Warming Mitigates the Impacts of Degradation on Nitrogen Allocation between Soil Microbes and Plants in Alpine Meadow
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Experimental Design
2.3. Sample Collection and Chemical Analysis
2.4. Calculations
2.5. Statistical Analysis
3. Results
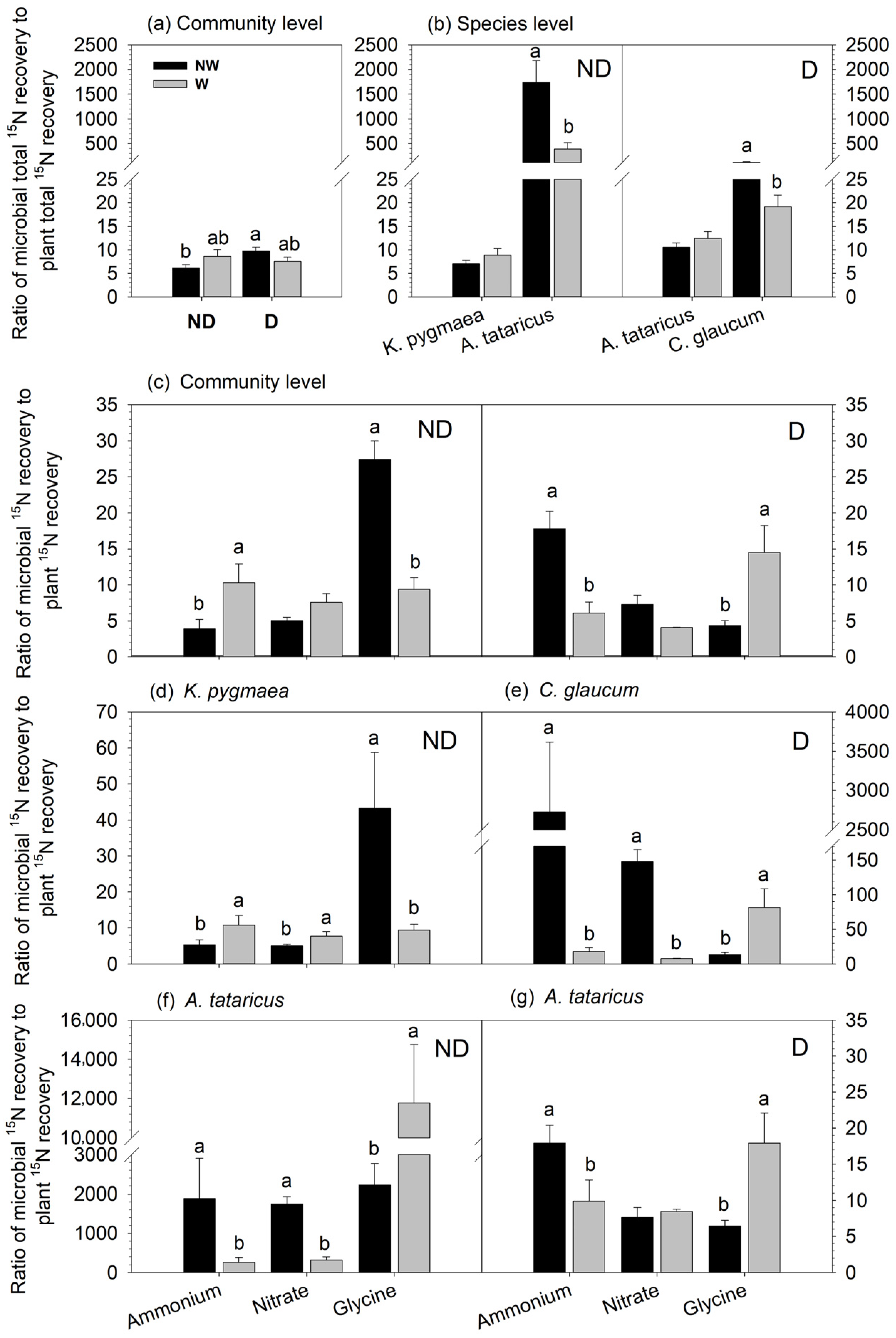
3.1. Effects of Degradation and Warming on the Recovery of 15N in Microbes
3.2. Effects of Degradation and Warming on the Recovery of 15N in Plants
3.3. Effects of Degradation and Warming on the Ratio of Microbial to Plant 15N Recovery
4. Discussion
4.1. N Allocation between Community-Level Plants and Microbes
4.2. N Distribution between Plant Species and Microbes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vitousek, P.M.; Howarth, R.W. Nitrogen limitation on land and in the sea: How can it occur? Biogeochemistry 1991, 13, 87–115. [Google Scholar] [CrossRef]
- Wang, Z. Plant Physiology; Scientific and Technical Documentation Press: Beijing, China, 2006. [Google Scholar]
- Zayed, O.; Hewedy, O.A.; Abdelmoteleb, A.; Ali, M.; Youssef, M.S.; Roumia, A.F.; Seymour, D.; Yuan, Z.-C. Nitrogen Journey in Plants: From Uptake to Metabolism, Stress Response, and Microbe Interaction. Biomolecules 2023, 13, 1443. [Google Scholar] [CrossRef] [PubMed]
- Robson, T.M.; Baptist, F.; Clément, J.-C.; Lavorel, S. Land use in subalpine grasslands affects nitrogen cycling via changes in plant community and soil microbial uptake dynamics. J. Ecol. 2010, 98, 62–73. [Google Scholar] [CrossRef]
- Holst, J.; Liu, C.; Brüggemann, N.; Butterbach-Bahl, K.; Zheng, X.; Wang, Y.; Han, S.; Yao, Z.; Yue, J.; Han, X. Microbial N Turnover and N-Oxide (N2O/NO/NO2) Fluxes in Semi-arid Grassland of Inner Mongolia. Ecosystems 2007, 10, 623–634. [Google Scholar] [CrossRef]
- Müller, C.; Rütting, T.; Kattge, J.; Laughlin, R.J.; Stevens, R.J. Estimation of parameters in complex 15N tracing models by Monte Carlo sampling. Soil Biol. Biochem. 2007, 39, 715–726. [Google Scholar] [CrossRef]
- Chen, J.; Carrillo, Y.; Pendall, E.; Dijkstra, F.A.; Dave Evans, R.; Morgan, J.A.; Williams, D.G. Soil Microbes Compete Strongly with Plants for Soil Inorganic and Amino Acid Nitrogen in a Semiarid Grassland Exposed to Elevated CO2 and Warming. Ecosystems 2015, 18, 867–880. [Google Scholar] [CrossRef]
- Afkhami, M.E.; Almeida, B.K.; Hernandez, D.J.; Kiesewetter, K.N.; Revillini, D.P. Tripartite mutualisms as models for understanding plant–microbial interactions. Curr. Opin. Plant Biol. 2020, 56, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Kuzyakov, Y.; Xu, X. Competition between roots and microorganisms for nitrogen: Mechanisms and ecological relevance. New Phytol. 2013, 198, 656–669. [Google Scholar] [CrossRef]
- Albano, L.J.; Turetsky, M.R.; Mack, M.C.; Kane, E.S. Deep roots of Carex aquatilis have greater ammonium uptake capacity than shallow roots in peatlands following permafrost thaw. Plant Soil 2021, 465, 261–272. [Google Scholar] [CrossRef]
- Alexandre, A.; Quintã, R.; Hill, P.W.; Jones, D.L.; Santos, R. Ocean warming increases the nitrogen demand and the uptake of organic nitrogen of the globally distributed seagrass Zostera marina. Funct. Ecol. 2020, 34, 1325–1335. [Google Scholar] [CrossRef]
- Kuster, T.M.; Wilkinson, A.; Hill, P.W.; Jones, D.L.; Bardgett, R.D. Warming alters competition for organic and inorganic nitrogen between co-existing grassland plant species. Plant Soil 2016, 406, 117–129. [Google Scholar] [CrossRef]
- Pang, Z.; Jiang, L.; Wang, S.; Xu, X.; Rui, Y.; Zhang, Z.; Luo, C.; Wang, Y. Differential response to warming of the uptake of nitrogen by plant species in non-degraded and degraded alpine grasslands. J. Soils Sediments 2019, 19, 2212–2221. [Google Scholar] [CrossRef]
- Reay, M.K.; Marsden, K.A.; Powell, S.; Chadwick, D.R.; Jones, D.L.; Evershed, R.P. Combining field and laboratory approaches to quantify N assimilation in a soil microbe-plant-animal grazing land system. Agric. Ecosyst. Environ. 2023, 346, 108338. [Google Scholar] [CrossRef]
- Liu, S.; Li, T.; Liu, B.; Xu, C.; Zhu, Y.; Xiao, L. Grassland vegetation decline is exacerbated by drought and can be mitigated by soil improvement in Inner Mongolia, China. Sci. Total Environ. 2024, 908, 168464. [Google Scholar] [CrossRef]
- Wang, X.; Dong, S.; Gao, Q.; Zhang, Y.; Hu, G.; Luo, W. The rate of soil nitrogen transformation decreased by the degradation of alpine grasslands in the Qinghai Tibet Plateau. Acta Pratacult. Sin. 2018, 27, 1–9. [Google Scholar]
- Chen, L.; Shi, J.; Wang, Y.; Ma, Y.; Dong, Q.; Hou, X. Study on Different Degraded Degrees Grassland Community Structure Characteristics of the Alpine Area. Acta Agrestia Sin. 2016, 24, 210–213. [Google Scholar]
- Lu, H.; Yao, T.; Li, J.; Ma, W.; Chai, X. Vegetation and soil microorganism characteristics of degraded grasslands. Acta Pratacult. Sin. 2015, 24, 34–43. [Google Scholar]
- Wang, J.; Zhang, D.; Cao, G.; Tian, Q. Regional characteristics of the alpine meadow degradation succession on the Qinghai-Tibetan Plateau. Acta Pratacult. Sin. 2013, 22, 1–10. [Google Scholar]
- Wen, L.; Dong, S.; Li, Y.; Wang, X.; Li, X.; Shi, J.; Dong, Q. The impact of land degradation on the C pools in alpine grasslands of the Qinghai-Tibet Plateau. Plant Soil 2013, 368, 329–340. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Lu, H.; Yao, T.; Wang, L.; Guo, C.; Shi, S. Characteristics of, and the correlation between, vegetation and N-fixing soil bacteria in alpine grassland showing various degrees of degradation. Acta Ecol. Sin. 2017, 37, 3647–3654. [Google Scholar]
- He, F.; Zeng, W.; Wang, Z.; Zeng, H.; Wang, W. Effect of temperate grassland deterioration on soil microbiological characteristics at different depths. Microbiol. China 2016, 43, 702–711. [Google Scholar]
- Bai, Y.; Li, X.; Wang, H.; Wen, W.; Li, R.; Li, G.; Wang, H. Nitrogen storage variations in typical steppe during grassland degradation progress—A case study of typical steppe in Xilin Hot City, lnner Mongolia. Pratacult. Sci. 2015, 32, 311–321. [Google Scholar]
- Månsson, K.F.; Olsson, M.O.; Falkengren-Grerup, U.; Bengtsson, G. Soil moisture variations affect short-term plant-microbial competition for ammonium, glycine, and glutamate. Ecol. Evol. 2014, 4, 1061–1072. [Google Scholar] [CrossRef]
- Xiang, X.; De, K.; Lin, W.; Feng, T.; Li, F.; Wei, X.; Wang, W. Different fates and retention of deposited NH4+ and NO3− in the alpine grasslands of the Qinghai-Tibet plateau. Ecol. Indic. 2024, 158, 111415. [Google Scholar] [CrossRef]
- Hill, P.W.; Farrar, J.; Roberts, P.; Farrell, M.; Grant, H.; Newsham, K.K.; Hopkins, D.W.; Bardgett, R.D.; Jones, D.L. Vascular plant success in a warming Antarctic may be due to efficient nitrogen acquisition. Nat. Clim. Chang. 2011, 1, 50–53. [Google Scholar] [CrossRef]
- Cort, C.E.; Stricker, E.; Crain-Wright, G.M.; Darrouzet-Nardi, A. Rapid foliar uptake of inorganic and amino acid nitrogen in three dryland plant species. Res. Sq. 2024; preprint. [Google Scholar] [CrossRef]
- McKane, R.B.; Johnson, L.C.; Shaver, G.R.; Nadelhoffer, K.J.; Rastetter, E.B.; Fry, B.; Giblin, A.E.; Kielland, K.; Kwiatkowski, B.L.; Laundre, J.A.; et al. Resource-based niches provide a basis for plant species diversity and dominance in arctic tundra. Nature 2002, 415, 68–71. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, T.; Guo, R.; Li, H.; Zhang, R.; Allan Degen, A.; Huang, K.; Wang, X.; Bai, Y.; Shang, Z. Fencing enclosure alters nitrogen distribution patterns and tradeoff strategies in an alpine meadow on the Qinghai-Tibetan Plateau. CATENA 2021, 197, 104948. [Google Scholar] [CrossRef]
- Yao, B.; Shi, G.; Zhou, H.; Zhao, X.; Peñuelas, J.; Sardans, J.; Wang, F.; Wang, Z. Uneven distributions of unique species promoting N niche complementarity explain the stability of degraded alpine meadow. Sci. Total Environ. 2024, 911, 168487. [Google Scholar] [CrossRef]
- Yu, C.; Liu, M.; Song, M.; Xu, X.; Zong, N.; Zhu, J.; Shi, P. Nitrogen enrichment enhances the competition for nitrogen uptake between Stipa purpurea and microorganisms in a tibetan alpine steppe. Plant Soil 2023, 488, 503–516. [Google Scholar] [CrossRef]
- Lai, C.; Peng, F.; Sun, J.; Zhou, J.; Li, C.; Xu, X.; Chen, X.; You, Q.; Sun, H.; Sun, J.; et al. Niche differentiation and higher uptake of available nitrogen maintained the productivity of alpine meadow at early degradation. Biol. Fertil. Soils 2023, 59, 35–49. [Google Scholar] [CrossRef]
- Xu, X.; Ouyang, H.; Cao, G.; Richter, A.; Wanek, W.; Kuzyakov, Y. Dominant plant species shift their nitrogen uptake patterns in response to nutrient enrichment caused by a fungal fairy in an alpine meadow. Plant Soil 2011, 341, 495–504. [Google Scholar] [CrossRef]
- Wu, B.; Ding, M.; Zhang, H.; Devlin, A.T.; Wang, P.; Chen, L.; Zhang, Y.; Xia, Y.; Wen, J.; Liu, L.; et al. Reduced soil multifunctionality and microbial network complexity in degraded and revegetated alpine meadows. J. Environ. Manag. 2023, 343, 118182. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, X.; Wang, J.; Liao, L.; Lei, S.; Liu, G.; Zhang, C. Alpine meadow degradation depresses soil nitrogen fixation by regulating plant functional groups and diazotrophic community composition. Plant Soil 2022, 473, 319–335. [Google Scholar] [CrossRef]
- Zhang, C.; He, J.; Shen, J. Global change field manipulative experiments and their applications in soil microbial ecology. Chin. J. Appl. Ecol. 2016, 27, 1663–1673. [Google Scholar]
- Plymale, A.E.; Boerner, R.E.J.; Logan, T.J. Relative nitrogen mineralization and nitrification in soils of two contrasting hardwood forests: Effects of site microclimate and initial soil chemistry. For. Ecol. Manag. 1987, 21, 21–36. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, Y.; Wang, D.; Ma, J.; Xue, K.; An, Z.; Luo, W.; Sheng, Y. Effects of Temperature and Humidity on Soil Gross Nitrogen Transformation in a Typical Shrub Ecosystem in Yanshan Mountain and Hilly Region. Life 2023, 13, 643. [Google Scholar] [CrossRef]
- Puri, G.; Ashman, M.R. Relationship between soil microbial biomass and gross N mineralisation. Soil Biol. Biochem. 1998, 30, 251–256. [Google Scholar] [CrossRef]
- Loiseau, P.; Soussana, J.F. Effects of elevated CO2, temperature and N fertilization on nitrogen fluxes in a temperate grassland ecosystem. Glob. Chang. Biol. 2000, 6, 953–965. [Google Scholar] [CrossRef]
- Mu, X.; Fan, X. A review on ecological models of soil N mineralization. Chin. J. Appl. Ecol. 1999, 10, 114–118. [Google Scholar]
- Calderón, F.J.; Jackson, L.E.; Scow, K.M.; Rolston, D.E. Microbial responses to simulated tillage in cultivated and uncultivated soils. Soil Biol. Biochem. 2000, 32, 1547–1559. [Google Scholar] [CrossRef]
- Bijoor, N.S.; Czimczik, C.I.; Pataki, D.E.; Billings, S.A. Effects of temperature and fertilization on nitrogen cycling and community composition of an urban lawn. Glob. Chang. Biol. 2008, 14, 2119–2131. [Google Scholar] [CrossRef]
- Liu, B.; Wang, C.; Huang, J.; He, N.; Wang, Q.; Dong, K. Applications of 15N Pool Dilution and 15N Tracer Techniques in the Quantifying N Transformations of Grasslands: Methodology and Advances. Acta Agrestia Sin. 2014, 22, 1153–1162. [Google Scholar]
- Jiang, L.; Wang, S.; Pang, Z.; Xu, X.; Kardol, P.; Li, Y.; Zhang, L.; Wang, Y.; Lei, Z.; Lan, Z.; et al. Plant organic N uptake maintains species dominance under long-term warming. Plant Soil 2018, 433, 243–255. [Google Scholar] [CrossRef]
- Piao, S.; Fang, J.; He, J. Variations in Vegetation Net Primary Production in the Qinghai-Xizang Plateau, China, from 1982 to 1999. Clim. Chang. 2006, 74, 253–267. [Google Scholar] [CrossRef]
- Shen, M.; Tang, Y.; Chen, J.; Zhu, X.; Zheng, Y. Influences of temperature and precipitation before the growing season on spring phenology in grasslands of the central and eastern Qinghai-Tibetan Plateau. Agric. For. Meteorol. 2011, 151, 1711–1722. [Google Scholar] [CrossRef]
- Rui, Y. Effects of Warming and Grazing on Soil Nitrogen Transformation and the Associated Functional Microorganisms in an Alpine Meadow of the Qinghai-Tibet Plateau. Ph.D. Thesis, Graduate University of Chinese Academy of Sciences, Beijing, China, 2012. [Google Scholar]
- Meng, F. Effects of Changing Temperature and Moisture on Phenological Sequences of Plant and Plant Community on the Alpine Meadow. Ph.D. Thesis, Institute of Tibetan Plateau Research Chinese Academy of Sciences, Beijing, China, 2016. [Google Scholar]
- Wu, Z. Flora of Tibet; Science Press: Beijing, China, 1987. [Google Scholar]
- Zhou, L. Process and Reasons of Rangeland Degeneration In Naqu Perfecture of Tibet Autonomous Region. Mt. Res. 1998, 16, 239–243. [Google Scholar]
- Li, Y.; Wang, S.; Jiang, L.; Zhang, L.; Cui, S.; Meng, F.; Wang, Q.; Li, X.; Zhou, Y. Changes of soil microbial community under different degraded gradients of alpine meadow. Agric. Ecosyst. Environ. 2016, 222, 213–222. [Google Scholar] [CrossRef]
- Cui, S.; Meng, F.; Suonan, J.; Wang, Q.; Li, B.; Liu, P.; Renzeng, W.; Lv, W.; Jiang, L.; Zhang, L.; et al. Responses of phenology and seed production of annual Koenigia islandica to warming in a desertified alpine meadow. Agric. Meteorol. 2017, 247, 376–384. [Google Scholar] [CrossRef]
- Pan, X.; Lin, B.; Liu, Q. Effects of elevated temperature on soil organic carbon and soil respiration under subalpine co-niferous forest in western Sichuan Province, China. Chin. J. Appl. Ecol. 2008, 19, 1637–1643. [Google Scholar]
- Li, N.; Wang, G.; Gao, Y.; Wang, J.; Liu, L. Effects of simulated warming on soil nutrients and biological characteristics of alpine meadow soil in the head waters region of the yangtze river. Acta Pedol. Sin. 2010, 47, 1214–1224. [Google Scholar]
- Lin, X.; Zhang, Z.; Wang, S.; Hu, Y.; Xu, G.; Luo, C.; Chang, X.; Duan, J.; Lin, Q.; Xu, B.; et al. Response of ecosystem respiration to warming and grazing during the growing seasons in the alpine meadow on the Tibetan plateau. Agric. For. Meteorol. 2011, 151, 792–802. [Google Scholar] [CrossRef]
- Owen, A.G.; Jones, D.L. Competition for amino acids between wheat roots and rhizosphere microorganisms and the role of amino acids in plant N acquisition. Soil Biol. Biochem. 2001, 33, 651–657. [Google Scholar] [CrossRef]
- Wilkinson, A.; Hill, P.W.; Farrar, J.F.; Jones, D.L.; Bardgett, R.D. Rapid microbial uptake and mineralization of amino acids and peptides along a grassland productivity gradient. Soil Biol. Biochem. 2014, 72, 75–83. [Google Scholar] [CrossRef]
- Bardgett, R.D.; Streeter, T.C.; Bol, R. Soil microbes compete effectively with plants for organic-nitrogen inputs to temperate grasslands. Ecology 2003, 84, 1277–1287. [Google Scholar] [CrossRef]
- Zogg, G.P.; Zak, D.R.; Pregitzer, K.S.; Burton, A.J. Microbial immobilization and the retention of anthropogenic nitrate in a northern hardwood forest. Ecology 2000, 81, 1858–1866. [Google Scholar] [CrossRef]
- Han, X. Effects of Soil Nitrogen to Plant-Microbial on Nitrogen Competition in Temperate Forest. Master’s Thesis, Beijing Forestry University, Beijing, China, 2015. [Google Scholar]
- Xu, X.; Bai, J.; Ouyang, H. Advances in Studies on Organic Nitrogen Uptake by Terrestrial Plants. J. Nat. Resour. 2011, 26, 715–724. [Google Scholar]
- Jiang, L.; Wang, S.; Pang, Z.; Wang, C.; Kardol, P.; Zhong, L.; Yu, Q.; Lan, Z.; Wang, Y.; Xu, X.; et al. Effects of grazing on the acquisition of nitrogen by plants and microorganisms in an alpine grassland on the Tibetan plateau. Plant Soil 2017, 416, 297–308. [Google Scholar] [CrossRef]
- Näsholm, T.; Huss-Danell, K.; Högberg, P. Uptake of organic nitrogen in the field by four agriculturally important plant species. Ecology 2000, 81, 1155–1161. [Google Scholar] [CrossRef]
- Zheng, H.; Liu, Y.; Chen, Y.; Zhang, J.; Li, H.; Wang, L.; Chen, Q. Short-term warming shifts microbial nutrient limitation without changing the bacterial community structure in an alpine timberline of the eastern Tibetan Plateau. Geoderma 2020, 360, 113985. [Google Scholar] [CrossRef]
- Guan, P.; Yang, J.; Yang, Y.; Wang, W.; Zhang, P.; Wu, D. Land conversion from cropland to grassland alleviates climate warming effects on nutrient limitation: Evidence from soil enzymatic activity and stoichiometry. Glob. Ecol. Conserv. 2020, 24, e01328. [Google Scholar] [CrossRef]
- Qiao, L. Effects of Long-Term Warming and Fertilization on Soil Aggregate Stability and Nutrient Accumulation Mechanism in Alpine Meadow. Master’s Thesis, Northwest A&F University, Xianyang, China, 2020. [Google Scholar]
- Wang, Q.; Chen, L.; Xu, H.; Ren, K.; Xu, Z.; Tang, Y.; Xiao, J. The effects of warming on root exudation and associated soil N transformation depend on soil nutrient availability. Rhizosphere 2021, 17, 100263. [Google Scholar] [CrossRef]
- Øien, D.-I.; Pedersen, B.; Kozub, Ł.; Goldstein, K.; Wilk, M. Long-term effects of nutrient enrichment controlling plant species and functional composition in a boreal rich fen. J. Veg. Sci. 2018, 29, 907–920. [Google Scholar] [CrossRef]



| Effect | df | 15N Recovered in Microbial Biomass | Plant 15N Recovery | Ratio of Microbial 15N Recovery to Plant 15N Recovery | |||
|---|---|---|---|---|---|---|---|
| F Value | p Value | F Value | p Value | F Value | p Value | ||
| Degradation | 1 | 56.41 | <0.001 | 85.15 | <0.001 | 2.03 | 0.17 |
| Warming | 1 | 11.73 | 0.002 | 26.89 | <0.001 | 4.38 | 0.05 |
| N type | 2 | 2.92 | 0.07 | 35.64 | <0.001 | 17.19 | <0.001 |
| Degradation × Warming | 1 | 3.93 | 0.06 | 28.62 | <0.001 | 0.45 | 0.51 |
| Degradation × N type | 2 | 1.35 | 0.28 | 13.68 | <0.001 | 13.26 | <0.001 |
| Warming × N type | 2 | 3.97 | 0.03 | 17.75 | <0.001 | 0.92 | 0.41 |
| Degradation × Warming × N type | 2 | 13.94 | <0.001 | 7.90 | 0.002 | 39.24 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pang, Z.; Wen, G.; Jiang, L.; Nie, X.; Wang, Z.; Pang, R.; Liu, W.; Chen, M.; Zhao, W.; Tang, L.; et al. Warming Mitigates the Impacts of Degradation on Nitrogen Allocation between Soil Microbes and Plants in Alpine Meadow. Agronomy 2024, 14, 508. https://doi.org/10.3390/agronomy14030508
Pang Z, Wen G, Jiang L, Nie X, Wang Z, Pang R, Liu W, Chen M, Zhao W, Tang L, et al. Warming Mitigates the Impacts of Degradation on Nitrogen Allocation between Soil Microbes and Plants in Alpine Meadow. Agronomy. 2024; 14(3):508. https://doi.org/10.3390/agronomy14030508
Chicago/Turabian StylePang, Zhe, Guoqi Wen, Lili Jiang, Xiaowei Nie, Zongsong Wang, Rui Pang, Wenjing Liu, Meirong Chen, Weiwai Zhao, Li Tang, and et al. 2024. "Warming Mitigates the Impacts of Degradation on Nitrogen Allocation between Soil Microbes and Plants in Alpine Meadow" Agronomy 14, no. 3: 508. https://doi.org/10.3390/agronomy14030508
APA StylePang, Z., Wen, G., Jiang, L., Nie, X., Wang, Z., Pang, R., Liu, W., Chen, M., Zhao, W., Tang, L., Zhang, B., Li, L., Zhou, S., Xu, X., Hao, Y., Cui, X., Wang, S., & Wang, Y. (2024). Warming Mitigates the Impacts of Degradation on Nitrogen Allocation between Soil Microbes and Plants in Alpine Meadow. Agronomy, 14(3), 508. https://doi.org/10.3390/agronomy14030508









