Abstract
Agroforestry systems offer clear environmental and agronomic advantages, but their effect on plant–biotic stressor interactions remains poorly understood. Specifically, the shade from companion trees can create microclimates favorable to fungal diseases on herbaceous crops. This potential drawback may offset other benefits, highlighting the urgent need for advanced plant health monitoring in these systems. This study assessed the potential of hyperspectral reflectance to detect the single and combined effects of simulated tree shading and infection by the fungal pathogen Alternaria alternata on grain sorghum (Sorghum bicolor L. Moench) under rainfed field conditions. Sorghum was grown either under full light or 50% shading conditions. Half of the plots were artificially inoculated with an A. alternata spore suspension (2 × 108 CFU mL−1), while the others served as controls. Leaf and ground-canopy measurements were acquired with a full range spectroradiometer (VNIR-SWIR, 400–2,400 nm) and UAV imagery covered the VIS-NIR range (400–1,000 nm) before the onset of visible symptoms. Permutational multivariate analysis of variance of leaf and ground-canopy data revealed significant effects of shading (Sh), infection (Aa), and their interaction (p < 0.05), allowing early detection of infection two days before symptom appearance, while UAV data showed only singular significant effects. Partial least squares discriminant analysis accuracy reached 78% at the leaf level, 90% at the ground-canopy level, and 74% (Sh) and 75% (Aa) at the UAV scale. Furthermore, vegetation spectral indices derived from the spectra confirmed greater physiological stress in shaded and infected plants, consistent with disease incidence assessments. Our results establish scale-specific hyperspectral reflectance spectroscopy as a powerful, non-destructive technique for early plant health surveillance in agroforestry. This advanced optical sensing capability is poised to illuminate complex stressor interactions, marking a significant step forward for precision agroforestry management.
1. Introduction
Defined by Nair [1] as “the deliberate association between perennial tree species, agricultural crops and/or an animal component, within the same management unit”, agroforestry combines trees and crops or livestock in the same space. This practice is recognized by the European Commission for its role in carbon sequestration, climate change mitigation and adaptation, soil degradation prevention, and biodiversity protection [2,3]. Trees in agroforestry systems enhance CO2 sequestration and soil nutrient availability [4]. The shade provided by tree canopies influences the microclimate, leading to cooler daytime temperatures and warmer nighttime ones, as well as higher air humidity and soil moisture [5,6]. These conditions create a more favorable environment for plant growth and the presence of other organisms [7,8,9]. Additionally, agroforestry influences the incidence and abundance of pests, pathogens, and weeds both through increased top-down regulation by natural enemies and via bottom-up factors such as the moderation of microclimate, soil nutrients, and water content [10,11,12]. Despite these known benefits, agroforestry faces several challenges that hinder its widespread adoption. For instance, farmers face practical challenges, including the inherent complexity of managing multiple plant and animal species within a single agroecosystem, high labor demands, and the need for context-specific long-term management strategies [13]. In this scenario, the integration of advanced digital technologies can provide real-time data and insights that are crucial for managing the complexity of agroforestry systems and help farmers optimize resource use, monitor ecological interactions, and implement precise management practices [14].
Vegetation spectroscopy utilizes the optical characteristics of plant leaves and canopies (e.g., their reflectance properties) to conduct rapid, high-throughput, and non-destructive evaluations of plant conditions. This approach allows for the concurrent assessment of multiple plant traits across extensive populations and over long durations [15,16,17]. Among the different optical techniques available, hyperspectral sensing is particularly notable for its ability to detect and quantify plant characteristics and diagnose diseases with high accuracy [18,19]. Its effectiveness stems from the interaction of light with molecular bonds, primarily C-H, N-H, and O-H, which causes vibrational excitation at distinct wavelengths within the visible (VIS: 400–700 nm), near-infrared (NIR: 700–1,100 nm), and short-wave infrared (SWIR: 1,100–2,400 nm) spectral ranges [20,21]. Compared to traditional optical tools like RGB or multispectral sensors that capture only a few wide bands, hyperspectral instruments collect data across many narrow spectral bands, resulting in more detailed and information-rich outputs [18]. This finer spectral resolution supports a variety of analytical techniques. Other than calculating simple vegetation spectral indices (VSIs), such as the normalized difference vegetation index (NDVI) [22], hyperspectral reflectance profiles, representing a composite of structural and chemical leaf traits in particular environmental contexts, can also be interpreted through machine learning algorithms to extract phenotypic information [16]. In addition, this approach can help us monitor plant function over large geographic regions if scaled to remote sensing collections from air- or spaceborne platforms (e.g., unmanned aerial vehicle, UAV) [20]. To the best of our knowledge, hyperspectral sensors have never been adopted to investigate interactions in agroforestry systems.
Sorghum (Poaceae family) is a versatile crop used for both human consumption and animal feed, and it is particularly well-suited to agroforestry systems due to its resilience in arid and semi-arid regions [23]. Sorghum bicolor L. Moench, primarily cultivated for its grain, has a deep and robust root system that can reach depths of 150–160 cm, allowing it to efficiently access water and nutrients [24]. Although sorghum is tolerant of various abiotic stresses, its productivity is often reduced by biotic factors, including grain mold caused by fungi of the genus Alternaria [25]. Alternaria alternata, a ubiquitous ascomycetous fungus, can infect sorghum during the flowering stage and spread internally. The infection typically causes irregular, light gray leaf lesions with distinct dark brownish-red margins [26], and it adversely affects grain quality by degrading starch and protein content. Moreover, it promotes the synthesis of harmful mycotoxins, which pose significant risks to human and animal health, including carcinogenicity, immunotoxicity, and developmental impairments in children [27,28]. Environmental stressors such as high humidity and drought exacerbate the disease [29,30,31]. The increasing global distribution and adaptability of Alternaria spp. underscore the urgent need for further research into their effects on crop health and food safety, particularly in the context of climate change [32,33]. While hyperspectral data offer promising tools for studying sorghum’s interactions with biotic stressors, their use has so far been limited, mainly to applications such as cultivar discrimination [34] and the estimation of biomass, nitrogen, chlorophyll content [35], or water stress [36], all within conventional agricultural systems. To date, no hyperspectral studies have been conducted in agroforestry settings or focused on disease detection in sorghum grown under different levels of shading.
This study aimed to evaluate the potential of hyperspectral data, collected at the leaf, ground-canopy, and UAV-canopy scales, to characterize the effects of simulated tree shading and A. alternata infection on sorghum under field conditions (agroforestry field studies are costly and slow, while artificial shading structures [37,38] provide a faster way to test light reduction effects, isolating them from other tree influences). Specifically, the objectives were to (i) determine whether shading affects A. alternata infection; (ii) assess the capability of hyperspectral signature analysis to accurately, rapidly, and non-destructively distinguish sorghum plants subjected to shading and/or A. alternata infection, even prior to visible symptom development (i.e., early detection); and (iii) elucidate sorghum responses to single and combined effects of shading and A. alternata infection through analysis of spectra-derived leaf features.
2. Materials and Methods
2.1. Experimental Material and Design
The research activities took place for two years (2023 and 2024) at the Centre for Agri-environmental Research “Enrico Avanzi” of the University of Pisa, located in San Piero a Grado, Pisa, Central Italy (43.67446° N, 10.31833° E, 0 m a.s.l.). The experimental area is characterized by a typical Mediterranean environment, with average annual maximum and minimum daily temperatures of 19.3 and 11.3 °C, respectively, and a mean rainfall of 971 mm per year (data collected by the TOS01005251 automatic meteorological station of Tuscany Region, situated close to the experimental site; historical series: 1992–2022; https://www.sir.toscana.it/, accessed on 1 August 2025). The experiment was temporally replicated on two neighboring fields with similar characteristics. On average, the soil type is silty clay loam (sand:silt:clay 15.5, 47.5, 37.0). Average soil pH, electrical conductivity, total N, organic matter, total assimilable P, total limestone, and active limestone were 7.91, 95.9 μS cm−1, 0.93 mg g−1, 1.88 g 100 g−1, 11.9 mg kg−1, 54.98 mg g−1, and 4.9 mg g−1, respectively.
The crop was established on 1 June 2023 and 2024 by sowing S. bicolor cultivar PR89Y79 at 20 seeds per linear meter and at a depth of 3–5 cm in a non-inversion tillage system (30 cm depth) with 50 cm row spacing (40 seeds m−2; dose of 12–14 kg ha−1). The experiments were established as a randomized complete block design with four replications as block factors and four treatments assigned into blocks (i.e., experimental plots): (i) unshaded and non-inoculated (Sh−/Aa−; i.e., control), (ii) shaded and non-inoculated (Sh+/Aa−), (iii) unshaded and A. alternata-inoculated (Sh−/Aa+), and (iv) shaded and A. alternata-inoculated (Sh+/Aa+). The experimental plot dimension was 2 by 2 m, and to minimize interplot interference, the plots were separated by 2 m alleys. Shading was produced using a metallic structure on which 15 cm wide, black polyethylene strips were mounted at a height of 1.50 m, targeting a 50% reduction in light availability (Figure S1 [39]). The shade intensity level applied was determined based on data from [40] on an olive tree (Olea europaea L.) agroforestry system in Tuscany. The polyethylene strips were specifically chosen for their ability to move with the wind, more accurately simulating the dynamic natural motion of tree canopies in agroforestry systems. The shading structures were positioned immediately following seed germination.
A. alternata conidia (i.e., strains A214 and 216 mixed in equal proportions) were produced at the Plant Pathology Lab of the Department of Agriculture, Food and Environment of the University of Pisa. Cultures were grown in Petri dishes on potato dextrose agar (PDA, 39 g L−1, Sigma-Aldrich, Milan, Italy) amended with streptomycin sulphate (0.1 g L−1, Gold Biotechnology, Saint Louis, MO, USA) for two weeks at 23 °C under a 12 h photoperiod [41]. Colonies were then flooded with sterile deionized water containing 0.01% (vol/vol) Tween-80 (Sigma-Aldrich, Saint Louis, MO, USA) surfactant and gently scraped with a sterile glass bar to dislodge conidia from conidiophores. The resulting suspension was then filtered through sterile gauze to remove hyphal fragments and obtain a clean conidial suspension. Inoculation was done late in the evening on 1 August 2023 and 6 August 2024, when sorghum was at the flowering growth stage (BBCH scale: 59). Inoculum concentration was adjusted to approximately 2 × 108 conidia ml−1 by a Bürker hemocytometer chamber (Henneberg-Sander, Giessen-Lützellinden, Düsseldorf, Germany) and applied with a dedicated Nebla electric backpack pump (15L, Li-ION to corresponding experimental units (i.e., inoculated plots) FPM, Strocker, Bolzano, Italy) until leaf and panicle run-off.
In 2023, leaf and ground-canopy hyperspectral measurements were conducted on 4th September (BBCH scale: 65), when early visible symptoms appeared at both leaf and panicle levels on a few plants. These symptoms then became clearly recognizable to the naked eye and were widespread by mid-September. In 2024, UAV-canopy spectral measurements were conducted on 30th September (BBCH scale: 65), immediately after the appearance of visible symptoms, which manifested on 23rd and 28th September at the leaf and panicle level, respectively. Typical grain mold symptoms were observed: leaves showed light gray lesions with distinct dark brownish-red margins [42], while panicles displayed reduced seed size, shriveling, and discoloration ranging from whitish to grayish-black, often accompanied by fungal fruiting bodies [31]. At the end of the experiments, the leaf and panicle samples were collected to confirm infection and pathogen presence through standard microscopic and molecular diagnostic methods [41].
2.2. Assessment of Disease Incidence
On 25 September 2023 and 30 September 2024 (around 55 days post-A. alternata inoculation), plants from each subplot were further monitored for A. alternata symptoms (Figure S2), and grain mold incidence was scored using a 0–4 scale, based on the percentage of plants showing symptoms at leaf and/or panicle levels: 0 = 0%; 1 = 1–9%; 2 = 10–24%; 3 = 25–49%; and 4 = ≥50%.
2.3. Collection of Leaf and Ground-Canopy Hyperspectral Data
Hyperspectral data were collected at leaf and ground-canopy scales using a full range (350–2,500 nm) ASD Field-Spec 4 HR spectroradiometer (Analytical Spectral Devices, Boulder, CO, USA). Leaf reflectance was measured by fitting the spectroradiometer’s fiber optic into a plant probe with a leaf-clip containing an internal halogen light source. For each plant, two randomly selected areas (Ø 1 cm, Table S1) on the adaxial surface of the youngest mature leaf were investigated, with one measurement per area; the collections were then averaged for each leaf/plant. Canopy reflectance measurements were conducted between 11:00 and 13:00 under clear sky conditions, fitting the spectroradiometer’s fiber optic into a pistol grip kept one meter above the canopy, with a foreoptic angle approximately at nadir of the target (around 10 cm2 area per measurement, Table S1). The experimental plots were visually divided into four cardinal subplots (i.e., NW, NE, SE, and SW) to facilitate systematic data collection: two plants and two canopy areas were randomly selected within each subplot. Relative reflectance for both leaf and canopy measurements was determined by dividing vegetation radiance by the radiance of a 99% reflectance Spectralon® white panel (Labsphere, North Sutton, NH, USA) that was collected either just above the canopy, for canopy measurements, or mounted within the internal leaf-clip, for leaf measurements, measured every 10 spectral collections. All spectral analyses and calculations were performed on untransformed reflectance profiles (only spectral jump correction and data interpolation were carried out).
2.4. Collection of UAV-Canopy Hyperspectral Data
During the 2024 experimental campaign, on 30 September, hyperspectral data were acquired using a UAV operated by the Department of Information Engineering at the University of Pisa. The UAV was equipped with a Headwall Nano-VNIR Hyperspec® imaging sensor (Headwall Photonics, Bolton, MA, USA), which employs pushbroom scanning technology to capture high-resolution hyperspectral imagery. The sensor records 640 spatial pixels per line across 270 contiguous spectral bands, spanning a spectral range from approximately 400 to 1,000 nm (Table S1). To reduce motion-induced artifacts and ensure image stability, the camera was mounted on an active electromechanical gimbal.
Prior to each flight, system calibration procedures were conducted. First, a dark current image was recorded by completely covering the sensor’s lens, allowing for the correction of sensor noise. Next, the exposure time was manually optimized by imaging a calibrated white reference panel under ambient lighting conditions to ensure an optimal signal-to-noise ratio and avoid pixel saturation.
A calibrated 2 m × 2 m reflectance tarp (Headwall Photonics, MA, USA) was placed within the field of view as an in-scene radiometric reference. This target was used post-flight to convert at-sensor radiance to surface reflectance by regressing the observed radiance values against the known reflectance spectrum of the tarp.
Flights were carried out between 11:00 and 12:00 local time under clear sky conditions to maintain consistent solar illumination. The UAV operated at an altitude of 22 m above ground level, yielding a ground sampling distance (GSD) of approximately 2.0 cm. Flight lines were planned to ensure a minimum of 60% lateral overlap between adjacent swaths, enabling complete spatial coverage and facilitating image mosaicking during post-processing. To allow data acquisition, all shading structures present in the experimental setup were temporarily removed just before each UAV pass and promptly reinstalled afterward.
Raw hyperspectral data were initially pre-processed using Headwall’s SpectralView software (Headwall Photonics, Bolton, MA, USA), including dark current subtraction, radiometric calibration, and spectral band alignment. Geometric correction and ortho-rectification were subsequently performed using a structure-from-motion (SfM) photogrammetry pipeline, which incorporated synchronized RGB images and a UAV onboard GNSS/IMU data. The final output consisted of georeferenced orthomosaics of surface reflectance across the full spectral range, corrected for radiometric and geometric distortions and suitable for downstream analysis.
2.5. Analyses of Hyperspectral Signatures
Percentage difference in reflectance relative to the control (Sh−/Aa−) for the hyperspectral profiles collected at leaf, ground- and UAV-canopy scales was calculated for each wavelength as Δ%= (RT) − (RC)/(RC) × 100. RT indicates reflectance of plants under “treated” conditions, i.e., Sh−/Aa−, Sh−/Aa−, and Sh−/Aa−; RC indicates reflectance of plants under “control” conditions, i.e., Sh−/Aa−.
The effects of shading (Sh), A. alternata infection (Aa), and their interaction (Sh × Aa) on the reflectance profiles of sorghum leaves and canopies collected at the ground and UAV scales were determined by permutational analysis of variance (PERMANOVA [43]), employing Euclidian measurements of dissimilarity and 10,000 permutations. Spectral responses were visualized using principal coordinate analysis (PCoA) on the same spectral data utilized for PERMANOVA, using the ‘vegan’ package in R (www.r-project.org [44]). Using Euclidean distances, PCoA was run only for the significant effects shown by PERMANOVA. Partial least squares discriminant analysis (PLS-DA) [45], was additionally performed to determine the ability of hyperspectral data to classify experimental groups that showed statistical significance by PERMANOVA. These analyses were applied 500 times by iteratively splitting observations into different groups of calibration (training) and validation (testing) sets, and the number of correct classifications in both the calibration and the validation sets were used to evaluate the accuracy of the tested model. The calibration:validation data ratio and the number of components (i.e., latent variables) used to obtain the models that would give the best discrimination accuracy were determined by iteratively running the PLS-DA models with different calibration:validation data ratios (i.e., 50:50, 70:30, 80:20) and numbers of components and were based on the highest Kappa values returned for the validation models. PLS-DA was performed using the ‘caret’ and ‘vegan’ packages in R (www.r-project.org) [44,46].
2.6. Leaf Trait Estimation by Vegetation Spectral Indices
Some widely used VSIs (commonly involved in abiotic/biotic stress–plant interaction) were calculated: anthocyanin reflectance index [ARI: (1/R550) − (1/R700) [47]; photochemical reflectance index [PRI: (R531 − R570)/(R531 + R570)] [48]; normalized difference vegetation index [NDVI: (R780 − R570)/(R780 + R570)] [22]; normalized difference water index [NDWI: (R860 − R1240)/(R860 + R1240)] [49]; chlorophyll index [CI: (R750 − R705)/(R750 + R705)] [47]; carotenoid reflectance index [CRI: (1/R510 − 1/R550)] [50]; normalized difference nitrogen index [NDNI: (log(1/R1510) − log(1/R1680))/(log(1/R1510) + log(1/R1680))] [51]; normalized difference lignin index [NDLI: (log(1/R1754) − log(1/R1680))/(log(1/R1754) + log(1/R1680))] [51]; and plant senescence reflectance index [PSRI: (R687 − R500)/(R687 + R500)] [52]. Rx indicates reflectance at x nm wavelength. These indices were calculated from spectra averaged per plant.
2.7. Statistical Analysis of Disease Incidence and Vegetation Spectral Indices
Disease incidence scores measured in 2023 and 2024 were merged and analyzed using the non-parametric Kruskal–Wallis test; when significant, pairwise comparisons among experimental conditions were performed using Dunn’s test. The Shapiro–Wilk test was used to evaluate the normal distribution of leaf VSIs. The effects of Sh, Aa, and their interaction (Sh × Aa) on these VSIs were assessed by two-way analysis of variance (ANOVA), followed by Tukey’s HSD post hoc test. Statistically significant effects were considered at p ≤ 0.05. These univariate statistical analyses were run in JMP Pro 14.0.0 (SAS Institute Inc., Cary, NC, USA).
3. Results
3.1. Disease Incidence (Experiments 2023 and 2024)
The Kruskal–Wallis test of disease incidence revealed that A. alternata infection increased under shading, with approximately twofold and threefold higher values in the Sh−/Aa+ and Sh+/Aa+ conditions, respectively, compared with the Sh−/Aa− controls (Figure 1).
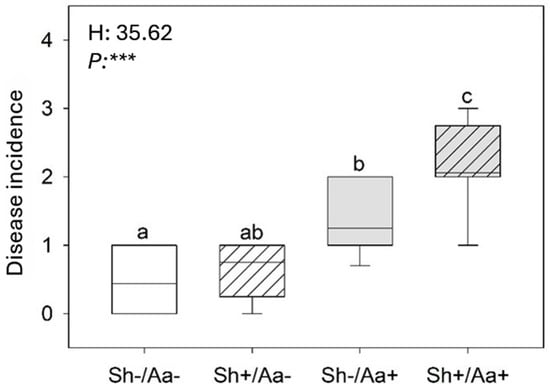
Figure 1.
Variation in grain mold disease incidence in Sorghum bicolor under four experimental conditions: (i) unshaded and non-inoculated (Sh−/Aa−; i.e., control), (ii) shaded and non-inoculated (Sh+/Aa−), (iii) unshaded and Alternaria alternata-inoculated (Sh−/Aa+), and (iv) shaded and A. alternata-inoculated (Sh+/Aa+). The box plots display the median (horizontal line), the 5th and 95th percentiles (boxes), and the range (whiskers). Different letters indicate significant differences among means according to Kruskal–Wallis and Dunn’s post hoc test (p ≤ 0.05). Asterisks indicate significance levels (p < 0.001, ***).
3.2. Variations in Leaf and Ground-Canopy Spectral Signatures
Several spectral ranges were initially evaluated to optimize the statistical performance of PERMANOVA on the leaf- and ground-canopy-level hyperspectral profiles. At the leaf level, the strongest statistical significance for the tested effects was obtained using the full wavelength range (400–2,400 nm), whereas for the ground-canopy spectra, the VIS-NIR range (400–1,000 nm) yielded the highest significance (Table 1, Figure 2A,B). Coefficients of variation were qualitatively higher across the VIS, NIR, and SWIR regions at the leaf level, and predominantly in the NIR region for the ground-canopy data (Figure 2C,D). At both levels, the final PERMANOVA revealed a significant Sh × Aa interaction effect on the reflectance profiles of sorghum. In fact, all tested effects were significant, except for Aa at the leaf level, which was marginally significant (p = 0.08; Table 1). The outcomes of the PCoA for the significant Sh × Aa interaction effects on the leaf- and ground-canopy-level hyperspectral profiles are shown in Figure 2E,F.

Table 1.
F values and p levels (***: p ≤ 0.001; **: p ≤ 0.01; *: p ≤ 0.05; ns: p > 0.05) of two-way permutational analyses of variance for the effects of shade (Sh), Alternaria alternata infection (Aa), and their interaction (Sh × Aa) on hyperspectral profiles of Sorghum bicolor, collected at the leaf (400–2,400 nm) and ground-canopy levels (400–1,000 nm). df: degrees of freedom.
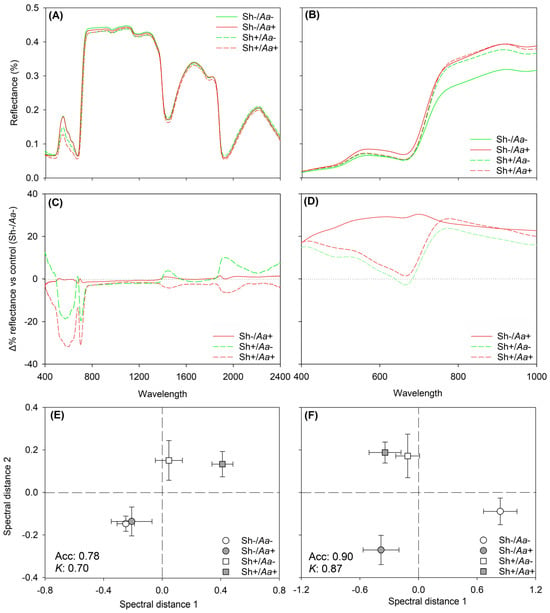
Figure 2.
Mean reflectance from leaf (A) and ground-canopy (B) hyperspectral measurements of Sorghum bicolor under four experimental conditions: (i) unshaded and non-inoculated (Sh−/Aa−; i.e., control), (ii) shaded and non-inoculated (Sh+/Aa−), (iii) unshaded and Alternaria alternata-inoculated (Sh−/Aa+), and (iv) shaded and A. alternata-inoculated (Sh+/Aa+). Percentage difference in reflectance of Sh−/A+, Sh+/Aa-, and Sh+/Aa+ plants relative to the control (Δ% reflectance of the singular treatment vs. control, Sh-/Aa-), computed at each wavelength, for the hyperspectral profiles collected at leaf (400–2,400 nm, (C)) and ground-canopy scales (400–1,000 nm, (D)). Scores (mean ± standard error) for the first and second principal components from principal coordinate analyses (PCoA) of reflectance data collected from sorghum at leaf (400–2,400 nm, (E)) and ground-canopy levels (400–1,000 nm, (F)), highlighting the capability of discriminating different experimental conditions by hyperspectral data. Average accuracy (Acc) and Kappa (K) values for validation from partial least squares discriminant analysis are reported on the bottom-left corners of the panels.
According to PLS-DA, the highest classification performance (i.e., highest Kappa) for distinguishing experimental conditions from spectral profiles was obtained using an 80:20 calibration-to-validation split, with 35 and 30 components (latent variables) for the leaf- and ground-canopy-level analysis, respectively. For leaf hyperspectral data, the validation accuracy and Kappa were 0.78 ± 0.10 and 0.70 ± 0.13 (using 500 random permutations of the data), respectively, with discrimination primarily driven by shading effects, but particularly for the Sh+/Aa+ plants (Figure 2E). Misclassifications occurred only between the Sh−/Aa− and Sh−/Aa+ plants (i.e., unshaded; Table 2). For ground-canopy hyperspectral data, the validation accuracy and Kappa increased to 0.90 ± 0.13 and 0.87 ± 0.27 (using 500 random permutations of the data), respectively, with discrimination primarily driven by A. alternata infection status, again especially for the Sh+/Aa+ plants (Figure 2F). Here, minor misclassifications occurred only between the Sh−/Aa− and Sh+/Aa− plants (i.e., non-inoculated; Table 2).

Table 2.
Confusion matrix summary showing, for each row, the proportion of correct (bold) and incorrect (non-bold) classifications obtained from partial least squares discriminant analysis (PLS-DA) for Sorghum bicolor under four experimental conditions: (i) unshaded and non-inoculated (Sh−/Aa−; control); (ii) shaded and non-inoculated (Sh+/Aa−), (iii) unshaded and Alternaria alternata-inoculated (Sh−/Aa+), and (iv) shaded and A. alternata-inoculated (Sh+/Aa+). Results are reported for both leaf (400–2,400 nm) and ground-canopy (400–1,000 nm) level analyses using validation datasets generated from an 80% calibration and 20% validation split. Values represent the mean of 500 PLS-DA permutations.
3.3. Variations in UAV-Canopy Spectral Signatures
Several spectral ranges were initially evaluated to optimize the statistical performance of PERMANOVA on the UAV-canopy-level hyperspectral profiles. As in the leaf- and ground-canopy-level analysis, the strongest statistical significance for the tested effects was obtained when using all the available wavelengths, i.e., the VIS-NIR range (400–1,000 nm; Table 3, Figure 3A). The coefficients of variation were qualitatively higher in the NIR region for UAV-canopy data as well (Figure 3B). The final PERMANOVA indicated significant unifactorial effects of Sh and Aa, but not their combined effect (Sh × Aa), on sorghum reflectance profiles at the UAV-canopy level (Table 3). The PCoA results for the significant Sh and Aa effects on the UAV-canopy-level hyperspectral profiles are shown in Figure 3C,D.

Table 3.
F values and p levels (**: p ≤ 0.001; *: p ≤ 0.05; ns: p > 0.05) of two-way permutational analyses of variance for the effects of shade (Sh), Alternaria alternata infection (Aa), and their interaction (Sh × Aa) on the hyperspectral profiles of Sorghum bicolor, collected at the UAV-canopy level (400–1,000 nm). df: degrees of freedom.
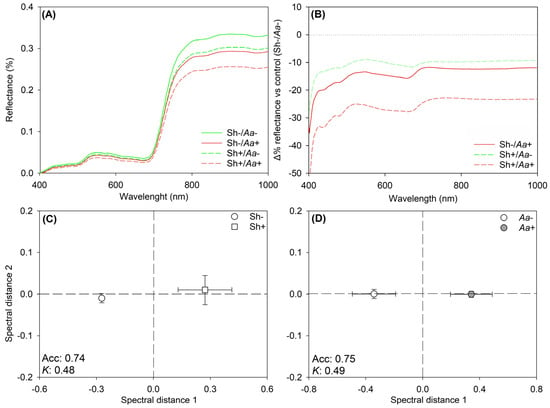
Figure 3.
Mean reflectance of UAV-canopy hyperspectral measurements collected from Sorghum bicolor under four experimental conditions (A): (i) unshaded and non-inoculated (Sh−/Aa−; i.e., control), (ii) shaded and non-inoculated (Sh+/Aa−), (iii) unshaded and Alternaria alternata-inoculated (Sh−/Aa+), and (iv) shaded and A. alternata-inoculated (Sh+/Aa+). Coefficient of variation in reflectance by wavelength for all the hyperspectral profiles collected at the UAV-canopy level (B). Scores (mean ± standard error) for the first and second principal components from principal coordinate analyses (PCoAs) of reflectance data collected from sorghum at the UAV-canopy level (400–1,000 nm), highlighting the capability of discriminating different shading (C) and A. alternata inoculation conditions (D) by hyperspectral data. Average accuracy (Acc) and Kappa (K) values for validation from partial least squares discriminant analysis are reported on the bottom-left corners of the panels.
According to PLS-DA, the highest classification performance (i.e., highest Kappa) for distinguishing shading and A. alternata infection conditions from spectral profiles was obtained using an 80:20 calibration-to-validation split, with 30 and 35 components (latent variables), respectively. The validation accuracy and Kappa for Sh were 0.75 ± 0.10 and 0.40 ± 0.07, respectively, and similarly, the validation accuracy and Kappa for Aa were 0.75 ± 0.06 and 0.49 ± 0.04, respectively.
3.4. Variations in Vegetation Spectral Indices
Table 4 summarizes the effects of Sh, Aa, and their interaction (Sh × Aa) on VSIs. A significant Sh × Aa interaction was observed for the NDVI, CRI, NDNI, and NDLI. Compared to the control (Sh−/Aa−), the NDVI, CRI, and NDNI increased by 24.79, 21.17, and 3.21%, respectively, in the Sh+/Aa+ plants. Notably, the NDVI increased by 14.82% also in the Sh+/Aa− plants, whereas the NDNI decreased by 5.12% under shading alone. In contrast, the NDLI showed only a significant decrease of 4.57% exclusively in the Sh+/Aa− plants (Figure 4). Significant main effects of both Sh and Aa were observed for the NDWI and CI, with values increasing under shade but decreasing following A. alternata infection. The PRI, ARI, and PSRI exhibited only a significant Aa effect, decreasing by 7.35 and 10.00 and increasing by 0.03% in response to A. alternata infection.

Table 4.
F values and p levels (***: p ≤ 0.001; **: p ≤ 0.001; *: p ≤ 0.05; ns: p > 0.05) of two-way analysis of variance for the effects of shading (Sh), A. alternata infection (Aa), and their interaction (Sh × Aa) on leaf traits derived from Sorghum bicolor spectra. df: degrees of freedom. Trait abbreviations: anthocyanin reflectance index, ARI; chlorophyll index, CI; carotenoid reflectance index, CRI; normalized difference lignin index, NDLI; normalized difference nitrogen index, NDNI; normalized difference vegetation index, NDVI; normalized difference water index, NDWI; photochemical reflectance index, PRI; plant senescence reflectance index, PSRI.
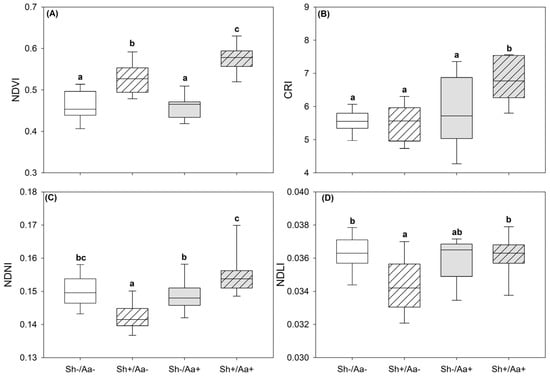
Figure 4.
Variations in NDVI (A), CRI (B), NDNI (C) and NDLI (D) calculated using spectra collected from Sorghum bicolor leaves under four experimental conditions: (i) unshaded and non-inoculated (Sh−/Aa−; i.e., control), (ii) shaded and non-inoculated (Sh+/Aa−), (iii) unshaded and Alternaria alternata-inoculated (Sh−/Aa+), and (iv) shaded and A. alternata-inoculated (Sh+/Aa+). The box plots display the median (horizontal line), the 5th and 95th percentiles (boxes), and the range (whiskers). Different letters indicate significant differences among means according to two-way analysis of variance and Tukey’s post hoc test (p ≤ 0.05). For abbreviations of VSIs, see Table 4.
4. Discussion
4.1. Shading Conditions Increase the Incidence of A. alternata Infection in Sorghum
Tree canopies substantially modify the microclimate beneath them by reducing photosynthetically active radiation, attenuating the day–night thermal range, and increasing relative humidity and leaf wetness duration, thus creating conditions known to favor the development of several pathogenic fungi [8,9,12]. Here, we showed an increase in grain mold incidence under simulated tree shading: typical grain mold symptoms were mostly observed in the Sh+/Aa+ leaves, showing light gray lesions with distinct dark brownish-red margins [42] and panicles, displaying reduced seed size, shriveling, and discoloration ranging from whitish to grayish-black, often accompanied by fungal fruiting bodies [31]. This finding confirms that although shade alone does not initiate infection, it creates microenvironments that enhance disease progression and severity when the pathogen is present.
From a mechanistic perspective, several complementary explanations are plausible. Increased leaf wetness duration and humidity promote spore germination and tissue penetration by A. alternata [53,54]. In parallel, reduced light availability may lower photosynthetic efficiency, thereby decreasing the energetic resources allocated to the synthesis of defensive metabolites (e.g., flavonoids and anthocyanins) [55,56]. In sorghum, compounds such as anthocyanins, flavonoids, and dhurrin (mainly produced during early developmental stages) are involved in antifungal responses [57]; their production may be compromised under low-light conditions, making the plants more vulnerable. Finally, changes in carbohydrate allocation and source–sink balance under shading can provide additional substrates to the pathogen or limit defense mechanisms, as documented in other host–pathogen systems [58].
These findings are particularly relevant for the integration of crops and trees in agroforestry systems. While tree cover provides multiple benefits (climate regulation, soil protection, biodiversity), it can also alter the dynamics of opportunistic pathogens, requiring targeted management strategies (e.g., varietal choice, pruning practices, microclimate control). It is noteworthy that in the present study, the use of artificial shading structures prevented the trees from manifesting any potential interaction with the companion sorghum, other than shading. In real agroforestry conditions, habitat modifications and improved biodiversity can lead to lower incidence of crop diseases due to, e.g., increased presence of pathogen biocontrol agents, lower spatial density and uniformity of the host crop plants, tree–crop root interactions, and stimulation of elicitor production [10,59].
4.2. Hyperspectral Data Differentiate Sorghum Responses to Shading and A. alternata Infection Across Scales
The hyperspectral profiles of plants were strongly influenced by both simulated tree shading and A. alternata infection, with the dominant drivers of discrimination varying across the spatial scales of measurement (leaf, ground-canopy, UAV-canopy). At the leaf level, the highest classification performance was achieved when the full VIS–NIR–SWIR range (400–2400 nm) was used, yielding a validation accuracy of around 80%. Discrimination was primarily driven by shading, especially in plants exposed to both shading and infection, while misclassification occurred only between unshaded plants. This suggests that shading produced strong, broadband spectral changes through alterations in pigment composition, chlorophyll density, leaf structure, and water relations [8], which outweighed the subtler leaf-scale optical effects of early or moderate A. alternata infection, which are often confined to localized lesions and do not dominate the average reflectance of a sampled leaf. The observed sensitivity of leaf spectra to shading is consistent with the physiological domains covered: the VIS (400–700 nm) reflects pigment changes [60,61], the NIR (700–1300 nm) responds to internal structure and turgor [62], and the SWIR [1300–2400 nm] captures water absorption and biochemical shifts linked to lignin, cellulose, and nitrogen metabolism [63,64].
At the ground-canopy scale, accuracy increased to 90%, with discrimination now primarily driven by infection status, again particularly in the Sh+/Aa+ plants. Misclassifications were limited to non-inoculated canopies. This shift in the dominant driver from shading (leaf level) to infection (canopy level) may result from symptom aggregation: while lesions and chlorotic patches are small and spatially scattered at the leaf scale, they tend to cluster within the canopy, forming larger contiguous areas with altered reflectance [65]. Infection can also reduce leaf area index, cause drooping or curling, and modify canopy architecture, thus changing light interception and NIR scattering [66,67]. By integrating reflectance from multiple leaves, stems, and plant residues, ground-canopy measurements reduce within-treatment variability (“signal averaging”) and enhance the detection of consistent stress-related patterns. Here, shading effects, although still detectable, were partially masked by vertical light gradients and self-shading, while panicle infection symptoms remained visually and spectrally conspicuous.
UAV-based canopy data, collected over a broader area with a narrower spectral range (VIS–NIR), retained sensitivity to the main effects of both shading and infection but did not capture their interaction. This attenuation likely reflects spatial and spectral averaging: UAV imagery blends signals from healthy and stressed tissues, non-foliar elements (soil, senescent residues, shadows), and atmospheric or bidirectional reflectance effects [68,69]. The absence of SWIR wavelengths also limits the detection of certain biochemical changes that may mediate stress interactions [70]. Nevertheless, shading signatures at the UAV scale likely result from reduced visible reflectance due to increased chlorophyll retention and NIR shifts linked to altered canopy structure [71], while infection signatures reflect reduced NIR scattering from mesophyll damage and increased VIS reflectance from chlorosis or necrosis [65].
Overall, the multi-scale patterns highlight that close-range hyperspectral measurements (leaf and ground-canopy) are better suited to detecting fine-scale physiological and biochemical changes, including interactive effects, while UAV observations provide spatially comprehensive assessments of the main stress drivers. In agroforestry contexts, where tree-induced microclimates can interact with pathogen dynamics in spatially heterogeneous ways [72], integrating these scales offers both mechanistic insight and practical monitoring potential. Specifically, the UAV-scale sensitivity to both shading and infection validates its use for wide-area surveillance, allowing for the identification of high-risk disease hotspots and the implementation of targeted, field-based precision management strategies [19,69]. Linking physiological processes to spectral signatures across scales can therefore inform precision crop health management strategies that address both abiotic and biotic stressors.
4.3. Vegetation Spectral Indices Reflect Combined Effects of Shading and A. alternata Infection on Sorghum Physiology and Stress
The observed variations in VSIs highlight the complex physiological responses of S. bicolor to both abiotic (i.e., shading) and biotic (i.e., A. alternata infection) stressors, revealing significant interactions that modulate pigment content, water status, and secondary metabolism [48,63,73]. Notably, indices such as the NDVI, CRI, NDNI, and NDLI exhibited significant Sh × Aa interactions, increasing markedly in plants subjected to both shading and infection (Sh+/Aa+). This suggests a synergistic response involving the enhanced accumulation of carotenoids, nitrogen-containing compounds, and lignin, likely reflecting defense activation and structural reinforcement under combined stress [26,64]. Specifically, the NDNI and NDLI decreased in shaded but not inoculated plants (Sh+/Aa−), indicating that shading alone may reduce these biochemical components in the absence of an infection process [54,65]. Notably, the NDVI increased under shading, likely due to pigment conservation in lower light conditions, and under the combination of shading and infection (Sh+/Aa+), suggesting a complex physiological interaction where the plant’s response to shading is amplified or altered by the presence of the pathogen [56,60,65]. Other indices, including the ARI, PRI, and PSRI, responded predominantly to shading, showing significant declines consistent with reduced synthesis of protective pigments and enhanced senescence processes triggered by shading conditions [8,9].
These results underscore how combined VSI analysis can provide an integrated and sensitive picture of sorghum’s physiological and biochemical responses to simultaneous environmental and pathogenic stresses. The interaction between shading and infection translates into complex metabolic and structural adjustments detectable via spectral indices, which can be leveraged for early diagnosis and targeted monitoring in agroforestry systems, where tree-induced microclimates influence disease dynamics [8,72]. Employing VSIs to distinguish and quantify these interactions offers valuable tools for precision management strategies aimed at enhancing crop resilience under multiple stress factors.
5. Conclusions
This study provides novel insights into the interactive effects of tree shading and A. alternata infection on sorghum in agroforestry-like conditions. Simulated shading significantly increased the incidence of grain mold by modifying microclimatic factors such as humidity and light availability, which in turn impacted pathogen development and plant defense mechanisms. Hyperspectral data collected across multiple spatial scales revealed distinct spectral signatures associated with both shading and infection. Leaf-level measurements were particularly sensitive to shading-induced physiological changes, while canopy-level data enhanced the detection of infection symptoms due to spatial aggregation. UAV-based hyperspectral data offered spatially extensive, though spectrally coarser, assessments of crop health. Vegetation spectral indices highlighted complex biochemical and structural adjustments under combined stress, emphasizing the value of spectral monitoring for early diagnosis. These findings underscore the importance of integrating advanced sensing technologies in agroforestry management to optimize crop health monitoring, improve disease prediction, and develop targeted interventions. Future research should explore long-term dynamics under real agroforestry conditions and the application of hyperspectral remote sensing for operational disease management.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/agronomy15112458/s1, Figure S1: Unshaded (A) and shaded (B) sorghum plots; Figure S2: Visible symptoms in sorghum leaves (A) and panicles (B) caused by infection with Alternaria alternata. Table S1. Scale, spectral range, spectral sampling, and pixel resolution for the sensors used in the experimental setup.
Author Contributions
Conceptualization, L.C., D.A., M.F., M.M., L.G.T. and S.R.; methodology, L.C., S.P. and D.A.; formal analysis, L.P., S.R., G.S. and L.G.T.; data curation, L.P. and M.A.; investigation, G.S.; writing—original draft preparation, L.P. and S.R.; writing—review and editing, N.A., M.A., G.C., M.F., M.M., C.N., S.P., E.P., A.P., N.S. and L.C.; supervision, N.A., G.C., C.N., E.P., A.P., N.S. and L.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the University of Pisa under the “PRA–Progetti di Ricerca di Ateneo” (Institutional Research Grants)—Project no. PRA_2022_42, “iAgroforestry: Application of digital techniques in management and defense of agroforestry systems”.
Data Availability Statement
The original contributions presented in this study are included in the Supplementary Material. Further inquiries can be directed to the corresponding author.
Acknowledgments
This paper and related research was conducted during, and with the support of, the Italian national inter-university PhD course in Sustainable Development and Climate Change (https://www.phd-sdc.it/). We gratefully acknowledge the staff of the “Enrico Avanzi” Agro-environmental Research Center of the University of Pisa for their invaluable support and assistance throughout the development of this research. This publication was produced while attending the PhD program in Sustainable Development and Climate Change at the University School for Advanced Studies IUSS Pavia, Cycle XXXVIII, with the support of a scholarship financed by Ministerial Decree no. 351 of 9 April 2022, based on the NRRP—funded by the European Union–NextGenerationEU–Mission 4 “Education and Research”, Component 1 “Enhancement of the offer of educational services: from nurseries to universities”, Investment 4.1 “Extension of the number of research doctorates and innovative doctorates for public administration and cultural”.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| Aa | Alternaria alternata infection |
| Acc | Accuracy |
| ANOVA | Analysis of variance |
| ARI | Anthocyanin reflectance index |
| C | Carbon |
| CFU | Colony-forming unit |
| CI | Chlorophyll index |
| CO2 | Carbon dioxide |
| CRI | Carotenoid reflectance index |
| H | Hydrogen |
| HSD | Honest significant difference |
| K | Kappa |
| N | Nitrogen |
| NDLI | Normalized difference lignin index |
| NDNI | Normalized difference nitrogen index |
| NDVI | Normalized difference vegetation index |
| NDWI | Normalized difference water index |
| NIR | Near-infrared |
| O | Oxygen |
| P | Phosphorus |
| PCoA | Principal coordinates analysis |
| PERMANOVA | Permutational multivariate analysis of variance |
| PSRI | Plant senescence reflectance index |
| PLS-DA | Partial least squares discriminant analysis |
| PDA | Potato dextrose agar |
| PRI | Photo-chemical reflectance index |
| RGB | Red, green, blue |
| Sh | Shading |
| SWIR | Short-wave infrared |
| UAV | Unmanned aerial vehicle |
| VSI | Vegetation spectral index |
| VIS | Visible |
References
- Nair, P.K.R. State-of-the-art of agroforestry research and education. Agrofor. Syst. 1993, 23, 95–119. [Google Scholar] [CrossRef]
- Antichi, D.; Mazzoncini, M.; Tramacere, L.G.; Sbrana, M.; Barberi, P.; Moonen, A.C.; Mele, M. Agroforestry systems for adaptation to and mitigation of climate change: Effects on soil fertility. Agrochimica 2019, 2019, 39–144. [Google Scholar]
- Mantino, A.; Pecchioni, G.; Tozzini, C.; Mele, M.; Ragaglini, G. Agronomic performance of soybean and sorghum in a short rotation poplar coppice alley-cropping system under Mediterranean conditions. Agrofor. Syst. 2023, 97, 1025–1039. [Google Scholar] [CrossRef]
- Friedlingstein, P.; O’Sullivan, M.; Jones, M.W.; Andrew, R.M.; Gregor, L.; Hauck, J.; Le Quéré, C.; Luijkx, I.T.; Olsen, A.; Peters, G.P.; et al. Global Carbon Budget 2022. Earth Syst. Sci. Data 2022, 14, 4811–4900. [Google Scholar] [CrossRef]
- Niether, W.; Armengot, L.; Andres, C.; Schneider, M.; Gerold, G. Shade trees and tree pruning alter throughfall and microclimate in cocoa (Theobroma cacao L.) production systems. Ann. For. Sci. 2018, 75, 38. [Google Scholar] [CrossRef]
- Lin, B.B. Agroforestry management as an adaptive strategy against potential microclimate extremes in coffee agriculture. Agr. For. Meteorol. 2007, 144, 85–94. [Google Scholar] [CrossRef]
- Tielbörger, K.; Kadmon, R. Indirect effects in a desert plant community: Is competition among annuals more intense under shrub canopies? Plant Ecol. 2000, 150, 53–63. [Google Scholar] [CrossRef]
- Valladares, F.; Niinemets, Ü. Shade tolerance, a key plant feature of complex nature and consequences. Annu. Rev. Ecol. Evol. Syst. 2008, 39, 237–257. [Google Scholar] [CrossRef]
- Valladares, F.; Laanisto, L.; Niinemets, Ü.; Zavala, M.A. Shedding light on shade: Ecological perspectives of understorey plant life. Plant Ecol. Divers. 2016, 9, 237–251. [Google Scholar] [CrossRef]
- Pumariño, L.; Sileshi, G.W.; Gripenberg, S.; Kaartinen, R.; Barrios, E.; Muchane, M.N.; Midega, C.; Jonsson, M. Effects of agroforestry on pest, disease and weed control: A meta-analysis. Basic Appl. Ecol. 2015, 16, 573–582. [Google Scholar] [CrossRef]
- Valladares, F.; Gianoli, E.; Gómez, J.M. Ecological limits to plant phenotypic plasticity. New Phytol. 2016, 219, 644–659. [Google Scholar] [CrossRef] [PubMed]
- Romero, F.; Cazzato, S.; Walder, F.; Vogelgsang, S.; Bender, S.F.; van der Heijden, M.G.A. Humidity and high temperature are important for predicting fungal disease outbreaks worldwide. New Phitol. 2022, 234, 1553–1556. [Google Scholar] [CrossRef] [PubMed]
- Tranchina, M.; Reubens, B.; Frey, M.; Mele, M.; Mantino, A. What challenges impede the adoption of agroforestry practices? A global perspective through a systematic literature review. Agrofor. Syst. 2024, 98, 1817–1837. [Google Scholar] [CrossRef]
- Tranchina, M.; Burgess, P.; Cella, F.G.; Cumplido-Marin, L.; Gosme, M.; den Herder, M.; Kay, S.; Lawson, G.; Lojka, B.; Palma, J.; et al. Exploring agroforestry limiting factors and digitalization perspectives: Insights from a European multi-actor appraisal. Agrofor. Syst. 2024, 98, 2499–2515. [Google Scholar] [CrossRef]
- Peñuelas, J.; Filella, I. Visible and near-infrared reflectance techniques for diagnosing plant physiological status. Trends Plant Sci. 1998, 3, 151–156. [Google Scholar] [CrossRef]
- Cotrozzi, L.; Couture, J.J. Hyperspectral assessment of plant responses to multi-stress environments: Prospects for managing protected agrosystems. Plants People Planet 2020, 2, 244–258. [Google Scholar] [CrossRef]
- Gongora-Canul, C.; Salgado, J.D.; Singh, D.C.; Cruz, A.P.; Cotrozzi, L.; Couture, J.; Rivadeneira, M.G.; Cruppe, G.; Valent, B.; Todd, T.; et al. Temporal dynamics of wheat blast epidemics and disease measurements using multispectral imagery. Phytopathology 2020, 110, 393–405. [Google Scholar] [CrossRef]
- Mahlein, A.K.; Kuska, M.T.; Behmann, J.; Polder, G.; Walter, A. Hyperspectral sensors and imaging technologies in phytopathology: State of the art. Annu. Rev. Phytopathol. 2018, 56, 535–558. [Google Scholar] [CrossRef]
- Cotrozzi, L. Spectroscopic detection of forest diseases: A review (1970–2020). J. For. Res. 2022, 33, 21–38. [Google Scholar] [CrossRef]
- Cotrozzi, L.; Townsend, P.A.; Pellegrini, E.; Nali, C.; Couture, J.J. Reflectance spectroscopy: A novel approach to better understand and monitor the impact of air pollution on Mediterranean plants. Environ. Sci. Pollut. Res. 2018, 25, 8249–8267. [Google Scholar] [CrossRef]
- Ustin, S.L.; Jacquemoud, S. How the optical properties of leaves modify the absorption and scattering of energy and enhance leaf functionality. In Remote Sensing of Plant Biodiversity; Cavender-Bares, J., Gamon, J.A., Townsend, P.A., Eds.; Springer: Cham, Switzerland, 2020; pp. 349–384. [Google Scholar] [CrossRef]
- Gamon, J.A.; Field, C.B.; Goulden, M.L.; Griffin, K.L.; Hartley, A.E.; Joel, G.; Peñuelas, J.; Valentini, R. Relationships between NDVI, canopy structure, and photosynthesis in three Californian vegetation types. Ecol. Appl. 1995, 5, 28–41. [Google Scholar] [CrossRef]
- Berenji, J.; Dahlberg, J. Perspectives of sorghum in Europe. J. Agron. Crop Sci. 2004, 190, 332–338. [Google Scholar] [CrossRef]
- Orlandi, F.; Bonofiglio, T.; Ruga, L.; Fornaciari, M. Meteorological influences on pheno–morpho–yield data of grain sorghum varieties in Central Italy. Agron. J. 2017, 109, 2182–2189. [Google Scholar] [CrossRef]
- Aichinger, G.; Del Favero, G.; Warth, B.; Marko, D. Alternaria toxins–Still emerging? Compr. Rev. Food Sci. Food Saf. 2021, 20, 4390–4406. [Google Scholar] [CrossRef]
- Agrios, G.N. Plant Pathology, 5th ed.; Academic Press: San Diego, CA, USA, 2005. [Google Scholar] [CrossRef]
- Ostry, V. Alternaria mycotoxins: An overview of chemical characterization, producers, toxicity, analysis and occurrence in foodstuffs. World Mycotoxin J. 2008, 1, 175–188. [Google Scholar] [CrossRef]
- European Food Safety Authority; Arcella, D.; Eskola, M.; Gómez Ruiz, J.A. Dietary exposure assessment to Alternaria toxins in the European population. EFSA J. 2016, 14, 4654. [Google Scholar] [CrossRef]
- Rodriguez-Herrera, R.; Rooney, W.L.; Rosenow, D.T.; Frederiksen, R.A. Inheritance of grain mold resistance in grain sorghum without a pigmented testa. Crop Sci. 2000, 40, 1573–1578. [Google Scholar] [CrossRef]
- Navi, S.S.; Bandyopadhyay, R.; Reddy, R.K.; Thakur, R.P.; Yang, X.B. Effects of wetness duration and grain development stages on sorghum grain mold infection. Plant Dis. 2005, 89, 872–878. [Google Scholar] [CrossRef]
- Ackerman, A.; Wenndt, A.; Boyles, R. The sorghum grain mold disease complex: Pathogens, host responses, and the bioactive metabolites at play. Front. Plant Sci. 2021, 12, 660171. [Google Scholar] [CrossRef]
- Van de Perre, E.; Jacxsens, L.; Liu, C.; Devlieghere, F.; De Meulenaer, B. Climate impact on Alternaria moulds and their mycotoxins in fresh produce: The case of the tomato chain. Food Res. Int. 2015, 68, 41–46. [Google Scholar] [CrossRef]
- Medina, A.; Akbar, A.; Baazeem, A.; Rodriguez, A.; Magan, N. Climate change, food security and mycotoxins: Do we know enough? Fungal Biol. Rev. 2017, 31, 143–154. [Google Scholar] [CrossRef]
- Chidoko, P.; Mhike, X.; Chemura, A.; Manyanga, M. Spectral characterization and discrimination of sorghum (Sorghum bicolor (L.) Moench) cultivars for remote sensing-based phenotyping and selection. J. Indian Soc. Remote Sens. 2025, 53, 1–13. [Google Scholar] [CrossRef]
- Li, J.; Shi, Y.; Veeranampalayam-Sivakumar, A.N.; Schachtman, D.P. Elucidating sorghum biomass, nitrogen and chlorophyll contents with spectral and morphological traits derived from unmanned aircraft system. Front. Plant Sci. 2018, 9, 1406. [Google Scholar] [CrossRef]
- Tunca, E.; Köksal, E.S.; Öztürk, E.; Akay, H.; Çetin Taner, S. Accurate estimation of sorghum crop water content under different water stress levels using machine learning and hyperspectral data. Environ. Monit. Assess. 2023, 195, 877. [Google Scholar] [CrossRef]
- Barion, G.; Hewidy, M.; Panozzo, A.; Aloia, A.; Vamerali, T. Effects of lightorientation and mechanical damage to leaves on isoflavone accumulation in soybean seeds. Agronomy 2021, 11, 589. [Google Scholar] [CrossRef]
- Tramacere, L.G.; Antichi, D.; Mele, M.; Ragaglini, G.; Mantino, A. Effects of intercropping on the herbage production of a binary grass-legume mixture (Hedysarum coronarium L. and Lolium multiflorum Lam.) under artificial shade in Mediterranean rainfed conditions. Agrofor. Syst. 2024, 98, 1445–1460. [Google Scholar] [CrossRef]
- Varella, A.C.; Moot, D.J.; Pollock, K.M.; Peri, P.L.; Lucas, R.J. Do light and alfalfa responses to cloth and slatted shade represent those measured under an agroforestry system? Agrofor. Syst. 2011, 81, 157–173. [Google Scholar] [CrossRef]
- Mantino, A.; Tozzini, C.; Bonari, E.; Mele, M.; Ragaglini, G. Competition for Light Affects Alfalfa Biomass Production More Than Its Nutritive Value in an Olive-Based Alley-Cropping System. Forests 2021, 12, 233. [Google Scholar] [CrossRef]
- Pisuttu, C.; Pellegrini, E.; Cotrozzi, L.; Nali, C.; Lorenzini, G. Ecophysiological and biochemical events associated with the challenge of Verticillium dahliae to eggplant. Eur. J. Plant Pathol. 2020, 158, 879–894. [Google Scholar] [CrossRef]
- Prom, L.K.; Garcia, M.F.; Herrington, M.C. Morphological and molecular characterization of sorghum grain mold pathogens in relation to disease severity. Plant Pathol. 2020, 69, 1765–1775. [Google Scholar] [CrossRef]
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Austral. Ecol. 2001, 26, 32–46. [Google Scholar] [CrossRef]
- Dixon, P. VEGAN, a package of R functions for community ecology. J. Veg. Sci. 2003, 14, 927–930. [Google Scholar] [CrossRef]
- Chevallier, S.; Bertand, D.; Kohler, A.; Courcoux, P. Application of PLS-DA in multivariate image analysis. J. Chemom. 2006, 20, 221–229. [Google Scholar] [CrossRef]
- Kuhn, M. Building predictive models in R using the caret package. J. Stat. Softw. 2008, 28, 5. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Merzlyak, M.N.; Chivkunova, O.B. Optical properties and nondestructive estimation of anthocyanin content in plant leaves. Photochem. Photobiol. 2001, 74, 38–45. [Google Scholar] [CrossRef]
- Gamon, J.A.; Serrano, L.; Surfus, J.S. The photochemical reflectance index: An optical indicator of photosynthetic radiation use efficiency across species, functional types, and nutrient levels. Oecologia 1997, 112, 492–501. [Google Scholar] [CrossRef]
- Gao, B.C. NDWI-a normalized difference water index for remote sensing of vegetation liquid from space. Remote Sens. Environ. 1996, 58, 257–266. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Zur, Y.; Chivkunova, O.B.; Merzlyak, M.N. Assessing carotenoid content in plant leaves with reflectance spectroscopy. Photochem. Photobiol. 2002, 75, 272–281. [Google Scholar] [CrossRef]
- Serrano, L.; Peñuelas, J.; Ustin, S.L. Remote sensing of nitrogen and lignin in Mediterranean vegetation from AVIRIS data: Decomposing biochemical from structural signals. Remote Sens. Environ. 2002, 81, 355–364. [Google Scholar] [CrossRef]
- Merzlyak, M.N.; Gitelson, A.A.; Chivkunova, O.B.; Rakitin, V.Y. Non-destructive optical detection of pigment changes during leaf senescence and fruit ripening. Physiol. Plant. 1999, 106, 135–141. [Google Scholar] [CrossRef]
- Katul, L.; Wan, S.; Hung, W.; Collins, S. Effect of microclimate on fungal spore germination and disease development. Plant Pathol. 2012, 61, 25–36. [Google Scholar] [CrossRef]
- Thomma, B.P.H.J. Alternaria spp.: From general saprophyte to specific parasite. Mol. Plant Pathol. 2003, 4, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Isah, T. Stress and defense responses in plant secondary metabolites production. Biol. Res. 2019, 52, 39. [Google Scholar] [CrossRef] [PubMed]
- Ballaré, C.L. Light regulation of plant defense. Annu. Rev. Plant Biol. 2014, 65, 335–363. [Google Scholar] [CrossRef] [PubMed]
- Weir, T.L.; Park, S.W.; Vivanco, J.M. Biochemical and physiological mechanisms mediated by allelochemicals. Curr. Opin. Plant Biol. 2004, 7, 472–479. [Google Scholar] [CrossRef]
- Doehlemann, G.; Ökmen, B.; Zhu, W.; Sharon, A. Plant pathogenic fungi and oomycetes: A case of cross-kingdom horizontal gene transfer? PLoS Pathog. 2008, 4, e1000165. [Google Scholar] [CrossRef]
- Cerda, R.; Avelino, J.; Harvey, C.A.; Gary, C.; Tixier, P.; Allinne, C. Coffee agroforestry systems capable of reducing disease-induced yield and economic losses while providing multiple ecosystem services. Crop Prot. 2020, 134, 105149. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Merzlyak, M.N.; Lichtenthaler, H.K. Detection of red edge spectral shift caused by chlorophyll decrease in plant leaves. Photochem. Photobiol. 2003, 71, 366–373. [Google Scholar] [CrossRef]
- Blackburn, G.A. Hyperspectral remote sensing of plant pigments. J. Exp. Bot. 2007, 58, 855–867. [Google Scholar] [CrossRef]
- Carter, G.A.; Knapp, A.K. Leaf optical properties in higher plants: Linking spectral characteristics to stress and chlorophyll concentration. Am. J. Bot. 2001, 88, 677–684. [Google Scholar] [CrossRef]
- Curran, P.J. Remote sensing of foliar chemistry. Remote Sens. Environ. 1989, 30, 271–278. [Google Scholar] [CrossRef]
- Serbin, S.P.; Singh, A.; McNeil, B.E.; Kingdon, C.C.; Townsend, P.A. Spectroscopic determination of leaf morphological and biochemical traits for northern temperate and boreal tree species. Ecol. Appl. 2014, 24, 1651–1669. [Google Scholar] [CrossRef] [PubMed]
- Mahlein, A.K. Plant disease detection by imaging sensors. Parallels and specific demands for precision agriculture and plant phenotyping. Plant Dis. 2016, 100, 241–251. [Google Scholar] [CrossRef]
- Carretero, R.; Serrago, R.A.; Bancal, M.O.; Perelló, A.E.; Miralles, D.J. Absorbed radiation and radiation use efficiency as affected by foliar diseases in relation to their vertical position into the canopy in wheat. Field Crops Res. 2010, 116, 184–195. [Google Scholar] [CrossRef]
- Liu, C.; Du, T.; Kang, S.; Zhang, J.; Ding, R. Effects of disease on plant canopy structure and light interception. Ecol. Indic. 2019, 99, 98–108. [Google Scholar] [CrossRef]
- Haboudane, D.; Miller, J.R.; Pattey, E.; Zarco-Tejada, P.J.; Strachan, I.B. Hyperspectral vegetation indices and novel algorithms for predicting green LAI of crop canopies: Modeling and validation in the context of precision agriculture. Remote Sens. Environ. 2004, 90, 337–352. [Google Scholar] [CrossRef]
- López-López, J.A.; Kokaly, R.F.; Asner, G.P. The challenge of monitoring complex biotic stress interactions using UAV hyperspectral data. Remote Sens. 2020, 12, 1423. [Google Scholar] [CrossRef]
- Thenkabail, P.S.; Lyon, J.G.; Huete, A. Hyperspectral Remote Sensing of Vegetation; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar] [CrossRef]
- Gamon, J.A.; Qiu, H.L.; Sanchez-Azofeifa, A. Ecological applications of remote sensing at multiple scales. In Functional Plant Ecology; CRC Press: Boca Raton, FL, USA, 2007; pp. 655–684. [Google Scholar] [CrossRef]
- Jose, S. Agroforestry for ecosystem services and environmental benefits: An overview. Agrofor. Syst. 2009, 76, 1–10. [Google Scholar] [CrossRef]
- Carter, G.A. Reflectance Wavebands and Indices for Remote Estimation of Photosynthesis and Stomatal Conductance in Pine Canopies. Remote Sens. Environ. 1998, 63, 77–85. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).