and decreased Claroideoglomus abundance (Figure 1, Figure 3, and Figure 4). In contrast, biomass under BMF treatment was driven by increased AMF spore density, notably higher Claroideoglomus abundance, and the positive effects of reduced Cd and As bioaccumulation (Figure 1, Figure 3 and Figure 4). These factors collectively highlight the contrasting stress levels and beneficial interactions associated with TMF and BMF treatments. Linear mixed models explained a significant portion of biomass variation, underscoring the importance of heavy metal bioaccumulation, AMF community characteristics, and soil properties (Figure 7).
Under TMF treatment, maize biomass decreased compared to the CK, although this reduction was not statistically significant (Figure 1f). This trend coincided with alterations in AMF community composition (Figure 4c) and a decreased relative abundance of beneficial Claroideoglomus (Figure 4d and Figure A4). The increased bioaccumulation of toxic heavy metals, particularly Cd and As, in maize roots under TMF (Figure 3 and Figure A2) likely contributed to this decline in maize growth. Cd and As disrupt essential metabolic processes, inhibit nutrient uptake, and induce oxidative stress, ultimately harming plant growth [72]. Additionally, TMF residue degradation can alter soil physicochemical properties, such as pH and organic matter content, thereby enhancing Cd and As bioavailability [70,73]. Furthermore, the physical barrier created by TMF around the root system can hinder water and nutrient absorption, indirectly affecting heavy metal behavior [29,74]. Our work uniquely highlights that the shift in the AMF community structure, despite increased network complexity (Figure 5a,b), compromises the plant’s resilience against environmental stresses, partly due to a reduction in Claroideoglomus, a key genus linked to improved nutrient acquisition and decreased heavy metal bioavailability through glomalin production [72,75].
In contrast to TMF, BMF treatment significantly enhanced maize biomass compared to the control (CK) (Figure 1f). This positive effect was associated with improved soil properties, including increased TC content and nutrient availability (Table 1). Furthermore, BMF treatment increased the relative abundance of Claroideoglomus (Figure A4). Our results provide novel insight by demonstrating that the enhanced growth under BMF treatment is fundamentally linked to these favorable changes in soil chemistry and AMF community dynamics. Mechanistically, the enhanced growth under BMF treatment can be attributed to increased TC content (Table 1), higher abundance of Claroideoglomus (Figure A4), and reduced BACs of toxic metals such as Cd and As (Figure 3b,d). This aligns with previous findings demonstrating the capacity of AMF to mitigate heavy metal toxicity in plants through extensive hyphal networks that capture pollutants [36]. AMF reduces pollutant accumulation via chelation and immobilization [38]. The release of organic compounds during BMF degradation can further limit the mobility and bioavailability of Cd and As through chelation or stabilization [40,41,72]. Claroideoglomus can also enhance plant tolerance to heavy metals via mechanisms such as glomalin production [65]. Despite exhibiting lower network complexity (Figure 5b,c), our original analysis reveals that the maintenance of effective plant–microbe interactions are the crucial factor driving biomass gains under BMF, rather than network complexity alone. Regression analysis further elucidated the relationship between plant growth and heavy metal stress (Figure 6), revealing significant negative correlations between maize biomass and BACs of Cd and As.
In summary, our study demonstrates that the contrasting effects of TMF and BMF on maize physiology are driven by an intricate interplay between heavy metal dynamics, AMF community structure, and soil organic matter. The findings stress that while TMF may exacerbate heavy metal stress through adverse changes in soil and root environments, BMF can actively mitigate these risks by enhancing beneficial soil properties and microbial interactions. These insights provide a fresh perspective on the potential of Biodegradable Mulch Films to improve crop resilience and environmental safety in agricultural systems.
5. Conclusions
This study reveals distinct impacts of traditional (TMF) and Biodegradable Mulch Films (BMF) on heavy metal dynamics in maize (Zea mays L.) cultivation. TMF significantly increased As and Cd bioavailability and accumulation in maize roots, corresponding with reduced biomass, decreased soil pH, and diminished organic matter content. Conversely, BMF effectively mitigated heavy metal uptake by modifying soil chemistry and enhancing beneficial arbuscular mycorrhizal fungi (AMF), particularly Claroideoglomus. BMF treatment improved soil total carbon and nutrient availability, contributing to enhanced maize growth. The interplay between heavy metal dynamics, AMF community structure, and soil organic matter significantly influenced maize physiology in plastic-affected soils. Notably, Claroideoglomus demonstrated potential for heavy metal filtration and chelation, suggesting applications for co-remediation of microplastics and heavy metals in agricultural systems. These findings highlight the crucial role of AMF in enhancing crop resilience against environmental stressors while addressing the challenges of plastic pollution in terrestrial ecosystems. As BMF emerges as a promising alternative to conventional films, it offers pathways toward sustainable agriculture by reducing heavy metal risks while maintaining productivity.
Author Contributions
Conceptualization, methodology, investigation, writing—original draft preparation, Q.S. and T.S.; data curation, methodology, M.W., M.X. and G.W.; conceptualization, funding acquisition, writing—review and editing, D.L. All authors have read and agreed to the published version of the manuscript.
Funding
The study was supported by the National Natural Science Foundation of China, grant number 31800425.
Data Availability Statement
All sequence data have been deposited in the National Center for Biotechnology Information Sequence Read Archive under accession number PRJNA1224526.
Acknowledgments
We thank Science Park of Sichuan Normal University for experimental field assistance.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Appendix A
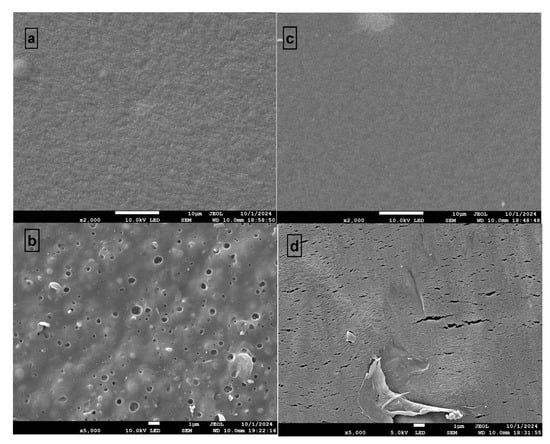
Figure A1.
Comparison of surface morphology of TMF and BMF before and after application to soil for one year and 20 days, respectively: (a) TMF before application, (b) TMF after application, (c) BMF before application, (d) BMF after application.
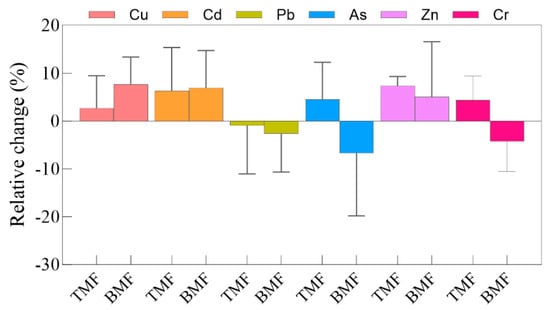
Figure A2.
Relative changes in Cu, Cd, Pb, As, Zn, and Cr concentration in TMF and BMF treatment soils.
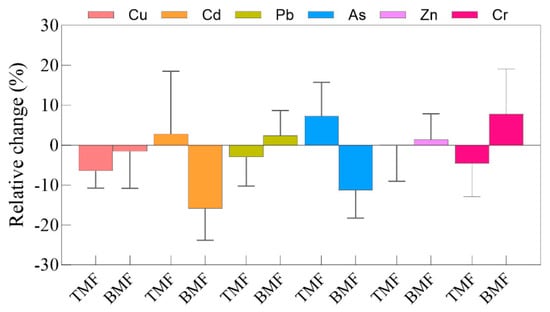
Figure A3.
Relative changes in Cu, Cd, Pb, As, Zn, and Cr bioaccumulation coefficients in maize root TMF and BMF treatments.
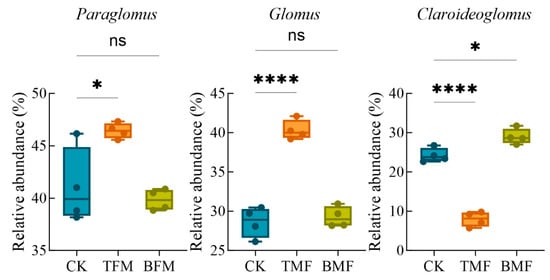
Figure A4.
Relative abundance of AMF genera of Paraglomus, Glomus, and Claroideoglomus under different treatments. Significance levels defined as follows: * p < 0.05 and **** p < 0.0001. ns denoting no significant difference.
References
- Sun, D.; Li, H.; Wang, E.; He, W.; Hao, W.; Yan, C.; Li, Y.; Mei, X.; Zhang, Y.; Sun, Z.; et al. An Overview of the Use of Plastic-Film Mulching in China to Increase Crop Yield and Water-Use Efficiency. Natl. Sci. Rev. 2020, 7, 1523–1526. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Wei, X.; Wang, C.; Zhao, R. Plastic Film Mulching Significantly Boosts Crop Production and Water Use Efficiency but Not Evapotranspiration in China. Agric. Water Manag. 2023, 275, 108023. [Google Scholar] [CrossRef]
- Zhang, L.; Wei, H.; Zhang, K.; Li, Z.; Li, F.-M.; Zhang, F. Plastic Film Mulching Increases Crop Yields and Reduces Global Warming Potential under Future Climate Change. Agric. For. Meteorol. 2024, 349, 109963. [Google Scholar] [CrossRef]
- Aging Processes of Polyethylene Mulch Films and Preparation of Microplastics with Environmental Characteristics | Bulletin of Environmental Contamination and Toxicology. Available online: https://link.springer.com/article/10.1007/s00128-020-02975-x (accessed on 11 February 2025).
- Huang, Y.; Liu, Q.; Jia, W.; Yan, C.; Wang, J. Agricultural Plastic Mulching as a Source of Microplastics in the Terrestrial Environment. Environ. Pollut. 2020, 260, 114096. [Google Scholar] [CrossRef] [PubMed]
- Qiang, L.; Hu, H.; Li, G.; Xu, J.; Cheng, J.; Wang, J.; Zhang, R. Plastic Mulching, and Occurrence, Incorporation, Degradation, and Impacts of Polyethylene Microplastics in Agroecosystems. Ecotoxicol. Environ. Saf. 2023, 263, 115274. [Google Scholar] [CrossRef]
- Ju, T.; Yang, K.; Ji, D.; Chang, L.; Alquiza, M.d.J.P.; Li, Y. Microplastics Influence Nutrient Content and Quality of Salt-Affected Agricultural Soil under Plastic Mulch. Environ. Res. 2025, 264, 120376. [Google Scholar] [CrossRef]
- Qi, Y.; Ossowicki, A.; Yang, X.; Huerta Lwanga, E.; Dini-Andreote, F.; Geissen, V.; Garbeva, P. Effects of Plastic Mulch Film Residues on Wheat Rhizosphere and Soil Properties. J. Hazard. Mater. 2020, 387, 121711. [Google Scholar] [CrossRef]
- Boots, B.; Russell, C.W.; Green, D.S. Effects of Microplastics in Soil Ecosystems: Above and Below Ground. Environ. Sci. Technol. 2019, 53, 11496–11506. [Google Scholar] [CrossRef]
- de Souza Machado, A.A.; Kloas, W.; Zarfl, C.; Hempel, S.; Rillig, M.C. Microplastics as an Emerging Threat to Terrestrial Ecosystems. Glob. Change Biol. 2018, 24, 1405–1416. [Google Scholar] [CrossRef]
- Dong, D.; Guo, Z.; Wu, F.; Yang, X.; Li, J. Plastic Residues Alter Soil Microbial Community Compositions and Metabolite Profiles under Realistic Conditions. Sci. Total Environ. 2024, 906, 167352. [Google Scholar] [CrossRef]
- Sintim, H.Y.; Bary, A.I.; Hayes, D.G.; Wadsworth, L.C.; Anunciado, M.B.; English, M.E.; Bandopadhyay, S.; Schaeffer, S.M.; DeBruyn, J.M.; Miles, C.A.; et al. In Situ Degradation of Biodegradable Plastic Mulch Films in Compost and Agricultural Soils. Sci. Total Environ. 2020, 727, 138668. [Google Scholar] [CrossRef] [PubMed]
- Campanale, C.; Galafassi, S.; Di Pippo, F.; Pojar, I.; Massarelli, C.; Uricchio, V.F. A Critical Review of Biodegradable Plastic Mulch Films in Agriculture: Definitions, Scientific Background and Potential Impacts. TrAC Trends Anal. Chem. 2024, 170, 117391. [Google Scholar] [CrossRef]
- Liao, J.; Chen, Q. Biodegradable Plastics in the Air and Soil Environment: Low Degradation Rate and High Microplastics Formation. J. Hazard. Mater. 2021, 418, 126329. [Google Scholar] [CrossRef]
- Huang, F.; Zhang, Q.; Wang, L.; Zhang, C.; Zhang, Y. Are Biodegradable Mulch Films a Sustainable Solution to Microplastic Mulch Film Pollution? A Biogeochemical Perspective. J. Hazard. Mater. 2023, 459, 132024. [Google Scholar] [CrossRef] [PubMed]
- Beltrán-Sanahuja, A.; Benito-Kaesbach, A.; Sánchez-García, N.; Sanz-Lázaro, C. Degradation of Conventional and Biobased Plastics in Soil under Contrasting Environmental Conditions. Sci. Total Environ. 2021, 787, 147678. [Google Scholar] [CrossRef]
- Song, D.; Jin, G.; Su, Z.; Ge, C.; Fan, H.; Yao, H. Influence of Biodegradable Microplastics on Soil Carbon Cycling: Insights from Soil Respiration, Enzyme Activity, Carbon Use Efficiency and Microbial Community. Environ. Res. 2025, 266, 120558. [Google Scholar] [CrossRef]
- He, S.; Wei, Y.; Li, Z.; Yang, C. Aging Microplastic Aggravates the Pollution of Heavy Metals in Rhizosphere Biofilms. Sci. Total Environ. 2023, 890, 164177. [Google Scholar] [CrossRef] [PubMed]
- Hüffer, T.; Hofmann, T. Sorption of Non-Polar Organic Compounds by Micro-Sized Plastic Particles in Aqueous Solution. Environ. Pollut. 2016, 214, 194–201. [Google Scholar] [CrossRef]
- Liu, B.; Zhao, S.; Qiu, T.; Cui, Q.; Yang, Y.; Li, L.; Chen, J.; Huang, M.; Zhan, A.; Fang, L. Interaction of Microplastics with Heavy Metals in Soil: Mechanisms, Influencing Factors and Biological Effects. Sci. Total Environ. 2024, 918, 170281. [Google Scholar] [CrossRef]
- Li, M.; Wu, D.; Wu, D.; Guo, H.; Han, S. Influence of Polyethylene-Microplastic on Environmental Behaviors of Metals in Soil. Environ. Sci. Pollut. Res. Int. 2021, 28, 28329–28336. [Google Scholar] [CrossRef]
- Khalid, N.; Aqeel, M.; Noman, A.; Khan, S.M.; Akhter, N. Interactions and Effects of Microplastics with Heavy Metals in Aquatic and Terrestrial Environments. Environ. Pollut. 2021, 290, 118104. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, M.J.; Ansari, A.J.; Hai, F.I. Antibiotic Sorption onto Microplastics in Water: A Critical Review of the Factors, Mechanisms and Implications. Water Res. 2023, 233, 119790. [Google Scholar] [CrossRef]
- The Types of Microplastics, Heavy Metals, and Adsorption Environments Control the Microplastic Adsorption Capacity of Heavy Metals|Environmental Science and Pollution Research. Available online: https://link.springer.com/article/10.1007/s11356-023-28131-6 (accessed on 11 February 2025).
- Huang, C.; Ge, Y.; Yue, S.; Zhao, L.; Qiao, Y. Microplastics Aggravate the Joint Toxicity to Earthworm Eisenia Fetida with Cadmium by Altering Its Availability. Sci. Total Environ. 2021, 753, 142042. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, X.; Song, N. Polyethylene Microplastics Increase Cadmium Uptake in Lettuce (Lactuca sativa L.) by Altering the Soil Microenvironment. Sci. Total Environ. 2021, 784, 147133. [Google Scholar] [CrossRef]
- Liu, Y.; Cui, W.; Li, W.; Xu, S.; Sun, Y.; Xu, G.; Wang, F. Effects of Microplastics on Cadmium Accumulation by Rice and Arbuscular Mycorrhizal Fungal Communities in Cadmium-Contaminated Soil. J. Hazard. Mater. 2023, 442, 130102. [Google Scholar] [CrossRef]
- Liu, Y.; Li, B.; Zhou, J.; Li, D.; Liu, Y.; Wang, Y.; Huang, W.; Ruan, Z.; Yao, J.; Qiu, R.; et al. Effects of Naturally Aged Microplastics on Arsenic and Cadmium Accumulation in Lettuce: Insights into Rhizosphere Microecology. J. Hazard. Mater. 2025, 486, 136988. [Google Scholar] [CrossRef]
- Grifoni, M.; Pellegrino, E.; Arrighetti, L.; Bronco, S.; Pezzarossa, B.; Ercoli, L. Interactive Impacts of Microplastics and Arsenic on Agricultural Soil and Plant Traits. Sci. Total Environ. 2024, 912, 169058. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Qiu, Y.L. Phylogenetic Distribution and Evolution of Mycorrhizas in Land Plants. Mycorrhiza 2006, 16, 299–363. [Google Scholar] [CrossRef]
- Evolutionary History of Mycorrhizal Symbioses and Global Host Plant Diversity—Brundrett—2018—New Phytologist—Wiley Online Library. Available online: https://nph.onlinelibrary.wiley.com/doi/full/10.1111/nph.14976 (accessed on 11 February 2025).
- Pearson, J.N.; Jakobsen, I. The Relative Contribution of Hyphae and Roots to Phosphorus Uptake by Arbuscular Mycorrhizal Plants, Measured by Dual Labelling with 32P and 33P. New Phytol. 1993, 124, 489–494. [Google Scholar] [CrossRef]
- Substantial Nitrogen Acquisition by Arbuscular Mycorrhizal Fungi from Organic Material Has Implications for N Cycling | PNAS. Available online: https://www.pnas.org/doi/10.1073/pnas.1005874107 (accessed on 11 February 2025).
- Wang, S.; Chen, A.; Xie, K.; Yang, X.; Luo, Z.; Chen, J.; Zeng, D.; Ren, Y.; Yang, C.; Wang, L.; et al. Functional Analysis of the OsNPF4.5 Nitrate Transporter Reveals a Conserved Mycorrhizal Pathway of Nitrogen Acquisition in Plants. Proc. Natl. Acad. Sci. USA 2020, 117, 16649–16659. [Google Scholar] [CrossRef]
- Riaz, M.; Kamran, M.; Fang, Y.; Wang, Q.; Cao, H.; Yang, G.; Deng, L.; Wang, Y.; Zhou, Y.; Anastopoulos, I.; et al. Arbuscular Mycorrhizal Fungi-Induced Mitigation of Heavy Metal Phytotoxicity in Metal Contaminated Soils: A Critical Review. J. Hazard. Mater. 2021, 402, 123919. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, Y.; Li, X.; Yan, J.; Wu, L.; Tang, Z.; He, Y.; Zhan, F. Mycorrhizal Extraradical Mycelium Can Reduce Cadmium Uptake by Maize and Cadmium Leaching from Contaminated Soil: Based on an in-Growth Core Experiment. Front. Microbiol. 2024, 15, 1507798. [Google Scholar] [CrossRef]
- Yang, F.; Han, J.; Lin, R.; Yin, Y.; Deng, X.; Li, Y.; Lin, J.; Wang, J. Regulation of the Rhizosphere Microenvironment by Arbuscular Mycorrhizal Fungi to Mitigate the Effects of Cadmium Contamination on Perennial Ryegrass (Lolium perenne L.). Microorganisms 2024, 12, 2335. [Google Scholar] [CrossRef]
- Li, H.H.; Chen, X.W.; Zhai, F.H.; Li, Y.T.; Zhao, H.M.; Mo, C.H.; Luo, Y.; Xing, B.; Li, H. Arbuscular Mycorrhizal Fungus Alleviates Charged Nanoplastic Stress in Host Plants via Enhanced Defense-Related Gene Expressions and Hyphal Capture. Environ. Sci. Technol. 2024, 58, 6258–6273. [Google Scholar] [CrossRef] [PubMed]
- Ahammed, G.J.; Shamsy, R.; Liu, A.; Chen, S. Arbuscular Mycorrhizal Fungi-Induced Tolerance to Chromium Stress in Plants. Environ. Pollut. 2023, 327, 121597. [Google Scholar] [CrossRef]
- Fang, X.; Lee, X.; Twagirayezu, G.; Cheng, H.; Lu, H.; Huang, S.; Deng, L.; Ji, B. A Critical Review of the Effectiveness of Biochar Coupled with Arbuscular Mycorrhizal Fungi in Soil Cadmium Immobilization. J. Fungi 2024, 10, 182. [Google Scholar] [CrossRef]
- Pan, J.; Cao, S.; Xu, G.; Rehman, M.; Li, X.; Luo, D.; Wang, C.; Fang, W.; Xiao, H.; Liao, C.; et al. Comprehensive Analysis Reveals the Underlying Mechanism of Arbuscular Mycorrhizal Fungi in Kenaf Cadmium Stress Alleviation. Chemosphere 2023, 314, 137566. [Google Scholar] [CrossRef] [PubMed]
- Leifheit, E.F.; Lehmann, A.; Rillig, M.C. Potential Effects of Microplastic on Arbuscular Mycorrhizal Fungi. Front. Plant Sci. 2021, 12, 626709. [Google Scholar] [CrossRef]
- Erenstein, O.; Jaleta, M.; Sonder, K.; Mottaleb, K.; Prasanna, B.M. Global maize production, consumption and trade: Trends and R&D implications. Food Sec. 2022, 14, 1295–1319. [Google Scholar] [CrossRef]
- FAO. Healthy Soils Are the Basis for Healthy Food Production; Food and Agriculture Organization of United Nations: Rome, Italy, 2015; Available online: http://www.fao.org/soils-2015/news/news-detail/en/c/277682/ (accessed on 11 February 2025).
- Li, J.; Zhou, L.; Chen, G.; Yao, M.; Liu, Z.; Li, X.; Yang, X.; Yang, Y.; Cai, D.; Tuerxun, Z.; et al. Arbuscular Mycorrhizal Fungi Enhance Drought Resistance and Alter Microbial Communities in Maize Rhizosphere Soil. Environ. Technol. Innov. 2025, 37, 103947. [Google Scholar] [CrossRef]
- Zhou, H.-Y.; Nian, F.-Z.; Chen, B.-D.; Zhu, Y.-G.; Yue, X.-R.; Zhang, N.-M.; Xia, Y.-S. Synergistic Reduction of Arsenic Uptake and Alleviation of Leaf Arsenic Toxicity in Maize (Zea mays L.) by Arbuscular Mycorrhizal Fungi (AMF) and Exogenous Iron through Antioxidant Activity. JoF 2023, 9, 677. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jiang, P.; Liao, C.; Fei, J.; Zhang, Y.; Xiangmin, R.; Peng, J.; Luo, G. Understanding the Increased Maize Productivity of Intercropping Systems from Interactive Scenarios of Plant Roots and Arbuscular Mycorrhizal Fungi. Agric. Ecosyst. Environ. 2025, 381, 109450. [Google Scholar] [CrossRef]
- Wang, G.; Sun, Q.; Wei, M.; Xie, M.; Shen, T.; Liu, D. Plastic Film Residue Reshaped Protist Communities and Induced Soil Nutrient Deficiency Under Field Conditions. Agronomy 2025, 15, 419. [Google Scholar] [CrossRef]
- Zhao, Z.-Y.; Wang, P.-Y.; Wang, Y.-B.; Zhou, R.; Koskei, K.; Munyasya, A.N.; Liu, S.-T.; Wang, W.; Su, Y.-Z.; Xiong, Y.-C. Fate of Plastic Film Residues in Agro-Ecosystem and Its Effects on Aggregate-Associated Soil Carbon and Nitrogen Stocks. J. Hazard. Mater. 2021, 416, 125954. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Ding, F.; Flury, M.; Wang, Z.; Xu, L.; Li, S.; Jones, D.L.; Wang, J. Macro- and Microplastic Accumulation in Soil after 32 Years of Plastic Film Mulching. Environ. Pollut. 2022, 300, 118945. [Google Scholar] [CrossRef]
- Bibi, S.; Khan, S.; Taimur, N.; Daud, M.K.; Azizullah, A. Responses of morphological, physiological, and biochemical characteristics of maize (Zea mays L.) seedlings to atrazine stress. Environ. Monit. Assess. 2019, 191, 717. [Google Scholar] [CrossRef]
- Phillips, J.M.; Hayman, D.S. Improved Procedures for Clearing Roots and Staining Parasitic and Vesicular-Arbuscular Mycorrhizal Fungi for Rapid Assessment of Infection. Trans. Br. Mycol. Soc. 1970, 55, 15818. [Google Scholar] [CrossRef]
- Brundrett, M.C.; Bougher, N.L.; Dell, B.; Grove, T.S.; Malajczuk, N. Working with Mycorrhizas in Forestry and Agriculture; Australian Centre for International Agricultural Research: Canberra, Australia, 1996. [Google Scholar]
- Ministry of Environmental Protection of People’s Republic of China. National Environmental Protection Standard HJ615-2011: Soil–Determination of Organic Carbon—Potassium Dichromate Oxidation Spectrophotometric Method; China Environmental Science Press: Beijing, China, 2011. [Google Scholar]
- Liu, W.J.; Zeng, F.X.; Jiang, H. Determination of Total Nitrogen in Solid Samples by Two-Step Digestion–Ultraviolet Spectrophotometry Method. Commun. Soil Sci. Plant Anal. 2013, 44, 1080–1091. [Google Scholar] [CrossRef]
- Liu, X.; Wu, D.; Abid, A.A.; Liu, Y.; Zhou, J.; Zhang, Q. Determination of Paddy Soil Ammonia Nitrogen Using Rapid Detection Kit Coupled with Microplate Reader. Toxics 2022, 10, 725. [Google Scholar] [CrossRef]
- Ministry of Environmental Protection of People’s Republic of China. National Environmental Protection Standard HJ 632-2011: Soil—Determination of Total Phosphorus—Alkali Fusion-Molybdenum Antimony Anti-Colorimetric Method; China Environmental Science Press: Beijing, China, 2011. [Google Scholar]
- Ministry of Ecology and Environment of the People’s Republic of China. China National Ecological Environment Standard HJ1315-2023: Soil and Sediment—Determination of 19 Total Metal Elements—Inductively Coupled Plasma Mass Spectrometry; China Environmental Science Press: Beijing, China, 2023. [Google Scholar]
- Cruzado-Tafur, E.; Bierla, K.; Torró, L.; Szpunar, J. Accumulation of As, Ag, Cd, Cu, Pb, and Zn by Native Plants Growing in Soils Contaminated by Mining Environmental Liabilities in the Peruvian Andes. Plants 2021, 10, 241. [Google Scholar] [CrossRef]
- Liu, D.; Nishida, M.; Takahashi, T.; Asakawa, S. Transcription of mcrA Gene Decreases Upon Prolonged Non-Flooding Period in a Methanogenic Archaeal Community of a Paddy-Upland Rotational Field Soil. Microb. Ecol. 2018, 75, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, S.; Young, J.P. Improved PCR primers for the detection and identification of arbuscular mycorrhizal fungi. FEMS Microbiol. Ecol. 2008, 65, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Xiang, D.; Chen, B.; Li, H. Specificity and selectivity of arbuscular mycorrhizal fungal polymerase chain reaction primers in soil samples by clone library analyses. Acta Agric. Scand. Sect. B—Soil. Plant Sci. 2015, 66, 333–339. [Google Scholar] [CrossRef]
- Hou, S.; Zheng, N.; Tang, L.; Ji, X.; Li, Y. Effect of Soil pH and Organic Matter Content on Heavy Metals Availability in Maize (Zea mays L.) Rhizospheric Soil of Non-Ferrous Metals Smelting Area. Environ. Monit. Assess. 2019, 191, 634. [Google Scholar] [CrossRef]
- Fei, J.; Zou, T.; Geng, M.; Luo, G.; Pang, C.; Huang, Y.; Yang, P.; Peng, J.; Jiang, Y. Residual Mulch-Film Characteristics Affect Heavy Metal Migration of Different Soil Layers in the Subtropical Croplands of China. Environ. Pollut. 2024, 360, 124702. [Google Scholar] [CrossRef]
- Colpaert, R.; de Vaufleury, A.; Rieffel, D.; Amiot, C.; Crini, N.; Gimbert, F. The Effects of Polystyrene Microparticles on the Environmental Availability and Bioavailability of As, Cd and Hg in Soil for the Land Snail Cantareus aspersus. Sci. Total Environ. 2024, 947, 174451. [Google Scholar] [CrossRef]
- Yu, H.; Hou, J.; Dang, Q.; Cui, D.; Xi, B.; Tan, W. Decrease in Bioavailability of Soil Heavy Metals Caused by the Presence of Microplastics Varies across Aggregate Levels. J. Hazard. Mater. 2020, 395, 122690. [Google Scholar] [CrossRef]
- Feng, X.; Wang, Q.; Sun, Y.; Zhang, S.; Wang, F. Microplastics Change Soil Properties, Heavy Metal Availability and Bacterial Community in a Pb-Zn-Contaminated Soil. J. Hazard. Mater. 2022, 424, 127364. [Google Scholar] [CrossRef] [PubMed]
- Naz, M.; Afzal, M.R.; Qi, S.S.; Dai, Z.; Sun, Q.; Du, D. Microbial-Assistance and Chelation-Support Techniques Promoting Phytoremediation under Abiotic Stresses. Chemosphere 2024, 365, 143397. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Y.; Wang, X.; Wang, L.; Meng, G.; Chen, Y. The Adsorption Behavior of Metals in Aqueous Solution by Microplastics Effected by UV Radiation. J. Environ. Sci. 2020, 87, 272–280. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, X.; Ji, C.; Deng, W.; Yang, G.; Hao, Z.; Chen, B. Physicochemical Properties of Environmental Media Can Affect the Adsorption of Arsenic (As) by Microplastics. Environ. Pollut. 2023, 338, 122592. [Google Scholar] [CrossRef] [PubMed]
- Hao, B.; Zhang, Z.; Bao, Z.; Hao, L.; Diao, F.; Li, F.Y.; Guo, W. Claroideoglomus etunicatum Affects the Structural and Functional Genes of the Rhizosphere Microbial Community to Help Maize Resist Cd and La Stresses. Environ. Pollut. 2022, 307, 119559. [Google Scholar] [CrossRef] [PubMed]
- Tan, Q.; Guo, Q.; Wei, R.; Zhu, G.; Du, C.; Hu, H. Influence of Arbuscular Mycorrhizal Fungi on Bioaccumulation and Bioavailability of As and Cd: A Meta-Analysis. Environ. Pollut. 2023, 316, 120619. [Google Scholar] [CrossRef]
- Qin, L.; Wang, M.; Sun, X.; Yu, L.; Wang, J.; Han, Y.; Chen, S. Formation of Ferrihydrite Induced by Low pe+pH in Paddy Soil Reduces Cd Uptake by Rice: Evidence from Cd Isotope Fractionation. Environ. Pollut. 2023, 328, 121644. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.-Y.; Chen, L.; Yang, X.; Jeyakumar, P.; Wang, Z.; Sun, S.; Qiu, T.; Zeng, Y.; Chen, J.; Huang, M.; et al. Unveiling the Impacts of Microplastics on Cadmium Transfer in the Soil-Plant-Human System: A Review. J. Hazard. Mater. 2024, 477, 135221. [Google Scholar] [CrossRef]
- Zhang, J.; Diao, F.; Hao, B.; Xu, L.; Jia, B.; Hou, Y.; Ding, S.; Guo, W. Multiomics reveals Claroideoglomus etunicatum regulates plant hormone signal transduction, photosynthesis and La compartmentalization in maize to promote growth under La stress. Ecotoxicol. Environ. Saf. 2023, 262, 115128. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).