Abstract
Avocado (Persea americana Miller), a crop of major economic importance in Mexico, is threatened by several quarantine pests, and recent reports have suggested that the lance fly Neosilba batesi (Diptera: Lonchaeidae) may be responsible for significant yield losses. To clarify the role of this species, we surveyed avocados from six localities in Veracruz State on the Gulf coast of Mexico and identified lance flies using both morphological and molecular tools. None of the symptoms previously attributed to N. batesi infestation in Hass avocado were observed in any of the fruits inspected across the six localities. However, 90 fruits displayed clear signs of borer attack by Conotrachelus spp. or other primary pests, and 64 of these damaged fruits (60%) yielded lance flies. Hass avocados were rarely infested and hosted only N. batesi, whereas creole avocados (P. americana var. drymifolia) were hosts to N. batesi, N. glaberrima, N. recurva, and N. flavitarsis and an undescribed species (Neosilba sp.3) that was detected by analysis of the COI gene sequences of males. Additionally, Lonchaea cristula was reported for the first time emerging from creole avocado. Each avocado yielded an average of between 2.3 and 21.0 adult lance flies. Infestation was more frequent and numerous in fruits collected from the ground than in those harvested directly from trees, supporting the idea that lance flies preferentially exploit pre-damaged or fallen fruits. Indeed, lonchaeid eggs were frequently observed deposited on the periphery or inside oviposition holes created by other pests. Overall, our results indicate that Neosilba spp. act as secondary invaders in Veracruz, with no evidence of N. batesi behaving as a primary pest in this region. None of the avocados were infested by species of Tephritidae and none of the Neosilba species we identified appear to pose a threat to avocado production in Mexico. This study highlights the value of combining morphological and molecular tools for species identification and underscores the importance of differentiating between primary and secondary invaders in the context of avocado pest management.
1. Introduction
Avocado (Persea americana Miller) is a member of the flowering plant family Lauraceae native to Mexico and Central America. Mexico is the world’s leading producer of avocado, with an annual production of 2.7 million tonnes in 2023, half of which was exported to the United States [1]. Several species and varieties of Persea are cultivated in Mexico, with P. americana var. Hass being of particular commercial importance. However, the ancestral variety P. americana var. drymifolia, known as creole avocado, is also grown widely in Mexico and is highly appreciated by local consumers. Several fruit-boring pests of quarantine significance infest avocado in this region, including Conotrachelus aguacatae (Barber), Conotrachelus perseae (Barber) (Coleoptera: Curculionidae), Helipus lauri Boheman (Coleoptera: Curculionidae), and Stenoma catenifer Walsingham (Lepidoptera: Elachistidae) [2].
Lance flies of the genus Neosilba Waddill & Weems are native to the Neotropics and are usually considered to be a polyphagous secondary invader of fruits that had previously been infested by tephritid fruit flies (Diptera, Tephritidae), or other primary pests [3,4,5]. However, Neosilba batesi (Curran, 1932) (Diptera: Lonchaeidae) has been identified as a potential pest of economic concern for avocado production in Colombia [6]. Recently, this pest was reported to be responsible for 30% crop losses in avocado in the central Mexican state of Michoacan [7,8], although this estimate was made in the absence of a rigorous assessment of the insect’s phytosanitary status. The genetic identity of this pest in central Mexico has now been confirmed by amplification and sequencing of the mitochondrial cytochrome c oxidase subunit I (COI) gene [9]. The main sign of N. batesi infestation in avocado in Michoacan was described as a ring of reddish discoloration around the peduncle or reddish spots, presumably caused by larval development following female oviposition in the junction of the pedicel with the fruit. According to Lemus-Soriano et al. [9], infestation accelerates ripening and leads to premature fruit drop.
Given the enormous economic importance of avocado production in Mexico and the paucity of published information on avocado infestations by N. batesi and other lance flies, this study surveyed avocado fruits across six localities in the state of Veracruz, Mexico, that borders the Gulf of Mexico. The aim of the study was to document the occurrence of lonchaeid fly infestations of avocado in this region using morphological and genetic techniques.
2. Materials and Methods
2.1. Fruit Collection
Avocado fruits were inspected in the central region of Veracruz state, between 16 May and 6 June 2024, and between 27 March, and 16 May in 2025. Fruits of the creole or Hass varieties were inspected and collected from isolated avocado trees in the localities of Tatatila, Tenexpanoya, Tlanehuayocan and Xalapa and from two orchards in Altotonga and Huatusco (Figure 1A,B).
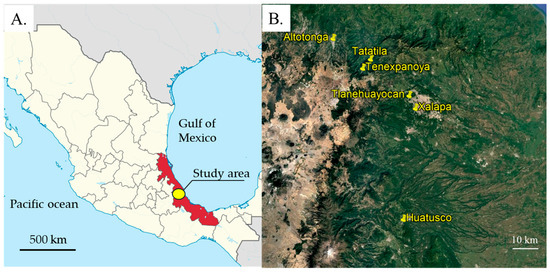
Figure 1.
Location of the study area (yellow circle) in Veracruz State (red fill) on the Gulf coast of Mexico (A). The sample locations of the study (yellow pins) were located close to the villages of Altotonga, Tatatila, Tenexpanoya, Tlanehuayocan, Xalapa and Huatusco (B). Image (A) was adapted from https://commons.wikimedia.org/wiki/File:Veracruz_in_Mexico_(location_map_scheme).svg (accessed on 15 August 2025) under the Creative Commons Attribution (CC BY-SA 3.0) (accessed on 15 August 2025). Image (B) was adapted from Google Earth (image date 12 December 2020), used under Google’s policies for non-commercial academic use.
Avocado fruits were collected directly from the tree or from the ground after shaking the branches with a stick to induce premature fruit drop. The five fruits collected in Tlanehuayocan were gathered from the ground as no fruits were present on trees.
2.2. Fruit Classification and Insect Rearing
All avocado fruits were carefully inspected for signs of damage. Fruits with no evident damage (intact fruits) and those showing signs of infestation by insect pests were separated. Avocados with signs of infestation were transported to the laboratory for a detailed inspection. The presence of eggs or larvae was assessed at 35× magnification using a stereomicroscope (Leica EZ4, Wetzlar, Germany). A slice was cut from the apical section of the fruit to detect signs of larval feeding damage (Figure 2A,D). Fruits with holes that presumably indicated the presence of a borer pest (Figure 2B,C,E,F), were taken to the laboratory, weighed, measured for equatorial diameter, and individually placed in a 470 mL plastic cup with a 5 mm layer of vermiculite as a pupation substrate. The cups were maintained at 24 ± 1 °C, 65 ± 10% relative humidity, and a 12:12 h L:D photoperiod in a climate-controlled laboratory. Adult lonchaeid emergence was recorded daily for 48 days following fruit collection. Emerged flies were euthanized by freezing at −20 °C and preserved in 70% ethanol for subsequent identification.

Figure 2.
Inspection of damaged and undamaged avocado fruits. The apical section at the peduncle insertion point was dissected for the presence of lonchaeid eggs and larvae in non-damaged fruits of the creole variety (A–C) and Hass variety (D–F). Avocado fruits with borer pest holes in the creole variety (B,C) and the Hass variety (E,F). The apical section around the pedicel (A,D) and the lateral borer holes of damaged fruits (B,C,E,F) are indicated by yellow arrows.
2.3. Species Identification, Sequencing and Phylogenic Analysis
Lonchaeid flies that emerged from avocados were examined under a stereomicroscope to determine species and sex [3,4,10]. Females were not identified due to the absence of taxonomic keys for Neosilba. Male identification was initially based on the morphological characteristics of the genitalia [11,12,13]. A subset of the specimens was sent to the National Museum of Scotland to confirm species-level identifications.
Nine males and one female of Neosilba spp. and two females of Lonchaea cristula McAlpine, 1964 were subjected to COI gene sequence analysis. For this, DNA extraction, PCR amplification, sequencing and sequence assembly were performed as described previously [5,13]. Briefly, DNA extraction was performed using the DNeasy Blood & Tissue Kit (Qiagen, Hilden, Germany) following the manufacturer’s instructions. COI gene amplification was performed using primers C1-J-1718 and C1-N-2191 [14] on a SureCycler 8800 thermocycler (Agilent Technologies, Santa Clara, CA, USA). The resulting amplicons (525 bp in length) were purified using the Wizard SV Gel and PCR System Clean-Up kit (Promega, Madison, WI, USA), and were sequenced at the Labsergen Langebio laboratory (Irapuato, Mexico). The sequences were assembled and edited using BioEdit v.7.0.5 software in line with previous studies [5,13].
For the phylogenetic analysis, COI gene sequences obtained in this study and those of the related species present in GenBank were aligned in MAFFT v.7 [15] and MEGA 7.0.26 software [16]. The best nucleotide substitution model was identified using the Akaike Information Criterion (AIC) within jModelTest 2.1.10 software [17]. The phylogenetic tree was generated by applying the maximum likelihood (ML) method in raxmlGUI 2.0.16 with the GTR + I + G evolutionary model [18]. Bootstrap analysis was performed on 1000 repetitions. Finally, a phylogenetic analysis was performed by Bayesian inference with the MrBayes v.3.2.6 software [19] for five million generations. The resulting phylogenetic tree was edited in FigTree v.1.4.3 [20]. Numbers at branch nodes indicate bootstrap values (BS ≥ 70) and Bayesian Posterior Probabilities (BPP ≥ 0.9). Silba fumosa (Egger, 1862), collected in Germany [21], was used as an outgroup for the phylogenetic tree, as it had previously been employed in the genetic identification of N. batesi from Mexican avocados [9].
2.4. Statistical Analysis
The mean diameter and weight of infested avocados, and the mean number of flies that developed in each infested fruit, were analyzed by fitting generalized linear models (GLM) with a quasi-Poisson distribution to account for overdispersion. The results of GLM analyses are presented as χ2 statistics. Means were compared using the Bonferroni test. Pairwise comparisons of the number of flies that emerged per fruit were subjected to the Holm-Bonferroni p-value adjustment in order to control the risk of a type I error. The prevalence (%) of fruits from which two or more Neosilba species emerged was analyzed using Fisher’s exact test. The median time elapsed for adults to emerge from field-collected avocados was determined by Kaplan–Meier analysis and compared by log-rank test. All analyses were performed using the R-based package Jamovi, version 2.3.28 [22].
3. Results
3.1. Fruit Inspection and Global Fly Emergence
A total of 181 avocado fruits were inspected across the six locations of which 90 (50%) showed clear signs of external damage (Table 1). Samples were obtained at six sites with elevations of 1608–2286 m.

Table 1.
Location of inspected creole and Hass avocados across various localities in Veracruz state. A different number of fruits were examined in each locality and categorized as either intact (no visible external damage) or damaged (showing clear signs of boring pest damage).
The external reddish ring discoloration at the peduncle, or the presence of eggs, larvae, or other signs of damage previously described for N. batesi in the pedicel region [9], were not observed in any of the inspected fruits. However, the 90 damaged fruits from Tatatila, Tenexpanoya, Tlanehuayocan, and Xalapa all showed external lateral damage, characterized by boring holes, likely caused by Conotrachelus spp. Examination of the lateral holes at 35× magnification revealed unhatched eggs or post-hatching egg chorion around and inside the pest borer holes (Figure 3A–C).
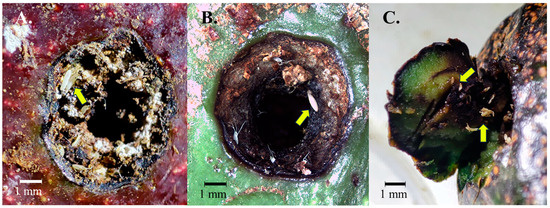
Figure 3.
Magnified images of boring insect damage to avocados with the presence of Neosilba eggs on the periphery of the hole (A,B) or inside the hole when the hole was lightly excavated and inspected (C). Neosilba eggs were easily visible when magnified and are indicated by yellow arrows. No eggs were observed in undamaged avocados.
3.2. Emergence of Lonchaeid Flies
A total of 355 Neosilba spp. adults (195 females and 160 males) emerged from 90 damaged avocado fruits collected in Veracruz, including 86 infested fruits of the creole variety and 4 of the Hass variety (Table 2). Additionally, 11 adults of Lonchaea cristula (5 females and 6 males) emerged exclusively from six creole avocados collected in Tatatila in 2024. No infestation by L. cristula was recorded in 2025 at any location.

Table 2.
Number, mean diameter, and mean weight of damaged avocados collected from different localities and the mean number of Neosilba spp. adults that emerged per fruit with the median emergence time and 95% confidence interval (CI) under laboratory conditions.
The diameter and weight of avocados differed significantly among localities, with fruits from Tatatila showing the smallest average diameter (GLM, χ2 = 59.1, df = 3, p < 0.001) and weight (GLM, χ2 = 138, df = 3, p < 0.001), followed by those from Tenexpanoya (Table 2). The largest fruits were collected from the ground at Tlanehuayocan, although their diameter and weight did not differ significantly from those of Hass avocados collected in Xalapa.
The mean percentage of damaged fruits from which at least one Neosilba adult emerged differed significantly among localities (GLM χ2 = 15.6, df = 3, p < 0.001). Hass avocados from Xalapa exhibited a significantly lower prevalence of infestation (21%) compared to creole avocados collected in Tatatila, Tenexpanoya, and Tlanehuayocan (68–80%) (Table 2).
Despite differences in fruit size, no correlation was found between the number of emerging flies and fruit diameter (Spearman’s R = −0.180, df = 52, p = 0.193) or weight (Spearman’s R = 0.175, df = 52, p = 0.205).
The mean number of Neosilba flies that emerged from fruits was highly variable and was higher for the creole avocados collected directly from the ground in Tlanehuayocan (χ2 = 8.74, df = 3, p = 0.033) than the damaged avocados fruits collected in Xalapa (Table 2). A maximum of 58 Neosilba spp. adults emerged from a single small creole avocado (30 cm diameter, 22 g) collected in Tatatila. Interestingly, L. cristula emerged from 19% of the fruits with a mean (±SE) of 1.8 ± 0.5 flies per fruit and a maximum of 4 adults emerging from a single creole fruit. This is the first record of L. cristula infestation of [creole] avocado. Of the six creole avocados that produced adults of L. cristula, five (83%) were also infested by Neosilba spp.
The median (95% CI) time from fruit collection to Neosilba spp. emergence in the laboratory was similar for males and females of fruits collected in all localities; Tatatila (log-rank test, p = 0.076), Tenexpanoya (log-rank test, p = 0.86), Tlanehuayocan (log-rank test, p = 0.63) and Xalapa (log-rank test, p = 0.23). However, the median emergence time of adults (both sexes) differed significantly among localities (log-rank test, p < 0.001), being shortest for Tatatila, longest for Tenexpanoya and Xalapa, and intermediate in samples from Tlanehuayocan (Table 2). The median emergence time of all Neosilba spp. (32 days) (all individuals pooled) was similar to that of L. cristula (31 days) (log-rank test, p = 0.54).
3.3. Species Identification, Sequencing and Phylogenic Analysis
Males of Neosilba spp. were identified based on examination of the genitalia. Four males from Tatatila were lost during handling, so a total of 156 males from the 160 males that emerged were examined. Among the emerged males, N. batesi, N. glaberrima (Wiedemann, 1830), N. recurva MacGowan & Lasa, 2025 and N. flavitarsis MacGowan & Lasa, 2025 were identified (Table 3). Neosilba batesi was the most abundant species, comprising 65% of the emerged males, followed by N. glaberrima (28%). Both species were present in samples from all four localities. A smaller number of males corresponded to N. recurva (6%) and N. flavitarsis (1%), which were only recorded in samples from Tatatila and Tenexpanoya.

Table 3.
Total number of Neosilba males identified by genital dissection and the species composition and prevalence of co-infestation by two or more species across the different localities.
Based on the identification of males, the prevalence of creole avocados containing two or more Neosilba species was similar among localities (χ2 = 3.83, df = 3, p = 0.280) (Table 3). Half of the fruits from Tlanehuayocan were co-infested, and it is worth bearing in mind that these fruits were collected from the ground. More importantly, the prevalence of co-infestation may be underestimated, as only males were used for species identification.
Taxonomic identification was confirmed through COI sequencing of males from each species group and a single female (INECOL_24/20F) that could not be identified from morphological characteristics (Table 4).

Table 4.
Species and host plant, specimen number, origin and GenBank accession numbers for COI gene nucleotide sequences obtained from Neosilba spp., Lonchaea cristula in the present and previous studies, which were used in the phylogenetic analysis. Bold text indicates individuals that were reared from avocado in the present study.
Two N. batesi males (INECOL_24/48, INECOL_24/23) showed clear genetic similarity to the specimens of N. batesi previously recorded from Hass avocados from Michoacan state in Mexico [9] and from figs in Veracruz state [5] (Figure 4). As noted previously [5,9], N. batesi specimens clustered into a clade that did not correspond to the N. batesi sequence reported from Florida (CSCA-17X541) (Figure 4). The sequences from two pairs of males identified morphologically as N. recurva (INECOL_24/47, INECOL_25/58) and N. flavitarsis (INECOL_25/55, INECOL_25/56) matched with their respective species. As these species had been previously reared from figs in Veracruz State [5,13], these records from avocado represent new host records for each of these species. Two males morphologically identified as N. glaberrima (INECOL_24/50, INECOL_25/57) clustered with N. glaberrima specimens from figs (INECOL_24/43 and INECOL_24/52) in Veracruz state [5] (Table 4, Figure 4). The sequenced female (INECOL_24/20F) also belonged to the N. glaberrima cluster. In contrast, another glaberrima-like male (INECOL_24/49) exhibited genetic divergence and formed a separate branch of the phylogenetic tree, suggesting the presence of an additional cryptic species in avocado (Neosilba sp.3) that was different from the unknown species reported previously in figs, which we previously named Neosilba sp.1 (INECOL_24/41, INECOL_24/42) and Neosilba sp.2? (INECOL_24/44) [5] (Figure 4). Neither Neosilba sp.1 or Neosilba sp.2? were detected infesting avocados in our study area.
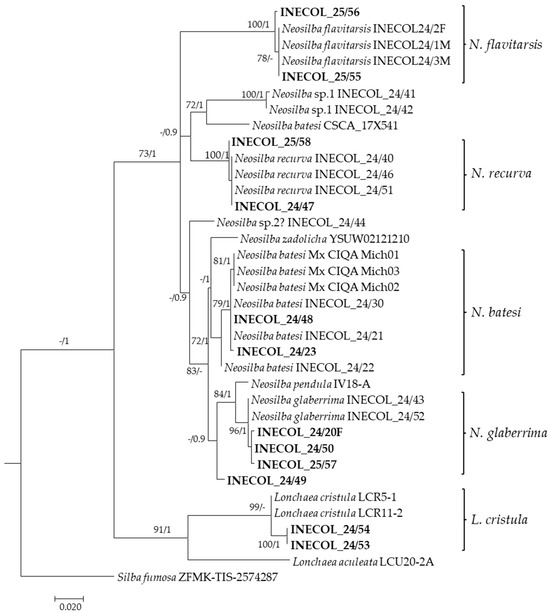
Figure 4.
Maximum likelihood phylogenetic tree based on COI gene partial sequences illustrating the evolutionary relationships among lonchaeid flies. Specimens sequenced in the present study are shown in bold text. The tree was reconstructed using raxmlGUI and GTR + I + G evolutionary model with 1000 rapid bootstrap replicates. The numbers at the nodes indicate bootstrap values for 1000 replicates (BS ≥ 70%)/Bayesian Posterior Probabilities (BPP ≥ 0.9).
The identity of L. cristula specimens that developed in avocado (INECOL_24/53, INECOL_24/54), was confirmed by one of us (I.M.) at the National Museum of Scotland. The amplified sequences of these individuals clustered (98.9% sequence identity), with two specimens of L. cristula from Colombia [23] (Figure 4). The presence of this species in the study area had been detected earlier in torula yeast baited traps used for monitoring the invasive pest Silba adipata McAlpine, 1956 in fig orchards in this region [5].
4. Discussion
Lonchaeid flies were reared from 86 creole and 4 Hass avocados collected from different sites in central Veracruz State, Mexico. The characteristic reddish ring discoloration around the peduncle, or reddish partial spots previously reported as symptoms of larval development following N. batesi oviposition in Hass avocado [9], were not observed in any of the avocados inspected during this study across any of the localities sampled in Veracruz State. In the neighboring state of Puebla, producers associate the reddish avocado symptomatology with a natural abortion of the fruits (R. Lasa, pers. obs.). A total of 90 of the inspected fruits in Veracruz exhibited damage caused by borer pests. The holes observed on the lateral surface of the avocados (Figure 2), sometimes accompanied by a white residue, resembled the signs of damage caused by the curculionid pests C. aguacatae and C. perseae [24]. Some additional weevil and lepidopteran pests also attack avocado in this region [25,26,27,28]. However, no emergence of Conotrachelus spp. or other insects was recorded from the collected avocados, with the exception of an unidentified moth that emerged from a single fruit from Tatatila in 2025. Overall, 64 of the damaged fruits (60%) were infested by lance flies (Neosilba spp.). During fruit inspections, lonchaeid eggs or egg chorions were frequently observed on the external surface and within the borer holes, indicating that Neosilba spp. act as secondary invaders in avocados in this region.
A recent study conducted in Michoacan State in central Mexico postulated that N. batesi females exhibit opportunistic oviposition behavior by exploiting existing wounds or openings at the pedicel-fruit junction that result from pathogen infection or other types of damage [9]. This idea aligns with earlier observations suggesting that N. batesi prefers to oviposit on damaged or fallen fruits rather than on healthy, intact fruits [29,30]. Our findings are consistent with these observations, as N. batesi and other Neosilba species only emerged from fruits exhibiting signs of physical damage. In this respect, it seems unlikely that differences in lonchaeid infestation would be related to differences in exocarp (skin) thickness or toughness between Hass and creole avocados, as the use of wounds for oviposition immediately bypasses the exocarp barrier. The finding that infestation was not correlated with fruit size or weight further indicates that physical damage is more important than fruit development, which contrasts markedly with the oviposition preferences of tephritid flies in which fruit phenology is highly influential [31]. Although based on a limited number of samples, it appears that creole avocados are a better quality of host than the Hass variety, as the mean number of N. batesi adults that developed in each Hass avocado (2.3 adults/fruit in the present study, or 1.7–3.8 adults/fruit reported in Michoacán state [9]) was lower than the number of Neosilba spp. flies that emerged from creole avocados (4.7–21.0 adults/fruit) (Table 2). Also, the development time in creole avocado was approximately 10 days shorter (29–31 days) than observed in the Hass variety (41 days).
Hass avocados were infested exclusively by N. batesi, whereas N. glaberrima, N. recurva, N. flavitarsis, and N. batesi emerged from creole avocados. The identity of all the species detected in our study was confirmed by sequencing of the COI gene. This procedure also detected the presence of an unidentified species (Neosilba sp.3) which resembled N. glaberrima (based on the apical segment of the male abdomen [5]) but which remains to be described and named.
Among the emerged flies, N. batesi was the most prevalent species (65%) at all surveyed localities. Neosilba batesi has been reared from avocado, mango (Mangifera indica L.), orange (Citrus sinensis L.), tabog (Swinglea glutinosa Blanco), papaya (Carica papaya L.), peach palm (Guilielma gasipaes Kunth), guava (Psidium guajava L.), passion fruit (Passiflora spp.), miconia (Miconia sp.), fig (Ficus carica L.) and various pawpaw species in the genus Annona [3,5,11,32,33,34]. This species has been recorded from Florida (USA), Mexico, Guatemala, El Salvador, Costa Rica, Panama, Colombia and Peru [4].
Neosilba glaberrima was the second most abundant species in avocado in our study. This fly is recognized as a polyphagous species in Brazil, where it has been recorded emerging from avocado and from fruits of sixteen other species across different families [35]. In Veracruz, N. glaberrima also develops in fig [5] and various Annona species [11].
The emergence of N. recurva and N. flavitarsis was only observed in creole avocados collected in the Tatatila locality. Both species have been reported emerging from figs that were previously used for oviposition by the black fig fly Silba adipata [5,13]. This study identifies creole avocado as a new host for both N. recurva and N. flavitarsis.
Most of the avocados collected directly from trees were infested by a single Neosilba species, although a fraction harbored two or more lonchaeid species. While the co-occurrence of Tephritidae and Lonchaeidae has been documented, in which lonchaeids exploit oviposition wounds created by tephritids, the coexistence of multiple lonchaeid species within a single fruit has been less studied. Given the saprophagous behavior of most lonchaeids, it is reasonable to expect that fruits collected from the ground have a higher probability of being co-infested by multiple species compared to fruits attached to the tree. In our study, half of the damaged fruits collected from the ground in Tlanehuayocan were co-infested by N. batesi and N. glaberrima, whereas 0–17% were co-infested by these species in fruits collected from trees.
The occurrence of multiple Neosilba species within the same fruit was previously noted in Colombia [32], although this was not quantified. A few avocados in our study were co-infested with Neosilba spp. and L. cristula. Members of the genus Lonchaea, especially in the Nearctic and Palearctic, have traditionally been considered saproxylic, the larvae being found under the bark of dead or dying trees, often in association with other insects [36]. However, some members of this genus infest the fruit of various plant species in Colombia [32,37,38]. To our knowledge, this is the first report of L. cristula emerging from avocado. In contrast, members of the tephritid genus Anastrepha Schiner 1868 fail to develop in avocado as the eggs are rapidly encapsulated [30], a plant response that we never observed when dissecting the apical region of avocados in the present study.
In general, lance flies exhibit diverse larval feeding behaviors, including saprophagy, zoophagy, and phytophagy, and are associated with a wide range of ecological niches, though they are primarily linked to forested environments [39]. Within this family, the genera Neosilba and Lonchaea Fallen, 1820 are notable for developing in fruits of commercial importance [32,40,41,42]. Although there is some evidence suggesting that certain species can oviposit in apparently healthy fruits [40,43,44,45], most species within these genera are associated with fruits that have been damaged by other primary pests [5,46,47].
Our study faced several limitations in the small number of avocados that could be sampled at some sites and the inability to identify female flies to species given the lack of taxonomic keys for females, an issue that affects the majority of studies on lonchaeids. It should also be noted that the damaged avocados appeared to have been infested by Conotrachelus spp., which are recognized avocado pests in this region, although no weevils were reared from our samples. Consequently, the relationship between Conotrachelus spp. and lonchaeid infestation could not be established with absolute certainty. One possibility is that the weevils responsible for the original infestation had completed their development and exited the fruit prior to our sampling, or alternatively that lonchaeid infestation made the avocado unsuitable for the development of Conotrachelus spp. progeny; an issue that merits future study.
The results of this study indicate that N. batesi and N. glaberrima act as secondary invaders of avocados in Veracruz State. We present the first evidence that N. recurva, N. flavitarsis and L. cristula also function as secondary pests of this crop. As none of the undamaged avocados produced lonchaeids, we found no evidence of N. batesi acting as a primary pest, which has been reported in central Mexico.
Our findings have several practical implications. First, it appears that N. batesi is not a major threat to avocado production in Mexico but rather an opportunistic species that exploits damaged or diseased avocados or those in the process of abortion, which is likely the reason why this fly has attracted the attention of plant protection workers in central Mexico and Colombia. The risks to the avocado export industry appear to be low as the Neosilba and Lonchaea species that we identified are not primary pests and damaged avocados are destroyed in packaging plants before they can be exported.
5. Conclusions
This study provides novel evidence on the diversity and infestation patterns of lonchaeid flies associated with avocados in central Veracruz, Mexico. Neosilba batesi and N. glaberrima were the most prevalent species, both functioning as secondary invaders by exploiting wounds or borer damage in fruits. In contrast, N. recurva, N. flavitarsis, and Lonchaea cristula were recovered at lower frequencies but are reported here for the first time as secondary colonizers of avocado, whereas an additional unidentified species (Neosilba sp.3) was detected by COI gene sequencing and remains to be described.
Overall, these findings highlight the value of integrating morphological and molecular tools for species identification and underscore the importance of differentiating between primary and secondary invaders in the context of avocado pest management. Given the paucity of studies on the diversity and host-plant relationships for the Lonchaeidae in North America, future studies should involve surveys over a wider geographic range, across different types of natural and agricultural ecosystems to identify areas of particular interest in which seasonal variation in lonchaeid populations and the phytosanitary status of this family could be better understood.
Author Contributions
Conceptualization, R.L., I.M. and T.W.; methodology, R.L., L.N.-d.-l.-F. and I.M.; formal analysis, R.L., L.N.-d.-l.-F., I.M. and T.W.; investigation, R.L., I.M. and T.W.; resources, R.L., L.N.-d.-l.-F. and T.W.; writing—original draft preparation, R.L., L.N.-d.-l.-F., I.M. and T.W.; writing—review and editing, R.L. and T.W.; visualization, R.L. and I.M.; supervision, R.L.; project administration, R.L. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by SECIHTI project number CBF-2025-I-3511.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors. Sequences reported in this study are available at https://data.mendeley.com/datasets/tr4zf7kzxz/1 (accessed on 13 October 2025).
Acknowledgments
We are grateful to Ricardo Díaz del Castillo for assistance in the field. We also thank growers for access to field sites and Jesús Roberto Córdova Garcia (Municipality of Tatatila) for facilitating contact with fig growers. Juan Sebastian Gómez Díaz (INECOL) kindly provided technical support in the laboratory.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- SIAP. Servicio de Información Agroalimentaria y Pesquera. Available online: https://www.gob.mx/agricultura/dgsiap/acciones-y-programas/produccion-agricola-404122 (accessed on 13 October 2025).
- SENASICA. Servicio Nacional de Sanidad, Inocuidad y Calidad Agroalimentaria. Plagas Reglamentadas del Aguacate. 2024. Available online: https://dj.senasica.gob.mx/ASIA/Fitosanitario/barrenadores_del_aguacate?tipoIngreso=public&tipoVista=public (accessed on 20 August 2025).
- McAlpine, J.F.; Steyskal, G.C. A revision of Neosilba McAlpine with a key to the world genera of Lonchaeidae. Can. Entomol. 1982, 114, 105–137. [Google Scholar] [CrossRef]
- MacGowan, I. World catalogue of the family Lonchaeidae (Diptera, Cyclorrhapha, Acalyptratae). Zootaxa 2023, 5307, 1–96. [Google Scholar] [CrossRef] [PubMed]
- Lasa, R.; Navarro-de-la-Fuente, L.; MacGowan, I.; Williams, T. A complex of lance flies (Diptera: Lonchaeidae) infesting figs in Veracruz, Mexico with the description of a new species. Insects 2025, 16, 458. [Google Scholar] [CrossRef]
- Campos-Rivera, J. Otra Plaga Que Nace al Aguacate. Desarrollo Rural, Universidad Nacional de Colombia. 13 December 2014. Available online: https://agenciadenoticias.unal.edu.co/detalle/otra-plaga-que-le-nace-al-aguacate-1 (accessed on 25 August 2025).
- Gómez, E. Alertan Sobre Plaga de Mosca de la Fruta que Daña Cultivos de Aguacate en Michoacán. La Jornada. 20 September 2024. Available online: https://www.jornada.com.mx/2024/09/20/ciencias/a06n1cie (accessed on 9 August 2025).
- Laboratorio de Tecnología Orgánica, Uruapan, Michoacán. Technical Note: La Mosquita del Fruto del Aguacate. 2024. Available online: https://www.quadratin.com.mx/www/wp-content/uploads/2024/08/FICHA-TECNICA-mosquita-del-aguacate-Neosilva-batesi-2-1.pdf (accessed on 1 October 2025).
- Lemus-Soriano, B.A.; Morales-Galván, O.; García-Gallegos, D.; García-Valderas, D.V.; Kassen, M.; Illescas-Riquelme, C.P. Neosilba batesi Curran (Diptera: Lonchaeidae): Identification, distribution, and its relationship with avocado fruits. Diversity 2025, 17, 499. [Google Scholar] [CrossRef]
- McAlpine, J.F. Old World lonchaeids of the genus Silba Macquart (=Carpolonchaea Bezzi), with descriptions of six new species (Diptera: Lonchaeidae). Can. Entomol. 1956, 88, 521–544. [Google Scholar] [CrossRef]
- Illescas-Riquelme, C.P.; Hernández, H.G.; Carrasco, J.V.; Cázares, M.C.M.L.; Montiel, C.R. Lonchaeidae (Diptera: Tephritoidea) associated with the genus Annona in Mexico. Southwest. Entomol. 2015, 40, 121–130. [Google Scholar] [CrossRef]
- Galeano-Olaya, P.E.; Canal, N.A. New species of Neosilba McAlpine (Diptera: Lonchaeidae) and new records from Colombia. Papéis Avulsos Zool. 2021, 52, 361–385. [Google Scholar] [CrossRef]
- MacGowan, I.; Navarro-de-la-Fuente, L.; Williams, T.; Lasa, R. A new species of Neosilba (Diptera, Lonchaeidae) associated with fig orchards in Mexico. Zootaxa 2025, 5583, 195–200. [Google Scholar] [CrossRef]
- Simon, C.; Frati, F.; Beckenbach, A.; Crespi, B.; Liu, H.; Flook, P. Evolution, weighting, and phylogenetic utility of mitochondrial gene sequences and a compilation of conserved polymerase chain reaction primers. Ann. Entomol. Soc. Am. 1994, 87, 651–701. [Google Scholar] [CrossRef]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Brief. Bioinf. 2019, 20, 1160–1166. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nature Meth. 2012, 9, 772. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Edler, D.; Klein, J.; Antonelli, A.; Silvestro, D. raxmlGUI 2.0: A graphical interface and toolkit for phylogenetic analyses using RAxML. Meth. Ecol. Evol. 2020, 12, 373–377. [Google Scholar] [CrossRef]
- Rambaut, A. FigTree v1.4.3 Software. Institute of Evolutionary Biology, University of Edinburgh. 2016. Available online: http://tree.bio.ed.ac.uk/software/figtree/ (accessed on 9 August 2025).
- Reimann, A.; Rulik, B. The Lonchaeidae (Diptera) of the GBOL project, with the description of a new Priscoearomyia species. Contrib. Entomol. 2024, 74, 165–179. [Google Scholar] [CrossRef]
- Jamovi. Jamovi Statistical Software v.2.3.28. 2024. Available online: https://www.jamovi.org (accessed on 15 October 2024).
- Balseiro, F.J.; Uribe, S.I. DNA barcoding for identification of species of the genus Lonchaea Fallen, 1820 (Diptera: Lonchaeidae) of Antioquia. Rev. Fac. Cienc. 2021, 10, 51–66. [Google Scholar] [CrossRef]
- SENASICA-DGSV. Barrenador Pequeño del Hueso del Aguacate Conotrachelus aguacatae (Barber) y Conotrachelus perseae (Barber) (Coleoptera: Curculionidae); Servicio Nacional de Sanidad, Inocuidad y Calidad Agroalimentaria Dirección General de Sanidad Vegetal—Centro Nacional de Referencia Fitosanitaria—Grupo Especialista Fitosanitario, Ficha Técnica: Tecámac, Mexico, 2016; 11p. Available online: https://www.gob.mx/cms/uploads/attachment/file/155683/2._Ficha_T_cnica_Barrenador_peque_o_del_hueso_del_aguacate_2025.pdf (accessed on 13 October 2025).
- SENASICA-DGSV. Barrenador Grande de la Semilla del Aguacate, Heilipus lauri Boheman. (Coleoptera: Curculionidae); Servicio Nacional de Sanidad, Inocuidad y Calidad Agroalimentaria-Dirección General de Sanidad Vegetal—Centro Nacional de Referencia Fitosanitaria—Grupo Especialista Fitosanitario, Ficha Técnica: Tecámac, Mexico, 2016; 10p. Available online: https://www.gob.mx/cms/uploads/attachment/file/155685/3._Ficha_T_cnica_Barrenador_grande_del_hueso_del_aguacate_2025.pdf (accessed on 20 August 2025).
- SENASICA-DGSV. Palomilla Barrenadora del Aguacate (Stenoma catenifer Walsingham); Servicio Nacional de Sanidad, Inocuidad y Calidad Agroalimentaria—Dirección General de Sanidad Vegetal—Centro Nacional de Referencia Fitosanitaria—Grupo Especialista Fitosanitario, Ficha Técnica: Tecámac, Mexico, 2016; 16p. Available online: https://www.gob.mx/cms/uploads/attachment/file/155686/1._Ficha_T_cnica_Stenoma_catenifer_2025.pdf (accessed on 20 August 2025).
- Fadda, L.A.; Lasa, R.; Vera, L.A.; Lira, A. Occurrence of Stenoma catenifer and first record of Cryptaspasma perseana in Veracruz, Mexico. Southwest. Entomol. 2023, 48, 1005–1010. [Google Scholar] [CrossRef]
- Sanhueza-Peñaranda, N.; González-Hernández, A.H.; Valdez-Carrasco, J.; Guzmán-Franco, A.; Castañeda-Vildózola, A.; Martínez-Ortega, J. New record of Cryptaspasma perseana (Lepidoptera: Tortricidae) in Puebla, México. Proc. Entomol. Soc. Wash. 2024, 126, 400–401. [Google Scholar] [CrossRef]
- Hernández-Ortiz, V. Diptera. In Catalogo de Insectos y Acaros Plaga de los Cultivos Agricolas de Mexico; Deloya-Lopez, A.C., Valenzuela-Gonzalez, J.E., Eds.; Sociedad Mexicana de Entomologia A.C.: Xalapa, Mexico, 1999; pp. 69–82. [Google Scholar]
- Aluja, M.; Diaz-Fleischer, F.; Arredondo, J. Nonhost status of commercial Persea americana “Hass” to Anastrepha ludens, Anastrepha obliqua, Anastrepha serpentina and Anastrepha striata (Diptera: Tephritidae) in Mexico. J. Econ. Entomol. 2004, 97, 293–309. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Fleischer, F.; Papaj, D.R.; Prokopy, R.J.; Norrbom, A.L.; Aluja, M. Evolution of fruit fly oviposition behavior. In Fruit Flies (Tephritidae): Phylogeny and Evolution of Behavior; Aluja, M., Norrbom, A.L., Eds.; CRC Press: Boca Raton, FL, USA, 1999; pp. 811–841. [Google Scholar]
- Saavedra-Díaz, J.; Galeano-Olaya, P.E.; Canal, D.N.A. Relaciones ecológicas entre frutos hospederos, moscas frugívoras y parasitoides en un fragmento de bosque seco tropical. Rev. Cienc. Agric. 2017, 34, 32. [Google Scholar] [CrossRef]
- Balseiro, F.J. Identificación de Lonchaeidae (Diptera: Tephritoidea) Asociados a Pasifloras en el Departamento de Antioquia, con Énfasis en el género Lonchaea Fallen1820. Master’s Thesis, Universidad Nacional de Colombia, Medellin, Colombia, 15 December 2020. Available online: https://repositorio.unal.edu.co/handle/unal/80076 (accessed on 15 August 2025).
- Herrera, A.M.; Canal, N.A.; Agudelo-Martinez, J.C.; Perez-Buitrago, N. Diversidad y ecologia de Tephritoidea (Insecta: Diptera) en el norte de la Orinoquia colombiana. Rev. Biol. Colomb. 2022, 70, 423–436. [Google Scholar] [CrossRef]
- Raga, A.; de Souza-Filho, M.F.; Strikis, P.C.; Montes, S.M.N.M. Lance fly (Diptera: Lonchaeidae) host plants in the state of São Paulo, southeast Brazil. Entomotropica 2015, 30, 57–68. [Google Scholar]
- MacGowan, I.; Rotheray, G.E. British Lonchaeidae (Diptera): Keys and Notes for the Identification of the Species, 1st ed.; Part 15; Royal Entomological Society: London, UK, 2008; Volume 10, pp. 1–142. [Google Scholar]
- Chacón, P.; Rojas, M. Entomofauna asociada a Passiflora mollisima, P. edulis f. flavicarpa y P. quadrangularis en el departamento del Valle del Cauca. Turrialba 1984, 34, 297–311. [Google Scholar]
- Medina, J.A.; Kondo, T. Listado taxonómico de organismos que afectan la pitaya amarilla, Selenicereus megalanthus (K. Schum. ex Vaupel) Moran (Cactaceae) en Colombia. Corporación Colomb. Investig. Agropecu. 2012, 13, 41–46. [Google Scholar] [CrossRef]
- MacGowan, I.; Rotheray, G.E. Lonchaeidae (lance flies). In Manual of Afrotropical Diptera. Volume 3. Brachycera–Cyclorrhapha, Excluding Calyptratae. Suricata; Kirk-Spriggs, A.H., Sinclair, B.J., Eds.; South African National Biodiversity Institute: Pretoria, South Africa, 2021; pp. 1587–1596. Available online: https://www.nhm.ac.uk/our-science/our-work/biodiversity/manual-afrotropical-diptera.html (accessed on 9 August 2025).
- Garcia, F.R.M.; Norrbom, A.L. Tephritoid flies (Diptera, Tephritoidea) and their plant hosts from the state of Santa Catarina in southern Brazil. Fla. Entomol. 2011, 94, 151–157. [Google Scholar] [CrossRef]
- Uchôa, M.A. Fruit flies (Diptera: Tephritoidea): Biology, host plants, natural enemies, and the implications to their natural control. In Integrated Pest Management and Pest Control: Current and Future Tactics; Larramendy, M.L., Soloneski, S., Eds.; InTech: Rijeka, Croatia, 2012; pp. 271–300. [Google Scholar]
- Lemos, L.N.; Adaime, R.; Costa-Neto, S.V.; Deus, E.G.; Jesus-Barros, C.R.; Strikis, P.C. New Findings on Lonchaeidae (Diptera: Tephritoidea) in the Brazilian Amazon. Fla. Entomol. 2015, 98, 1227–1237. [Google Scholar] [CrossRef]
- Uchôa-Fernandes, M.A.; de Oliveira, I.; Molina, R.M.S.; Zucchi, R.A. Species diversity of frugivorous flies (Diptera: Tephritoidea) from hosts in the cerrado of the state of Mato Grosso do Sul, Brazil. Neotrop. Entomol. 2002, 31, 515–524. [Google Scholar] [CrossRef]
- Strikis, P.; Prado, A. A new species of the genus Neosilba (Diptera: Lonchaeidae). Zootaxa 2005, 828, 1–4. [Google Scholar] [CrossRef]
- Raga, A.; Souza-Filho, M.F.; Machado, R.A.; Sato, M.E.; Siloto, R.C. Host ranges and infestation indices of fruit flies (Tephritidae) and lance flies (Lonchaeidae) in São Paulo State, Brazil. Fla. Entomol. 2011, 94, 787–794. [Google Scholar] [CrossRef]
- Pitkin, B.R. Family Lonchaeidae. In Catalog of the Diptera of the Australasian and Oceanic Regions; Pitkin, B.R., Ed.; Bishop Museum Press: Honolulu, HI, USA, 1996; pp. 476–478. [Google Scholar]
- Ahlmark, K.; Steck, G.J. A new U.S. record for a secondary fruit infester, Neosilba batesi (Curran) (Diptera: Lonchaeidae). Insecta Mundi 1997, 11, 116. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).