Yield Stability and Antioxidant Response of Wheat Under Multi-Environment Conditions: Insights from AMMI and GGE Biplot Analyses
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Design
2.2. Soil Characteristics of the Experimental Localities
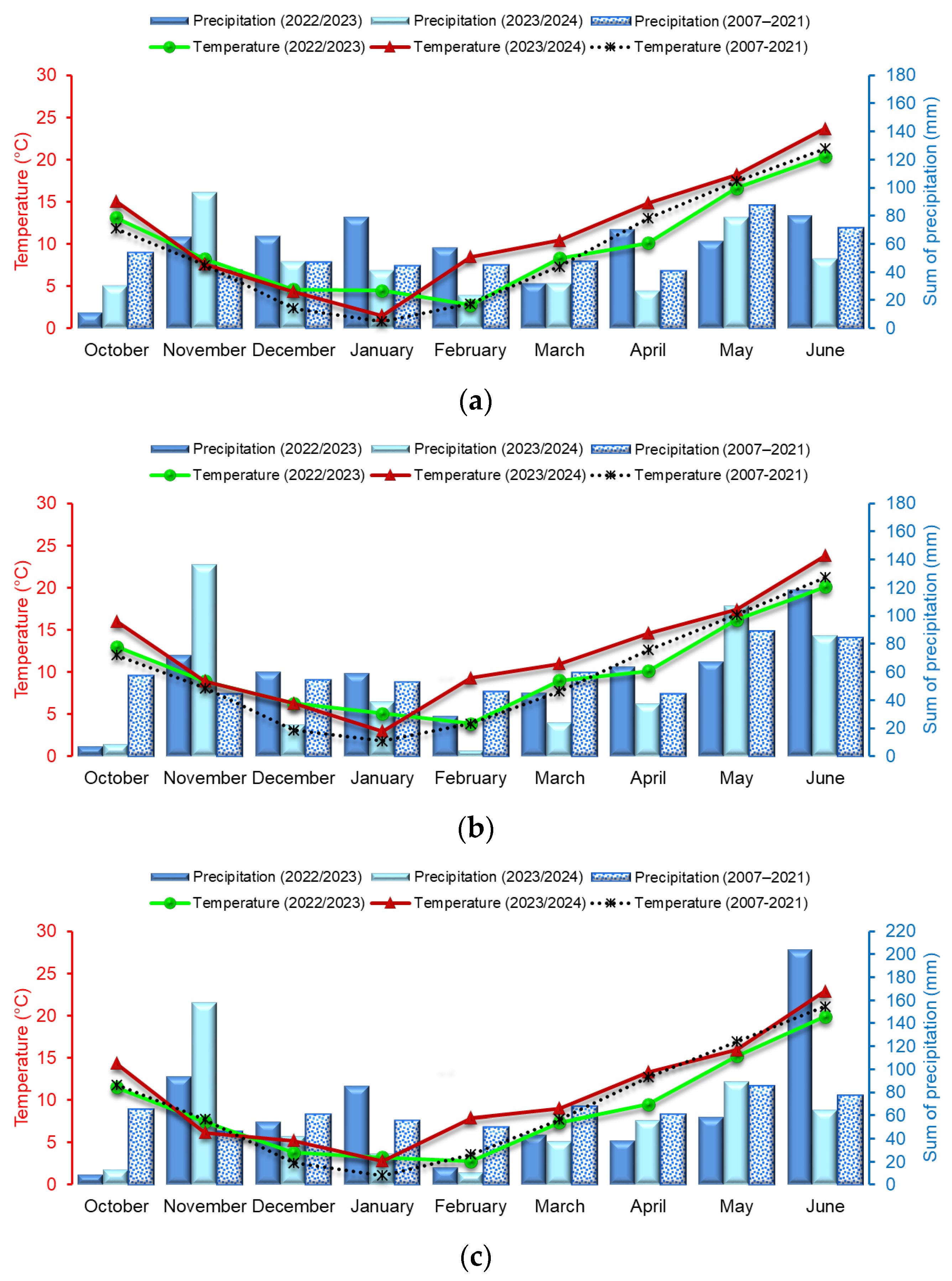
2.3. Meteorological Conditions
2.4. Agro-Morphological Traits
2.5. Biochemical Parameters
2.5.1. DPPH Radical Scavenging Activity
2.5.2. Total Phenolic Content
2.6. Statistical Analysis
3. Results
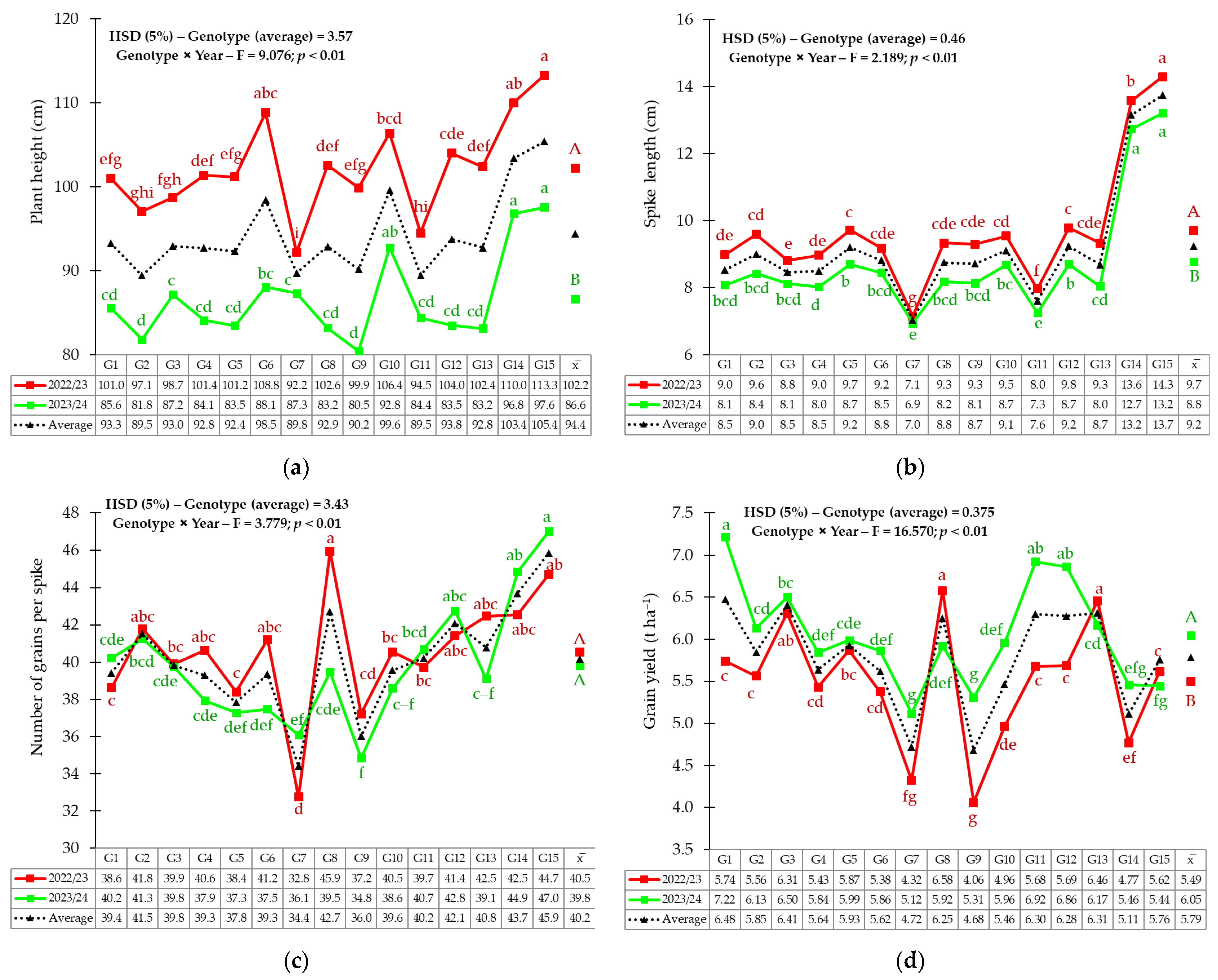
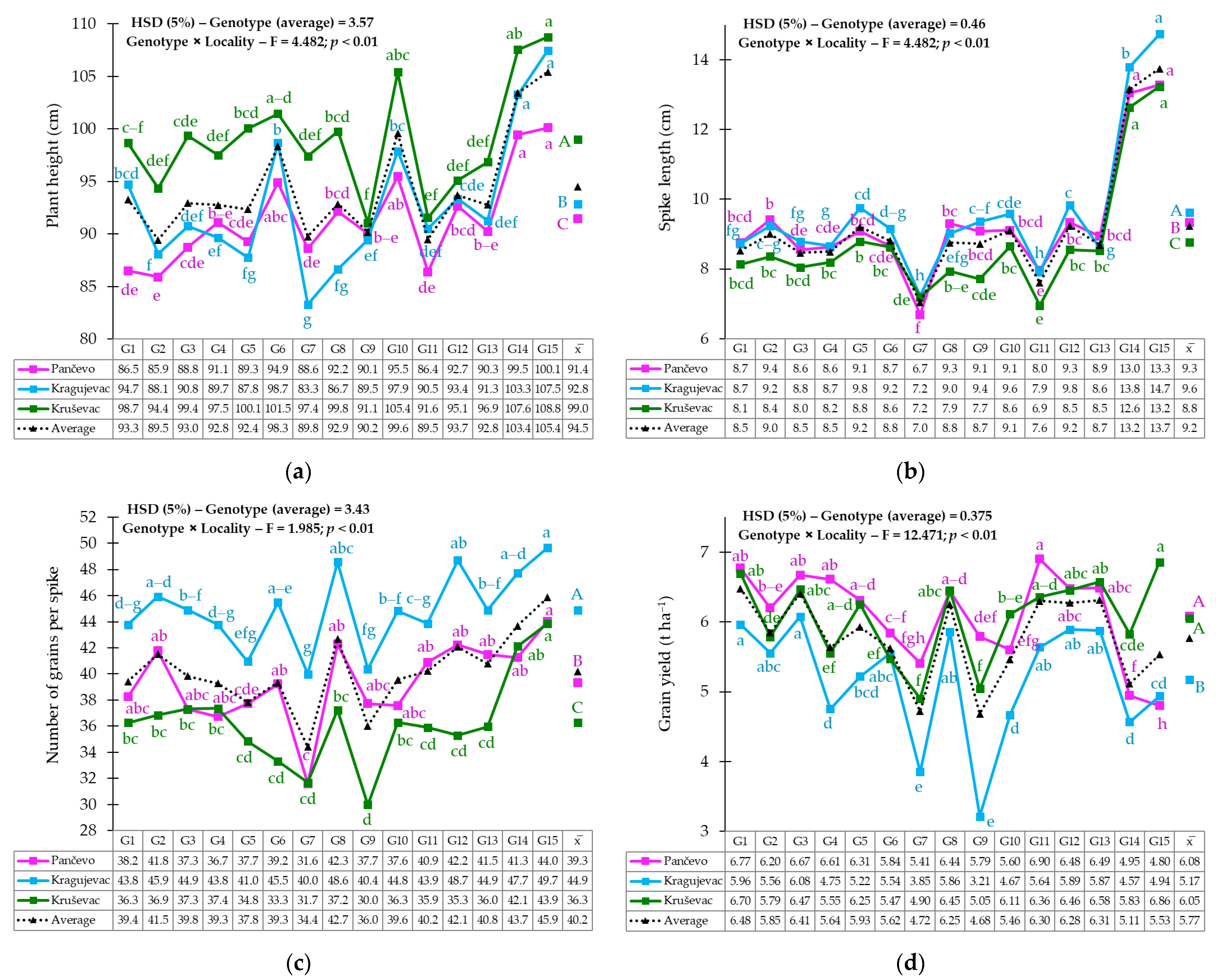
3.1. Phenotypic Variability of Yield and Yield-Related Traits
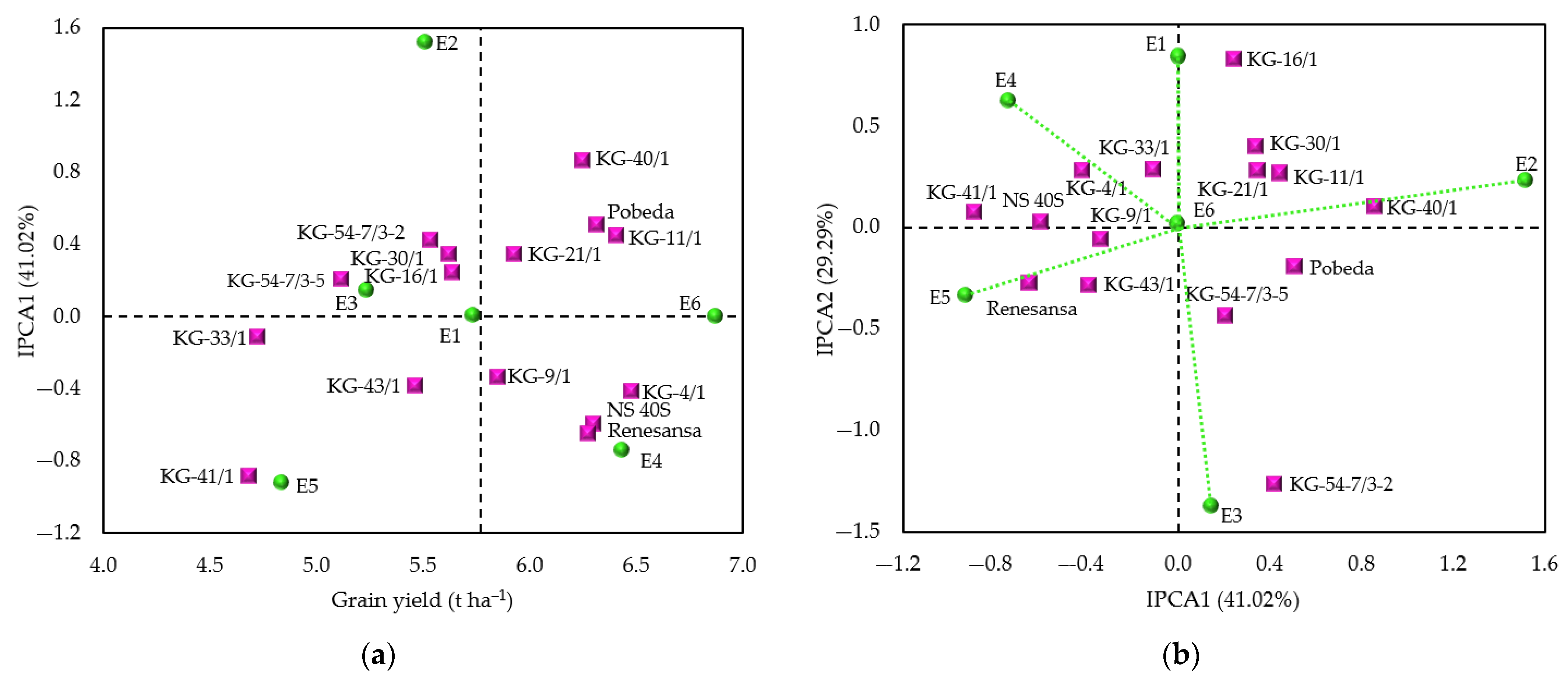
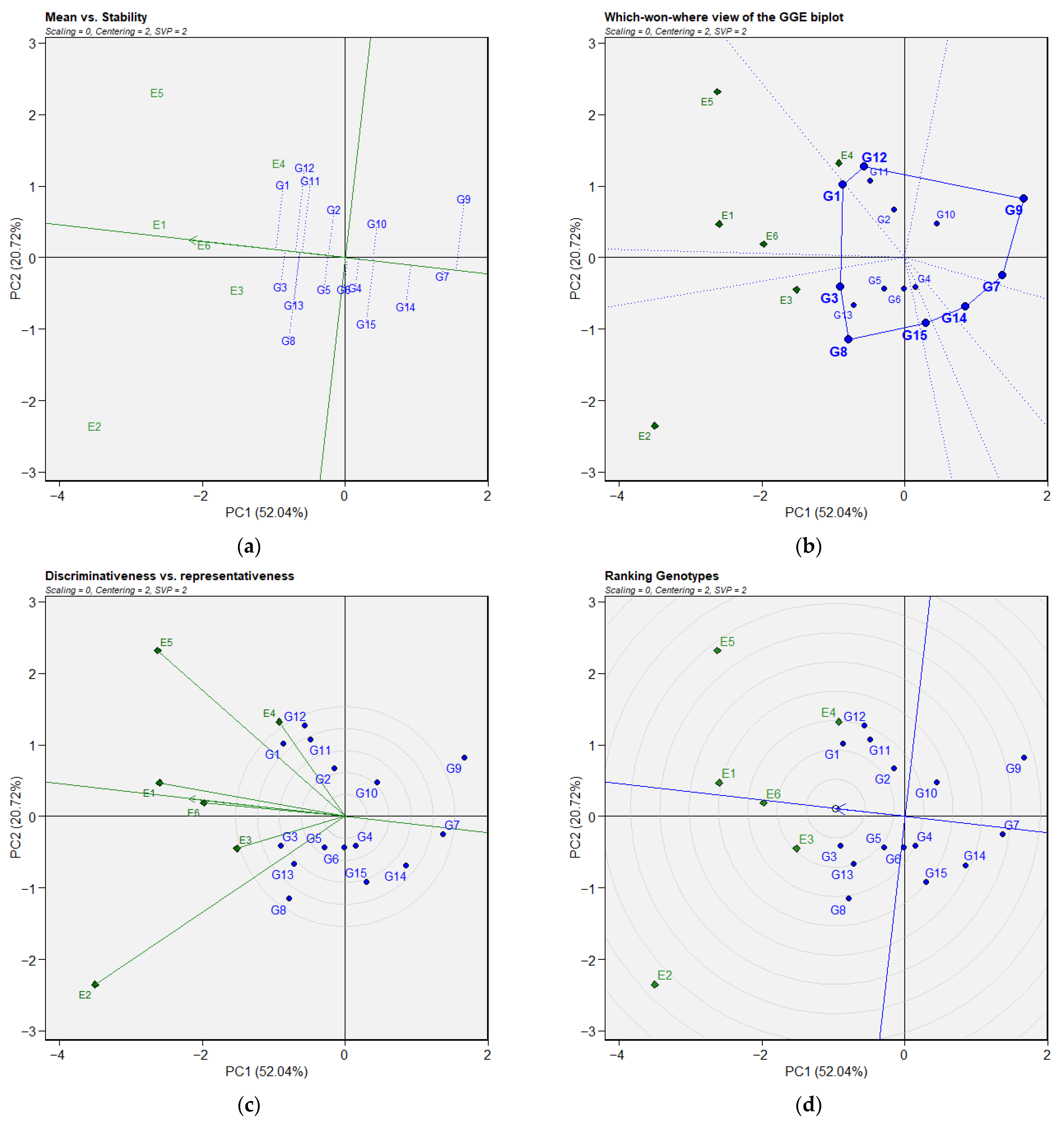
3.2. Genotype × Environment Interaction
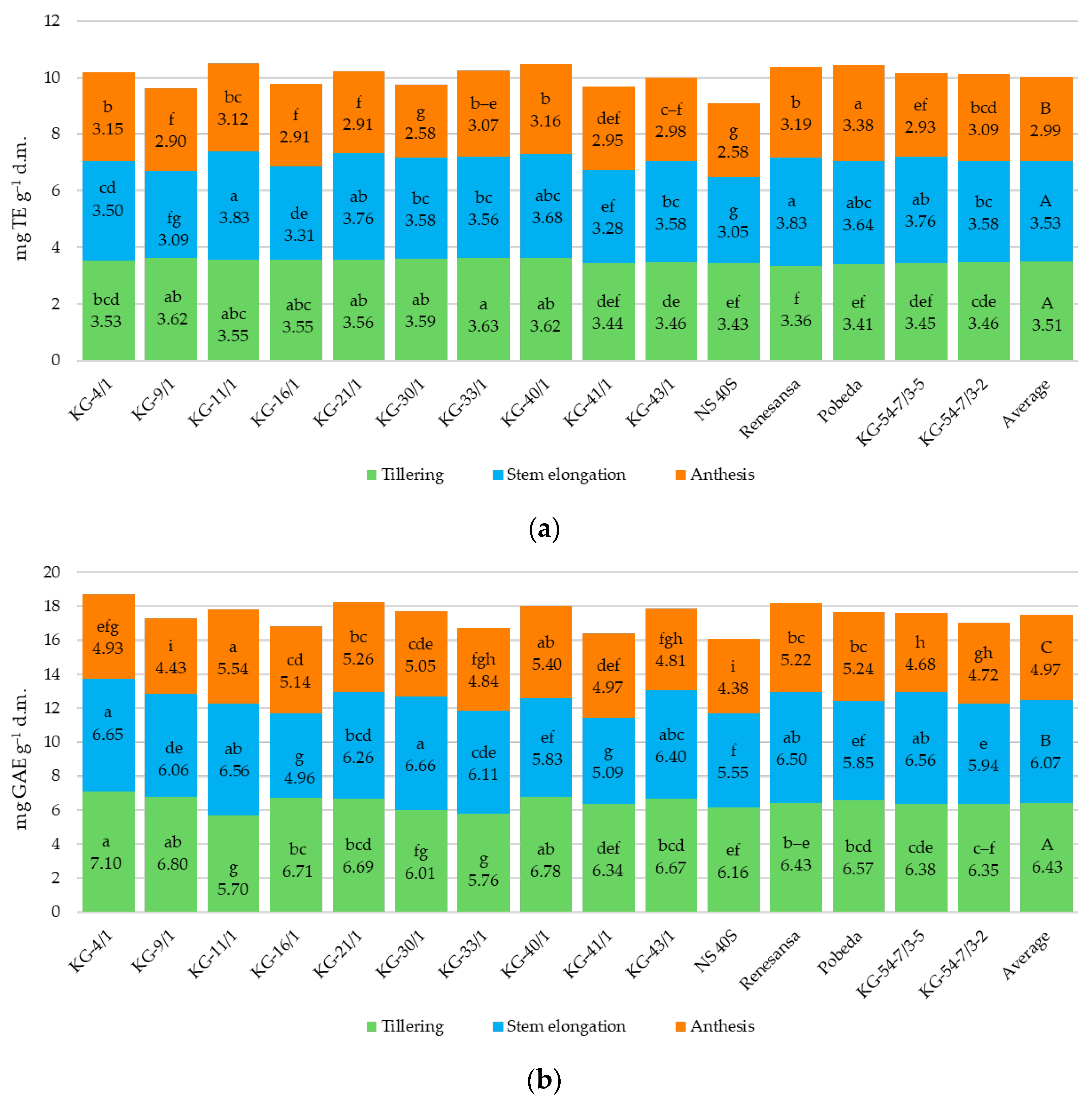
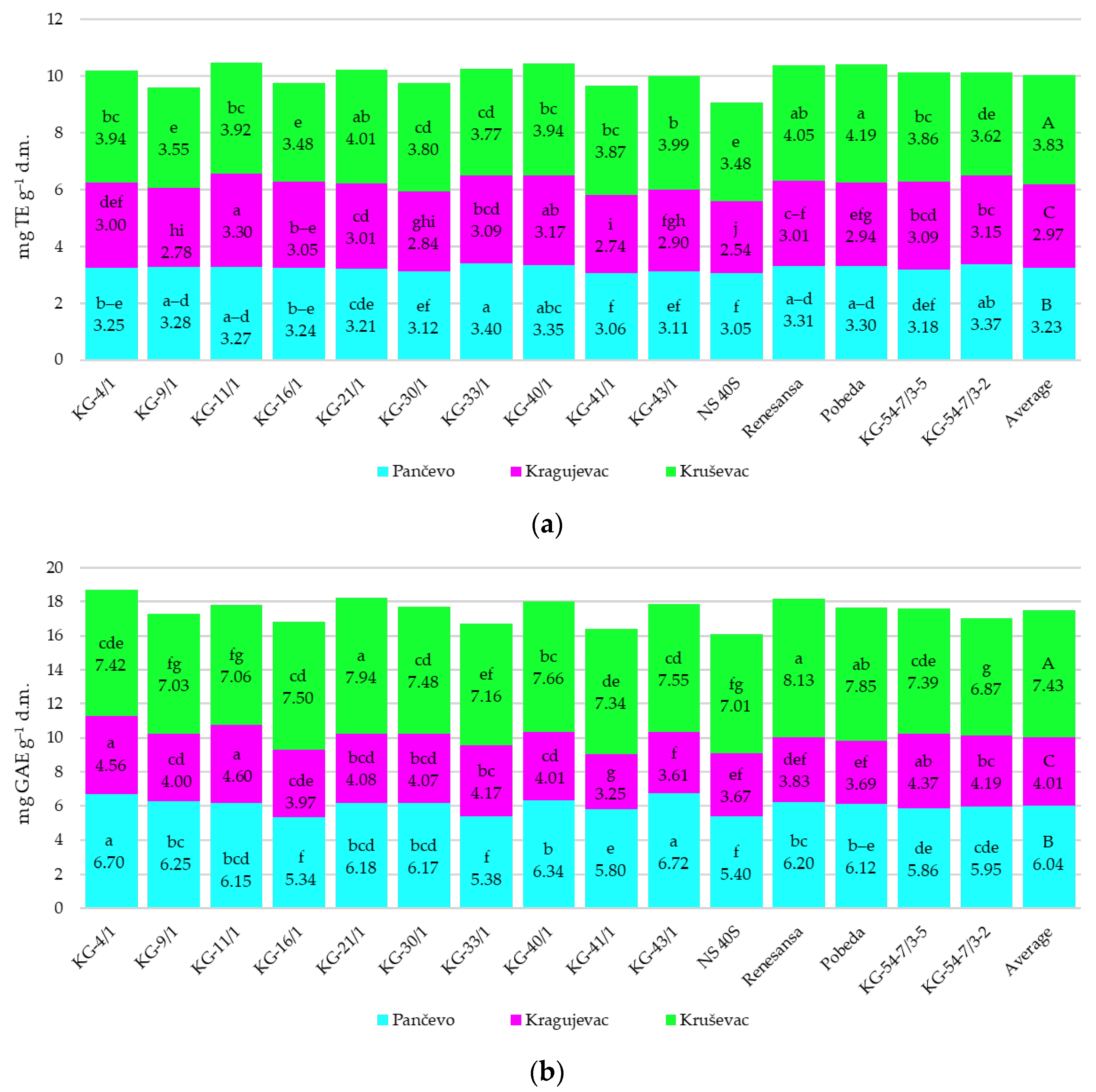
3.3. Antioxidant Activity Parameters
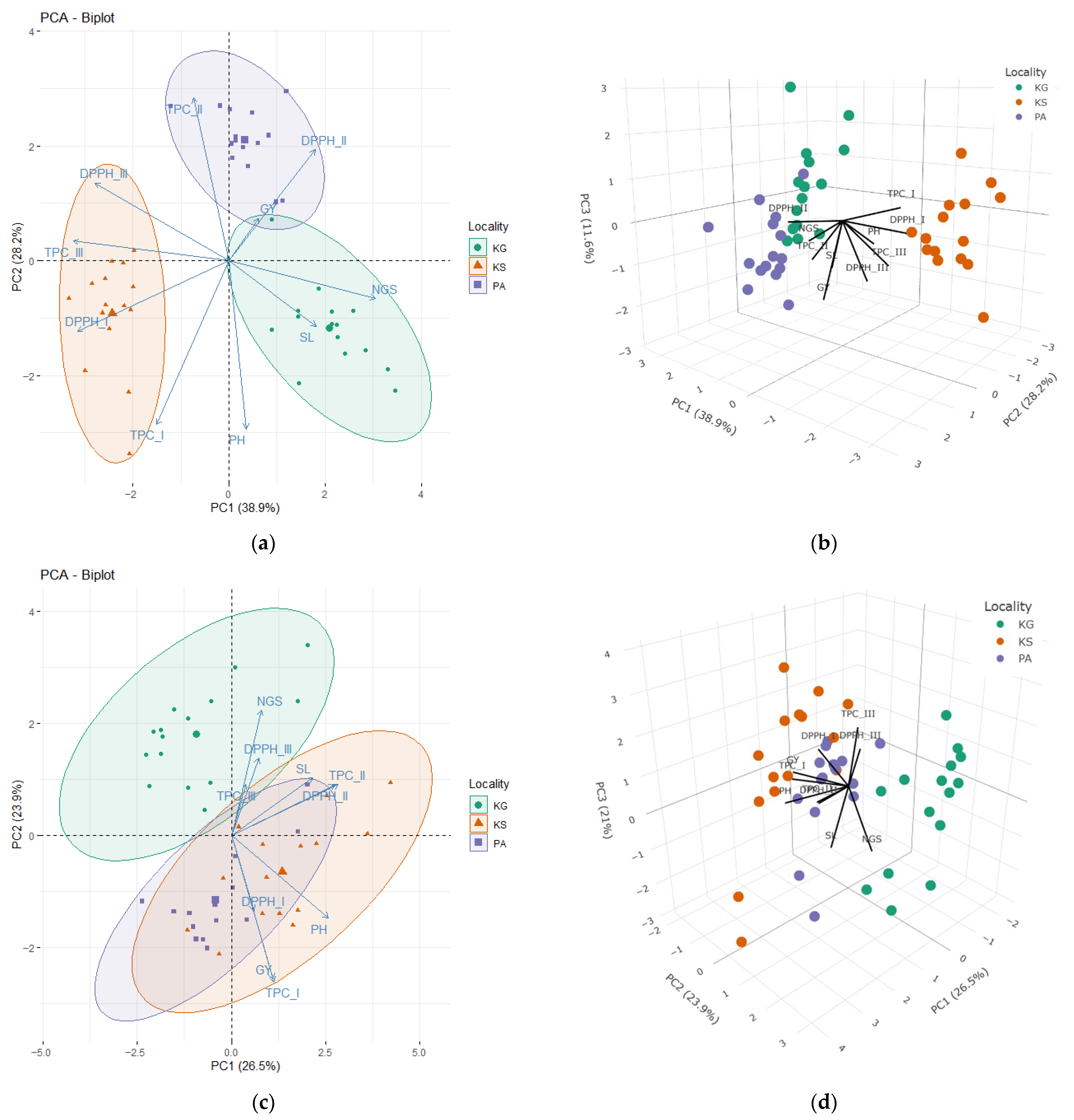
3.4. Relationship Among Analyzed Traits
4. Discussion
4.1. Phenotypic Variability of Agro-Morphological Traits
4.2. Grain Yield Stability
4.3. Antioxidant Activity
4.4. Relationship Among Agro-Morphological and Biochemical Parameters
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shiferaw, B.; Smale, M.; Braun, H.J.; Duveiller, E.; Reynolds, M.; Mauricho, G. Crops that feed the world 10. Past scuccesses and future challenges to the role played by wheat in global food security. Food Secur. 2013, 5, 291–317. [Google Scholar] [CrossRef]
- Giraldo, P.; Benavente, E.; Manzano-Agugliaro, F.; Gimenez, E. Worldwide Research Trends on Wheat and Barley: A Bibliometric Comparative Analysis. Agronomy 2019, 9, 352. [Google Scholar] [CrossRef]
- Cao, S.; Xu, D.; Hanif, M.; Xia, X.; He, Z. Genetic Architecture Underpinning Yield Component Traits in Wheat. Theor. Appl. Genet. 2020, 133, 1811–1823. [Google Scholar] [CrossRef]
- Albajes, R.; Cantero-Martínez, C.; Capell, T.; Christou, P.; Farre, A.; Galceran, J.; López-Gatius, F.; Marin, S.; Martín-Belloso, O.; Motilva, M.-J.; et al. Building Bridges: An Integrated Strategy for Sustainable Food Production throughout the Value Chain. Mol. Breed. 2013, 32, 743–770. [Google Scholar] [CrossRef]
- Slafer, G.A.; Savin, R.; Sadras, V.O. Wheat Yield Is Not Causally Related to the Duration of the Growing Season. Eur. J. Agron. 2023, 148, 126885. [Google Scholar] [CrossRef]
- Reynolds, M.; Foulkes, M.J.; Slafer, G.A.; Berry, P.; Parry, M.A.J.; Snape, J.W.; Angus, W.J. Raising Yield Potential in Wheat. J. Exp. Bot. 2009, 60, 1899–1918. [Google Scholar] [CrossRef] [PubMed]
- Hall, A.J.; Richards, R.A. Prognosis for Genetic Improvement of Yield Potential and Water-Limited Yield of Major Grain Crops. Field Crops Res. 2013, 143, 18–33. [Google Scholar] [CrossRef]
- Senapati, N.; Semenov, M.A. Large Genetic Yield Potential and Genetic Yield Gap Estimated for Wheat in Europe. Glob. Food Secur. 2020, 24, 100340. [Google Scholar] [CrossRef]
- Mao, H.; Jiang, C.; Tang, C.; Nie, X.; Du, L.; Liu, Y.; Cheng, P.; Wu, Y.; Liu, H.; Kang, Z.; et al. Wheat Adaptation to Environmental Stresses under Climate Change: Molecular Basis and Genetic Improvement. Mol. Plant 2023, 16, 1564–1589. [Google Scholar] [CrossRef]
- Frantová, N.; Rábek, M.; Elzner, P.; Středa, T.; Jovanović, I.; Holková, L.; Martinek, P.; Smutná, P.; Prášil, I.T. Different Drought Tolerance Strategy of Wheat Varieties in Spike Architecture. Agronomy 2022, 12, 2328. [Google Scholar] [CrossRef]
- Sareen, S.; Budhlakoti, N.; Mishra, K.K.; Bharad, S.; Potdukhe, N.R.; Tyagi, B.S.; Singh, G.P. Resilience to Terminal Drought, Heat, and Their Combination Stress in Wheat Genotypes. Agronomy 2023, 13, 891. [Google Scholar] [CrossRef]
- Aberkane, H.; Belkadi, B.; Kehel, Z.; Filali-Maltouf, A.; Tahir, I.S.A.; Meheesi, S.; Amri, A. Assessment of Drought and Heat Tolerance of Durum Wheat Lines Derived from Interspecific Crosses Using Physiological Parameters and Stress Indices. Agronomy 2021, 11, 695. [Google Scholar] [CrossRef]
- Alemu, A.; Åstrand, J.; Montesinos-López, O.A.; Isidro y Sánchez, J.; Fernández-Gónzalez, J.; Tadesse, W.; Vetukuri, R.R.; Carlsson, A.S.; Ceplitis, A.; Crossa, J.; et al. Genomic Selection in Plant Breeding: Key Factors Shaping Two Decades of Progress. Mol. Plant 2024, 17, 552–578. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kumar, A.; Pandey, D.; Reddy, U.G.; Parmar, H.K.; Singh, P.; Garg, N.K.; He, X.; Tarekegn, Z.T.; Singh, G.; et al. Breeding and Selection Strategies to Accelerate Genetic Gains in Wheat for South Asia. VeriXiv 2025, 2, 110. [Google Scholar] [CrossRef]
- Crouch, J.H.; Payne, T.S.; Dreisigacker, S.; Wu, H.; Braun, H.-J. Improved Discovery and Utilization of New Traits for Breeding. In Wheat Facts and Futures; Dixon, J., Braun, H.-J., Kosina, P., Crouch, J., Eds.; CIMMYT: Mexico City, Mexico, 2009; pp. 42–51. [Google Scholar]
- Balkan, A. Genetic variability, heritability and genetic advance for yield and quality traits in M2-4 generations of bread wheat (Triticum aestivum L.) genotypes. Turk. J. Field Crops 2018, 23, 173–179. [Google Scholar] [CrossRef]
- Yagdi, K.; Sozen, E. Heritability, Variance Components and Correlations of Yield and Quality Traits in Durum Wheat (Triticum durum Desf.). Pak. J. Bot. 2009, 41, 753–759. [Google Scholar]
- Matković Stojšin, M.; Zečević, V.; Petrović, S.; Dimitrijević, M.; Mićanović, D.; Banjac, B.; Knežević, D. Variability, correlation, path analysis and stepwise regression for yield components of different wheat genotypes. Genetika 2018, 50, 817–828. [Google Scholar] [CrossRef]
- Banjac, B.; Mladenov, V.; Petrović, S.; Matković-Stojšin, M.; Krstić, Đ.; Vujić, S.; Mačkić, K.; Kuzmanović, B.; Banjac, D.; Jakšić, S.; et al. Phenotypic Variability of Wheat and Environmental Share in Soil Salinity Stress (3S) Conditions. Sustainability 2022, 14, 8598. [Google Scholar] [CrossRef]
- Urošević, D.; Knežević, D.; Ðurić, N.; Matković Stojšin, M.; Kandić, V.; Mićanović, D.; Stojiljković, J.; Zečević, V. Assessing the Potential of Old and Modern Serbian Wheat Genotypes: Yield Components and Nutritional Profiles in a Comprehensive Study. Agronomy 2023, 13, 2426. [Google Scholar] [CrossRef]
- Qasemi, S.H.; Mostafavi, K.; Khosroshahli, M.; Bihamta, M.R.; Ramshini, H. Genotype and Environment Interaction and Stability of Grain Yield and Oil Content of Rapeseed Cultivars. Food Sci. Nutr. 2022, 10, 4308–4318. [Google Scholar] [CrossRef] [PubMed]
- Amelework, A.B.; Bairu, M.W.; Marx, R.; Laing, M.; Venter, S.L. Genotype × Environment Interaction and Stability Analysis of Selected Cassava Cultivars in South Africa. Plants 2023, 12, 2490. [Google Scholar] [CrossRef]
- Taherian, M.; Saeidnia, F.; Hamid, R.; Nazeri, S.M. Identification of High-Yielding and Stable Cultivars of Wheat under Different Sowing Dates: Comparison of AMMI and GGE-Biplot Analyses. Heliyon 2024, 10, e39599. [Google Scholar] [CrossRef]
- Olivoto, T.; Lúcio, A.D.C.; da Silva, J.A.G.; Marchioro, V.S.; Souza, V.Q.; Jost, E. Mean Performance and Stability in Multi-Environment Trials I: Combining Features of AMMI and BLUP Techniques. Agron. J. 2019, 111, 1–12. [Google Scholar] [CrossRef]
- Pour-Aboughadareh, A.; Poczai, P.; Etminan, A.; Jadidi, O.; Kianersi, F.; Shooshtari, L. An Analysis of Genetic Variability and Population Structure in Wheat Germplasm Using Microsatellite and Gene-Based Markers. Plants 2022, 11, 1205. [Google Scholar] [CrossRef]
- Wang, T.-C.; Rose, T.; Zetzsche, H.; Ballvora, A.; Friedt, W.; Kage, H.; Léon, J.; Lichthardt, C.; Ordon, F.; Snowdon, R.J.; et al. Multi-environment field trials for wheat yield, stability and breeding progress in Germany. Sci. Data 2025, 12, 64. [Google Scholar] [CrossRef]
- Demydov, O.; Zamlila, N.; Novytska, N.; Kirilenko, V.; Miliar, B. Assessment of the Stability of Common Winter Wheat Breeding Lines in Multi-Environment Tests. Sci. Horiz. 2024, 27, 62–74. [Google Scholar] [CrossRef]
- Mohammadi, R.; Roostaei, M.; Armion, M.; Abdipour, M.; Rahmati, M.; Shahbazi, K. Deciphering Genotype × Environment Interaction for Grain Yield in Durum Wheat: An Integration of Analytical and Empirical Approaches for Increased Yield Stability and Adaptability. Eur. J. Agron. 2025, 168, 127656. [Google Scholar] [CrossRef]
- Luković, K.; Prodanović, S.; Perišić, V.; Milovanović, M.; Perišić, V.; Rajičić, V.; Zečević, V. Multivariate analysis of morphological traits and the most important productive traits of wheat in extreme wet conditions. Appl. Ecol. Environ. Res. 2020, 18, 5857–5871. [Google Scholar] [CrossRef]
- Matković Stojšin, M.; Petrović, S.; Banjac, B.; Roljević Nikolić, S.; Zečević, V.; Bačić, J.; Đorđević, R.; Knežević, D. Development of selection criteria for improving grain yield in wheat grown in different agro-ecological environments. Acta Agric. Serb. 2022, 27, 79–87. [Google Scholar] [CrossRef]
- Nikolić, O.; Živanović, T.; Kraljević-Balalić, M.; Milovanović, M. Interrelationship between Grain Yield and Physiological Parameters of Winter Wheat Nitrogen Nutrition Efficiency. Genetika 2011, 43, 91–100. [Google Scholar] [CrossRef]
- Caverzan, A.; Casassola, A.; Brammer, S.P. Antioxidant Responses of Wheat Plants under Stress. Genet. Mol. Biol. 2016, 39, 1–6. [Google Scholar] [CrossRef]
- Štolfa Čamagajevac, I.; Vuković, R.; Vuković, K.; Vuković, A.; Ivezić, V.; Žuna Pfeiffer, T.; Krstin, L.; Lončarić, Z. Wheat Leaf Antioxidative Status—Variety-Specific Mechanisms of Zinc Tolerance during Biofortification. Plants 2021, 10, 2223. [Google Scholar] [CrossRef]
- Nardino, M.; Perin, E.C.; Aranha, B.C.; Carpes, S.T.; Fontoura, B.H.; de Sousa, D.J.P.; Freitas, D.S.D. Understanding Drought Response Mechanisms in Wheat and Multi-Trait Selection. PLoS ONE 2022, 17, e0266368. [Google Scholar] [CrossRef]
- Oguz, M.C.; Aycan, M.; Oguz, E.; Poyraz, I.; Yildiz, M. Drought Stress Tolerance in Plants: Interplay of Molecular, Biochemical and Physiological Responses in Important Development Stages. Physiologia 2022, 2, 180–197. [Google Scholar] [CrossRef]
- Matković Stojšin, M.; Petrović, S.; Banjac, B.; Zečević, V.; Roljević Nikolić, S.; Majstorović, H.; Đorđević, R.; Knežević, D. Assessment of Genotype Stress Tolerance as an Effective Way to Sustain Wheat Production under Salinity Stress Conditions. Sustainability 2022, 14, 6973. [Google Scholar] [CrossRef]
- Republic Hydrometeorological Service of Serbia. Available online: http://www.hidmet.gov.rs/ (accessed on 15 June 2025).
- Meier, U. (Ed.) Growth Stages of Mono- and Dicotyledonous Plants—BBCH Monograph; Federal Biological Research Centre for Agriculture and Forestry: Bonn, Germany, 2001. [Google Scholar]
- Molyneux, P. The Use of Stable Free Radical Diphenylpicrylhydrazyl (DPPH) for Estimating Antioxidant Activity. Songklanakarin J. Sci. Technol. 2004, 26, 211–219. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- R Project for Statistical Computing; Version 4.3.2 (2023-10-31 ucrt); R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 20 July 2025).
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 3rd ed.; Sage: Thousand Oaks, CA, USA, 2019; Available online: https://www.john-fox.ca/Companion/ (accessed on 20 July 2025).
- de Mendiburu, F. agricolae: Statistical Procedures for Agricultural Research; R Package Version 1.3-7. 2023. Available online: https://CRAN.R-project.org/package=agricolae (accessed on 20 July 2025).
- Gauch, H.G.; Zobel, R.W. AMMI Analysis of Yield Trials. In Genotype by Environment Interaction; Kang, M.S., Gauch, H.G., Eds.; CRC Press: Boca Raton, FL, USA, 1996; pp. 85–122. [Google Scholar]
- Olivoto, T.; Lúcio, A.D. metan: An R package for multi-environment trial analysis. Methods Ecol. Evol. 2020, 11, 783–789. [Google Scholar] [CrossRef]
- Purchase, J.L.; Hatting, H.; van Deventer, C.S. Genotype × Environment Interaction of Winter Wheat (Triticum aestivum L.) in South Africa: II. Stability Analysis of Yield Performance. S. Afr. J. Plant Soil. 2000, 17, 101–107. [Google Scholar] [CrossRef]
- Kassambara, A.; Mundt, F. factoextra: Extract and Visualize the Results of Multivariate Data Analyses; R Package Version 1.0.7. 2020. Available online: https://CRAN.R-project.org/package=factoextra (accessed on 25 July 2025).
- Sievert, C. Interactive Web-Based Data Visualization with R, Plotly, and Shiny; Chapman and Hall/CRC: London, UK, 2020; Available online: https://plotly-r.com/ (accessed on 22 August 2025).
- Achilli, A.L.; Roncallo, P.F.; Echenique, V. Genetic Gains in Grain Yield and Agronomic Traits of Argentinian Durum Wheat from 1934 to 2015. Agronomy 2022, 12, 2151. [Google Scholar] [CrossRef]
- Yan, Q.; Zeng, Z.; Wang, C.; Li, J.; Song, J.; Li, Q.; Zhao, Y.; Liu, C.; Jing, X. Genetic Diversity and Population Structure of Wheat Germplasm for Grain Nutritional Quality Using Haplotypes and KASP Markers. Agriculture 2025, 15, 1986. [Google Scholar] [CrossRef]
- Pour-Aboughadareh, A.; Ahmadi, J.; Mehrabi, A.A.; Moghaddam, M.; Etminan, A. Evaluation of agro-morphological diversity in wild relatives of wheat collected in Iran. J. Agric. Sci. Technol. 2017, 19, 943–956. [Google Scholar]
- Mladenov, V.; Dimitrijević, M.; Petrović, S.; Boćanski, J.; Banjac, B.; Kondić-Špika, A.; Trkulja, D. Genetic Analysis of Spike Length in Wheat. Genetika 2019, 51, 167–178. [Google Scholar] [CrossRef]
- Ullah, I.M.; Mahpara, S.; Bibi, R.; Shah, R.U.; Ullah, R.; Abbas, S.; Ullah, M.I.; Hassan, A.M.; El-Shehawi, A.M.; Bresric, M.; et al. Grain Yield and Correlated Traits of Bread Wheat Lines: Implications for Yield Improvement. Saudi J. Biol. Sci. 2021, 28, 5714–5719. [Google Scholar] [CrossRef] [PubMed]
- Shamsabadi, E.E.; Sabouri, H.; Soughi, H.; Sajadi, S.J. Genetic Analysis of Spike Traits in Wheat (Triticum aestivum L.). Genetika 2020, 52, 559–569. [Google Scholar] [CrossRef]
- Lacko-Bartošová, M.; Lacko-Bartošová, L.; Kobida, Ľ.; Kaur, A.; Moudrý, J. Phenolic Acids Profiles and Phenolic Concentrations of Emmer Cultivars in Response to Growing Year under Organic Management. Foods 2023, 12, 1480. [Google Scholar] [CrossRef]
- Popović, V.; Ljubičić, N.; Kosrić, M.; Radulović, M.; Blagojević, D.; Ugrenović, V.; Popović, D.; Ivošević, B. Genotype × environment interaction for wheat yield traits suitable for selection in different seed priming conditions. Plants 2020, 9, 1804. [Google Scholar] [CrossRef]
- Ljubičić, N.; Popović, V.; Ćirić, V.; Kostić, M.; Ivošević, B.; Popović, D.; Pandžić, M.; El Musafah, S.; Janković, S. Multivariate Interaction Analysis of Winter Wheat Grown in Environment of Limited Soil Conditions. Plants 2021, 10, 604. [Google Scholar] [CrossRef] [PubMed]
- Chrpová, J.; Grausgruber, H.; Weyermann, V.; Buerstmayr, M.; Palicová, J.; Kozová, J.; Trávníčková, M.; Nguyen, Q.T.; Moreno Amores, J.E.; Buerstmayr, H.; et al. Resistance of Winter Spelt Wheat [Triticum aestivum subsp. spelta (L.) Thell.] to Fusarium Head Blight. Front. Plant Sci. 2021, 12, 661484. [Google Scholar] [CrossRef]
- Serrago, R.A.; Carrera, C.S.; Savin, R.; Slafer, G.A. Is the Relationship between Grain Number and Spike Dry Weight Linear? Insights from Larger Spikes in Wheat. Crop J. 2025, 13, 636–640. [Google Scholar] [CrossRef]
- Ferrante, A.; Savin, R.; Slafer, G.A. Floret Development and Spike Fertility in Wheat: Differences between Cultivars of Contrasting Yield Potential and Their Sensitivity to Photoperiod and Soil N. Field Crops Res. 2020, 256, 107908. [Google Scholar] [CrossRef]
- Mądry, W.; Studnicki, M.; Rozbicki, J.; Golba, J.; Gozdowski, D.; Pecio, A.; Oleksy, A. Ontogenetic-Based Sequential Path Analysis of Grain Yield and Its Related Traits in Several Winter Wheat Cultivars. Acta Agric. Scand. Sect. B Soil Plant Sci. 2015, 65, 605–618. [Google Scholar] [CrossRef]
- Al-Ashkar, I.; Sallam, M.; Al-Suhaibani, N.; Ibrahim, A.; Alsadon, A.; Al-Doss, A. Multiple Stresses of Wheat in the Detection of Traits and Genotypes of High-Performance and Stability for a Complex Interplay of Environment and Genotypes. Agronomy 2022, 12, 2252. [Google Scholar] [CrossRef]
- Spanic, V.; Jukic, G.; Zoric, M.; Varnica, I. Some Agronomic Properties of Winter Wheat Genotypes Grown at Different Locations in Croatia. Agriculture 2024, 14, 4. [Google Scholar] [CrossRef]
- Luković, K.; Rakonjac, A.; Zečević, V.; Matković Stojšin, M.; Rajičić, V.; Stojšin, M.; Bratković, K. Wheat Yield Stability Across Years with Varying Climate Conditions. In Proceedings of the 3rd International Symposium on Biotechnology, Čačak, Serbia, 13–14 March 2025; pp. 111–117. [Google Scholar] [CrossRef]
- Sugár, E.; Fodor, N.; Sándor, R.; Bónis, P.; Vida, G.; Árendás, T. Spelt Wheat: An Alternative for Sustainable Plant Production at Low N-Levels. Sustainability 2019, 11, 6726. [Google Scholar] [CrossRef]
- Pospišil, A.; Pospišil, M.; Svečnjak, Z.; Matotan, S. Influence of Crop Management upon the Agronomic Traits of Spelt (Triticum spelta L.). Plant Soil Environ. 2011, 57, 435–440. [Google Scholar] [CrossRef]
- Dumalasová, V.; Grausgruber, H.; Zelba, O.; Hanzalová, A.; Buerstmayr, H.; Weyermann, V.; dell’Avo, F.; Cuendet, C.; Koppel, R.; Sooväli, P. Spelt Wheat Resistance to Rusts, Powdery Mildew, Leaf Blotch and Common Bunt. Cereal Res. Commun. 2025, 53, 451–467. [Google Scholar] [CrossRef]
- Roljević Nikolić, S.; Kovačević, D.; Cvijanović, G.; Dolijanović, Ž.; Marinković, J. Grain Yield and Rhizosphere Microflora of Alternative Types of Wheat in Organic Production. Rom. Biotechnol. Lett. 2018, 23, 13301–13309. [Google Scholar]
- Vojnov, B.; Manojlović, M.; Latković, D.; Milošev, D.; Dolijanović, Ž.; Simić, M.; Babec, B.; Šeremešić, S. Grain Yield, Yield Components and Protein Content of Organic Spelt Wheat (Triticum spelta L.) Grown in Different Agro-Ecological Conditions of Northern Serbia. Ratar. Povrt. 2020, 57, 1–7. [Google Scholar] [CrossRef]
- Zečević, V.; Bošković, J.; Milenković, S.; Matković Stojšin, M.; Balijagić, J.; Đukić, N.; Knežević, D. Phenotypic Variability of Yield Components of Triticum spelta in Organic Production. Agric. For. 2018, 64, 71–78. [Google Scholar] [CrossRef]
- Begna, T. The Role of Genotype by Environmental Interaction in Plant Breeding. Int. J. Agric. Biosci. 2020, 9, 209–215. [Google Scholar]
- Bratković, K.; Luković, K.; Perišić, V.; Savić, J.; Maksimović, J.; Adžić, S.; Rakonjac, A.; Matković Stojšin, M. Interpreting the Interaction of Genotype with Environmental Factors in Barley Using Partial Least Squares Regression Model. Agronomy 2024, 14, 194. [Google Scholar] [CrossRef]
- Abebe, A.T.; Adewumi, A.S.; Adebayo, M.A.; Shaahu, A.; Mushoriwa, H.; Alabi, T.; Derera, J.; Agbona, A.; Chigeza, G. Genotype × Environment Interaction and Yield Stability of Soybean (Glycine max L.) Genotypes in Multi-Environment Trials (METs) in Nigeria. Heliyon 2024, 10, e38097. [Google Scholar] [CrossRef]
- Kebede, G.; Worku, W.; Feyissa, F.; Jifar, H. Genotype by Environment Interaction and Stability Analysis for Selection of Superior Fodder Yield Performing Oat (Avena sativa L.) Genotypes Using GGE Biplot in Ethiopia. Ecol. Genet. Genom. 2023, 28, 100192. [Google Scholar] [CrossRef]
- Saeidnia, F.; Taherian, M.; Nazeri, S.M. Graphical Analysis of Multi Environmental Trials for Wheat Grain Yield Based on GGE-Biplot Analysis under Diverse Sowing Dates. BMC Plant Biol. 2023, 23, 198. [Google Scholar] [CrossRef]
- Pour-Aboughadareh, A.; Khalili, M.; Poczai, P.; Olivoto, T. Stability Indices to Deciphering the Genotype-by-Environment Interaction (GEI) Effect: An Applicable Review for Use in Plant Breeding Programs. Plants 2022, 11, 414. [Google Scholar] [CrossRef] [PubMed]
- Sabir, K.; Rose, T.; Wittkop, B.; Stahl, A.; Snowdon, R.J.; Ballvora, A.; Friedt, W.; Kage, H.; Léon, J.; Ordon, F.; et al. Stage-Specific Genotype-by-Environment Interactions Determine Yield Components in Wheat. Nat. Plants 2023, 9, 1688–1696. [Google Scholar] [CrossRef]
- Mullualem, D.; Tsega, A.; Mengie, T.; Fentie, D.; Kassa, Z.; Fassil, A.; Wondaferew, D.; Gelaw, T.A.; Astatkie, T. Genotype-by-Environment Interaction and Stability Analysis of Grain Yield of Bread Wheat (Triticum aestivum L.) Genotypes Using AMMI and GGE Biplot Analyses. Heliyon 2024, 10, e32918. [Google Scholar] [CrossRef] [PubMed]
- Yue, H.; Wang, Y.; Chen, Z.; Zhu, J.; Behera, P.P.; Liu, P.; Yang, H.; Wei, J.; Bu, J.; Jiang, X.; et al. Assessing the Role of Genotype by Environment Interaction of Winter Wheat Cultivars Using Envirotyping Techniques in North China. Front. Plant Sci. 2025, 16, 1538661. [Google Scholar] [CrossRef]
- Mohammadi, R.; Armion, M.; Zadhasan, E.; Ahamdi, M.M.; Amir, A. The Use of AMMI Model for Interpreting Genotype × Environment Interaction in Durum Wheat. Exp. Agric. 2018, 54, 670–683. [Google Scholar] [CrossRef]
- Bishwas, K.C.; Poudel, M.R.; Regmi, D. AMMI and GGE Biplot Analysis of Yield of Different Elite Wheat Line under Terminal Heat Stress and Irrigated Environments. Heliyon 2021, 7, e07206. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Kumar, M.; Singh, V.; Chaudhary, L.; Yashveer, S.; Sheoran, R.; Dalal, M.S.; Nain, A.; Lamba, K.; Gangadharaiah, N.; et al. Genotype by Environment Interaction Analysis for Grain Yield of Wheat (Triticum aestivum (L.) em.Thell) Genotypes. Agriculture 2022, 12, 1002. [Google Scholar] [CrossRef]
- Perišić, V.; Perišić, V.; Luković, K.; Bratković, K.; Zečević, V.; Babić, S.; Matković Stojšin, M. Stability of Grain Yield Performance of Winter Wheat Genotypes. Sel. Sem. 2022, 28, 52–60. [Google Scholar] [CrossRef]
- Omrani, A.; Omrani, S.; Khodarahmi, M.; Shojaei, S.H.; Illés, Á.; Bojtor, C.; Mousavi, S.M.N.; Nagy, J. Evaluation of Grain Yield Stability in Some Selected Wheat Genotypes Using AMMI and GGE Biplot Methods. Agronomy 2022, 12, 1130. [Google Scholar] [CrossRef]
- Wodebo, K.Y.; Tolemariam, T.; Demeke, S.; Garedew, W.; Tesfaye, T.; Zeleke, M.; Gemiyu, D.; Bedeke, W.; Wamatu, J.; Sharma, M. AMMI and GGE Biplot Analyses for Mega-Environment Identification and Selection of Some High-Yielding Oat (Avena sativa L.) Genotypes for Multiple Environments. Plants 2023, 12, 3064. [Google Scholar] [CrossRef]
- Hossain, M.A.; Sarker, U.; Azam, M.G.; Kobir, M.S.; Roychowdhury, R.; Ercisli, S.; Ali, D.; Oba, S.; Golokhvast, K.S. Integrating BLUP, AMMI, and GGE Models to Explore GE Interactions for Adaptability and Stability of Winter Lentils (Lens culinaris Medik.). Plants 2023, 12, 2079. [Google Scholar] [CrossRef]
- Elbasyoni, I.S. Performance and Stability of Commercial Wheat Cultivars under Terminal Heat Stress. Agronomy 2018, 8, 37. [Google Scholar] [CrossRef]
- Wardofa, G.; Mohammed, H.; Asnake, D.; Alemu, T. Genotype × Environment Interaction and Yield Stability of Bread Wheat Genotypes in Central Ethiopia. J. Plant Breed. Genet. 2019, 7, 87–94. [Google Scholar] [CrossRef]
- Jędzura, S.; Bocianowski, J.; Matysik, P. The AMMI Model Application to Analyze the Genotype–Environmental Interaction of Spring Wheat Grain Yield for the Breeding Program Purposes. Cereal Res. Commun. 2023, 51, 197–205. [Google Scholar] [CrossRef]
- Yan, W.; Hunt, L.A.; Sheng, Q.; Szlavnics, Z. Cultivar evaluation and mega-environment investigation based on the GGE biplot. Crop Sci. 2000, 40, 597–605. [Google Scholar] [CrossRef]
- Yan, W.; Kang, M.S.; Ma, B.; Woods, S.; Cornelius, P.L. GGE Biplot vs. AMMI Analysis of Genotype-by-Environment Data. Crop Sci. 2007, 47, 643–655. [Google Scholar] [CrossRef]
- Bosi, S.; Negri, L.; Fakaros, A.; Oliveti, G.; Whittaker, A.; Dinelli, G. GGEBiplot Analysis to Explore the Adaption Potential of Italian Common Wheat Genotypes. Sustainability 2022, 14, 897. [Google Scholar] [CrossRef]
- Yan, W. Two Types of Biplots to Integrate Multi-Trial and Multi-Trait Information for Genotype Selection. Crop Sci. 2024, 64, 1608–1618. [Google Scholar] [CrossRef]
- Aktaş, H. Tracing Highly Adapted Stable Yielding Bread Wheat (Triticum aestivum L.) Genotypes for Greatly Variable South-Eastern Turkey. Appl. Ecol. Environ. Res. 2016, 14, 159–176. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Fritschi, F.B.; Mittler, R. Global Warming, Climate Change, and Environmental Pollution: Recipe for a Multifactorial Stress Combination Disaster. Trends Plant Sci. 2021, 26, 588–599. [Google Scholar] [CrossRef] [PubMed]
- Banjac, B.; Dimitrijević, M.; Petrović, S.; Mladenov, V.; Banjac, D.; Kiprovski, B. Antioxidant Variability of Wheat Genotypes under Salinity Stress in situ. Genetika 2020, 52, 1145–1160. [Google Scholar] [CrossRef]
- Chugh, V.; Kaur, N.; Gupta, A.K. Differential Antioxidative Response of Tolerant and Sensitive Maize (Zea mays L.) Genotypes to Drought Stress at Reproductive Stage. Indian J. Biochem. Biophys. 2013, 50, 150–158. [Google Scholar]
- Kiani, R.; Arzani, A.; Mirmohammady Maibody, S.A.M. Polyphenols, Flavonoids, and Antioxidant Activity Involved in Salt Tolerance in Wheat, Aegilops sylandrica and Their Amphidiploids. Front. Plant Sci. 2021, 12, 646221. [Google Scholar] [CrossRef]
- Zhang, A.; Liu, M.; Gu, W.; Wang, J.; Zhang, Y.; Ma, Z.; Zhao, T.; Li, Y.; Bai, Y. Effect of Drought on Photosynthesis, Total Antioxidant Capacity, Bioactive Component Accumulation, and the Transcriptome of Atractylodes lancea. BMC Plant Biol. 2021, 21, 293. [Google Scholar] [CrossRef]
- Nawaz, H.; Hussain, N.; Yasmeen, A. Growth, Yield and Antioxidants Status of Wheat (Triticum aestivum L.) Cultivars under Water Deficit Conditions. Pak. J. Agric. Sci. 2015, 52, 953–959. [Google Scholar]
- Barros Santos, M.C.; da Silva Lima, L.R.; Nascimento, F.R.; do Nascimento, T.P.; Cameron, L.C.; Ferreira, M.S.L. Metabolomic Approach for Characterization of Phenolic Compounds in Different Wheat Genotypes during Grain Development. Food Res. Int. 2019, 124, 118–128. [Google Scholar] [CrossRef]
- Brandolini, A.; Castoldi, P.; Plizzari, L.; Hidalgo, A. Phenolic Acids Composition, Total Polyphenols Content and Antioxidant Activity of Triticum monococcum, Triticum turgidum and Triticum aestivum: A Two-Years Evaluation. J. Cereal Sci. 2013, 58, 123–131. [Google Scholar] [CrossRef]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of Phenylpropanoid Pathway and the Role of Polyphenols in Plants under Abiotic Stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef] [PubMed]
- Melios, S.; Ninou, E.; Irakli, M.; Tsivelika, N.; Sistanis, I.; Papathanasiou, F.; Didos, S.; Zinoviadou, K.; Karantonis, H.C.; Argiriou, A.; et al. Effect of Genotype, Environment, and Their Interaction on the Antioxidant Properties of Durum Wheat: Impact of Nitrogen Fertilization and Sowing Time. Agriculture 2024, 14, 328. [Google Scholar] [CrossRef]
- Buczek, J.; Jańczak-Pieniążek, M.; Harasim, E.; Kwiatkowski, C.A.; Kapusta, I. Effect of Cropping Systems and Environment on Phenolic Acid Profiles and Yielding of Hybrid Winter Wheat Genotypes. Agriculture 2023, 13, 834. [Google Scholar] [CrossRef]
- Salekjalali, M.; Haddad, R.; Jafari, B. Analysis of Antioxidant Enzyme Activity during Reproductive Stages of Barley under Drought Stress. J. Ecobiotechnol. 2011, 3, 40–47. [Google Scholar]
- Mathew, I.; Shimelis, H.; Mwadzingeni, L.; Chaplot, V. Variance Components and Heritability of Traits Related to Root:Shoot Biomass Allocation and Drought Tolerance in Wheat. Euphytica 2018, 214, 225. [Google Scholar] [CrossRef]
- Sewore, B.M.; Abe, A. Genetic Variability and Trait Associations in Bread Wheat (Triticum aestivum L.) Genotypes under Drought-Stressed and Well-Watered Conditions. CABI Agric. Biosci. 2024, 5, 64. [Google Scholar] [CrossRef]
- Javed, M.; Ali, A.; Kashif, M.; Ali, M.; Ullah, S.; Alam, A. Estimation of Heritability, Genotypic Variability and Correlations Analysis for Yield and Yield Attributing Traits among Bread Wheat (Triticum aestivum L.) Genotypes. J. Appl. Life Sci. Environ. 2024, 57, 91–106. [Google Scholar] [CrossRef]
- Ghafoor, A.Z.; Ceglińska, A.; Karim, H.; Wijata, M.; Sobczyński, G.; Derejko, A.; Studnicki, M.; Rozbicki, J.; Cacak-Pietrzak, G. Influence of Genotype, Environment, and Crop Management on the Yield and Bread-Making Quality in Spring Wheat Cultivars. Agriculture 2024, 14, 2131. [Google Scholar] [CrossRef]
- Sangha, J.S.; Wang, W.; Knox, R.; Ruan, Y.; Cuthbert, R.D.; Isidro-Sánchez, J.; Li, L.; He, Y.; DePauw, R.; Singh, A.; et al. Phenotypic Plasticity of Bread Wheat Contributes to Yield Reliability under Heat and Drought Stress. PLoS ONE 2025, 20, e0312122. [Google Scholar] [CrossRef]
- Shokat, S.; Großkinsky, D.K.; Roitsch, T.; Liu, F. Activities of Leaf and Spike Carbohydrate-Metabolic and Antioxidant Enzymes Are Linked with Yield Performance in Three Spring Wheat Genotypes Grown under Well-Watered and Drought Conditions. BMC Plant Biol. 2020, 20, 400. [Google Scholar] [CrossRef]
- Niroula, A.; Khatri, S.; Khadka, D.; Timilsina, R. Total phenolic contents and antioxidant activity profile of selected cereal sprouts and grasses. Int. J. Food Prop. 2019, 22, 427–437. [Google Scholar] [CrossRef]
- Spanic, V.; Drezner, G.; Dvojkovic, K.; Horvat, D. Traits of 25 Winter wheat varieties grown in Croatia in the last 100 years. Agron. Glas. 2016, 78, 3–16. [Google Scholar]
- Ding, H.; Wang, C.; Cai, Y.; Yu, K.; Zhao, H.; Wang, F.; Shi, X. Characterization of a Wheat Stable QTL for Spike Length and Its Genetic Effects on Yield-Related Traits. BMC Plant Biol. 2024, 24, 292. [Google Scholar] [CrossRef]
- Philipp, N.; Weichert, H.; Bohra, U.; Weschke, W.; Schulthess, A.W.; Weber, H. Grain number and grain yield distribution along the spike remain stable despite breeding for high yield in winter wheat. PLoS ONE 2018, 13, e0205452. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Sparkes, D.L. Dissecting the Trade-Off of Grain Number and Size in Wheat. Planta 2021, 254, 3. [Google Scholar] [CrossRef] [PubMed]
| No. | Genotype 1 | Pedigree | Institution |
|---|---|---|---|
| 1. | KG-4/1 | Pobeda × Aleksandra | Center for Small Grains and Rural Development, Kragujevac |
| 2. | KG-9/1 | Cipovka × Aleksandra | |
| 3. | KG-11/1 | Oda × Aleksandra | |
| 4. | KG-16/1 | Planeta × Aleksandra | |
| 5. | KG-21/1 | Srna × Aleksandra | |
| 6. | KG-30/1 | Evropa 90 × Kruna | |
| 7. | KG-33/1 | Zenit × Kruna | |
| 8. | KG-40/1 | Milica × Kruna | |
| 9. | KG-41/1 | Kruna × Venera | |
| 10. | KG-43/1 | Aleksandra × Ana Morava | |
| 11. | NS 40S (standard) | NA 694 × NSA 88-3141 | Institute for Field and Vegetable Crops, Novi Sad |
| 12. | Renesansa (standard) | Jugoslavija × NS 55-25 | |
| 13. | Pobeda (standard) | Sremica × Balkan | |
| 14. | KG-54-7/3-5 (spelt) | KG-100 × SSK 2001/02 | Center for Small Grains and Rural Development, Kragujevac |
| 15. | KG-54-7/3-2 (spelt) | KG-100 × SSK 2001/02 |
| Debth (cm) | pH in H2O | pH in KCl | Humus (%) | N (%) | P2O5 (mg 100 g−1) | K2O (mg 100 g−1) | CaCO3 (%) |
|---|---|---|---|---|---|---|---|
| Pančevo | |||||||
| 0–30 | 8.27 | 7.57 | 3.39 | 0.20 | 20.6 | 25.53 | 12.59 |
| Kragujevac | |||||||
| 0–30 | 6.93 | 5.81 | 3.95 | 0.20 | 9.67 | 19.40 | ˂ 0.42 |
| Kruševac | |||||||
| 0–30 | 6.17 | 5.35 | 2.14 | 0.228 | 8.56 | 19.24 | 0.80 |
| Environment 1 | Mean (t ha−1) | IPCA1 Score | Rank of Genotypes | |||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |||
| E1 | 5.74 | 0.005 | KG-11/1 | KG-4/1 | KG-40/1 | NS 40S |
| E2 | 5.51 | 1.516 | KG-40/1 | KG-11/1 | Pobeda | KG-21/1 |
| E3 | 5.24 | 0.146 | KG-54-7/3-2 | Pobeda | Renesansa | KG-40/1 |
| E4 | 6.43 | −0.744 | NS 40S | KG-4/1 | KG-16/1 | KG-41/1 |
| E5 | 4.84 | −0.925 | Renesansa | KG-4/1 | KG-9/1 | NS 40 S |
| E6 | 6.87 | 0.001 | KG-4/1 | KG-11/1 | KG-21/1 | NS 40 S |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brković, P.; Matković Stojšin, M.; Nikolić, O.; Perišić, V.; Luković, K.; Babić, S.; Roljević Nikolić, S. Yield Stability and Antioxidant Response of Wheat Under Multi-Environment Conditions: Insights from AMMI and GGE Biplot Analyses. Agronomy 2025, 15, 2684. https://doi.org/10.3390/agronomy15122684
Brković P, Matković Stojšin M, Nikolić O, Perišić V, Luković K, Babić S, Roljević Nikolić S. Yield Stability and Antioxidant Response of Wheat Under Multi-Environment Conditions: Insights from AMMI and GGE Biplot Analyses. Agronomy. 2025; 15(12):2684. https://doi.org/10.3390/agronomy15122684
Chicago/Turabian StyleBrković, Predrag, Mirela Matković Stojšin, Olivera Nikolić, Vladimir Perišić, Kristina Luković, Snežana Babić, and Svetlana Roljević Nikolić. 2025. "Yield Stability and Antioxidant Response of Wheat Under Multi-Environment Conditions: Insights from AMMI and GGE Biplot Analyses" Agronomy 15, no. 12: 2684. https://doi.org/10.3390/agronomy15122684
APA StyleBrković, P., Matković Stojšin, M., Nikolić, O., Perišić, V., Luković, K., Babić, S., & Roljević Nikolić, S. (2025). Yield Stability and Antioxidant Response of Wheat Under Multi-Environment Conditions: Insights from AMMI and GGE Biplot Analyses. Agronomy, 15(12), 2684. https://doi.org/10.3390/agronomy15122684









