Abstract
In this study, the origin of Spanish durum wheat and olive tree landrace collections (preserved in seed banks and ex situ field collections, respectively) was traced throughout different studies on genetic structure analysis of different landrace collections and historical records of plant material circulation of these two crops in the south and east of Spain (the main cultivation areas). Although there were several groups of Spanish durum wheat landraces, they did not cluster geographically and likely came from intense grain circulation within the country and abroad (due to a high seeding rate of about 100 kg/ha and an unstable production) from the 15 to 19th centuries (especially the Maghreb and Sicily). However, Spanish olive tree landraces experienced lower genetic circulation over time and space due to the longevity of the species and the large size of the reproductive material. They can be clearly divided into two groups: those from the south, of Maghrebi origin, which most likely originated during the Islamic expansion of the 8–15th centuries, and those from the east, arriving most likely during Roman times from Italy. The genetic circularity levels of the plant material of these two crops are different.
1. Introduction
Durum wheat (DW) (Triticum turgidum L. subsp. durum (Desf.) Husn.) and olive tree (OT) (Olea europaea L. ssp. europaea var. sativa) are two important crops in Spain, with a long history in the country. Currently, the national DW acreage is approximately 250,000 ha, while approximately 2,624,000 ha was devoted to OT in 2020. Both crops are principally established in southern Spain, mainly in the region of Andalusia [1]. Regarding their plant type, reproductive nature, and cultivation, the two crops are different. DW is an annual, herbaceous, self-pollinated cereal, whereas OT is a perennial (and long-lived) and generally self-incompatible woody crop. DW cultivars consist of pure lines that can be easily multiplied by seed with hardly any segregation. In contrast, OT is vegetatively propagated to maintain the cultivar traits lost upon sexual reproduction, i.e., individuals of the same variety are clones (Table 1). Interestingly, OT and DW share similar historical evolution patterns: domestication in the Mediterranean Levant, restriction to this region over millennia, and expansion throughout the Mediterranean Rim well into the first millennium BCE (Before Common Era) [2], where the role played by Phoenicians, Carthaginians, Greeks, and above all Romans (they penetrated deep inland, not just along the coast) was of prime importance [3,4].

Table 1.
Differences between durum wheat and olive tree.
Although there may have been DW in Spain during the Chalcolithic and Bronze Age, it was a minority component of a mixture of wheat species that were cultivated, and hulled wheats (einkorn and emmer) were the prevailing species [5]. However, DW has naked seeds (which facilitate grain grinding and milling) and adapts very well to Mediterranean climate conditions (their spring varieties compete well with those of other wheat species [6]), so it spread throughout the Mediterranean Basin, especially in the Roman age (where it became the main wheat species), beginning in the 5th century BCE [3]. On the Iberian Peninsula, DW spread widely to the south (Andalusia and Extremadura) and to the east (Murcia, Valencia, and the Balearic Islands), especially from the 2nd century BCE. The high demand for bread and other food led to the introduction of new wheat landraces (LRs) in Spain from the different regions under Roman control. Tito Livio wrote c. 9 BCE in his decades that for many years, Italy supplied wheat grain to Spain [7], and Roman historian Strabo described c. 7 CE (Common Era) that Baetica Province (which was approximately the region of Andalusia) was one of the Roman Empire granaries [8]. In the Islamic expansion on the Iberian Peninsula (Al-Andalus age, 8–15th centuries), DW cultivation increased, and most likely, new LRs were introduced from the Mediterranean Levant and North Africa [3,9].
In the case of OT, and unlike DW, wild OT or oleaster (O. europaea L. ssp. europaea var. sylvestris) existed along the Mediterranean coast and was used by the locals long before domestication [10,11]. It is supposed that the arrival of the first domesticated OT occurred during the Phoenician and Greek colonization of the southern and eastern Iberian Peninsula from 600 to 500 BCE. The Phoenician (and later Carthaginian) colonies were established in the south (Adra, Almuñecar, Malaga, Cadiz, etc.), while the Greeks settled in the northeast, mainly in present-day Catalonia (Empuries and Roses). However, it was during the Roman age that cultivation expanded and penetrated through the south and east of the Iberian Peninsula. Pliny the Elder [12] wrote that by 581 BCE, there were only a few OTs in Italy, Spain, and Africa; however, by the 2nd century BCE, Cato described that olive cultivation was commonplace in the Roman Republic [13]. According to Remesal et al. [14], olive cultivation spread along the Guadalquivir (or Betis) banks in the time of Julius Cesar (~50 BCE), who promoted the influx of Italic settlers to Baetica Province. Strabo reported that from Turdetania (southwest Spain), ‘wheat, wine are exported in quantity, and olive oil not only in quantity, but also of the best quality‘ [8]. Hispania (in particular Baetica) experienced a period of OT expansion and specialization during the 1st century CE driven by the high oil and olive consumption of the city of Rome and its surroundings, as evidenced by the impressive remains of amphorae preserved in the anthropic Mount Testaccio (Rome) and the pottery discovered along the Guadalquivir and Genil banks in southern Spain [15]. In Islamic culture, the OT was a blessed tree, and its oil was a symbol of light. Abu Zacaria (or Al-Awwam), in his Book of Agriculture, refers to the olive groves in Aljarafe (near Seville), and al-Idrisi wrote that ‘the oil of Seville comes from a district called al-Sharaf (Aljarafe, meaning ‘upland’ in Arabic) that extends 60 km to Niebla and is planted with olive and fig trees. It is 18 km wide’. It was one of the largest olive orchards in the world at the time [16].
Both crops were of great importance for various uses, especially food. Different types of bread (rather flat) were made with DW semolina but also puls (gruel) and laganum (a kind of lasagna). Bread wheat (Triticum aestivum L. ssp. aestivum), which permitted the production of spongier leavened bread, was introduced later through the north (from France) at the time of the Empire. Muslims introduced new food types from DW semolina, such as pasta and couscous [3]. Olives were processed for obtaining oil (culinary use, lighting, body use, industrial oil, anointing, etc.), although table olives (prepared and dressed in several ways) were also a common food [17].
LRs of DW and OT (in OT, LRs are often called traditional cultivars) were cultivated until well into the 20th century. In Figure 1, the main cultivating Spanish provinces by 1898 (a year where cultivation of the two crops was still performed with LRs) of both crops are shown. In that year, there were c. 770,000 ha of DW and 1,092,300 ha of OT. The most important provinces for both crops were in the south and the east, especially the provinces along the Guadalquivir valley (Jaen, Cordova, and Seville). For DW, the most relevant provinces were Jaen, Seville, Malaga, and Cordova. Cadiz, Murcia, Granada, and Badajoz were also important provinces [18]. Regarding OT cultivation, the main provinces were also Jaen, Cordova, and Seville, followed by Tarragona, Lleida, Malaga, and Badajoz [18]. The three most important provinces accounted (by 1898) for 45.7% and 49.5% of the national area of DW and OT, respectively, but some differences were also found. Thus, the eastern provinces (corresponding to the regions of Valencia, Catalonia, and the Balearic Islands) were more important for OT cultivation than for DW. In contrast, the inland northern provinces and the Canary Islands (counted as one province in Figure 2) did not appear among the main OT-cultivating provinces, while there was some DW cultivation [19,20].
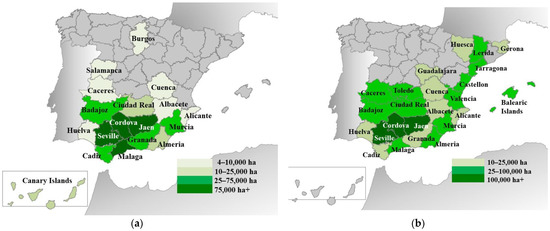
Figure 1.
Provinces cultivating main durum wheat (a) and olive tree (b) in Spain in 1898. Data from [18,19].
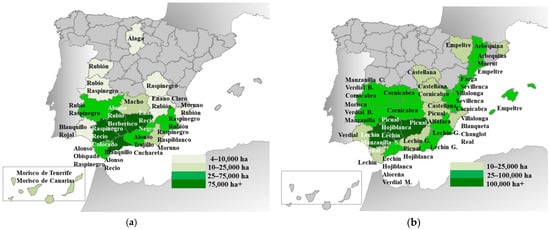
Figure 2.
Main provinces cultivating durum wheat (a) and olive tree (b) landraces in Spain in 1898. In Figure 2b, G. = Granada, M. = Malaga, S. = Seville. Data from [18,20,21].
The diversity of LRs of both crops in Spain was quite high. The National Center for Plant Genetic Resources (CRF-INIA) maintains a large collection of Spanish DW LRs comparable to those in countries with a long tradition of this crop, such as Italy, Algeria, Turkey, Tunisia, or Morocco [3]. In the CRF database, 403 entries of DW LRs can be found. The collection is preserved in the facilities of the Agricultural Research Station at La Canaleja (Alcalá de Henares, Madrid, Spain). Seeds of each entry are kept in plastic bags and conserved under refrigeration. LR names such as ‘Recio’, ‘Raspinegro’, and ‘Rubio’ appear frequently. Other names that appear are ‘Fanfarrón’, ‘Claro’, ‘Durillo’, ‘Morillo’, ‘Moro’, ‘Rojal’, ‘Bascuñana’, and ‘Siciliano’ [22,23]. Modern cultivars are shorter and mature earlier than LRs (Figure 3). The varietal denominations usually refer to the high grain quality of some LRs or the hardness of the species (‘Fino’, ‘Recio’). Other traits also give name to the LRs, such as ‘Enano’ for its short plant height; ‘Azulejo’, for the bluish tone of the spike; ‘Blanco’, ‘Blanquillo’, and ‘Colorado’, for the color of the grain; ‘Raspinegro’, ‘Rubio’, or ‘Rojal’ for the color of the awns and glumes; and for the geographical origin, normally Maghrebi (‘Moro’, ‘Morisco’, ‘Moruno’, ‘Berberisco’, ‘Africano’) but also Sicilian (‘Siciliano’) [18,24].

Figure 3.
Plot trial at Escacena (Huelva) comparing a cultivar (left) and a landrace (right) of durum wheat. Note the difference in height and earliness.
Regarding OT, LRs are still the basis of most plantations worldwide since very few bred cultivars have been released thus far [25]. Nonetheless, despite the large number of LRs conserved in Spain, only a few of them (Picual, Arbequina, Manzanilla, Hojiblanca, Cornicabra, etc.) are currently under cultivation in extensive areas, while others are only planted on a small scale and are in danger of disappearing (Figure 4). For the first time in the history of olive growing, there is a serious risk of genetic erosion [25]. Thus, an integrated effort to establish an international network of germplasm banks has been led by the FAO and the International Olive Council (IOC), in which the role of the Olive World Germplasm Bank (OWGB) of Cordoba (Spain) stands out. This bank was established in 1970, and the initial collection has been enlarged through different national and international prospective surveys and exchanges with other germplasm banks. The OWGB of Cordoba has a backup copy at Mengibar (Jaen) and a duplicate of a true-to-type LR at the University of Cordoba in a soil free of Verticillium, a fungus causing an important disease of OT. It is integrated into the Network of Collections of the National Plant Genetic Resources Program (INIA) and included in the National (CRF-INIA) and European (ESP046) inventories. At present, it has more than 1000 accessions from 29 countries, of which 330 come from Spain. The collection is maintained in the field, and each accession is usually represented by two trees. Currently, 264 newly acquired accessions (71 of Spanish origin) at different propagation facilities are under study before their introduction to the field collection [26].

Figure 4.
Some of the main Spanish olive tree landraces: (a) ‘Arbequina’; (b) ‘Blanqueta’; (c) ‘Cornicabra’; (d) ‘Gordal Sevillana’; (e) ‘Manzanilla de Sevilla’; (f) ‘Picual’. Adapted from [27] with permission by GRUPO UCOLIVO (Dep. Agronomia, University of Cordoba).
As mentioned for DW, OT LRs have been named after different morphological traits, such as the shape of the fruit (‘Redondilla’ meaning round shape; ‘Picual’ and ‘Picudo’ referring to fruit with an acute apex; ‘Manzanilla’ as apple-shaped; or ‘Cornicabra’ and ‘Cornezuelo’ meaning having a horn shape); the (large) size of the fruit (‘Gordal’); or the color of fruits and or leaves (‘Blanqueta’, ‘Blanquilla’, ‘Hojiblanca’). Toponyms (‘Marteño’, ‘Lopereño’, ‘Andaluza’) and practical uses of the fruit (‘de aceite’ and ‘Corriente’) have also been widely employed to name them [27]. This varietal denomination system has led to extraordinary confusion in the identification of LRs due to the occurrence of synonyms (different names for the same variety) and homonyms (same name for different varieties). In fact, these two phenomena are among the main constraints in managing olive germplasm collections. An extraordinary effort has been made over the years to identify the real number of LRs based on morphological traits, which led to the discrimination of 272 different varieties in the Spanish catalog [27]. Recent extensive works have been performed on the Spanish accessions preserved at the OWGB of Cordoba with 33 SSR markers, 11 endocarp traits [28], and 96 EST-SNPs [25], identifying 239 genotypes out of 279 accessions and 254 out of 330, respectively.
Little is known about the origin and history of the varieties of these two crops. Rapid assumptions simply mention the Roman or Arab role in the origin of the Spanish LRs, but little has been published on this topic [18,21]. With the advent of new molecular marker technologies, we can better examine the similarities and differences between DW and OT LRs. Second, an Internet search permits us to look for historical articles and documents more easily. The objective of this study was to integrate findings from articles on the genetic structure of DW and OT LRs with information from historical records of plant circulation to gain insight into the origin of the LRs of these two crops in Spain.
2. Methodology
Different studies on genetic structure analysis in ample LR collections and historical records of plant material circulation of the two crops have been extensively reviewed in the present article. Several international germplasm collections of LRs have been genotyped with different DNA markers (SSRs, SNPs, ETS-SNPs, etc.) and, recently, applying high-throughput sequencing techniques to assess their genetic structure for DW [29,30] and for OT [4,26,28,31,32]. These analyses focused on the phylogenetic reconstruction of the relationships between samples after estimating their genetic distances and grouping them according to different clustering methods. In addition, population genetic analyses were applied using two different but complementary approaches: first, establishing a priori populations/partitions with samples sharing one or several characteristics (e.g., geographical origin), and subsequently, testing the significance of these partitions by applying an AMOVA or similar test. A second approach consisted of finding the most likely number of populations or genetic clusters (K) in a large dataset with no ‘a priori’ partitions. Specific software, such as Structure®, and its different versions, were used to this end [33]. The program is run multiple times until the most likely number of groups is found. Then, the algorithm is run again with this constraint, and the genetic ancestry of every sample is depicted in terms of admixture coefficients. Thus, admixture appears when a genotype background is shared by different genetic clusters [31]. Often, a PCA or PCoA is also conducted to contrast the Structure and AMOVA results since these methods are based on different approaches, but they should yield similar results if the genetic structure is robust.
On the other hand, historical records on the movement and circulation of plant material of both crops were searched in historical references. This circulation was divided between circulation within Spain (focusing on the south and the east, the core area of both crops) and circulation between Spain and neighboring countries of the Mediterranean Rim.
3. Analysis of the Information Obtained for DW
3.1. Analysis of DW Genetic Structure
Several studies have noted that Spanish DW LRs are genetically similar to their North African counterparts (mainly from Morocco and Algeria) [9] and Italian counterparts, especially those from Sicily [29,30] (Figure 5A). In a genetic study of 63 DW LRs from the Mediterranean Basin based on SSR markers, Moragues et al. [9] found that genotypes from the Iberian Peninsula and North Africa (Egypt, Algeria, and Morocco) grouped together. This result supports a south dispersal of DW from northern Africa to the Iberian Peninsula. In a broader study with 172 LRs from 21 Mediterranean countries and 44 SSR markers, Soriano et al. [29] found that western Mediterranean LRs (including those from Spain) formed a consistent subpopulation, one of the five in which the LRs of the Mediterranean Basin were grouped. Gliadin Gli-B2h, which is frequently found among the LRs of southern and eastern Spain, was also found in material from northern Africa, especially Algeria [34,35]. However, within the country, the distribution of Spanish LRs by genetic groups does not show a clear geographical pattern. According to Ruiz et al. [35], there is slight east–south differentiation of LRs, and a genetic group (or population) of LRs called Pop 2 (phenotypically characterized by lateness) is frequently located in mountainous areas of the provinces of Jaen, Malaga, and Granada (east Andalusia). In the same way, another population (Pop 9) was associated with warm and dry areas of Almeria–Murcia (southeast Spain), while a subgroup of Pop3 (characterized by earliness) was found in western Andalusia (Cordova, Seville, Cadiz, Huelva). However, the genetic variation in the different Spanish populations was larger within than between zones, which suggests dynamic circulation of LRs across the various areas of DW cultivation in the country [35,36].
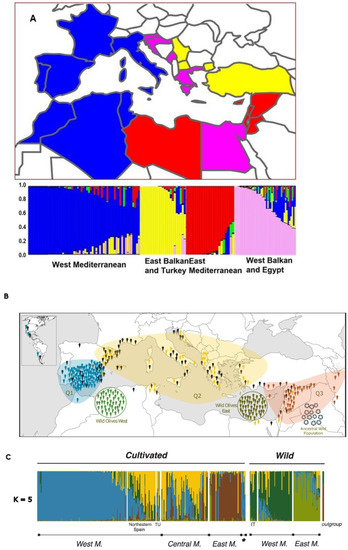
Figure 5.
(A) Analysis of the genetic structure of a population of 192 genotypes of durum wheat from the Mediterranean Rim with four groups (each depicted by a color) and geographical distribution of the groups [29]. (B) Map indicating the colored grouping of 289 olive cultivars, each color representing the three different gene pools, as inferred by Structure. Wild olives are also colored according to geographical origin and gene pool. (C) The proportions of ancestry of olive cultivars and wild olives according to Structure for K = 5 subdivisions. The geographical origin as well as the Iputative status of the samples (cultivated or wild) is specified. East M = eastern Mediterranean Basin; Central M = central Mediterranean Basin; West M = western Mediterranean Basin; * denotes America; outgroup = Olea europaea subsp. cuspidata [31].
3.2. Historical Records of DW Circulation
Although Alonso de Herrera, in 1645, referred to Trechel as the most appreciated variety for DW [37], the historical evolution of DW (and wheat in general) in Spain from the 15th to 18th centuries is largely unknown [7]. However, numerous historical references on wheat grain circulation in southern and eastern Spain (the most important DW areas) in the period 1450–1850 were recorded, and in this study, many of them were consulted. LRs have recently been defined as crop populations in a constant state of evolution [38]. Wheat LRs perfectly fit with this concept, as they are annually adapting (to climate and soil conditions), mixing with other LRs, and being selected for yield stability and the quality of their products [39]. Therefore, the period of approximately 500 years before the advent of modern breeding is key to understanding the formation of the DW LRs in Spain. This circulation was classified into two sections: external (from several areas, out of the country, from the west and south of the Mediterranean Basin to Spain) and internal (within locations to the south and east of Spain).
3.2.1. External Circulation of DW Grain: The ‘Sea Wheats’
Table 2 (above) describes the maritime traffic of wheat grain from the south and west of the Mediterranean Basin (where almost all the cultivated wheat was DW) to the south and east of Spain. This traffic was commonplace during the Middle Ages and modern period, despite the policy of Spanish raids (political tension with some surprise attacks and piracy) in the Maghreb, which also included the occupation of important Maghrebi coastal cities by the Spaniards [40]. Algeria (especially Oran) appears to be the main grain origin area. Other sites referenced as trading with Spain were Algiers, Bugia, and Dellys (in Algeria), Casablanca (Morocco), and Cape Negro (Tunisia) [40,41]. Sicily (most of the time, cities and ports are not mentioned) and Sardinia were also described. Sicilian wheat was also very important [42] since island wheat production frequently exceeded domestic consumption [43], and almost all wheat was DW of extraordinary diversity [44].
The main southern and eastern Spanish receiving ports were Palma de Mallorca, Valencia, Seville, and Cadiz. There were various causes for this constant maritime traffic of wheat grain (the so-called ‘sea wheats’): low production in the destination areas due to droughts (recurrent in the Mediterranean climate areas), diseases (common bunt, rusts) or pests (locust) of wheat, or even human diseases (e.g., the Black Death eliminated labor and limited crop harvest). The low prices and availability of wheat grain in the region of origin and grain supply for the army or prisons were other causes of grain circulation [40,45,46]. Droughts in wheat-producing areas were the most frequent cause of these movements. The Genoese merchants were the main maritime transport agents in the western part of the Mediterranean Basin in the 14–18th centuries [47]. It must be said that in the aforementioned regions of origin (Maghreb, Sicily), the most cultivated wheat species was DW [3].
3.2.2. Internal Circulation of DW Grain
Table 2 (at the bottom) describes DW grain circulation (by land and sea) within southern and eastern Spain. Movements of grain to supply the producing areas of the most populated cities were frequently recorded (e.g., from Lorca to Murcia, from Orihuela to Valencia) [48,49]. There was also transport between ports of the Spanish Mediterranean coast (e.g., from Cartagena to Málaga, Cadiz, and Seville) and from ports toward the interior (e.g., from Cadiz to Carmona). The internal grain circulation in carts or ships took place from the interior of the country to the ports or from the ports to the interior. There were several factors (many described above) that affected the internal circulation of DW seeds in Spain:
- Droughts and floods. When there was a lack of grain in one area due to drought, grain had to be imported from another area. Droughts were common in the south and east of the country. For example, the years 1473 and 1521 are cited as drought years in Carmona, near Seville [50], and 1497 was a year of drought in Murcia and nearby Lorca [48]. Floods could also affect wheat grain production (to a lesser extent compared to drought) in a given area, either through damage by waterlogging or through an increase in some diseases such as common bunt and/or rusts [3]. For example, the period 1760–1800 was characterized by high climatic instability, known as the Maldà Anomaly [51,52]. During this time, drought periods alternated with persistent rains. Thus, for example, in the 1760s, there were frequent droughts in eastern Spain (e.g., Elche, Orihuela), while in the 1770s, 1780s, and 1790s, there were years of floods, which alternated with years of severe drought [53].
- Diseases and pests of wheat, such as common bunt, rusts, or locusts. Between 1783 and 1788 (in the midst of the Maldà Anomaly), there were many reports and newspaper articles that refer to common bunt, a seed-borne disease (caused by the fungus Tilletia tritici) that was endemic to the country and neighboring countries, such as France. The affected grain carried the disease to the following season. Bringing new and clean grain (despite common bunt also being soil-borne) was a reason for wheat seed circulation [54]. Strong locust attacks were also recorded in the period 1770–1800 in several regions of southern and eastern Spain [55].
- Grain trade. Wheat grain could be bought in the different cereal markets located throughout Spain. The wheat price was established by city halls in special book lists or ‘mercuriales’. Regarding DW trade, Granada and Lorca (Murcia) markets located in southeastern Spain stood out [56]. When a certain village or town had a surplus of wheat grain that could be sold, this gave rise to a takeout or ‘saca’, which often required a royal permit [57,58]. When wheat production was low, many municipalities in Spain banned such takeouts [47].
- Varietal degeneration. When harvesting DW grain, it could be physically mixed with grain from other cereals in adjacent fields or in unclean carts or ships (bread wheat, barley, rye, grass weed seeds, etc.). In addition, spontaneous crosses (infrequent due to the self-pollinating nature of the crop but cannot be ruled out) may have occurred with other varieties, as well as some natural mutations. As part of this grain was used for sowing, this mixture could lead to the degeneration of the variety over several seasons, giving rise to another population variety with lower yield and quality. The solution was to bring clean seeds of the same or another variety from elsewhere [59].

Table 2.
Circulation of durum wheat grain between southern and eastern Spain and Mediterranean Basin regions (above), and between southern and eastern Spain (below) (1450–1850).
Table 2.
Circulation of durum wheat grain between southern and eastern Spain and Mediterranean Basin regions (above), and between southern and eastern Spain (below) (1450–1850).
| Origin | Region/Current Country | Destination | Motivation | Year | Conveyance | Reference |
|---|---|---|---|---|---|---|
| Oran | Algeria | Mallorca | Low production (drought) | 1530 | Sea | [40] |
| Oran | Algeria | Mallorca | Low production in origin | 1588 | Sea | [40] |
| Oran | Algeria | Mallorca | Low production, low price | 1591 | Sea | [40] |
| Algiers | Algeria | Mallorca | Low production, low price | ~1450 | Sea | [40] |
| Sicily | Italy | Motril (Granada) | Commercial | 1599 | Sea | [42] |
| Bugia | Algeria | Mallorca | Low production, low price | ~1450 | Sea | [40] |
| Dellys | Algeria | Mallorca | Low production, low price | ~1450 | Sea | [40] |
| Cape Negro | Tunisia | Mallorca | Low production (Black Death) | 1652 | Sea | [40] |
| North Africa | Maghreb | Seville | Low price in place of origin | 1521 | Sea | [41] |
| Caller, Oristano | Sardinia (Italy) | Valencia | Commercial | 1626–1638 | Sea | [45] |
| Sicily | Italy | Valencia | Commercial | 1626–1638 | Sea | [45] |
| Greece | Ottoman Empire (Greece) | Cadiz | Commercial, yellow fever (1804) | 1798–1807 | Sea | [46] |
| Casablanca | Morocco | Cadiz | Commercial | 1787–1788 | Sea | [46] |
| Oran | Argelia | Seville | Commercial | 1665–1666 | Sea | [42] |
| Lorca | Murcia | Murcia | Black Death | 1489 | Land | [48] |
| Baza, Velez Rubio | Andalusia | Lorca | Drought | 1503–1508 | Sea | [48] |
| Cadiz, Puerto de Santa María | Andalusia | Carmona | Drought | 1506, 1520–1522 | Land | [50] |
| Orihuela | Valencia | Valencia | Low price in place of origin | 1404 | Sea | [49] |
| Cartagena | Murcia | Cadiz, Malaga, Sevilla | Commercial | 1662–1667 | Sea | [47] |
3.2.3. Mechanisms of Genetic Change in DW LRs
As mentioned above, the seeds of DW LRs preserved in the CRF are derived from different collection missions carried out between the 1920s and 1960s [20,24,35]. For instance, between 1948 and 1957, E. Sánchez-Monge classified 109 Spanish DW varieties at the Aula Dei Station (Zaragoza), mostly from the INIA cereal research centers and the Elvas Breeding Station (Portugal) but also from the breeding stations of Jerez de la Frontera (Cadiz), Zaragoza, and Ejea de los Caballeros (Zaragoza) [24]. In many instances, the LRs were mixtures of several genotypes. With the information provided in this study, several situations could explain the origin of the genotype mixing of Spanish DW LRs:
- Situation 1: In a certain town or village, there was no wheat grain to be sown due to, for example, a drought. Wheat was imported from another population or brought from a Mediterranean port (which may have come from another Spanish port, from the Maghreb or from Sicily).
- Situation 2: In a certain town or village, a part of the grain was available for sowing, but the seeds were not sufficient. Wheat was then imported in the same way as in situation 1. A mixture of two genotypes occurred.
- Situation 3: A part of the grain was available for sowing, but the seeds again were not sufficient. DW grain was then imported, but from two different sources (e.g., one from the harvest of a neighboring town, another from a maritime import from Sicily or the Maghreb). Since each source was not sufficient to obtain all the seeds for sowing, a mixture of three genotypes was used, and a different population originated.
Other situations might have occurred where genotype mixtures became even more complex, and in the end, a variety population was formed that evolved according to the laws of population genetics, giving rise to the LRs that were collected in southern and eastern Spain in the early and mid-20th centuries [29,60]. Natural selection of the genotypes best adapted to soil, climate, and pathogen conditions also modified the population, as well as selection by farmers of the most suitable grains for the products (bread, fideos noodles, semolina type, etc.). Genetic drift, which can occur when small grain samples with different genetic proportions are taken from the original population, can also alter wheat populations [61]. During the 19th century, selection of the best DW LRs in terms of yield, yield stability, and quality was carried out in different villages of Spain (e.g., ‘Recio’ and ‘Fanfarrón’ were among the preferred LRs). In addition, meticulous farmers selected grains from individual plants according to their size and semolina quality [59].
4. Analysis of the Information Obtained in OT
4.1. Analysis of OT Genetic Structure
Most studies analyzing large OT germplasm collections to assess their genetic structure agree in three ways. First, the OT LRs cluster into three main groups corresponding to the western, central, and eastern Mediterranean Basin (Q1, Q2, and Q3, respectively, as Diez et al. [62] reported in a study with 387 cultivated and wild accessions genotyped with 25 SSR markers) (Figure 5B). Second, the central Mediterranean LRs show a significant level of admixture with local oleasters. Finally, the relationships between these clusters and their wild ancestor seem to be defined by a primary domestication event in the Levant [31,63]. Indeed, 90% of the evaluated OT LRs shared a chorotype from this area [63]. Only a reduced number of LRs, mainly from eastern Spain and Italy, showed a chorotype only present in the same geographical area. This group of LRs might be the result of a minor and independent domestication event, although this hypothesis is still under debate [62,63]. The difficulty of sampling true oleasters hinders the possibility of tracing the origin of the LRs. Several studies have failed to sample true oleasters in the eastern Mediterranean Basin, a key geographical area for olive domestication [31,32,64,65]. This fact highlights the urgent need to preserve the remaining oleaster areas in situ and their seeds in ex situ collections.
Selection of adapted OT genotypes by farmers and the recurrent crosses between them and with local oleasters could explain the origin of the LRs that are currently known [66]. These LRs have remained in the field for centuries due to the longevity of OT [67]. Relatively recent historical events might have also shaped the genetic structure and distribution of olive LRs. According to the study performed by Diez et al. [31], fingerprinting a large international collection of LRs and wild accessions with DNA markers, the LRs from southern Spain and Portugal showed a close link with those from the Near East. This pattern was later confirmed by analyzing the complete genomes of a selection of representative LRs [32]. The early contact with Phoenicians (and later Carthaginians), who founded several cities in southern Spain, and the Islamic expansion in Spain of the 8–15th centuries CE, which lasted several centuries, could be responsible for this pattern. In addition, most LRs from America showed shared ancestry with those from Portugal and southern Spain, reflecting the early commercial trade between these areas. Indeed, after the Christian Reconquest (completed in 1492), the southern part of Spain was integrated into the kingdom of Castile (part of Spain, but maintaining a certain level of independence), which traded with America, especially from the city of Seville, during the 15–18th centuries. Thus, American varieties (from California, Mexico, Chile, and Argentina) originated from the western Mediterranean LR group. Conversely, LRs from eastern Spain (Aragon, Catalonia, Valencia, and the Balearic Islands, up to the early 18th-century Kingdom of Aragon) were more similar to those from Italy, Greece, and eastern Turkey. Recent studies reached the same conclusion by analyzing the genome of a large set of cultivated and wild olives [4,32]. Although this pattern was consistent, some odd cases were found, such as ‘Cirujal’, a local cultivar from northeastern Spain that was identified as the Syrian cultivar ‘Safrawi’ [58].
4.2. Historical Records of OT Circulation
The long lifespan of OT, which can reach several centuries [68,69], favored reduced circulation of LRs and the permanence of olive groves over time. The movement and multiplication of olive plants were performed by means of clonal propagation using large propagules such as hardwood cuttings and trunk ovules taken from the basal part of old trunks. The large size of this material restricted movement over long distances [21]. However, there are records of remote human dispersion, such as the introduction of olive plants in Peru collected from the Aljarafe (Seville) in 1560 by Antonio de Rivera [70]. Grafting was also performed and was historically important for the long-distance diffusion of olive LRs due to the small size of the material. In fact, the name of the variety ‘Empeltre’ comes from the Catalonian word ‘empelt’, meaning to graft. It is likely that this name originated from the fact that this variety was grafted onto other older ones from southern Aragon and the Ebro Valley to distant locations such as the Balearic Islands, where it was grafted onto local oleasters [71]. ‘Manzanilla de Sevilla’ was also grafted in several areas of Badajoz onto the LR ‘Morisca’ [21]. OT was also propagated through seeds, with many drawbacks, i.e., difficult germination of the seed, segregation of traits, and appearance of atavistic traits (e.g., small fruit size), giving the resulting OT an oleaster-like appearance. Thus, Hispanic–Muslim Ibn al-Awwam in the 12th century wrote that ‘most trees planted from seed produced a fruit of its species, except the OT, whose seed produced the oleaster’ [72]. Plants originating from seeds were generally grafted with the producing variety and seldom gave rise to a new variety. In any case, the circulation of OT plant material occurred, and in this study, it was divided into external and internal circulation.
4.2.1. External Circulation of OT Genotypes
It is not easy to find movement records of olive plant material (cuttings or fruit) arriving or leaving Spain. Most trade was conducted with oil, and less with olive fruits, which could indeed constitute a planting material. One factor to consider in the circulation of OT plant material, as occurred in DW, is drought, although OT has traditionally been considered a crop tolerant to this abiotic stress. Two examples of OT exchanges with other regions of the Mediterranean Basin are represented in Table 3 (above). For example, a prolonged drought in the Guadalquivir Valley that occurred at the time of Visigoths on the Iberian Peninsula in 641 or in Al-Andalus around the year 1030 made it necessary to bring olive plants by sea in ships from Ifriqiyya (present-day Tunisia) [16,73]. The wars of the Reconquista could also affect the Andalusian olive grove. For instance, one of the strategies of the Christian King of Castile Alfonso VI to reduce the power of Muslim Al-Andalus was to cut down OTs, as this king did in 1081 when he sieged Seville [16].
4.2.2. Internal Circulation of OT Genotypes
The internal circulation of olive material within the country is presented in Table 3 (at the bottom). In the mountains (sierra) of the localities of Priego de Cordova and Carcabuey (Cordova), OTs from the Castilian variety ‘Picudo Castellano’ were introduced a little before 1840 [74]. People from Ayelo de Malferit (Valencia) brought materials from a small oleaster (or perhaps wild OT) forest in the Sierra of Enguera (Valencia), an individual with high production and fruits showing a clustered shape. That tree was uprooted and planted in Ayelo de Malferit, giving way to the ‘Changlot Real’ variety [75]. Some LRs were selected for their frost tolerance. Thus, ‘Arbequina’ was the only LR that survived the snow in summer 1795 in Solsona (Lleida), killing olives of other varieties [76]. The same happened in Alava because of an intense snow in 1914 that killed almost all ‘Empeltre’ trees but not ‘Bermejuelo’ [77].

Table 3.
Circulation of olive trees (external and internal) in Spain.
Table 3.
Circulation of olive trees (external and internal) in Spain.
| Origin 1 | Region/ Current Country | Destination | Motivation | Landrace | Year | Reference |
|---|---|---|---|---|---|---|
| Italy | Italy | Baetica (Andalusia) | Demographic expansion in Hispania | - | ~100–44 BCE 2 | [8,14] |
| Ifriqiyya | Tunisia | Andalusia | Severe drought | - | ~1030 | [73] |
| Arbeca (Lleida) | Balearic Islands | Mallorca | Olive orchards in new land (by King of Aragon Jaume I) | ‘Arbequina’ | 13th century | [71] |
| Aragon region | Aragon | Balearic Islands | Crop expansion (by grafting) | ‘Empeltre’ | 13–15th century | [27] |
| Solsona | Catalonia | Solsona (Lleida) | Natural selection by frost tolerance | ‘Arbequina’ | 1795 | [76] |
| Sierra de Enguera (Valencia) | Valencia | Ayelo de Malferit (Valencia) | High-yield genotype (from oleaster or feral olive tree) | ‘Changlot Real’ | 19th century | [75] |
| Castile | Castile–La Mancha, Castile and Leon | Priego, Carcabuey (Cordova) | Olive orchards in the mountains (sierra) | ‘Picudo castellano’ | A little before 1840 | [74] |
| Seville | Andalusia | Badajoz | Crop expansion (by grafting) | ‘Manzanilla de Sevilla’ | - | [27] |
| Seville | Andalusia | Seville | Better frost tolerance | ‘Hojiblanca’ replaced ‘Lechin’ | 1941–1942 | [73] |
1 Internal circulation is represented in the upper part of the table, while at the bottom, the historical traffic (mainly maritime) of olive trees between Spain and the Mediterranean Basin regions is represented. 2 Strabo mentioned that olive tree cultivation spread in the times of Julius Cesar [8]. Therefore, the years of his birth and death are written.
4.2.3. Mechanisms of Genetic Change in OT LRs
As mentioned above, the replacement of LRs in OT was slow due to the longevity of the tree and the selection of LRs adapted to local conditions and with high oil and/or olive quality. Interestingly, frost appears to be more important than drought as the main factor driving varietal replacement, despite OT being a crop located in a Mediterranean climate. OT is not as tolerant to frost as it is to drought, and temperatures below −8 °C for short periods of time (days) can be fatal [73]. Many attempts to grow OTs farther north or into the inner Iberian Peninsula were made in the past, with the aim of supplying oil and/or olives to the local population. However, the strongest frosts, which could occur every 10–40 years (less frequently toward the south and the coast, but still possible), could be lethal for the olive trees. During the 14–19th centuries (in the Little Ice Age period), frequent frost episodes occurred in the Northern Hemisphere. In Spain, four periods of climatic instability at that time characterized by intense rains, snowfalls, and storms at sea have been recorded (approximately 1450, 1570–1610, 1769–1800, and 1820–1860) [78], with the most well known being the Maldà Anomaly (1760–1800) [51,52]. During some of those years, there could have been episodes of OT death by frost but also selection of the most frost-tolerant genotypes, which likely gave rise to new LRs in Spain, such as ‘Arbequina’ or ‘Bermejuelo’, as mentioned above. In years outside these periods, there were also times of strong frosts, such as those that occurred in southern Spain in 1891 and in 1941–1942. The latter caused partial replacement of the Lechin variety by Hojiblanca in Seville. Diseases and pests seem to play a secondary role with respect to abiotic stresses in causing tree death and variety replacement, although sooty mold (caused by the fungal species Capnodium spp. and Limacinula spp.) was an important problem in Spain in the early 19th century, prompting substitution of the crop in some cases [73].
Regarding breeding, selection of individuals derived from olive seeds (in open pollination) grown beside their own orchard was possibly the main method applied [71]. Planting two or three varieties in an orchard was quite common to allow a staged harvest, to combine oil and table olive varieties, and to improve the pollination process [25]. These poly-varietal OT orchards gave rise to seeds with higher genetic diversity.
5. Discussion
5.1. Reduced vs. Intense Circulation of Plant Material
The distribution patterns of DW and OT LRs in Spain are clearly different. While OT LRs clustered into two well-defined genetic groups, DW LRs did not exhibit a structured distribution pattern. In OT, the groups corresponding to the LRs from southern and eastern Spain are genetically different [31]. The eastern varieties (Aragon, Catalonia, Valencia, and the Balearic Islands), of which Arbequina is a good example, are very similar to those from Italy and Greece and likely originated from local selection from natural crosses of varieties introduced in Roman times or crossed with local oleasters [73]. This central Mediterranean Basin group might have originated from a minor local domestication event or from crossing between precultivated forms and local oleaster. The latter case, spontaneous crossing with ancient olive varieties, may have played an important role in the current genetic diversity of the cultivated olive, especially in LRs from the central Mediterranean Basin [31]. Remarkably, LRs from southern Spain (Andalusia and Extremadura), of which Picual, Hojiblanca, or Manzanilla de Sevilla are representative, are more closely related to those from North Africa and the eastern Mediterranean Basin, likely originating during the Islamic expansion of the Iberian Peninsula between the 8th and 15th centuries. Although the first cultivated olives in southern Spain could be derived from eastern varieties (from the westward spread by Phoenicians, Greeks, and Romans), this material could be largely replaced by North African varieties introduced during the Islamic expansion to southern Spain [4,73]. The extraordinary lifespan of olive and its occasional replacement using large propagules from the same orchard may have guaranteed the maintenance of the traditional OT LRs over time [68]. Actually, the spread of LRs outside of their original cultivation areas was marginal until the development of the nursery industry in the 1970s. Historical evidence of long-distance movement of OT LRs is not abundant, and it is especially related to their grafting onto oleasters or previous LRs [31,79].
In contrast, DW LRs from Spain originated from intense circulation of genotypes from the western Mediterranean and within the country itself, especially in the 16–19th centuries, as described by Pascual et al. [36]. The need for a significant number of seeds to sow annually (approximately 100 kg/ha, ~20% of the harvest) and the stresses to which the production was subjected (e.g., droughts, causing losses that prevented the sowing of the following season) led to high circulation of plant material and a high probability of genotype mixing. The seeds of DW LRs collected during the different missions of the early and mid-20th centuries mirrored that diversity.
The definition of a landrace as a living and evolutionary entity [38] is clear in the case of DW. However, in OT, the genotypic variation in the LRs over time and space was very small, preserving the genetic identity and stability of the material until well into the 20th century. In this sense, the genetic circularity level of a landrace (GCLL) as a concept could be coined. The GCLL represents the genetic and genotypic changes in a landrace over time before the advent of modern plant breeding. This change depends on various crop factors: herbaceous (annual) or woody (perennial), cross or self-pollinated crop, the existence of wild relatives for spontaneous crosses, physical seed or clone mixing with other species, seed production rate, etc. In the present study, the GCLLs of the two crops were different, being high for DW and low for OT.
5.2. The Genetic Structure of Spanish LRs of Other Mediterranean Crops
There have been studies on the genetic structure of Spanish LRs of other crops. The distribution of einkorn (Triticum monococcum L.) LRs in the Mediterranean Basin distinguished accessions from Morocco and the Iberian Peninsula from those from the rest of Europe and the Near East, suggesting that each group had its own regional dynamics [80]. In an analysis of the core collection of Spanish barley LRs, southern LRs grouped with eastern ones [81], and both groups of LRs also resembled those of the western Mediterranean Rim, specifically those of Morocco, which can itself be considered a secondary center of barley diversity [82]. In a study on DNA markers in Mediterranean (albeit mainly Greek) melon LRs, Staub et al. [83] found that some LRs of the Inodorus group grouped with Spanish ‘Piel de Sapo’ LRs, suggesting an exchange between Mediterranean regions. Ganopoulos et al. [20] found two genetic groups in fig tree LRs from the Mediterranean Rim, although in this case, they did not correspond exactly to geographical origin. One group had entries from Greece, Cyprus, and Italy, while the other also had entries from these countries plus Spain, France, and Turkey. In addition, synonymy was identified between the Greek landrace ‘Rigota’ and the Spanish LRs ‘Mission 1’ and ‘Mission 2’. Likewise, Pérez-Jiménez et al. [84], while characterizing the LRs of fig trees in southern Spain, found that some of the white early figs showed genetic similarity with a French LR. Cericola et al. [85] genotyped a set of 238 accessions of eggplant from the Mediterranean Basin and Asia. Although Spanish LRs clustered with their Mediterranean counterparts, the genetic correlation was lower than the phenotypic correlation, and in fact, some Spanish accessions also clustered genetically with Asian LRs, a feature shared by those of other Mediterranean countries.
All these examples show that the agricultural heritage of Spain (especially the south and the east) maintained close links with Mediterranean agriculture until well into the 20th century, especially on the western side. An example is the case of the melon, as it is known today (sweet and globose). This type of melon first arrived in Spain (Andalusia) in the second half of the 11th century, coming from Central Asia (Khorasan and Persia), as a consequence of the trade routes and the agricultural development of the first Islamic expansion. From Andalusia, the melon spread to the rest of Europe, such as Italy [86].
6. Conclusions
The methodology used in this study was simple but effective in unearthing the origin of LRs of DW and OT in Spain but could also be used for other crops in other countries or regions. The aim was to combine insight from genetic structure analysis of LRs using DNA markers with information from historical records of movement or circulation of varieties or plant material in a given country or region. Two crops where the GCLL was different were examined. In DW, where the seeding rate was high, the recurrent lack of seeds in particular areas of Spain made it necessary to obtain new seeds with neighboring or more distant origins, which may have belonged to a different genotype, giving rise to genetic change in the landrace population. In OT, the longevity and large size of the propagation material caused much slower circulation of plant material, leading to persistence in a certain area over a long period. It would be interesting to know the GCLL of other crops with historical importance in Spain, such as barley, melon, eggplant, fava bean, common grape vine, orange tree, or fig tree. In addition, new lines of research have been opened to determine the relationships between varieties and historical periods, to understand not only their geographic–genetic closeness relationship but also their historical similarity.
Author Contributions
Conceptualization, F.M.-M.; methodology, F.M.-M., J.R.G.-Á., C.M.D. and P.R.; validation, J.R.G.-Á. and P.R.; resources, C.M.D.; data curation, F.M.-M., J.R.G.-Á., C.M.D. and P.R.; writing—original draft preparation, F.M.-M.; writing—review and editing, J.R.G.-Á., C.M.D. and P.R.; visualization, F.M.-M. and C.M.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Acknowledgments
The authors acknowledge J.M. Soriano for his assistance towards making the durum wheat part of Figure 5, and I. Solís and D. Barranco for providing the photographs of durum wheat and olive tree, respectively.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| durum wheat | (DW) |
| olive tree | (OT) |
| Landrace | (LR) |
| genetic circularity level of a landrace | (GCLL) |
References
- MAPA (Ministerio de Agricultura, Pesca y Alimentación), Anuario de Estadística. Available online: https://www.mapa.gob.es/es/estadistica/temas/publicaciones/anuario-de-estadistica/default.aspx (accessed on 26 April 2023).
- Zohary, D.; Hopf, M.; Weiss, E. Domestication of Plants in the Old World: The Origin and Spread of Cultivated Plants in West Asia, Europe, and the Nile Valley, 4th ed.; Oxford University Press: Oxford, UK, 2012; pp. 41–51, 116–121. [Google Scholar]
- Martínez-Moreno, F.; Solís, I.; Noguero, D.; Blanco, A.; Özberk, İ.; Nsarellah, N.; Elias, E.; Mylonas, I.; Soriano, J.M. Durum wheat in the Mediterranean Rim: Historical evolution and genetic resources. Genet. Resour. Crop Evol. 2020, 67, 1415–1436. [Google Scholar] [CrossRef]
- Jiménez-Ruiz, J.; Ramírez-Tejero, J.A.; Fernández-Pozo, N.; Leyva-Pérez, M.d.l.O.; Yan, H.; Rosa, R.d.l.; Belaj, A.; Montes, E.; Rodríguez-Ariza, M.O.; Navarro, F.; et al. Transposon activation is a major driver in the genome evolution of cultivated olive trees (Olea europaea L.). Plant Genome 2020, 13, e20010. [Google Scholar] [CrossRef]
- Peña-Chocarro, L. Prehistoric Agriculture in Southern Spain during the Neolithic and the Bronze Age; the Application of Ethnographic Models; British Archaeological Reports International Series; BAR Publishing: Oxford, UK, 1995; pp. 86–90. [Google Scholar]
- Frankin, S.; Roychowdhury, R.; Nashef, K.; Abbo, S.; Bonfil, D.J.; Ben-David, R. In-field comparative study of landraces vs. modern wheat genotypes under a Mediterranean climate. Plants 2021, 10, 2612. [Google Scholar] [CrossRef]
- Royo, C.; Briceño-Félix, G.A. Wheat breeding in Spain. In The World Wheat Book: A History of Wheat Breeding; Bonjean, A.P., Angus, W.J., van Ginkel, M., Eds.; Lavoisier: Paris, France, 2011; Volume 2, pp. 121–154. [Google Scholar]
- Strabo. Geography; books III–IV; Meana, M.J.; Piñero, F., Translators; Gredos: Madrid, Spain, 1992. [Google Scholar]
- Moragues, M.; Moralejo, M.; Sorrells, M.E.; Royo, C. Dispersal of durum wheat [Triticum turgidum L. ssp. turgidum convar. durum (Desf.) MacKey] landraces across the Mediterranean basin assessed by AFLPs and microsatellites. Genet. Resour. Crop Evol. 2007, 54, 1133–1144. [Google Scholar] [CrossRef]
- Breton, C.; Terral, J.F.; Pinatel, C.; Médail, F.; Bonhomme, F.; Bervillé, A. The origins of the domestication of the olive tree. Comptes Rendus-Biol. 2009, 332, 1059–1064. [Google Scholar] [CrossRef]
- Belaj, A.; Muñoz-Diez, C.; Baldoni, L.; Satovic, Z.; Barranco, D. Genetic diversity and relationships of wild and cultivated olives at regional level in Spain. Sci. Hortic. 2010, 124, 323–330. [Google Scholar] [CrossRef]
- Pliny, (the Elder). Natural History; Books 12–16; Manzanero, F.; García, I.; Arribas, M.L.; Moure, A.M.; Sancho, J.L., Translators; Gredos: Madrid, Spain, 2010; Volume 15, pp. 1–8. [Google Scholar]
- Cato, (the Elder). On Farming; Dalby, A., Translator; Prospect Books: London, UK, 1998; pp. 1–148. [Google Scholar]
- Remesal, J. El aceite bético en el Imperio Romano. In Tierras del Olivo; Páez, J., Remesal, J., Parras, M., Mataix, J., Gaforio, J.J., Rallo, L., Castro, I., Viguera, M.J., Guzmán-Álvarez, J.R., Sánchez, V., Eds.; Fundación El Legado Andalusí: Granada, Spain, 2007; pp. 67–81. [Google Scholar]
- Guzmán Álvarez, J.R. El Palimpsesto Cultivado. Historia de los Paisajes del Olivar Andaluz; Junta de Andalucía: Sevilla, Spain, 2004; pp. 37–43. Available online: https://www.juntadeandalucia.es/export/drupaljda/1337165052El_Palimpsesto_cultivado.pdf (accessed on 26 April 2023).
- Tahiri, A. Agricultura y Poblamiento Rural en Sevilla Durante la Época ‘Abâdî; Pub. Ayuntamiento de Sevilla: Sevilla, Spain, 2001; pp. 180–199. [Google Scholar]
- IOC (International Olive Council). Available online: https://www.internationaloliveoil.org/ (accessed on 26 April 2023).
- Martínez-Moreno, F.; Solís, I. Decrease in durum wheat area and varietal change in Spain from 1888–1963. Int. J. Agric. Nat. Resour. 2022, 49, 123–129. [Google Scholar] [CrossRef]
- Zambrana, J.F. Crisis y Modernización del Olivar Español, 1870–1930; Ministerio de Agricultura, Pesca y Alimentación: Madrid, Spain, 1987; pp. 397–405. [Google Scholar]
- Gadea, M. Trigos Españoles; Instituto Nacional de Investigaciones Agronómicas: Madrid, Spain, 1954; pp. 237–346. [Google Scholar]
- Rallo, L. Variedades de olivo en España: Una aproximación cronológica. In Variedades de Olivo en España; Rallo, L., Barranco, D., Caballero, J.M., Del Río, C., Martín, A., Tous, J., Trujillo, I., Eds.; Mundi-Prensa: Madrid, Spain, 2005; pp. 15–44. [Google Scholar]
- Martínez-Moreno, F.; Solís, I. Evolución histórica de variedades de trigo duro en España. Vida Rural 2017, 9, 60–66. [Google Scholar]
- CRF (Centro Nacional de Recursos Fitogenéticos, INIA). Available online: https://www.inia.es/serviciosyrecursos/Recursos%20gen%C3%A9ticos/fitogeneticos/Paginas/Home.aspx (accessed on 26 April 2023).
- Sánchez-Monge, E. Catálogo Genético de Trigos Españoles; Ministerio de Agricultura: Madrid, Spain, 1957; pp. 572–807. Available online: https://digital.csic.es/bitstream/10261/126861/1/Sanchez-MongeE_CatGenTrigEsp_1957.pdf (accessed on 26 April 2023).
- Rallo, L.; Barranco, D.; Díez, C.M.; Rallo, P.; Suárez, M.P.; Trapero, C.; Pliego-Alfaro, F. Strategies for olive (Olea europaea L.) breeding: Cultivated genetic resources and crossbreeding. In Advances in Plant Breeding Strategies: Fruits; Al-Khayri, J.M., Ed.; Springer International Publishing AG: New York, NY, USA, 2018; pp. 535–600. [Google Scholar]
- Belaj, A.; Ninot, A.; Gómez-Gálvez, F.J.; El Riachy, M.; Gurbuz-Veral, M.; Torres, M.; Lazaj, A.; Klepo, T.; Paz, S.; Ugarte, J.; et al. Utility of EST-SNP Markers for Improving Management and Use of Olive Genetic Resources: A Case Study at the Worldwide Olive Germplasm Bank of Córdoba. Plants 2022, 11, 921. [Google Scholar] [CrossRef]
- Barranco, D.; Rallo, L.; Trujillo, I. Elaiografía hispánica. In Variedades de Olivo en España; Rallo, L., Barranco, D., Caballero, J.M., Del Río, C., Martín, A., Tous, J., Trujillo, I., Eds.; Mundi-Prensa: Madrid, Spain, 2005; pp. 80–231. [Google Scholar]
- Trujillo, I.; Ojeda, M.A.; Urdiroz, N.M.; Potter, D.; Barranco, D.; Rallo, L.; Diez, C.M. Identification of the Worldwide Olive Germplasm Bank of Córdoba (Spain) using SSR and morphological markers. Tree Genet. Genomes 2014, 10, 141–155. [Google Scholar] [CrossRef]
- Soriano, J.M.; Villegas, D.; Aranzana, M.J.; García Del Moral, L.F.; Royo, C. Genetic structure of modern durum wheat cultivars and mediterranean landraces matches with their agronomic performance. PLoS ONE 2016, 11, e0160983. [Google Scholar] [CrossRef] [PubMed]
- Maccaferri, M.; Harris, N.S.; Twardziok, S.O.; Pasam, R.K.; Gundlach, H.; Spannagl, M.; Ormanbekova, D.; Lux, T.; Prade, V.M.; Milner, S.G.; et al. Durum wheat genome highlights past domestication signatures and future improvement targets. Nat. Genet. 2019, 51, 885–895. [Google Scholar] [CrossRef] [PubMed]
- Diez, C.M.; Trujillo, I.; Martinez-Urdiroz, N.; Barranco, D.; Rallo, L.; Marfil, P.; Gaut, B.S. Olive domestication and diversification in the Mediterranean Basin. New Phytol. 2015, 206, 436–447. [Google Scholar] [CrossRef]
- Julca, I.; Marcet-Houben, M.; Cruz, F.; Gómez-Garrido, J.; Gaut, B.S.; Díez, C.M.; Gut, I.G.; Alioto, T.S.; Vargas, P.; Gabaldón, T. Genomic evidence for recurrent genetic admixture during the domestication of Mediterranean olive trees (Olea europaea L.). BMC Biol. 2020, 181, 148. [Google Scholar] [CrossRef]
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of population structure using multilocus genotype data. Genetics 2000, 155, 945–959. [Google Scholar] [CrossRef]
- Melnikova, N.V.; Mitrofanova, O.P.; Liapounova, O.A.; Kudryavtsev, A.M. Global diversity of durum wheat Triticum durum Desf. for alleles of gliadin-coding loci. Russ. J. Genet. 2010, 46, 43–49. [Google Scholar] [CrossRef]
- Ruiz, M.; Giraldo, P.; Royo, C.; Villegas, D.; Jose Aranzana, M.; Carrillo, J.M. Diversity and genetic structure of a collection of Spanish durum wheat landraces. Crop Sci. 2012, 52, 2262–2275. [Google Scholar] [CrossRef]
- Pascual, L.; Ruiz, M.; López-Fernández, M.; Pérez-Penã, H.; Benavente, E.; Vázquez, J.F.; Sansaloni, C.; Giraldo, P. Genomic analysis of Spanish wheat landraces reveals their variability and potential for breeding. BMC Genom. 2020, 21, 122. [Google Scholar] [CrossRef]
- Alonso de Herrera, G. Agricultura General; Real Sociedad Económica Matritense: Madrid, Spain, 1818; (original published in 1513); pp. 70–89. Available online: https://bibdigital.rjb.csic.es/records/item/9699-agricultura-general-de-gabriel-alonso-de-herrera-tomo-i (accessed on 26 April 2023).
- Casañas, F.; Simó, J.; Casals, J.; Prohens, J. Toward an evolved concept of landrace. Front. Plant Sci. 2017, 8, 145. [Google Scholar] [CrossRef] [PubMed]
- Zeven, A.C. Landraces: A review of definitions and classifications. Euphytica 1998, 104, 127–139. [Google Scholar] [CrossRef]
- Vidal, J.J. El comercio del trigo entre Mallorca y África del Norte en los siglos XVI y XVII. Mayurca 1976, 15, 73–92. Available online: https://raco.cat/index.php/Mayurqa/article/view/117582 (accessed on 26 April 2023).
- Borrero, M. Crisis de cereales y alzas de precios en la Sevilla de la primera mitad del siglo XVI. Hist. Inst. Doc. 1991, 18, 39–56. [Google Scholar]
- Montojo, V.M. The trade with Granada’s Kingdom: Activity of Merchants of Cartagena (16th–17th centuries). In El Reino de Granada en el Siglo XVII; Sánchez, V., Ed.; Instituto de Estudios Almerienses, Diputación de Almería: Almeria, Spain, 1997; pp. 237–252. [Google Scholar]
- Iodice, A.; Piccinno, L. Whatever the cost: Grain trade and the Genoese dominating minority in Sicily and Tabarka (16th–18th centuries). Bus. Hist. 2021. [Google Scholar] [CrossRef]
- De Cillis, E. I Grani d’Italia; Ministero Dell’economia Nazionale: Roma, Italy, 1927; pp. 119–132. [Google Scholar]
- Blanes, R. Mercaderes italianos en las importaciones marítimas valencianas en el segundo cuarto del seiscientos (1626–1650). In I Coloquio Internacional “Los Extranjeros en la España Moderna”; Villar, M.B., Pezzi, P., Eds.; Ministerio de Ciencia e Innovación: Málaga, Spain, 2003; Volume 1, pp. 217–227. [Google Scholar]
- Martínez-Ruiz, J.I. El mercado internacional de cereales y harinas y el abastecimiento de la periferia española en la segunda mitad del siglo XVIII: Cádiz, entre la regulación y el mercado. Investig. Hist. Económica 2005, 1, 45–79. [Google Scholar] [CrossRef]
- Montojo, V.M. El comercio de Alicante a mitad del siglo XVII según los derechos y sisas locales de 1658–1662 y su predominio sobre el de Cartagena. Murgetana 2010, 122, 43–66. [Google Scholar]
- Martínez, M. Producción y comercio de cereales en Lorca durante la Baja Edad Media. Anu. Estud. Mediev. 1989, 19, 635–667. [Google Scholar]
- Barrio, J.A. La producción, el consumo y la especulación de los cereales en una ciudad de frontera, Orihuela, siglos XIII–XV. In Alimentar la Ciudad en la Edad Media/Encuentros Internacionales del Medievo; Arízaga, B., Solórzano, J.A., Eds.; Instituto de Estudios Riojanos: Logroño, Spain, 2009; pp. 59–86. [Google Scholar]
- González, M. Las crisis cerealistas en Carmona a fines de la Edad Media. Hist. Inst. Doc. 1976, 3, 283–308. [Google Scholar] [CrossRef]
- Barriendos, M.; Llasat, M.C. The Case of the ‘Maldá’ Anomaly in the Western Mediterranean Basin (AD 1760–1800): An Example of a Strong Climatic Variability. Clim. Change 2003, 61, 191–216. [Google Scholar] [CrossRef]
- Alberola-Romá, A. Tiempo, clima y enfermedad en la prensa española de la segunda mitad del siglo XVIII: Diarios meteorológicos y crónicas de desastres en el. Meml. Lit. el Argon. Español 2015, 12, 1–25. Available online: http://argonauta.revues.org/2142 (accessed on 26 April 2023). [CrossRef]
- García-Torres, A. Extremismo climático y peligro biológico en el sureste español (1780–1800). Rev. de Hist. Mod. An. de la Univ. de Alicante 2017, 35, 345–376. [Google Scholar] [CrossRef]
- Martínez-Moreno, F.M.; Solís, I.; Barriendos, M.; Tejedor, E. Correlations between historical climate data and incidents of common bunt in Spanish wheat, 1755–1801. Hist. Agrar. 2020, 82, 68–97. [Google Scholar] [CrossRef]
- Muñoz, J. Riesgo y Catástrofe: El Impacto de las Plagas de Langosta en la España de Finales del Siglo XVIII (1770–1800). Ph.D. Thesis, Universidad de Alicante, Alicante, Spain, 2019. Available online: https://rua.ua.es/dspace/bitstream/10045/90648/1/tesis_jesus_munoz_pertierra.pdf (accessed on 26 April 2023).
- Barquín, R. El precio del trigo en España (1814–1883). Hist. Agrar. 1999, 17, 177–218. Available online: https://www.historiaagraria.com/en/issues/rafael-barquin-gil-el-precio-del-trigo-en-espana-1814-1883 (accessed on 26 April 2023).
- Otte, E. El comercio exterior andaluz a fines de la Edad Media. In Actas del II Coloquio de Historia Medieval Andaluza; Hacienda y Comercio: Sevilla, Spain, 1981. [Google Scholar]
- Escribano, J.M. El trigo de la discordia. Antequera frente a la administración militar a principios del siglo XVI. Chron. Nov. 2018, 44, 243–286. Available online: https://hdl.handle.net/10481/58713 (accessed on 26 April 2023).
- Rivero, J. Los Cambios Técnicos del Cultivo de Cereal en España (1800–1930); Ministerio de Agricultura, Alimentación y Medio Ambiente: Madrid, Spain, 2013; pp. 694–697. [Google Scholar]
- Jaradat, A.A. Wheat landraces: A mini review. Emir. J. Food Agric. 2013, 25, 20–29. [Google Scholar] [CrossRef]
- Raman, H.; Stodart, B.J.; Cavanagh, C.; MacKay, M.; Morell, M.; Milgate, A.; Martin, P. Molecular diversity and genetic structure of modern and traditional landrace cultivars of wheat (Triticum aestivum L.). Crop Pasture Sci. 2010, 61, 222–229. [Google Scholar] [CrossRef]
- Díez, C.M.; Gaut, B.S. The jury may be out, but it is important that it deliberates: A response to Besnard and Rubio de Casas about olive domestication. New Phytol. 2016, 209, 471–473. [Google Scholar] [CrossRef]
- Besnard, G.; Rubio de Casas, R. Single vs. multiple independent olive domestications: The jury is (still) out. New Phytol. 2016, 209, 466–470. [Google Scholar] [CrossRef]
- Lumaret, R.; Ouazzani, N.; Michaud, H.; Vivier, G.; Deguilloux, M.-F.F.; Di Giusto, F. Allozyme variation of oleaster populations (wild olive tree) (Olea europaea L.) in the Mediterranean Basin. Heredity 2004, 92, 343–351. [Google Scholar] [CrossRef]
- Unver, T.; Wu, Z.; Sterck, L. Genome of wild olive and the evolution of oil biosynthesis. Proc. Natl. Acad. Sci. USA 2017, 114, E9413–E9422. [Google Scholar] [CrossRef]
- Fontanazza, G. Aspectos genéticos y técnicas de propagación para una plantación intensiva. In Enciclopedia Mundial del Olivo; Consejo Oleícola Internacional: Barcelona, Spain, 1996; pp. 111–144. Available online: https://www.internationaloliveoil.org/product/enciclopedia-mundial-del-olivo/?lang=es (accessed on 26 April 2023).
- Etiam, D. El cultivo del olivo en la Antigua Israel. In Enciclopedia Mundial del Olivo; Consejo Oleícola Internacional: Barcelona, Spain, 1996; pp. 36–41. [Google Scholar]
- Arnan, X.; López, B.C.; Martínez-Vilalta, J.; Estorach, M.; Poyatos, R. The age of monumental olive trees (Olea europaea) in northeastern Spain. Dendrochronologia 2012, 30, 11–14. [Google Scholar] [CrossRef]
- Bombarely, A.; Doulis, A.G.; Lambrou, K.K.; Zioutis, C.; Margaritis, E.; Koubouris, G. Elucidation of the Origin of the Monumental Olive Tree of Vouves in Crete, Greece. Plants 2021, 10, 2374. [Google Scholar] [CrossRef] [PubMed]
- Del Río, J. La transformación ecológica indiana. In La Agricultura Viajera. Cultivos y Manufactureras de Plantas Industriales y Alimentarias en España y en la América Virreinal; Fernández, J., González, I., Eds.; Real Jardín Botánico CSIC: Barcelona, Spain, 1990; p. 24. [Google Scholar]
- Guzmán-Álvarez, J.R.; Hernández, P.; Gómez, J.A.; Lora, A. Olivares de España. Recorrido por la Biografía del Olivar, su Memoria y sus Paisajes; Almuzara: Córdoba, Spain, 2020; pp. 59–69. [Google Scholar]
- Cubero, J.I. El Libro de Agricultura de Al Awam; Consejería de Agricultura y Pesca, Junta de Andalucía: Seville, Spain, 2003; pp. 757–792. [Google Scholar]
- Guzmán-Álvarez, J.R. Los Paisajes del Olivar en Andalucía; Diputación de Jaén: Jaén, Spain, 2018; Volume 2, pp. 725–734. Available online: https://www.dipujaen.es/export/files/paisajes-del-olivar/propuesta-POAs-Vol2-anexos.pdf (accessed on 26 April 2023).
- Alcalá-Zamora, P. Observaciones sobre el beneficio de la aceituna. Sem. Ind. 1840, 2, 1–10. [Google Scholar]
- Alberola y Serra, E. Olive grove situation in Valencia. In Proceedings of the VII Congreso Internacional de Oleicultura, Sevilla, Spain, 5–9 December 1924; p. 119. [Google Scholar]
- Anonymous. Carta de un párroco del obispado de Solsona. Sem. de Agric. y Otras Artes Dirigido a los Párrocos 1797, 2, 340. [Google Scholar]
- Díaz de Mendivil, J.M. Olive grove situation in Alava. In Proceedings of the VII Congreso Internacional de Oleicultura, Sevilla, Spain, 5–9 December 1924; p. 550. [Google Scholar]
- Sousa, A.; Garcia-Murillo, P. Changes in the wetlands of Andalusia (Doñana Natural Park, SW Spain) at the end of the Little Ice Age. Clim. Change 2003, 58, 193–217. [Google Scholar] [CrossRef]
- Sandalio de Arias, A. Adiciones a la Agricultura General. In Agricultura General; Alonso de Herrera, G. Real Sociedad Económica Matritense: Madrid, Spain, 1818; (original published in 1513); Volume 2, p. 349. Available online: https://bibdigital.rjb.csic.es/records/item/9699-agricultura-general-de-gabriel-alonso-de-herrera-tomo-i (accessed on 26 April 2023).
- Oliveira, H.R.; Jones, H.; Leigh, F.; Lister, D.L.; Jones, M.K.; Peña-Chocarro, L. Phylogeography of einkorn landraces in the Mediterranean basin and Central Europe: Population structure and cultivation history. Archaeol. Anthropol. Sci. 2011, 3, 327–341. [Google Scholar] [CrossRef]
- Yahiaoui, S.; Igartua, E.; Moralejo, M.; Ramsay, L.; Molina-Cano, J.L.; Ciudad, F.J.; Lasa, J.M.; Gracia, M.P.; Casas, A.M. Patterns of genetic and eco-geographical diversity in Spanish barleys. Theor. Appl. Genet. 2008, 116, 271–282. [Google Scholar] [CrossRef]
- Igartua, E.; Moralejo, M.; Casas, A.M.; Torres, L.; Molina-Cano, J.L. Whole-genome analysis with SNPs from BOPA1 shows clearly defined groupings of Western Mediterranean, Ethiopian, and Fertile Crescent barleys. Genet. Resour. Crop Evol. 2013, 60, 251–264. [Google Scholar] [CrossRef]
- Staub, J.E.; López-Sesé, A.I.; Fanourakis, N. Diversity among melon landraces (Cucumis melo L.) from Greece and their genetic relationships with other melon germplasm of diverse origins. Euphytica 2004, 136, 151–166. [Google Scholar] [CrossRef]
- Perez-Jiménez, M.; López, B.; Dorado, G.; Pujadas-Salvá, A.; Guzmán, G.; Hernandez, P. Analysis of genetic diversity of southern Spain fig tree (Ficus carica L.) and reference materials as a tool for breeding and conservation. Hereditas 2012, 149, 108–113. [Google Scholar] [CrossRef]
- Cericola, F.; Portis, E.; Toppino, L.; Barchi, L.; Acciarri, N.; Ciriaci, T.; Sala, T.; Rotino, G.L.; Lanteri, S. The population structure and diversity of eggplant from Asia and the Mediterranean Basin. PLoS ONE 2013, 8, e73702. [Google Scholar] [CrossRef]
- Paris, H.S.; Amar, Z.; Lev, E. Medieval emergence of sweet melons, Cucumis melo (Cucurbitaceae). Ann. Bot. 2012, 110, 23–33. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).