Suitability of Lupinus albus L. Genotypes for Organic Farming in Central Northern Bulgaria
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Experiment and Site Characteristics
2.2. Plant Measurements
2.3. Tolerance to Fusarium oxysporum f. sp. Lupini
2.4. Chemical Composition and Feeding Value
2.5. Parameters of Ecological Stability and Statistical Analysis
3. Results
3.1. Morphological Characteristics
3.2. Forage Yield and Ecological Stability
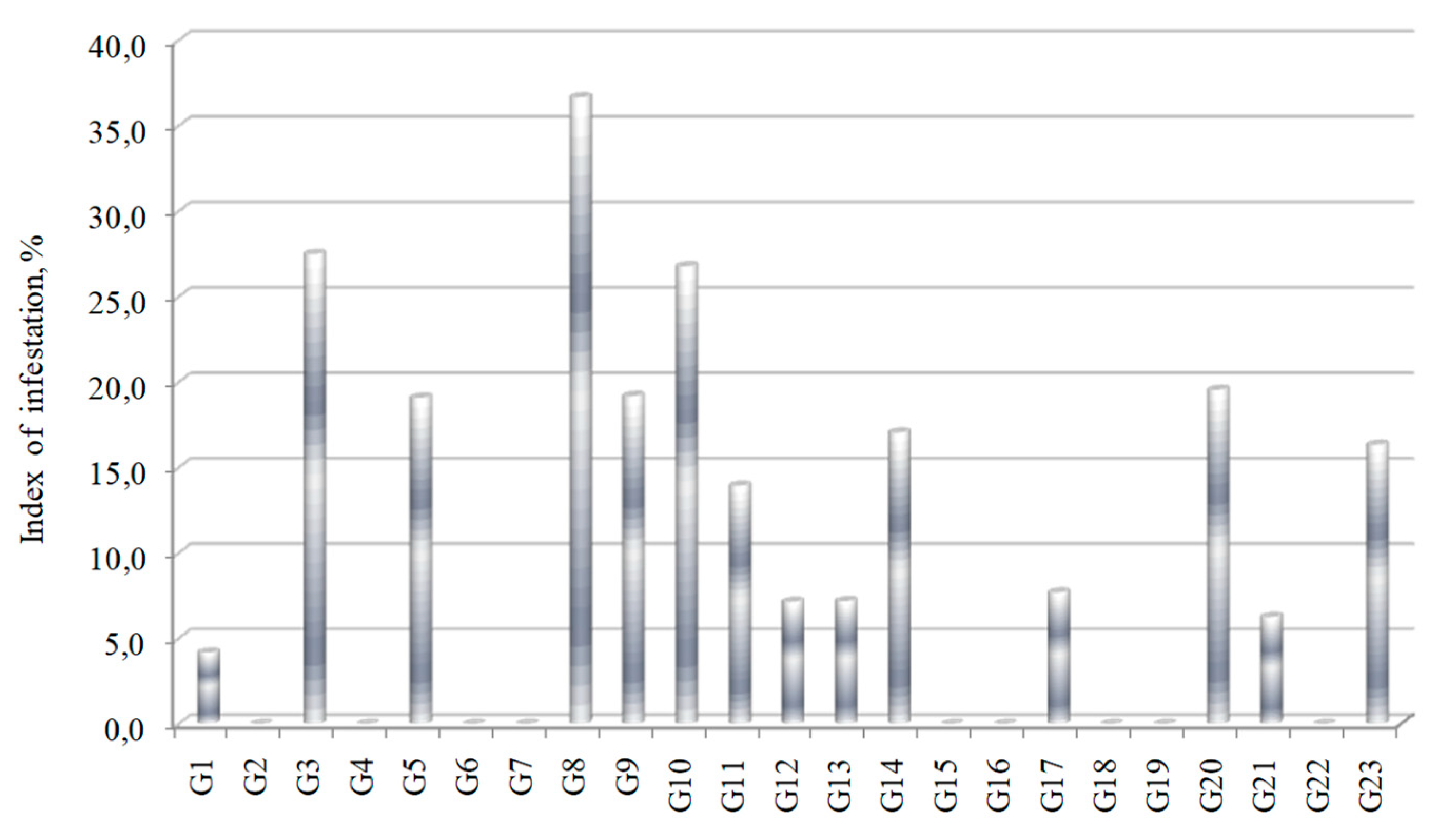
3.3. Tolerance to Fusarium oxysporum
3.4. Chemical Composition and Feeding Value
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kurlovich, B.S.; Kartuzova, L.T.; Cheremisov, B.M.; Emeljanenko, T.A.; Tikhonovich, I.A.; Kozhemyakov, A.P.; Tchetkova, S.A. Evaluation of the biological nitrogen-fixing ability. Plant Genet. Resour. Newsl. 2000, 123, 68–77. [Google Scholar]
- Eastwood, R.J.; Drummond, C.S.; Schifino-Wittmann, M.T.; Hughes, C.E. Diversity and Evolutionary History of Lupins—Insights from New Phylogenies. In Proceedings of the 12th International Lupin Conference—Lupins for Health and Wealth, Fremantle, Australia, 14–18 September 2008; pp. 346–354. [Google Scholar]
- Prusinski, J. White lupin (Lupinus albus L.)-Nutritional and health values in human nutrition—A review. Czech J. Food Sci. 2017, 35, 95–105. Available online: https://cjfs.agriculturejournals.cz/artkey/cjf-201702-0001_white-lupin-lupinus-albus-l-nutritional-and-health-values-in-human-nutrition-a-review.php (accessed on 1 January 2024). [CrossRef]
- Yakovenko, G.L.; Lukashevisn, M.I.; Ageeva, P.A.; Novik, N.V.; Zakharova, M.V. Status and prospects of breeding of cultivated species of Lupin in Russia. IOP Conf. Ser. Earth Environ. Sci. 2021, 663, 012014. Available online: https://iopscience.iop.org/article/10.1088/1755-1315/663/1/012014 (accessed on 1 January 2024). [CrossRef]
- Beyene, C. Genetic variation among white lupin (Lupinus albus L.) landraces from Northwestern and Southern Ethiopia for agronomic traits and nutrient contents of grain. J. Plant Breed. Crop Sci. 2020, 12, 156–169. [Google Scholar]
- Fumagalli, P.; Comolli, R.; Ferrè, C.; Ghiani, A.; Gentili, R.; Citterio, S. The rotation of white lupin (Lupinus albus L.) with metal-accumulating plant crops: A strategy to increase the benefits of soil phytoremediation. J. Environ. Manag. 2014, 145, 35–42. [Google Scholar] [CrossRef]
- Yagovenko, G.L.; Lukashevich, M.I.; Ageeva, P.A.; Novik, N.V.; Misnikova, N.V. Evaluation of the modern lupine varieties developed in the All-Russian Lupin Scientific Research Institute. IOP Conf. Ser. Earth Environ. Sci. 2022, 1010, 012096. Available online: https://iopscience.iop.org/article/10.1088/1755-1315/1010/1/012096/pdf (accessed on 1 January 2024). [CrossRef]
- Aleksiev, G. Sustainable development of bulgarian organic agriculture. Trakia J. Sci. 2020, 18, 603–606. Available online: http://tru.uni-sz.bg/tsj/TJS%20-%20Suppl.1,%20Vol.18,%202020/96_G.Alexiev2.pdf (accessed on 1 January 2024). [CrossRef]
- Gresta, F.; Oteri, M.; Scordia, D.; Costale, A.; Armone, R.; Meineri, G.; Chiofalo, B. White Lupin (Lupinus albus L.), an Alternative Legume for Animal Feeding in the Mediterranean Area. Agriculture 2023, 13, 434. [Google Scholar] [CrossRef]
- Milleville, C. Cultiver des Cultures Associées. Projet Reine Mathilde, Chambre d’Agriculture de la Manche. 2014. Available online: http://partage.cra-normandie.fr/bio/cultiver-des-cultures-associees.pdf (accessed on 1 January 2024).
- Macholdt, J.; Honermeier, B. Importance of variety choice: Adapting to climate change in organic and conventional farming systems in Germany. Outlook Agric. 2017, 46, 178–184. [Google Scholar] [CrossRef]
- Konvalina, P.; Stehno, Z.; Moudrý, J. The critical point of conventionally bred soft wheat varieties in organic farming systems. Agron. Res. 2009, 7, 801–810. Available online: https://orgprints.org/id/eprint/20772/1/The_critical_point_if_conventionally_bred_soft_wheat_varieties_in_organic_farming_systems.pdf (accessed on 1 January 2024).
- Kalapchieva, S.; Yankova, V. Influence of growing factors on seed characteristics of garden pea in the conditions of biological production. In Proceedings of the Ecology and Health, X Jubilee National Scientific Conference, Plovdiv, Bulgaria, 5 June 2014; pp. 163–168. Available online: https://hst.bg/bulgarian/ECOLOGY_AND_HEALTH.pdf (accessed on 1 January 2024).
- Lammerts van Bueren, E.T. Ethics of Plant Breeding: The IFOAM Basic Principles as a Guide for the Evolution of Organic Plant Breeding. Ecol. Farming 2010, 2010, 7–10. [Google Scholar]
- Chanev, M. Problems and perspectives in organic cultivation of cereals—Overview. Ecol. Eng. Environ. Prot. 2021, 2, 66–75. Available online: https://orgprints.org/id/eprint/43330/1/Chanev_%20OF_66-75.pdf (accessed on 1 January 2024). [CrossRef]
- Barov, V. Analysis and Schemes of Field Trials; NAPS: Sofia, Bulgaria, 1982; 668p. [Google Scholar]
- Ishikawa, R.; Shirouzu, K.; Nakashita, H.; Lee, H.Y.; Motoyama, T.; Yamaguchi, I.; Teraoka, T.; Arie, T. Foliar Spray of Validamycin A or Validoxylamine A controls tomato Fusarium wilt. Am. Phytopathol. Soc. 2005, 95, 1209–1216. [Google Scholar] [CrossRef]
- Shaban, W.I.; El-Barougy, E.; Zian, A.H. Control of lupine Fusarium wilt by biofumigation with mustard and canola seed meal. Tunis. J. Plant Prot. 2011, 6, 87–98. [Google Scholar]
- McKinney, H.H. A new system of grading plant diseases. J. Agric. Res. 1923, 26, 195–218. [Google Scholar]
- AOAC. Official Methods of Analysis, 18th ed.; Association of Analytical Chemists: Gaithersburg, MD, USA, 2010; Available online: https://law.resource.org/pub/us/cfr/ibr/002/aoac.methods.1.1990.pdf (accessed on 1 January 2024).
- Nikolova, I.; Georgieva, N.; Naydenova, Y. Forage quality and energy feeding value estimation of alfalfa (Medicago sativa L.),treated by biological active compounds. J. Mt. Agric. Balk. 2016, 19, 78–95. [Google Scholar]
- INRA. Alimentation des Bovins, Ovins et Caprins; Jarrige, R., Ed.; INRA: Paris, France, 1988; p. 471. Available online: https://belinra.inrae.fr/index.php?lvl=notice_display&id=8610 (accessed on 1 January 2024).
- Finlay, K.W.; Wilkinson, G.N. Adaptation in a plant breeding programme. Aust. J. Agric. Res. 1963, 14, 742–754. Available online: https://pdf.usaid.gov/pdf_docs/PNAAS139.pdf (accessed on 1 January 2024). [CrossRef]
- Wricke, G. Übereine Methode zur Erfassung der ökologischen Streubreite in Feldversuchen. Z. Für Pflanzenzüchtung 1962, 47, 92–96. [Google Scholar]
- Kang, M.S. Genotype-by-Environment Interaction and Plant Breeding; Louisiana State University: Baton Rouge, LA, USA, 1988. [Google Scholar]
- Francis, T.R.; Kannenberg, L.W. Yield stability studies in short-season maize: I. A descriptive method for grouping genotypes. Can. J. Plant Sci. 1978, 58, 1029–1034. [Google Scholar] [CrossRef]
- Cruz, C.D. Programa Genes—Biometria; Editora UFV: Viçosa, Brazil, 2006. [Google Scholar]
- Hossain, M.A.; Sarker, U.; Azam, M.G.; Kobir, M.S.; Roychowdhury, R.; Ercisli, S.; Ali, D.; Oba, S.; Golokhvast, K.S. Integrating BLUP, AMMI, and GGE Models to Explore GE Interactions for Adaptability and Stability of Winter Lentils (Lens culinaris Medik.). Plants 2023, 12, 2079. [Google Scholar] [CrossRef]
- Kanatas, P.; Travlos, I.; Papastylianou, P.; Gazoulis, I.; Kakabouki, I.; Tsekoura, A. Yield, quality and weed control in soybean crop as affected by several cultural and weed management practices. Not. Bot. Horti Agrobot. Cluj-Napoca 2020, 48, 329–341. Available online: https://www.notulaebotanicae.ro/index.php/nbha/article/view/11823 (accessed on 1 January 2024). [CrossRef]
- Al-Tawaha, A.M.; Farrokhi, Z.; Yoga, N.; Roshan, P.; Amanullah, I.; Al-Tawaha, A.M.; Aleksanyan, A.; Khanum, S.; Thangadurai, D.; Sangeetha, J.; et al. Weed Management in Organic Cropping Systems. In Organic Farming for Sustainable Development; Sangeetha, J., Soytong, K., Thangadurai, D., Al-Tawaha, A.R.M., Eds.; Apple Academic Press: Cambridge, MA, USA, 2022; p. 436. [Google Scholar] [CrossRef]
- Uhr, Z.; Ivanov, G. Opportunities for increased yields in condition of biological farming system in wheat. New Knowl. J. Sci. 2015, 4, 35–41. Available online: https://science.uard.bg/index.php/newknowledge/article/view/92/86 (accessed on 1 January 2024).
- Paolini, R.; Faustini, F. Organic cropping systems: Strategic choices and weed control. Inf. Fitopatol. 2005, 55, 24–29. Available online: https://typeset.io/papers/organic-cropping-systems-strategic-choices-and-weed-control-2q1h759yra (accessed on 1 January 2024).
- Georgieva, N. Suitability of vetch (Vicia sativa L. and V. villosa Roth.) cultivars for organic farming conditions. Pak. J. Bot. 2018, 50, 161–167. Available online: https://www.semanticscholar.org/paper/Suitability-of-vetch-(Vicia-sativa-L.-and-V.-Roth)-Georgieva/86fb71d2b9b093b5f946ba4d111d5858648c5a2b#cited-papers (accessed on 1 January 2024).
- Georgieva, N. Suitability of pea cultivars for organic farming conditions. Biol. Agric. Hortic. 2017, 33, 225–234. [Google Scholar] [CrossRef]
- Olle, M.; Lepse, L.; Williams, I. Organic farming of pea in the northern hemisphere—A review. Acta Hortic. 2016, 1123, 137–142. [Google Scholar] [CrossRef]
- Moll, R.H.; Stuber, C.W. Quantitative genetics-empirical results relevant to plant breeding. Adv.Agron. 1974, 26, 277–313. Available online: https://www.sciencedirect.com/science/article/abs/pii/S0065211308608743?via%3Dihub (accessed on 1 January 2024).
- Kazarina, A.V.; Abramenko, I.S. Assessment of soybean raw material in regard to plant adaptability in the climatic conditions of the Middle Volga Region. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Volume 1284, II International Conference on Environmental Technologies and Engineering for Sustainable Development, Tashkent, Uzbekistan, 13–15 September 2023; Available online: https://iopscience.iop.org/article/10.1088/1755-1315/1284/1/012022 (accessed on 1 January 2024).
- Shcherbyna, O.Z.; Levchenko, T.M.; Holodna, A.V.; Baidiuk, T.O.; Kurhak, V.H.; Tymoshenko, O.O.; Romaniuk, L.S.; Lubchych, O.H.; Tkachenko, N.V.; Polishchuk, S.V.; et al. Evaluation of plasticity and yield stability in white lupin and soybean varieties. Ukr. J. Ecol. 2021, 11, 360–365. Available online: https://www.ujecology.com/articles/evaluation-of-plasticity-and-yield-stability-in-white-lupin-and-soybean-varieties.pdf (accessed on 1 January 2024).
- Kazydub, N.G.; Kuz’mina, S.P.; Plenteva, M.M.; Smirnov, I.V. Evaluation of the adaptability of dry bean varieties grown under conditions of organic farming. IOP Conf. Ser. Earth Environ. Sci. 2021, 624, 012068. Available online: https://iopscience.iop.org/article/10.1088/1755-1315/624/1/012068/meta (accessed on 1 January 2024). [CrossRef]
- Abou-Zeid, N.M.; El-Garhy, A.M.; Mokhtar, S.A. Biological and chemical control of root-rot/wilt diseases in legume crops under green house conditions in Egypt. Egypt. J. Agric. Res. 2002, 80, 1493–1501. Available online: https://journals.ekb.eg/article_313044.html (accessed on 1 January 2024).
- Jensen, B.; Jornsgaard, B.; Knudsen, J.C. Lupin grows well where pea is destroyed by soil borne diseases and vice versa. In Newsletter from Danish Research Centre for Organic Farming September; Danish Research Centre for Organic Farming: Foulum, Denmark, 2004; p. 3. [Google Scholar]
- Horoszkiewicz-Janka, J.; Jajor, E.; Korbas, M. Potential risk of infection of pathogenic fungi to legumes (Fabales) and possibilities of their control. Prog. Plant Prot. 2013, 53, 762–767. Available online: https://www.cabidigitallibrary.org/doi/full/10.5555/20133419853 (accessed on 1 January 2024).
- Zian, A.H.; El-Demardash, I.S.; El-Mouhamady, A.A.; El-Barougy, E. Studies the Resistance of Lupine for Fusarium oxysporum F. sp Lupini) through Molecular Genetic Technique. World Appl. Sci. J. 2013, 26, 1064–1069. Available online: https://www.idosi.org/wasj/wasj26(8)13/12.pd (accessed on 1 January 2024).
- Golubev, A.A.; Kurlovich, B.S. Diseases and pests. In Lupins (Geography, Classification, Genetic Resources and Breeding); Kurlovich, B.S., Ed.; OY International North Express: St. Petersburg, Russia, 2002; pp. 205–225. [Google Scholar]
- Fraser, M.D.; Fychan, R.; Jones, R. Comparative yield and chemical composition of two varieties of narrow-leafed lupin (Lupinus angustifolius) when harvested as whole-crop, moist grain and dry grain. Anim. Feed. Sci. Technol. 2005, 120, 43–50. Available online: https://www.sciencedirect.com/science/article/pii/S0377840105000039?casa_token=cPe8aUnx5NAAAAAA:lR8E-aYvlrIAHQ46y5zpJJ0yK6chhlXWRR5K87TYcOJoFMG4fktZygYE0rT_LOh0Ziz3jCS8oqcV (accessed on 1 January 2024). [CrossRef]
- Brink, G.E.; Casler, M.D.; Hall, M.B. Canopy structure and neutral detergent fiber differences amond temperate perennial grasses. Crop Sci. 2007, 47, 2182–2189. [Google Scholar] [CrossRef]
- Adugna, T.; Alem, Y.; Dawit, A. Livestock Feed Resources in Ethiopia: Challenges, Opportunities and the Need for Transformation; Ethiopia Animal Feed Industry Association: Addis Ababa, Ethiopia, 2012; Available online: https://www.academia.edu/92090508/Livestock_Feed_Resources_in_Ethiopia_Challenges_Opportunities_and_the_Need_for_Transformation (accessed on 1 January 2024).
- Petkova, R.; Stoyanova, A. Nutritional value of grains of wintering pea variety “Peace” in the light of the increase mineral doses nitrogen and growth regulators. In Proceedings of the International Science Conference, “Economics and Society Development on the Base of Knowledge”, Stara Zagora, Bulgaria, 4–5 June 2009; pp. 482–487. [Google Scholar]
- Bozhanova, V.; Koteva, V.; Savova, T.; Marcheva, M.; Panayotova, G.; Nedyalkova, S.; Rachovska, G.; Kostov, K.; Mihova’, G. Choice of appropriate cereals varieties and seed production for the needs of organic farming in Bulgaria—Problems and answers. In Proceedings of the National Conference “Biological Plant Science, Animal Science and Foods”, Troyan, Bulgaria, 27–28 November 2014; pp. 68–77. [Google Scholar]
| Genotypes | ADGR, cm/Day | PH, cm | AGB, g DM/Plant |
|---|---|---|---|
| Astra | 0.70 hi | 72.3 jk | 37.76 l |
| Nahrquell | 0.71 i | 75.9 lm | 31.00 g |
| Ascar | 0.70 i | 71.3 ij | 33.41 h |
| BGR 6305 | 0.68 fg | 69.9 h | 35.85 jk |
| Shienfield Gard | 0.82 l | 87.1 n | 34.40 hi |
| WAT | 0.60 e | 57.7 f | 27.64 ef |
| Kijewskij Mutant | 0.59 e | 57.2 f | 21.68 b |
| Hetman | 0.43 a | 41.7 a | 12.77 a |
| Start | 0.50 c | 48.2 c | 28.35 f |
| Amiga | 0.53 d | 51.4 d | 23.48 c |
| Garant | 0.53 d | 51.1 d | 26.85 e |
| Tel Keram | 0.69 gh | 70.9 hi | 41.68 m |
| Bezimenii 1 | 0.70 hi | 75.2 l | 35.90 k |
| Bezimenii 2 | 0.75 k | 76.8 m | 35.06 ij |
| Pflugs Ultra | 0.69 gh | 70.9 hi | 36.08 jk |
| Termis Mestnii | 0.73 j | 76.1 lm | 46.00 n |
| Horizont | 0.68 fg | 70.2 hi | 31.66 g |
| Solnechnii | 0.74 jk | 72.9 k | 46.50 n |
| Pink Mutant | 0.67 f | 65.9 g | 36.62 kl |
| Manovitskii | 0.54 d | 52.3 d | 23.80 c |
| Barde | 0.59 e | 55.4 e | 28.82 f |
| Dega | 0.47 b | 45.3 b | 25.31 d |
| Desnyanskii | 0.44 a | 42.5 a | 24.41 cd |
| CV(%) | 16 | 20 | 25 |
| Source of Variation | df | Sum of Squares | |||||
|---|---|---|---|---|---|---|---|
| PH | % of Total Variation | ADGR | % of Total Variation | AGB | % of Total Variation | ||
| Environment | 1 | 11,897.79 ** | 66.0 | 0.99 ** | 69.2 | 4591.97 ** | 64.7 |
| Genotype | 22 | 498.93 ** | 30.4 | 0.034 ** | 26.2 | 191.79 ** | 29.7 |
| Genotype × environment | 22 | 29.13 ** | 3.6 | 0.003 ns | 4.6 | 17.86 ** | 5.6 |
| Genotypes | Yield, t DM/ha | bi | Wi2 | KR | CVi | |
|---|---|---|---|---|---|---|
| Astra | 12.88 | k | 1.26 | 27.53 | 8 | 47.25 |
| Nahrquell | 10.65 | g | 0.90 | 3.81 | 5 | 41.13 |
| Ascar | 11.32 | h | 1.24 | 23.87 | 14 | 52.63 |
| BGR 6305 | 12.27 | ij | 1.13 | 6.41 | 2 | 44.41 |
| Shienfield Gard | 11.96 | i | 0.74 | 26.72 | 14 | 30.45 |
| WAT | 9.44 | ef | 0.90 | 3.75 | 6 | 46.16 |
| Kijewskij Mutant | 7.34 | b | 0.83 | 12.05 | 19 | 53.86 |
| Hetman | 4.42 | a | 0.31 | 187.99 | 23 | 34.74 |
| Start | 9.87 | f | 0.60 | 62.83 | 20 | 30.07 |
| Amiga | 8.08 | c | 0.66 | 46.37 | 22 | 39.67 |
| Garant | 9.21 | e | 0.86 | 8.59 | 17 | 45.95 |
| Tel Keram | 14.23 | l | 1.37 | 53.98 | 12 | 46.37 |
| Bezimenii 1 | 12.35 | ij | 1.01 | 0.06 | 1 | 39.82 |
| Bezimenii 2 | 11.98 | i | 1.14 | 8.04 | 4 | 46.02 |
| Pflugs Ultra | 12.27 | ij | 1.26 | 27.31 | 10 | 49.41 |
| Termis Mestnii | 15.64 | m | 1.63 | 157.35 | 14 | 50.00 |
| Horizont | 10.92 | gh | 0.85 | 9.55 | 12 | 37.73 |
| Solnechnii | 15.95 | m | 1.39 | 62.05 | 10 | 42.37 |
| Pink Mutant | 12.54 | jk | 1.15 | 8.61 | 2 | 44.25 |
| Manovitskii | 8.17 | cd | 0.71 | 32.56 | 21 | 42.41 |
| Barde | 9.87 | f | 0.90 | 4.18 | 6 | 44.02 |
| Dega | 8.56 | d | 0.98 | 0.20 | 8 | 54.58 |
| Desnyanskii | 8.13 | cd | 1.17 | 12.05 | 18 | 67.93 |
| Genotypes | CP | CF | Ash | GE | ME | UFL | UFV | PBD | PDIN | PDIE |
|---|---|---|---|---|---|---|---|---|---|---|
| g/kg DM | MJ/kg DM | Feed units | g/kg DM | |||||||
| Astra | 171.2 | 206.6 | 78.30 | 11.583 | 6.489 | 0.855 | 0.768 | 127 | 108 | 98 |
| Nahrquell | 143.2 | 228.2 | 76.60 | 11.446 | 6.397 | 0.849 | 0.764 | 100 | 90 | 93 |
| Ascar | 165.3 | 227.9 | 77.60 | 11.546 | 6.327 | 0.816 | 0.724 | 122 | 104 | 95 |
| BGR 6305 | 192.0 | 211.1 | 86.90 | 11.675 | 6.403 | 0.82 | 0.726 | 148 | 121 | 101 |
| Shienfield Gard | 166.2 | 233.2 | 72.80 | 11.546 | 6.419 | 0.836 | 0.747 | 122 | 104 | 96 |
| WAT | 182.3 | 226.8 | 82.20 | 11.627 | 6.352 | 0.811 | 0.717 | 138 | 115 | 98 |
| Kijewskij Mutant | 194.2 | 209.7 | 91.00 | 11.689 | 6.380 | 0.814 | 0.719 | 150 | 122 | 101 |
| Hetman | 185.3 | 193.9 | 90.00 | 11.647 | 6.357 | 0.817 | 0.723 | 141 | 116 | 100 |
| Start | 176.6 | 215.1 | 86.60 | 11.582 | 6.398 | 0.833 | 0.743 | 128 | 108 | 98 |
| Amiga | 155.9 | 224.1 | 73.40 | 11.501 | 6.247 | 0.803 | 0.710 | 112 | 98 | 92 |
| Garant | 187.7 | 202.1 | 81.70 | 11.651 | 6.508 | 0.849 | 0.760 | 143 | 118 | 102 |
| Tel Keram | 192.3 | 244.1 | 80.20 | 11.670 | 6.039 | 0.727 | 0.619 | 148 | 121 | 95 |
| Bezimenii 1 | 159.8 | 221.4 | 76.30 | 11.520 | 6.339 | 0.824 | 0.733 | 116 | 100 | 94 |
| Bezimenii 2 | 177.9 | 222.6 | 68.70 | 11.595 | 6.693 | 0.895 | 0.815 | 133 | 112 | 102 |
| Pflugs Ultra | 174.0 | 227.5 | 77.60 | 11.585 | 6.333 | 0.811 | 0.718 | 130 | 109 | 96 |
| Termis Mestnii | 174.8 | 202.8 | 71.70 | 11.584 | 6.290 | 0.802 | 0.707 | 130 | 110 | 95 |
| Horizont | 163.9 | 177.7 | 71.30 | 11.535 | 6.541 | 0.875 | 0.793 | 120 | 103 | 98 |
| Solnechnii | 161.5 | 234.2 | 73.00 | 11.525 | 6.312 | 0.813 | 0.721 | 118 | 101 | 94 |
| Pink Mutant | 156.5 | 164.1 | 71.60 | 11.502 | 6.409 | 0.843 | 0.756 | 113 | 98 | 94 |
| Manovitskii | 199.8 | 199.9 | 85.90 | 11.709 | 6.608 | 0.871 | 0.785 | 155 | 125 | 105 |
| Barde | 159.0 | 196.3 | 74.20 | 11.515 | 6.441 | 0.851 | 0.766 | 115 | 100 | 96 |
| Dega | 180.6 | 201.5 | 78.30 | 11.616 | 6.512 | 0.855 | 0.767 | 136 | 113 | 100 |
| Desnyanskii | 186.1 | 221.7 | 82.10 | 11.644 | 6.452 | 0.836 | 0.746 | 142 | 117 | 100 |
| Min | 143.2 | 164.1 | 68.70 | 11.446 | 6.039 | 0.727 | 0.619 | 100 | 90 | 92 |
| Max | 199.8 | 244.1 | 91.00 | 11.709 | 6.693 | 0.895 | 0.815 | 155 | 125 | 105 |
| Mean | 174.2 | 212.7 | 78.60 | 11.587 | 6.402 | 0.831 | 0.740 | 130 | 109 | 98 |
| CV, % | 14.70 | 18.90 | 6.30 | 0.10 | 0.10 | 0.03 | 0.04 | 14.40 | 9.30 | 3.40 |
| LSD0.05 | 26.21 | 31.04 | 9.02 | 0.21 | 0.25 | 0.06 | 0.07 | 23.66 | 16.53 | 5.76 |
| Genotypes | CP | CF | GE | PBD | ARS | R |
|---|---|---|---|---|---|---|
| Astra | 14 | 9 | 11 | 12 | 46 | 10 |
| Nahrquell | 23 | 20 | 17 | 20 | 80 | 18 |
| Ascar | 16 | 19 | 12 | 13 | 60 | 13 |
| BGR 6305 | 4 | 11 | 3 | 3 | 21 | 3 |
| Shienfield Gard | 15 | 21 | 12 | 13 | 61 | 14 |
| WAT | 8 | 17 | 7 | 7 | 39 | 6 |
| Kijewskij Mutant | 2 | 10 | 2 | 2 | 16 | 2 |
| Hetman | 7 | 3 | 5 | 6 | 21 | 3 |
| Start | 11 | 12 | 11 | 11 | 45 | 9 |
| Amiga | 22 | 16 | 16 | 19 | 73 | 17 |
| Garant | 5 | 7 | 5 | 4 | 21 | 3 |
| Tel Keram | 3 | 23 | 4 | 3 | 33 | 5 |
| Bezimenii 1 | 19 | 13 | 15 | 16 | 63 | 15 |
| Bezimenii 2 | 10 | 15 | 9 | 9 | 43 | 8 |
| Pflugs Ultra | 13 | 18 | 10 | 10 | 51 | 11 |
| Termis Mestnii | 12 | 8 | 11 | 10 | 41 | 7 |
| Horizont | 17 | 2 | 13 | 14 | 46 | 10 |
| Solnechnii | 18 | 22 | 14 | 15 | 69 | 16 |
| Pink Mutant | 21 | 1 | 16 | 18 | 56 | 12 |
| Manovitskii | 1 | 5 | 1 | 1 | 8 | 1 |
| Barde | 20 | 4 | 15 | 17 | 56 | 12 |
| Dega | 9 | 6 | 8 | 8 | 31 | 4 |
| Desnyanskii | 6 | 14 | 6 | 5 | 31 | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Georgieva, N.; Kosev, V.; Vasileva, I. Suitability of Lupinus albus L. Genotypes for Organic Farming in Central Northern Bulgaria. Agronomy 2024, 14, 506. https://doi.org/10.3390/agronomy14030506
Georgieva N, Kosev V, Vasileva I. Suitability of Lupinus albus L. Genotypes for Organic Farming in Central Northern Bulgaria. Agronomy. 2024; 14(3):506. https://doi.org/10.3390/agronomy14030506
Chicago/Turabian StyleGeorgieva, Natalia, Valentin Kosev, and Ivanina Vasileva. 2024. "Suitability of Lupinus albus L. Genotypes for Organic Farming in Central Northern Bulgaria" Agronomy 14, no. 3: 506. https://doi.org/10.3390/agronomy14030506
APA StyleGeorgieva, N., Kosev, V., & Vasileva, I. (2024). Suitability of Lupinus albus L. Genotypes for Organic Farming in Central Northern Bulgaria. Agronomy, 14(3), 506. https://doi.org/10.3390/agronomy14030506





