Abstract
Excessive nitrogen addition in farmland on the Loess Plateau reduces soil quality and endangers the atmospheric environment. We designed an experiment to investigate the effects of different nitrogen application rates on the soil physicochemical properties and microbial diversity of spring wheat fields on the Loess Plateau, aiming to identify the optimal nitrogen application rate and avoid the detrimental effects of excessive nitrogen addition. A field experiment was conducted from 2022 to 2023 with four nitrogen (N) application rates (0, 55, 110, and 220 kg·N·ha−1·y−1). This study aimed to assess the changes in soil properties, nutrient contents, enzyme activities, and bacterial community structure. The results showed that increasing N application generally enhanced soil bulk density, nitrate nitrogen (NO3−-N), ammonium nitrogen (NH4+-N), and microbial biomass nitrogen (MBN) (p < 0.05). In contrast, soil water content initially increased and then decreased. Soil organic carbon and total nitrogen rose markedly with higher N inputs, particularly in the 0–20 cm layer, whereas total phosphorus was less affected. Nitrogen addition stimulated soil enzyme activities (protease, urease, nitrate reductase, and nitrite reductase), though excessive input (220 kg·N·ha−1·y−1) produced inhibitory effects. Actinobacteria (relative abundance: 29–35%) and Proteobacteria (relative abundance: 14–22%) were the dominant phyla in all treatments. Alpha diversity peaked at low nitrogen input (55 kg·N·ha−1·y−1), while high N level reduced evenness and species richness (p < 0.05). Principle Coordinate Analysis (PCoA) revealed that both N application and soil depth shaped microbial community assembly, with deeper layers (20–40 cm) being more sensitive to N input. Correlation analysis indicated that soil moisture, bulk density, and C:N:P stoichiometry were key drivers of bacterial community variation. Overall, moderate nitrogen input (110 kg·N·ha−1·y−1) improved soil fertility and supported microbial functionality, whereas excessive application degraded soil structure and reduced biodiversity. These findings highlight the need for balanced N management strategies in rain-fed agriculture of the Loess Plateau to sustain both productivity and ecological stability.
1. Introduction
Nitrogen is a primary limiting factor for productivity in most terrestrial ecosystems, making crop yield and quality heavily dependent on fertilization []. Since the first application of synthetic N fertilizers in the 19th century, advances—particularly the Haber–Bosch process—have facilitated large-scale production, driving sustained increases in global use throughout the 20th century [,]. Cereal crops alone account for 55% of global N demand. In China, where food demand is particularly high, excessive nitrogen fertilization is widely practiced to maximize grain yields []. However, over-application accelerates soil degradation and compaction, increases greenhouse gas emissions, and undermines long-term sustainability [,,]. Optimizing nitrogen use is therefore essential for maintaining soil health and ecological integrity [].
Nitrogen inputs strongly influence soil physicochemical properties, enzyme activities, and microbial communities [,]. Moderate N application enhances carbon (C) and nitrogen (N) sequestration, while excessive application increases bulk density, deteriorates soil structure, and suppresses sequestration efficiency []. Soil N content typically increases with higher fertilizer application, whereas phosphorus (P) often declines []. Although moderate N promotes microbial growth and residue accumulation, boosting soil enzyme activity, excessive N inhibits enzymatic function []. Importantly, long-term overfertilization diminishes bacterial abundance and diversity [] but has minimal impact on soil fungi []. Thus, soil properties, enzyme activity, and microbial communities serve as critical indicators of farmland quality, directly influencing crop yield and quality.
The Loess Plateau in north–central China is the world’s largest loess deposit, covering 640,000 km2 [,]. Its ecosystem is fragile, with Loessial soil as the most representative type. Loessial soils are highly erodible, with inherently low organic matter and nutrient content, including organic carbon (OC), total nitrogen (TN), and total phosphorus (TP) [,]. To compensate, local farmers frequently apply excessive N fertilizer, which alters soil pH and nutrient balance, reducing microbial diversity, impairing symbiotic functions, and increasing crop dependence on chemical inputs [,]. However, despite its agricultural importance and ecological vulnerability, most existing studies have not specifically addressed the response of the dry-farmed spring wheat systems on the Loess Plateau to urea application. Consequently, there is a critical lack of mechanistic understanding regarding how different N application levels precisely affect soil stoichiometry, enzyme activities, and microbial community structure in this unique and fragile agroecosystem.
In order to resolve these uncertainties, we conducted a 2-year field experiment in 2022 and 2023 with different N application levels in the Loess Plateau region of China, using dry-farmed spring wheat soil as the research object. We studied the changing rules of soil stoichiometric characteristics, enzyme activity, and microbial diversity of spring wheat soil under different N application levels and explored the main factors affecting changes in soil nutrients in dry-farmed spring wheat soil. The objectives of this experiment were as follows: (1) evaluate the effects of N application levels on soil stoichiometric characteristics of spring wheat and its interactions with enzyme activity; (2) analyze the changes in yield indicators of spring wheat under different N application levels; and (3) examine the changes in the diversity of soil microorganisms and microbial community structure under different N application levels. We hypothesized that (1) N application treatments would reduce the soil C:N and increase soil chemometric characteristics in the spring wheat ecosystems; and (2) N application treatments would increase the soil microbial diversity and relative abundance of dominant species, but excessive application will ultimately reduce diversity, with changes in soil microorganisms affecting changes in soil nutrients.
2. Materials and Methods
2.1. Experimental Site
This study was conducted in 2022 and 2023 in Anjiapo Village, Dingxi City, Gansu Province, China (35°64′ N, 104°64′ E), located within the hilly–gully region of the central Loess Plateau. The site has a temperate continental climate with an average elevation of 2000 m and pronounced diurnal temperature variation. The mean annual temperature is 7.2° C, with approximately 140 frost-free days. Precipitation is relatively low (377 mm) and concentrated between July and September, while annual evaporation reaches 1531 mm. As a semi-arid, rain-fed system, the region experiences frequent drought due to insufficient rainfall in spring and autumn, coupled with scarce groundwater resources. The soils in the study area are primarily classified as Huangmian soil, which is developed from loess-like parent material. The soil texture is characterized as uniform and fine-loamy. The monthly mean temperature and precipitation for both years are presented in Figure 1.
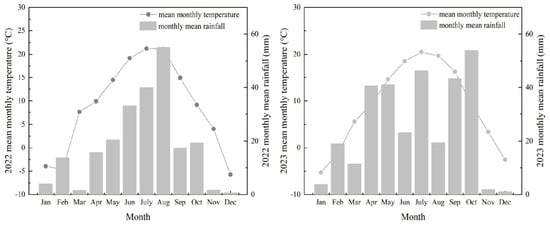
Figure 1.
Monthly mean temperature and precipitation in 2022 and 2023.
2.2. Experimental Design
The fixed experiment on different N application levels for spring wheat adopted a randomized block design, with no nitrogen fertilizer as the control group (CK). Three different N application levels were set according to the local fertilizer application amount, as follows: low N (LN, 55 kg·N·ha−1·y−1, accounting for 1/4 of the local nitrogen fertilizer application rate, N use efficiency is 13.22 kg·kg−1), medium N (MN, 110 kg·N·ha−1·y−1, accounting for 1/2 of the local nitrogen fertilizer application rate, N use efficiency is 13.91 kg·kg−1), and high N (HN, 220 kg·N·ha−1·y−1, local fertilizer application rate, N use efficiency is 9.43 kg·kg−1). The randomization process was implemented using a random number table method. Each treatment was repeated three times, for a total of 12 experimental plots (the area was 4 m × 6 m, with 25 cm row spacing for spring wheat planted in the plots). Plots were spaced 5 m apart. To avoid potential edge effects, a 0.5 m buffer zone was established for each plot. The local spring wheat ‘Ganchun 27’ was selected as the research target, sown in late March 2022 and 2023 (at a sowing rate of 187.5 kg·ha−1), harvested in early August. The remaining field management measures were the same as the local practice. The base fertilizer applied in the experiment was calcium superphosphate (containing 16% P) at a rate of 150 kg·N·hm−1·y−1. For the nitrogen treatments, the fertilizer was split into two equal applications, as follows: the first was applied basally at the sowing stage of spring wheat, and the second was topdressed during the tillering stage. To meet the water demand of the spring wheat during its growth period, all treatments were supplemented with 150 mm of irrigation at the jointing stage. The irrigation water was sourced from harvested natural rainfall stored in water cellars. It was delivered to the experimental plots via plastic flexible pipes, and the exact amount of irrigation water was measured using a water meter with a precision of 0.001 m3.
2.3. Sample Collection and Determination
Three replicates were performed for each experimental plot. Soil samples were collected from the experimental plots using a 5 cm-diameter soil auger following an S-shaped zigzag pattern during the 2022 and 2023 growing seasons. Sampling was conducted separately at three depth intervals: 0–10 cm, 10–20 cm, and 20–40 cm. For each depth, soil from multiple cores was combined to form a composite sample. These composite samples were then thoroughly homogenized, cleared of visible roots, stones, and other debris, and promptly transported to the laboratory under refrigeration (4 °C) for storage and subsequent analysis.
Two sampling schemes were adopted as follows: (1) Annual sampling for basic soil properties. Key indicators, including soil bulk density (core method) [], water content (gravimetric method with aluminum boxes) [], mineral nitrogen (NO3−-N and NH4+-N measured by KCl extraction and Continuous Flow Analysis, CFA) [], and microbial biomass nitrogen (chloroform fumigation–extraction) [], were measured once per year. (2) Growth stage-specific sampling for comprehensive soil characterization. Soil was collected at three critical stages (early, middle, and late) of wheat development. These samples were analyzed for total nitrogen (TN; semi-micro Kjeldahl method) [], total phosphorus (TP; molybdenum antimony anti-colorimetric method), soil organic carbon (SOC; dichromate oxidation), and the following four enzyme activities: protease (ninhydrin colorimetry) [], urease (phenol–sodium hypochlorite colorimetry) [], nitrate reductase and nitrite reductase (α-naphthylamine colorimetry and sulfanilic acid colorimetry, respectively) [,]. All other parameters were measured using soil samples collected from the three depth intervals of 0–10 cm, 10–20 cm, and 20–30 cm.
In addition, soil samples from the 0–10 cm layer were collected using the same method during the jointing stage of spring wheat in June 2023 and stored in a −80 °C freezer for the detection of soil microbial diversity. The specific measurement method was as follows: DNA was extracted from 0.25 g of soil samples using the DNA extraction kit (MoBio Laboratories, Carlsbad, CA, USA), and 1% agarose gel electrophoresis solution was used to detect DNA quality. PCR amplification of soil V3–V4 DNA genes was performed using 16S rRNA technology (primer 338F/806R) [], and the amplification products, stained using fluorescent agent Qubit3.0, were recovered for quantitative analysis. The sequencing work was performed using the NovaSeq 6000 system (Illumina, San Diego, CA, USA). The Illumina MiSeq sequencing platform was used to sequence and analyze the PCR amplification products. The sequencing work was contracted to Shanghai Paisenuo Biotechnology Co., Ltd. (Shanghai, China).
Observed Species (Observed OTUs): Counts the number of unique Operational Taxonomic Units (OTUs) in each sample and is the most intuitive metric for species richness. Chao1: A richness statistical estimator, based on the abundance of rare species (such as singletons and doubletons) in a sample, predicts the total number of species in a community and is particularly useful for compensating for rare species that may not have been sequenced. Shannon: The Shannon diversity index integrates both species richness and evenness; a higher index value indicates greater community diversity. Simpson: The Simpson diversity index measures dominance in a community, i.e., the probability that two individuals randomly selected belong to the same species. It is often converted to 1-D or -ln(D), where higher values indicate greater diversity. Pielou’s Evenness: Derived from the Shannon index, this metric specifically describes the uniformity of abundance distribution among different species in a community, ranging from 0 to 1, with values closer to 1 indicating a more even community.
Sobs in Formula (1) represents the Observed Species richness. In Formula (2) for the Chao1 index, F1 is the number of singletons (OTUs with only one read) and F2 is the number of doubletons (OTUs with exactly two reads). In Formula (3) for the Shannon index, pi is the proportion of the total sequences represented by OTUi. In Formula (4) for the Simpson index, pi is the proportion of the total sequences represented by OTU. In Formula (5), H’ is the Shannon index and ln(S) is the natural logarithm of the total number of species. In Formula (6), n1 is the number of singletons (OTUs with only one read) and N is the total number of reads in the sample [,].
2.4. Data Statistical Analysis
Data were analyzed using IBM SPSS Statistics 26.0. One-way analysis of variance (ANOVA) was applied to examine the two-year mean values of soil bulk density, water content, nitrate nitrogen (NO3−-N), ammonium nitrogen (NH4+-N), and microbial biomass nitrogen (MBN), with Duncan’s post hoc test used to assess significant differences (p < 0.05). One-way ANOVA was also performed to evaluate the effects of fertilization, growth stage, and soil depth on total nitrogen (TN), total phosphorus (TP), soil organic carbon (SOC), and enzyme activities, followed by Duncan’s test for multiple comparisons (p < 0.05). Principal Component Analysis (PCoA) was used to explore relationships between bacterial community composition (relative abundance) and soil physicochemical and nutrient properties. Pearson correlation analysis was additionally conducted in SPSS to quantify associations among soil properties, soil nutrient contents, and enzyme activities. The Redundancy Analysis (RDA) was performed and visualized using Canaco 5.0 software.
3. Results and Analysis
3.1. Effects of Nitrogen Levels on Basic Soil Properties
Across the nitrogen application gradient (0 to 220 kg·N·ha−1·y−1), Soil bulk density (BD), nitrate nitrogen (NO3−-N), ammonium nitrogen (NH4+-N), and microbial biomass nitrogen (MBN) all increased with higher nitrogen application rates, whereas soil water content (SWC) initially rose and then declined. Nitrogen addition significantly increased NO3−-N and NH4+-N contents in the entire 0–40 cm layer (p < 0.05), while SWC, BD, and MBN did not differ significantly (p > 0.05). Across the soil profile, BD and SWC increased with depth (0–10, 10–20, 20–30 cm), whereas NO3−-N, NH4+-N, and MBN declined significantly (p < 0.05, Table 1).

Table 1.
Soil basic properties at different soil depths under various fertilization treatments.
3.2. Effects of Nitrogen Application on Soil Chemical Stoichiometric Characteristics
Nitrogen application markedly influenced soil chemical properties (p < 0.05, Figure 2 and Figure 3). Across the nitrogen gradient (0 to 220 kg·N·ha−1·y−1), total nitrogen (TN), total phosphorus (TP), and soil organic carbon (SOC) all increased with higher N inputs. In the four fertilization treatments (0, 55, 110, and 220 kg·N·ha−1·y−1), TN, TP, and SOC decreased with soil depth, with significant interlayer differences (p < 0.05). In 2022, the HN treatment resulted in SOC increases of 1.1689, 0.9988, and 0.4736 in the 0–10 cm, 10–20 cm, and 20–40 cm soil layers, respectively, compared to CK. The corresponding TN increases under HN were 0.1159, 0.0466, and 0.0678 across these same layers. During 2023, the HN treatment led to SOC increments of 0.7398, 0.6264, and 0.0108 in the respective soil depths, while TN increases were 0.0644 and 0.0697 in the 0–10 cm and 10–20 cm layers, with no notable difference observed in the 20–40 cm layer. Furthermore, a consistent declining trend in TN, TP, and SOC was observed across the wheat growth stages, from the initial to mid and final growth phases.
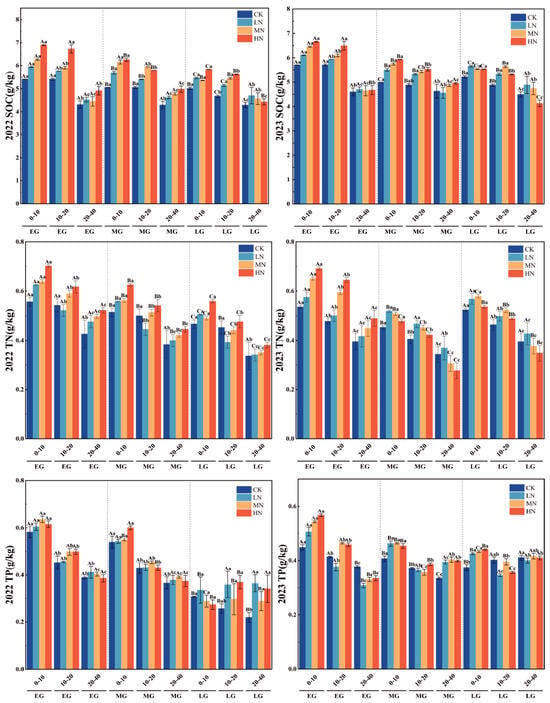
Figure 2.
Changes in SOC, TN, and TP across different soil layers at various growth stages of spring wheat under different nitrogen application treatments. Note: SOC: soil organic carbon; TN: total nitrogen; and TP: total phosphorus. Capital letters indicate significant differences in the indicators across different growth stages of wheat within the same soil layer depth, while lowercase letters indicate significant differences in the indicators across different soil layer depths at the same wheat growth stage.
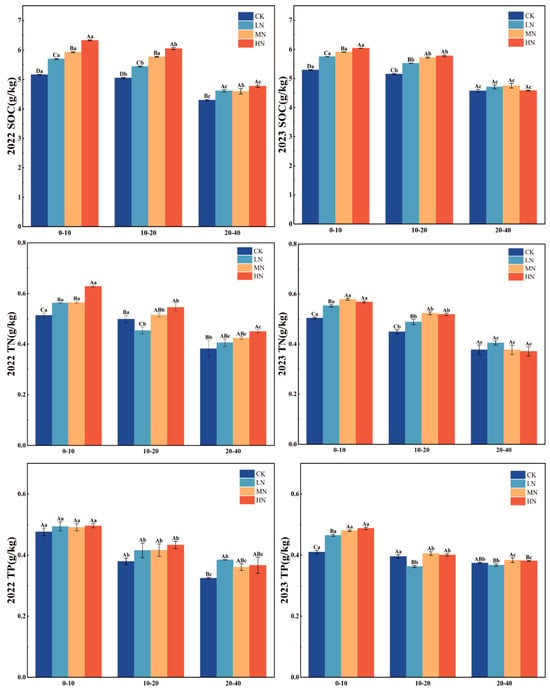
Figure 3.
Variations in SOC, TN, and TP across different soil layers under various nitrogen treatments. Note: capital letters indicate significant differences in the indicators among different fertilization treatments of wheat at the same soil depth, while lowercase letters denote significant differences across various soil depths under the same fertilization treatment. SOC: soil organic carbon; TN: total nitrogen; and TP: total phosphorus.
At the 0−10 cm depth, the C:N ratio under the moderate nitrogen (MN) treatment was significantly higher than under the control (CK) (p < 0.05, Figure 4). In contrast, no significant differences in the C:N ratio were observed among the four treatments at the 10−40 cm depth. The soil C:N ratio increased significantly with depth under both low (LN) and high (HN) nitrogen treatments (p < 0.05), whereas no significant variation was observed in the control (CK) and medium nitrogen (MN) treatments (p > 0.05). For the soil C:P ratio, no significant differences were observed among soil layers across all treatments, with the exception of under low nitrogen (LN) application (p > 0.05). Similarly, the N:P ratio was significantly affected by depth only under HN application (p < 0.05), showing no stratification under other conditions.
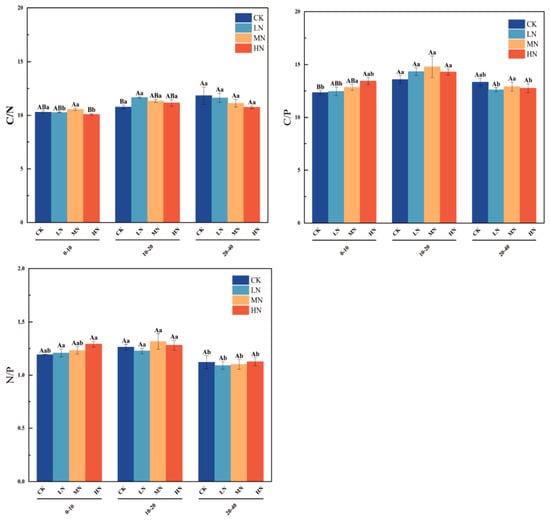
Figure 4.
Variations in C/N, C/P, and N/P ratios across different soil layers under various nitrogen treatments. Note: capital letters indicate significant differences in the indicators among different fertilization treatments of wheat at the same soil depth, while lowercase letters denote significant differences across various soil depths under the same fertilization treatment. Soil ecological stoich- ometry was represented by the following three key ratios: C/N, carbon-to-nitrogen ratio; C/P, ca- bon-to-phosphorus ratio; and N:P, nitrogen-to-phosphorus ratio.
3.3. Effects of Nitrogen Application Levels on the Soil Enzyme Activity
Activities of protease (PRO), urease (URE), nitrate reductase (NR), and nitrite reductase (NIR) increased significantly with higher nitrogen levels (p < 0.05), reaching their maximum under the high nitrogen (HN) treatment. Their activities were 1.3–1.7 times and 1.2–1.4 times that of the control (CK), respectively. In the 0–10 cm soil layer, the activities of nitrate reductase (NR) and nitrite reductase (NIR) under the HN treatment were also significantly higher than under CK, both being 1.1 times that of CK. However, in the 10–40 cm soil layer, the activities of nitrate reductase (NR) and nitrite reductase (NIR) showed no significant differences among the different nitrogen application treatments (p > 0.05). All four enzymes showed a pronounced decline with soil depth (p < 0.05). The activities of PRO, URE, and NR exhibited similar patterns, as follows: they increased significantly from the early to mid-growth stages, peaked at the mid-growth stage, and then declined during the late growth stage. In contrast, the activity of NIR showed a continuous and steady increase throughout the entire wheat development period (Figure 5 and Figure 6).
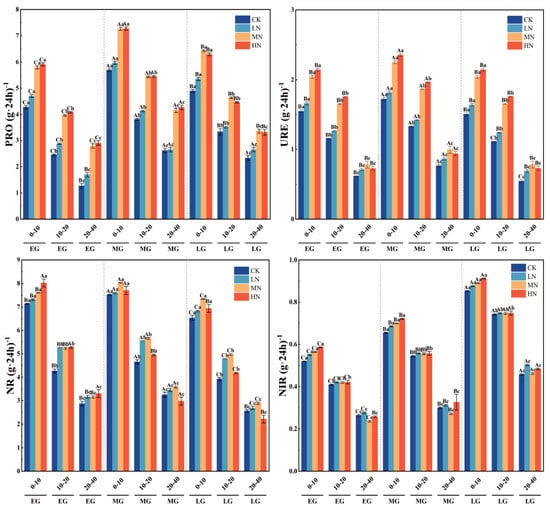
Figure 5.
Variations in soil enzyme activities across different soil layers during various growth stages of spring wheat. Note: capital letters indicate significant differences in the indicators across different growth stages of wheat within the same soil layer depth, while lowercase letters indicate significant differences in the indicators across different soil layer depths at the same wheat growth stage. PRO: soil protease; URE: urease; NR: nitrate reductase; and NIR: nitrite reductase.
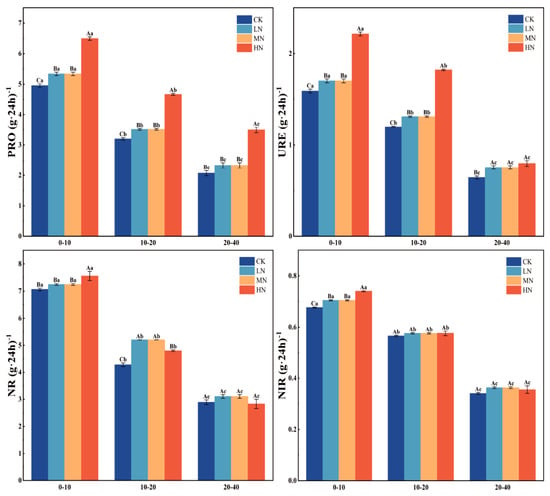
Figure 6.
Variations in soil enzyme activities across different soil layers under various nitrogen treatments. Note: capital letters indicate significant differences in the indicators among different fertil ization treatments of wheat at the same soil depth, while lowercase letters denote significant di −ferences across various soil depths under the same fertilization treatment. PRO: soil protease; URE: urease; NR: nitrate reductase; and NIR: nitrite reductase.
3.4. Effects of Nitrogen Application Levels on the Soil Microorganism
The dominant microbial groups were consistent across treatments and depths, with Actinobacteriota being the most abundant, followed by Proteobacteria, Acidobacteriota, and Gemmatimonadota. Significant differences were observed in the relative abundances of specific phyla, as follows: the relative abundance of Acidobacteriota under MN treatment was 2–5% higher than that under LN and HN conditions. Similarly, the abundance of Gemmatimonadota in LN treatment was 2–4% higher than that under MN, HN, and CK treatments (Figure 7).
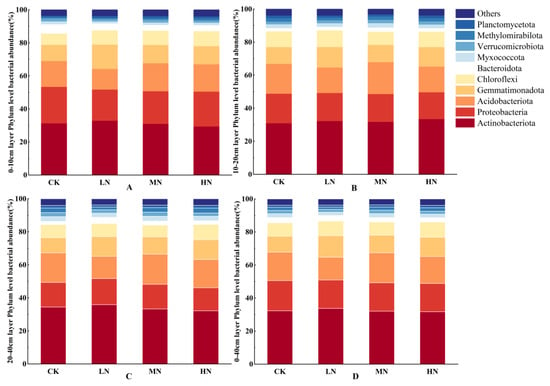
Figure 7.
Variations in relative abundance of bacterial phyla across different soil layers under various nitrogen treatments. Note: in the figure, panels (A–D) represent the relative abundances of bacterial phyla at depths of 0–10 cm, 10–20 cm, 20–40 cm, and 0–40 cm, respectively.
Alpha diversity analysis indicated that Good’s coverage of all treatments exceeded 0.99, demonstrating that the sequencing depth captured over 99% of the community diversity. Under the LN treatment, the values of Chao1, Faith’s PD, Shannon index, and Observed Species were significantly higher (p < 0.05) than those under the CK, MN, and HN treatments, with increases ranging from 1.08- to 1.19-fold, 1.28- to 1.85-fold, 1.07-fold, and 1.19- to 1.23-fold, respectively (Figure 8).
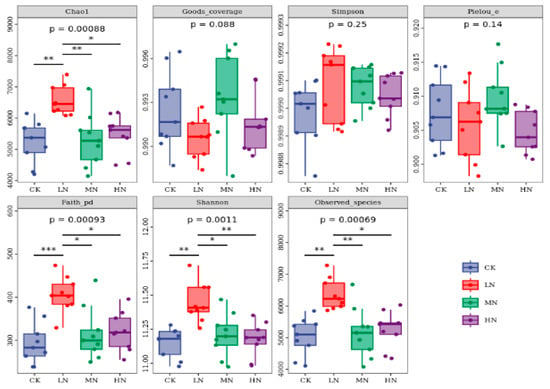
Figure 8.
Significance analysis of alpha diversity indices under different nitrogen treatments. Note: asterisks indicate significant differences between treatments: * p < 0.05, ** p < 0.01, *** p < 0.001. Observed Species: the number of unique operational taxonomic units (OTUs) or amplicon sequence variants (ASVs) observed in a sample, serving as a direct measure of species richness. Chao1 Index: an estimator of total species richness. Shannon Index: a composite metric that reflects community diversity by incorporating both species richness and evenness. Simpson Index: a measure of dominance, representing the probability that two randomly selected individuals belong to the same species. Faith’s Phylogenetic Diversity (Faith’s PD): a measure of phylogenetic diversity that quantifies. Pielou’s Evenness: s measure of species evenness that quantifies the uniformity of species abundance distributions in a community. Good’s Coverage: an index of sequencing depth that estimates the probability that a randomly selected sequence in a sample belongs to a species that has already been sequenced; values > 0.97 generally indicate sufficient sequencing depth to reliably characterize the community.
3.5. Relationship Analysis
Significant correlations were observed between soil physicochemical properties and enzyme activities. SWC was positively correlated with BD, negatively correlated with MBN, TN, TP, PRO, NR, and NIR (p < 0.05, Table 2), and showed no significant correlation with NO3−-N, NH4+-N, SOC, or URE.

Table 2.
Correlations between soil basic properties, nutrients, and soil enzyme activities.
The RDA model was statistically significant (p < 0.001). The first two RDA axes together accounted for [52.26]% of the constrained variance (RDA1: [38.46]%, RDA2: [13.8]%). Correlation analysis revealed distinct ecological preferences for key bacterial phyla. Actinobacteria exhibited significant positive correlations with the soil C:N ratio, bulk density (BD), and soil water content (SWC), but a significant negative correlation with the C:P ratio, which emerged as its strongest predictor. In contrast, Proteobacteria showed positive correlations with total phosphorus (TP), ammonium nitrogen (NH4+-N), microbial biomass nitrogen (MBN), total nitrogen (TN), soil organic carbon (SOC), and nitrate nitrogen (NO3−-N), while being negatively correlated with the C:N ratio, BD, and SWC. Among these, NH4+-N was the most influential factor. Furthermore, Acidobacteria demonstrated a strong positive correlation with the C:P ratio, whereas Gemmatimonadota and Bacteroidota were both significantly negatively correlated with it (Figure 9).
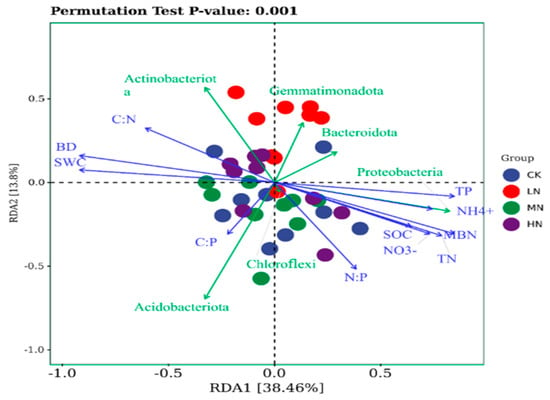
Figure 9.
Redundancy analysis (RDA) of soil basic properties and stoichiometric ratios with dominant bacterial phyla. Note: SWC: soil water content; BD: soil bulk density; NO3−-N: nitrate nitrogen; NH4+-N: ammonium nitrogen; C:N: carbon-to-nitrogen ratio; C:P: carbon-to-phosphorus ratio; and N:P: nitrogen-to-phosphorus ratio.
4. Discussion
In this study, soil bulk density (BD), nitrate nitrogen (NO3−-N), and ammonium nitrogen (NH4+-N) increased with rising nitrogen application rates under the four nitrogen application treatments (CK: 0 kg·N·ha−1·y−1, LN: 55 kg·N·ha−1·y−1, MN: 110 kg·N·ha−1·y−1, HN: 220 kg·N·ha−1·y−1), reaching their maximum values under the high nitrogen (HN) condition. Microbial biomass nitrogen (MBN) exhibited a similar trend in the 0–20 cm soil layer, also peaking under the HN treatment. However, no significant differences in MBN were observed among the four nitrogen treatments in the 20–40 cm soil layer. In contrast, soil water content (SWC) followed a different pattern: SWC was higher under the control (CK) and moderate nitrogen (MN) treatments compared to the other fertilization treatments, and SWC in the deeper soil layers was greater than that in the surface layers. Elevated N input accelerates soil acidification, which reduces porosity and consequently increases BD. Excessive application of nitrogen fertilizer can lead to soil acidification, which subsequently induces soil compaction and thereby increases soil bulk density. Studies have shown that applying 112 kg/ha of nitrogen fertilizer significantly acidifies farmland soils, while 168 kg/ha causes severe acidification. Long-term nitrogen fertilization also leads to such outcomes []. In contrast, reducing nitrogen application can ameliorate soil compaction and lower soil bulk density []. Under the moderate nitrogen (MN) condition, soil water content was higher than that under both low nitrogen (LN) and high nitrogen (HN) treatments. This phenomenon may be attributed to an “optimal point” of water-use efficiency being achieved under the MN treatment []. At this level, nitrogen application is sufficient to significantly promote plant growth, yet it is likely not to induce the extreme plant growth and water demand observed under the HN treatment, while also avoiding the potential negative impacts of high nitrogen on soil structure. Consequently, a relative balance between crop yield and water use is achieved, resulting in higher soil water content compared to both LN and HN treatments [].Urea-derived nitrogen is hydrolyzed by urease to NH4+-N and further oxidized to NO3−-N via nitrification, explaining the observed accumulation of both inorganic N forms. Moreover, additional nitrogen supports microbial metabolism, thereby promoting MBN accumulation under higher fertilization levels [,]. Both BD and SWC increased with depth, whereas NO3−-N, NH4+-N, and MBN declined. The increase in BD with depth reflects the depletion of organic matter in subsoils, leaching process, and overburden compaction. Subsoils also retained higher moisture due to reduced evaporation and root activity, as most root systems are concentrated in surface horizons [,]. Conversely, NO3−-N decreased with depth because nitrification is predominantly driven by strictly aerobic bacteria, which are less active in deeper, oxygen-limited layers. Similarly, the lower availability of urease enzymes in subsoils restricted NH4+-N accumulation [,]. MBN also declined with depth, consistent with the reduced organic matter supply that fuels microbial activity in deeper layers [].
In this study, nitrogen fertilization significantly increased soil organic carbon (SOC) and total nitrogen (TN) in both the first (2022) and second (2023) years, although the effects were less pronounced in the 20–40 cm soil layer. During the first year, nitrogen addition had little influence on soil phosphorus, with no significant differences among treatments. By the second year, however, nitrogen application enhanced total phosphorus (TP) in the 0–10 cm layer. These results align with previous reports that nitrogen fertilization markedly elevates SOC and TN while exerting only limited effects on TP. Nitrogen addition not only directly increased TN but also stimulated wheat growth, root exudation, and residue input while suppressing organic matter decomposition, thereby enhancing SOC accumulation. In contrast, nitrogen fertilizer does not directly alter soil phosphorus but exerts indirect effects through microbial processes, which remain limited in the short term []. Fertilization treatments most strongly influenced the soil C:N ratio, particularly in the surface layer. In contrast, effects on C:P and N:P ratios were minor and largely reflected depth-dependent variations influenced by nitrogen application rates. Under medium nitrogen (MN) conditions, microbial growth was enhanced, leading to greater immobilization of exogenous nitrogen and an increase in soil nitrogen. Meanwhile, organic carbon was consumed at a slower rate, collectively raising the C:N ratio. Because the soil phosphorus pool remained relatively stable and unresponsive to short-term nitrogen inputs, the C:P and N:P ratios showed little change [,].
Enzyme activity patterns further reflected these dynamics. PRO and URE activities increased with nitrogen application across all three layers but declined with depth under the same fertilization regime. In contrast, NR and NIR activities increased with nitrogen input only in the surface layer and declined with depth across all treatments. Since soil enzymes largely derive from microbial and root secretions, their activities are the highest in organic-rich topsoil and diminish with depth []. Soil microorganisms drive the synthesis of enzymes. The high enzyme activity in the surface soil (0–20 cm), particularly for nitrate reductase (NR) and nitrite reductase (NIR), is not merely a result of environmental conditions but is also driven by a rich and diverse microbial community []. Nitrogen addition increases substrate availability for PRO and URE, thereby enhancing their activities []. Elevated NR and NIR activities in surface soils likely reflect sufficient energy supply and micro-anaerobic conditions that favor their function []. Notably, compared with CK, MN, and HN, the LN treatment significantly enhanced microbial richness, phylogenetic diversity, evenness, and OTU number []. This suggests that moderate nitrogen inputs stimulate microbial diversity, whereas excessive nitrogen (MN and HN) suppresses it. The dominant bacterial phyla were consistent across different fertilization treatments, though their relative abundances varied. Specifically, the relative abundance of Acidobacteriota under the MN treatment was higher than that under both LN and HN conditions. Similarly, the abundance of the phylum Gemmatimonadota in the LN treatment exceeded that in the MN, HN, and CK treatments. The key role of moderate nitrogen application lies in its dual function: it provides essential substrates while sustaining high microbial diversity, thereby supporting comprehensive enzymatic functions. In contrast, high nitrogen application (HN) indirectly suppresses certain enzyme activities by disrupting the community structure [,].
Strong correlations emerged between soil physicochemical properties and enzyme activities, which in turn shaped the distribution of key bacterial phyla. SWC and BD were critical physical factors, both of which were negatively correlated with most nutrients and enzyme activities. SWC and BD were positively correlated with each other, but negatively correlated with MBN, TN, TP, and enzyme activities (PRO, NR, NIR). This pattern reflects physical constraints: high BD reduced porosity and oxygen diffusion, limiting aerobic microbial metabolism and enzymatic efficiency, particularly for nitrogen-cycle enzymes such as NR and NIR []. Concurrently, elevated SWC in compacted soils may result in anaerobic conditions, further suppressing microbial metabolism and nutrient mineralization. Thus, even when TN and TP are sufficient, nutrient bioavailability and transformation remain low under unfavorable physical conditions [].
Different bacterial groups showed distinct responses to soil stoichiometry and structure. Actinobacteriota were positively correlated with C:N and BD but negatively with C:P, consistent with their oligotrophic (K-strategist) nature and adaptability to nutrient-poor, stressful conditions []. High C:N signals nitrogen limitation, and high BD imposes physical stress—conditions under which Actinobacteriota thrive. Their negative correlation with C:P suggests sensitivity to phosphorus scarcity. In contrast, Proteobacteria, typical copiotrophs (r-strategists), correlated positively with nearly all nutrient indicators (TN, TP, SOC, NH4+-N, NO3−-N, MBN) but negatively with C:N, BD, and SWC, indicating preference for nutrient-rich soils with favorable structure []. Ammonium nitrogen emerged as the strongest determinant of their abundance, highlighting their key role in rapid nitrogen cycling. Acidobacteriota and Gemmatimonadota both responded strongly to C:P, but in opposite directions: Acidobacteriota correlated positively, consistent with their oligotrophic strategy and adaptation to low phosphorus availability [], while Gemmatimonadota correlated negatively, consistent with their ability to accumulate polyphosphates and competitive advantage under high phosphorus (low C:P) conditions [,,].
In summary, soil structure (SWC, BD) directly constrains nutrient bioavailability and enzymatic activity, while soil stoichiometry (C:N, C:P, N:P) regulates microbial succession. Together, these factors govern the assembly and function of soil bacterial communities under different nitrogen fertilization regimes.
5. Conclusions
This study demonstrated that nitrogen (N) fertilization significantly altered soil physicochemical properties, enzyme activities, and microbial community structure in spring wheat fields of the Loess Plateau, with effects varying by N rate and soil depth. Soil Properties: Compared to the control (CK), high N application (HN, 220 kg·N·ha−1·y−1) significantly increased soil bulk density (BD) by 0.01–0.03 g/cm3, nitrate nitrogen (NO3−-N) by 7.08–4.70 mg/kg, and ammonium nitrogen (NH4+-N) by 5.91–4.53 mg/kg across the 0–40 cm profile. Soil water content (SWC) was highest under moderate N (MN, 110 kg·N·ha−1·y−1), exceeding LN and HN by 0.63–1.09%. Soil Nutrients and Stoichiometry: Total nitrogen (TN) and soil organic carbon (SOC) increased with N input, particularly in the 0–10 cm layer under MN (TN: +12.4%; SOC: +9.8% vs. CK). The C:N ratio was significantly higher under MN at 0–10 cm (by 6.5%, p < 0.05), while C:P and N:P ratios remained stable across treatments. Enzyme Activities: Protease and urease activities under HN were 1.3–1.7 times higher than CK, while nitrate and nitrite reductase activities peaked under MN and declined under HN in subsoil layers, indicating inhibitory effects of excessive N. Microbial Diversity: Alpha diversity was highest under LN (Chao1: +19%; Shannon: +7% vs. CK), but declined under HN. Actinobacteria and Proteobacteria dominated across treatments, with Acidobacteriota abundance higher under MN (+3–5% vs. LN/HN). These findings hold the following implications for our initial hypotheses:
- (1)
- Nitrogen application increased the C:N ratio in the surface soil and altered soil stoichiometry;
- (2)
- Low to moderate nitrogen levels enhanced microbial diversity and enzyme activity, whereas excessive nitrogen led to a decline;
- (3)
- Changes in microbial community structure were closely associated with variations in soil nutrient availability and physical conditions.
In practice, moderate N application (110 kg·N·ha−1·y−1) is recommended to maintain soil fertility, microbial functionality, and sustainable productivity in the Loess Plateau spring wheat system.
Author Contributions
J.L.: Methodology and Writing—Original Draft Preparation. G.L.: Methodology, Supervision, Funding Acquisition, and Writing—Reviewing and Editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (NSFC) (32360438), the Top-notch Leading Talent Project in Gansu Province (GSBJLJ-2023-09).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
We appreciate and thank the anonymous reviewers for helpful comments that led to an overall improvement of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zhou, Z.; Wang, C.; Zheng, M.; Jiang, L.; Luo, Y. Patterns and mechanisms of responses by soil microbial communities to nitrogen addition. Soil. Biol. Biochem. 2017, 115, 433–441. [Google Scholar] [CrossRef]
- Hawkesford, M.J. Reducing the reliance on nitrogen fertilizer for wheat production. J. Cereal Sci. 2014, 59, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Frink, C.R.; Waggoner, P.E.; Ausubel, J.H. Nitrogen fertilizer: Retrospect and prospect. Proc. Natl. Acad. Sci. USA 1999, 96, 1175–1180. [Google Scholar] [CrossRef]
- Zhou, J.; Jiang, X.; Wei, D.; Zhao, B.; Ma, M.; Chen, S.; Cao, F.; Shen, D.; Guan, D.; Li, J. Consistent effects of nitrogen fertilization on soil bacterial communities in black soils for two crop seasons in China. Sci. Rep. 2017, 7, 3267. [Google Scholar] [CrossRef]
- Zhou, J.; Xia, F.; Liu, X.; He, Y.; Xu, J.; Brookes, P.C. Effects of nitrogen fertilizer on the acidification of two typical acid soils in South China. J. Soils Sediments 2014, 14, 415–422. [Google Scholar] [CrossRef]
- Wang, Y.; Ying, H.; Yin, Y.; Zheng, H.; Cui, Z. Estimating soil nitrate leaching of nitrogen fertilizer from global meta-analysis. Sci. Total Environ. 2019, 657, 96–102. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, F. Nitrogen fertilizer induced greenhouse gas emissions in China. Curr. Opin. Environ. Sustain. 2011, 3, 407–413. [Google Scholar] [CrossRef]
- Krasilnikov, P.; Taboada, M.A.; Amanullah. Fertilizer use, soil health and agricultural sustainability. Agriculture 2022, 12, 462. [Google Scholar] [CrossRef]
- Jia, X.; Zhong, Y.; Liu, J.; Zhu, G.; Shangguan, Z.; Yan, W. Effects of nitrogen enrichment on soil microbial characteristics: From biomass to enzyme activities. Geoderma 2020, 366, 114256. [Google Scholar] [CrossRef]
- Wang, Q.; Ma, M.; Jiang, X.; Guan, D.; Wei, D.; Zhao, B.; Chen, S.; Cao, F.; Li, L.; Yang, X.; et al. Impact of 36 years of nitrogen fertilization on microbial community composition and soil carbon cycling-related enzyme activities in rhizospheres and bulk soils in northeast China. Appl. Soil. Ecol. 2019, 136, 148–157. [Google Scholar] [CrossRef]
- Hu, Q.; Liu, T.; Ding, H.; Li, C.; Tan, W.; Yu, M.; Liu, J.; Cao, C. Effects of nitrogen fertilizer on soil microbial residues and their contribution to soil organic carbon and total nitrogen in a rice-wheat system. Appl. Soil. Ecol. 2023, 181, 104648. [Google Scholar] [CrossRef]
- Touhami, D.; McDowell, R.W.; Condron, L.M.; Bouray, M. Nitrogen fertilization effects on soil phosphorus dynamics under a grass-pasture system. Nutr. Cycl. Agroecosyst. 2022, 124, 227–246. [Google Scholar] [CrossRef]
- Yevdokimov, I.; Gattinger, A.; Buegger, F.; Munch, J.C.; Schloter, M. Changes in microbial community structure in soil as a result of different amounts of nitrogen fertilization. Biol. Fertil. Soils 2008, 44, 1103–1106. [Google Scholar] [CrossRef]
- Wu, J.; Liu, W.; Zhang, W.; Shao, Y.; Duan, H.; Chen, B.; Wei, X.; Fan, H. Long-term nitrogen addition changes soil microbial community and litter decomposition rate in a subtropical forest. Appl. Soil. Ecol. 2019, 142, 43–51. [Google Scholar] [CrossRef]
- Marschner, P. Structure and function of the soil microbial community in a long-term fertilizer experiment. Soil. Biol. Biochem. 2003, 35, 453–461. [Google Scholar] [CrossRef]
- Liu, G. Soil conservation and sustainable agriculture on the Loess Plateau: Challenges and prospects. Ambio 1999, 8, 663–668. [Google Scholar]
- Wang, J.; Fu, B.; Qiu, Y.; Chen, L. Analysis on soil nutrient characteristics for sustainable land use in Danangou catchment of the Loess Plateau, China. Catena 2003, 54, 17–29. [Google Scholar] [CrossRef]
- Wang, J.; Fu, B.; Qiu, Y.; Chen, L. Soil nutrients in relation to land use and landscape position in the semi-arid small catchment on the loess plateau in China. J. Arid Environ. 2001, 48, 537–550. [Google Scholar] [CrossRef]
- Xu, M.; Wang, J.; Zhu, Y.; Han, X.; Ren, C.; Yang, G. Plant biomass and soil nutrients mainly explain the variation of soil microbial communities during secondary succession on the Loess Plateau. Microb. Ecol. 2022, 83, 114–126. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, X.; Liu, L.; Li, T.; Dou, Y.; Qiao, J.; Wang, Y.; An, S.; Chang, S.X. Nitrogen fertilization weakens the linkage between soil carbon and microbial diversity: A global meta-analysis. Glob. Change Biol. 2022, 28, 6446–6461. [Google Scholar] [CrossRef]
- Beltran-Garcia, M.J.; Martínez-Rodríguez, A.; Olmos-Arriaga, I.; Valdes-Salas, B.; Di Mascio, P.; White, J.F. Nitrogen fertilization and stress factors drive shifts in microbial diversity in soils and plants. Symbiosis 2021, 84, 379–390. [Google Scholar] [CrossRef]
- Nath, C.P.; Dutta, A.; Hazra, K.K.; Praharaj, C.S.; Kumar, N.; Singh, S.S.; Singh, U.; Das, K. Long-term impact of pulses and organic amendments inclusion in cropping system on soil physical and chemical properties. Sci. Rep. 2023, 13, 6508. [Google Scholar] [CrossRef]
- Sadiq, M.; Rahim, N.; Tahir, M.M.; Alasmari, A.; Alqahtani, M.M.; Albogami, A.; Ghanem, K.Z.; Abdein, M.A.; Ali, M.; Mehmood, N.; et al. Conservation tillage: A way to improve yield and soil properties and decrease global warming potential in spring wheat agroecosystems. Front. Microbiol. 2024, 15, 1356426. [Google Scholar] [CrossRef]
- Yuan, J.; Yan, L.; Li, G.; Sadiq, M.; Rahim, N.; Wu, J.; Ma, W.; Xu, G.; Du, M. Effects of conservation tillage strategies on soil physicochemical indicators and N2O emission under spring wheat monocropping system conditions. Sci. Rep. 2022, 12, 7066. [Google Scholar] [CrossRef] [PubMed]
- Herai, Y.; Kouno, K.; Hashimoto, M.; Nagaoka, T. Relationships between microbial biomass nitrogen, nitrate leaching and nitrogen uptake by corn in a compost and chemical fertilizer-amended regosol. Soil. Sci. Plant Nutr. 2006, 2, 186–194. [Google Scholar] [CrossRef]
- Du, M.; Yao, Y.; Liu, S.; Li, G.; Yuan, J. Reducing nitrogen application rates and straw mulching can alleviate greenhouse gas emissions from wheat field soil and improve soil quality. Agronomy 2024, 14, 2087. [Google Scholar] [CrossRef]
- Tan, Y.; Chai, Q.; Li, G.; Hu, F.; Yu, A.; Zhao, C.; Fan, Z.; Yin, W.; Fan, H. No-till and nitrogen fertilizer reduction improve nitrogen translocation and productivity of spring wheat (Triticum aestivum L.) via promotion of plant transpiration. Front. Plant Sci. 2022, 13, 988211. [Google Scholar] [CrossRef]
- Wu, J.; Wang, H.; Li, G.; Chen, N. Effects of nitrogen deposition on soil nitrogen fractions and enzyme activities in wet meadow of the Qinghai-Tibet Plateau. Sci. Rep. 2024, 14, 31848. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.K.; Kumar, S. Nitrate reductase, arginine deaminase, urease and dehydrogenase activities in natural soil (ridges with forest) and in cotton soil after acetamiprid treatments. Chemosphere 2008, 71, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Yin, C.; Fan, X.; Gao, Z.; Chen, H.; Tan, L.; Chang, S.X.; Zhao, Y.; Liang, Y. Procyanidin inhibited N2O emissions from paddy soils by affecting nitrate reductase activity and nirS- and nirK-denitrifier populations. Biol. Fertil. Soils 2021, 57, 935–947. [Google Scholar] [CrossRef]
- Arunrat, N.; Sansupa, C.; Kongsurakan, P.; Sereenonchai, S.; Hatano, R. Soil Microbial Diversity and Community Composition in Rice–Fish Co-Culture and Rice Monoculture Farming System. Biology 2022, 11, 1242. [Google Scholar] [CrossRef]
- Williams, A.; Birt, H.W.G.; Raghavendra, A.; Dennis, P.G. Cropping System Diversification Influences Soil Microbial Diversity in Subtropical Dryland Farming Systems. Microb. Ecol. 2023, 85, 1473–1484. [Google Scholar] [CrossRef]
- Barak, P.W.U.M.; Jobe, B.O.; Krueger, A.R.; Peterson, L.A.; Laird, D.A. Effects of long-term soil acidification due to nitrogen fertilizer inputs in Wisconsin. Plant Soil. 1997, 197, 61–69. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, Z.; Jiang, B.; Baoyin, B.; Cui, Z.; Wang, H.; Li, Q.; Cui, J. Effects of Long-Term Application of Nitrogen Fertilizer on Soil Acidification and Biological Properties in China: A Meta-Analysis. Microorganisms 2024, 12, 1683. [Google Scholar] [CrossRef]
- Fu, C.; Ma, W.; Qiang, B.; Jin, X.; Zhang, Y.; Wang, M. Effect of Chemical Fertilizer with Compound Microbial Fertilizer on Soil Physical Properties and Soybean Yield. Agronomy 2023, 13, 2488. [Google Scholar] [CrossRef]
- Tong, Y.; Dong, Q.; Yu, Y.; Cao, Q.; Yang, X.; Liu, W.; Yang, Z.; Zhang, X.; Liu, Y.; Zhang, C. Nitrogen application increases the productivity of perennial alpine cultivated grassland by improving soil physicochemical properties and microbial community characteristics. Plant Soil. 2024, 505, 559–579. [Google Scholar] [CrossRef]
- Mori, H.; Maruyama, F.; Kato, H.; Toyoda, A.; Dozono, A.; Ohtsubo, Y.; Nagata, Y.; Fujiyama, A.; Tsuda, M.; Kurokawa, K. Design and experimental application of a novel non-degenerate universal primer set that amplifies prokaryotic 16S rRNA genes with a low possibility to amplify eukaryotic rRNA genes. DNA Res. 2014, 21, 217–227. [Google Scholar] [CrossRef]
- McAndrew, D.W.; Malhi, S.S. Long-term N fertilization of a solonetzic soil: Effects on chemical and biological properties. Soil. Biol. Biochem. 1992, 7, 619–623. [Google Scholar] [CrossRef]
- Alvarez, R.; Alvarez, R. A review of nitrogen fertilizer and conservation tillage effects on soil organic carbon storage. Soil. Use Manag. 2005, 21, 38–52. [Google Scholar] [CrossRef]
- Wang, J.; Liu, W.Z.; Dang, T.H.; Sainju, U.M. Nitrogen fertilization effect on soil water and wheat yield in the Chinese Loess Plateau. Agron. J. 2013, 105, 143–149. [Google Scholar] [CrossRef]
- Sun, J.; Li, W.; Li, C.; Chang, W.; Zhang, S.; Zeng, Y.; Zeng, C.; Peng, M. Effect of different rates of nitrogen fertilization on crop yield, soil properties and leaf physiological attributes in banana under subtropical regions of China. Front. Plant Sci. 2020, 11, 613760. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Chen, X.; Zhang, F. Current nitrogen management status and measures to improve the intensive wheat–maize system in China. Ambio 2010, 39, 376–384. [Google Scholar] [CrossRef]
- El-Sharkawi, H.M. Effect of nitrogen sources on microbial biomass nitrogen under different soil types. Int. Sch. Res. Not. 2012, 2012, 310727. [Google Scholar] [CrossRef]
- Kowalczyk, W.; Wrona, D.; Przybyłko, S. Effect of Nitrogen Fertilization of Apple Orchard on Soil Mineral Nitrogen Content, Yielding of the Apple Trees and Nutritional Status of Leaves and Fruits. Agriculture 2022, 12, 2169. [Google Scholar] [CrossRef]
- Li, Y.; Long, Y.; Liu, T.; Zhang, D.; He, M.; Xie, Q.; Zhang, Z. The role of soil microorganisms and physicochemical properties in determining the germinate of invasive Solidago canadensis L. Plant Soil. 2025, 507, 897–914. [Google Scholar] [CrossRef]
- Rutkowski, K.; Łysiak, G.P. Effect of Nitrogen Fertilization on Tree Growth and Nutrient Content in Soil and Cherry Leaves (Prunus cerasus L.). Agriculture 2023, 13, 578. [Google Scholar] [CrossRef]
- Hu, W.; Jiao, Z.; Wu, F.; Liu, Y.; Dong, M.; Ma, X.; Fan, T.; An, L.; Feng, H. Long-term effects of fertilizer on soil enzymatic activity of wheat field soil in Loess Plateau, China. Ecotoxicology 2014, 23, 2069–2080. [Google Scholar] [CrossRef]
- Pintarič, M.; Štuhec, A.; Tratnik, E.; Langerholc, T. Specific Fertilization Practices Reveal Important Insights into the Complex Interaction Between Microbes and Enzymes in Soils of Different Farming Systems. Life 2024, 14, 1562. [Google Scholar] [CrossRef]
- Tian, X.; Hu, H.; Ding, Q.; Song, M.; Xu, X.; Zheng, Y.; Guo, L. Influence of nitrogen fertilization on soil ammonia oxidizer and denitrifier abundance, microbial biomass, and enzyme activities in an alpine meadow. Biol. Fertil. Soils 2014, 50, 703–713. [Google Scholar] [CrossRef]
- Balotf, S.; Kavoosi, G.; Kholdebarin, B. Nitrate reductase, nitrite reductase, glutamine synthetase, and glutamate synthase expression and activity in response to different nitrogen sources in nitrogen-starved wheat seedlings. Biotechnol. Appl. Biochem. 2016, 63, 220–229. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, X.; Zhang, L.; Zeng, L.; Liu, Y.; Wang, X.; He, P.; Li, S.; Liang, G.; Zhou, W.; et al. The stronger impact of inorganic nitrogen fertilization on soil bacterial community than organic fertilization in short-term condition. Geoderma 2021, 382, 114752. [Google Scholar] [CrossRef]
- Huang, L.; Gao, X.; Liu, M.; Du, G.; Guo, J.; Ntakirutimana, T. Correlation among soil microorganisms, soil enzyme activities, and removal rates of pollutants in three constructed wetlands purifying micro-polluted river water. Ecol. Eng. 2012, 46, 98–106. [Google Scholar] [CrossRef]
- Li, Y.; Tu, Q.; Liu, S.; Ding, W.; Min, X.; Zhou, S.; Zhang, J.; Li, J.; Yuan, C. Effects of the combined compost of grape branches and sheep manure on a soil-microorganism-chardonnay (Vitis vinifera L.) plant ecosystem. Sci. Hortic. 2024, 336, 113430. [Google Scholar] [CrossRef]
- Ren, N.; Wang, Y.; Ye, Y.; Zhao, Y.; Huang, Y.; Fu, W.; Chu, X. Effects of continuous nitrogen fertilizer application on the diversity and composition of rhizosphere soil bacteria. Front. Microbiol. 2020, 11, 1948. [Google Scholar] [CrossRef]
- Li, C.H.; Ma, B.L.; Zhang, T.Q. Soil bulk density effects on soil microbial populations and enzyme activities during the growth of maize (Zea mays L.) planted in large pots under field exposure. Can. J. Plant Sci. 2002, 82, 147–154. [Google Scholar] [CrossRef]
- Udawatta, R.P.; Kremer, R.J.; Garrett, H.E.; Anderson, S.H. Soil enzyme activities and physical properties in a watershed managed under agroforestry and row-crop systems. Agric. Ecosyst. Environ. 2009, 131, 98–104. [Google Scholar] [CrossRef]
- Barka, E.A.; Vatsa, P.; Sanchez, L.; Gaveau-Vaillant, N.; Jacquard, C.; Klenk, H.; Clément, C.; Ouhdouch, Y.; van Wezel, G.P. Taxonomy, physiology, and natural products of Actinobacteria. Microbiol. Mol. Biol. Rev. 2016, 80, 1–43. [Google Scholar] [CrossRef]
- Kim, H.; Lee, S.; Jo, H.Y.; Finneran, K.T.; Kwon, M.J. Diversity and composition of soil Acidobacteria and Proteobacteria communities as a bacterial indicator of past land-use change from forest to farmland. Sci. Total Environ. 2021, 797, 148944. [Google Scholar] [CrossRef]
- Kalam, S.; Basu, A.; Ahmad, I.; Sayyed, R.Z.; El-Enshasy, H.A.; Dailin, D.J.; Suriani, N.L. Recent understanding of soil acidobacteria and their ecological significance: A critical review. Front. Microbiol. 2020, 11, 580024. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, K.S.; Lauber, C.L.; Knight, R.; Bradford, M.A.; Fierer, N. Consistent effects of nitrogen fertilization on soil bacterial communities in contrasting systems. Ecology 2010, 91, 3463–3470. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, X.; Wang, H.; Hui, X.; Wang, Z.; Qiu, W. Long-term nitrogen fertilization impacts soil fungal and bacterial community structures in a dryland soil of Loess Plateau in China. J. Soils Sediments 2018, 18, 1632–1640. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).