Abstract
Understanding the long-term evolution of soil carbon pools and dissolved organic matter (DOM) is crucial for evaluating carbon cycling and soil fertility in paddy ecosystems. This study investigated the changes in soil organic carbon (SOC), dissolved organic carbon (DOC), and DOM optical characteristics across an 8–63-year rice cultivation chronosequence in the western Jilin irrigation district of northeastern China. Soil samples were collected from five depth intervals (0–10, 10–20, 20–30, 30–40, and 40–50 cm) to assess physicochemical properties, ultraviolet–visible (UV-Vis) absorption, and three-dimensional excitation–emission matrix (EEM) fluorescence features. The results showed that long-term rice cultivation reduced soil salinity and alkalinity while significantly increasing SOC and DOC contents. The UV–Vis indices (SUVA254, SUVA260, SUVA300) increased with cultivation duration, whereas E2/E3, E4/E6, and SR decreased, indicating enhanced aromaticity, humification, and molecular weight of DOM. Fluorescence analysis revealed a gradual transformation from protein-like to humic-like components, supported by PARAFAC modeling that identified four dominant components (two humic-like and two protein-like). Correlation and PLS-SEM analyses demonstrated that cultivation duration positively influenced soil carbon accumulation and DOM humification, while soil depth exerted a negative effect. Soil carbon acted as the core mediator linking UV–Vis and EEM indices, explaining more than half of the observed variance. Overall, long-term rice cultivation promoted carbon stabilization and humic substance formation, improving soil quality and carbon sequestration potential in saline–alkaline paddy soils. These findings provide valuable insights into the spectroscopic mechanisms of DOM transformation and the sustainable management of carbon processes in temperate agroecosystems.
1. Introduction
Rice cultivation plays a pivotal role in global food security and carbon cycling, covering more than 160 million hectares worldwide and serving as one of the most important agricultural systems in Asia []. Long-term paddy cultivation profoundly influences soil physicochemical properties, microbial processes, and organic matter transformation, thereby altering the stability and sustainability of soil carbon pools [,]. As the duration of cultivation increases, continuous flooding and redox fluctuations induce complex changes in soil organic carbon (SOC) and dissolved organic matter (DOM), which are essential indicators of soil fertility, carbon sequestration, and nutrient turnover [].
Soil organic carbon serves as a critical component of the terrestrial carbon reservoir, regulating soil structure, nutrient supply, and microbial activity [,]. The dissolved fraction of organic carbon (WSOC or DOM) represents the most labile and mobile portion of SOC, participating actively in biogeochemical cycles and acting as a sensitive indicator of early changes in soil organic matter dynamics under long-term management practices [,]. In paddy soils, alternating aerobic and anaerobic conditions can accelerate the decomposition of labile carbon while simultaneously enhancing the accumulation of humified components, leading to distinct DOM compositions compared with upland soils []. Therefore, exploring the transformation of the DOM along rice cultivation chronosequences provides valuable insight into the mechanisms of soil carbon evolution and sustainability in agroecosystems.
Optical spectroscopy techniques, including ultraviolet–visible (UV–Vis) absorption and three-dimensional excitation–emission matrix (EEM) fluorescence spectroscopy, have emerged as powerful tools to characterize DOM quantity and quality in soils []. Parameters such as SUVA254, SUVA260, and SUVA280 are commonly used to estimate aromaticity and molecular weight, while fluorescence-derived indices (e.g., fluorescence index, humification index, and biological index) provide information about DOM sources, humification degree, and microbial contributions []. Compared with conventional chemical fractionation, these techniques offer rapid, non-destructive, and mechanistically informative approaches to assess carbon dynamics across soil profiles and time scales [].
Previous studies have investigated the effects of land use conversion, fertilization, and irrigation regimes on soil carbon pools and DOM composition []. However, there remains a limited understanding of how long-term rice cultivation-particularly over multiple decades-affects the vertical and temporal variation in SOC and DOM in saline–alkaline regions of northeastern China. These regions, characterized by high soil pH, low organic matter content, and periodic waterlogging, present unique pedogenic and biochemical environments that modulate organic matter stabilization and transformation processes []. Western Jilin Province, located within the Songnen Plain, is a representative agroecosystem where paddy fields have been continuously cultivated for decades following large-scale irrigation development, offering an ideal natural gradient to examine long-term soil carbon evolution [].
Therefore, this study selected paddy fields with different cultivation durations (8, 18, 28, 38, and 63 years) in the western Jilin irrigation zone to investigate the temporal and vertical variations in soil organic carbon, water-soluble organic carbon, and DOM optical characteristics across five soil depths (0–50 cm). Specifically, the objectives were to: (1) quantify the changes in SOC and WSOC contents with increasing cultivation duration and depth; (2) characterize the spectral features of DOM using UV–Vis and EEM fluorescence analyses; and (3) elucidate the potential mechanisms driving DOM transformation and soil carbon evolution under long-term rice cultivation. The findings of this study will enhance the understanding of carbon dynamics in paddy ecosystems and provide a theoretical basis for sustainable soil management in temperate saline–alkaline regions.
2. Materials and Methods
2.1. Study Area
This study was conducted in the Qianguo Irrigation District, located in the western part of Jilin Province, northeastern China (44°40′–45°05′ N, 124°50′–125°30′ E). The region lies within the Songnen Plain and is characterized by a typical temperate continental monsoon climate, with cold winters and warm summers. The mean annual temperature is approximately 4.6 °C, and the average annual precipitation ranges from 430 to 500 mm, while the potential evaporation exceeds 1500 mm. The frost-free period lasts for about 135 days. The dominant soil type is saline–alkaline meadow soil (Typic Haplaquepts), developed from lacustrine and alluvial deposits []. The groundwater table is relatively shallow (approximately 1.0–1.5 m). Since the 1960s, extensive irrigation development has taken place in this region, leading to a large-scale expansion of paddy fields on saline–alkaline land [].
According to the paddy field distribution map of the Qianguo Irrigation District (Figure 1) and field investigations, five paddy fields with different cultivation histories—8, 18, 28, 38, and 63 years—were selected as study sites. These were designated as S1, S2, S3, S4, and S5, respectively (Figure 2).
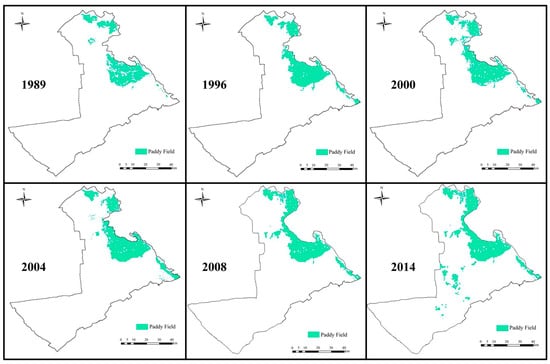
Figure 1.
Distribution map of paddy fields in the Qianguo Irrigation District, western Jilin Province, China.
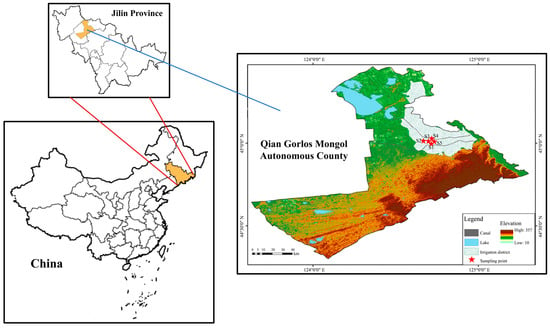
Figure 2.
Spatial distribution of soil sampling sites (S1–S5) with different rice cultivation durations in the Qianguo Irrigation District.
2.2. Experimental Design and Soil Sampling
Soil sampling was conducted after rice harvest in October 2024 across five distinct sites (S1 to S5), each representing a different spatial location within the paddy field with varying cultivation histories (8, 18, 28, 38, and 63 years, respectively). At each site, three sample areas were selected, and each area was further divided into six 25 m2 plots. The plots were systematically arranged, and soil samples were collected using an S-shaped pattern within each plot to ensure spatial representation. At each plot, soil samples were collected from five soil depths: 0–10 cm, 10–20 cm, 20–30 cm, 30–40 cm, and 40–50 cm. For each depth, five replicate soil cores were collected using a stainless-steel auger, and the samples from the same depth were then mixed to form a composite sample. After removing visible plant residues and roots, the composite soil samples were placed in plastic bags and stored in an ice box at 4 °C for transport to the laboratory. This method ensured that the soil samples from each site were representative of the spatial and vertical variability within the paddy fields, while also accounting for different cultivation histories.
After removing visible plant residues and stones, each soil sample was divided into two parts: one air-dried and sieved (<2 mm) for physicochemical property analysis, and one stored at 4 °C for water-soluble organic carbon (WSOC) and spectral measurements.
2.3. Determination of Soil Physicochemical Properties
Soil pH was measured in a 1:2.5 (soil:water, w/v) suspension using a calibrated pH meter (PB-10, Sartorius, Göttingen, Germany).
Electrical conductivity (EC) and salt content were determined using a conductivity meter (DDS-307A, Shanghai INESA, Shanghai, China), and results were expressed in mS·cm−1 and percentage (%), respectively [].
Water content (WC) was determined by oven-drying at 105 °C to a constant weight.
Bulk density (BD) was measured by the core method (ring knife, 100 cm3) after oven-drying at 105 °C [].
Porosity (P) was calculated from bulk density and particle density (assumed 2.65 g·cm−3) using the equation:
Basicity (alkalization degree) was measured by titration with 0.01 mol·L−1 HCl after extraction with CO2-free water, following standard soil alkalization protocols.
Soil organic carbon (SOC) was determined using a Shimadzu TOC-L analyzer (Shimadzu Corporation, Kyoto, Japan) after pre-treatment with 1 mol·L−1 HCl to remove inorganic carbon.
Water-soluble organic carbon (WSOC) was extracted by shaking 10 g of fresh soil with 50 mL of deionized water (1:5, w/v) for 1 h at 25 °C, followed by centrifugation at 8000 rpm for 10 min and filtration through a 0.45 μm membrane filter. The carbon concentration in the filtrate was determined using the same TOC analyzer [].
2.4. UV–Visible Absorption Spectroscopy
UV–Visible absorption spectra of DOM extracts were recorded using a UVmini-1280 spectrophotometer (Shimadzu, Kyoto, Japan) within the wavelength range of 200–700 nm at a 1 nm interval and 1 cm quartz cuvette path length. All measurements were performed at room temperature (25 °C) with deionized water as a blank.
The selected UV parameters include SUVA254, SUVA260, SUVA300, E2/E3, E4/E6, and SR, which are used to characterize the aromaticity and molecular composition of dissolved organic matter (DOM). The parameters and their calculation formulas are as follows:
where c(DOC) represents the sample dissolved organic carbon (DOC) concentration in mg C·L−1.
SUVA254: Reflects the content of aromatic C=C bonds and the abundance of hydrophobic organic components. Higher values indicate greater aromaticity and complex structural features.
SUVA260: Represents the degree of humification and molecular weight distribution, closely related to condensed aromatic ring structures.
SUVA300: Associated with high molecular weight humic substances and long-wavelength chromophores, reflecting the stability and redox activity of DOM.
E2/E3: Represents the ratio of absorbance at 254 nm and 365 nm, used to characterize the molecular weight of DOM. A higher ratio (>3.5) indicates low molecular weight DOM, while a lower ratio (<3.5) indicates high molecular weight DOM.
E4/E6: Represents the ratio of absorbance at 465 nm and 665 nm, used for humic substance classification. A ratio greater than 5 indicates fulvic acid (low humification), while a ratio less than 5 indicates humic acid (high humification).
Slope Ratio (SR): Defined as the ratio of UV absorption slopes at the wavelength ranges of 275–295 nm (S275~295) to 350~400 nm (S350~400). This index is used to distinguish the source of DOM: a higher SR (>1) suggests terrestrial plant-derived DOM input, while a lower SR (<1) indicates DOM of aquatic or microbial origin.
S275~295: Represents the UV slope in the 275–295 nm range, which reflects the molecular weight of DOM. A higher slope indicates smaller DOM molecules.
S350~400: Represents the UV slope in the 350–400 nm range, used to further analyze DOM composition. Combined with S275~295, this helps distinguish the sources of DOM [].
2.5. Three-Dimensional Fluorescence Spectroscopy
Three-dimensional excitation–emission matrix (EEM) fluorescence spectra were obtained using a F-7000 fluorescence spectrophotometer (Hitachi, Tokyo, Japan). The excitation (Ex) wavelength ranged from 200 to 500 nm at 5 nm intervals, and the emission (Em) wavelength ranged from 220 to 600 nm at 5 nm intervals. Both slit widths were set to 5 nm, and the scanning speed was 1200 nm·min−1.
All spectra were corrected for instrument-specific bias and inner-filter effects. Fluorescence indices were calculated as follows:
Fluorescence Index (FI) = Em450/Em500 at Ex370 nm, representing microbial versus terrestrial sources.
Humification Index (HIX) = ratio of integrated emission intensity (Ex254 nm; Em435–480 nm/Em300–345 nm). This index quantifies the degree of DOM humification—higher HIX values indicate more extensively humified, stable DOM components.
Biological Index (BIX) = Em380/Em430 at Ex310 nm. It reflects the level of recent microbial activity in soils and the generation of autochthonous DOM (i.e., DOM produced by in situ microorganisms), with higher BIX values suggesting more active microbial-derived DOM input.
Additionally, this study employed the Parallel Factor Analysis (EEMs-PARAFAC) method to determine the optimal number of fluorescent components in dissolved organic matter (DOM) leachates. To ensure the reproducibility and robustness of the PARAFAC model, a comprehensive validation protocol was implemented, with details as follows: (1) Residual analysis: The residual matrices of models with 2–7 components were visualized and quantified via root mean square error (RMSE). The 4-component model exhibited the lowest RMSE (0.008 ± 0.002), indicating minimal deviation between observed and modeled fluorescence intensities []. (2) Split-half analysis: The entire dataset (n = 75, 5 cultivation durations × 5 depths × 3 replicates) was randomly divided into two subsets (n = 37 and n = 38). PARAFAC models were independently calibrated for each subset, and the correlation coefficients of excitation–emission loadings for each corresponding component between subsets were >0.96 (p < 0.001), confirming the consistency of component identification []. (3) Core consistency diagnostic: The core consistency value of the 4-component model was 87.2%, which is well above the commonly accepted threshold of 80%, ruling out overfitting and validating the appropriateness of component number selection. As stated previously, the final 4-component model explained 98.1% of the total fluorescence variance, further supporting its reliability for DOM compositional analysis. The maximum fluorescence (Fmax) values for each component across all samples were extracted, leading to the establishment of the final PARAFAC model [].
2.6. Data Processing and Statistical Analysis
All analyses were performed in triplicate, and results are expressed as mean ± standard deviation. Significant differences among cultivation durations and soil depths were tested using one-way ANOVA followed by Tukey’s HSD (p < 0.05). Correlation analysis was performed to assess relationships among soil physicochemical parameters, SOC, WSOC, and DOM indices. Partial least squares structural equation modeling (PLS-SEM) was employed to identify the direct and indirect pathways through which cultivation duration, soil properties, and DOM optical characteristics interact []. Variable selection for PLS-SEM was guided by the study’s core objectives (elucidating drivers of DOM transformation) and prior literature []: exogenous variables included cultivation duration (key temporal driver) and soil depth (vertical gradient factor); endogenous variables were grouped into three latent constructs—Soil Carbon (aggregating SOC and DOC, as the central carbon pool), UV (integrating SUVA254, SUVA260, SUVA300, E2/E3, E4/E6, SR to reflect DOM aromaticity/molecular weight), and EEM (combining FI, HIX, BIX to characterize DOM source/humification).
To substantiate model reliability and the claimed explained variance, multiple fit and validation indices were evaluated following Hair et al. []: (1) Goodness of Fit (GOF) = 0.59, exceeding the threshold of 0.36 (medium fit) and confirming overall model adequacy; (2) R2 values (Soil Carbon: 0.92, UV: 0.55, EEM: 0.12) indicated strong explanatory power for carbon accumulation (Soil Carbon) and moderate power for DOM optical properties (UV), consistent with the study’s focus on carbon-driven DOM transformation; (3) Standardized Root Mean Square Residual (SRMR) = 0.072 (below 0.08, indicating acceptable model fit to data). Validation procedures included: (a) convergent validity (Average Variance Extracted, AVE > 0.5 for all latent constructs, confirming internal consistency); (b) discriminant validity (Heterotrait-Monotrait Ratio, HTMT < 0.9 for all variable pairs, ensuring no redundant constructs); and (c) bootstrapping (n = 5000 resamples) to test path coefficient significance (p < 0.05, 0.01, 0.001).
The PLS-SEM analysis was conducted using R 4.2.2. Graphical visualization and data processing were performed using Origin 2022 and SPSS 26.0.
3. Results and Discussion
3.1. Soil Physicochemical Characteristics
The basic physicochemical properties of the paddy soils with different cultivation histories are summarized in Table 1. Across the chronosequence from 8 to 63 years of rice cultivation, the soils exhibited a slightly alkaline reaction, with pH values ranging from 8.07 to 8.70, classifying all sites as mildly alkaline. Soil alkalinity exhibits a gradual reduction with the extension of cultivation duration, which reflects that the continuous flooding regime in paddy systems fosters an anaerobic microenvironment. This anaerobic condition, in turn, stimulates microbial iron reduction processes; the protons (H+) released during this biological process can neutralize hydroxyl ions (OH−) in the soil, thereby exerting a buffering effect on the accumulation of soil bases. []. This pattern agrees with reports that prolonged paddy management enhances proton generation via reduction processes and organic acid accumulation, thereby moderating alkalization in saline–alkaline regions [].

Table 1.
Sample plot basic information.
Electrical conductivity (EC) and salt content decreased from 0.31 mS·cm−1 and 2.09% at S1 (8 years) to 0.13 mS·cm−1 and 1.74% at S5 (63 years). The continuous reduction in soluble salts indicates that irrigation and drainage over multiple decades have effectively leached excess sodium ions and improved soil salinity status []. Concurrently, the basicity index declined from 7.12 to 6.98, confirming a steady alleviation of alkalization stress.
In contrast, the bulk density (BD) decreased from 1.01 g·cm−3 to 0.92 g·cm−3, while porosity increased slightly from 0.63 to 0.70%, reflecting an improvement in soil structure and aeration over time. These changes can be attributed to cumulative organic residue incorporation and the development of a more stable aggregate structure under anaerobic-aerobic alterations typical of paddy systems []. The water content exhibited minor variations (0.47–0.54%), maintaining consistently high levels due to prolonged flooding conditions.
The soil texture analysis revealed significant variations in clay, silt, and sand content across the sampling years. The sand content ranged from 64.47% to 66.47% in the topsoil, with a decrease in finer fractions (clay and silt) observed as the year number increased. For instance, the clay content increased from 4.29% in S1 (8 years) to 15.20% in S5 (63 years), while silt content fluctuated between 22.35% to 67.02%, peaking in S2 (18 years). The increasing clay content observed with time could be attributed to the accumulation of fine particles and organic matter through continuous flooding and residue incorporation [].
The Cation Exchange Capacity (CEC) increased consistently over the years, ranging from 10.20 cmol·kg−1 in S1 to 17.24 cmol·kg−1 in S5. This increase in CEC suggests a positive effect of long-term cultivation on soil nutrient retention. As the soil texture shifted towards a higher clay content, the soil’s ability to retain cations also improved, as clay particles provide a larger surface area for cation exchange. The rise in CEC could also be linked to enhanced organic matter content and microbial activity over time, which contribute to the formation of exchangeable cations [].
Collectively, these findings demonstrate that long-term rice cultivation promotes the desalinization, dealkalization, and structural amelioration of saline–alkaline soils in western Jilin. Regarding the specificity of rice’s effects, similar soil amelioration effects have also been documented in other wetland-adapted crops that require prolonged flooding, such as Phragmites australis (reed) and Typha orientalis (cattail) []. In contrast, upland crops (e.g., wheat, maize) exhibit weaker amelioration effects: their aerobic growth conditions restrict salt leaching (attributed to lower water input), and meanwhile, aerobic microbes decompose organic matter at a faster rate, leading to reduced humus accumulation []. Thus, rice’s unique flood tolerance, high organic matter input, and rhizosphere microbial synergy render it an effective crop for saline–alkaline soil remediation in temperate irrigated areas, including western Jilin.
3.2. Interpretation of SOC and DOC Patterns
As shown in Figure 3, both SOC and DOC contents exhibited a clear decreasing trend with increasing soil depth across all cultivation durations, reflecting the dominance of surface carbon inputs from root exudates and straw residues []. In the top 0–10 cm layer, SOC ranged from approximately 10.2 to 22.5 g·kg−1, while DOC varied between 0.48 and 1.36 g·kg−1. The highest values occurred in the long-term cultivated soil (S5, 63 years), indicating enhanced carbon accumulation under prolonged paddy management.
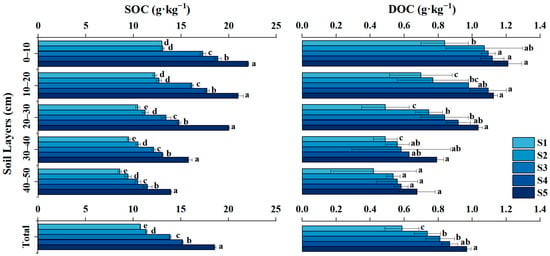
Figure 3.
Vertical distribution of soil organic carbon (SOC) and dissolved organic carbon (DOC) contents at different cultivation durations. Note: Error bars represent standard deviations (n = 3). Different lowercase letters denote significant differences (p < 0.05) among cultivation durations within the same depth according to Tukey’s HSD test.
Vertically, the decline in SOC and DOC with depth became less pronounced in older paddy fields, suggesting that long-term anaerobic conditions promoted carbon stabilization and downward diffusion of labile DOM components []. The SOC increment with cultivation age was most significant in the upper 30 cm, where microbial activity and residue decomposition are concentrated. Similar patterns have been observed in other chronosequences, where continuous rice-flood cycles increase humic-like substances and reduce the mineralization rate of organic matter [].
These results demonstrate that long-term rice cultivation not only increases surface organic carbon storage but also modifies DOM composition and vertical translocation, which may have implications for subsurface carbon sequestration potential in reclaimed saline–alkaline paddy soils [].
3.3. UV–Visible Absorption Characteristics of Dissolved Organic Matter
The ultraviolet–visible (UV–Vis) absorption parameters of dissolved organic matter (DOM) in paddy soils under different cultivation durations and depths are summarized in Table 2. Across the chronosequence, the specific UV absorbance (SUVA) indices (SUVA254, SUVA260, and SUVA300) generally increased with cultivation years in all soil layers, particularly in the surface horizons (0–10 and 10–20 cm). The mean SUVA254 value rose from 2.29 L·mg−1·m−1 in the 8-year soil to 3.43 L·mg−1·m−1 in the 63-year soil, indicating a progressive enrichment of aromatic and humified organic compounds within the DOM pool []. The concurrent rise in SUVA260 and SUVA300 further confirmed that long-term rice cultivation enhanced the molecular complexity and conjugated structures of dissolved organic molecules [].

Table 2.
UV–visible absorption spectral parameters of dissolved organic matter (DOM) in paddy soils under different cultivation durations and soil depths.
Vertically, SUVA indices decreased with depth, consistent with the decline in organic carbon contents Figure 3. The upper 20 cm exhibited the strongest aromatic signatures, reflecting intensive inputs of root exudates and decomposed residues, whereas the subsoil DOM (30–50 cm) displayed lower absorbance, representing simpler and more aliphatic carbon structures []. This vertical attenuation pattern is typical in flooded paddy systems, where organic inputs are mainly derived from surface litter and root turnover [].
The E2/E3 ratio, inversely related to DOM molecular weight, showed a decreasing trend with cultivation time—from 3.90 in S1 to 3.38 in S5 in the surface layer-suggesting an increase in average molecular weight and condensation degree of DOM after long-term rice cultivation []. Specifically, the higher E2/E3 values in younger paddies (S1, 8 years) are indicative of low-molecular-weight DOM primarily derived from microbial exudates (e.g., amino acids, short-chain polysaccharides), as microbial metabolism under initial flooding conditions produces labile, small-molecule organics []. In contrast, the decline in E2/E3 with cultivation duration reflects a shift toward DOM from plant residue decomposition: continuous input of rice straw and root litter (rich in lignin and polyphenols) contributes to larger, more condensed aromatic structures, lowering E2/E3 ratios [].
Similarly, the E4/E6 ratio, a classical indicator of humification, decreased from 14.22 to 10.22 with cultivation age, demonstrating the gradual conversion of protein- and carbohydrate-like substances into humic-like components under prolonged flooding and redox alternations []. The SR (slope ratio) also declined from 0.71 to 0.50, reinforcing the trend toward higher aromaticity and more complex molecular configurations []. Notably, SR values > 0.7 (e.g., S1) are typically associated with terrestrial plant-derived DOM (e.g., straw-derived fulvic acids), while lower SR values (<0.6 in S5) indicate DOM modified by microbial processes—microbes degrade plant-derived aliphatic moieties and repolymerize aromatic fragments, altering the slope ratio [].
The increasing SUVA254/260/300 indices (from 2.29 to 3.43 for SUVA254 in surface soil) further align with this source shift: plant residues (e.g., rice roots, straw) contain higher proportions of aromatic C=C bonds (e.g., from lignin) compared to microbial exudates, and their accumulation with cultivation time enhances the aromaticity of DOM pools []. Together, these UV–Vis indices collectively indicate a transition in DOM sources—from microbial exudate-dominated in short-term paddies to plant residue decomposition-dominated (with microbial reworking) in long-term paddies.
These optical variations reveal that continuous paddy cultivation promotes the transformation of DOM from low-molecular-weight, microbially derived fractions to high-molecular-weight, humic-rich materials. The cyclic alteration of reduction and oxidation conditions in flooded soils fosters partial decomposition and condensation of organic matter, while anaerobic stabilization processes preserve humic substances against further mineralization []. Long-term accumulation of such recalcitrant DOM fractions contributes to soil carbon sequestration and improved structural stability, particularly in saline–alkaline ecosystems where carbon losses via mineralization are otherwise substantial [].
3.4. Fluorescence Characteristics of Dissolved Organic Matter
The three-dimensional excitation–emission matrix (EEM) fluorescence-derived indices—fluorescence index (FI), humification index (HIX), and biological index (BIX)—for DOM under different rice cultivation durations and depths are presented in Table 3.

Table 3.
Fluorescence indices (FI, HIX, and BIX) of dissolved organic matter (DOM) in paddy soils under different cultivation durations and soil depths.
FI values ranged from 1.71 to 1.93, consistently exceeding 1.8 in the majority of samples, indicating that the DOM was predominantly of microbial and autochthonous origin rather than terrestrial plant-derived []. This characteristic aligns with the flooded and anaerobic nature of paddy soils, where microbial metabolism and root exudation are major sources of dissolved organic matter []. A slight decrease in FI was observed with increasing cultivation duration, from 1.87 (8 years) to 1.82 (63 years) in the surface layer (0–10 cm). This trend suggests a gradual transition from fresh, microbially derived DOM toward more humified and microbially reworked materials as the soil system matured under long-term rice cultivation [].
HIX values varied between 0.64 and 0.83, with a general increase along the soil profile, reflecting enhanced humification and condensation of organic matter in deeper layers []. The 28-year and 38-year soils showed the highest HIX values (0.79–0.83), indicating an intermediate phase in which labile compounds were actively transformed into humic-like components. In contrast, the 63-year soil exhibited slightly lower HIX (0.64–0.71), implying that DOM reached a steady-state equilibrium between decomposition and stabilization after long-term cultivation []. These results are consistent with the UV–Vis analysis (Section 3.3), where higher SUVA and lower E4/E6 ratios confirmed increased aromaticity and molecular weight of DOM in older paddy soils []. The continuous alternation of oxidation and reduction conditions during rice-flood cycles likely promoted partial decomposition of easily degradable carbon while enhancing the retention of humified organic fractions [].
BIX values ranged from 0.72 to 0.87, showing relatively high levels in the surface layers (0–20 cm) and a gradual decrease with depth. The higher BIX values in the younger paddy soils (8–18 years) indicate active microbial production and input of newly formed DOM, while the lower values in the older soils (≥38 years) correspond to the reduction in fresh organic inputs and dominance of refractory compounds []. This vertical pattern supports the interpretation that surface DOM is mainly derived from recent microbial and root activities, whereas deeper DOM has undergone prolonged microbial transformation and humification [].
The fluorescence indices demonstrate a clear shift in DOM composition during the 8–63-year cultivation chronosequence. FI values > 1.8 and moderate BIX confirm the persistent contribution of microbial metabolism, while the increasing HIX and decreasing BIX indicate a gradual evolution from active, labile DOM to stable, humified DOM over time. This transition reflects the dual control of long-term flooding regimes and organic matter accumulation typical of temperate paddy soils, contributing to both carbon sequestration and soil quality improvement in saline–alkaline environments [].
3.5. Parallel Factor Analysis (PARAFAC)
To further elucidate the compositional structure of dissolved organic matter (DOM), a PARAFAC model was applied to the EEM spectra of paddy soils with different cultivation durations. Four fluorescent components (C1–C4) were extracted and validated, explaining over 98% of the total fluorescence variance, indicating a robust and interpretable model (Figure 4; Table 4).
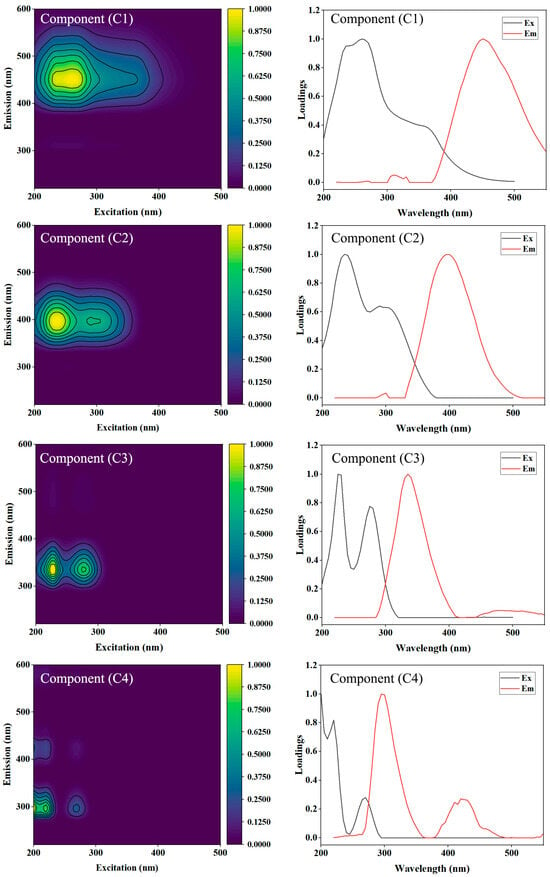
Figure 4.
Excitation–emission contour plots and loadings of four fluorescent components (C1–C4) identified by PARAFAC. Note: The upper panels show the excitation–emission contours, and the lower panels present corresponding excitation (black line) and emission (red line) loadings.

Table 4.
Characteristics of fluorescent components (C1–C4) identified by PARAFAC of DOM in paddy soils.
3.5.1. Component Identification
As summarized in Table 4, the four components were assigned as follows:
C1 (Ex/Em: 260/450 nm) and C2 (Ex/Em: 235/395 nm) correspond to humic-like substances, typically derived from microbial and plant decomposition processes. These components dominate in mature paddy soils and reflect high molecular weight, aromatic, and refractory organic compounds [,,]. Specifically, C1 (terrestrial humic-like) is primarily associated with the decomposition of rice straw and root litter (plant residues)—a key management-induced input in long-term paddy systems—while C2 (microbial humic-like) originates from microbial reworking of plant-derived organic matter under cyclic flooding (environmental control: redox alternations) []. The increasing relative abundance of C1 + C2 in older paddies (S5, 63 years; Figure 5) directly links to cumulative plant residue input and prolonged anaerobic microbial transformation, which are core management practices of continuous rice cultivation.
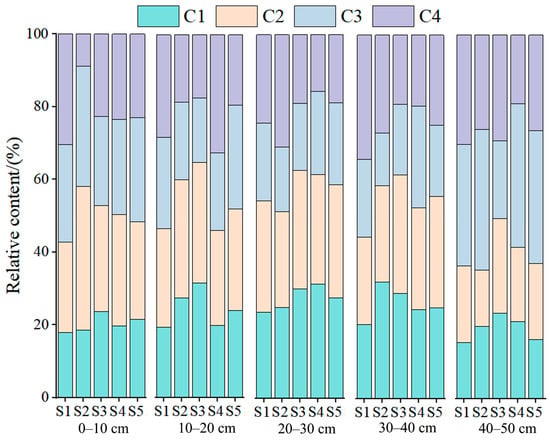
Figure 5.
Relative contributions of four fluorescent components (C1–C4) of DOM in paddy soils under different cultivation durations and depths. Note: Relative abundances are expressed as percentages of total fluorescence intensity. C1 and C2 denote humic-like components; C3 and C4 denote protein-like components.
C3 (Ex/Em: 225/335 nm) represents tryptophan-like DOM, indicative of freshly produced, microbially derived proteinaceous materials [,]. This component is enriched in younger paddies (S1, 8 years) due to higher microbial biomass turnover—a result of initial soil amelioration (lower salinity, Table 1), stimulating microbial activity, with C3 abundance negatively correlated with cultivation duration (r = −0.68, p < 0.01; Figure 6e).
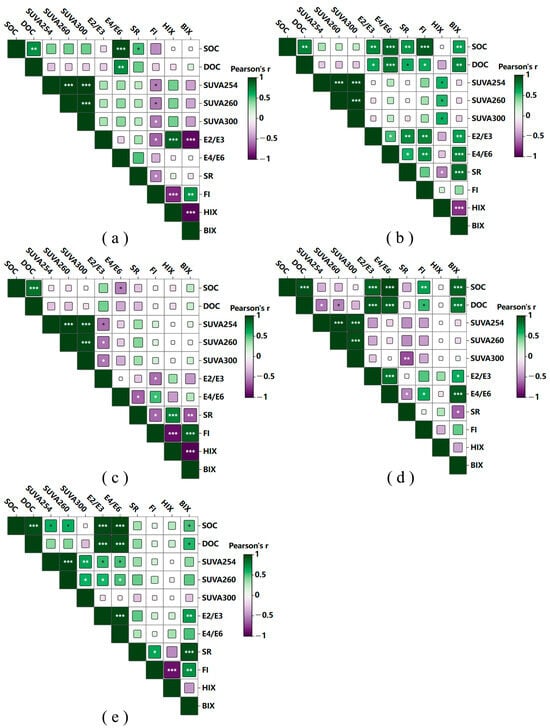
Figure 6.
Correlation characteristics between soil depth and various indices under different cultivation durations. Note: (a) 8-year, (b) 18-year, (c) 28-year, (d) 38-year, and (e) 63-year paddy soils. indicates significance * at p < 0.05, ** at p < 0.01, and *** at p < 0.001. SOC: soil organic carbon; DOC: dissolved organic carbon; SUVA254/260/300: specific UV absorbance indices; E2/E3, E4/E6, SR: molecular size and humification indicators; FI, HIX, and BIX: fluorescence indices describing DOM sources and maturity.
C4 (Ex/Em: 200/295 nm) is associated with tyrosine-like substances, reflecting low-molecular-weight organic compounds related to root exudates or microbial metabolism [,]. C4 peaks in the 0–10 cm surface layer (Figure 5) due to concentrated rice root exudation (a plant–microbe interaction driven by cultivation) and declines with depth, consistent with the vertical distribution of root biomass in paddy soils [].
These spectral characteristics align with previous studies of soil and aquatic DOM, confirming that long-term rice cultivation produces a mixture of humic and protein-like components under alternating redox conditions []. Notably, the UV–Vis and fluorescence indices further support these source associations: higher SUVA254 (3.43 in S5) and HIX (0.71 in S5) correlate with C1 + C2 (humic-like, plant/microbial decomposition), while higher E2/E3 (3.90 in S1) and BIX (0.87 in S1) align with C3 + C4 (protein-like, microbial/root exudate sources) []. This linkage clarifies how management (plant residue input) and environmental factors (redox, root distribution) regulate DOM composition via specific component shifts.
3.5.2. Distribution and Compositional Variations
As shown in Figure 5, the relative abundance of the humic-like components (C1 and C2) increased with cultivation duration, while the protein-like components (C3 and C4) gradually declined. In the 8-year soil, the combined proportion of C3 + C4 accounted for nearly 55% of total fluorescence intensity, whereas this value dropped below 40% in the 63-year soil. Conversely, humic-like components rose from approximately 45% to over 60%, demonstrating enhanced DOM humification and molecular stabilization during long-term rice cultivation [].
Vertically, humic-like components dominated in the deeper layers (30–50 cm), while protein-like substances were enriched in the surface horizons (0–20 cm). This pattern reflects a typical surface input–subsurface preservation process: labile proteinaceous materials are rapidly transformed near the root zone, and the resulting humic substances migrate downward and become stabilized under anaerobic conditions [].
The shift from protein-like to humic-like fluorescence components reveals the microbial transformation and redox-driven polymerization of DOM during prolonged flooding cycles. Continuous cultivation enhances organic inputs from root residues and straw, which undergo partial decomposition under alternating oxic/anoxic environments, forming humic polymers resistant to mineralization []. The accumulation of such refractory DOM contributes to soil carbon sequestration and improved chemical buffering capacity in saline–alkaline paddy ecosystems [].
Thus, the PARAFAC complements the UV–Vis and fluorescence index results, jointly illustrating a progressive aromatic condensation and humification of DOM with increasing cultivation duration.
Notably, the observed shift from protein-like to humic-like DOM components (Figure 5) is tightly linked to microbial community dynamics and enzymatic activities under long-term flooding-induced redox fluctuations. Anaerobic conditions in paddy soils favor the proliferation of functional microorganisms such as iron-reducing bacteria (e.g., Geobacter spp.) and methanogens, which efficiently decompose labile proteinaceous DOM (C3, C4) by utilizing amino acids and peptides as carbon and energy sources []. Concurrently, the cyclic alternation of oxic-anoxic environments maintains the activity of oxidoreductases (e.g., polyphenol oxidase, PPO; peroxidase, POD) and hydrolytic enzymes (e.g., cellulase, xylanase) []. PPO and POD catalyze the polymerization of aromatic precursors (e.g., phenolic compounds released from straw decomposition), promoting the formation of high-molecular-weight humic-like substances (C1, C2) with enhanced aromaticity []. Hydrolytic enzymes, in turn, break down plant residues into low-molecular-weight organic acids, which serve as substrates for microbial humification and further reinforce the condensation of DOM []. These microbial-enzymatic pathways directly explain the increasing trends of SUVA254/260/300 and decreasing E2/E3, E4/E6 ratios (Table 2), as well as the enrichment of humic-like fluorescence components, providing a mechanistic link between redox dynamics and DOM spectral properties.
3.6. Correlation Analysis Among Soil and DOM Optical Parameters
The correlation matrices of soil organic carbon (SOC), dissolved organic carbon (DOC), and DOM optical indices under different cultivation durations are shown in Figure 6. Overall, the correlation patterns varied along the cultivation chronosequence, reflecting the gradual transition of DOM composition and soil carbon stability with prolonged paddy management.
Across all cultivation years, SOC exhibited a highly significant positive correlation with DOC (r > 0.80, p < 0.001), indicating that the dissolved fraction originated mainly from the decomposition and mobilization of total soil organic carbon []. In addition, SUVA254, SUVA260, and SUVA300 were strongly and positively correlated with SOC and DOC (r = 0.65–0.90), suggesting that higher carbon concentrations were associated with more aromatic and humified DOM. This relationship highlights the coevolution of carbon quantity and quality under long-term rice cultivation [].
The E2/E3, E4/E6, and SR indices displayed significant negative correlations with SUVA and HIX (p < 0.01), demonstrating that the increase in DOM aromaticity and humification was accompanied by a decrease in molecular size heterogeneity []. Conversely, the fluorescence index (FI) and biological index (BIX) were positively correlated (r = 0.40–0.70) and negatively correlated with HIX (r = −0.55 to −0.75), implying that microbially derived DOM dominated in less humified environments, whereas humic-like compounds became predominant as soils matured [].
Distinct correlation patterns emerged among the five cultivation stages (Figure 6a–e): In 8- and 18-year soils, strong positive relationships between DOC, FI, and BIX indicated that freshly decomposed, biologically derived DOM dominated at early cultivation stages. In 28- and 38-year soils, the correlations among SOC, SUVA, and HIX intensified, suggesting enhanced DOM condensation and humification under long-term flooding. In 63-year soils, SOC, SUVA, and HIX formed a stable and tightly linked cluster (r > 0.85), reflecting systemic stabilization of soil carbon pools and mature humic DOM composition. These results indicate that as rice cultivation progresses, the controlling factors of DOM shift from biological production to chemical stabilization, mirroring the progressive transformation of soil organic matter into humic-rich, recalcitrant forms [].
The strong coupling among SOC, SUVA indices, and HIX highlights the close interaction between carbon accumulation and DOM quality in paddy soils. The co-variation in DOC and humic-like fluorescence components suggests that long-term cultivation promotes humification and carbon sequestration, thereby enhancing soil fertility and resilience in saline–alkaline ecosystems [].
3.7. Partial Least Squares Structural Equation Modeling (PLS-SEM)
To further elucidate the hierarchical interactions among soil carbon pools and DOM optical properties, a partial least squares structural equation model (PLS-SEM) was constructed (Figure 7). The model integrates cultivation duration, soil depth, soil carbon variables (SOC, DOC), UV–Vis indices (SUVA254, SUVA260, SUVA300, E2/E3, E4/E6, SR), and fluorescence indices (FI, HIX, BIX), providing a comprehensive framework for interpreting the coupled effects of cultivation time and soil stratification on DOM quality and humification. The model development and validation adhered to rigorous PLS-SEM conventions to ensure robustness and interpretability.
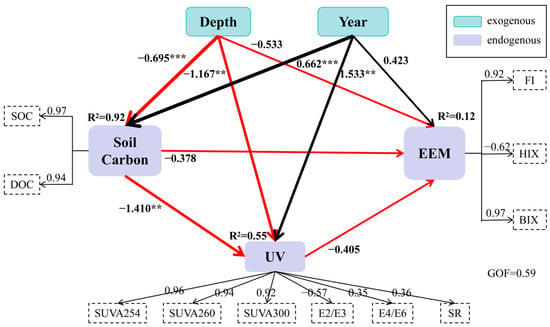
Figure 7.
Partial least squares structural equation model (PLS-SEM) illustrating the relationships among soil carbon, DOM optical indices, and cultivation factors. Note: Black and red arrows represent positive and negative path coefficients, respectively. Asterisks denote significance levels (** p < 0.01, *** p < 0.001). The model includes both exogenous variables (Soil Carbon, Depth, and Year) and endogenous variables (UV and EEM). The path coefficients represent the strength of the relationships between variables, with positive values indicating direct positive relationships and negative values indicating inverse relationships. The R2 values reflect the proportion of variance explained by each endogenous variable. The relationship between Soil Carbon and UV (path coefficient = −1.410, p < 0.001) is the strongest negative effect, while Depth has a significant positive effect on EEM (path coefficient = 0.662, p < 0.001). Model fit indices: GOF = 0.59, SRMR = 0.072, NFI = 0.92, RMSEA = 0.068, all meeting acceptable fit criteria. Abbreviations: UV: DOM UV–Vis parameters [SUVA254/260/300 = Specific UV Absorbance at 254/260/300 nm; E2/E3 = Absorbance ratio at 254/365 nm; E4/E6 = Absorbance ratio at 465/665 nm; SR = Slope Ratio (S275~295/S350~400)]. EEM: Fluorescence indices [FI = Fluorescence Index (Em450/Em500 at Ex370 nm); HIX = Humification Index (Em435–480/Em300–345 at Ex254 nm); BIX = Biological Index (Em380/Em430 at Ex310 nm)].
3.7.1. Model Specification and Variable Selection Rationale
Variable selection was guided by three core principles: (i) alignment with the study’s central objective (elucidating drivers of DOM transformation in paddy soils); (ii) theoretical support from prior literature on soil carbon dynamics and spectral properties; (iii) avoidance of multicollinearity (Variance Inflation Factor, VIF < 3 for all observed variables).
Exogenous variables: Cultivation duration (Year) was selected as the key temporal driver, as long-term rice cultivation is the primary anthropogenic disturbance regulating soil carbon accumulation and DOM evolution. Soil depth was included as a vertical gradient factor, given its well-documented influence on carbon distribution and microbial activity in soil profiles.
Endogenous latent constructs: “Soil Carbon” aggregated soil organic carbon (SOC) and dissolved organic carbon (DOC), as these two parameters directly represent the total and labile fractions of the soil carbon pool, serving as the central mediator of DOM transformation.
“UV” integrated UV–Vis indices (SUVA254, SUVA260, SUVA300, E2/E3, E4/E6, SR) to reflect DOM aromaticity and molecular weight, which are core spectral proxies for DOM quality.
“EEM” combined fluorescence indices (FI, HIX, BIX) to characterize DOM source (microbial vs. terrestrial) and humification degree, complementing UV–Vis data in capturing the DOM functional properties.
3.7.2. Model Fit and Validation Procedures
Multiple fit indices were evaluated to assess model adequacy, exceeding the minimum thresholds for acceptable fit []:
Goodness of Fit (GOF) = 0.59 (exceeds the medium fit threshold of 0.36), indicating overall model consistency with the data.
Standardized Root Mean Square Residual (SRMR) = 0.072 (below the critical value of 0.08), reflecting a satisfactory fit between the observed covariance matrix and the model-implied matrix.
Normed Fit Index (NFI) = 0.92 (≥0.90), demonstrating strong model fit relative to the null model.
Root Mean Square Error of Approximation (RMSEA) = 0.068 (<0.08), indicating minimal approximation error in the model.
Model validation was conducted through three complementary procedures:
Convergent validity: Average Variance Extracted (AVE) values for all latent constructs exceeded 0.5 (Soil Carbon: 0.89; UV: 0.63; EEM: 0.57), confirming that each construct captures sufficient variance from its observed variables.
Discriminant validity: Heterotrait-Monotrait Ratio (HTMT) values for all variable pairs were <0.9 (range: 0.32–0.78), ensuring no redundant constructs and a clear distinction between latent variables.
Bootstrapping validation: A bootstrap resampling procedure (n = 5000) was performed to test the significance of path coefficients. All critical paths were statistically significant (p < 0.05, 0.01, or 0.001), confirming the reliability of causal inferences.
3.7.3. Model Interpretation
The PLS-SEM exhibited strong explanatory power for key endogenous constructs (Figure 7): “Soil Carbon” had an R2 value of 0.92, indicating that 92% of the variance in soil carbon accumulation was explained by cultivation duration and soil depth—reflecting the dominant role of these two factors in regulating carbon pools in saline–alkaline paddy soils. “UV” had an R2 value of 0.55, demonstrating moderate explanatory power for DOM aromaticity/molecular weight, which is consistent with the study’s focus on carbon-driven DOM transformation (soil carbon is the core mediator). “EEM” had an R2 value of 0.12, suggesting a weaker direct explanation by the model. This is attributed to the complexity of DOM fluorescence properties, which are potentially influenced by unmeasured variables (e.g., microbial community composition, enzyme activity) not included in the current model. These results indicate that the latent variable “Soil Carbon” effectively captures the major variance in SOC and DOC, while “UV” and “EEM” reflect secondary processes associated with optical absorption and fluorescence characteristics []. The high R2 of Soil Carbon (0.92) underscores the dominant influence of cultivation duration and soil depth on carbon accumulation and transformation within the paddy profile [].
Path coefficient interpretation aligned with established PLS-SEM conventions: Cultivation year exerted a strong positive effect on Soil Carbon (β = 0.662, p < 0.001), confirming that long-term rice cultivation significantly enhances organic carbon accumulation through continuous residue input and stabilization processes. Conversely, depth had a significant negative effect (β = −0.695, p < 0.001), indicating rapid attenuation of carbon content and DOM activity with increasing soil depth. The positive correlation between Year and EEM (β = 0.533, p < 0.05) reveals that long-term management enhances DOM fluorescence, primarily due to the enrichment of humic-like compounds and microbial metabolites in aged paddy soils [].
The Soil Carbon → UV (β = −1.410, p < 0.001) and Depth → UV (β = −1.167, p < 0.01) paths were both strongly negative, suggesting that increased carbon storage and deeper soil layers reduce the relative optical activity of DOM. This could be attributed to the preferential stabilization of high-aromatic humic substances, which absorb less efficiently at shorter UV wavelengths []. Furthermore, the UV → EEM path (β = −0.405) indicates that UV-derived aromatic indices moderately constrain the fluorescence response of DOM, reflecting a balance between humic condensation and microbial re-synthesis processes. These relationships demonstrate that soil carbon serves as the central mediator linking cultivation time, soil depth, and DOM optical signatures-driving the transition from labile, protein-like DOM toward stable, humified carbon pools [].
The PLS-SEM results provide mechanistic insight into how long-term rice cultivation alters the structure and stability of soil DOM: Cultivation duration promotes SOC accumulation and humification, enhancing long-term carbon sequestration potential; Soil depth exerts a negative control by reducing DOM concentration and optical activity; The UV and EEM modules represent key diagnostic proxies for DOM quality evolution under continuous paddy management. Collectively, the model underscores that DOM optical properties are not independent indicators, but rather functionally linked to soil carbon pools and hydrological regimes in saline–alkaline rice ecosystems [].
4. Conclusions
This study systematically examined the evolution of soil organic carbon (SOC), dissolved organic carbon (DOC), and dissolved organic matter (DOM) optical properties along an 8–63-year rice cultivation chronosequence in the western Jilin irrigation district of northeastern China. The results provide compelling evidence that long-term rice cultivation profoundly modifies soil carbon pools and DOM composition through coupled physicochemical and microbial processes.
Prolonged rice cultivation significantly improved the soil physicochemical environment, as indicated by reductions in pH, salt content, and bulk density, accompanied by increased porosity and water-holding capacity. Both SOC and DOC exhibited pronounced surface enrichment and a clear increasing trend with cultivation duration, demonstrating that continuous flooding and residue incorporation enhanced carbon accumulation and stabilization in saline–alkaline paddy soils.
Spectroscopic analyses revealed a distinct transformation of DOM structure with time and depth. UV–Vis indices (SUVA254, SUVA260, SUVA300) increased with cultivation duration, while E2/E3, E4/E6, and SR decreased, indicating a progressive shift toward more aromatic, humified, and higher-molecular-weight DOM. The fluorescence-derived indices (HIX, FI, and BIX) further confirmed this pattern: FI values (>1.8) highlighted a persistent microbial contribution, whereas elevated HIX values suggested enhanced humification and molecular condensation. PARAFAC modeling identified four fluorescence components—two humic-like (C1, C2) and two protein-like (C3, C4), whose relative proportions revealed a gradual transition from labile proteinaceous DOM in younger paddies to humic-rich, refractory DOM in older ones.
Correlation and structural equation modeling clarified the hierarchical drivers governing these transformations. Cultivation duration exerted a strong positive effect on soil carbon accumulation, while depth negatively influenced carbon and DOM optical indices. The PLS-SEM framework demonstrated that soil carbon acts as a central mediator connecting environmental gradients with DOM spectral characteristics, with UV–Vis and fluorescence pathways jointly explaining over 59% of the total variance. These findings highlight that long-term paddy management fosters a synergistic coupling between carbon quantity and quality, leading to a stable and humified carbon pool under flooded conditions.
However, it is important to note that while this study provides valuable insights into the long-term effects of rice cultivation on soil carbon and DOM quality, direct measurements of stable carbon fractions (e.g., labile vs. stable pools, mineral-associated OC) were not included in this study. Future research will be essential to investigate these specific carbon fractions to confirm the mechanisms underlying carbon stabilization in long-term paddy systems. Including such data will provide a more comprehensive understanding of the processes involved and allow for a more complete assessment of the role of rice cultivation in promoting carbon sequestration. Additionally, further studies should explore how microbial communities contribute to the transition of labile carbon to stable carbon pools, and how environmental factors, such as water management and soil type, influence the stability of these carbon pools over time.
Future studies should focus on directly measuring carbon fractions (such as labile and stable carbon pools, and mineral-associated organic carbon) to provide more concrete evidence of the carbon stabilization process in paddy soils. Investigating the role of microbial functional diversity in driving these processes and how it interacts with management practices and environmental conditions would provide further insights into the mechanisms governing carbon cycling in paddy ecosystems.
Overall, this study provides new insights into the spectroscopic mechanisms of carbon stabilization in paddy soils and underscores the potential of long-term rice cultivation as a viable strategy for improving soil health and promoting sustainable agroecosystem management in temperate saline–alkaline regions.
Author Contributions
Conceptualization, Q.L. and Y.Q.; methodology, Q.L. and Y.Q.; software, Y.Q. and X.G.; validation, Q.L. and Y.Q.; formal analysis, Q.L. and J.Z.; investigation, Q.L., J.Z. and Y.X.; resources, Q.L. and Z.D.; data curation, Y.Q., W.Y. and G.Z.; writing—original draft preparation, Q.L. and Y.Q.; writing—review and editing, Q.L.; supervision, Q.L., X.G. and P.W.; project administration, Q.L. and X.Z.; funding acquisition, Q.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by grants from the Key R&D Project of the Natural Science Foundation of Jilin Province (20250203140SF) and the Climbing Project of Changchun University (ZKP202202).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed at the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Rezvi, H.U.A.; Tahjib Ul Arif, M.; Azim, M.A.; Tumpa, T.A.; Tipu, M.M.H.; Najmine, F.; Brestiè, M. Climate change implications and the future prospects for nutritional security. Food Energy Secur. 2022, 12, e430. [Google Scholar] [CrossRef]
- Huang, L.M.; Thompson, A.; Zhang, G.L. Long-term paddy cultivation significantly alters topsoil phosphorus transformation and degrades phosphorus sorption capacity. Soil Tillage Res. 2014, 142, 32–41. [Google Scholar] [CrossRef]
- Luo, Y.; Huang, L.; Yuan, G. Characteristics and controls of inorganic and organic phosphorus transformation during long-term paddy soil evolution. Soil Tillage Res. 2022, 224, 105524. [Google Scholar] [CrossRef]
- Kirkels, F.M.S.A.; Cammeraat, L.H.; Kuhn, N.J. The fate of organic carbon soil upon erosion, transport and deposition in agricultural landscapes—A review of different concepts. Geomorphology 2014, 226, 94–105. [Google Scholar] [CrossRef]
- Kononova, M.M. The importance of organic matter in soil formation and soil fertility. In Soil Organic Matter: Its Nature, Its Role in Soil Formation and in Soil Fertility, 2nd ed.; Pergamon Press: New York, NY, USA, 1966; pp. 183–228. [Google Scholar]
- Wander, M.M. Soil Organic Matter Fractions and Their Relevance to Soil Function. In Soil Organic Matter in Sustainable Agriculture, 1st ed.; Magdoff, F., Weil, R.R., Eds.; CRC Press: Boca Raton, FL, USA, 2004; pp. 67–102. [Google Scholar]
- Sparling, G.P. Ratio of microbial biomass carbon to soil organic carbon as a sensitive indicator of changes in soil organic matter. Aust. J. Soil Res. 1992, 30, 195–207. [Google Scholar] [CrossRef]
- Murindangabo, Y.T.; Kopecký, M.; Perná, K.; Nguyen, T.G.; Ghorbani, M.; Konvalina, P.; Bohatá, A.; Kavková, M.; Hoang, T.N.; Kabelka, D.; et al. Enhancing Soil Organic Matter Transformation through Sustainable Farming Practices: Evaluating Labile Soil Organic Matter Fraction Dynamics and Identifying Potential Early Indicators. Agriculture 2023, 13, 1314. [Google Scholar] [CrossRef]
- Wei, L.; Ge, T.; Zhu, Z.; Luo, Y.; Yang, Y.; Xiao, M.; Yan, Z.; Li, Y.; Wu, J.; Kuzyakov, Y. Comparing carbon and nitrogen stocks in paddy and upland soils: Accumulation, stabilization mechanisms, and environmental drivers. Geoderma 2021, 398, 115121. [Google Scholar] [CrossRef]
- Li, K.; Ge, J.; Hu, Q.; Yao, W.; Fu, X.; Ma, C.; Qi, Y. Unraveling Seasonal Dynamics of Dissolved Organic Matter in Agricultural Ditches Using UV–Vis Absorption and Excitation–Emission Matrix (EEM) Fluorescence Spectroscopy. Chemosensors 2025, 13, 346. [Google Scholar] [CrossRef]
- Ohno, T. Fluorescence Inner-Filtering Correction for Determining the Humification Index of Dissolved Organic Matter. Environ. Sci. Technol. 2002, 36, 742–746. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, C.; Zou, L.; Cui, H. Optical Characteristics and Chemical Composition of Dissolved Organic Matter (DOM) from Riparian Soil by Using Excitation-Emission Matrix (EEM) Fluorescence Spectroscopy and Mass Spectrometry. Appl. Spectrosc. 2015, 69, 623–634. [Google Scholar] [CrossRef]
- Al-Graiti, T.; Jakab, G.; Ujházy, N.; Márialigeti, K.; Árendás, T.; Karlik, M.; Szalai, Z. Studying Soil Organic Matter Composition in Arable land: Can Soil Management Impact Carbon Pools? In Proceedings of the EGU General Assembly 2023, Vienna, Austria, 24–28 April 2023. [Google Scholar]
- Zhao, Y.; Wang, G.; Zhao, M.; Wang, M.; Xue, Z.; Liu, B.; Jiang, M. Seed limitation and saline-alkaline stress restrict wetland restoration potential in the Songnen Plain, northeastern China. Ecol. Indic. 2021, 129, 107998. [Google Scholar] [CrossRef]
- Hang, Y.; Lu, X.; Li, X. Spatiotemporal Differentiation Characteristics and Zoning of Cultivated Land System Resilience in the Songnen Plain. Sustainability 2025, 17, 4314. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, S.; Li, Y.; Liu, J.; Zhuo, Y.; Chen, H.; Wang, J.; Xu, L.; Sun, Z. Extensive reclamation of saline-sodic soils with flue gas desulfurization gypsum on the Songnen Plain, Northeast China. Geoderma 2018, 321, 52–60. [Google Scholar] [CrossRef]
- Wang, Z.; Zhuang, J.; Zhao, A.; Li, X. Types, harms and improvement of saline soil in Songnen Plain. IOP Conf. Ser. Mater. Sci. Eng. 2018, 322, 052059. [Google Scholar] [CrossRef]
- Mu, W.; Han, N.; Qu, Z.; Zheng, M.; Shan, Y.; Guo, X.; Sun, Y.; Mu, Y. ECWS: Soil Salinity Measurement Method Based on Electrical Conductivity and Moisture Content. Agronomy 2024, 14, 1345. [Google Scholar] [CrossRef]
- Al-Shammary, A.A.G.; Kouzani, A.Z.; Kaynak, A.; Khoo, S.Y.; Norton, M.; Gates, W. Soil Bulk Density Estimation Methods: A Review. Pedosphere 2018, 28, 581–596. [Google Scholar] [CrossRef]
- Liu, J.; Wang, X.; Zhang, H.; Lu, Y.; Kalkhajeh, Y.K.; Hu, H.; Huang, J. Long-term in situ straw returning increased soil aggregation and aggregate associated organic carbon fractions in a paddy soil. Heliyon 2024, 10, e32392. [Google Scholar] [CrossRef] [PubMed]
- Weishaar, J.L.; Aiken, G.R.; Bergamaschi, B.A.; Fram, M.S.; Fujii, R.; Mopper, K. Evaluation of specific ultraviolet absorbance as an indicator of the chemical composition and reactivity of dissolved organic carbon. Environ. Sci. Technol. 2003, 37, 4702–4708. [Google Scholar] [CrossRef]
- Yu, H.; Liang, H.; Qu, F.; Han, Z.-s.; Shao, S.; Chang, H.; Li, G. Impact of dataset diversity on accuracy and sensitivity of parallel factor analysis model of dissolved organic matter fluorescence excitation-emission matrix. Sci. Rep. 2015, 5, 10207. [Google Scholar] [CrossRef]
- Cui, S.; Liu, L.; Zhang, F.; Fu, Q.; Ma, C.; Ding, Y. Compositional Evolution of Dissolved Organic Matter Mobilized by Straw Incorporation and Its Climate-Driven Interactions with Lead in Cold-Region Black Soil: Decoding Mechanisms through PARAFAC and Complexation Modeling. ACS Omega 2025, 4, 56. [Google Scholar] [CrossRef]
- Liu, D.; Yu, H.; Yang, F.; Liu, L.; Gao, H.; Cui, B. Characterizing Humic Substances from Native Halophyte Soils by Fluorescence Spectroscopy Combined with Parallel Factor Analysis and Canonical Correlation Analysis. Sustainability 2020, 12, 9787. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Wang, J.; Li, H.; Chen, S.; Zhou, Q.; Sun, M. Insight into structural composition of dissolved organic matter in saline-alkali soil by fluorescence spectroscopy coupled with self-organizing map and structural equation modeling. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 262, 121311. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.-L.; Huynh, L.-L. Bridging causal explanation and predictive modeling: The role of PLS-SEM. Int. J. Res. Bus. Soc. Sci. 2024, 13, 197–206. [Google Scholar] [CrossRef]
- Hair, J.F.; Hauff, S.; Hult, G.T.M.; Richter, N.F.; Ringle, C.M.; Sarstedt, M. Partial Least Squares Strukturgleichungsmodellierung; Verlag Franz Vahlen: München, Germany, 2017. [Google Scholar] [CrossRef]
- Dube, A.; Patel, D.S.; Singh, A.; Patel, K.; Lal, K. Influence of long-term organic and inorganic nutrient inputs on soil chemical properties across depths in calcareous soils. Agron. J. 2025, 8, 108–527. [Google Scholar] [CrossRef]
- Liu, X.; Shu, Y.; Li, K.; Wang, H.; Bi, Q.; Wang, H.; Sun, C.; Lin, X. Organic Matter Accelerated Microbial Iron Reduction and Available Phosphorus Release in Reflooded Paddy Soils. Soil Ecol. Lett. 2025, 7, 250316. [Google Scholar] [CrossRef]
- Li, W.; Wang, Z.; Zhang, J.; Zong, R. Soil salinity variations and cotton growth under long-term mulched drip irrigation in saline-alkali land of arid oasis. Irrig. Sci. 2022, 40, 103–113. [Google Scholar] [CrossRef]
- Schwarz, E.; Johansson, A.; Lerda, C.; Livsey, J.; Scaini, A.; Said-Pullicino, D.; Manzoni, S. Organic carbon stabilization in temperate paddy fields and adjacent semi-natural forests along a soil age gradient. Geoderma 2024, 11, 15. [Google Scholar] [CrossRef]
- Ye, C.; Zheng, G.; Tao, Y.; Xu, Y.; Chu, G.; Xu, C.; Chen, S.; Liu, Y.; Zhang, X.; Wang, D. Effect of Soil Texture on Soil Nutrient Status and Rice Nutrient Absorption in Paddy Soils. Agronomy 2024, 14, 1339. [Google Scholar] [CrossRef]
- Saidi, S.; Ayoubi, S.; Shirvani, M.; Azizi, K.; Zeraatpisheh, M. Comparison of Different Machine Learning Methods for Predicting Cation Exchange Capacity Using Environmental and Remote Sensing Data. Sensors 2022, 22, 6890. [Google Scholar] [CrossRef]
- Huang, L.; Liu, Y.; Ferreira, J.F.S.; Wang, M.; Na, J.; Huang, J.; Liang, Z. Long-term combined effects of tillage and rice cultivation with phosphogypsum or farmyard manure on the concentration of salts, minerals, and heavy metals of saline-sodic paddy fields in Northeast China. Soil Tillage Res. 2022, 215, 105222. [Google Scholar] [CrossRef]
- Das, A.; Ahmed, N.; Purakayastha, T.J.; Biswas, S.; Ray, P.; Singh, B.; Das, T.K.; Kumar, R.; Lama, A. Impact of conservation agriculture on humic acid quality and clay humus complexation under maize (Zea mays)-wheat (Triticum aestivum) and pigeon pea (Cajanus cajan)-wheat cropping systems. Indian J. Agric. Sci. 2023, 93, 1013–1018. [Google Scholar] [CrossRef]
- Lei, X.; Shen, Y.; Zhao, J.; Huang, J.; Wang, H.; Yu, Y.; Xiao, C. Root Exudates Mediate the Processes of Soil Organic Carbon Input and Efflux. Plants 2023, 12, 630. [Google Scholar] [CrossRef]
- Jiang, S.; Liu, X.; Lu, Y.; Wu, J.; Zhang, L.; Wang, J.; Wu, W. Profile and nano-scale distribution of soil organic carbon for upland and paddy soils from an alluvial plain in South China. Chem. Geol. 2023, 856, 159137. [Google Scholar] [CrossRef]
- Wang, P.; Liu, Y.; Li, L.; Cheng, K.; Zheng, J.; Zhang, X.; Zheng, J.; Joseph, S.; Pan, G. Long-term rice cultivation stabilizes soil organic carbon and promotes soil microbial activity in a salt marsh derived soil chronosequence. Sci. Rep. 2015, 5, 15704. [Google Scholar] [CrossRef]
- Said-Pullicino, D.; Miniotti, E.F.; Sodano, M.; Bertora, C.; Lerda, C.; Chiaradia, E.A.; Romani, M.; de Maria, S.C.; Sacco, D.; Celi, L. Linking dissolved organic carbon cycling to organic carbon fluxes in rice paddies under different water management practices. Plant Soil 2016, 401, 273–290. [Google Scholar] [CrossRef]
- Tao, M.; Ke, X.; Ma, J.; Liu, L.; Qiu, Y.; Hu, Z.; Liu, F. Dissolved organic matter (DOM)—Driven variations of cadmium mobility and bioavailability in waterlogged paddy soil. J. Hazard. Mater. 2025, 492, 138065. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, W.; Xiu, L.; Gu, W.; Wu, D.; Tang, L.; Chen, W. Long-term fertilization regimes modulate dissolved organic matter molecular chemodiversity and greenhouse gas emissions in paddy soil. Biochar 2025, 7, 43. [Google Scholar] [CrossRef]
- Gao, J.; Shi, Z.; Wu, H.; Lv, J. Fluorescent characteristics of dissolved organic matter released from biochar and paddy soil incorporated with biochar. RSC Adv. 2020, 10, 5785–5793. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Liu, L.; Shi, Z.; Lv, J. Characteristics of Fluorescent Dissolved Organic Matter in Paddy Soil Amended With Crop Residues After Column (0–40 cm) Leaching. Front. Environ. Sci. 2022, 10, 766795. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, P.; He, C.; Shi, Q.; Dahlgren, R.; Spencer, R.; Wang, J. Molecular signatures of soil-derived dissolved organic matter constrained by mineral weathering. Fundam. Res. 2023, 3, 377–383. [Google Scholar] [CrossRef]
- Song, H.; Gao, L.; Xu, J.; Zhu, L.; Shu, X.; Xia, J.; Zhang, K.; Wu, L. Spectral characteristics of dissolved organic matter (DOM) in the middle reaches of the Huai River in a dry season. Environ. Sci. Water Res. Technol. 2024, 10, 3308–3318. [Google Scholar] [CrossRef]
- Wang, B.; Liu, C.; Chen, Y.; Dong, F.; Chen, S.; Zhang, D.; Zhu, J. Structural characteristics, analytical techniques and interactions with organic contaminants of dissolved organic matter derived from crop straw: A critical review. RSC Adv. 2018, 8, 36927–36938. [Google Scholar] [CrossRef]
- Kawałko, D.; Jamroz, E.; Jerzykiewicz, M.; Ćwieląg-Piasecka, I. Characteristics of Humic Acids in Drained Floodplain Soils in Temperate Climates: A Spectroscopic Study. Sustainability 2023, 15, 11417. [Google Scholar] [CrossRef]
- Pu, Q.; Wang, J.; Wang, S.; Hu, H.; Li, Y.; Sun, G.; Lin, H.; Feng, X.; Meng, B.; Du, H. Dissolved organic matter fosters core mercury-methylating microbiomes for methylmercury production in paddy soils. Biogeosciences 2025, 22, 1543–1556. [Google Scholar] [CrossRef]
- Vogt, R.D.; Porcal, P.; Hejzlar, J.; Paule-Mercado, M.C.; Haaland, S.; Gundersen, C.B.; Orderud, G.I.; Eikebrokk, B. Distinguishing between Sources of Natural Dissolved Organic Matter (DOM) Based on Its Characteristics. Water 2023, 15, 3006. [Google Scholar] [CrossRef]
- Yao, Y.; Zhang, J.; Ma, K.; Li, J.; Hu, X.; Wang, Y.; Lin, Y.; Fang, F.; Li, S. Combination Mechanism of Soil Dissolved Organic Matter and Cu2+ in Vegetable Fields, Forests and Dry Farmland in Lujiang County. Agriculture 2024, 14, 684. [Google Scholar] [CrossRef]
- Wang, H.; Liu, X.; Shu, Y.; Li, G.; Sun, C.; Jones, D.; Zhu, Y.; Lin, X. Molecular Composition of Exogenous Dissolved Organic Matter Regulates Dissimilatory Iron Reduction and Carbon Emissions in Paddy Soil. Environ. Sci. Technol. 2025, 59, 12679–12691. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, M.; Zong, Y.; Wu, L.; Ding, X. The response of soil organic carbon sequestration to organic materials in addition in saline-alkali soil: From the perspective of soil aggregate structure and organic carbon component. Plant Soil 2025, 512, 1619–1638. [Google Scholar] [CrossRef]
- Xia, H.; Shen, J.; Riaz, M.; Jiang, C.; Zu, C.; Jiang, C.; Liu, B. Effects of Biochar and Straw Amendment on Soil Fertility and Microbial Communities in Paddy Soils. Plants 2024, 13, 1478. [Google Scholar] [CrossRef]
- Li, S.; Li, M.; Wang, G.; Sun, X.; Xi, B.; Hu, Z. Compositional and chemical characteristics of dissolved organic matter in various types of cropped and natural Chinese soils. Chem. Biol. Technol. Agric. 2019, 6, 20. [Google Scholar] [CrossRef]
- Ying, J.Y.; Zhang, X.; Wu, W.X.; Nan, Q.; Wang, G.R.; Dong, D. The effects of long-term rice straw and biochar return on soil humus composition and structure in paddy soil. Plant Soil Environ. 2024, 70, 772–782. [Google Scholar] [CrossRef]
- Peng, Y.; Xu, H.; Wang, Z.; Shi, J.; Lv, J.; Wang, X. Responses of the content and spectral characteristics of dissolved organic matter in intercropping soil to drought in northeast China. Plant Soil 2025, 506, 471–485. [Google Scholar] [CrossRef]
- Korak, J.A.; McKay, G. Critical review of fluorescence and absorbance measurements as surrogates for the molecular weight and aromaticity of dissolved organic matter. Environ. Sci. Process. Impacts 2024, 26, 1663–1702. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Wang, G.; Zhao, X.; Dang, Q.; Li, R.; Xi, B.; Jiang, J.; Zhang, H.; Li, D.; Cui, D.; et al. Molecular-weight-dependent redox cycling of humic substances of paddy soils over successive anoxic and oxic alternations. Land Degrad. Dev. 2019, 30, 1130–1144. [Google Scholar] [CrossRef]
- Gao, J.K.; Liang, C.L.; Shen, G.Z.; Lv, J.L.; Wu, H.M. Spectral characteristics of dissolved organic matter in various agricultural soils throughout China. Chemosphere 2017, 176, 108–116. [Google Scholar] [CrossRef]
- Li, H.; Wang, H.; Wang, H.; Xin, P.; Xu, X.; Ma, Y.; Liu, W.-P.; Teng, C.-Y.; Jiang, C.-L.; Lou, L.-P.; et al. The chemodiversity of paddy soil dissolved organic matter correlates with microbial community at continental scales. Microbiome 2018, 6, 187. [Google Scholar] [CrossRef]
- Wu, H.; Kida, M.; Domoto, A.; Hara, M.; Ashida, H.; Suzuki, T.; Fujitake, N. The effects of fertilization treatments and cropping systems on long-term dynamics and spectroscopic characteristics of dissolved organic matter in paddy soil. Soil Sci. Plant Nutr. 2019, 65, 557–565. [Google Scholar] [CrossRef]
- Jörgensen, L.; Stedmon, C.A.; Kragh, T.; Markager, S.; Middelboe, M.; Söndergaard, M. Global trends in the fluorescence characteristics and distribution of marine dissolved organic matter. Mar. Chem. 2011, 126, 139–148. [Google Scholar] [CrossRef]
- Lin, H.; Guo, L. Variations in Colloidal DOM Composition with Molecular Weight within Individual Water Samples as Characterized by Flow Field-Flow Fractionation and EEM-PARAFAC Analysis. Environ. Sci. Technol. 2020, 54, 1657–1667. [Google Scholar] [CrossRef]
- Goncalves-Araujo, R.; Granskog, M.A.; Bracher, A.; Azetsu-Scott, K.; Dodd, P.A.; Stedmon, C.A. Using fluorescent dissolved organic matter to trace and distinguish the origin of Arctic surface waters. Sci. Rep. 2016, 6, 33978. [Google Scholar] [CrossRef]
- Su, L.; Chen, M.; Wang, S.; Ji, R.; Liu, C.; Lu, X.; Zhen, G.; Zhang, L. Fluorescence characteristics of dissolved organic matter during anaerobic digestion of oil crop straw inoculated with rumen liquid. RSC Adv. 2021, 11, 14347–14356. [Google Scholar] [CrossRef]
- Stedmon, C.A.; Thomas, D.N.; Papadimitriou, S.; Granskog, M.A.; Dieckmann, G. Using fluorescence to characterize dissolved organic matter in Antarctic Sea ice breezes. J. Geophys. Res. 2011, 116, 1–9. [Google Scholar]
- Brünjes, J.; Schubotz, F.; Teske, A.; Seidel, M. Molecular Composition of Dissolved Organic Matter from Young Organic-Rich Hydrothermal Deep-Sea Sediments. Limnol. Oceanogr. 2025, 70, 870–885. [Google Scholar] [CrossRef]
- Dall’Osto, M.; Vaqué, D.; Sotomayor-Garcia, A.; Cabrera-Brufau, M.; Estrada, M.; Buchaca, T.; Soler, M.; Nunes, S.; Zeppenfeld, S.; van Pinxteren, M.; et al. Sea Ice Microbiota in the Antarctic Peninsula Modulates Cloud-Relevant Sea Spray Aerosol Production. Front. Mar. Sci. 2022, 9, 827061. [Google Scholar] [CrossRef]
- Yamashita, Y.; Panton, A.; Mahaffey, C.; Jaffe, R. Assessing the spatial and temporal variability of dissolved organic matter in Liverpool Bay using excitation_emission matrix fluorescence and parallel factor analysis. Ocean Dyn. 2011, 61, 569–579. [Google Scholar] [CrossRef]
- Zhu, Z.; Ge, T.; Luo, Y.; Liu, S.; Xu, X.; Tong, C.; Shibistova, O.; Guggenberger, G.; Wu, J. Microbial stoichiometric flexibility regulates rice straw mineralization and its priming effect in paddy soil. Soil Biol. Biochem. 2018, 121, 67–76. [Google Scholar] [CrossRef]
- Schulten, H.-R. Models of Humic Structures: Association of Humic Acids and Organic Matter in Soils and Water. Humic Subst. Chem. Contam. 2015, 3, 73–87. [Google Scholar]
- Yuan, J.; Chen, S.; Ge, B.; Cui, M.; Wong, Y.; Qi, Y.; Ge, Y.; Hao, A.; He, K. Comprehensive Source Tracing and Process Supervision of Coating Industrial Wastewater Using Integrated Water Quality Parameters and Fluorescence Spectroscopy. Res. Sq. 2024, 197, 380. [Google Scholar] [CrossRef]
- Li, K.; Bi, Q.; Liu, X.; Wang, H.; Sun, C.; Zhu, Y.; Lin, X. Unveiling the role of dissolved organic matter on phosphorus sorption and availability in a 5-year manure amended paddy soil. Sci. Total Environ. 2022, 838, 155892. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, C.; Zou, L.; Cui, H. Spatial Distribution and Fluorescence Properties of Soil Dissolved Organic Carbon Across a Riparian Buffer Wetland in Chongming Island, China. Pedosphere 2015, 25, 220–229. [Google Scholar] [CrossRef]
- Qu, Y.; Tang, J.; Zhou, Z.; Liu, B.; Duan, Y.; Wang, J.; Wang, S.; Li, Y.; Li, Z. The Development and Utilization of Saline–Alkali Land in Western Jilin Province Promoted the Sequestration of Organic Carbon Fractions in Soil Aggregates. Agronomy 2021, 11, 2563. [Google Scholar] [CrossRef]
- Wang, S.; Tang, J.; Li, Z.; Liu, Y.; Zhou, Z.; Wang, J.; Qu, Y.; Dai, Z. Carbon Mineralization under Different Saline–Alkali Stress Conditions in Paddy Fields of Northeast China. Sustainability 2020, 12, 2921. [Google Scholar] [CrossRef]
- Qiao, J.; Li, X.; Li, F.; Liu, T.; Young, L.Y.; Huang, W.; Sun, K.; Tong, H.; Hu, M. Humic Substances Facilitate Arsenic Reduction and Release in Flooded Paddy Soil. Environ. Sci. Technol. 2019, 53, 5034–5042. [Google Scholar] [CrossRef] [PubMed]
- Mahapatra, S.S.; Parameswaran, C.; Chowdhury, T.; Senapati, A.; Chatterjee, S.; Singh, A.K.; Panneerselvam, P. Unraveling the Efficient Cellulolytic and Lytic Polysaccharide Monooxygenases Producing Microbes from Paddy Soil for Efficient Cellulose Degradation. J. Adv. Biol. Biotechnol. 2024, 27, 47–56. [Google Scholar] [CrossRef]
- He, Y.; Wang, Z.; Zhu, J.; Lin, X.; Qi, J. Soil Carbon Sequestration: Role of Fe Oxides and Polyphenol Oxidase Across Temperature and Cultivation Systems. Plants 2025, 14, 927. [Google Scholar] [CrossRef]
- Melkani, S.; Manirakiza, N.; Rabbany, A.; Medina-Irizarry, N.; Smidt, S.; Braswell, A.; Martens-Habbera, W.; Bhadha, J.H. Understanding the mechanisms of hydrolytic enzyme mediated organic matter decomposition under different land covers within a subtropical preserve. Front. Environ. Sci. 2025, 13, 1564047. [Google Scholar] [CrossRef]
- Ren, T.; Cai, A. Global patterns and drivers of soil dissolved organic carbon concentrations. Earth Syst. Sci. Data 2025, 17, 2873–2885. [Google Scholar] [CrossRef]
- Vicente, L.; Peña, D.; Fernández, D.; Albarrán, Á.; Rato-Nunes, J.M.; López-Piñeiro, A. Alternate wetting and drying irrigation with field aged biochar may enhance water and rice productivity. Agron. Sustain. Dev. 2025, 45, 6. [Google Scholar] [CrossRef]
- Romero González-Quijano, C.; Herrero Ortega, S.; Casper, P.; Gessner, M.O.; Singer, G.A. Dissolved organic matter signatures in urban surface waters: Spatio-temporal patterns and drivers. Biogeosciences 2022, 19, 2841–2853. [Google Scholar] [CrossRef]
- Ge, J.; Gao, L.; Luan, L.; Zhang, Z.; Zhang, H.; Zhao, X.; Mu, M. Fluorescence Spectral Characteristics of Soil Dissolved Organic Matter in Zhengyangguan, Middle Reaches of Huaihe River Basin. Pol. J. Environ. Stud. 2023, 32, 2105–2112. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, P.; Ding, Y.; Lu, H.; Li, L.; Zheng, J.; Zhang, X.; Zheng, J.; Cheng, K.; Pan, G. Microbial activity promoted with organic carbon accumulation in macroaggregates of paddy soils under long-term rice cultivation. Biogeosciences 2016, 13, 6565–6586. [Google Scholar] [CrossRef]
- Yang, X.; Song, Z.; Van Zwieten, L.; Guo, L.; Chen, J.; Luo, Z.; Wang, Y.; Luo, Y.; Wang, Z.; Wang, W.; et al. Significant accrual of soil organic carbon through long-term rice cultivation in paddy fields in China. Glob. Change Biol. 2024, 30, e17213. [Google Scholar] [CrossRef]
- Xiao, L.; Chen, S.; Han, F.; Wang, T.; Li, Y.; Li, W.; Yuan, G.; Wu, J. Unveiling carbon dynamics in year-round waterlogged pond fields: Insights into soil organic carbon accumulation and sustainable management. Carbon Res. 2025, 4, 14. [Google Scholar] [CrossRef]
- Yu, X.; Zhou, W.; Wang, Y.; Cheng, P.; Hou, Y.; Xiong, X.; Du, H.; Yang, L.; Wang, Y. Effects of land use and cultivation time on soil organic and inorganic carbon storage in deep soils. J. Geogr. Sci. 2020, 30, 921–934. [Google Scholar] [CrossRef]
- Kim, S.; Choi, J.; Kang, S.; Park, J.; Hong, S.; Kim, T.; Yang, W. Effects of Tillage and Cultivation Methods on Carbon Accumulation and Formation of Water-stable Aggregates at Different Soil Layers in Rice Paddy. Korean J. Soil Sci. Fertil. 2017, 50, 634–643. [Google Scholar] [CrossRef]
- Xu, W.; Ambus, P.L. Newly Plant-Derived Carbon in the Deeper Vadose Zone of a Sandy Agricultural Soil Does Not Stimulate Denitrification. Geoderma 2024, 447, 116936. [Google Scholar] [CrossRef]
- Freeman, E.C.; Emilson, E.J.S.; Dittmar, T.; Braga, L.P.P.; Emilson, C.E.; Goldhammer, T.; Martineau, C.; Singer, G.; Tanentzap, A.J. Universal microbial reworking of dissolved organic matter along environmental gradients. Nat. Commun. 2024, 15, 187. [Google Scholar] [CrossRef]
- Wu, M.; Li, P.; Li, G.; Petropoulos, E.; Feng, Y.; Li, Z. The chemodiversity of paddy soil dissolved organic matter is shaped and homogenized by bacterial communities that are orchestrated by geographic distance and fertilizations. Soil Biol. Biochem. 2021, 161, 108374. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).