Abstract
Rhizobial associations with leguminous plants are some of the most important symbioses on Earth, and they have economic relevance in agriculture. Because their interactions are positive and have advantages for both partners, nitrogen-fixing rhizobia also demand significant carbohydrate allocation in exchange for key nutrients, and this demand is reflected in the anatomy of roots. In the current scenario of climate change, rhizobia–legume interactions can be affected, and plants may need to compensate for carbon loss when light availability is not correct. Under such conditions, roots can modify their anatomy to accommodate symbionts’ needs, and the outcome of an interaction can switch from mutualism to parasitism, resulting in changes in root allocation. We experimented with two legume species originating from well-irradiated environments (Coronilla juncea L. and Ornithopus compressus L.) and two species from shaded environments (Trifolium repens L. and Vicia sativa L.). We applied high radiation, intermediate radiation, and low radiation to two treatments of microbial inoculation (inoculation and control). After an incubation period of 105 days, we quantified the root area, size, and complexity, as well as the nodule production and mass, plant relative growth, and below-ground allocation. For plants originating in shaded environments, nodulation, root complexity, and below-ground allocation were enhanced in inoculated plants when they were transferred to conditions of high irradiance. Strikingly, plants from environments exposed to high light radiation were less plastic when exposed to changing light availability, and the symbionts were less beneficial than expected in stress-free environments. Our study proved that the stress imposed on plants due to high irradiance is overcome when plants are inoculated, and the positive effect is more evident in plants that are usually grown in shaded environments (e.g., Trifolium repens and Vicia sativa).
1. Introduction
Mutualistic interactions between plants and soil-borne microorganisms are an important component of agricultural productivity [1]. One of the most important groups of plant-associated microbial mutualists is that of rhizobia. It is estimated that symbiotic interactions can produce 90 Tg of nitrogen per year [2]. The symbiosis between leguminous plants and rhizobia is widespread, and the mechanism of action is well known. Root colonization with rhizobia results in the formation of nodules [3] in which bacteria fix atmospheric nitrogen (N2). Nodulation and nitrogen fixation are dependent on the adequate supply of P to a plant [4,5,6]. Nitrogen-fixing rhizobia and their legume counterparts have been demonstrated to critically determine the productivity and species composition of ecosystems [7]; because of their key function in global nitrogen cycles, legumes are considered ecosystem engineers [8,9] and keystone species in both natural ecosystems and agriculture [10]. The benefits of symbiotic interactions include increased plant growth in terms of increases in height [11,12], total biomass production [13,14] shoot–root ratio [14,15,16], and the production of flowers. Increases in seed yield and total crop biomass have been observed in Cicer arietinum, Phaseolus vulgaris, and Glycine max, among others [1,11,15,17]. Similarly, the presence of legumes and their symbionts critically enhances the sustainability of agroforestry systems [18] and pastures [19]. In legumes, the effects of rhizobial nitrogen fixation on plant growth and crop yield [7], as well as plant chemical defensive traits, have been well-studied [17,20,21].
Although legume–rhizobia associations can be clearly beneficial for plants in most cases, they can incur costs for plants, as microbial symbionts may consume up to 16% of the photosynthetically fixed carbon to maintain their growth and reproductive functions [22]. However, recent research has demonstrated that plants are able to compensate for this cost through the sink stimulation of photosynthesis, which is thought to be an adaptation that takes advantage of the nutrient supply that is provided without compromising the total amount of photosynthates available for plant growth and development [23]. The tradeoff between benefits and costs can be modified due to environmental changes. In addition, cultural practices have been shown to cause strong variations in plant performance [24], resulting in variations in root branching with critical effects on crop productivity. Variability in root architecture can be of use in agriculture for the selection of genotypes that require less nutrients by using root architecture to increase production and focusing on the lateral development of roots [25]. In legumes, when N is limited, the first known adaptation is the formation of nodules to increase BNF at the expense of total root biomass production. Apart from inorganic N, free amino acids, such as glutamine, can modify root architecture. High glutamine contents inhibit root growth by acting as an internal N-status signal for the mediation of root development [26]. Soil salinity stress modifies root anatomy and function [27]. Salt stress is responsible for the formation and development of root hairs [28]. In phosphorus-deficiency-tolerant Oryza sativa, protein kinase acts as an enhancer of early root growth, enabling the plant to maximize its uptake of P and other nutrients [25]. In the current situation of global climate change, in which less rainfall is expected, coupled with increased radiation and temperatures [29], plants need to adapt to the new system, and farmers have to replace old cultivars with better-adapted ones. In this sense, knowledge of how crop plants respond to increased radiation would help to identify better cropping practices.
Of particular importance in plant development is light availability. In both natural and agricultural ecosystems, light availability is not constant for plants due to competition among plant species. However, the effect of light on root architecture has only been marginally considered, despite the fact that this will be one of the greatest threats that plants will have to face due to the already obvious effects of climate change [21]. We propose that legume–Rhizobium interactions could serve as an extra aid for plants to thrive when the irradiance goes beyond the ideal for plant performance.
In the present study, we carried out an experiment to study the relationships between the amount of irradiance and the root architecture of four legumes that would be ideal candidates as new crops for human food and animal fodder. Two of the chosen species, Coronilla juncea L. and Ornithopus compressus L., have their natural range of distribution in well-irradiated areas, whereas the other two, Trifolium repens L. and Vicia sativa L., are usually found in shaded areas. The four species are native to the Mediterranean region. V. sativa prefers temperate climates and has a vegetative period of 74–120 days. The intensity of radiation is also an important variable in plant performance, as densely planted crops have reduced productivity. These plants are moisture-loving, producing strong crops in areas where precipitation for May–June is no less than 175–200 mm. The soil pH should be neutral or close to neutral, as acidic soils suppress nodule formation. T. repens is best adapted to humid temperate climates and has a vegetative period of 90–100 days [30]. It flowers from May to July or throughout the summer in cool, moist areas [31], but it becomes semidormant in hot, dry, and highly illuminated areas. It grows in the partial shade of aspen and oak woodlands [32,33]. It can grow in soils with a pH from 5.5 to 8.0. C. juncea grows well on stony and sandy soils with a large range of pH values. It is common in dry and sunny scrublands from sea level to about 800 m above sea level. Plants of this species may have difficulty thriving and drop leaves without ample sunlight. They do not tolerate shadow and can be resistant to drought and calcareous soils [34]. O. compressus has a Mediterranean–Atlantic distribution. It is usually found in open pasturelands with high light intensity. In fact, this species does not tolerate shadow. It is resistant to very pronounced thermal variations, with a preference for high temperatures and low rainfall. The species is considered a drought and acidity indicator. It has a vegetative period of around 110 days [35].
These four species are commonly used in pasturelands and are well-adapted to grazing. They contribute protein to animal fodder. In the scenario of climate change, the lack of rainfall and the increase in irradiation reduce the amount of fodder available for animals, thus jeopardizing meat production.
We firstly hypothesized that morphological and symbiotic root traits associated with the four legumes would change in response to light irradiance, with plants originating from shaded environments, showing better performance than their counterparts. Secondly, we hypothesized that these responses would vary between inoculated and non-inoculated plants, with rhizobia enhancing plant performance when plants were outside of their natural level of irradiance. The results of our study allowed us to choose appropriate plant species with appropriate inoculants to establish new crops under varying levels of light intensity due to global changes. Thirdly, we hypothesized that the stress imposed on plants by high irradiance is easily overcome when plants are inoculated, and the positive effect is more evident in plants that usually grow in shaded environments (i.e., Trifolium repens L. and Vicia sativa L.). Conversely, plants growing in highly irradiated areas benefit less from inoculation when exposed to light conditions that are different from those under which they grow in nature (i.e., Coronilla juncea L. and Ornithopus compressus L.).
2. Materials and Methods
A total of 24 tanks (45 cm in length × 32 cm in width × 40 cm in depth) were set up. Eight tanks were placed in a growing cabinet under a 12 h light–dark regime at 900 µmol·m−2s−1 (intermediate irradiance intensity). Another eight tanks were located in a second cabinet with the same light–darkness regime and with a light intensity of 1800 µmol·m−2s−1 (high irradiance intensity). The remaining eight tanks were placed on a lab bench in front of a window to have natural irradiance, which was measured with a lux meter (Uceri MT 912 Light Meter); irradiance was 450 µmol·m−2s−1. Light was supplied by lamps covering the whole range of photosynthetically active range (Samsung LED LM301, MN-W450, and MN-W1800, respectively). The lab and the two cabinets had similar temperatures of 24 ± 1 °C during the light period and 17 ± 1 °C during the night, mimicking the average spring temperature in the Iberian Peninsula [36] (Figure 1). Every two tanks in each treatment harbored one of the four plant species under study: Vicia sativa L., Trifolium repens L., Coronilla juncea L., and Ornithopus compressus L.
Figure 1.
Setup of C. juncea, O. compressus, T. repens, and V. sativa in tanks for growth at three different radiation levels—high radiation (1800 µmol·m−2s−1), intermediate radiation (900 µmol·m−2s−1), and low natural radiation (450 µmol·m−2s−1)—with inoculation with rhizobia.
Seeds were sterilized with NaClO at 50% for 5 min and germinated on plain agar plates at 22 °C in full darkness. Thirty-three-day-old seedlings were placed in each tank and suspended in a distilled water and culture solution with an autoclaved square sponge rubber that acted as a seedling holder. Each individual sponge rubber that held a seedling was introduced into autoclaved plastic cylinders (4 cm in diameter × 30 cm) that had open bottoms to let the solution freely circulate and to guarantee that the roots received enough oxygen. Every tank was filled with a Hoagland nutrient solution at 5% in distilled water (Table 1). The use of N in the Hoagland solution was necessary because it is well-documented that the formation of effective symbiosis is hindered when nitrogen is absent or very high in soils or in growing media. On the other hand, low nitrogen levels in soils trigger nodule formation and N2-fixing symbioses [37,38,39]. This fact was taken into account in the current study, so legumes were supplied with a low concentration of N to guarantee plant growth and the formation of effective nodules throughout the experiment.

Table 1.
Concentrations of stock Hoagland nutrient solution applied to seedlings of C. juncea, O. compressus, T. repens, and V. sativa, which were grown in hydroponics at three different radiation levels—high radiation (1800 µmol·m−2s−1), intermediate radiation (900 µmol·m−2s−1), and low radiation (450 µmol·m−2s−1)—with inoculation with rhizobia.
Groups of 60 seedlings each were treated with one of the three different radiation levels, which were those of high radiation (1800 µmol·m−2s−1), intermediate radiation (900 µmol·m−2s−1), and low natural radiation (450 µmol·m−2s−1); the different radiation levels were imposed by using different lightbulbs that were placed above the plants (Figure 1). Half of each tank (i.e., 30 seedlings) was inoculated with the appropriate rhizobial strain, and the other half remained non-inoculated as a control. The inoculants were Rhizobium leguminosarum USDA2370T for V. sativa and C. juncea, Rhizobium leguminosarum and Rhizobium leguminosarum subsp. trifolii ATCC1440 for T. repens, and Rhizobium fredii for O. compressus. The bacteria were chosen according to their affinity to plant species found in the literature and according to their availability in our bacterial collection. The bacterial inoculants consisted of a heavy suspension of YMA broth with an average cell count of 9 × 109 mL, with an amount of 330 mL per plant. The tanks used for the three treatments were randomly placed on benches and relocated every week to guarantee the similarity of conditions throughout the experiment.
After 75 days of growth, 15 seedlings per species and treatment were harvested so as to be able to calculate the relative growth rate.
The final harvest occurred after a total of 105 days of plant growth in the tanks. Below-ground allocation was calculated for plants in all treatments. Below-ground allocation is the fraction of new biomass formed in terms of roots and nodules over the growth period. This was calculated according to [40]:
where RGR is the relative growth rate (mg·g−1·day−1) and ð is the fraction of new biomass gained during the growth period.
df/dt = RGR(ð − Br/Bt)
Br/Bt is the root weight ratio, which is based on the total plant biomass (Bt) and root biomass (Br).
All plants were measured for root dry mass production, size, and complexity. The size and complexity were estimated after scanning the roots immediately after harvest. The images that were obtained were analyzed using the J-Image program [41], which is an image-processing program for multidimensional image data; scanning the roots allowed for the images to be transformed into pixels and the calculation of the root complexity. The pixels were transformed into linear units so they could be reported as cm or mm. The fractal index calculated with the J-Image program was used as a measurement of the root complexity. The total number of nodules per plant and the dry weight were also taken.
Statistical Analysis
Statistical analyses were performed using the free SPSS software (Version 26) [42]. The analysis used was the Kruskal–Wallis test, as the data did not meet normality according to the Shapiro–Wilk test or equal variance after Levene’s test. Comparisons between pairs were performed using the Kruskal–Wallis ANOVA (one factor). Significant differences were accepted at the level of p ≤ 0.05.
3. Results
Visual analyses of the roots revealed that there was no nodulation on the non-inoculated plants, thus proving the maintenance of the sterile conditions throughout the experiment. Inoculated roots were successfully nodulated, and there were significant differences related to the light treatments under which the plants grew. The nodulation in the high-radiation (HR) treatment was significantly higher than in any of the other treatments, regardless of the species considered, except for T. repens, which did not show differences in nodulation between high and intermediate levels of radiation. Variations in the nodule number and nodule mass were observed among the legumes originating from light (C. juncea and O. compressus) and shaded environments (T. repens and V. sativa) (Figure 2a) (Table S1). A similar pattern was observed in the nodule biomass (weight), which was significantly lower in the low-radiation (LR) treatment for the four species, with the minimum nodule biomass being found in the two legumes from well-irradiated areas (Figure 2b) (Table S1).
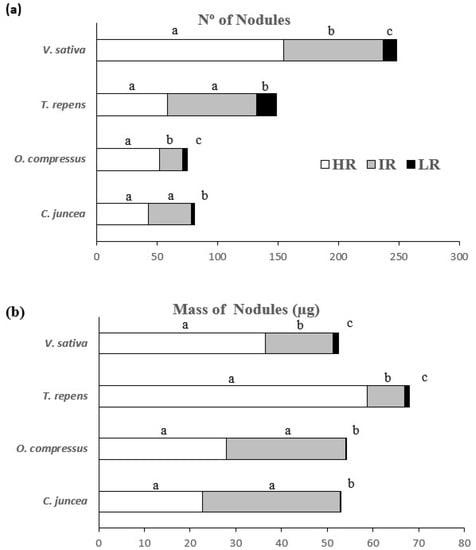
Figure 2.
Number of nodules (a) and nodule biomass (b) in the roots of C. juncea, O. compressus, T. repens, and V. sativa when grown under three regimes of light (HR: high radiation; IR: intermediate radiation; LR: low radiation). The results are only for inoculated plants. The values are the means ± SE of 15 individuals per treatment. Different letters indicate significant differences among treatments (p ≤ 0.05).
The root area of the species originating from areas with high radiation was generally lower than that found in species coming from shaded areas. The former significantly increased their root masses in the intermediate radiation (IR) treatment when inoculated, whereas the latter produced significantly more biomass when grown with HR and IR after inoculation (Figure 3). This very same pattern was followed by the shoot biomass (Table 2). In general, inoculation better enhanced the root area at intermediate levels of radiation for all species, as well as at high radiation for species originating in shaded environments (Table S1). Once again, this was the pattern followed by shoot biomass production (Table 2). In C. juncea, the pattern of root size was similar to that of root area (Figure 4). For the other three species, the root size was significantly enhanced with the inoculation treatment, particularly in the intermediate- and low-irradiance treatments.
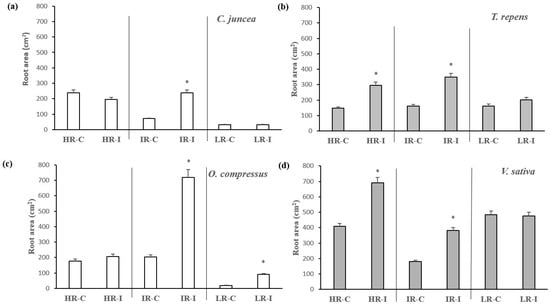
Figure 3.
Average root area per plant of C. juncea (a), O. compressus (c), T. repens (b), and V. sativa (d) when grown under high (HR), intermediate (IR), and low irradiance (LR) levels with (I) and without (C) rhizobial inoculation. The bars represent means ± sd (n = 15). The asterisks on top of the columns indicate significant differences in root area among treatments according to the Kruskal–Wallis test (p < 0.05).

Table 2.
Biomass production of C. juncea, O. compressus, T. repens, and V. sativa when grown at three different radiation levels (high radiation: HR; intermediate radiation: IR; low radiation: LR) and with inoculation with rhizobia.
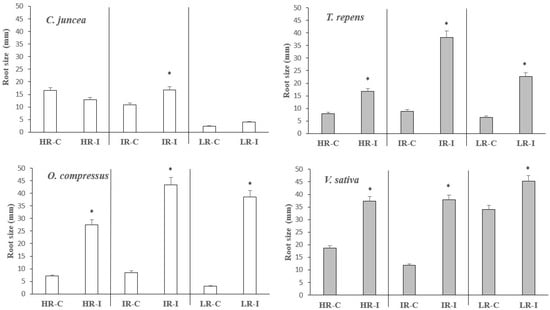
Figure 4.
Root size per plant of C. juncea, O. compressus, T. repens, and V. sativa when grown at high (HR), intermediate (IR), and low irradiance (LR) levels with (I) and without (C) rhizobial inoculation. The bars represent means ± sd (n = 15). The asterisks on top of the columns indicate significant differences in root area among treatments according to the Kruskal–Wallis test (p < 0.05).
The root complexity, which was measured with the fractal index in images of the scanned roots, varied among the types of plants and the light and microbial treatments. Although the root complexity in the plants from shaded environments remained constant regardless of the treatment, the plants originating from well-illuminated environments responded differently to both the light regime and inoculation (Figure 5). C. juncea and O. compressus had more complex root systems in the HI treatment with and without inoculation than T. repens and V. sativa did. Intermediate and low levels of light reduced the root complexity, which reached its minimum when the plants underwent the IR-inoculated treatment (Figure 5). The root complexity in the plants from shaded environments did not seem to be affected by the light intensity (Table S1).
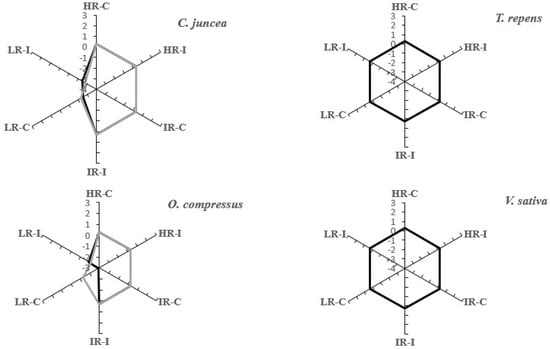
Figure 5.
Root complexity measured as the fractal index for four leguminous species grown under varying light conditions and rhizobial inoculation. Gray line: root complexity after 75 days of plant growth. Solid line: root complexity after 105 days of plant growth.
The relative growth rates (RGRs) for roots (Figure 6a) (Table S2) were much higher in the plants grown with the inoculation treatment for all species. The exception was C. juncea, for which we did not identify a benefit from either the light or the inoculation treatment (Figure 6a). In general, the relative root growth in plants from shaded environments was greater than that in plants from well-irradiated ones. The plants of O. compressus and T. repens reached a significantly greater RGR with the intermediate radiation and inoculation, whereas V. sativa grew significantly more quickly with high radiation and inoculation. This pattern was exactly the same as that for below-ground allocation. In addition, the below-ground allocation was significantly lower in plants coming from well-irradiated environments, with the exception of the inoculated plants of O. compressus with IR. The plants from shaded environments allocated significantly more of their resources to roots with HR or IR and inoculation than those undergoing the other treatments (Figure 6b) (Table S2).
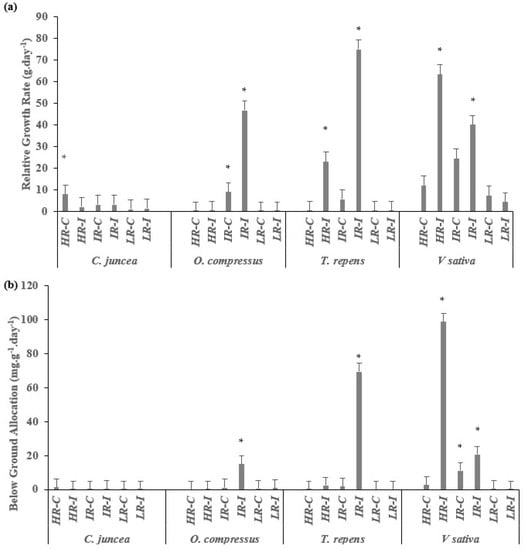
Figure 6.
(a) Relative root growth rate and (b) allocation of biomass to roots in plants of C. juncea, O. compressus, T. repens, and V. sativa when grown under three regimes of light with and without rhizobial inoculation. The values of 15 replicates are presented as means ± SE. The asterisks on top of the columns indicate significant differences among treatments according to the Kruskal–Wallis test (p ≤ 0.05).
4. Discussion
We analyzed the simultaneous effects of light availability and rhizobial inoculation on root anatomical indicators in 75-and 105-day-old seedlings of four legumes, with two originating from well-irradiated areas and two originating from shaded areas. The experimental design included inoculation and no inoculation of seedlings that were treated with three levels of radiation.
We found that the root architecture, growth rate, and biomass allocation were clearly enhanced by the inoculation. We also observed different growth patterns for the well-irradiated and shaded plants. These patterns contribute to the plants’ adaptation to changing environments, which were simulated with increased levels of sunlight. Our results showed that rhizobia had the effect of enhancing both shoot and root growth and below-ground allocation under conditions with high and intermediate irradiance in plants coming from shaded environments. There was a reduction in root performance in the plants originating from well-irradiated environments, and inoculation resulted in higher biomass production in plants irradiated with intermediate levels of light.
Nodulation was reduced by low radiation, regardless of the origin of the plants (well-irradiated or shaded environments). However, globally, nodule production was greater in the plants originating from shaded environments, although the nodules from plants originating from well-irradiated environments were heavier when the plants were exposed to high and intermediate levels of radiation, indicating that there was an interactive effect of rhizobia, light, and plant growth. This uncommon interactive effect was previously observed in plants of Phaseolus lunatus L. [21], and it implies that light availability mediates plant growth in the presence of different root symbionts [43,44,45]. Heavier nodules were produced at the expense of the devotion of more resources to the below-ground part of the plant, as was the case in V. sativa and T. repens, where heavier roots and nodules coincided with greater below-ground allocation. It is possible that these two species, which originated from shaded environments, needed to compensate plant growth due to reduced photosynthesis. Compensation is accomplished by devoting more carbon resources to root-associated symbionts [33]. Compensation would provide a plant with extra nitrogen from the soil, thus contributing to the global plant performance in the so-called sink stimulation (up-regulation) of photosynthesis [22,46].
In plants from well-irradiated environments, inoculation triggered plant root growth. This growth was modulated by the amount of light. While under intermediate radiation (IR), root growth and biomass were enhanced; under extreme light conditions (i.e., too low or too high), the symbiont acted as a parasite by reducing root allocation and promoting greater root mass. When plants from shaded environments were exposed to greater levels of light, the presence of the symbionts seemed to prepare them to be ready to cope with water and nutrient scarcity by devoting more resources to below-ground allocation and the production of more and heavier nodules. The mutualism/parasitism continuum in legumes can explain how they have evolved with regard to the environment over time [47]. It is known that rhizobial infection and nodule occupancy are due to plant sanctions on symbionts [48]. Although plants have control of the inoculation and nodulation processes via selective partitioning within root systems [47], changes in the abiotic environment can disrupt the symbiotic process, as has been discussed for pH [39] and light [21]. Again, this study points toward the fact that, under modified environmental conditions (e.g., changing levels of radiation), mutualistic cooperation is selected by both plants and bacteria. Hence, we propose a modification of mutualistic behavior under unfavorable abiotic conditions that induce a symbiotic switch in the outcome of the interactions. Our study shows for the first time that, for plants coming from predominantly shaded environments, light availability is a key factor in determining (i) biomass allocation, (ii) root size and complexity, and (iii) the threshold between mutualism and parasitism in plant–rhizobia interactions.
Nutrient acquisition under intermediate levels of light and temperature may limit a plant’s sanctions on rhizobia, thus preventing infection. In fact, it is well-documented that the benefits of root symbionts are limited when sufficient nutrients are available [39,49,50]. The reduced nodulation that we observed in plants grown under intermediate levels of light can be explained by the plants’ rejection of symbionts when they were not required [47].
What we also proved is that plant species of different origins (well-irradiated or shaded environments) respond differently to changes in light availability in terms of root development. Whereas plants originating in shaded environments seem to be more plastic in the way in which they produce roots and nodules when transferred to high irradiance, plants from well-irradiated areas are less prone to investing in roots and symbionts when exposed to either very high or very low radiation. We believe that plants from shaded environments, in which they have to search for light, are ready to lavish their photosynthate when light is not limited; instead of devoting most of their resources to their shoots, they are homogeneously distributed throughout the entire plant [51]. Differences in light availability are expected due to climate change, and they will have an effect on the growth of crops; humans are already in the position of searching for alternative crops that are adapted to changing environments. In terms of how legumes will maintain active biological nitrogen fixation for agriculture and the production of enough fodder, we understand that shaded plants would be more plastic and adaptable to changing environments than those that are already stressed by high temperatures. When searching for the best positive and effective nodulation, variations in light availability can be interpreted as an important mediator of such mutualistic relationships.
We are well aware of the fact that we only tested four leguminous species and their specific symbionts at three levels of light, and more questions need to be addressed before we can provide a general conclusion for legumes. However, we have learned that variations between mutualism and parasitism in symbiotic interactions have significant implications regarding the stability of mutualism and the productivity of agro-ecological systems. This is of particular interest for legumes, as they represent around 30% of agricultural production worldwide [52], and alternative crops of this species are needed due to the variations in natural conditions as a result of climate change.
In conclusion, we proved our first hypothesis by showing that the morphology and nodule production in the four studied species are strongly dependent on the level of irradiation. The root biomass allocation, size, and complexity in plants originating from predominantly shaded environments are determined by the amount of light. Our second conclusion is that the below-ground allocation and relative growth rate are greater in inoculated plants originating from shaded environments. In these plants, light determines whether the rhizobia act as mutualistic organisms or parasites. Finally, we conclude that, as proposed, the stress imposed on plants by high irradiance is overcome when the plants are inoculated, and the positive effect is more evident in plants that usually grow in shaded environments (i.e., Trifolium repens L. and Vicia sativa L.).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy13082058/s1, Table S1: Comparison by pairs with the Kruskal–Wallis test for the number of nodules, nodule biomass, average root area, and fractal index; Table S2: Comparison by pairs with the Kruskal–Wallis test for relative growth rate (RGR) and below-ground allocation (BGR).
Author Contributions
Both authors equally contributed to both the research and the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data will be made available on request.
Acknowledgments
We thank Jesús del Gran Poder Rodríguez-Sánchez for his valuable assistance during lab work and continuous support in the design of the experimental setting.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ramana, V.; Ramakrishna, M.; Purushotham, K.; Reddy, K.B. Effect of bio-fertilizers on growth, yield attributes and yield of french bean (Phaseolus vulgaris L.). Legume Res. 2010, 33, 178–183. [Google Scholar]
- Sadowsky, M.J.; Cregan, P.B.; Keyser, H.H. Nodulation and Nitrogen Fixation Efficacy of Rhizobium fredii with Phaseolus vulgaris Genotypes. Appl. Environ. Microbiol. 1988, 54, 1907–1910. [Google Scholar] [CrossRef] [PubMed]
- Franche, C.; Lindström, K.; Elmerich, C. Nitrogen-Fixing Bacteria Associated with Leguminous and Non-Leguminous Plants. Plant Soil 2009, 321, 35–59. [Google Scholar] [CrossRef]
- Magadlela, A.; Vardien, W.; Kleinert, A.; Dreyer, L.L.; Valentine, A.J. The Role of Phosphorus Deficiency in Nodule Microbial Composition, and Carbon and Nitrogen Nutrition of a Native Legume Tree in the Cape Fynbos Ecosystem. Aust. J. Bot. 2015, 63, 379. [Google Scholar] [CrossRef]
- Pérez-Fernández, M.; Míguez-Montero, Á.; Valentine, A. Phosphorus and Nitrogen Modulate Plant Performance in Shrubby Legumes from the Iberian Peninsula. Plants 2019, 8, 334. [Google Scholar] [CrossRef]
- Míguez-Montero, M.A.; Valentine, A.; Pérez-Fernández, M.A. Regulatory Effect of Phosphorus and Nitrogen on Nodulation and Plant Performance of Leguminous Shrubs. AoB Plants 2019, 12, plz047. [Google Scholar] [CrossRef]
- Sprent, J.I.; Sprent, P. Nitrogen Fixing Organisms: Pure and Applied Aspects, 1st ed.; Chapman and Hall: London, UK, 1990. [Google Scholar]
- Carney, K.M.; Matson, P.A. Plant Communities, Soil Microorganisms, and Soil Carbon Cycling: Does Altering the World Belowground Matter to Ecosystem Functioning? Ecosystems 2005, 8, 928–940. [Google Scholar] [CrossRef]
- Van Der Heijden, M.G.A.; Bardgett, R.D.; Van Straalen, N.M. The Unseen Majority: Soil Microbes as Drivers of Plant Diversity and Productivity in Terrestrial Ecosystems. Ecol. Lett. 2008, 11, 296–310. [Google Scholar] [CrossRef]
- Lodge, D.J.; Hawksworth, D.L.; Ritchie, B.J. Microbial diversity and tropical forest functioning. In Biodiversity and Ecosystem Processes in Tropical Forests; Orians, G.H., Dirzo, R., Cushman, J.H., Eds.; Springer: Berlin/Heidelberg, Germany, 1996; Volume 122, pp. 69–100. [Google Scholar]
- Safapour, M.; Ardakani, M.; Khaghani, S.; Rejali, F.; Zargari, K.; Changizi, M.; Teimuri, M. Response of Yield and Yield Components of Three Red Bean (Phaseolus vulgaris L.) Genotypes to Co-Inoculation with Glomus intraradices and Rhizobium phaseoli. Am.-Eurasian J. Agric. Environ. Sci. 2011, 11, 398–405. [Google Scholar]
- Hayman, D.S. Mycorrhizae of Nitrogen-Fixing Legumes. Mircen J. 1986, 2, 121–145. [Google Scholar] [CrossRef]
- Mathur, N.; Vyas, A. Influence of Arbuscular Mycorrhizae on Biomass Production, Nutrient Uptake and Physiological Changes in Ziziphus Mauritiana Lam. under Water Stress. J. Arid Environ. 2000, 45, 191–195. [Google Scholar] [CrossRef]
- Vejsadova, H.; Siblikova, D.; Gryndler, M.; Simon, T.; Miksik, I. Influence of inoculation with Bradyrhizobium japonicum and Glomus claroideum on seed yield of soybean under greenhouse and field conditions. J. Plant Nutr. 1993, 16, 619–629. [Google Scholar] [CrossRef]
- Veresoglou, S.D.; Menexes, G.; Rillig, M.C. Do Arbuscular Mycorrhizal Fungi Affect the Allometric Partition of Host Plant Biomass to Shoots and Roots? A Meta-Analysis of Studies from 1990 to 2010. Mycorrhiza 2012, 22, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Gavito, M.E.; Curtis, P.S.; Mikkelsen, T.N.; Jakobsen, I. Atmospheric CO2 and Mycorrhiza Effects on Biomass Allocation and Nutrient Uptake of Nodulated Pea (Pisum sativum L.). Plants 2000, 51, 1931–1938. [Google Scholar] [CrossRef]
- Pérez-Fernández, M.; Calvo-Magro, E.; Ramírez-Rojas, I.; Moreno-Gallardo, L.; Alexander, V. Patterns of Growth Costs and Nitrogen Acquisition in Cytisus striatus (Hill) Rothm. and Cytisus balansae (Boiss.) Ball Are Mediated by Sources of Inorganic N. Plants 2016, 5, 20. [Google Scholar] [CrossRef]
- Muleta, D. Legume Responses to Arbuscular Mycorrhizal Fungi Inoculation in Sustainable Agriculture. Microbes for Legume Improvement; Springer: Vienna, Austria, 2010; pp. 293–323. [Google Scholar] [CrossRef]
- Haystead, A.; Malajczuk, N.; Grove, T.S. Underground Transfer of Nitrogen between Pasture Plants Infected with Vesicular-Arbuscular Mycorrhizal Fungi. New Phytol. 1988, 108, 417–423. [Google Scholar] [CrossRef]
- Thamer, S.; Schädler, M.; Bonte, D.; Ballhorn, D.J. Dual Benefit from a Belowground Symbiosis: Nitrogen Fixing Rhizobia Promote Growth and Defense against a Specialist Herbivore in a Cyanogenic Plant. Plant Soil 2011, 341, 209–219. [Google Scholar] [CrossRef]
- Ballhorn, D.J.; Schädler, M.; Elias, J.D.; Millar, J.A.; Kautz, S. Friend or Foe—Light Availability Determines the Relationship between Mycorrhizal Fungi, Rhizobia and Lima Bean (Phaseolus lunatus L.). PLoS ONE 2016, 11, e0154116. [Google Scholar] [CrossRef]
- Kaschuk, G.; Kuyper, T.W.; Leffelaar, P.A.; Hungria, M.; Giller, K.E. Are the Rates of Photosynthesis Stimulated by the Carbon Sink Strength of Rhizobial and Arbuscular Mycorrhizal Symbioses? Soil Biol. Biochem. 2009, 41, 1233–1244. [Google Scholar] [CrossRef]
- Stevens, G.G.; Pérez-Fernández, M.A.; Morcillo, R.J.L.; Kleinert, A.; Hills, P.; Brand, D.J.; Steenkamp, E.T.; Valentine, A.J. Roots and Nodules Response Differently to P Starvation in the Mediterranean-Type Legume Virgilia Divaricata. Front. Plant Sci. 2019, 10, 73. [Google Scholar] [CrossRef]
- Chirko, C.P.; Gold, M.A.; Nguyen, P.V.; Jiang, J.P. Influence of Direction and Distance from Trees on Wheat Yield and Photosynthetic Photon Flux Density (Qp) in a Paulownia and Wheat Intercropping System. For. Ecol. Manag. 1996, 83, 171–180. [Google Scholar] [CrossRef]
- Villordon, A.; Ginzberg, I.; Firon, N. Root Architecture and Root and Tuber Crop Productivity. Trends Plant Sci. 2014, 19, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Jennings, A.; Barlow, P.W.; Forde, B.G. Dual Pathways for Regulation of Root Branching by Nitrate. Proc. Natl. Acad. Sci. USA 1999, 96, 6529–6534. [Google Scholar] [CrossRef] [PubMed]
- Arif, M.R.; Islam, M.T.; Robin, A.H.K. Salinity Stress Alters Root Morphology and Root Hair Traits in Brassica Napus. Plants 2019, 8, 192. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, W.; Li, K.; Sun, F.; Han, C.; Wang, Y.; Li, X. Salt-Induced Plasticity of Root Hair Development Is Caused by Ion Disequilibrium in Arabidopsis Thaliana. J. Plant Res. 2008, 121, 87–96. [Google Scholar] [CrossRef]
- IPCC; IPCC5 WGII. Climate Change 2013. The Fifth Assessment Report; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2014; Volume 5. [Google Scholar]
- Gibson, P.B.; Cope, W.A. White clover. In Agronomy Monographs; American Society of Agronomy: Madison, WI, USA, 1985; Chapter 20. [Google Scholar] [CrossRef]
- Abbasi, M.K.; Khan, M.N. Introduction of White Clover for Herbage Production and Nitrogen Fixation in the Hilly Areas of Azad Jammu and Kashmir. Mt. Res. Dev. 2004, 24, 134–140. [Google Scholar] [CrossRef][Green Version]
- Wasser, C.H.; Shoemaker, J.W. Ecology and Culture of Selected Species Useful in Revegetating Disturbed Lands in the West; Clinton, H.W., Ed.; Fish and Wildlife Service, U.S. Department of the Interior: Washington, DC, USA, 1982; 347p. [Google Scholar]
- Matlack, G.R.; Gibson, D.J.; Good, R.E. Clonal Propagation, Local Disturbance, and the Structure of Vegetation: Ericaceous Shrubs in the Pine Barrens of New Jersey. Biol. Conserv. 1993, 63, 1–8. [Google Scholar]
- Coca Pérez, M. Árboles, Arbustos y Matas Del Parque Natural Los Alcornocales (Cádiz-Málaga): Clave de Determinación, Descripción, Usos, 2nd ed.; ORNI TOUR: Cádiz, Spain, 2001. [Google Scholar]
- Menéndez Valderrey, J.L. Ornithopus compressus. 2013. Available online: https://www.asturnatura.com/especie/ornithopus-perpusillus (accessed on 9 May 2023).
- Pérez-Fernández, M.A.; Calvo-Magro, E.; Rodríguez-Sánchez, J.; Valentine, A. Differential growth costs and nitrogen fixation in Cytisus multiflorus (L0H_er.) Sweet and Cytisus scoparius (L.) Link are mediated by sources of inorganic N. Plant Biol. J. 2017, 19, 742–748. [Google Scholar] [CrossRef]
- Valladares, F.; Villar-Salvador, P.; Domínguez, S.; Fernandez, M.; Penuelas, J.L.; Pugnaire, F.I. Enhancing the Early Performance of the Leguminous Shrub Retama sphaerocarpa (L.) Boiss.: Fertilisation versus Rhizobium Inoculation. Plant Soil 2002, 240, 253–262. [Google Scholar] [CrossRef]
- Valverde, C.; Ferrari, A.; Gabriel Wall, L. Effects of Calcium in the Nitrogen-Fixing Symbiosis between Actinorhizal Discaria Trinervis (Rhamnaceae) and Frankia. Symbiosis 2009, 49, 151–155. [Google Scholar] [CrossRef]
- Pérez-Fernández, M.A.; Hill, Y.J.; Calvo-Magro, E.; Valentine, A. Competing Bradyrhizobia Strains Determine Niche Occupancy by Two Native Legumes in the Iberian Peninsula. Plant Ecol. 2015, 216, 1537–1549. [Google Scholar] [CrossRef]
- Piceno, Y.M.; Lovell, C.R. Stability in natural bacterial communities: II. Plant resource allocation effects on rhizosphere diazotroph assemblage competition. Microb. Ecol. 2000, 39, 41–48. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- IBM SPSS Software. Available online: https://www.ibm.com/analytics/spss-statistics-software (accessed on 9 May 2023).
- Ames, R.N.; Bethlenfalvay, G.J. Localized increase in nodule activity but no competitive interaction of cowpea rhizobia due to pre-establishment of vesicular-arbuscular mycorrhiza. New Phytol. 1987, 106, 207–215. [Google Scholar] [CrossRef]
- Ballhorn, D.J.; Kautz, S.; Schädler, M. Induced Plant Defense via Volatile Production Is Dependent on Rhizobial Symbiosis. Oecologia 2013, 172, 833–846. [Google Scholar] [CrossRef]
- Marschner, P. Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Mineral Nutrition of Higher Plants; Academic Press: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Millar, J. Effect of Mycorrhizal Colonization and Light Limitation on Growth and Reproduction of Lima Bean (Phaseolus lunatus L.). J. Appl. Bot. Food Qual. 2013, 86, 172–179. [Google Scholar] [CrossRef]
- Kiers, E.T.; Denison, R.F. Sanctions, Cooperation, and the Stability of Plant-Rhizosphere Mutualisms. Annu. Rev. Ecol. Evol. Syst. 2008, 39, 215–236. [Google Scholar] [CrossRef]
- Gubry-Rangin, C.; Garcia, M.; Béna, G. Partner Choice in Medicago Truncatula–Sinorhizobium Symbiosis. Proc. R. Soc. B 2010, 277, 1947–1951. [Google Scholar] [CrossRef]
- Reinhard, S.; Weber, E.; Martin, P.; Marschner, H. Influence of Phosphorus Supply and Light Intensity on Mycorrhizal Response in Pisum-Rhizobium-Glomus Symbiosis. Experientia 1994, 50, 890–896. [Google Scholar] [CrossRef]
- Ronsheim, M.L. The Effect of Mycorrhizae on Plant Growth and Reproduction Varies with Soil Phosphorus and Developmental Stage. Am. Midl. Nat. 2012, 167, 28–39. [Google Scholar] [CrossRef]
- Mortimer, P.E.; Pérez-Fernández, M.A.; Valentine, A.J. The Role of Arbuscular Mycorrhizal Colonization in the Carbon and Nutrient Economy of the Tripartite Symbiosis with Nodulated Phaseolus vulgaris. Soil Biol. Biochem. 2008, 40, 1019–1027. [Google Scholar] [CrossRef]
- Graham, P.H.; Vance, C.P. Legumes: Importance and Constraints to Greater Use. Plant Physiol. 2003, 131, 872–877. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).