Abstract
Long-term application of chemical fertilizer poses an environmental threat to belowground ecosystems. However, the impact of nitrogen (N) or phosphorus (P) fertilizers on soil biodiversity and the conditions of soil food web remains largely unknown. Soil nematodes are the most abundant multicellular soil animals and serve as excellent bioindicators of soil. Here, we investigated soil nematode communities and food web structure in a long-term experiment with different application rates of N and P fertilizers in northeast China. The application of N and P fertilizers increased the abundance of bacterivores but suppressed the abundance of omnivores and predators. The abundance of bacterivores exhibited an increasing trend, while that of omnivores and predators showed a decreasing trend with increasing rates of N and P fertilizers. Plant parasites displayed a decreasing trend in response to N fertilizer, but not to P fertilizer. N and P fertilizers also altered nematode functional guild composition, with N fertilizer increasing the abundance of Ba1, and P fertilizer increasing the abundance of Fu2 and Ba3. Nonmetric multidimensional scaling (NMDS) analysis revealed apparent successions of nematode communities from no fertilizer soils to high rates of N or P fertilizer soils at both the genus and functional guild levels. Furthermore, N and P fertilizers resulted in different nematode communities. In terms of nematode food web indices, N fertilizer increased the enrichment index (EI) but reduced the channel index (CI) and structure index (SI), whereas P fertilizer only reduced the SI value. High rates of N and P fertilizers increased the respired carbon of bacterivores but reduced the respired carbon of predators. Mantel tests revealed significant correlations between soil properties and the community composition of both fungivores and omnivores. Among all soil properties, available phosphorus (AP) had the greatest influence on the community structure of soil nematodes. Our findings indicate that N fertilizer has a powerful effect on nematode food web structure, while P fertilizer exerts a stronger effect on soil nematode community composition.
1. Introduction
In order to enhance grain yield, excessive application of mineral fertilizer has frequently been implemented, resulting in a variety of issues, particularly the intensification of nitrogen (N) deposition [1]. The escalation of N deposition is a global concern and has been found to trigger a range of ecological and environmental problems [2]. While phosphorus (P) is a vital element for plant growth, only 20% of the applied P is absorbed by plants in the early phase, with the remaining amount accumulating in the soil [3]. The extensive use of N and P fertilizers has dramatically altered terrestrial ecosystem processes, including the dynamics of the soil biota community and nutrient cycling [4,5]. Hence, it is crucial to enhance our comprehension of the impacts of N enrichment on soil ecosystem components and functions.
Nematodes play a vital role in terrestrial ecosystems as they occupy all trophic levels of soil food webs [6]. Their communities are useful as environmental and food web indicators, as they respond to environmental changes, and reveal soil conditions [7,8]. Nematodes can be categorized into multiple trophic groups based on their feeding habits, such as plant parasites, bacterivores, fungivores, omnivores, and predators [9]. Previous studies have demonstrated that inorganic N fertilizer has considerable impacts on the abundance and community composition of soil nematodes [10,11]. Long-term fertilizer application has been found to increase the abundance of plant parasites and bacterivores in a tallgrass prairie ecosystem in Kansas, USA [12], while reducing the abundance of omnivores in a grass sward in British Columbia [13]. Meanwhile, van Eekeren et al. [14] reported that a lower amount of inorganic fertilizer was associated with a higher abundance and proportion of plant feeder nematodes in a sandy soil grassland ecosystem in the Netherlands. Additionally, a study conducted in a pasture in New Zealand showed that fertilization increased the abundance of total and plant parasites, while reducing the abundance of fungivores and predators [15]. Nitrogen fertilizer was found to significantly increase the relative abundance of plant parasites and reduce the abundance of omnivores in a subtropical farm in Florida, USA [16], but had no significant impact on soil nematode communities in a temperate farm in northeast China [17]. In contrast, studies on the effects of mineral phosphorus fertilizers on soil nematode communities are scarce. In one study, phosphorus addition was found to significantly suppress the density of total nematodes and omnivore-predators in tropical secondary forests [18]. Other studies have focused on how phosphorus fertilizer affects specific nematode species such as cyst nematode, Heterodera avenae, and H. glycines [19,20,21]. Currently, there is a lack of understanding of the effects of phosphorus fertilizer on the overall composition and structure of soil nematode communities. Despite ongoing debate regarding the extent and direction of nematode responses to nitrogen, it is clear that variations occur based on levels of N addition and specific ecosystems. Therefore, a long-term fertilizer experiment is needed to reveal general patterns of soil nematode community composition and structure under different rates of the N and P fertilizers.
Ecological indices of nematodes are helpful to understand the nematode communities and their relationships with the ecosystem and soil food webs [22,23]. The Shannon–Wiener Index (H′) is used to describe species diversity in a community, and is a more reliable indicator of the evenness and abundance of the species present, as well as the disturbance of the habitat [24]. The maturity index (MI and PPI) reflects the degree of disturbance in an ecosystem and is closely related to primary production [7]. The channel index (CI) indicates the primary decomposition pathway of the soil food web; the enrichment index (EI) is a sensitive indicator of the soil food web’s response to available resources; and the structure index (SI) is used to indicate the status of the structure of the soil food web during soil disturbance or restoration [8]. The addition of fertilizer typically reduces MI values [10,25]. However, Sarathchandra et al. [15] reported that MI values did not change after phosphorus fertilization in pasture soils in New Zealand. The EI, SI, and CI values have been reported to increase, decrease, or remain stable in response to nitrogen addition [22,25,26,27]. However, there is a study found that fertilizer can decrease the channel index 35% [28]. Phosphorus addition significantly suppressed nematode faunal indices EI and SI, but increased two faunal indices in tropical secondary forests. Previous studies have extensively reported the effects of N addition on the ecological indices of soil nematodes, but the responses of soil nematode communities to N addition are directly affected by the form of N, the rate of N addition, and soil properties [13,29,30,31]. In addition, soil nematode communities are often overlooked in studies of phosphorus addition.
Examining the impacts of nitrogen and phosphorus additions on soil nematode communities can significantly enhance our understanding of how mineral fertilizers modify soil food webs and ecosystem processes. In this study, we investigated the responses of soil nematode communities to rates of N and P additions in a long-term fertilizer experiment in a mollisol region. Specifically, the objectives of the study were (1) to ascertain the effect of N and P addition rates on soil nematode communities and food web structure, and (2) to explore how N and P fertilizers impact soil nematode communities. We hypothesize that the addition of N and P may have negative effects on the abundance, diversity, and food web complexity of soil nematodes. Additionally, we anticipate that these effects may intensify with increasing rates of N and P addition.
2. Materials and Methods
2.1. Experimental Site
The long-term experimental plots, with varying levels of fertilization, were established in Shengli Village, Hailun City, Heilongjiang Province, China (47°26′ N, 126°47′ E) in 2002. The experimental site is situated in a typical black soil area, characterized by a flat terrain at an elevation of 210 m. The region experiences a temperate continental monsoon climate, with an average annual precipitation range of 500–600 mm. The average annual temperature is 1.5 °C, with an accumulated annual temperature of 2450 °C. The annual sunshine duration spans from 2600 to 2800 h. The frost-free period lasts for 125 days each year.
2.2. Experimental Design
Six long-term fertilizer treatments were implemented, with varying nitrogen (N) and phosphorus (P) levels, including a control without any fertilizer (N0P0), and different combinations of N and P rates (N1P1, N2P1, N3P1, N1P2, and N1P3). The different rates of N and P were represented by N0, N1, N2, N3, P0, P1, P2, and P3, indicating increasing rates of N and P. Urea was used as nitrogen fertilizer, while ammonium dihydrogen phosphate was used as phosphorus fertilizer, with the application amount calibrated using triple superphosphate. Potassium sulfate, containing potassium oxide (K2O), was utilized for potassium fertilizer. A 2-year crop rotation of soybean and maize was implemented from the beginning of the experiment. For maize: nitrogen was applied at rates of 100 kg/ha, 160 kg/ha, and 220 kg/ha, while the P2O5 rates were 20 kg/ha, 60 kg/ha, and 100 kg/ha. For soybean, nitrogen was applied at rates of 15 kg/ha, 35 kg/ha, and 55 kg/ha, while the P2O5 rates were 30 kg/ha, 60 kg/ha, and 90 kg/ha. The K2O rate was set at 30 kg/ha. Each treatment consisted of three replicate plots, with each plot being 32 m2 (4 m × 8 m). The N0P0 treatment did not receive any chemical fertilizer application for maize and soybean. Crops were sown in early May and harvested in early October every year, with regular weeding and tillage conducted for each treatment.
2.3. Soil Sampling
In September 2022, soil samples were collected from plots during the maize phase of the soybean and maize rotation. Seven soil cores, with 20 cm in depth, were manually collected from each plot using a sampling shovel. The soil samples were taken within the maize row and between the maize plants. Subsequently, the soil collected from each plot was combined and manually mixed to ensure homogeneity of the composite sample. To eliminate roots and large stones, the soil samples were passed through a 2 mm mesh soil sieve. Finally, about 400 g of soil from each sample was stored in a plastic bag and refrigerated at 4 °C.
2.4. Nematode Extraction and Identification
Nematodes were extracted from a 100 g fresh soil for a period of 48 h using the modified Baermann funnel method, which is based on the hydrotaxis and gravity of nematodes [32]. The extracted soil nematodes were then enumerated under an Olympus microscope (Olympus BX43, Tokyo, Japan). The first 100 nematodes encountered were identified at the genus level and classified into different trophic groups, namely plant parasites (Pp), bacterivores (Ba), fungivores (Fu), omnivores (Om), and predators (Pr) [9]. Additionally, the nematodes were assigned to functional guilds based on their trophic behavior and ecological life strategy [33,34]. In cases where the nematode population in the sample was less than 100 individuals, all nematodes present were identified. The abundance of soil nematodes was converted to individuals per 100 g dry soil.
2.5. Calculation of Community Structure Indices and Respired Carbon
The ecological indices used in this study included the Shannon–Wiener index (H’) and trophic diversity (TD), which were used to assess the environmental disturbance on community diversity and trophic groups [35,36]. Maturity indices of free-living nematodes (MI) and plant-parasitic nematodes (PPI) were utilized to evaluate the life-history characteristics of free-living nematodes and plant-parasitic nematodes, respectively [7]. The channel index (CI), enrichment index (EI), and structure index (SI) were calculated to determine the influence of fertilizer application on the soil food web [8]. We obtained the respired carbon of nematode taxa from the database of Nemaplex Main Menu (http://nemaplex.ucdavis.edu/Uppermnus/topmnu.htm, accessed on 10 May 2023). These indices were calculated as follows:
where pi is the proportion of individuals in the ith taxon, vi represents the c–p value of the ith taxon, fi represents the frequency of the ith taxon of free-living nematodes or plant parasites, and Ti represents the relative abundance of each trophic group.
H′ = −∑pi lnpi
MI = ∑vi fi
PPI = ∑vi f′i
TD = 1/∑(Ti)2
CI = 100 × 0.8 Fu2/(3.2 Ba1 + 0.8 Fu2)
EI = 100 × e/(e + b)
SI = 100 × s/(s + b)
e = ∑(3.2 Ba1 + 0.8 Fu2)
b = ∑0.8(Ba2 + Fu2)
s = ∑(1.8 N3 + 3.2 N4 + 0.8 Pr2 + 3.2 N4 + 5.0 N5)
In the above equations, N represents the nematode trophic groups (Ba3–Ba5, Fu3–Fu5, Om3–Om5, Pr2–Pr5). The numerical value attached to each group (e.g., Ba1) represents the respective colonizer-persistor c-p value, which is used to categorize the functional guilds of bacterivores, fungivores, omnivores, and predators [8]. For example, Ba1 is bacterivores with a c-p value of 1.
2.6. Soil Properties
In this study, soil total carbon (TC) and total nitrogen (TN) contents were measured using an elemental analyzer (VarioELIII, Elementar, Heraeus, Germany). Soil total phosphorus (TP) and soil available phosphorus (AP) contents were extracted using HClO4-H2SO4 digestion and 0.5 M NaHCO3 solution, respectively, followed by the use of molybdenum antimony resistance colorimetric in a UV-Vis spectrophotometer (UV-6000, Metash Instruments Co., Ltd., Shanghai, China) to determine their contents. The soil ammonium nitrogen (NH4+–N) and nitrate nitrogen (NO3−–N) contents were extracted through a 2 mol L−1 KCl solution at a 1:10 (wt: volume) ratio of soil to KCl solution, and their contents were determined using a continuous flow analyzer (SKALAR San++, Skalar, Breda, The Netherlands). Soil total potassium (TK) content was extracted using the acetic acid and ammonium leaching method and was quantified by flame photometry (ICPS-7500, Shimadzu, Kyoto, Japan). Soil pH was measured using a pH meter (Mettler Toledo Delta320, Greifensee, Switzerland) in soil water suspensions (1:2.5 g/mL). Soil water content (WT) was determined by drying soil at 105 °C until a constant weight was obtained.
2.7. Data Analysis
One-way analysis of variance (ANOVA) was used to compare the effects of fertilizer gradients on soil nematode variables. Statistical significance was determined at a significance level of p < 0.05. The ANOVAs were performed using IBM SPSS 27.0 (IBM Corporation, Armonk, NY, USA). Nonmetric multidimensional scaling (NMDS) plots were employed to assess the change trends in soil nematode community composition (represented by genus and functional guilds) along different N and P gradients of fertilizer application. The NMDS analysis was performed using the weighted Bray–Curtis distance matrices generated with CANOCO 4.5 (Ithaca, NY, USA). To determine the contribution of each soil property variable to variations in soil nematode community, a random forest (RF) analysis with 1000 permutations was conducted to evaluate the most important factors using the randomForest package. Mantel tests were carried out between the community composition of nematode trophic groups and soil properties (9999 permutations) using the “linket” package in R software 4.2.3.
3. Results
3.1. Effect of N and P Fertilizers on Abundance of Soil Nematodes
The abundance of soil nematodes across trophic groups and functional guilds was influenced by the application of N and P fertilizers (Figure 1). There were significant increasing trends in the abundance of bacterivores with increasing rates of N and P fertilizers. Both N3P1 and N1P3 resulted in a significantly higher abundance of bacterivores compared with N0P0 (Figure 1c,d). Contrastingly, omnivores and predators exhibited decreasing trends in their abundance with increasing rates of N and P fertilizers. Notably, only the high rate of P fertilizer (N1P2 and N1P3) significantly reduced the abundance of omnivores compared to N0P0 (Figure 1h). The rate of N and P fertilizers had no significant impact on the abundance of plant parasites, fungivores, and total soil nematodes, while the abundance of plant parasites exhibited a decreasing trend with increasing rates of N fertilizer (Figure 1a,b,e,f,k,l). In this study, a total of 14 functional guilds were observed (Table S1). The application of N fertilizer significantly increased the abundance of Ba1 (p < 0.05), while the application of P fertilizer significantly increased the abundance of Ba3 and Fu2 but reduced the abundance of Fu4 and Om4 (p < 0.05).
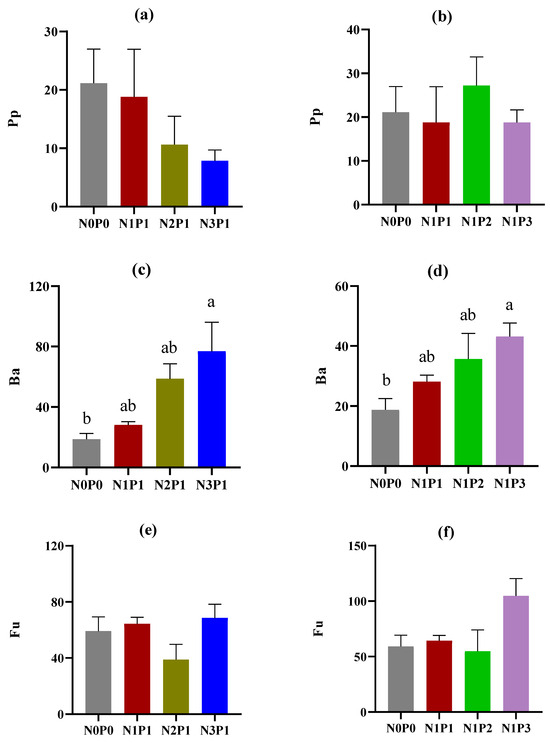
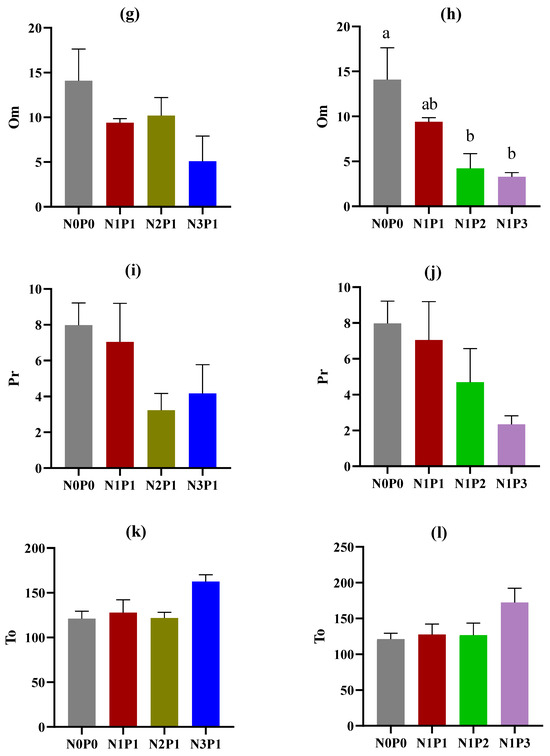
Figure 1.
Abundance (individuals per 100 g dry soil) of soil nematodes under different rates of N and P fertilizers. Pp, plant parasites; Ba, bacterivores; Fu, fungivores; Om, omnivores; Pr, predators; and To, total soil nematodes. (a,c,e,g,i,k) indicate changes in nematode trophic groups with increasing rates of N fertilizer; (b,d,f,h,j,l) indicate changes in nematode trophic groups with increasing rates of P fertilizer. The rates of N and P fertilizers are denoted as N0P0, N1P1, N2P1, N3P1, N1P2, and N1P3. The lines above the bars represent the standard errors of the means. Different letters above the bars indicate significant differences at the p < 0.05 level.
3.2. Effect of N and P Fertilizers on Soil Nematode Community Diversity
A total of 38 taxa were detected in this study (Table 1). The application of N and P fertilizers had no significant effects on nematode community indices, including H’, TD, and PPI (Figure 2a–d,g,h). However, a trend was observed in which TD decreased with increasing rates of N and P fertilizers (Figure 2c,d). High rates of N and P fertilizers resulted in a reduction in the MI value (Figure 2e,f). The application rates of N and P fertilizers had an impact on the beta diversity of soil nematodes at both the genus and functional guild levels (Figure 3). NMDS analysis revealed that the nematode community developed along the direction from N0P0 to N1P1, N2P1, and N3P1, or from N0P0 to N1P1, N1P2, and N1P3 at both the genus and functional guild levels. Furthermore, N2P1 and N3P1 were clearly separated from N1P2 and N1P3 along axis 2 (Figure 3a,b). At the genus level, the soils treated with N and P fertilizers were clearly separated from those of the N0P0 (Figure 3a).

Table 1.
Abundance of soil nematode genus under different rates of N and P fertilizers. The rates of N and P fertilizers are denoted as N0P0, N1P1, N2P1, N3P1, N1P2, and N1P3. p-value represents statistical probability.
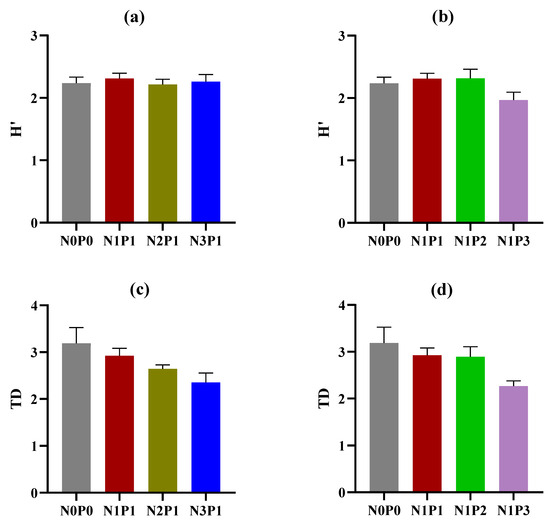
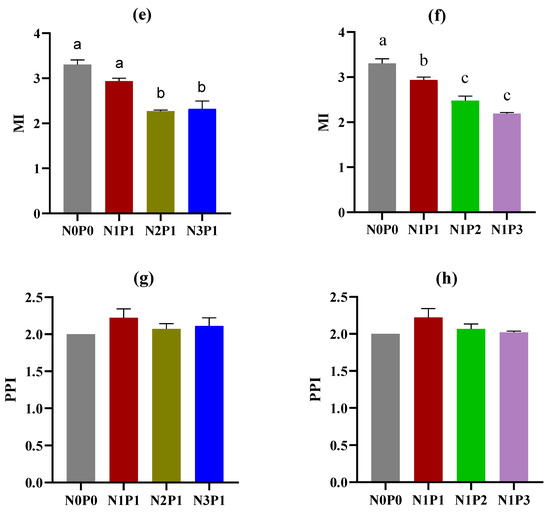
Figure 2.
Nematode community indices under different rates of N and P fertilizers. H’, Shannon–Wiener index; TD, trophic diversity; MI, maturity index of free-living nematodes; and PPI, maturity index of plant parasites. (a,c,e,g) indicate changes in nematode community indices with increasing rates of N fertilizer; (b,d,f,h) indicate changes in nematode community indices with increasing rates of P fertilizer. The rates of N and P fertilizers are denoted as N0P0, N1P1, N2P1, N3P1, N1P2, and N1P3. The lines above the bars represent the standard errors of the means. Different letters above the bars indicate significant differences at the p < 0.05 level.
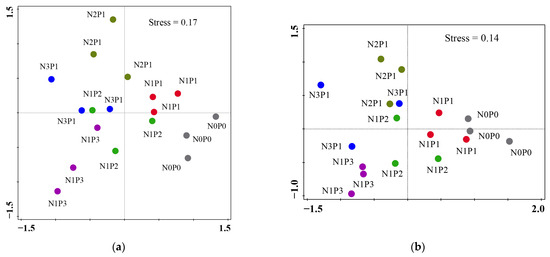
Figure 3.
Nonmetric multidimensional scaling plots for nematode genus (a,b) functional guilds. The rates of N and P fertilizers are denoted as N0P0, N1P1, N2P1, N3P1, N1P2, and N1P3.
3.3. Effect of N and P Fertilizers on Nematode Food Web Structure and Respired Carbon
The application of phosphorus fertilizer did not have any effect on the EI value. However, the N fertilizer had a significant impact on the EI value, with a significantly higher value in N2P1 as compared with N0P0 (Figure 4a). The SI demonstrated a decreasing trend with higher rates of both N and P fertilizers, and the values were significantly lower in N3P1, N1P2, and N1P3 than in N0P0 (Figure 4c,d). Additionally, the CI value was significantly lower in N2P1 than in N0P0, but no significant difference was observed in P fertilizers (Figure 4e,f).
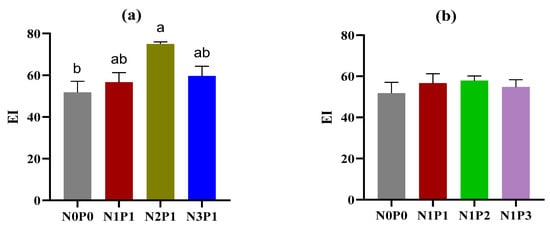
Figure 4.
Soil nematode food web indices under different rates of N and P fertilizers. EI, enrichment index; SI, structure index; and CI, channel index. (a,c,e) indicate changes in nematode food web indices with increasing rates of N fertilizer; (b,d,f) indicate changes in nematode food web indices with increasing rates of P fertilizer. The rates of N and P fertilizers are denoted as N0P0, N1P1, N2P1, N3P1, N1P2, and N1P3. The lines above the bars represent the standard errors of the means. Different letters above the bars indicate significant differences at the p < 0.05 level.
Furthermore, both N and P fertilizers affected the respired carbon of soil nematodes (Table 2). Bacterivores showed significantly higher respired carbon in N3P1 and N1P3 compared to N0P0. In contrast, N2P1 significantly reduced the respired carbon of predators and of total soil nematodes compared to N0P0. The respired carbon of predators was significantly lower in N1P3 than in N0P0. However, neither N fertilizer nor P fertilizer had a significant effect on the respired carbon of plant parasites, fungivores, and omnivores.

Table 2.
Respired C of soil nematodes under different rates of N and P fertilizers. Pp, plant parasites; Ba, bacterivores; Fu, fungivores; Om, omnivores; Pr, predators; and To, total soil nematodes. The rates of N and P fertilizers are denoted as N0P0, N1P1, N2P1, N3P1, N1P2, and N1P3. Different letters within the same column indicate significant differences at the p < 0.05 level among different rates of N or P fertilizers.
3.4. Relationship between Soil Properties and Soil Nematode Community Structure
The application of both N and P fertilizers significantly affected the concentrations of NH4+–N, TP, AP, TK, and pH (Table S2). Specifically, increasing the rate of N fertilizer resulted in higher NH4+–N concentrations, while increasing the rate of P fertilizer led to higher TP and AP concentrations.
There was a significant negative correlation between Pp and TN, while a positive correlation was found between Pp and WT and pH (Table 3). Ba only had a negative correlation with WT, whereas Fu demonstrated positive correlations with NH4+–N and AP. The Om was negatively correlated with NH4+–N, TP, AP, and TK, while the Pr was negatively correlated with TC and TK. The To was only correlated with NH4+–N. The TD, MI, and SI had significantly negative correlations with NH4+–N, AP, and TK. The MI and SI also showed a negative correlation with TP. Furthermore, WT was negatively correlated with Ba and EI, but positively correlated with MI and CI.

Table 3.
Correlation between soil nematode traits and soil properties. Pp, plant parasites; Ba, bacterivores; Fu, fungivores; Om, omnivores; Pr, predators; To, total soil nematodes; H’, Shannon–Weaver index; TD, trophic diversity; PPI, maturity index of plant parasites; MI maturity index of free-living nematodes; CI, channel index; EI, enrichment index; SI, structure index; TN, total nitrogen; TC, soil total carbon; C/N, ratio of soil organic carbon to total nitrogen; NH4+–N, ammonium-nitrogen; NO3−–N, nitrate nitrogen; TK, total potassium; AK; available potassium; TP, total phosphorus; AP, available phosphorus, WT, soil water content; and pH, soil pH. Correlation coefficients labeled with ‘*’ and ‘**’ indicate significant difference at the levels of p < 0.05 and p < 0.01, respectively.
Mantel tests revealed significant correlations between soil properties and the community composition of both fungivores and omnivores (Figure 5a). However, no significant correlations were observed for the community composition of plant parasites, bacterivores, and predators. Specifically, Fu was significantly correlated with TP, AP, and TK, while Om was significantly correlated with TP and TC. Additionally, the random forest analysis showed that AP exerted the greatest influence on the community structure of soil nematodes, followed by pH, TP, TK, and NO3−–N (Figure 5b).
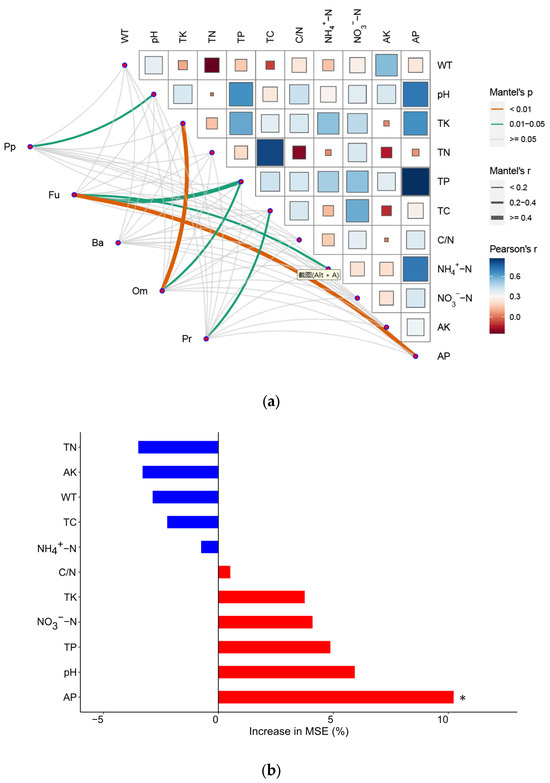
Figure 5.
Pairwise comparisons of the trophic composition of soil nematode communities with soil properties (a) and assessment of the importance of soil properties to variations in soil nematode communities by random forest analysis (b). The color squares indicate Pearson correlation coefficients. The correlation between nematode trophic groups and soil properties was evaluated using Mantel test. The edge width indicates the strength of the correlation, and the color indicates its statistical significance. * indicates significant influence at p < 0.05.
4. Discussion
4.1. Effect of N and P Fertilizers on Abundance of Soil Nematodes
In the current study, N and P fertilizers had different influences on nematode trophic groups. The abundance of plant parasites decreased with increasing rates of N fertilizer, but not P fertilizer (Figure 1a). This finding is consistent with previous studies, which found that N fertilizer reduces the abundance of plant parasites and that the negative effect is intensified with increased rates of N fertilizer [18,30,37]. However, it differs from other studies that report N fertilizer increases the abundance of plant parasites [38,39]. The discrepancy in results may be attributed to differences in the type of fertilizer applied, soil properties, or the soil ecosystem [40]. The reason for the decrease in the abundance of plant parasites with an increase in N fertilizer may be due to the toxic effect of NH4+ accumulation in the soil, which affects plant parasites [41], especially ectoparasitic nematodes, as most of their bodies are exposed to the soil. Moreover, N fertilizer can increase soil NH4+ that can be absorbed by crops and accumulate in their root systems [42]. When plant parasites parasitize the roots and consume ammonium-rich cell fluids, ammonium poisoning may be induced [30,43]. Our study revealed that the application of high rates of N fertilizer had a significant positive impact on the abundance of bacterivores. Furthermore, there was a clear increasing trend in the abundance of bacterivores in response to higher rates of N fertilizer (Figure 1c). This is consistent with previous studies, which reported an increase in the abundance of bacterivores with N fertilizer [10,44], but differs from a study finding that the relative abundance of bacterivores showed minor changes among different rates of N fertilizer [45]. Increases in soil N can lead to an increase in microbial activity and numbers, which may subsequently promote bacterivores [9]. The different findings may be attributed to differences in the species composition of bacterivores; for example, Caenorhabditis showed a gradual increase, while Acrobeloides displayed a declining trend with an increasing rate of N fertilizer [45]. There is limited research on the effect of P fertilizer on soil nematode community and abundance. Our study demonstrated a clear increasing trend in the abundance of bacterivores in response to higher rates of P fertilizer in maize fields (Figure 1d). Sarathchandra et al. [15] also reported that the abundance of nematodes increased after P fertilizer application in pasture soils. In contrast, omnivores and predators exhibited decreasing trends in their abundance with increasing rates of N and P fertilizers (Figure 1g–j). This finding is consistent with previous studies that have shown a reduction in the abundance of omnivores and predators with N addition [44,45]. Furthermore, P fertilizer was found to suppress the abundance of omnivores and predators in a secondary tropical forest [18]. This can be attributed to the toxic effects of N and P fertilizers on soil nematodes [21,43]. Additionally, the long lifespan and low reproductive rate of omnivores and predators may limit their ability to recover from disturbances [7,8]. Our results indicate that application N and P fertilizers induce stresses on the soil environment. Moreover, compared with N fertilizer, P fertilizer significantly reduced the abundance of omnivores (Figure 1h), suggesting that omnivores are more sensitive to P fertilizer than N fertilizer in maize fields in mollisol region. Furthermore, the application of N fertilizer increased the abundance of Ba1 (Table S1), while P fertilizer changed the abundance of Ba3, Fu2, Fu4, and Om4 (Table S1). This finding suggests that P fertilizer mainly influences functional guild with higher c-p values compared with N fertilizer, which typically impacts nematodes with low c-p values.
4.2. Effect of N and P Fertilizers on Soil Nematode Community Diversity
Nematode community indices are frequently used to illustrate the structure of nematode communities in soil. H’ and TD indices are linked to the diversity of soil nematodes, while PPI and MI indices indicate fertilization disturbance in soil ecosystems [40,46]. In our study, we did not observe significant changes in nematode community indices H′, TD, and PPI (Figure 2). This differs from previous findings that N fertilizer application reduced H’ but increased PPI [10,40,45]. These discrepancies may be attributed to differences in crop soils and their respective nematode community compositions. However, our results suggest that the genus diversity of soil nematodes only fluctuates slightly along with N and P fertilizers. Meanwhile, the MI decreased with increasing rates of N and P fertilizers, likely due to the greater abundance of enrichment bacterivores and general-opportunistic bacterivores in higher fertilizer soils. This finding is in line with most previous research, which has found that N fertilizer reduces MI [10,40]. Nonetheless, some studies have shown no significant effect of N fertilizer on MI [11]. The discrepancy in results may be due to variations in climate and soil ecosystems [47]. The nematode composition of genus and functional guild developed along the direction from low to high rates of N and P fertilizers (Figure 3a), which indicates that the addition rates of both N and P fertilizers impact the community succession of soil nematodes. Some studies reported that N or P addition caused significant changes in the composition of soil nematode communities in temperate forest and secondary tropical forests [11,18]. Additionally, the NMDS analysis of nematode genus and functional guild data showed that higher rates of N fertilizer soils were separated from higher rates of P fertilizer soils via axis 2 (Figure 3b), suggesting that N and P fertilizers have different impacts on the structure of soil nematode communities.
4.3. Effect of N and P Fertilizers on Nematode Food Web Structure and Respired Carbon
The food web indices EI, SI, and CI are well-established indicators of resource availability, structure, and decomposition pathway in detritus food webs [48]. This study demonstrated that the application of P fertilizer did not affect EI and CI values, whereas N fertilizer had an impact on both food web indices (Figure 4). The results of N fertilizer on EI and CI are consistent with Song et al. [45], who observed increasing and decreasing trends, respectively, after the application of N fertilizer. However, these results contradict those of Zhong et al. [49], likely due to differences in experiment duration. A meta analysis indicated that long-term experiments have a greater influence on the ecological indices of soil nematodes compared to short-term experiments [40]. The higher EI and lower CI values suggest an enriched availability of resources and a succession of decomposition pathways towards bacterial domination following the application of a higher rate of N fertilizer. Furthermore, our results indicate that the application of P fertilizer at the current rate does not have a significant effect on resource availability and decomposition pathways within the soil food web. The SI exhibited a decreasing trend with increasing rates of both N and P fertilizers (Figure 4c,d). Previous studies have shown that nitrogen addition can reduce SI [10,11]. Our findings further suggest that high application rates of N and P fertilizers intensify the degradation of the soil food web, leading to fewer linkages in the soil food web. The higher rates of N and P fertilizers increased the respired carbon of bacterivores, but reduced the respired carbon of predators (Table 2). The extent of respired carbon of soil nematodes indicates the utilization of carbon in metabolic activity or the process of CO2 evolution [48]. In light of this, our findings imply that the application of N and P fertilizers may enhance the contribution of r-strategy nematodes to soil CO2 evolution while reducing the contribution of K-strategy nematodes.
4.4. Relationship between Soil Properties and Soil Nematode Community Structure
Our studies revealed that NH4+–N, TP, AP, and TK were all crucial soil environmental factors that affect the structure of soil nematode communities (Table 3). The total abundance of soil nematodes was positively correlated with NH4+–N. This is likely due to the increase in the abundance of r-strategy nematodes, such as bacterivores and fungivores, following N addition [38,50]. Additionally, the abundance of r-strategy nematodes exhibited a significant positive correlation with NH4+–N or NO3−–N [51,52]. This was also corroborated by our results, which showed a positive correlation between the abundance of fungivores and NH4+–N (Table 3). In contrast, the abundance of Om exhibited a negative correlation with NH4+–N, TP, AP, and TK, and the abundance of Pr showed a negative correlation with TC and TK, suggesting that Om is more sensitive to readily available nutrients than Pr. This could be attributed to Pr occupying the highest trophic level in the food web, where the readily available nutrient primarily impacts the nematodes at the lower trophic levels, whose food sources are more influenced by soil available nutrients [9,53]. In addition, the indices TD, MI, and SI showed significant negative correlations with the contents of NH4+–N and AP. This indicates that the increase in NH4+–N and AP in the soil following the application of N and P fertilizers reduces the trophic diversity of soil nematodes and the stability of the soil food web. Previous studies have similarly shown that the addition of N fertilizer decreases MI and SI in the soil [10,45].
We observed a significant correlation between soil properties and the community composition of fungivores and omnivores (Figure 5a). Previous studies have shown that the nitrogen and phosphorus are vital environmental factors influencing the structure of nematode communities [10,38]. However, our study suggests that the application of N and P fertilizers mainly affects the community composition of fungivores and omnivores. At the community level, AP had the strongest effect on the community structure of soil nematodes, followed by pH and TP (Figure 5b). This indicates that P fertilizer has a stronger effect on the community structure of nematodes in comparison to N fertilizer in this study. Similarly, Zhao et al. [18] reported that the effects of phosphorus addition on soil nematode communities are more powerful than the effects of nitrogen addition in tropical secondary forests. Our findings align with previous studies indicating that soil pH is a principal determinant of soil nematode distribution and community composition [54,55]. In this study, TK was identified as one of major factors influencing soil nematode communities (Figure 5b). This can be attributed to the fact that K+ affects growth or development, such as egg laying [56].
5. Conclusions
This study showed that the application of N and P fertilizers had a negative effect on omnivores and predators, and a positive effect on bacterivores, which was intensified with increasing application rates of N and P fertilizers. Specifically, the long-term application of N and P fertilizers resulted in a nematode community with a low MI and SI, which suggests disturbed and degraded trophic links within the soil food web. Compared to P fertilizer, N fertilizer significantly affected the EI and CI, suggesting that low-level trophic nematodes and decomposition pathways are more sensitive to N fertilizers. N and P fertilizers increased the respired C of bacterivores but decreased the respired C of predators, indicating that N and P fertilizers may increase the contribution of bacterivores to C evolution while reducing the contribution of predators. At the levels of nematode genera and functional groups, NMDS analysis indicated that the nematode community structure gradually changed with increasing rates of fertilizer application, and the effects of N and P fertilizer on the nematode community structure were different. Random forest analysis indicated that P fertilizer had a stronger effect on the community structure of nematodes than did N fertilizer. These findings could provide a better understanding of the responses of soil biodiversity and the soil food web to chemical fertilizer additions in mollisols.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy14030507/s1. Table S1. Nematode functional guilds under different rates of N and P fertilizers. Note: Pp, plant parasites; Ba, bacterivores; Fu, fungivores; Om, omnivores; and Pr, predators. The number following the abbreviations is nematode c-p value. The rates of N and P fertilizers are denoted as N0P0, N1P1, N2P1, N3P1, N1P2, and N1P3. Table S2. Soil properties under different rates of N and P fertilizers. Note: TN, total nitrogen; TC, soil total carbon; C/N, ratio of soil organic carbon to total nitrogen; NH4+-N, ammonium-nitrogen; NO3−-N, nitrate nitrogen; TK, total potassium; AK; available potassium; TP, total phosphorus; AP, available phosphorus, WT, soil water content; and pH, soil pH. The rates of N and P fertilizers are denoted as N0P0, N1P1, N2P1, N3P1, N1P2, and N1P3.
Author Contributions
F.P. designed the research and wrote the paper; X.N. and X.Z. performed experiments; Q.F. and D.Z. helped in data analysis; Q.F. and W.H. helped in soil property analysis. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (32071636) and the Strategic Priority Research Program of the Chinese Academy of Sciences (XDA28010102).
Data Availability Statement
The data presented in this study are available on request from the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Galloway, J.N.; Townsend, A.R.; Erisman, J.W.; Bekunda, M.; Cai, Z.C.; Freney, J.R.; Martinelli, L.A.; Seitzinger, S.P.; Sutton, M.A. Transformation of the nitrogen cycle: Recent trends, questions, and potential solutions. Science 2008, 320, 889–892. [Google Scholar] [CrossRef] [PubMed]
- Hungate, B.A.; Dukes, J.S.; Shaw, M.R.; Luo, Y.Q.; Field, C.B. Nitrogen and climate change. Science 2003, 302, 1512–1513. [Google Scholar] [CrossRef] [PubMed]
- Turner, B.L.; Cheesman, A.W.; Condron, L.M.; Reitzel, K.; Richardson, A.E. Introduction to the special issue: Developments in soil organic phosphorus cycling in natural and agricultural ecosystems. Geoderma 2015, 257–258, 1–3. [Google Scholar] [CrossRef]
- Treseder, K.K. Nitrogen additions and microbial biomass: A meta-analysis of ecosystem studies. Ecol. Lett. 2008, 11, 1111–1120. [Google Scholar] [CrossRef] [PubMed]
- Leff, J.W.; Jones, S.E.; Prober, S.M.; Barberán, A.; Borer, E.T.; Firn, J.L.; Harpole, W.S.; Hobbie, S.E.; Hofmockel, K.S.; Knops, J.M.H.; et al. Consistent responses of soil microbial communities to elevated nutrient inputs in grasslands across the globe. Proc. Natl. Acad. Sci. USA 2015, 112, 10967–10972. [Google Scholar] [CrossRef] [PubMed]
- Van den Hoogen, J.; Geisen, S.; Routh, D.; Ferris, H.; Traunspurger, W.; Wardle, D.A.; de Goede, R.G.M.; Adams, B.J.; Ahmad, W.; Andriuzzi, W.S.; et al. Soil nematode abundance and functional group composition at a global scale. Nature 2019, 572, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Bongers, T. The maturity index: An ecological measure of environmental disturbance based on nematode species composition. Oecologia 1990, 83, 14–19. [Google Scholar] [CrossRef]
- Ferris, H.; Bongers, T.; de Goede, R.G.M. A framework for soil food web diagnostics: Extension of the nematode faunal analysis concept. Appl. Soil Ecol. 2001, 18, 13–29. [Google Scholar] [CrossRef]
- Yeates, G.W.; Bongers, T.; De Goede, R.G.M.; Freckman, D.W.; Georgieva, S.S. Feeding habits in soil nematode families and genera-an outline for soil ecologists. J. Nematol. 1993, 25, 315–331. [Google Scholar]
- Hu, J.; Chen, G.R.; Hassan, W.M.; Chen, H.; Li, J.Y.; Du, G.Z. Fertilization influences the nematode community through changing the plant community in the Tibetan Plateau. Eur. J. Soil Biol. 2017, 78, 7–16. [Google Scholar] [CrossRef]
- Wang, H.L.; Liu, G.C.; Huang, B.B.; Wang, X.C.; Xing, Y.J.; Wang, Q.G. Long-term nitrogen addition and precipitation reduction decrease soil nematode community diversity in a temperate forest. Appl. Soil Ecol. 2021, 162, 103895. [Google Scholar] [CrossRef]
- Todd, T. Effects of management practices on nematode community structure in tallgrass prairie. Appl. Soil Ecol. 1996, 3, 235–246. [Google Scholar] [CrossRef]
- Forge, T.A.; Bittman, S.; Kowalenko, C.G. Responses of grassland soil nematodes and protozoa to multi-year and single-year applications of dairy manure slurry and fertilizer. Soil Biol. Biochem. 2005, 37, 1751–1762. [Google Scholar] [CrossRef]
- Van Eekeren, N.; de Boer, H.; Bloem, J.; Schouten, T.; Rutgers, M.; de Goede, R.; Brussaard, L. Soil biological quality of grassland fertilized with adjusted cattle manure slurries in comparison with organic and inorganic fertilizers. Biol. Fertil. Soils 2009, 45, 595–608. [Google Scholar] [CrossRef]
- Sarathchandra, S.U.; Ghani, A.; Yeates, G.W.; Burch, G.; Cox, N.R. Effect of nitrogen and phosphate fertilizers on microbial and nematode diversity in pasture soils. Soil Biol. Biochem. 2001, 33, 953–964. [Google Scholar] [CrossRef]
- Wang, K.H.; McSorley, R.; Marshall, A.; Gallaher, R.N. Influence of organic Crotalaria juncea hay and ammonium nitrate fertilizers on soil nematode communities. Appl. Soil Ecol. 2006, 31, 186–198. [Google Scholar] [CrossRef]
- Liang, W.; Lou, Y.; Li, Q.; Zhong, S.; Zhang, X.; Wang, J. Nematode faunal response to long-term application of nitrogen fertilizer and organic manure in Northeast China. Soil Biol. Biochem. 2009, 41, 883–890. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, F.M.; Li, J.; Zou, B.; Wang, X.L.; Li, Z.A.; Fu, S.L. Effects of experimental nitrogen and/or phosphorus additions on soil nematode communities in a secondary tropical forest. Soil Biol. Biochem. 2014, 75, 1–10. [Google Scholar] [CrossRef]
- Simon, A.; Rovira, A. The influence of phosphate fertilizer on the growth and yield of wheat in soil infested with cereal cyst nematode (Heterodera avenae Woll.). Aust. J. Exp. Agric. 1985, 25, 191–197. [Google Scholar] [CrossRef]
- Price, N.S.; Roncadori, R.W.; Hussey, R.S. The growth of nematode ‘tolerant’ and ‘intolerant’ soyabeans as affected by phosphorus, Glomus intraradices and light. Plant Pathol. 1995, 44, 597–603. [Google Scholar] [CrossRef]
- Coyne, D.L.; Sahrawat, K.L.; Plowright, R.A. The influence of mineral fertilizer application and plant nutrition on plant-parasitic nematodes in upland and lowland rice in Cote d’Ivoire and its implications in long term agricultural research trials. Exp. Agric. 2004, 40, 245–256. [Google Scholar] [CrossRef]
- Ferris, H.; Matute, M.M. Structural and functional succession in the nematode fauna of a soil food web. Appl. Soil Ecol. 2003, 23, 93–110. [Google Scholar] [CrossRef]
- Du Preez, G.; Daneel, M.; De Goede, R.; Du Toit, M.J.; Ferris, H.; Fourie, H.; Geisen, S.; Kakouli-Duarte, T.; Korthals, G.; Sánchez-Moreno, S.; et al. Nematode-based indices in soil ecology: Application, utility, and future directions. Soil Biol. Biochem. 2022, 169, 108640. [Google Scholar] [CrossRef]
- Roth, D.S.; Perfecto, I.; Rathcke, B. The effects of management systems on ground-foraging ant diversity in Costa Rica. Ecol. Appl. 1994, 4, 423–436. [Google Scholar] [CrossRef]
- Shaw, E.A.; Boot, C.M.; Moore, J.C.; Wall, D.H.; Barone, J.S. Long-term nitrogen addition shifts the soil nematode community to bacterivore-dominated and reduces its ecological maturity in a subalpine forest. Soil Biol. Biochem. 2019, 130, 177–184. [Google Scholar] [CrossRef]
- Cesarz, S.; Reich, P.B.; Scheu, S.; Ruess, L.; Schaefer, M.; Eisenhauer, N. Nematode functional guilds, not trophic groups, reflect shifts in soil food webs and processes in response to interacting global change factors. Pedobiologia 2015, 58, 23–32. [Google Scholar] [CrossRef]
- Liu, T.; Mao, P.; Shi, L.L.; Eisenhauer, N.; Liu, S.J.; Wang, X.L.; He, X.X.; Wang, Z.Y.; Zhang, W.; Liu, Z.F.; et al. Forest canopy maintains the soil community composition under elevated nitrogen deposition. Soil Biol. Biochem. 2020, 143, 107733. [Google Scholar] [CrossRef]
- Tonjer, L.R.; Nybakken, L.; Birkemoe, T.; Renčo, M.; Ferdous, Z.; Asplund, J. Condensed tannins mediate the effect of long-term nitrogen addition on soil nematodes in a boreal spruce forest. Forest Ecol. Manag. 2023, 545, 121248. [Google Scholar] [CrossRef]
- Gruzdeva, L.I.; Matveeva, E.M.; Kovalenko, T.E. Changes in soil nematode communities under the impact of fertilizers. Eurasian Soil Sci. 2007, 40, 681–693. [Google Scholar] [CrossRef]
- Wei, C.; Zheng, H.; Li, Q.; Lü, X.; Yu, Q.; Zhang, H.; Chen, Q.; He, N.; Kardol, P.; Liang, W.; et al. Nitrogen addition regulates soil nematode community composition through ammonium suppression. PLoS ONE 2012, 7, e43384. [Google Scholar] [CrossRef]
- Pan, K.; Gong, P.; Wang, J.; Wang, Y.; Liu, C.; Li, W.; Zhang, L. Applications of nitrate and ammonium fertilizers alter soil nematode food webs in a continuous cucumber cropping system in Southwestern Sichuan, China. Eur. J. Soil Sci. 2015, 4, 287–300. [Google Scholar] [CrossRef]
- Barker, K.R. Nematode Extraction and Bioassays. In An Advanced Treatise on Meloidogyne; Barker, K.R., Carter, C.C., Sasser, J.N., Eds.; North Carolina State University Graphics: Raleigh, NC, USA, 1985; pp. 19–35. [Google Scholar]
- Bongers, T.; Bongers, M. Functional diversity of nematodes. Appl. Soil Ecol. 1998, 10, 239–251. [Google Scholar] [CrossRef]
- Bongers, T.; Ferris, H. Nematode community structure as a bioindicator in environmental monitoring. Trends Ecol. Evol. 1999, 14, 224–228. [Google Scholar] [CrossRef] [PubMed]
- Shannon, C.E.; Weaver, W. The Mathematical Theory of Communication; University of Illinois: Champaign, IL, USA, 1949. [Google Scholar]
- Neher, D.A. Role of nematodes in soil health and their use as indicators. J. Nematol. 2001, 33, 161–168. [Google Scholar] [PubMed]
- Sun, X.; Zhang, X.; Zhang, S.; Dai, G.; Han, S.; Liang, W. Soil nematode responses to increases in nitrogen deposition and precipitation in a temperate forest. PLoS ONE 2013, 8, e82468. [Google Scholar] [CrossRef] [PubMed]
- Nisa, R.U.; Tantray, A.Y.; Kouser, N.; Allie, K.A.; Wani, S.M.; Alamri, S.A.; Alyemeni, M.N.; Wijaya, L.; Shah, A.A. Influence of ecological and edaphic factors on biodiversity of soil nematodes. Saudi J. Biol. Sci. 2021, 28, 3049–3059. [Google Scholar] [CrossRef] [PubMed]
- De Melo Santana-Gomes, S.; Dias-Arieira, C.R.; Roldi, M.; Santo Dadazio, T.; Marini, P.M.; de Oliveira Barizão, D.A. Mineral nutrition in the control of nematodes. Afr. J. Agric. Res. 2013, 8, 2413–2420. [Google Scholar]
- Zhou, J.; Lu, M.; Sheng, X.J.; Wu, J.P. Effects of nitrogen addition and warming on nematode ecological indices: A meta-analysis. Eur. J. Soil Biol. 2022, 110, 103407. [Google Scholar] [CrossRef]
- Chen, D.; Lan, Z.; Hu, S.; Bai, Y. Effects of nitrogen enrichment on belowground communities in grassland: Relative role of soil nitrogen availability vs. soil acidification. Soil. Biol. Biochem. 2015, 89, 99–108. [Google Scholar] [CrossRef]
- Wall, M.E.; Tiedjens, V.A. Potassium deficiency in ammonium-and nitrate-fed tomato plants. Science 1940, 91, 221–222. [Google Scholar] [CrossRef]
- Kronzucker, H.J.; Britto, D.T.; Davenport, R.J.; Tester, M. Ammonium toxicity and the real cost of transport. Trends Plant Sci. 2001, 6, 335–337. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Xiang, Y.; Li, D.; Luo, X.; Wu, J. Global patterns and controls of soil nematode responses to nitrogen enrichment: A meta-analysis. Soil Biol. Biochem. 2021, 163, 108433. [Google Scholar] [CrossRef]
- Song, M.; Jing, S.; Zhou, Y.; Hui, Y.; Zhu, L.; Wang, F.; Hui, D.; Jiang, L.; Wan, S. Dynamics of soil nematode communities in wheat fields under different nitrogen management in Northern China Plain. Eur. J. Soil Biol. 2015, 71, 13–20. [Google Scholar] [CrossRef]
- Bongers, T.; van der Meulen, H.; Korthals, G. Inverse relationship between the nematode maturity index and plant parasite index under enriched nutrient conditions. Appl. Soil Ecol. 1997, 6, 195–199. [Google Scholar] [CrossRef]
- Xing, W.; Lu, X.M.; Niu, S.L.; Chen, D.M.; Wang, J.S.; Liu, Y.; Wang, B.X.; Zhang, S.; Li, Z.L.; Yao, X.J.; et al. Global patterns and drivers of soil nematodes in response to nitrogen enrichment. Catena 2022, 213, 106235. [Google Scholar] [CrossRef]
- Ferris, H. Form and function: Metabolic footprints of nematodes in the soil food web. Eur. J. Soil Biol. 2010, 46, 97–104. [Google Scholar] [CrossRef]
- Zhong, S.; Zeng, H.C.; Jin, Z.Q. Influences of different tillage and residue management systems on soil nematode community composition and diversity in the tropics. Soil Biol. Biochem. 2017, 107, 234–243. [Google Scholar] [CrossRef]
- Cheng, J.; Ma, W.; Hao, B.; Liu, X.; Li, F.Y. Divergent responses of nematodes in plant litter versus in top soil layer to nitrogen addition in a semi-arid grassland. Appl. Soil Ecol. 2021, 157, 103719. [Google Scholar] [CrossRef]
- Forge, T.A.; Simard, S.W. Structure of nematode communities in forest soils of southern British Columbia: Relationships to nitrogen mineralization and effects of clearcut harvesting and fertilization. Biol. Fert. Soils 2001, 34, 170–178. [Google Scholar]
- Postma-Blaauw, M.B.; de Vries, F.T.; de Goede, R.G.M.; Bloem, J.; Faber, J.H.; Brussaard, L. Within-trophic group interactions of bacterivorous nematode species and their effects on the bacterial community and nitrogen mineralization. Oecologia 2005, 142, 428–439. [Google Scholar] [CrossRef]
- Ren, N.; Wang, Y.; Ye, Y.; Zhao, Y.; Huang, Y.; Fu, W.; Chu, X. Effects of Continuous Nitrogen Fertilizer Application on the Diversity and Composition of Rhizosphere Soil Bacteria. Front. Microbiol. 2020, 11, 1948. [Google Scholar] [CrossRef]
- Fiscus, D.A.; Neher, D.A. Distinguishing sensitivity of free-living soil nematode genera to physical and chemical disturbances. Ecol. Appl. 2002, 12, 565–575. [Google Scholar] [CrossRef]
- Räty, M.; Huhta, V. Earthworms and pH affect communities of nematodes and enchytraeids in forest soil. Biol. Fert. Soils 2003, 38, 52–58. [Google Scholar] [CrossRef]
- Yao, L.; Ruan, M.Y.; Yea, S.W.; Cai, S.Q. DNA topoisomerase 2-associated proteins PATL1 and PATL2 regulate the biogenesis of hERG K+ channels. Proc. Natl. Acad. Sci. USA 2023, 120, e2216146120. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).