Pre-Breeding of Promising Coffea canephora Genotypes
Abstract
1. Introduction
2. Materials and Methods
2.1. Morphological Analysis of Conilon Coffee Seedlings
2.1.1. Experimental Location
2.1.2. Genetic Material
2.1.3. Seedling Propagation and Experimental Design
2.1.4. Morphophysiological Assessments
2.1.5. Statistical Analysis
2.2. Molecular Analysis
2.2.1. Sample Collection
2.2.2. DNA Extraction
2.2.3. PCR Amplification
2.2.4. Electrophoresis and Data Analysis
2.2.5. Statistical Analysis
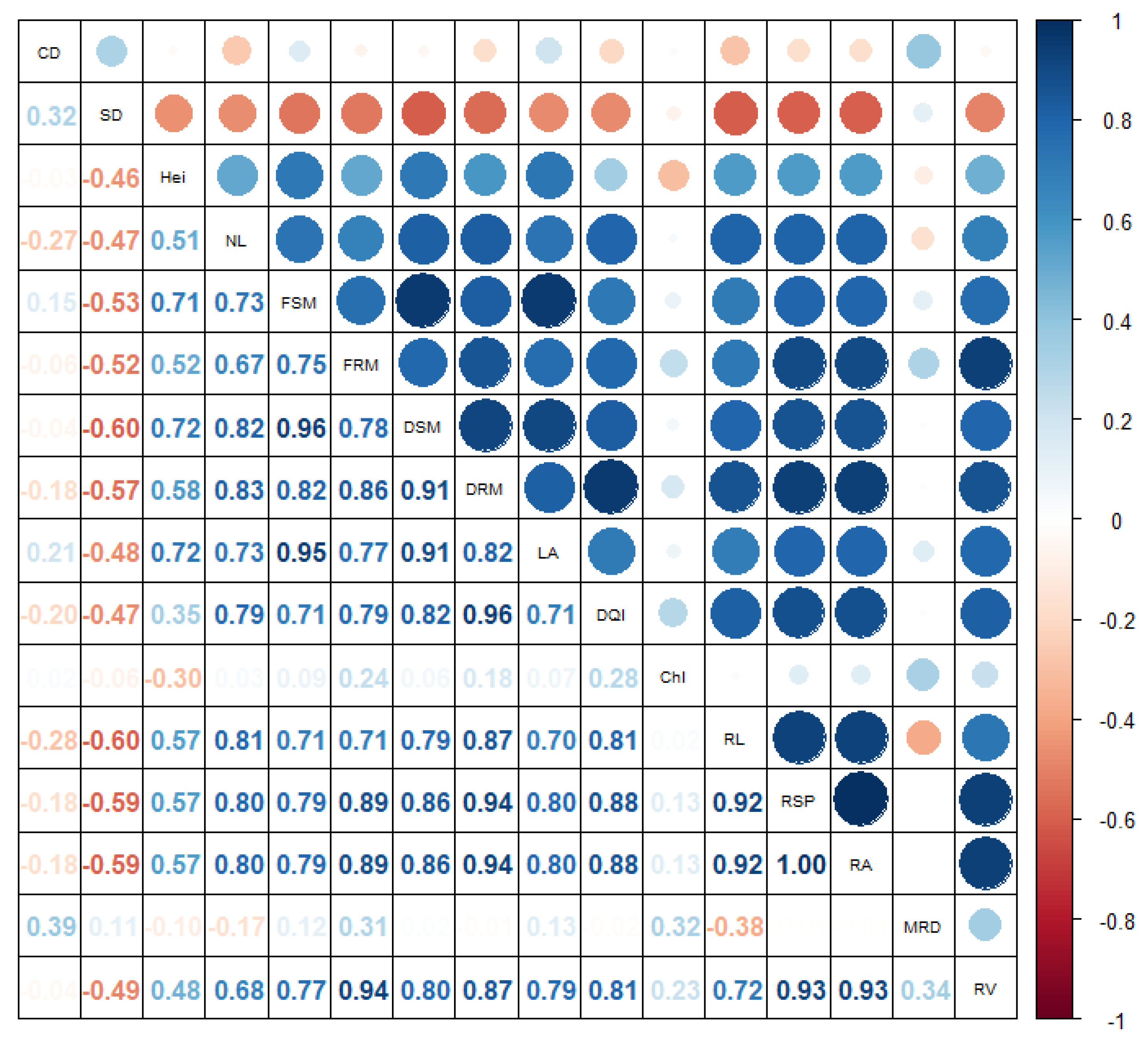
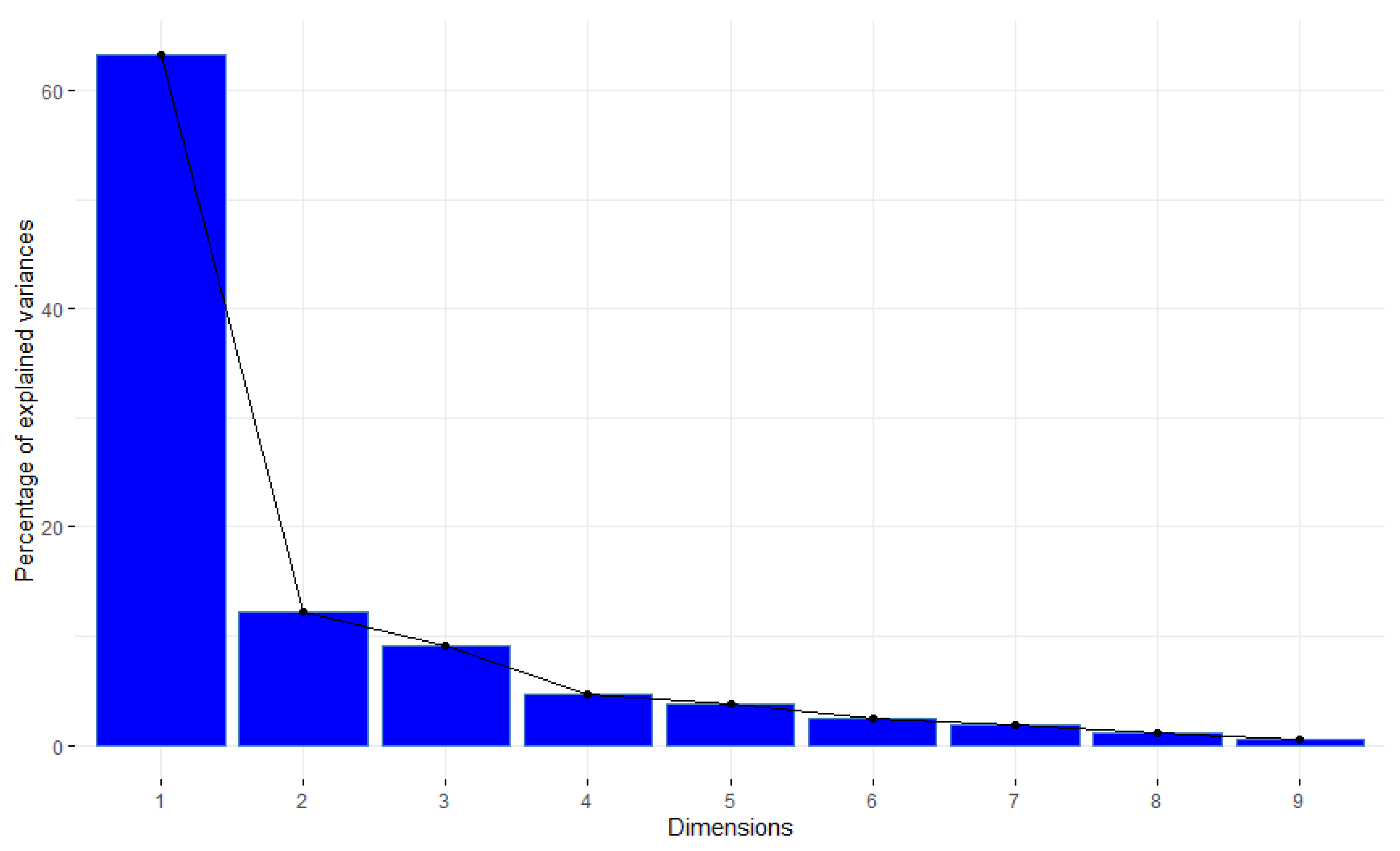
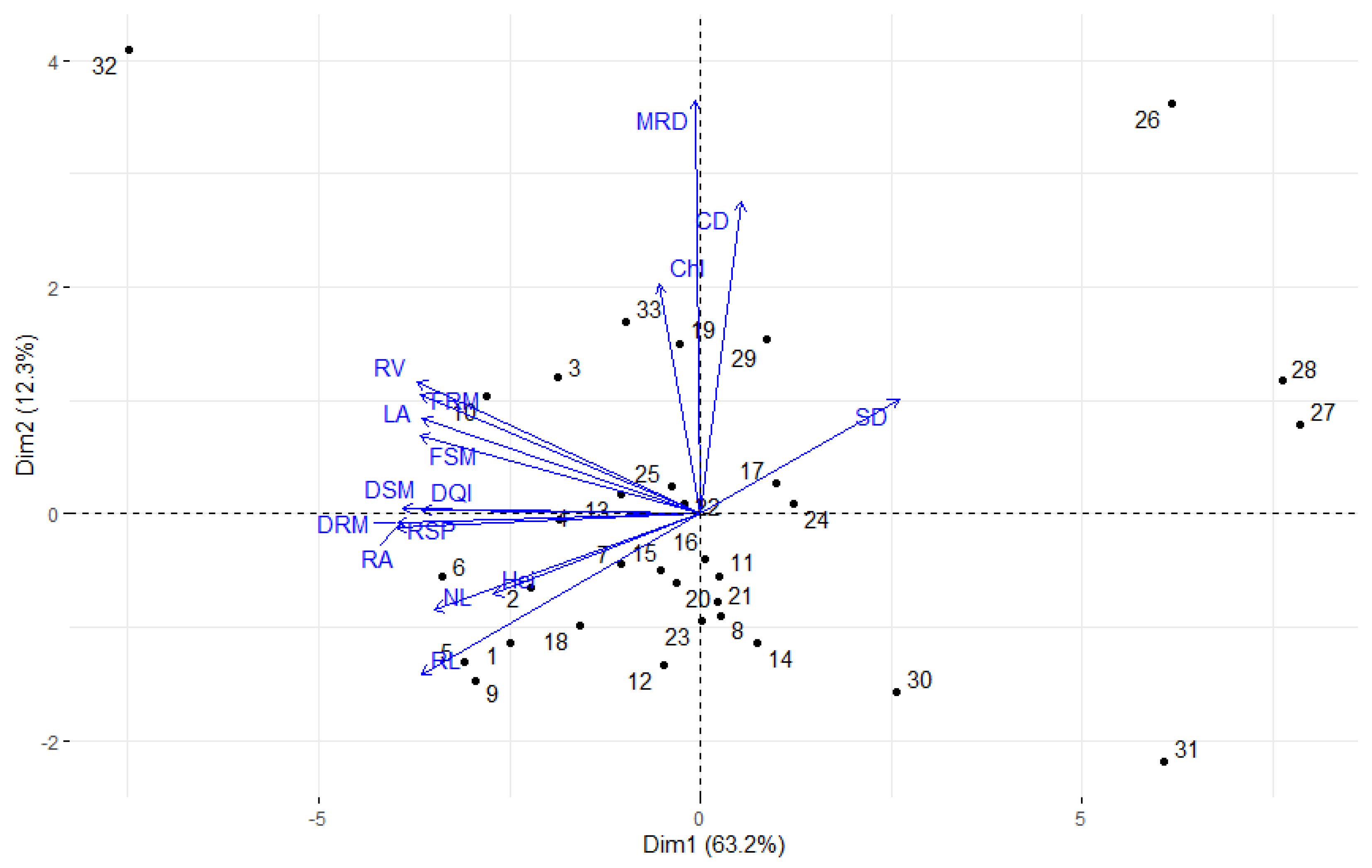
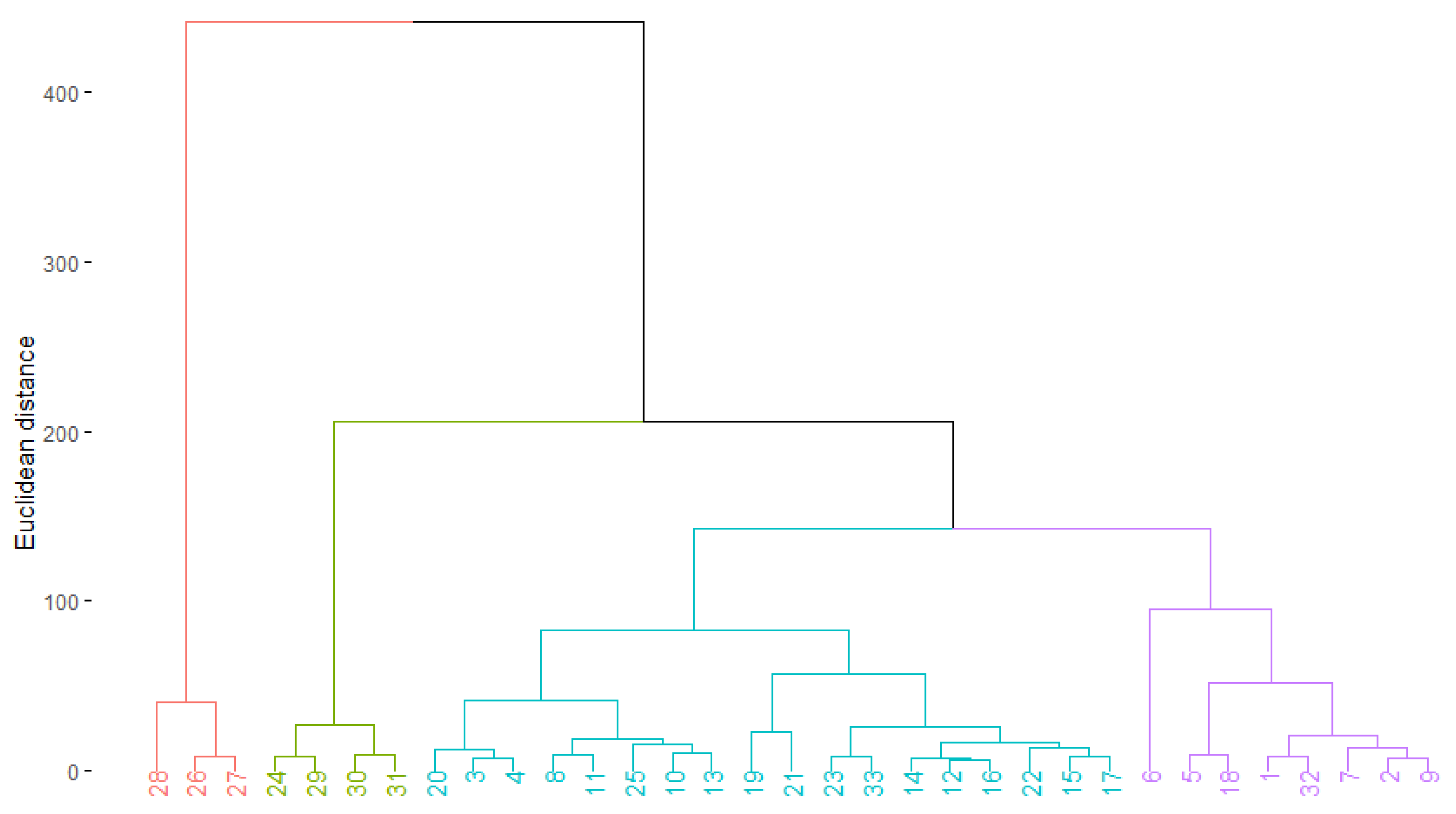
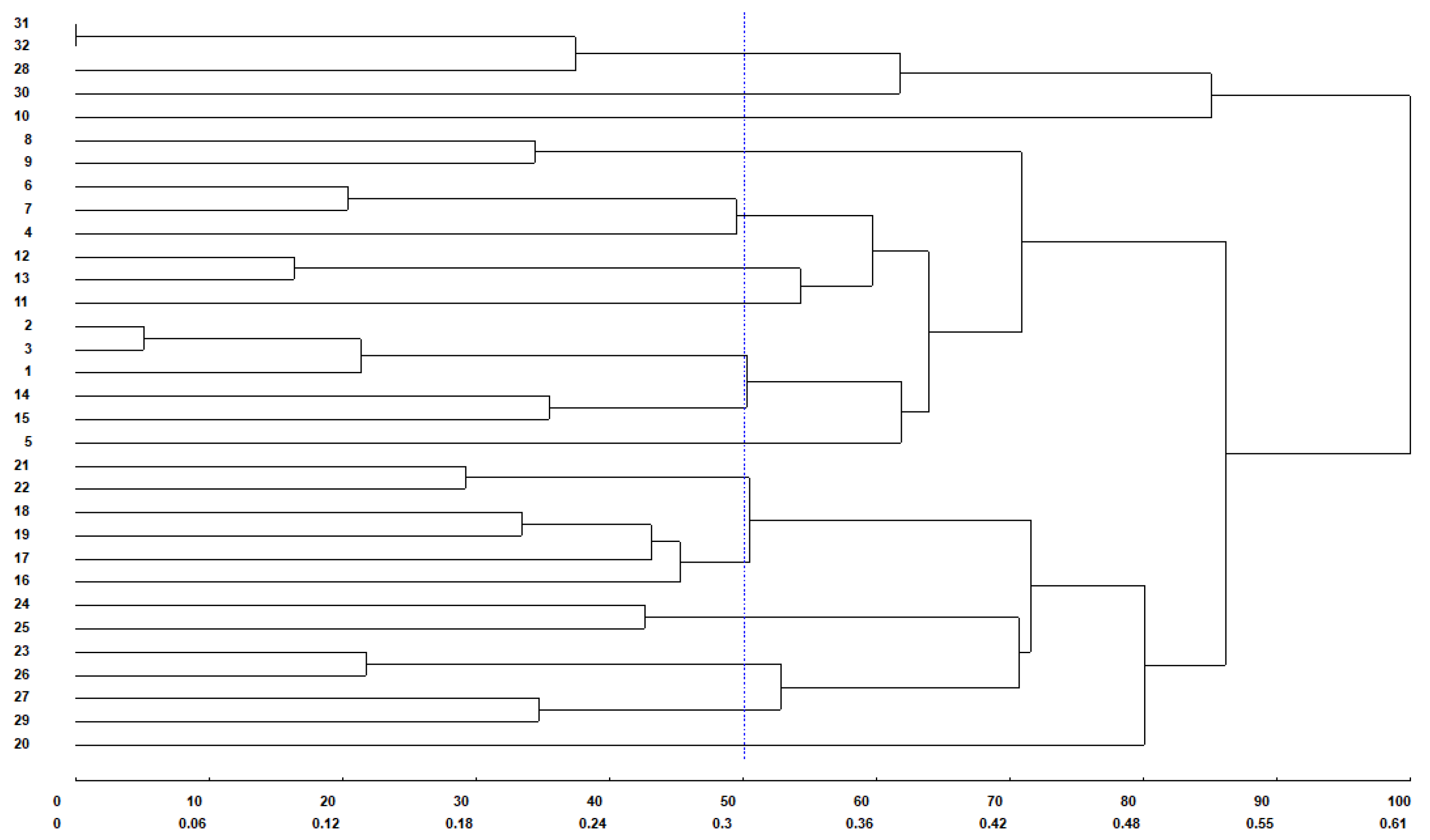
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sala, P.I.A.L.; Amabile, R.F.; Veiga, A.D.; Malaquias, J.V.; Fagioli, M.; Brige, F.A.A. Response of Conilon Coffee Cultivars in the Central Cerrado in Irrigated Management Pivot Sprinkler. Contrib. A Las Cienc. Soc. 2023, 16, 8408–8422. [Google Scholar] [CrossRef]
- Brige, F.A.A.; Amabile, R.F.; Malaquias, J.V.; Veiga, A.D.; Maciel, N.B.A.; Fialho, A.R.; Sala, P.I.A.L.; Fagioli, M. Genetic Parameters in Conilon Coffee in an Irrigated Systema in Cerrado. Contrib. A Las Cienc. Soc. 2023, 16, 1140–1156. [Google Scholar] [CrossRef]
- Companhia Nacional de Abastecimento (Conab) Série Histórica Café Conilon. Available online: http://www.conab.gov.br/ (accessed on 23 October 2024).
- Facco, A.G.; Salomão, B.A.; Andrade, R.G. Zoning of Coffee Growing Areas Suitable for Agricultural Mechanization in the State of Espírito Santo; Seven Editora: São José dos Pinhais, Brazil, 2023. [Google Scholar]
- Reis, G.d.O.S.; Melo, B.M.R.d.; Ferreira, S.; Pinto, L.V.A. Growth, Nutrition and Health of Coffee Seedlings Grown with Increasing Limestone Doses in the Substrate. Rev. Agrogeoambiental 2023, 15, e20231729. [Google Scholar] [CrossRef]
- Lani, J.A.; Benasssi, A.C.; Fanton, C.J.; Bravim, A.J.B. Influência de Tipos de Mudas e Preparo de Covas No Desenvolvimento e Produção Do Cafeeiro Conilon (Coffea canephora L. Pierre Ex. Froehner). In Proceedings of the Anais do IV Simpósio de Pesquisa dos Cafés do Brasil, Londrina, Brasil, 2–5 May 2005; pp. 1–4. [Google Scholar]
- Dardengo, M.C.J.D.; Sousa, E.F.; Reis, E.F.D.; Gravina, G. de A. Qualidade de Mudas de Café Conilon Produzidas Em Diferentes Recipientes e Níveis de Sombreamento. Coffee Sci. 2013, 8, 500–509. [Google Scholar]
- Bezerra, C.d.S.; Tomaz, J.S.; Valente, M.S.F.; Espindula, M.C.; Marques, R.L.S.; Tadeu, H.C.; Ferreira, F.M.; Silva, G.d.S.; Meneses, C.H.S.G.; Lopes, M.T.G. Phenotypic Diversity and Genetic Parameters of Coffea Canephora Clones. Plants 2023, 12, 4052. [Google Scholar] [CrossRef]
- Silva, L.O.E.; Schmidt, R.; Valani, G.P.; Ferreira, A.; Ribeiro-Barros, A.I.; Partelli, F.L. Root Trait Variability in Coffea Canephora Genotypes and Its Relation to Plant Height and Crop Yield. Agronomy 2020, 10, 1394. [Google Scholar] [CrossRef]
- Senra, J.F.d.B.; da Silva, J.A.; Ferrão, M.A.G.; Esposti, M.D.D.; Milheiros, I.S.; Fassarella, K.M. Genetic Variability of Conilon Coffee Population from Cultivar ‘ES8152’ Based on Morphoagronomic Variables. Coffee Sci. 2022, 17, e171986. [Google Scholar] [CrossRef]
- Giles, J.A.D.; Partelli, F.L.; Ferreira, A.; Rodrigues, J.P.; Oliosi, G.; E Silva, F.H.L. Genetic Diversity of Promising ‘Conilon’ Coffee Clones Based on Morpho-Agronomic Variables. An. Acad. Bras. Cienc. 2018, 90, 2437–2446. [Google Scholar] [CrossRef] [PubMed]
- Zolet, A.C.T.; Turchetto, C.; Guzman, F.; Arias, G.A.S.; Ludwig, F.S.; Veto, N.M. Polimorfismo de Nucleotídeo Único (SNP): Metodologias de Identificação, Análise e Aplicações. In Marcadores Moleculares na Era Genômica: Metodologias e Aplicações; Zolet, A.C.T., Turchetto, C., Zanella, C.M., Passaia, G., Eds.; Sociedade Brasileira de Genética: Ribeirão Preto, Brazil, 2017; pp. 132–179. ISBN 9788589265263. [Google Scholar]
- Alotaibi, M.O.; Abd-Elgawad, M.E. ISSR and SCoT for Evaluation of Hereditary Differences of 29 Wild Plants in Al Jubail Saudi Arabian. Saudi J. Biol. Sci. 2022, 29, 3223–3231. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.R.d.; Oliveira, M.S.P.d.; Moura, E.F.; Rodrigues, S.M. Seleção de Primers ISSR Para Uso Em Genomas de Camucamuzeiro. In Proceedings of the 17° Seminário de Iniciação Científica e 1° Seminário de Pós-graduação da Embrapa Amazônia Oriental, Belém, Brazil, 23 August 2013. [Google Scholar]
- Amruthakumar, S.; Manivel, B.; Sivamani, K.; Sethuraman, T.; Rao, N.S.P.; Ganesh, D. Molecular Identity for Commercially Important Inter-Specific Hybrids of Coffea Using ISSR-DNA Marker: Implication on Genetic Improvement. Plant Biotechnol. Rep. 2024, 18, 425–436. [Google Scholar] [CrossRef]
- Panaligan, A.C.; Baltazar, M.D.; Alejandro, G.J.D. Short Communication: Genetic Polymorphism of Registered and Popularly Cultivated Coffee (Coffea spp.) in the Philippines Using Inter-Simple Sequence Repeats Markers. Biodiversitas 2020, 21, 4228–4233. [Google Scholar] [CrossRef]
- Motta, L.B.; Soares, T.C.B.; Ferrão, M.A.G.; Caixeta, E.T.; Lorenzoni, R.M.; Neto, J.D. de S. Molecular Characterization of Arabica and Conilon Coffee Plants Genotypes by SSR and ISSR Markers. Braz. Arch. Biol. Technol. 2014, 57, 728–735. [Google Scholar] [CrossRef]
- Valadares, F.V.; Santos, R.M.; Dutra, I.P.; Neto, J.D.d.S.; Moulin, M.M. Análise Morfoagronômica e Molecular Das Cultivares de Café Do Ifes Campus de Alegre. Enciclopédia Biosf. 2016, 13, 87–102. [Google Scholar] [CrossRef]
- Verleysen, L.; Bollen, R.; Kambale, J.L.; Ebele, T.; Katshela, B.N.; Depecker, J.; Poncet, V.; Assumani, D.M.; Vandelook, F.; Stoffelen, P.; et al. Characterization of the Genetic Composition and Establishment of a Core Collection for the INERA Robusta Coffee (Coffea Canephora) Field Genebank from the Democratic Republic of Congo. Front. Sustain. Food Syst. 2023, 7, 1239442. [Google Scholar] [CrossRef]
- Rodrigues, M.J.L.; da Silva, C.A.; Braun, H.; Partelli, F.L. Nutritional Balance and Genetic Diversity of Coffea Canephora Genotypes. Plants 2023, 12, 1451. [Google Scholar] [CrossRef] [PubMed]
- Pamphile, N.N.; Eric, E.E.; Raphaelle, W.O. Caractérisation de La Diversité Génétique Des Caféiers Robusta (Coffea Canephora L.) Exploités Au Sud-Est Du Gabon à Travers l’analyse Par Microsatellites Des Génotypes. Rev. Afr. d’environnement et d’agriculture 2024, 6, 111–119. [Google Scholar] [CrossRef]
- Pereira, M.G.; Pereira, T.N.S.; Costa, F. Marcadores Moleculares no Pré-Melhoramento. In Marcadores Moleculares; Borém, A., Caixeta, E., Eds.; Editora UFV: Viçosa, Brazil, 2016; pp. 94–128. ISBN 9788572695558. [Google Scholar]
- Covre, A.M.; Canal, L.; Partelli, F.L.; Alexandre, R.S.; Ferreira, A.; Vieira, H.D. Development of Clonal Seedlings of Promising Conilon Coffee (Coffea canephora) Genotypes. Aust. J. Crop Sci. 2016, 10, 385–392. [Google Scholar] [CrossRef]
- Silva, F.L.D.; Baffa, D.C.F.; Rezende, J.C.D.; Oliveira, A.V.B.d.; Pereira, A.A.; Cruz, C.D. Genetic Variability among Robusta Coffee Genotypes in the State of Minas Gerais. Coffee Sci. 2015, 10, 20–27. [Google Scholar]
- Kiwuka, C.; Goudsmit, E.; Tournebize, R.; De Aquino, S.O.; Douma, J.C.; Bellanger, L.; Crouzillat, D.; Stoffelen, P.; Sumirat, U.; Legnate, H.; et al. Genetic Diversity of Native and Cultivated Ugandan Robusta Coffee (Coffea Canephora Pierre Ex A. Froehner): Climate Influences, Breeding Potential and Diversity Conservation. PLoS ONE 2021, 16, e0245965. [Google Scholar] [CrossRef]
- Zaidan, I.R.; Ferreira, A.; Noia, L.R.; Santos, J.G.; de Arruda, V.C.; do Couto, D.P.; Braz, R.A.; de Brites Senra, J.F.; Partelli, F.L.; Azevedo, C.F.; et al. Diversity and Structure of Coffea Canephora from Old Seminal Crops in Espírito Santo, Brazil: Genetic Resources for Coffee Breeding. Tree Genet. Genomes 2023, 19, 19. [Google Scholar] [CrossRef]
- Verdin Filho, A.C.; Volpi, P.S.; Ferrão, R.G.; Ferrão, M.A.G.; Fonseca, A.F.A.d.; Freitas, S.d.J.; Comério, M.; Rodrigues, W.N.; Colodetti, T.V.; Andrade Júnior, S.d.; et al. Produção de Mudas Clonais de Cafeeiro: Avanços Na Padronização Dos Cortes e Dimensões de Estacas; SIDALC: Vitória, ES, Spain, 2022. [Google Scholar]
- Ferrão, R.G.; Fonseca, A.F.A.d.; Ferrão, M.A.G.; Verdin Filho, A.C.; Volpi, P.S.; Muner, L.H.d.; Lani, J.A.; Prezotti, L.C.; Ventura, J.A.; Martins, D. dos S.; et al. Conilon Coffee: Techniques for Production with Improved Varieties, 4th ed.; Technical Circular n° 03-I; Incaper: Vitória, Spain, 2012. [Google Scholar]
- DICKSON, A.; LEAF, A.L.; HOSNER, J.F. Quality Appraisal of White Spruce and White Pine Seedling Stock in Nurseries. For. Chron. 1960, 36, 10–13. [Google Scholar] [CrossRef]
- R Core Team R. R Core Team R: A Language and Environment for Statistical Computing; Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Doyle, J.J. Isolation of Plant DNA from Fresh Tissue. Focus 1990, 12, 13–15. [Google Scholar]
- Berilli, A.P.C.G.; Pereira, M.G.; dos Santos Trindade, R.; da Costa, F.R.; da Cunha, K.S. Resposta a Seleção No 11o Ciclo de Seleção Recorrente Recíproca Entre Famílias de Irmãoscompletos de Milho. Acta Sci. Agron. 2013, 35, 435–441. [Google Scholar] [CrossRef][Green Version]
- Zietkiewicz, E.; Rafalsk, A.; Labuda, D. Genome Fingerprinting by Simple Sequence Repeat (SSR)-Anchored Polymerase Chain Reaction Amplification. Genomics 1994, 20, 176–183. [Google Scholar] [CrossRef]
- Jaccard, P. Étude Comparative de La Distribution Florale Dans Une Portion Des Alpes et Des Jura. Bull. Soc. Vaudoise Sci. Nat. 1901, 37, 547–579. [Google Scholar]
- Mojena, R. Hierarchical Grouping Methods and Stopping Rules: An Evaluation. Comput. J. 1977, 20, 359–363. [Google Scholar] [CrossRef]
- Milligan, G.W.; Cooper, M.C. An Examination of Procedures for Determining the Number of Clusters in a Data Set. Psychometrika 1985, 50, 159–179. [Google Scholar] [CrossRef]
- Cruz, C.D. GENES—Software Para Análise de Dados Em Estatística Experimental e Em Genética Quantitativa. Acta Sci. Agron. 2013, 35, 271–276. [Google Scholar] [CrossRef]
- Mantel, N. The Detection of Disease Clustering and a Generalized Regression Approach. Cancer Res. 1967, 27, 209–220. [Google Scholar]
- Santos, M.M.d.; Oliveira, M.G.; Cassol, D.; Rodrigues, W.P.; Falqueto, A.R.; Ramalho, J.C.; Partelli, F.L. Genotypic Diversity of Coffea Canephora Cv. Conilon Identified through Leaf Morpho- and Eco-Physiological Traits. Sci. Hortic. 2024, 324, 112603. [Google Scholar] [CrossRef]
- Ferrão, M.A.G.; Mendonça, R.F.d.; Fonseca, A.F.A.; Ferrão, R.G.; Senra, J.F.B.; Volpi, P.S.; Filho, C.A.V.; Comério, M. Characterization and Genetic Diversity of Coffea Canephora Accessions in a Germplasm Bank in Espírito Santo, Brazil. Crop Breed. Appl. Biotechnol. 2021, 21, e36132123. [Google Scholar] [CrossRef]
- de Lima, A.E.; Castro, E.d.M.; da Cunha, S.H.B.; Guimarães, R.J.; Chalfun, N.N.J.; de Carvalho, A.M.; Alves, E.; Carvalho, M.A.F. Production of Coffea Canephora Seedlings through Cuttings in a Nursery and Hydroponics Using Different Containers. Coffee Sci. 2023, 18, e182084. [Google Scholar] [CrossRef]
- Silva, C.A.d.; Martins, J.K.D.; Damaceno, J.B.D.; Enck, B.F.; Silva, D.R.d.; Domingues, C.G.; Luz, S.R.O.T.d.; Oliveira, V.d.S.; Moraes, F.d.; Altoé, M.S.; et al. Formation of Seedlings of Coffea Canephora Pierre Ex Froehner and Weed Control Under Application of Herbicides Oxyfluorfen and Pendimethalin. J. Agric. Sci. 2019, 11, 312. [Google Scholar] [CrossRef]
- Moncada, M.D.P.; Tovar, E.; Montoya, J.C.; González, A.; Spindel, J.; McCouch, S. A Genetic Linkage Map of Coffee (Coffea Arabica L.) and QTL for Yield, Plant Height, and Bean Size. Tree Genet. Genomes 2016, 12, 5. [Google Scholar] [CrossRef]
- Gartner, G.A.L.; McCouch, S.R.; Moncada, M.D.P. A Genetic Map of an Interspecific Diploid Pseudo Testcross Population of Coffee. Euphytica 2013, 192, 305–323. [Google Scholar] [CrossRef]
- Affleck-Graves, J.F.; Sobowale, I.O.; Money, A.H. A Principal Component Index Subject to Constraints. Invest. Anal. J. 1979, 8, 45–50. [Google Scholar] [CrossRef]
- Barbosa, I.d.P.; da Costa, W.G.; Nascimento, M.; Cruz, C.D.; de Oliveira, A.C.B. Recommendation of Coffea Arabica Genotypes by Factor Analysis. Euphytica 2019, 215, 178. [Google Scholar] [CrossRef]
- Amidou, N.; Michel, N.; Serge, H.; Valérie, P. Genetic Basis of Species Differentiation between Coffea Liberica Hiern and C. Canephora Pierre: Analysis of an Interspecific Cross. Genet. Resour. Crop Evol. 2007, 54, 1011–1021. [Google Scholar] [CrossRef]
- Filho, A.C.V.; Freitas, S.D.J.; Comério, M.; Volpi, P.S.; Colodetti, T.V.; Rodrigues, W.N.; Fonseca, A.F.A.D.; Posse, S.C.P.; Fontes, A.G.; Christo, B.F.; et al. Implications of the Cut Type and Apex Length of Stem Cuttings Used for the Production of Plantlets of Conilon Coffee. Coffee Sci. 2020, 15, e151770. [Google Scholar] [CrossRef]
- Bollen, R.; Verleysen, L.; Katshela, B.N.; Kambale, J.L.; Ebele, T.; Ruttink, T.; Vandelook, F.; Honnay, O.; Stoffelen, P. Sensory Profiles of Robusta Coffee (Coffea canephora) Genetic Resources from the Democratic Republic of the Congo. Front. Sustain. Food Syst. 2024, 8, 1382976. [Google Scholar] [CrossRef]
- Souza, F.d.F.; Caixeta, E.T.; Ferrão, L.F.V.; Pena, G.F.; Sakiyama, N.S.; Zambolim, E.M.; Zambolim, L.; Cruz, C.D. Molecular Diversity in Coffea Canephora Germplasm Conserved and Cultivated in Brazil. Crop Breed. Appl. Biotechnol. 2013, 13, 221–227. [Google Scholar] [CrossRef]
- Solórzano, R.G.L.; Bellis, F.; Leroy, T.; Plaza, L.; Guerrero, H.; Subia, C.; Calderón, D.; Fernández, F.; Garzón, I.; Lopez, D.; et al. Revealing the Diversity of Introduced Coffea Canephora Germplasm in Ecuador: Towards a National Strategy to Improve Robusta. Sci. World J. 2017, 2017, 1248954. [Google Scholar] [CrossRef]
- Huded, A.K.C.; Jingade, P.; Bychappa, M.; Mishra, M.K. Genetic Diversity and Population Structure Analysis of Coffee (Coffea canephora) Germplasm Collections in Indian Gene Bank Employing SRAP and SCoT Markers. Int. J. Fruit. Sci. 2020, 20, S757–S784. [Google Scholar] [CrossRef]
- Lahai, P.M.; Bah, M.A.; Lahai, M.T.; Aikpokpodion, P.O.; Johnson, R.A.B. Genetic Diversity of Coffee Germplasm in Sierra Leone: Implications for Conservation and Breeding Programs. Beverage Plant Res. 2023, 3, 26. [Google Scholar] [CrossRef]
- Adepoju, A.F.; Sobowale, I.O.; Adenuga, O.O. Genetic Diversity of Coffee Genotypes Using Simple Sequence Repeat (SSR) Markers. Eur. J. Med. Plants 2023, 34, 14–20. [Google Scholar] [CrossRef]
- Hemphill, J.F. Interpreting the Magnitudes of Correlation Coefficients. Am. Psychol. 2003, 58, 78–79. [Google Scholar] [CrossRef]
- Ferrão, L.F.V.; Caixeta, E.T.; Souza, F.d.F.; Zambolim, E.M.; Cruz, C.D.; Zambolim, L.; Sakiyama, N.S. Comparative Study of Different Molecular Markers for Classifying and Establishing Genetic Relationships in Coffea Canephora. Plant Syst. Evol. 2013, 299, 225–238. [Google Scholar] [CrossRef]
- Leroy, T.; De Bellis, F.; Legnate, H.; Musoli, P.; Kalonji, A.; Loor Solórzano, R.G.; Cubry, P. Developing Core Collections to Optimize the Management and the Exploitation of Diversity of the Coffee Coffea Canephora. Genetica 2014, 142, 185–199. [Google Scholar] [CrossRef]
- Putranto, R.A. Molecular Markers and Their Application for DNA Fingerprinting and Genetic Diversity Studies in Coffea Species. Menara Perkeb. 2014, 82, 39–50. [Google Scholar]
- Ruas, P.M.; Ruas, C.F.; Rampim, L.; Carvalho, V.P.; Ruas, E.A.; Sera, T. Genetic Relationship in Coffea Species and Parentage Determination of Interspecific Hybrids Using ISSR (Inter-Simple Sequence Repeat) Markers. Genet. Mol. Biol. 2003, 26, 319–327. [Google Scholar] [CrossRef]
| Variable | Acronym | Measurement |
|---|---|---|
| Plant height | Hei | Measurement with graduated ruler |
| Stem diameter | SD | Measured with a digital caliper in the middle third of the stem |
| Crown diameter | CD | Measurement with graduated ruler |
| Number of leaves | NL | Counted manually |
| Leaf area size | LA | Measured with the automatic leaf area meter model Li-cor®, model: Li-3100C (Manufacturer LI-COR Biosciences, Sao Paulo, Brazil). |
| Fresh and dry shoot and root mass | FSM, FRM, DSM and DRM | Estimated on a precision scale. |
| Relative chlorophyll content | Chl | Determined using a portable chlorophyll meter (model SPAD-502, Minolta) |
| Root length, root volume, root surface projection, root surface area, and Mean root diameter | RL, RV, RSP, RA and MRD | Analyzed by EPSON STD 4800 root scanner and WinRHIZO Pro 2022a software. |
| Dickson Quality Index | DQI | Calculated by the equation [29]: DQI = [(RDM + SDM)/(Hei/SD + SDM/RDM)] |
| Variable | MS | Mean | CV (%) |
|---|---|---|---|
| Crown diameter (cm) | 75.71 ** | 20.31 | 27.48 |
| Stem diameter (mm) | 3.30 ** | 10.78 | 8.99 |
| Height (cm) | 109.02 ** | 7.41 | 13.40 |
| Number of leaves | 59.62 ** | 9.60 | 33.59 |
| Fresh shoot mass (g) | 31.87 ** | 10.15 | 22.13 |
| Fresh root mass (g) | 8.59 ** | 4.10 | 29.51 |
| Dry shoot mass (g) | 4.15 ** | 3.01 | 25.18 |
| Dry root mass (g) | 1.07 ** | 1.27 | 31.42 |
| Leaf area (cm2) | 29,527.51 ** | 228.98 | 26.19 |
| Dickson Quality Index | 0.62 ** | 1.06 | 29.7 |
| Chlorophyll | 45.87 ** | 24.93 | 18.65 |
| Root length (cm) | 211,208 ** | 671.32 | 18.18 |
| Root surface projection (cm2) | 952.9 ** | 44.34 | 20.94 |
| Root surface area (cm2) | 9404.7 ** | 139.3 | 20.94 |
| Mean root diameter (mm) | 0.03 ** | 0.67 | 12.03 |
| Root volume (cm3) | 3.78 ** | 2.34 | 28.76 |
| Treat | CD | SD | Hei | NL | FSM | FRM | DSM | DRM | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 18.06 | b | 9.25 | b | 18.00 | c | 12.38 | a | 10.65 | b | 5.42 | b | 3.24 | b | 1.31 | c |
| 2 | 19.00 | b | 10.30 | b | 21.00 | b | 9.00 | b | 12.17 | a | 4.90 | b | 3.67 | a | 1.44 | c |
| 3 | 23.81 | a | 12.09 | a | 19.00 | b | 12.38 | a | 12.09 | a | 5.07 | b | 3.41 | b | 1.40 | c |
| 4 | 19.75 | b | 10.45 | b | 20.44 | b | 9.38 | b | 10.68 | b | 5.18 | b | 3.10 | b | 1.55 | b |
| 5 | 20.38 | b | 10.26 | b | 25.81 | a | 12.75 | a | 12.11 | a | 4.58 | b | 3.78 | a | 1.53 | b |
| 6 | 20.00 | b | 10.27 | b | 18.06 | c | 11.75 | a | 13.17 | a | 4.80 | b | 3.63 | a | 1.60 | b |
| 7 | 22.44 | a | 10.79 | b | 18.31 | c | 9.88 | a | 10.26 | c | 4.04 | b | 3.25 | b | 1.52 | b |
| 8 | 18.19 | b | 10.83 | b | 16.38 | c | 10.75 | a | 9.36 | c | 3.25 | c | 2.74 | b | 1.29 | c |
| 9 | 18.94 | b | 10.71 | b | 17.88 | c | 15.00 | a | 12.79 | a | 3.96 | b | 4.20 | a | 1.76 | b |
| 10 | 22.94 | a | 10.17 | b | 24.06 | a | 9.50 | b | 12.89 | a | 4.33 | b | 3.73 | a | 1.51 | b |
| 11 | 22.88 | a | 10.87 | b | 19.00 | b | 8.38 | b | 9.99 | c | 4.24 | b | 2.91 | b | 1.28 | c |
| 12 | 15.50 | b | 10.51 | b | 23.38 | a | 8.63 | b | 11.00 | b | 4.21 | b | 3.39 | b | 1.28 | c |
| 13 | 17.63 | b | 10.62 | b | 17.06 | c | 8.38 | b | 9.75 | c | 4.72 | b | 2.98 | b | 1.58 | b |
| 14 | 21.56 | a | 10.93 | b | 18.44 | c | 10.75 | a | 10.59 | b | 2.99 | c | 3.01 | b | 1.07 | c |
| 15 | 19.44 | b | 10.73 | b | 19.50 | b | 10.50 | a | 11.05 | b | 3.97 | b | 3.27 | b | 1.26 | c |
| 16 | 17.56 | b | 11.24 | a | 19.38 | b | 11.13 | a | 8.67 | c | 4.46 | b | 2.59 | b | 1.30 | c |
| 17 | 22.56 | a | 10.76 | b | 12.69 | e | 9.13 | b | 9.38 | c | 3.66 | b | 2.60 | b | 1.14 | c |
| 18 | 20.38 | b | 10.74 | b | 17.31 | c | 12.25 | a | 10.25 | c | 4.25 | b | 3.24 | b | 1.46 | c |
| 19 | 28.38 | a | 10.72 | b | 19.69 | b | 8.50 | b | 11.66 | b | 4.01 | b | 3.54 | a | 1.36 | c |
| 20 | 17.63 | b | 10.69 | b | 11.88 | e | 8.88 | b | 8.79 | c | 4.40 | b | 2.80 | b | 1.44 | c |
| 21 | 17.38 | b | 10.68 | b | 16.31 | c | 11.50 | a | 9.60 | c | 3.95 | b | 3.01 | b | 1.31 | c |
| 22 | 15.31 | b | 10.67 | b | 12.31 | e | 11.00 | a | 9.18 | c | 4.10 | b | 3.03 | b | 1.43 | c |
| 23 | 16.13 | b | 10.68 | b | 17.63 | c | 11.50 | a | 9.33 | c | 4.22 | b | 2.94 | b | 1.30 | c |
| 24 | 19.63 | b | 11.06 | b | 20.13 | b | 8.63 | b | 9.18 | c | 4.16 | b | 2.79 | b | 1.21 | c |
| 25 | 18.38 | b | 10.51 | b | 15.13 | d | 8.38 | b | 9.83 | c | 4.40 | b | 3.07 | b | 1.33 | c |
| 26 | 27.06 | a | 11.55 | a | 11.25 | e | 4.38 | c | 8.01 | c | 2.94 | c | 1.72 | c | 0.53 | e |
| 27 | 22.75 | a | 11.83 | a | 10.94 | e | 4.13 | c | 6.05 | d | 2.07 | c | 1.24 | c | 0.38 | e |
| 28 | 21.75 | a | 11.35 | a | 11.88 | e | 3.25 | c | 6.88 | d | 1.98 | c | 1.60 | c | 0.37 | e |
| 29 | 23.00 | a | 10.92 | b | 15.44 | d | 8.50 | b | 10.14 | c | 4.20 | b | 2.89 | b | 1.27 | c |
| 30 | 17.75 | b | 10.79 | b | 17.19 | c | 9.00 | b | 9.21 | c | 2.82 | c | 2.76 | b | 0.94 | d |
| 31 | 18.19 | b | 11.54 | a | 11.88 | e | 4.63 | c | 4.87 | d | 2.26 | c | 1.35 | c | 0.63 | e |
| 32 | 22.50 | a | 10.53 | b | 19.13 | b | 14.38 | a | 14.35 | a | 7.42 | a | 4.47 | a | 2.07 | a |
| 33 | 23.38 | a | 10.71 | b | 17.94 | c | 8.25 | b | 11.00 | b | 4.46 | b | 3.34 | b | 1.29 | c |
| Treat | LA | DQI | Chl | RL | RSP | RA | MRD | RV | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 244.24 | c | 1.02 | b | 23.64 | b | 823.75 | a | 54.19 | b | 170.25 | b | 0.66 | c | 2.82 | b |
| 2 | 281.45 | b | 1.10 | b | 24.95 | b | 806.74 | a | 50.96 | b | 160.10 | b | 0.63 | d | 2.55 | b |
| 3 | 287.13 | b | 1.18 | b | 24.00 | b | 771.08 | a | 53.08 | b | 166.76 | b | 0.68 | c | 2.90 | b |
| 4 | 241.41 | c | 1.17 | b | 24.49 | b | 765.86 | a | 51.92 | b | 163.11 | b | 0.68 | c | 2.81 | b |
| 5 | 285.38 | b | 1.05 | b | 22.58 | b | 861.79 | a | 53.67 | b | 168.60 | b | 0.62 | d | 2.64 | b |
| 6 | 308.17 | b | 1.31 | a | 27.31 | a | 920.50 | a | 55.09 | b | 173.05 | b | 0.60 | d | 2.63 | b |
| 7 | 226.04 | c | 1.25 | a | 24.95 | b | 795.56 | a | 49.29 | b | 154.86 | b | 0.61 | d | 2.42 | c |
| 8 | 233.24 | c | 1.11 | b | 28.33 | a | 738.36 | b | 44.14 | c | 138.66 | c | 0.60 | d | 2.08 | c |
| 9 | 270.37 | b | 1.48 | a | 22.04 | b | 807.96 | a | 49.60 | b | 155.81 | b | 0.61 | d | 2.40 | c |
| 10 | 311.83 | b | 1.09 | b | 23.80 | b | 724.32 | b | 53.00 | b | 166.51 | b | 0.74 | b | 3.10 | b |
| 11 | 220.63 | c | 1.03 | b | 23.95 | b | 733.08 | b | 43.92 | c | 137.97 | c | 0.60 | d | 2.07 | c |
| 12 | 213.84 | c | 0.96 | b | 23.88 | b | 682.05 | b | 44.07 | c | 138.46 | c | 0.65 | c | 2.25 | c |
| 13 | 226.75 | c | 1.31 | a | 27.55 | a | 720.44 | b | 48.83 | b | 153.41 | b | 0.68 | c | 2.63 | b |
| 14 | 252.09 | c | 0.91 | b | 22.25 | b | 681.49 | b | 41.02 | c | 128.85 | c | 0.60 | d | 1.95 | c |
| 15 | 263.81 | c | 1.03 | b | 22.59 | b | 667.81 | b | 44.65 | c | 140.28 | c | 0.67 | c | 2.35 | c |
| 16 | 223.84 | c | 1.04 | b | 25.36 | b | 684.99 | b | 45.72 | c | 143.62 | c | 0.67 | c | 2.40 | c |
| 17 | 222.13 | c | 1.08 | b | 25.29 | b | 665.01 | b | 43.16 | c | 135.59 | c | 0.65 | c | 2.24 | c |
| 18 | 239.42 | c | 1.23 | b | 25.31 | b | 863.43 | a | 51.88 | b | 162.97 | b | 0.60 | d | 2.45 | c |
| 19 | 288.22 | b | 1.12 | b | 24.93 | b | 603.77 | b | 39.88 | c | 125.28 | c | 0.67 | c | 2.09 | c |
| 20 | 170.05 | d | 1.37 | a | 23.68 | b | 761.82 | a | 50.96 | b | 160.11 | b | 0.67 | c | 2.70 | b |
| 21 | 213.61 | c | 1.12 | b | 24.05 | b | 622.53 | b | 41.40 | c | 130.06 | c | 0.66 | c | 2.18 | c |
| 22 | 184.46 | d | 1.36 | a | 31.76 | a | 674.07 | b | 44.80 | c | 140.73 | c | 0.66 | c | 2.35 | c |
| 23 | 200.01 | d | 1.09 | b | 24.29 | b | 650.38 | b | 43.44 | c | 136.48 | c | 0.67 | c | 2.31 | c |
| 24 | 226.11 | c | 0.97 | b | 21.38 | b | 522.09 | c | 37.30 | d | 117.18 | e | 0.72 | b | 2.10 | c |
| 25 | 192.36 | d | 1.16 | b | 27.76 | a | 709.23 | b | 48.42 | b | 152.11 | b | 0.68 | c | 2.64 | b |
| 26 | 145.29 | d | 0.53 | d | 29.53 | a | 285.69 | d | 21.84 | e | 68.62 | e | 0.80 | a | 1.37 | d |
| 27 | 107.29 | e | 0.38 | d | 23.55 | b | 281.43 | d | 18.94 | e | 59.51 | e | 0.70 | b | 1.02 | d |
| 28 | 107.41 | e | 0.36 | d | 24.46 | b | 243.56 | d | 17.41 | e | 54.68 | e | 0.75 | b | 1.00 | d |
| 29 | 255.94 | c | 1.14 | b | 26.23 | b | 518.72 | c | 37.11 | d | 116.59 | d | 0.72 | b | 2.12 | c |
| 30 | 187.58 | d | 0.82 | c | 24.29 | b | 547.52 | c | 32.50 | d | 102.11 | d | 0.59 | d | 1.52 | d |
| 31 | 87.01 | e | 0.62 | c | 21.13 | b | 543.97 | c | 31.10 | d | 97.69 | d | 0.57 | d | 1.40 | d |
| 32 | 366.00 | a | 1.65 | a | 28.94 | a | 822.34 | a | 70.99 | a | 223.03 | a | 0.86 | a | 4.85 | a |
| 33 | 273.13 | b | 1.10 | b | 24.38 | b | 652.04 | b | 48.93 | b | 153.73 | b | 0.75 | b | 2.98 | b |
| Primers | Sequence | TNB | NPB |
|---|---|---|---|
| UBC 819 | GTGTGTGTGTGTGTGTA | 5 | 5 |
| UBC 834 | GAGAGAGAGAGAGYT | 7 | 6 |
| UBC 841 | GAGAGAGAGAGAGAGAYC | 5 | 5 |
| UBC 842 | GAGAGAGAGAGAGAGAYG | 5 | 5 |
| UBC 845 | CTCTCTCTCTCTCTCTRG | 4 | 4 |
| UBC 848 | CACACACACACACACARG | 3 | 3 |
| UBC 849 | GTGTGTGTGTGTGTGTYA | 5 | 4 |
| UBC 854 | TCTCTCTCTCTCTCTCRG | 5 | 5 |
| UBC 855 | ACACACACACACACACYT | 4 | 4 |
| UBC 825 | ACACACACACACACACT | 13 | 13 |
| UBC 857 | ACACACACACACACACYG | 5 | 5 |
| UBC 859 | TGTGTGTGTGTGTGTGRC | 6 | 6 |
| UBC 868 | GAAGAAGAAGAAGAAGAA | 3 | 2 |
| UBC 875 | ACACACACACACACACYG | 10 | 7 |
| UBC 879 | CTTCACTTCACTTCA | 10 | 7 |
| UBC 889 | DBDACACACACACACAC | 10 | 8 |
| UBC 895 | AGGTCGCGGCCGCNNNNNNAT | 10 | 8 |
| Echt 5 | AGACAGACGC | 7 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alves, D.I.; Freitas, S.d.J.; Freitas, S.d.P.; Vettorazzi, J.C.F.; Pereira, L.L.; Moreli, A.P.; Partelli, F.L.; Berilli, S.d.S.; Peluzio, J.B.E.; Barbosa, P.d.O.; et al. Pre-Breeding of Promising Coffea canephora Genotypes. Agronomy 2025, 15, 2477. https://doi.org/10.3390/agronomy15112477
Alves DI, Freitas SdJ, Freitas SdP, Vettorazzi JCF, Pereira LL, Moreli AP, Partelli FL, Berilli SdS, Peluzio JBE, Barbosa PdO, et al. Pre-Breeding of Promising Coffea canephora Genotypes. Agronomy. 2025; 15(11):2477. https://doi.org/10.3390/agronomy15112477
Chicago/Turabian StyleAlves, Danielle Inácio, Silvio de Jesus Freitas, Silvério de Paiva Freitas, Julio Cesar Fiorio Vettorazzi, Lucas Louzada Pereira, Aldemar Polonini Moreli, Fábio Luiz Partelli, Sávio da Silva Berilli, João Batista Esteves Peluzio, Poliany de Oliveira Barbosa, and et al. 2025. "Pre-Breeding of Promising Coffea canephora Genotypes" Agronomy 15, no. 11: 2477. https://doi.org/10.3390/agronomy15112477
APA StyleAlves, D. I., Freitas, S. d. J., Freitas, S. d. P., Vettorazzi, J. C. F., Pereira, L. L., Moreli, A. P., Partelli, F. L., Berilli, S. d. S., Peluzio, J. B. E., Barbosa, P. d. O., Adão, J. E. A., Lima, M. d. S. P., & Berilli, A. P. C. G. (2025). Pre-Breeding of Promising Coffea canephora Genotypes. Agronomy, 15(11), 2477. https://doi.org/10.3390/agronomy15112477







