Abstract
The development of maize varieties with enhanced tolerance to drought stress has become a high-priority goal for maize breeding programs worldwide. In order to assess the variability of root and shoot traits in response to drought at an early vegetative stage, a set of 32 maize single-cross hybrids was grown under polyethylene glycol 8000-induced drought stress and well-watered control treatments. Drought stress significantly reduced hybrid seedling root and shoot lengths (RL and SL) as well as root and shoot fresh weights (RFW and SFW), while an increase in seedling root and shoot dry matter (RDM and SDM) and root fresh weight-to-shoot fresh weight ratio (RFW/SFW) was observed. The high heritability estimates for the four directly and easily measured traits, namely, RL, SL, RFW, and SFW (0.83, 0.83, 0.74, and 0.74, respectively), and medium-to-very-strong positive correlations among these traits under drought conditions indicate their applicability for the assessment of maize drought tolerance at the seedling stage and may represent a practical contribution to maize breeding programs for improved drought tolerance. Among the studied hybrids, hybrids 30, 3, and 23 were characterized by the largest RL under drought conditions and small relative change in RL between control and drought treatments. Hybrid 30 also showed one of the smallest relative reductions in SL, RFW, and SFW between the two treatments, while hybrids 3 and 23 were among those which exhibited the highest relative RL/SL and RFW/SFW increase between the two treatments, which supports their potential as parental lines in drought-tolerant breeding.
1. Introduction
Drought, a period without significant rainfall or a condition where water is limited, has been identified as the most common abiotic stress limiting crop performance worldwide in terms of growth and productivity []. It is also considered the most significant environmental factor affecting the productivity of maize (Zea mays L.) []. In terms of production quantity, maize is the most important cereal in the world [], and, together with wheat and rice, provides at least 30% of food calories in developing countries []. The demand for maize is increasing as a source of food, oil, and biofuel for the growing world population [], with estimates suggesting that by 2050 the demand for maize in developing countries will almost double the current demand. During its life cycle, which lasts depending on the genotype from 80 to 150 days, maize requires 500–800 mm of water [], which, according to Sah et al. [], is near or below the critical level for achieving a good yield. Since 15% to 20% of maize grain yield is lost annually due to drought, with the prospect of further increases as droughts become more frequent and severe [], developing maize varieties with enhanced tolerance to drought stress and higher water use efficiency has become a high-priority goal for breeding programs, both in the private and public sectors.
Maize is most sensitive to water stress during pollination and the early grain-filling period [,]. Therefore, the most widely used strategy for the improvement of maize drought tolerance is direct selection for yield under non-stress conditions and then evaluation of the selected genotypes at many sites with variable moisture availability []. But phenotyping for drought tolerance is laborious and resource-intensive, requiring multiple locations and years of testing to accurately characterize the traits involved, and also often inefficient because the heritability on an entry-mean basis (h2) for grain yield is much lower under stress [,]. Another suggestion for increasing the efficiency of selection for drought tolerance was to select secondary traits, which are easy to measure, have high h2, and are strongly correlated with grain yield under drought [,,], such as anthesis-silking interval (ASI). By selecting genotypes with short ASI under drought conditions, increases in grain yield have been reported [,], but Bruce et al. [] argued that the observed gains in yield were largely achieved due to reduced bareness under drought and not due to increased biomass production. As drought is a multidimensional stress, affecting plants at various organizational levels over space and time, with complex and unpredictable physiological responses [], it requires an understanding of genetic mechanisms controlling various plant traits for adopting different breeding approaches [].
The assessment of crop genotypes at seedling stage is an imperative feature of plant breeding for developing drought-tolerant cultivars. Vigorous maize seedlings lead to healthy crops and ultimately good production under water-deficit conditions []. According to Aslam et al. [], the relationship between root and shoot at seedling establishment largely influences the time of physiological maturity and yield. Denmead and Shaw [] found that drought during the vegetative growth stage reduced yield by 25%, while Edmeades et al. [], according to Akinwale et al. [], stated that drought at the seedling and early vegetative stage may result in reduced crop establishment, zero yield, or even complete crop failure. Duvick [] and Duvick and Cassman [] showed that the change in yield potential on a per plant basis from older to newer hybrids was not significant when measured on non-stressed plants grown at very low plant density, but that the newer hybrids outperformed the older ones at higher plant densities. Imposing higher plant densities induces stress responses in maize and reduces yield on a per plant basis, but this is compensated by the increased number of plants per unit area, resulting in higher net yield []. Therefore, establishing high plant density at the seedling and early vegetative stage after germination is a prerequisite for successful maize production.
The seedling stage of maize begins immediately after emergence and continues until the five-leaf stage, which occurs around 14 days after emergence. This stage is also referred to as the early vegetative stage, during which the plants are highly sensitive to environmental stress such as drought, which at this time can result in total crop failure []. Although earlier studies reported that seedling drought tolerance mechanisms are independent of drought responses during the flowering period [], information on the response of maize genotypes to drought at the seedling and early vegetative stages is essential for achieving rapid progress in selection for drought tolerance in maize [].
Under field conditions, it is not possible to accurately determine the effects of drought on root development []. In that regard, the application of polyethylene glycol (PEG) under controlled laboratory conditions has been used as an alternative to field trials for assessing maize drought stress resistance at germination and early vegetative stages [,,,,,,,,,,,]. PEG is considered an ideal regulator of water potential because its molecules, due to their high molecular weight (6000 and above), cannot penetrate cell walls []. This allows for precise assessment of the condition of a plant exposed to a specific osmotic potential []. These properties have made it the most widely used osmoticum to mimic decreases in soil water potential []. The use of PEG is suitable for screening large numbers of genotypes for drought tolerance at early stages [] and can serve as a selection criterion for improving crop resistance to drought []. Phenotyping young seedlings under controlled conditions is a useful approach to identify candidate drought-tolerant genotypes [], and can reduce, at least in part, the labor-intensive selection required under field conditions [].
The aim of the present study was to investigate the variability of maize hybrids available on the Croatian market for root and shoot traits under PEG-induced drought stress at an early vegetative growth stage.
2. Materials and Methods
2.1. Plant Material and Experimental Design
A total of 32 single-cross maize hybrids available on the Croatian market were used as plant material. The hybrids belong to different developers and FAO groups, but their commercial names were deliberately omitted (Table S1). The 32 hybrids were grown under two water treatments (control and drought). Within each water treatment, hybrids were arranged in a randomized complete block design (RCBD) with three replicates. Five adjacent plants per hybrid (representing experimental unit for analysis) were grown in each replicate (block), within each water treatment, for a total of 192 experimental units (2 water treatments × 32 hybrids × 3 replicates).
The hybrid seeds were sown in quartz sand in plastic containers with lids, and each container was watered with the same amount of water. The containers were covered for two days to prevent moisture loss and enhance germination, after which the lids were removed. Seeds were germinated in a growth chamber at 22 °C with a 16 h day and 8 h night photoperiod.
On the seventh day after sowing, seedlings, mostly at the one-leaf stage, were carefully separated and transplanted into plastic tubes (one plant per tube). Five adjacent tubes containing plants of the same hybrid constituted the experimental unit for analysis. The tubes had a diameter of 5 cm and a length of 40 cm, and were filled with vermiculite (0–3 mm) (Pull Rhenen B.V., Rhenen, The Netherlands). Each tube had a piece of white water-permeable, polypropylene nonwoven agrotextile (BR Garden, Nottingham, UK), secured at the bottom with a rubber band to prevent vermiculite leakage. Before transplanting, 60 mL of water was added to each tube, followed by an additional 10 mL on the next day and again on the second day after planting. The tubes were placed in trays filled with either half-strength Hoagland solution (0.8 g L−1 water, Phyto Technology Laboratories, Shawnee Mission, KS, USA) (control) or the same Hoagland solution supplemented with 6% polyethylene glycol (PEG) 8000 (Acros Organics, Geel, Belgium) to simulate drought conditions. This concentration of PEG (0.06 g g−1 water) was based on a preliminary experiment that showed a significant reduction in plant growth compared to the control. The osmotic pressure of the PEG solution prepared in this way was −0.064 MPa (at 22 °C), calculated according to the formula of Michel []. For each tube, 300 mL of solution was added to the tray (determined sufficient in preliminary tests). Fine-grained vermiculite, as used here, has a significant moisture retention capacity, which is why the solution was not subsequently added to either the control or the drought treatment. Through capillary action, the solution reached the top of the tubes. Plants were grown in a growth chamber at 22 °C, 60% relative humidity, under white light (fluorescent tubes), with a 16 h day/8 h night photoperiod for 26 days (Figure 1).

Figure 1.
Plant growth in the growth chamber on the second day after transplanting (A) and at the end of the experiment (B).
2.2. Trait Measurements
Twenty-six days after transplanting, plants (including roots) were removed from the tubes, and the roots were carefully separated from the vermiculite using a wire mesh. Taproot length (RL) and shoot length (SL) were measured on five plants of each hybrid per replicate, and the five plant mean was used as a raw data point for statistical analysis. Roots were thoroughly rinsed under tap water to remove all vermiculite and dried with paper towels. Root fresh weight (RFW) and shoot fresh weight (SFW) were measured on pooled samples of five plants per replicate. The shoot and root samples were then placed in paper bags and dried in an oven for 48 h at 70 °C, after which root dry weight and shoot dry weight were determined. Root dry matter (RDM) and shoot dry matter (SDM) contents were calculated from the fresh and dry weights of root and shoot, and expressed as a percentage of RFW and SFW, respectively. The two derived traits, root-to-shoot length ratio (RL/SL) and root-to-shoot fresh weight ratio (RFW/SFW), were calculated from the measured traits.
2.3. Statistical Analysis
Before performing analysis of variance (ANOVA), the assumptions of normality and homogeneity of variances within treatments (control and drought) were checked in SAS version 9.4 [] using the Kolmogorov–Smirnov and Brown–Forsythe tests, respectively. All traits met the assumptions except SFW and RDM. For these two traits, the raw data were transformed using the square root and reciprocal transformation, respectively (Table S2). Transformed data of SFW and RDM and raw data of six other traits were also checked for assumptions of ANOVA across treatments.
Analysis of variance (ANOVA) across treatments (control and drought) and within treatments was performed using the GLM procedure of SAS version 9.4 [], with treatment (T) as fixed and genotype (G) and G × T as random effects. Variance components for the traits were determined by equating the observed mean squares from the within-treatment ANOVA to their expectations and resolving for the desired variance components. Broad-sense heritability (h2) by treatment was calculated using the formula h2 = σ2G/(σ2G + σ2ε/r), where σ2G and σ2ε are the genotypic and error variances, respectively, and r is the number of replicates []. The statistical package Meta-R version 6.00 [] was used to calculate genotype BLUP values for the studied traits under both control and drought treatments. For SFW and RDM, calculated BLUPs were back-transformed to actual values.
The BLUPs were then used to calculate Pearson correlation coefficients between traits within each treatment using PROC CORR from the SAS version 9.4 []. Threshold levels of significance for correlation coefficients were adjusted for multiple comparisons by a false discovery rate test according to the Benjamini–Hochberg procedure (BH-FDR) using PROC MULTTEST from SAS version 9.4 []. Correlogram figures between the pairs of traits under both experimental conditions were obtained by using the corrplot() function of the package corrplot version 0.95 [] in R version 4.4.2 [], following the procedure described by Soetewey [].
3. Results
3.1. Analysis of Variance
Analysis of variance across water treatments (control and drought) revealed a significant effect of genotype (G) for all studied traits (Table 1) except for root length (RL). Similarly, the effect of water treatment (T) was significant for all traits except the root-to-shoot length ratio (RL/SL). The G × T interaction was significant for all traits except root dry matter content (RDM).

Table 1.
Combined analysis of variance (ANOVA) for eight seedling traits across 32 maize hybrids and two water treatments (control and drought).
ANOVA by treatment (Table 2) showed a significant effect of genotype for all traits under both control and drought conditions. Broad-sense heritability estimates were similar under both treatments, except for RL and the RFW/SFW ratio, for which heritability was much higher under drought than under control conditions (0.83 vs. 0.62 and 0.85 vs. 0.59, respectively) and RDM, for which higher heritability was observed under control conditions (0.61 vs. 0.41, respectively). Under the control treatment, heritability ranged from 0.59 (RFW/SFW) to 0.90 (SFW). Under drought conditions, heritability ranged from 0.41 (RDM) to 0.85 (RFW/SFW). Higher heritability estimates were generally observed for shoot traits than for root traits.

Table 2.
Analysis of variance (ANOVA) by treatment for eight seedling traits of 32 maize hybrids.
3.2. Effect of Drought on Trait Means
Means of eight seedling traits varied widely among the 32 maize genotypes under both control and drought conditions (Table 3 and Table S3).

Table 3.
Descriptive statistics for eight seedling traits in 32 maize genotypes under control and drought conditions.
Under drought conditions RL and SL were reduced by 33% and 34%, respectively, compared to the control, with a wide range of reduction among the genotypes (from 12% to 56% for RL and from 23% to 45% for SL). The mean reductions in plant fresh weight were even higher, reaching 51% for RFW and 66% for SFW. Variation in reduction among the genotypes for these two traits was also wide, ranging from 34% to 61% for RFW and from 49% to 74% for SFW. A contrasting genotypic response to drought was observed for the RL/SL ratio, with the relative change in individual genotypes ranging from −28% to +34%, resulting in no difference in means between the control and drought treatment for this trait. Mean RDM content increased under drought conditions by 117%, and all genotypes showed a consistent and substantial increase in RDM content under drought conditions, ranging from 97% to 136%. For SDM, genotypes also showed a consistent and strong increase in values under drought conditions, resulting in large differences in trait means between the control and drought conditions (Table 3). CV was lowest for RL in control (3.2%) and for RDM in drought (4.3%). The highest CV under control conditions was observed for SFW (23.3), and under drought conditions for RL (19.7%). For RL, SDM, RL/SL, and RFW/SFW, higher CVs were observed under drought conditions, while for SFW higher CV was observed under control conditions. For the remaining three traits (SL, RFW, and RDM), CVs under control and drought conditions were of similar magnitude.
Means (BLUPs) of eight traits in 32 maize genotypes under control and drought conditions are shown in Figure 2 and Figure 3 and in Table S3.
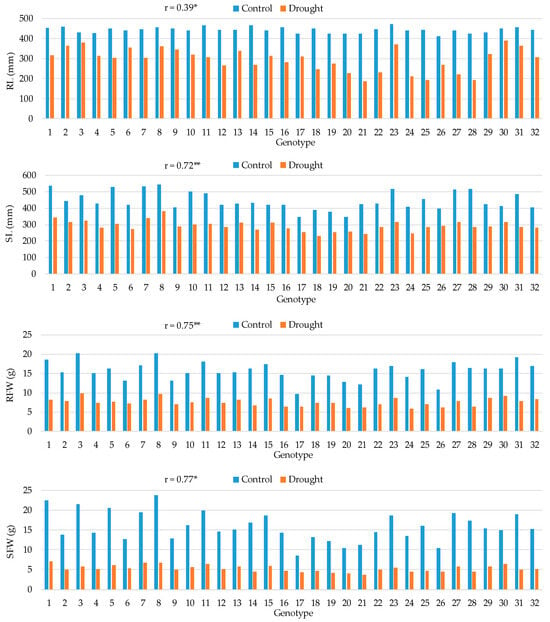
Figure 2.
Means for root length (RL), shoot length (SL), root fresh weight (RFW), and shoot fresh weight (SFW) in 32 maize hybrids in control and drought conditions and the Pearson correlation coefficients (r) between the two treatments. *, ** Correlation coefficient significant at the 0.05 and 0.01 probability levels, respectively.
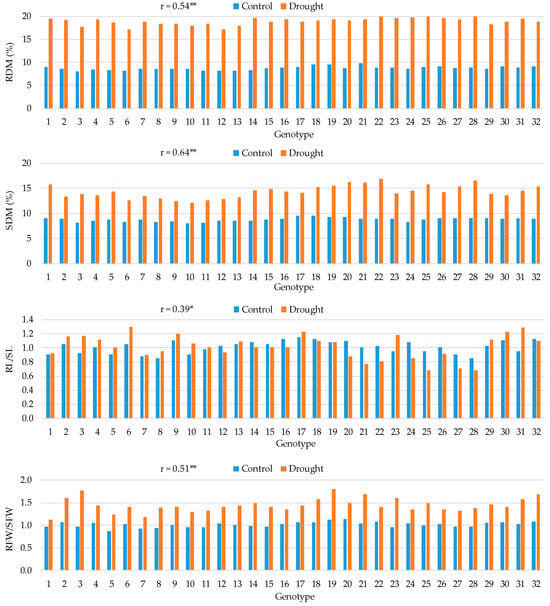
Figure 3.
Means for root dry matter (RDM), shoot dry matter (SDM), root-to-shoot length ratio (RL/SL), and root-to-shoot fresh weight ratio (RFW/SFW) in 32 maize hybrids in control and drought conditions and the Pearson correlation coefficients (r) between the two treatments. *, ** Correlation coefficient significant at the 0.05 and 0.01 probability levels, respectively.
The three hybrids with the longest root under control conditions were 23, 11, and 14, while under drought conditions the hybrids with the longest root were 30, 3, and 23 (Figure 2). All hybrids showed a reduction in root length under drought conditions, with the smallest reductions observed in hybrids 3, 30, and 6 (12%, 14%, and 19%, respectively). The correlation between the two treatments for root length (RL) was weak-to-moderate and positive (0.39 *). A strong and positive correlation between the two treatments was observed for shoot length (SL), 0.72 **. All hybrids exhibited a reduction in SL under drought conditions, ranging from 23% to 45%. Three hybrids, 8, 1, and 7, had the longest shoots under both control and drought conditions, while the hybrid with the smallest reduction in SL between the two treatments was hybrid 30. Hybrids 1, 8, and 31 had the highest root fresh weight (RFW) under control conditions, and hybrids 8, 3, 30, and 23 had the highest RFW under drought conditions. Hybrids 17, 26, and 30 were among the hybrids with the smallest reduction in RFW between the two conditions (34%, 43%, and 44%, respectively). A decrease in shoot fresh weight (SFW) under drought conditions, ranging from 49% to 74%, was observed for all studied hybrids. Hybrids 1 and 8 had the highest SFW under both control and drought conditions but hybrids 17, 30, and 26 were again the three hybrids with the smallest change between the two conditions. A strong and positive correlation was found between the two treatments for SFW (0.77 *).
A substantial increase in RDM and SDM contents was observed for all hybrids under drought conditions, with a greater increase in DM content in roots (ranging from 97% to 136%) than in shoots (ranging from 48% to 89%) (Figure 3). Hybrids 28 and 22 were among those with the highest RDM (20.3% and 20.2%, respectively) and SDM (16.8% and 16.5%, respectively) contents under drought conditions, as well as among those with the greatest relative increase in RDM and SDM contents between the control and drought conditions. The correlation between the two treatments was moderate and positive (0.54 **) for RDM and strong and positive (0.64 **) for SDM. The correlation between the two treatments for RL/SL was weak and positive (0.39 *), and for this trait, hybrids showed contrasting responses: some hybrids reduced while others increased the value of this ratio under drought conditions. The hybrids showing the highest increase in RL/SL under drought conditions were 31, 3, and 23 (34%, 26%, and 24%, respectively). A moderate and positive correlation between the two treatments was observed for the RFW/SFW (0.51 **), and an increase in this ratio under drought conditions, ranging from 16% to 83%, was observed for all studied hybrids. The two hybrids with the highest relative increase in this ratio under drought conditions were 3 (83%) and 23 (69%).
3.3. Correlation Between Traits
The correlations between the pairs of traits under control and drought conditions are presented in Figure 4. The direction of correlation coefficients between the pairs of traits detected simultaneously under control and drought conditions was similar, with the exception of correlation coefficients between the RFW and SFW, and between SFW and RFW/SFW, which were of higher magnitude under the control conditions (0.92 vs. 0.80 and −0.76 vs. −0.52, respectively).
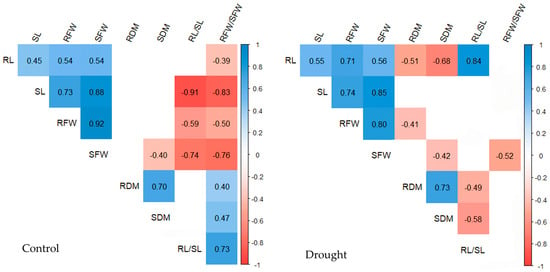
Figure 4.
Correlation coefficients between maize seedling traits in 32 maize genotypes under control and drought conditions. Traits: root length (RL), shoot length (SL), root fresh weight (RFW), shoot fresh weight (SFW), root dry matter (RDM), shoot dry matter (SDM), root-to-shoot length ratio (RL/SL), and root-to-shoot fresh weight ratio (RFW/SFW).
The majority of correlation coefficients detected only under control conditions were of negative direction, ranging from −0.40, as found between SFW and SDM, to −0.91, as found between SL and RL/SL, but the three correlation coefficients between the RFW/SFW and RDM, SDM, and RL/SL were positive and medium or strong (0.40, 0.47, and 0.73, respectively). The correlation coefficients detected between the pairs of traits exclusively under drought conditions were all negative and ranged from −0.41 (between RFW and RDM) to −0.68 (between RL and SDM), except for the one detected between RL and RL/SL, which was positive and very strong (0.84).
4. Discussion
In the present study, polyethylene glycol 8000 was used for assessing maize drought stress resistance at the seedling stage. One of the problems encountered when using PEG solutions in studies simulating the effect of drought on plant growth is the occurrence of hypoxia because the high viscosity of PEG solutions limits the movement of O2, thus increasing the likelihood of O2 deficiency in the roots []. To avoid hypoxia, vermiculite was used as the growth medium in this study because it has large air spaces that allow free movement of O2 to the root surface []. Consequently, according to Verslues et al. [], vermiculite-grown roots appear to be optimal for growth and metabolism. In our preliminary experiment, PEG 8000 at a concentration of 0.06 g g−1 water (6%) showed a clear slowdown in plant growth compared to the control. Ul Islam et al. [] used an even lower concentration of PEG, 5% PEG 6000, as one of the treatments, and achieved a significant reduction in primary root length.
The effect of PEG-induced drought on seedling traits was examined in 32 commercial maize hybrids. The effect of genotype (G) was significant for all traits except for RL, while the effect of water treatment (T) was significant for all traits except for the root-to-shoot length ratio (RL/SL). The G × T interaction was significant for all traits except RDM. A significant effect of genotype as well as water treatment has been previously reported for maize seedling traits in various germplasm, including inbreds [,,], hybrids [,,,], and local varieties/landraces [,,,,]. The significant G × T interaction for all traits except RDM indicates that hybrids showed distinct responses when exposed to drought stress and may reflect the existing genetic variability for drought tolerance in the studied germplasm. The response of the studied hybrids to simulated drought stress was typical, resulting in decreased values of the four directly measured traits: RL, SL, RFW, and SFW. According to Álvarez-Iglesias et al. [], mild and moderate stress conditions typically reduce shoot growth more than root growth in maize seedlings and the functional equilibrium between root and shoot in seedling growth under stress conditions is of great importance for achieving high yields [,]. Maintenance of main root growth is an adaptive mechanism for optimizing soil water uptake [], enabling roots to penetrate deeper into the soil and increasing the likelihood of finding water sources. In the set of maize hybrids studied here, root and shoot lengths were affected to a similar extent, with hybrid 30 standing out for maintaining both root and shoot growth under drought conditions. As mentioned earlier, a typical response under drought conditions is an increase in the root-to-shoot length ratio [], which, according to Queiroz et al. [], indicates that shoot development was indeed more affected by drought than root development. The hybrids which in the present study expressed an increase in RL/SL (hybrids 31, 3, and 23) under drought conditions were, together with hybrid 30, among those with the longest RL and the smallest relative RL reduction under drought. The similar magnitude of RL and SL reduction in this study might be explained by the relatively mild drought stress applied (−0.064 MPa), which may also account for the non-significant difference observed between the two conditions for the root-to-shoot length ratio. Nevertheless, it was expected that the osmotic potential in the substrate (despite the fact that vermiculite has a high moisture retention capacity) gradually decreases with time. This decrease is consistent with the gradual decrease in osmotic potential in field conditions during the dry period, starting from the surface towards the deeper soil layers. In the present study, a milder effect of drought was observed for RFW than for SFW (51% vs. 66% decrease under drought stress, respectively), resulting in an increased RFW/SFW. According to Álvarez-Iglesias et al. [], under drought conditions, initial growth of both main and secondary roots is necessary, as they increase the surface area for water uptake and could ensure subsequent water supply to the main root before water deficit becomes severe. In this regard, hybrid 30 ranked second in terms of the smallest relative reduction in SFW and third in terms of the smallest relative reduction in RFW under drought conditions.
Under drought stress, all hybrids showed a strong increase in RDM and SDM content, with average increases of 117% and 63%, respectively. The very high average increase in RDM under drought conditions, which ranged from 97% to 136%, implies a similar reaction of the studied hybrids to drought stress for this trait and may explain the non-significant G × T interaction observed for this trait. The increase in DM in roots and shoots may indicate that another potentially valuable stress tolerance mechanism has been activated in the studied germplasm: the adjustment of osmotic potential achieved by active accumulation of solutes in cells [,]. The accumulation of solutes, like proline and trehalose, has been well documented in roots and shoots of maize seedlings under dry conditions [,,,,]. This accumulation enables the retention of water during episodes of low external water potential, limiting turgor loss and damage from cell shrinkage. Under more prolonged or severe moisture deficit, these solutes are also implicated in the stabilization of various macromolecular structures []. The accumulation of osmolytes under drought conditions is usually more pronounced in roots than in shoots, suggesting that osmotic adjustment is an adaptation not only for surviving stress, but also for growth under such conditions []. The more pronounced accumulation of RDM than SDM, as well as the milder effect of drought on RFW than SFW, observed in our study, is aligned with this.
The calculated trait heritability values under control and drought conditions were of similar magnitude and generally high. In Badr et al. [], the broad-sense trait heritability coefficients calculated under control and 10% PEG drought stress treatment, which is comparable to the PEG treatment applied in the present study, were also of similar magnitude. High broad-sense heritability coefficients for seedling traits such as germination percentage, root and shoot length, and root fresh and dry weight were also found in the study of Mustamu et al. [], who investigated PEG-induced drought stress at the early seedling stage of local Indonesian maize varieties, as well as in the study of Khan et al. [], who investigated drought tolerance of 40 Pakistani maize inbreds at seedling and maturity stages. The results from these studies, as well as from the present study, indicate that the selection of maize-tolerant genotypes may be based on the high h2 seedling traits such as shoot length and shoot and root fresh and dry weight.
In the present study, strong positive correlations between control and drought conditions were found for SL, RFW, and SFW (0.72 **, 0.75 **, and 0.77 **, respectively). These traits also exhibited high heritabilities under both conditions. Between the same three directly measured traits, including RL, and between RDM and SDM, positive and moderate-to-strong correlations were observed under both control and drought conditions (Figure 4). Adhikari et al. [] reported similar correlations between RL and RFW and SFW under both control and drought conditions, while Badr et al. [] found correlations between the same traits under control and 10% PEG treatment to be generally weaker, but slightly stronger under drought stress conditions. Conversely, Khan et al. [] observed a higher number of strong and significant correlations among RL, SL, RFW, and SFW under control than under drought conditions. In the present study, correlations between directly measured and derived traits, as well as among derived traits, showed contrasting patterns and were specific to the designated water stress treatment. Therefore, using four easily and directly measured traits, namely, RL, SL, RFW, and SFW, for assessing drought tolerance at the maize seedling stage appears appropriate.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy15112624/s1, Table S1: Code, FAO group and breeders of 32 maize single-cross hybrids used as experimental material; Table S2: Kolmogorov–Smirnov test for normality and Brown–Forsythe test for homogeneity of variances for eight maize seedling traits; Table S3: Hybrid means (BLUPs) root length (RL), shoot length (SL), root fresh weight (RFW), shoot fresh weight (SFW), root dry matter (RDM), shoot dry matter (SDM), root-to-shoot length ratio (RL/SL), and root fresh weight-to-shoot fresh weight ratio (RFW/SFW) values under control and drought conditions.
Author Contributions
Conceptualization, I.P. and S.K.; methodology, S.K. and H.Š.; software, M.B. and H.Š.; validation, I.P. and H.Š.; formal analysis, S.K., M.B., and H.Š.; investigation, S.K. and A.L.; resources, I.P.; data curation, S.K. and H.Š.; writing—original draft preparation, M.B. and H.Š.; writing—review and editing, I.P., S.K., and A.L.; visualization, S.K., M.B., and H.Š.; supervision, I.P. and H.Š.; project administration, I.P.; funding acquisition, I.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Environmental Protection and Energy Efficiency Fund with the support of the Croatian Science Foundation of the Republic of Croatia (project AGRODROUGHT-ADAPT, PKP-2016-06-8290) and by the project PK.1.1.10.0008 (Research and Development of Plant Genetic Resources for Sustainable Agriculture), Centre of Excellence for Biodiversity and Molecular Plant Breeding (CoE CroP-Bio-Div), Zagreb, Croatia.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
Conflicts of Interest
Author Ana Lovrić was employed by the company Bc Institute for Breeding and Production of Field Crops. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Deribe, H. Review on Effects of Drought Stress on Maize Growth, Yield and Its Management Strategies. Commun. Soil Sci. Plant Anal. 2025, 56, 123–143. [Google Scholar] [CrossRef]
- Yin, X.; Olesen, J.E.; Wang, M.; Kersebaum, K.-C.; Chen, H.; Baby, S.; Öztürk, I.; Chen, F. Adapting maize production to drought in the northeast farming region of China. Eur. J. Agron. 2016, 77, 47–58. [Google Scholar] [CrossRef]
- FAOSTAT. Food and Agriculture Organization of the United Nations. FAOSTAT Statistical Database. Available online: https://www.fao.org/faostat/en/#data (accessed on 15 September 2025).
- Maazou, A.R.S.; Tu, J.L.; Qiu, J.; Liu, Z.Z. Breeding for Drought Tolerance in Maize (Zea mays L.). Am. J. Plant Sci. 2016, 7, 1858–1870. [Google Scholar] [CrossRef]
- Rasheed, A.; Jie, H.; Ali, B.; He, P.; Zhao, L.; Ma, Y.; Xing, H.; Qari, S.H.; Hassan, M.U.; Hamid, M.R.; et al. Breeding Drought-Tolerant Maize (Zea mays) Using Molecular Breeding Tools: Recent Advancements and Future Prospective. Agronomy 2023, 13, 1459. [Google Scholar] [CrossRef]
- Critchley, W.; Siegert, K. Water Harvesting: A Manual for the Design and Construction of Water Harvesting Schemes for Plant Production; FAO: Rome, Italy, 1991. [Google Scholar]
- Sah, R.P.; Chakraborty, M.; Prasad, K.; Pandit, M.; Tudu, V.K.; Chakravarty, M.K.; Narayan, S.C.; Rana, M.; Moharana, D. Impact of water deficit stress in maize: Phenology and yield components. Sci. Rep. 2022, 10, 2944. [Google Scholar] [CrossRef]
- Akinwale, R.O.; Awosanmi, F.E.; Ogunniyi, O.; Fadoju, A.O. Determinants of drought tolerance at seedling stage in early and extra-early maize hybrids. Maydica 2017, 62, M4. [Google Scholar]
- Bruce, W.B.; Edmeades, G.O.; Barker, T.C. Molecular and physiological approaches to maize improvement for drought resistance. J. Exp. Bot. 2002, 53, 13–25. [Google Scholar] [CrossRef]
- Zyomo, C.; Bernardo, R. Drought Tolerance in Maize: Indirect Selection through Secondary Traits versus Genomewide Selection. Crop Sci. 2012, 52, 1269–1275. [Google Scholar] [CrossRef]
- Álvarez-Iglesias, L.; de la Roza-Delgado, B.; Reigosa, M.J.; Revilla, P.; Pedrol, N. A simple, fast and accurate screening method to estimate maize (Zea mays L.) tolerance to drought at early stages. Maydica 2017, 62, M34. [Google Scholar]
- Ribaut, J.M.; Betran, J.; Monneveux, P.; Setter, T. Drought Tolerance in Maize. In Handbook of Maize: Its Biology; Bennetzen, J., Hake, S., Eds.; Springer: New York, NY, USA, 2009; pp. 311–344. [Google Scholar] [CrossRef]
- Bolaños, J.; Edmeades, G.O. Eight cycles of selection for drought tolerance in lowland tropical maize. I. responses in grain yield, biomass and radiation utilization. Field Crops Res. 1993, 31, 233–252. [Google Scholar] [CrossRef]
- Edmeades, G.O.; Bolaños, J.; Chapman, S.C.; Lafitte, H.R.; Bänziger, M. Selection improves drought tolerance in tropical maize populations. I. Gains in biomass, grain yield and harvest index. Crop Sci. 1999, 39, 1306–1315. [Google Scholar] [CrossRef]
- Khan, N.H.; Ahsan, M.; Naveed, M.; Sadaqat, H.A.; Javed, I. Genetics of drought tolerance at seedling and maturity stages in Zea mays L. Span. J. Agric. Res. 2016, 14, e0705. [Google Scholar] [CrossRef]
- Aslam, M.; Maqbool, M.A.; Cengiz, R. Drought Stress in Maize (Zea mays L.). Effects, Resistance Mechanisms, Global Achievements and Biological Strategies for Improvement; Springer Briefs in Agriculture; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar] [CrossRef]
- Denmead, O.T.; Shaw, R.H. The effects of soil moisture stress at different stages of growth on the development and yield of corn. Agron. J. 1960, 52, 272–274. [Google Scholar] [CrossRef]
- Edmeades, G.O.; Lafitte, H.R.; Bolaños, J.; Chapman, S.C.; Bänziger, M.; Deutsch, J.A. Developing maize that tolerates drought or low nitrogen conditions. In Stress Tolerance Breeding: Maize that Resists Insects, Drought, Low Nitrogen, and Acid Soils; Edmeades, G.O., Deutsch, J.A., Eds.; CIMMYT: El Batán, Mexico, 1994; pp. 21–84. [Google Scholar]
- Duvick, D.N. What is yield. In Developing Drought and Low-N Tolerant Maize; Edmeades, G.O., Bänziger, M., Mickelson, H.R., Peña-Valdivia, C.B., Eds.; CIMMYT: El Batan, Mexico, 1997; pp. 332–335. [Google Scholar]
- Duvick, D.N.; Cassman, K.G. Post-green revolution trends in yield potential of temperate maize in the North-Central United States. Crop Sci. 1999, 39, 1622–1630. [Google Scholar] [CrossRef]
- Adewale, S.A.; Akinwale, R.O.; Fakorede, M.A.B.; Badu-Apraku, B. Genetic analysis of drought-adaptive traits at seedling stage in early-maturing maize inbred lines and field performance under stress conditions. Euphytica 2018, 214, 145. [Google Scholar] [CrossRef]
- Meeks, M.; Murray, S.C.; Hague, S.; Hays, D. Measuring Maize Seedling Drought Response in Search of Tolerant Germplasm. Agronomy 2013, 3, 135–147. [Google Scholar] [CrossRef]
- Djemel, A.; Álvarez-Iglesias, L.; Pedrol, N.; López-Malvar, A.; Ordás, A.; Revila, P. Identification of drought tolerant populations at multi-stage growth phases in temperate maize germplasm. Euphytica 2018, 214, 138. [Google Scholar] [CrossRef]
- Mustamu, N.E.; Tampubolon, K.; Alridiwirsah, A.; Basyuni, M. Preliminary Identification of Local Maize Under Drought Stress By PEG-6000. BIO Web Conf. 2023, 69, 01018. [Google Scholar] [CrossRef]
- Mustamu, N.E.; Tampubolon, K.; Alridiwirsah, A.; Basyuni, M.; AL-Taey, D.K.A.; Janabi, H.J.K.; Mehdizadeh, M. Drought stress induced by polyethylene glycol (PEG) in local maize at the early seedling stage. Heliyon 2023, 9, e20209. [Google Scholar] [CrossRef]
- Badr, A.; El-Shazly, H.H.; Tarawneh, R.A.; Börner, A. Screening for Drought Tolerance in Maize (Zea mays L.) Germplasm Using Germination and Seedling Traits under Simulated Drought Conditions. Plants 2020, 9, 565. [Google Scholar] [CrossRef]
- Magar, M.M.; Atit Parajuli, A.; Sah, B.P.; Shrestha, J.; Sakha, B.M.; Koirala, K.B.; Dhital, S.P. Effect of PEG Induced Drought Stress on Germination and Seedling Traits of Maize (Zea mays L.) Lines. Turk. J. Agric. Nat. Sci. 2019, 6, 196–205. [Google Scholar] [CrossRef]
- Ul Islam, N.; Ali, G.; Dar, Z.A.; Maqbool, S.; Khulbe, R.K.; Bhat, A. Effect of PEG Induced Drought Stress on Maize (Zea mays L.) Inbreds. Plant Arch. 2019, 19, 1677–1681. [Google Scholar]
- Queiroz, M.; da Silva Oliveira, C.E.; Steiner, F.; Zuffo, A.M.; Zoz, T.; Vendruscolo, E.P.; Silva Mennes, V.; Mello, B.F.F.R.; Cabral, R.C.; Menis, F.T. Drought Stresses on Seed Germination and Early Growth of Maize and Sorghum. J. Agric. Sci. 2019, 11, 310. [Google Scholar] [CrossRef]
- Partheeban, C.; Chandrasekhar, C.N.; Jeyakumar, P.; Ravikesavan, R.; Gnanam, R. Effect of PEG Induced Drought Stress on Seed Germination and Seedling Characters of Maize (Zea mays L.) Genotypes. Int. J. Curr. Microbiol. App. Sci. 2017, 6, 1095–1104. [Google Scholar] [CrossRef]
- Khodarahmpour, Z. Effect of drought stress induced by polyethylene glycol (PEG) on germination indices in corn (Zea mays L.) hybrids. Afr. J. Biotechnol. 2011, 10, 18222–18227. [Google Scholar] [CrossRef]
- Khayatnezhad, M.; Gholamin, R.; Jamaati-e-Somarin, S.; Zabihi-e-Mahmoodabad, R. Effects of PEG Stress on Corn Cultivars (Zea mays L.) at Germination Stage. World Appl. Sci. J. 2010, 11, 504–506. [Google Scholar]
- Zhang, L.J.; Fan, J.J.; Ruan, Y.Y. Application of polyethylene glycol in the study of plant osmotic stress physiology. Plant Physiol. Commun. 2004, 40, 361–368. [Google Scholar]
- Guan, Z.; Wang, L.; Duan, L.; Zhou, Z.; Zhang, F.; Wang, Y. Effects of PEG simulated drought stress on seed germination of Abutilon theophrasti medicus. Seed 2022, 41, 66–70. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Zhao, W.; Hou, X.; Dong, S. Current views of drought research: Experimental methods, adaptation mechanisms and regulatory strategies. Front. Plant Sci. 2024, 15, 1371895. [Google Scholar] [CrossRef] [PubMed]
- Avramova, V.; Nagel, K.A.; AbdElgawad, H.; Bustos, D.; DuPlessis, M.; Fiorani, F.; Beemster, G.T.S. Screening for drought tolerance of maize hybrids by multi-scale analysis of root and shoot traits at the seedling stage. J. Exp. Bot. 2016, 67, 2453–2466. [Google Scholar] [CrossRef] [PubMed]
- Michel, B.E. Evaluation of the water potentials of solutions of polyethylene glycol 8000. Plant Physiol. 1983, 72, 66–70. [Google Scholar] [CrossRef] [PubMed]
- SAS Institute. Statistical Analysis Software (SAS) User’s Guide, Version 9.4; SAS Institute Inc.: Cary, NC, USA, 2016. [Google Scholar]
- Hallauer, A.R.; Carena, M.J.; Filho, J.B.M. Quantitative Genetics in Maize Breeding; Springer: New York, NY, USA, 2010. [Google Scholar]
- Alvarado, G.; López, M.; Vargas, M.; Pacheco, Á.; Rodríguez, F.; Burgueño, J.; Crossa, J. META-R (Multi Environment Trail Analysis with R for Windows), Version 6.00; CIMMYT Research Data & Software Repository Network: Texcoco, Mexico, 2016. [Google Scholar]
- Wei, T.; Simko, V. R Package ‘Corrplot’: Visualization of a Correlation Matrix (Version 0.95). 2024. Available online: https://github.com/taiyun/corrplot (accessed on 5 September 2025).
- R Core Team. R: A Language and Environment for Statistical Computing, Version 4.4.2; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.R-project.org/ (accessed on 5 September 2025).
- Soetewey, A. Correlation Coefficient and Correlation Test in R, Stats and R. Available online: https://statsandr.com/blog/correlation-coefficient-and-correlation-test-in-r/ (accessed on 16 September 2025).
- Verslues, P.E.; Ober, E.S.; Sharp, R.E. Root growth and oxygen relations at low water potentials. Impact of oxygen availability in polyethylene glycol solutions. Plant Physiol. 1998, 116, 1403–1412. [Google Scholar] [CrossRef] [PubMed]
- Findenegg, G.R. Effect of varied shoot/root ratio on growth of maize (Zea mays) under nitrogen-limited conditions: Growth experiment and model calculations. Plant Nutr. Physiol. Appl. 1990, 41, 21–27. [Google Scholar]
- Sharp, R.E.; Davies, W.J. Regulation of growth and development of plants growing with a restricted supply of water. In Plants under Stress. Biochemistry, Physiology and Ecology and Their Application to Plant Improvement; Jones, H.G., Flowers, T.J., Jones, M.B., Eds.; Cambridge University Press: Cambridge, UK, 1989. [Google Scholar]
- McCubbin, T.J.; Greeley, L.A.; Mertz, R.A.; Sidharth, S.; Griffith, A.E.; King-Miller, S.K.; Riggs, K.; Niehues, N.D.; Pareek, A.; Bryan, V.J.; et al. Maize nodal root growth maintenance during water deficit: Metabolic acclimation and the role of increased solute deposition in osmotic adjustment. Front. Plant Sci. 2025, 16, 1566453. [Google Scholar] [CrossRef]
- Velázquez-Márquez, S.; Conde-Martínez, V.; Trejo, C.; Delgado-Alvarado, A.; Carballo, A.; Suárez, R.; Mascorro, J.O.; Trujillo, A.R. Effects of water deficit on radicle apex elongation and solute accumulation in Zea mays L. Plant Physiol. Biochem. 2015, 96, 29–37. [Google Scholar] [CrossRef]
- Studer, C.; Hu, Y.; Schmidhalter, U. Evaluation of the differential osmotic adjustments between roots and leaves of maize seedlings with single or combined NPK-nutrient supply. Funct. Plant Biol. 2007, 34, 228–236. [Google Scholar] [CrossRef]
- Michelena, V.A.; Boyer, J.S. Complete turgor maintenance at low water potentials in the elongating region of maize leaves. Plant Physiol. 1982, 69, 1145–1149. [Google Scholar] [CrossRef]
- Sharp, R.E.; Davies, W.J. Solute regulation and growth by roots and shoots of water-stressed maize plants. Planta 1979, 147, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, B.; Sa, K.J.; Lee, J.K. Drought tolerance screening of maize inbred lines at an early growth stage. Plant Breed. Biotechnol. 2019, 7, 326–339. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).