Abstract
Treatments that increase the germination potential and vigor of Glycine max seedlings are continuously being stimulated, with the aim of achieving a higher percentage of emergence and better performance in the field. Considering the relationship of tryptophan with germination-associated phytohormones, this study tested the hypothesis that exogenous supply of tryptophan to soybean seeds can affect germination, physiological vigor, and the accumulation of primary and oxidative metabolism molecules in seedlings. Soybean seeds were exposed to soaking solutions containing different concentrations of the amino acid (0, 25, 50, 100, and 200 µM), and the seedlings were evaluated at three time periods, at 8 h after sowing (HAS), and 5 and 14 days after sowing (DAS). Treated seeds showed better germination fitness and seedlings showed greater vigor, and these parameters increased with increasing concentrations of tryptophan. In the initial hours and days of germination process evaluation (14 HAS and 5 DAS), the activities of starch metabolism enzymes (α- and β-amylase) tended to be higher, resulting in increased contents of sucrose, reducing sugars, and total soluble solids at 8 DAS, constituting an important metabolic effect for seedling growth. On the other hand, the induction of germination and vigor promoted by exogenous tryptophan in soybean seedlings occurred by stimulating the metabolic pathways of oxidative stress, resulting in increased concentrations of H2O2, malondialdehyde, and proline in the tissues. Additionally, it led to increased activities of the antioxidant enzymes superoxide dismutase and ascorbate peroxidase. These parameters were responsive to increasing supplied concentrations of tryptophan. Thus, the metabolic stress in soybean seeds induced by auxin seems to be an important inductive pathway for germination and vigor of G. max seeds.
1. Introduction
Research is being continuously conducted to find treatments that can enhance the vigor of soybean seeds. The goal is to achieve a higher percentage of seed emergence in cultivable environments and promote the uniform development of seedlings. This, in turn, results in stronger seedlings that exhibit superior performance under field conditions [1,2,3]. However, it is worth noting that seed development involves highly coordinated yet complex signaling pathways associated with reserve mobilization, cell elongation and division, and elimination of reactive oxygen species (ROS) [4,5]. This signaling stimulates the growth of the embryonic axis, leading to the development of a seedling from the seed that is in a latent/dormant state and comprises an ordered sequence of metabolic activities initiated with soaking [6]. The metabolic activation induced by water input decreases the stiffness of the integument, intensifies respiratory rate, gas exchange, transcription and translation of new mRNAs, and synthesis and activity of enzymes and hormones [7].
The activation of enzymes during soaking is associated with the metabolism of primary molecules. Thus, there is a reduction in the starch content of the endosperm and an increase in the levels of soluble sugars, such as glucose, fructose, and maltose [8,9]. These important carbohydrates ensure the energy levels necessary for the germination process to occur. On the other hand, breaking dormancy by water uptake triggers biochemical and cellular events accompanied by the generation of ROS (especially H2O2 and malondialdehyde—MDA) [10,11,12]. These ROS appear to play an important role in the mobilization of reserves through oxidative modifications of proteins. Thus, the seed proteome and transcriptome can be remodeled by selective oxidation, causing reserves to be reallocated to the fast-growing axis and embryo, stimulating germination [13].
In addition to primary molecule and oxidative metabolism, phytohormones also play important roles in the germination process. Among them, auxins act directly on the mechanisms of elongation and cell division, allowing the growth of the embryonic axis [14]. However, the direct application of phytohormones as germination inducers is highly questioned, since small imbalances can result in drastic effects that compromise seedling establishment [15]. Thus, this study tested the hypothesis that exogenous supply of tryptophan, which is the main precursor of the IAA biosynthesis pathways, to G. max seeds can affect germination, physiological vigor, and the accumulation of primary and oxidative metabolism molecules in seedlings. Tryptophan has been indicated as a mitogenic factor, which regulates cell proliferation through serotonin [16], modulating plant growth, development, and morphogenesis [17,18,19].
Research has confirmed that tryptophan can increase IAA levels in plant tissues and ensure growth of various crops [20,21,22,23,24,25]. However, these studies have only considered germination under different conditions, without metabolic evaluations. In a broader analysis, Mustafa et al. [26] suggested that seed biopriming with tryptophan may constitute a strategy to increase the yield of agricultural crops, as this amino acid is directly related to germination potential, growth, induction of the enzymatic antioxidant system, and biosynthesis of osmoregulatory molecules in plants. In addition to its relationship with the elevation of tissue auxin levels, Roychoudhury [27] demonstrated that tryptophan can be hydroxylated in the presence of tryptophan-5-hydroxylase to form 5-hydroxytryptophan. Furthermore, the aromatic amino acid decarboxylase enzyme converts 5-hydroxytryptophan into serotonin. Serotonin has been identified as a molecule that modulates cellular metabolism in plants, being involved in various morphophysiological processes [28], including stress responses, growth, and development [29]. Studies highlight its role in shoot organogenesis, root architecture, growth, flowering, senescence, and defense responses [29,30,31].
Considering this relationship of tryptophan with phytormones, it is possible that this amino acid plays a role in signaling processes and regulation of plant metabolism. Thus, aiming to provide a treatment that results in higher germination and vigor of soybean seeds, and seeking to increase the understanding about the actions of tryptophan on seed biochemistry of the germination process, this study sought to understand the effect of different concentrations on the number of normal seedlings, vigor, growth, and synthesis of primary and oxidative metabolism molecules in G. max seedlings.
2. Materials and Methods
2.1. Preparation of Plant Material, Germination, and Seedling Vigor and Growth
The tests were conducted in the Biodiversity Metabolism and Genetics Laboratory in partnership with the Seed Laboratory of the Instituto Federal Goiano—Rio Verde Campus, with the geographical coordinates 17°48′15.9″ S—50°54′19.5″ W. Glycine max seeds of cultivar BÔNUS were used, with IPRO—8579 RSF technology, which is adapted to the Brazilian Cerrado region and provides resistance to the four main caterpillars that attack the crop.
The seeds were subjected to five treatments, which consisted of different solutions of the amino acid tryptophan (0, 25, 50, 100, and 200 µM). The basic procedures for sample collection, evaluation, and interpretation of results required by the Seed Analysis Rule [32] were followed. Thus, the seeds were developed on “germitest” type paper, with different concentrations of tryptophan provided as moistening solutions for these papers, applied at a ratio of 2.5 times the mass of the dry paper, at a temperature of 27 °C. The sowing was conducted immediately after the distribution of the solutions on the germination papers using a ruler. The seeds covered in “germitest” were organized in rolls and placed in plastic bags that were properly identified. The bags were kept in a BOD-type germinator at a temperature of 25 ± 1 °C, relative humidity controlled between 90% and 95%, and photoperiod of 12–12 h.
For these evaluations, the seeds were sampled from the first to the eighth day after sowing. Those with radicle protrusion were considered germinated. On the eighth day, total germination was evaluated, considering the percentages of seeds that formed normal seedlings (seedlings with normal development of the hypocotyl and radicle), and abnormal and dead seedlings. The germination speed index was calculated according to Maguire [33], where GSI = E1/N1 + E2/N2 + … + En/Nn. In which: E1, E2, and En—number of normal seedlings computed on the first, second, and last count. N1, N2, and Nn—number of days after test implementation.
Seedling growth was monitored throughout development and biometric evaluations were performed on the eighth day after sowing. Total seedling length (cm) and total fresh weight (g) data were obtained.
2.2. Extraction and Quantification of Biomolecules from Primary Metabolism
For these tests the seeds were sampled at three different periods: 14 h after sowing (HAS), 5 days after sowing (DAS), and 8 days after sowing (DAS). The biomolecules of primary metabolism (Reducing Sugars—RS, Sucrose, Total Soluble Sugars—TSS, and Starch) were extracted from the fresh material that had been previously frozen in liquid nitrogen, ground, and stored in an ultra-freezer at −80 °C. We used 200 mg of the plant material, which had been previously processed in MCW solution (methanol, chloroform, and water). The mixture was left in contact for 24 h, and then the extracts were centrifuged at 1300 rpm for 30 min, with the supernatant collected. For starch extraction, the pellet from the previous extraction was resuspended with 30% perchloric acid [34] and after 24 h, the mixture was centrifuged at 1300 rpm for 30 min. Aliquots of these supernatants were used for the analyses. The concentration of reducing sugars was determined by the dinitrosalicylic acid method [35], the TSS and starch was determined by the anthrone method [36], and the sucrose content was estimated by the difference between the concentration of TSS and reducing sugars. The standard curve for the spectrophotometric determination of these carbohydrates was prepared with D-glucose.
2.3. Extraction and Enzyme Activity of Starch Metabolism
We used 250 mg of plant material macerated with liquid N2 and homogenized in 2000 μL of potassium phosphate buffer (100 mM) (pH 6.8) containing 0.1 mM ethylenediaminetetraacetic acid (EDTA), 5% (w/v) polyvinylpyrrolidone (PVPP), and 1 mM phenylmethylsulfonic fluoride (PMSF). The homogenate was kept overnight for 14 h in the refrigerator at 10 °C and then centrifuged at 12,000× g for 15 min at 4 °C, and the supernatant was used as extract for enzyme determinations.
The activity of α-amylase was determined by adding 250 μL of the obtained supernatant to a reaction medium containing 150 μL of 3 mM CaCl2. The mixture was incubated at 70 °C for 5 min to inactivate β-amylase. A total of 250 μL of 100 mM sodium citrate buffer (pH 5.0), and 125 μL of a 2% starch solution were added to the aliquot of 250 μL of β-amylase inactivation extract. The mixture was then incubated at 30 °C. After 10 min, the reaction was stopped by adding 1 mL of the reading reagent [3,5-dinitrosalicylic acid 1% (DNS), 2M NaOH, potassium sodium tartrate] and heating it to 60 °C for 10 min [37,38,39].
Determination of β-amylase enzyme activity was initiated by adding 250 μL of enzyme extract to a reaction medium containing 153 μL of 0.1 M EDTA for inactivation of α-amylase. A total of 250 μL of α-amylase inactivation extract was added to 250 μL of 100 mM sodium citrate buffer, pH 3.4, and 125 μL of 1% starch solution. The mixture was incubated at 30 °C for 20 min. Subsequently, 1 mL of reading reaction [3,5-dinitrosalicylic acid 1% (DNS), 2M NaOH, potassium sodium tartrate] was added and heated at 60 °C for 10 min, and then diluted as described above.
The reducing sugars formed by the action of α- and β- amylase were quantified by reading the absorbance at 540 nm and calculations performed using the standard curve of maltose 0.5 mg/mL 2% [37,38,39].
2.4. Extraction and Activity of Enzymes of the Antioxidant Metabolism
Enzyme was also extracted from fresh material previously stored in an ultrafreezer. They were macerated from 200 mg, in liquid nitrogen with 50% PVPP, following the protocol proposed by Biemelt et al. [40]. The enzyme extract was used to evaluate the activities of the enzymes catalase (CAT), superoxide dismutase (SOD), ascorbate peroxidase (APX), and guaiacol peroxidase (POD).
SOD activity was determined based on the methodology developed by Giannopolitis and Ries [41], which assesses the enzyme’s ability to inhibit nitro-blue tetrazolium (NBT) photoreduction. Readings were taken in a spectrophotometer at 560 nm. SOD activity was determined in U mg−1 protein, where 1U corresponds to the amount of enzyme required to inhibit NBT photoreduction by 50%. The CAT activity was evaluated according to the methodology proposed by Havir and Mchale [42] based on the consumption of H2O2 every 15 s, for 3 min, at 240 nm in a spectrophotometer. The molar extinction coefficient used was 36 mM−1 cm−1. CAT activity was quantified in µmol H2O2 min−1 mg−1 protein. APX activity was evaluated based on the Nakano and Asada [43] methodology by the consumption of ascorbate every 15 s for 3 min at 280 nm in a spectrophotometer. The molar extinction coefficient used was 2.8 mM−1 cm−1. APX activity was determined in µmol AsA min−1 mg−1 protein. POD activity was determined using the methodology developed by Fang and Kao [44], in which the formation of tetraguaiacol is observed through an increase in absorbance. The molar extinction coefficient used was 26.6 mM−1 cm−1. POD activity was determined in µmol H2O2 min−1 mg−1 protein.
2.5. Extraction and Quantification of Hydrogen Peroxide (H2O2) and Malondialdehyde (MDA) and Proline
To quantify H2O2 and MDA, 200 mg of plant material were macerated in liquid nitrogen and PVPP. The resulting mixture was homogenized in 0.1% (w/v) trichloroacetic acid (TCA), followed by centrifugation at 10,000× g for 15 min at 4 °C. The concentration of H2O2 was determined by reading at 390 nm using a spectrophotometer, following the method described by Velikova et al. [45]. The concentration of MDA was determined using the methodology proposed by Buege and Aust [46], where readings are taken in a spectrophotometer at 535 and 600 nm.
The proline concentration was quantified based on the methodology proposed by Bates et al. [47]. To do this, 100 mg of plant material was macerated in 3% sulfosalicylic acid. The obtained extracts were homogenized at room temperature for 60 min and then filtered with filter paper. The aliquots obtained after filtration were reacted with a solution consisting of 2 mL acid ninhydrin and 2 mL glacial acetic acid in a test tube for 1 h at 100 °C. The concentration of proline was determined according to the absorbance of the samples at 520 nm and based on a standard curve prepared with known concentrations of proline.
2.6. Experimental Design and Statistical Analysis
The experiments were conducted in an entirely randomized design, and the germination, GSI, and growth data were evaluated over 8 days. The concentrations of primary metabolism molecules, enzyme activities and synthesis of H2O2, MDA and proline were evaluated considering a double factorial scheme (5 × 3), i.e., five concentrations of tryptophan (0, 25, 50, 100, and 200 µM) and three evaluation times: 14 HAS, 5 DAS, and 8 DAS. Each treatment was evaluated in 8 repetitions, each one consisting of 25 seeds, totaling 200 experimental units per treatment.
The observed data for the parameters of germination, GSI, growth, and metabolism were subjected to a normality test and analysis of variance (ANOVA), using an F-test at a 5% probability level. To achieve this, the means observed for each repetition were compared independently within tryptophan concentrations and evaluation times, as well as for the interaction concentration × time. In significant cases, regression analysis was conducted to test the effect of amino acid concentrations at the different evaluated times. To better understand the relationship between the exploratory variables and the variables of germination, GSI, growth, and metabolism, they were analyzed together using correlation matrices. Additionally, independent matrices were generated specifically for the amino acid concentration data and the evaluation period. From these matrices, the response variables were combined and subjected to a principal component analysis (PCA). Since these variables had different units of measurement, correlation-based PCAs were performed. The data were standardized by setting the mean to 0 and the standard deviation to 1 before constructing the PCAs. The number of components was chosen based on eigenvalues (>1.0) and explained variance (above 80%). All analyses were conducted using R 4.2.3 software [48].
3. Results
3.1. Germination, GSI, and Growth
The concentrations of exogenous tryptophan affected the percentages of normal and abnormal seedlings obtained from the treated seeds, with the highest mean percentages of normal seedlings, as well as the lowest mean percentages of abnormal seedlings, observed in the seeds treated with concentrations equal to or greater than 50 µM of tryptophan (Figure 1a). These higher means correspond to 83.50, 82.85, and 84.85% normal seedlings for the concentrations of 50, 100, and 200 µM and 12.12, 16.00, and 12.00% abnormal seedlings for these same concentrations, respectively. Conversely, the lowest average percentages of normal seedlings (37%) and the highest percentages of abnormal plants (57%) were observed in the control plants treated with 0 µM tryptophan. However, the average percentage of dead seedlings was not affected by the tryptophan treatments and remained at approximately 4.15% across the different concentrations.
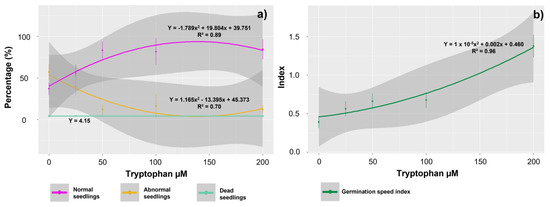
Figure 1.
Germination percentage (%) of Glycine max L. seedlings, determined by the percentage of normal, abnormal, and dead seedlings (a) and Germination speed index (b). Data obtained from seeds treated with different concentrations of tryptophan (0, 25, 50, 100, and 200 µM).
The GSI was also affected by the exogenous tryptophan concentrations, with the highest mean observed in seeds treated with 200 µM of the amino acid (1.27) (Figure 1b).
The tryptophan treatments affected the root and shoot growth of G. max seedlings (Figure 2a–c). The highest averages for both root and shoot were observed in the seeds treated with 50 µM (7.46 and 12.35 cm, respectively). Overall, the seedlings invested more in root than in shoot, with an average ratio (shoot/root) of approximately 1.77 cm (Figure 2b). The exogenous application of the amino acid also affected the biomass accumulation in the shoot of the seedlings and also the shoot/root ratio. The highest weight as well as the lowest ratio was observed in the treatment with 50 µM (respectively 3.26 and 3.78 g), indicating higher root development in seedlings receiving this tryptophan concentration. However, the average root weight was not affected by the amino acid, and was maintained at approximately 2.80 g throughout all concentrations (Figure 2c).
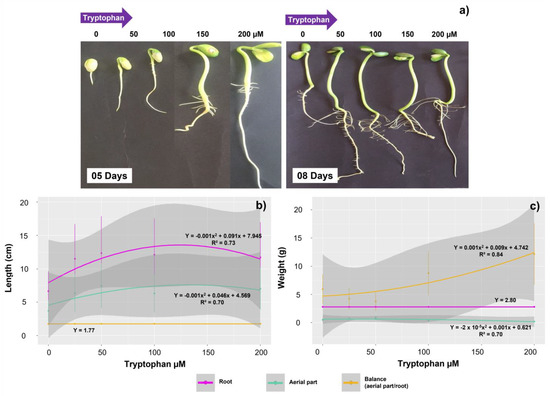
Figure 2.
Growth of Glycine max L. seedlings obtained from seeds treated with different concentrations of tryptophan at 5 and 8 days after sowing (a), length (b), and fresh biomass (c) of the root, aerial part, and shoot/root ratio of the seedlings. Data obtained from seeds treated with the concentrations (0, 25, 50, 100, and 200 µM) of tryptophan.
3.2. Biomolecules of Primary Metabolism
Tryptophan concentrations, evaluation times, and the interaction between these factors affected the reducing sugars content observed in the seedlings. In control seedlings, the highest RA contents were observed at 14 HAS (41.71 µg g−1 DW) and the lowest, at 5 DAS (20.41 µg g−1 DW) (Figure 3a). After exposure to tryptophan, these values began to reverse, with a decreasing trend in the contents with increasing tryptophan concentration at 14 HAS and an increasing trend in RA contents with increasing tryptophan concentrations at 5 DAS. Thus, the lowest RA contents at 14 HAS (19.34 µg g−1 DW) were observed in seedlings treated with 200 µM tryptophan, and the highest RA contents at 5 DAS (41.33 µg g−1 DW) were also observed in seedlings treated with tryptophan at the highest concentration tested. At 8 DAS, the highest mean RA content (50.85 µg g−1 DW) was observed in seedlings treated with 100 µM of the amino acid.
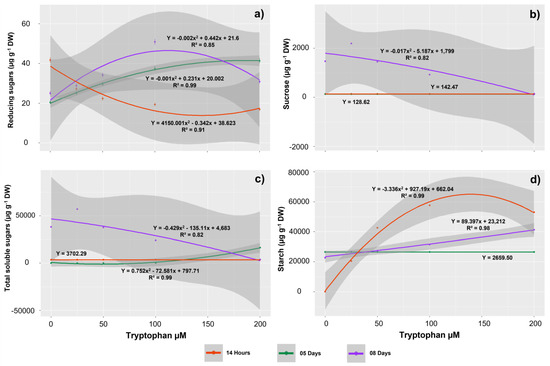
Figure 3.
Contents of the primary metabolism molecules, reducing sugars (RS) (a), sucrose (b), total soluble sugars (TSS) (c), and starch (d) in Glycine max L. seedlings obtained from seeds treated with different concentrations of tryptophan (0, 25, 50, 100, and 200 µM).
Sucrose tissue contents were affected by tryptophan concentrations only in the seedlings evaluated at 8 DAS (Figure 3b). These contents tended to decrease with increasing exposure concentrations, with the lowest average values observed in seedlings treated with 200 µM tryptophan (145.17 µg g−1 DW). Similarly, TSS contents tended to reduce with increasing tryptophan concentrations at 8 DAS, with the lowest content observed at 200 µM tryptophan (3772.77 µg g−1 DW) (Figure 3c). However, in this evaluation period, starch content tended to increase linearly with increasing exposure concentrations, with the highest contents observed in seedlings receiving the highest tryptophan concentration evaluated (41,357.84 µg g−1 DW) (Figure 3d). In the seedlings evaluated at 14 HAS, starch contents also tended to increase with increasing concentrations of tryptophan, with the highest averages observed in seedlings treated with 100 µM (57,637.73 µg g−1 DW).
3.3. Enzyme Activity of Starch Metabolism
Tryptophan concentrations affected the activity of α-amylase enzymes in seedlings evaluated at 14 HAS and 5 DAS. In contrast, β-amylase activity was affected only in seedlings evaluated at 14 HAS. The decrease in activity of starch metabolism enzymes in relation to the increase in tryptophan concentrations at 14 HAS was consistent with the observed increase in starch content in the seedling tissues. Thus, the lowest activities for these enzymes were observed in the seedlings treated with 200 µM (7.52 and 10.68 mg maltose mg−1 protein for α-amylase and β-amylase, respectively) (Figure 4a,b). At 5 DAS, there was a tendency for α-amylase activity to increase in relation to increasing tryptophan concentrations. This observation explains the observed increase in tissue TSS content. Thus, the highest activity for this enzyme was observed in the treatments with 200 µM (9.74 mg maltose mg−1 protein).
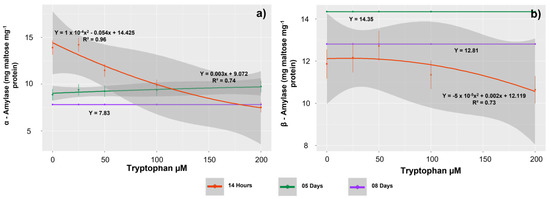
Figure 4.
Activities of starch metabolism enzymes, α-amylase (a) and β-amylase (b) in Glycine max L. seedlings obtained from seeds treated with different concentrations of tryptophan (0, 25, 50, 100, and 200 µM).
3.4. Activity of Enzymes of Antioxidant Metabolism
The effect of tryptophan concentrations, evaluation periods, and the interaction between these factors on SOD enzyme activity was also observed. Although the plants assessed at 5 DAS tended to exhibit higher activities for this enzyme, the seedlings tended to increase SOD activity with increasing exposure doses, regardless of the evaluation period (Figure 5a). Thus, in seedlings treated with 200 µM, the highest averages were observed at 5 DAS, 14 HAS, and 8 DAS, respectively (0.07, 0.04, and 0.03 U mg−1 protein). However, at 14 HAS, the activity of CAT and APX enzymes in the seedlings rose dramatically with increasing tryptophan concentrations (Figure 5b,c). Thus, the highest activities for these enzymes also occur in seedlings treated with 200 µM (1374.68 µmol of H2O2 min−1 mg−1 protein for CAT and 34,376.79 µmol of AsA min−1 mg−1 protein for APX, respectively).
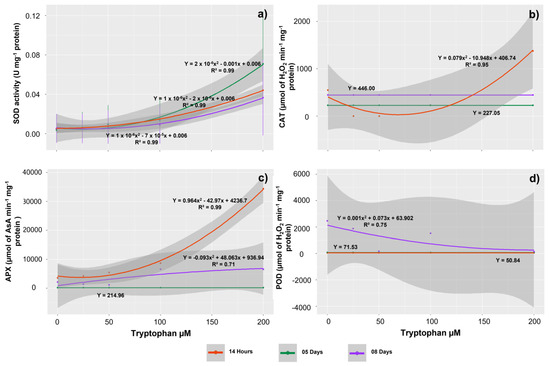
Figure 5.
Activities of the antioxidant enzymes superoxide dismutase (SOD) (a), catalase (CAT) (b), ascorbate peroxidase (APX) (c), and guaiacol peroxidase (POD) (d) in Glycine max L. seedlings obtained from seeds treated with different concentrations of tryptophan (0, 25, 50, 100, and 200 µM).
APX activity increased by increasing tryptophan concentration in the seedlings evaluated at 8 DAS, with the highest activities observed at 100 µM (6587.99 µmol AsA min−1 mg−1 protein). When the POD enzyme activity was evaluated, also there was an effect of tryptophan concentrations in the seedlings evaluated at 8 DAS. However, the behavior of this enzyme was in contrast with that observed for SOD and APX, as a decrease in activity was found in response to the increase in amino acid concentrations. Thus, the highest POD activity was observed in control seedlings (2457.53 µmol of H2O2 min−1 mg−1 protein) and the lowest activity in seedlings treated with 200 µM (138.01 µmol of H2O2 min−1 mg−1 protein) (Figure 5d).
3.5. Hydrogen Peroxide (H2O2), Malondialdehyde (MDA), and Proline
The concentrations of tryptophan, the evaluation period, as well as the interaction of these factors also influenced the tissue H2O2 content in G. max seedlings. This content tended to rise with increasing exposure concentrations at 14 HAS and 5 DAS, with the highest contents occurring in seedlings treated with 200 µM (9.76 and 9.70 mmol mg−1 FW, respectively) (Figure 6a). In the seedlings evaluated at 8 DAS, the peroxide content decreased as tryptophan concentrations increased. Thus, the lowest concentrations of this oxidant were observed at 200 µM (7.35 mmol mg−1 FW).
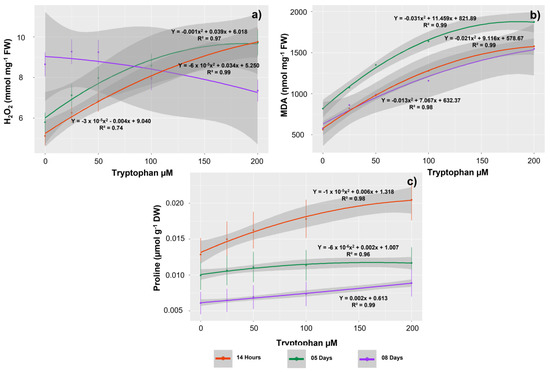
Figure 6.
Contents of the oxidants hydrogen peroxide (H2O2) (a) and malondialdehyde (MDA) (b), as well as proline (c) in Glycine max L. seedlings obtained from seeds treated with different concentrations of tryptophan (0, 25, 50, 100, and 200 µM).
The MDA contents were lower in the seedlings evaluated at 8 DAS and higher in those evaluated at 5 DAS. These contents rose with increasing concentrations of tryptophan in the sampled seedlings at all evaluation periods, with the highest averages observed at 200 µM (1874.15, 1577.10, and 1546.63 ηmol mg−1 FW for 5 DAS, 14 HAS, and 8 DAS, respectively) (Figure 6b).
Proline values tended to increase with increasing tryptophan concentrations. Thus, the highest contents were observed in seedlings treated with 200 µM, regardless of the evaluation period (0.024, 0.011, and 0.008 µmol g−1 DW for 14 HAS, 5 DAS, and 8 DAS, respectively) (Figure 6c).
3.6. Principal Component Analysis (PCA)
The two-dimensional plot of principal components revealed a positive relationship between the early developmental period of seedlings (14 HAS) and high values for proline synthesis and activities of the enzymes α-amylase and APX (Figure 7a). At 5 DAS, the seedlings tended to accumulate more starch than in the other evaluation periods and exhibited highest β-amylase activity and MDA content. Conversely, primary metabolism molecules (sucrose, RS, and TSS) and H2O2 synthesis tended to correlate positively with the last evaluation period (8 DAS).
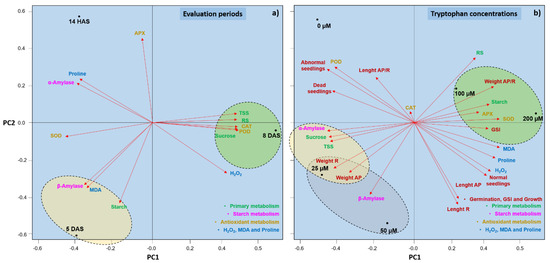
Figure 7.
Principal component analysis of the variables of germination, GSI, growth, primary metabolism molecules, starch metabolism enzymes, antioxidant metabolism enzymes, H2O2, MDA, and proline in Glycine max L. seedlings evaluated at three developmental periods (14 h after sowing (HAS), and 5 and 8 days after sowing (DAS) (a) and treated with different concentrations of tryptophan (0, 25, 50, 100, and 200 µM) (b). S = Shoot, R = Root, GSI = germination speed index, RS = reducing sugars, TSS = total soluble sugars, MDA = malondialdehyde.
When the dispersion of the response variables was evaluated in relation to the different concentrations of tryptophan tested, there was a tendency for the tryptophan-treated seedlings to be more metabolically stimulated than the untreated ones. The latter seedlings were associated with the highest ratios for the shoot length/root length. This indicates that the plants that received tryptophan tended to develop larger roots than the untreated plants. Conversely, the highest percentages of dead and abnormal seedlings were also associated with seeds not treated with the amino acid (Figure 7b). The 25 µM concentration correlated positively with the highest sucrose and TSS contents and the highest α-amylase activity. This concentration and the 50 µM stimulated the increase in biomass and β-amylase activity. However, the accumulation of starch, RS, H2O2, MDA, and proline, as well as the activity of the antioxidant enzymes APX and SOD, were more strongly correlated with the highest analyzed concentrations of tryptophan, i.e., 100 and 200 µM. These concentrations also correlated with higher percentages of normal seedlings and higher germination speed indices.
4. Discussion
4.1. Exogenous Tryptophan Improved Seed Germination Fitness and Seedling Vigor of G. Max
These positive effects resulted in increased seedling shoot and root length. This is because tryptophan, even if exogenously supplied, increases the tissue levels of auxin in soybeans. Auxins belong to the class of hormones activating plant growth and development [49], directly affecting cell division, elongation, and differentiation [50]. There is clear evidence that these hormones induce H+ ion excretion and consequent tissue elongation of soybean hypocotyls [51]. Some studies have also showed that auxin regulates gene expression in the hypocotyl region of soybean by increasing circulating RNA levels [52]. This indicates metabolic activation in the region and consequent stimulation of radicle growth, which may result in higher germination speed rates, since seed vigor can be directly correlated with the rate of oxygen metabolism [53].
Overall, the speed at which a seed germinates depends on the metabolic rate of the embryo, which gives it the ability to access reserves. Thus, the rate of metabolism, determined by O2 consumption, needs to increase during germination [54]. There is clear evidence that IAA accelerates seed metabolism. Zhao et al. [55] demonstrated that the direct use of IAA in cotton seed priming can accelerate seed germination by regulating endogenous hormones and sucrose metabolism. In addition, during the seedling stage, seedlings from IAA primed seeds showed more vigorous growth, with better root length, seedling height, biomass, and leaf photosynthesis capacity. This was also accompanied by higher sugar contents and activities of key enzymes in sucrose metabolism.
The metabolism and consequently germination and growth of other species was affected by IAA as well as by tryptophan. Rekoslavskaya et al. [56] suggest that DTrp and MTrp participate in IAA biosynthesis during wheat germination, which is essential for seedling growth during the initial days of heterotrophic growth, or respiration-dependent growth. We also found a trend of increasing root and shoot size as a function of increasing concentrations of tryptophan supplied to the seeds. Thus, seed priming with tryptophan ensures better performance in germination and subsequent plant growth, and is potentially effective for seeds subjected to salt or water stress conditions as well [57,58,59].
4.2. The High Activity of Starch Metabolizing Enzymes at 14 HA and 5 DAS Resulted in Increased Sucrose, RS, and TSS Contents Observed at 8 DAS
In seeds treated with exogenous tryptophan, the activity of α- and β-amylase enzymes in the early stages of seedling development made sugars available for metabolism in the subsequent stage. In these seeds, uptake of the solutions containing H2O and tryptophan promoted the metabolic activation necessary to break down the reserve starch. This constitutes a significant event for seedling development, as soybeans contain a large amount of starch and lipids. When these components are degraded, they can modulate a metabolic signaling cascade associated with radicle protrusion [60]. The starch in the early stages tends to be more abundant, as the seed is not yet sufficiently hydrated. However, in the next stages the starch tends to decrease, and it is made available to the seedling in the form of energy carbohydrates. Research shows that seed reserve carbohydrates are hydrolyzed to glucose by amylases and glucosidases. Therefore, glucose can be used as a form of energy or be transported to the embryonic axis in the form of sucrose [61,62,63]. After embryonic axis formation, the amount of sucrose increases in seedlings given its mobility, which is higher than that of starch, and its relevance, not only as an energy source, but also as a direct or indirect regulator of gene and phytohormone expression [64,65,66].
In some seeds, it is suggested that sucrose synthesis is exerted by auxin because high endogenous levels of this phytohormone have been correlated with peaks in the activity of hydrolases, which are responsible for the mobilization of this disaccharide [67,68,69]. In this study, sugar levels varied during the collection seasons. However, a relationship was observed between higher starch contents and increased tryptophan concentrations, as well as higher TSS and sucrose contents. Additionally, high α- and β-amylase activities were associated with low tryptophan concentrations (25 µM). Therefore, the higher concentrations seem to affect the activity of starch metabolism enzymes, especially at 14 HAS, stimulating plant metabolism and growth by other pathways unrelated to starch metabolism.
4.3. Exogenous Tryptophan Induces Germination and Vigor of Soybean Seedlings by Metabolic Pathways of Oxidative Stress
H2O2, MDA, and proline, as well as the activities of the enzymes SOD and APX increased in the seedlings treated with the highest doses of tryptophan. Research suggests that increased levels of H2O2 and MDA are essential for the metabolic signaling required for seed germination to take place. Recent studies claim that selective oxidation of proteins and mRNAs will act as a positive effector of seed germination [70,71]. In addition, H2O2 also protects seeds from pathogen attack during germination, owing to its antimicrobial properties [72]. Thus, it is suggested that there is a balance between oxidative communication that promotes germination and oxidative damage that interrupts or delays germination [73]. Barba-Espín et al. [74] showed that H2O2 increases pea seed germination percentage as well as seedling growth in a concentration-dependent manner. In the same study, peroxide treatment also increased APX activity and induced the synthesis of proteins related to plant signaling and development, cell elongation and division, and cell cycle control. Conversely, it is worth noting the importance of crosstalk between H2O2 and various signaling molecules, including plant phytohormones such as abscisic acid, gibberellins, and ethylene, and reactive molecules such as nitric oxide and hydrogen sulfide, which act in cell communication and signaling during seed germination [75].
The accumulation of H2O2 and other ROS, and consequently MDA, occurs mainly during soaking and the early stages of germination [76,77,78] as these species help degrade reserve tissue components and promote the loosening of lipid membranes, which is necessary for cell growth [79]. However, in this study, exposure to tryptophan was found to increase the tissue concentration of these molecules. This is because auxins also destabilize membranes. They decrease their condensation and weaken the interactions of the molecules, thus inducing strong disorders in the lipid system [80]. Oxidative damage to membranes is often accompanied by the production of MDA, which functions as a marker of lipid peroxidation [81,82]. Under these conditions, proline levels tend to increase, contributing to redox homeostasis. This is because this amino acid can stabilize proteins, membranes, and subcellular structures, protecting them from damage [83,84].
During germination, the accumulation of compatible solutes, which includes proline, increases the osmotic pressure inside the cells, and causes them to maintain water uptake and cell turgor pressure, thus allowing embryo growth and seedling formation [85,86]. Proline metabolism plays a key role in the oxidative pentose phosphate pathway (OPPP) by generating NAD(P)+ in the cytosol [87]. Since OPPP is involved in triggering the germination process, proline metabolism is believed to have a beneficial effect on seed germination [88,89]. Studies have identified the expression of genes encoding enzymes involved in proline metabolism in immature/mature seeds [90]. Hare et al. [91] observed an approximately fourfold increase in free proline during radicle emergence in Arabidopsis thaliana seeds, suggesting that proline metabolism plays a regulatory role in stimulating germination. Overall, studies associate increased concentrations of proline, oxidizing agents, and enzymes of the antioxidant system with stressful conditions imposed on seed germination [11,89,92,93].
For their own protection against toxic effects mediated by the abundant ROS, cells employ enzymes such as SOD and APX. SOD is one of the most effective intracellular antioxidant enzymes, being the first line of defense [94], mainly against the increase in superoxide anion (O2−) [95]. However, after the dismutation of O2− into H2O occurs by SOD, the complementary action of enzymes such as CAT and APX is necessary, promoting detoxification and maintaining the balance of H2O2 in the cellular medium, as this byproduct in large quantities is toxic [96]. The action of these enzymes also helps us understand the high levels of H2O observed in seedlings treated with the highest concentrations of tryptophan. Thus, we understand that increasing these concentrations induced metabolic stress in soybean seeds, and that this stress functioned as a germination and vigor-inducing pathway. However, more comprehensive studies involving not only the development of seedlings from tryptophan-treated seeds, but also the establishment of the plants in field systems, should be conducted for a better understanding of the effects of exogenous tryptophan on the physiology and production of G. max plants.
5. Conclusions
This study confirmed the hypothesis that exogenous supply of tryptophan to soybean seeds can affect germination, vigor, synthesis of primary metabolism molecules, and oxidative metabolism in G. max seedlings. Thus, treated seeds have better germinative fitness and the seedlings show greater vigor, and these parameters increase with increasing concentrations of tryptophan supplied. We also concluded that in the initial hours and days of germination process evaluation (14 HAS and 5 DAS), the activities of starch metabolism enzymes tend to be higher. This results in increased sucrose, RS, and TSS contents at 8 DAS, constituting an important metabolic effect for seedling growth. Conversely, the induction of germination and vigor promoted by exogenous tryptophan in soybean seedlings occurs by stimulating the metabolic pathways of oxidative stress, with an increase in the concentration of H2O2, MDA, and proline in the tissues, as well as an increase in the activities of the antioxidant enzymes SOD and APX. These parameters were responsive to increasing supplied concentrations of tryptophan. Thus, the metabolic stress in soybean seeds induced by auxin seems to be an important induction pathway for germination and vigor of seeds of G. max.
Author Contributions
Conceptualization, L.C.V., R.G.Á. and L.A.B.; methodology, R.G.Á.; validation, R.B.Q.; formal analysis, R.B.Q. and D.S.S.A.; investigation, R.B.Q., D.S.S.A. and M.S.O.; resources, R.B.Q. and M.S.O.; writing—original draft preparation, R.B.Q.; writing—review and editing, L.C.V. and L.A.B.; visualization, M.S.O.; supervision, L.C.V.; project administration, L.A.B.; funding acquisition, L.A.B. All authors have read and agreed to the published version of the manuscript.
Funding
The research that yielded these results was funded with resources from Public Notice No. 19 on 9 July 2021, from the Pro-Rectory of Research, Graduate Programs, and Innovation (PROPPI—IFGoiano), an internal call for support for RD&I projects aimed at strengthening applied research and innovation at IFGoiano. This research was also funded by funds from CAPES public notice nº 88887.342460/2019-00, through the postdoctoral fellowship for Marilene Silva Oliveira.
Data Availability Statement
All the data relevant to this manuscript are available on request from the corresponding author.
Acknowledgments
The authors would like to thank the Coordination for the Improvement of Higher Education Personnel (CAPES) and the Foundation for Research Support of the State of Goiás (FAPEG) for the Master’s grant awarded to Raphael Barros Queiroz, and the IFGoiano, Rio Verde campus for the infrastructure and the students involved in the study.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Reed, R.C.; Bradford, K.J.; Khanday, I. Seed germination and vigor: Ensuring crop sustainability in a changing climate. Heredity 2022, 128, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Damalas, C.A.; Koutroubas, S.D.; Fotiadis, S. Hydro-priming effects on seed germination and field performance of faba bean in spring sowing. Agriculture 2019, 9, 201. [Google Scholar] [CrossRef]
- Silva, E.C.; Viçosi, K.A.; Oliveira, L.A.B.; Galvão, C.S. Estresse salino na germinação e vigor de sementes de repolho. Sci. Agrar. Paran. 2018, 17, 374–377. [Google Scholar]
- Domergue, J.B.; Abadie, C.; Limami, A.; Way, D.; Tcherkez, G. Seed quality and carbon primary metabolism. Plant Cell Environ. 2019, 42, 2776–2788. [Google Scholar] [CrossRef]
- Oracz, K.; Karpiński, S. Phytohormones signaling pathways and ROS involvement in seed germination. Front. Plant Sci. 2016, 7, 864. [Google Scholar] [CrossRef] [PubMed]
- Rosental, L.; Nonogaki, H.; Fait, A. Activation and regulation of primary metabolism during seed germination. Seed Sci. Res. 2014, 24, 1–15. [Google Scholar] [CrossRef]
- Bewley, J.D.; Bradford, K.J.; Hilhorst, H.W.; Nonogaki, H. Germination. In Seeds: Physiology of Development, Germination and Dormancy, 3rd ed.; Bewley, J.D., Bradford, K.J., Hilhorst, H.W., Nonogaki, H., Eds.; Springer: New York, NY, USA, 2013; pp. 133–181. [Google Scholar] [CrossRef]
- Schreier, T.B.; Fahy, B.; David, L.C.; Siddiqui, H.; Castells-Graells, R.; Smith, A.M. Introduction of glucan synthase into the cytosol in wheat endosperm causes massive maltose accumulation and represses starch synthesis. Plant J. 2021, 106, 1431–1442. [Google Scholar] [CrossRef]
- Huang, P.; Li, C.; Liu, H.; Zhao, Z.; Liao, W. Hydrogen gas improves seed germination in cucumber by regulating sugar and starch metabolisms. Horticulturae 2021, 7, 456. [Google Scholar] [CrossRef]
- Jurdak, R.; Rodrigues, G.D.A.G.; Chaumont, N.; Schivre, G.; Bourbousse, C.; Barneche, F.; Kharrat, M.B.D.; Bailly, C. Intracellular reactive oxygen species trafficking participates in seed dormancy alleviation in Arabidopsis seeds. New Phytol. 2022, 234, 850–866. [Google Scholar] [CrossRef]
- Verma, D.; Jalmi, S.K.; Bhagat, P.K.; Verma, N.; Sinha, A.K. A bHLH transcription factor, MYC2, imparts salt intolerance by regulating proline biosynthesis in Arabidopsis. FEBS J. 2020, 287, 2560–2576. [Google Scholar] [CrossRef]
- Leymarie, J.; Vitkauskaité, G.; Hoang, H.H.; Gendreau, E.; Chazoule, V.; Meimoun, P.; Corbineau, F.; El-Maarouf-Bouteau, H.; Bailly, C. Role of reactive oxygen species in the regulation of Arabidopsis seed dormancy. Plant Cell Physiol. 2012, 53, 96–106. [Google Scholar] [CrossRef]
- Diaz-Vivancos, P.; Barba-Espín, G.; Hernández, J.A. Elucidating hormonal/ROS networks during seed germination: Insights and perspectives. Plant Cell Rep. 2013, 32, 1491–1502. [Google Scholar] [CrossRef]
- Wu, M.; Wu, J.; Gan, Y. The new insight of auxin functions: Transition from seed dormancy to germination and floral opening in plants. Plant Growth Regul. 2020, 91, 169–174. [Google Scholar] [CrossRef]
- Robert-Seilaniantz, A.; Navarro, L.; Bari, R.; Jones, J.D. Pathological hormone imbalances. Curr. Opin. Plant Biol. 2007, 10, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Azmitia, E.C. Modern views on an ancient chemical: Serotonin effects on cell proliferation, maturation, and apoptosis. Brain Res. Bull. 2001, 56, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Kahveci, H.; Bilginer, N.; Diraz-Yildirim, E.; Kulak, M.; Yazar, E.; Kocacinar, F.; Karaman, S. Priming with salicylic acid, β-carotene and tryptophan modulates growth, phenolics and essential oil components of Ocimum basilicum L. grown under salinity. Sci. Hortic. 2021, 281, 109964. [Google Scholar] [CrossRef]
- Erland, L.A.E.; Saxena, P.K. Beyond a neurotransmitter: The role of serotonin in plants. Neurotransmitter 2017, 4, e1538. [Google Scholar] [CrossRef]
- Pelagio-Flores, R.; Ruiz-Herrera, L.F.; López-Bucio, J. Serotonin modulates Arabidopsis root growth via changes in reactive oxygen species and jasmonic acid–ethylene signaling. Physiol. Plant. 2016, 158, 92–105. [Google Scholar] [CrossRef] [PubMed]
- Hanci, F.; Çingi, M.; Akinci, H. Influence of L-tryptophan and melatonin on germination of onion and leek seeds at different temperatures. Türkiye Tarımsal Araştırmalar Derg. 2019, 6, 214–221. [Google Scholar] [CrossRef]
- Antony, E.; Sridhar, K.; Kumar, V. Effect of chemical sprays and management practices on Brachiaria ruziziensis seed, production. Field Crops Res. 2017, 211, 19–26. [Google Scholar] [CrossRef]
- Mustafa, A.; Hussain, A.; Naveed, M.; Ditta, A.; Nazli, Z.E.H.; Sattar, A. Response of okra (Abelmoschus esculentus L.) to soil and foliar applied L-tryptophan. Soil Environ. 2016, 35, 76–84. [Google Scholar]
- Abbas, S.H.; Sohail, M.; Saleem, M.; Mahmood, T.; Aziz, I.; Qamar, M.; Majeed, A.; Arif, M. Effect of L-tryptophan on plant weight and pod weight in chickpea under rainfed conditions. Sci. Tech. Dev. 2013, 32, 277–280. [Google Scholar]
- Parvez, M.A.; Muhammad, F.; Ahmad, M. Effect of auxin precursor (L-tryptophan) on the growth and yield of tomato (Lycopersicon esculentum). Pak. J. Biol. Sci. 2000, 3, 11541155. [Google Scholar] [CrossRef]
- Khodary, S.E.A. Effect of salinity and tryptophan on growth and some metabolic changes in wheat and sorghum plants. Biol. Plant. 1992, 34, 439–443. [Google Scholar] [CrossRef]
- Mustafa, A.; Imran, M.; Ashraf, M.; Mahmood, K. Perspectives of using l-tryptophan for improving productivity of agricultural crops: A review. Pedosphere 2018, 28, 16–34. [Google Scholar] [CrossRef]
- Roychoudhury, A. Multifaceted roles of serotonin in plants. Young Sci.-Tomorrow’s Sci. Begins Today 2021, 5, 26–35. [Google Scholar]
- Raza, A.; Salehi, H.; Rahman, M.A.; Zahid, Z.; Madadkar Haghjou, M.; Najafi-Kakavand, S.; Charagh, S.; Osman, H.S.; Albaqami, M.; Zhuang, Y.; et al. Plant hormones and neurotransmitter interactions mediate antioxidant defenses under induced oxidative stress in plants. Front. Plant Sci. 2022, 13, 961872. [Google Scholar] [CrossRef]
- Erland, L.A.E.; Yasunaga, A.; Li, I.T.S.; Murch, S.J.; Saxena, P.K. Direct visualization of location and uptake of applied melatonin and serotonin in living tissues and their redistribution in plants in response to thermal stress. J. Pineal Res. 2019, 66, e12527. [Google Scholar] [CrossRef]
- Abbasi, B.H.; Younas, M.; Anjum, S.; Ahmad, N.; Ali, M.; Fazal, H.; Hano, C. Serotonin in plant signalling and communication. In Neurotransmitters in Plant Signaling and Communication; Baluška, F., Mukherjee, S., Ramakrishna, A., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2020; pp. 75–92. [Google Scholar] [CrossRef]
- Akula, R.; Mukherjee, S. New insights on neurotransmitters signaling mechanisms in plants. Plant Signal. Behav. 2020, 15, 1737450. [Google Scholar] [CrossRef]
- Brasil, Ministério da Agricultura, Pecuária e Abastecimento. Regras para Análise de Sementes; MAPA/ACS: Brasília, Brazil, 2009; pp. 1–395. [Google Scholar]
- Maguire, J.D. Speed of germination-aid in selection and evaluation for seedling emergence and vigor. Crop Sci. 1962, 2, 176–177. [Google Scholar] [CrossRef]
- Rabêlo, V.M.; Magalhães, P.C.; Bressanin, L.A.; Carvalho, D.T.; Reis, C.O.D.; Karam, D.; Doriguetto, A.C.; Santos, M.H.; Santos Filho, P.R.S.; Souza, T.C.D. The foliar application of a mixture of semisynthetic chitosan derivatives induces tolerance to water deficit in maize, improving the antioxidant system and increasing photosynthesis and grain yield. Sci. Rep. 2019, 1, 8164. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.L. Modified DNS method for reducing sugars. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Yemm, E.W.; Willis, A.J. The estimation of carbohydrates in plant extracts by anthrone. Biochem. J. 1954, 57, 508. [Google Scholar] [CrossRef] [PubMed]
- Tárrago, J.F.; Nicolás, G. Starch degradation in the cotyledons of germinating lentils. Plant Physiol. 1976, 58, 618–621. [Google Scholar] [CrossRef] [PubMed]
- Bernfeld, P. Amylase α and β. Methods Enzymol. 1955, 1, 149–151. [Google Scholar]
- Kishorekumar, A.; Jaleel, C.A.; Manivannan, P.; Sankar, B.; Sridharan, R.; Panneerselvam, R. Comparative effects of different triazole compaunds on growth, photosynthetic pigments and carbohydrate metabolism of Solenostemon rotundifolius. Colloids Surf. 2007, 60, 207–212. [Google Scholar] [CrossRef]
- Biemelt, S.; Keetman, U.; Albrecht, G. Re-aeration following hypoxia or anoxia leads to activation of the antioxidative defense system in roots of wheat seedlings. Plant Physiol. 1998, 116, 651–658. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol. 1997, 59, 309–314. [Google Scholar] [CrossRef]
- Havir, E.A.; Mchale, N.A. Biochemical and developmental characterization of multiple forms of catalase in tobacco leaves. Plant Physiol. 1987, 84, 450–455. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Fang, W.-C.; Kao, C.H. Enhanced peroxidase activity in rice leaves in response to excess iron, copper and zinc. Plant Sci. 2000, 158, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. In Methods in Enzymology; Qin, P.Z., Ed.; Academic Press: New York, NY, USA, 1978; pp. 302–310. [Google Scholar]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria. Available online: https://www.R-project.org/ (accessed on 27 March 2023).
- Sosnowski, J.; Truba, M.; Vasileva, V. The impact of auxin and cytokinin on the growth and development of selected crops. Agriculture 2023, 13, 724. [Google Scholar] [CrossRef]
- Teale, W.D.; Paponov, I.A.; Palme, K. Auxin in action: Signalling, transport and the control of plant growth and development. Nat. Rev. Mol. Cell Biol. 2006, 7, 847–859. [Google Scholar] [CrossRef]
- Bawa, G.; Feng, L.; Chen, G.; Chen, H.; Hu, Y.; Pu, T.; Cheng, Y.; Shi, J.; Xiao, T.; Zhou, W.; et al. Gibberellins and auxin regulate soybean hypocotyl elongation under low light and high-temperature interaction. Physiol. Plant. 2020, 170, 345–356. [Google Scholar] [CrossRef]
- Hagen, G.; Kleinschmidt, A.; Guilfoyle, T. Auxin-regulated gene expression in intact soybean hypocotyl and excised hypocotyl sections. Planta 1984, 162, 147–153. [Google Scholar] [CrossRef]
- Zhao, G.W.; Cao, D.D.; Chen, H.Y.; Ruan, G.H.; Yang, M.J. A study on the rapid assessment of conventional rice seed vigour based on oxygen-sensing technology. Seed Sci. Technol. 2013, 41, 257–269. [Google Scholar] [CrossRef]
- Raju, V.C.S.; Anand, K.B.P. Evaluation of the effect of oxygen sensing on seed germination and metabolic rate in five different seed varieties. Int. J. Res. Rev. Pharm. Appl. Sci. 2011, 1, 295–312. [Google Scholar]
- Zhao, T.; Deng, X.; Xiao, Q.; Han, Y.; Zhu, S.; Chen, J. IAA priming improves the germination and seedling growth in cotton (Gossypium hirsutum L.) via regulating the endogenous phytohormones and enhancing the sucrose metabolism. Ind. Crops Prod. 2020, 155, 112788. [Google Scholar] [CrossRef]
- Rekoslavskaya, N.I.; Yurjeva, O.V.; Salyaev, R.K.; Mapelli, S.; Kopytina, T.V. D-Tryptophan as IAA source during wheat germination. Bulg. J. Plant Physiol. 1999, 25, 39–49. [Google Scholar]
- Godoy, F.; Olivos-Hernández, K.; Stange, C.; Handford, M. Abiotic stress in crop species: Improving tolerance by applying plant metabolites. Plants 2021, 10, 186. [Google Scholar] [CrossRef] [PubMed]
- Jamil, M.; Kharal, M.A.; Ahmad, M.; Abbasi, G.H.; Nazli, F.; Hussain, A.; Akhtar, M.F.-Z. Inducing salinity tolerance in red pepper (Capsicum annuum L.) through exogenous application of proline and L-tryptophan. Soil Environ. 2018, 37, 160–168. [Google Scholar]
- Iqbal, M.; Ashraf, M. Seed treatment with auxins modulates growth and ion partitioning in salt-stressed wheat plants. J. Integr. Plant Biol. 2007, 49, 1003–1015. [Google Scholar] [CrossRef]
- Gutierrez, L.; Van Wuytswinkel, O.; Castelain, M.; Bellini, C. Combined networks regulating seed maturation. Trends Plant Sci. 2007, 12, 294–300. [Google Scholar] [CrossRef]
- Damaris, R.N.; Lin, Z.; Yang, P.; He, D. The rice alpha-amylase, conserved regulator of seed maturation and germination. Int. J. Mol. Sci. 2019, 20, 450. [Google Scholar] [CrossRef]
- López-Coria, M.; Sánchez-Sánchez, T.; Martínez-Marcelo, V.H.; Aguilera-Alvarado, G.P.; Flores-Barrera, M.; King-Díaz, B.; Sánchez-Nieto, S. SWEET transporters for the nourishment of embryonic tissues during maize germination. Genes 2019, 10, 780. [Google Scholar] [CrossRef]
- Oliveira, M.C.; Ferreira, G.; Guimarães, V.F.E.; Dias, G.B. Germinação de sementes de atemóia (Annona cherimola Mill. x A. squamosa L.) cv ‘Gefner’ submetidas a tratamentos com ácido giberélico (GA3) e ethephon. Rev. Bras. Frutic. 2010, 32, 544–554. [Google Scholar] [CrossRef]
- Ćosić, T.; Motyka, V.; Savić, J.; Raspor, M.; Marković, M.; Dobrev, P.I.; Ninković, S. Sucrose interferes with endogenous cytokinin homeostasis and expression of organogenesis-related genes during de novo shoot organogenesis in kohlrabi. Sci. Rep. 2021, 11, 1–16. [Google Scholar] [CrossRef]
- Zhang, S.; Peng, F.; Xiao, Y.; Wang, W.; Wu, X. Peach PpSnRK1 participates in sucrose-mediated root growth through auxin signaling. Front. Plant Sci. 2020, 11, 409. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, Y.S.; Jo, Y.D.; Kang, S.Y.; Ahn, J.W.; Kang, B.C.; Kim, J.B. Sucrose and methyl jasmonate modulate the expression of anthocyanin biosynthesis genes and increase the frequency of flower-color mutants in chrysanthemum. Sci. Hortic. 2019, 256, 108602. [Google Scholar] [CrossRef]
- Mishra, B.S.; Sharma, M.; Laxmi, A. Role of sugar and auxin crosstalk in plant growth and development. Physiol. Plant. 2022, 174, e13546. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Xu, X.; Gong, Z.; Tang, Y.; Wu, M.; Yan, F.; Zhang, X.; Zhang, Q.; Yang, F.; Hu, X.; et al. Auxin response factor 6A regulates photosynthesis, sugar accumulation, and fruit development in tomato. Hortic. Res. 2019, 6, 85. [Google Scholar] [CrossRef]
- Buckeridge, M.S.; Aidar, M.P.M.; Santos, H.P.; Tiné, M.A.S. Acúmulo de reserva. In Germinação: Do Básico ao Aplicado; Ferreira, A.G., Borghetti, F., Eds.; Artmed: Porto Alegre, Brazil, 2004; pp. 31–50. [Google Scholar]
- Barba-Espín, G.; Diaz-Vivancos, P.; Job, D.; Belghazi, M.; Job, C.; Hernandez, J.A. Understanding the role of H2O2 during pea seed germination: A combined proteomic and hormone profiling approach. Plant Cell Environ. 2011, 34, 1907–1919. [Google Scholar] [CrossRef] [PubMed]
- Bazin, J.; Langlade, N.; Vincourt, P.; Arribat, S.; Balzergue, S.; El-Maarouf Bouteau, H. Targeted mRNA oxidation regulates sunflower seed dormancy alleviation during dry after-ripening. Plant Cell 2011, 23, 2196–2208. [Google Scholar] [CrossRef]
- Coll, N.S.; Epple, P.; Dang, J.L. Programmed cell death in the plant immune system. Cell Death Differ. 2011, 18, 1247–1256. [Google Scholar] [CrossRef]
- Sharma, S.A.M.E.E.R.; Yadav, S.A.R.O.J.; Sibi, G. Seed germination and maturation under the influence of hydrogen peroxide—A review. J. Crit. Rev. 2020, 7, 6–10. [Google Scholar] [CrossRef]
- Barba-Espin, G.; Diaz-Vivancos, P.; Clemente-Moreno, M.J.; Albacete, A.; Faize, L.; Faize, M.; Pérez-Alfocea, F.; Hernández, J.A. Interaction between hydrogen peroxide and plant hormones during germination and the early growth of pea seedlings. Plant Cell Environ. 2010, 33, 981–994. [Google Scholar] [CrossRef]
- Wojtyla, Ł.; Lechowska, K.; Kubala, S.; Garnczarska, M. Different modes of hydrogen peroxide action during seed germination. Front. Plant Sci. 2016, 7, 66. [Google Scholar] [CrossRef]
- Kranner, I.; Roach, T.; Beckett, R.P.; Whitaker, C.; Minibayeva, F.V. Extracellular production of reactive oxygen species during seed germination and early seedling growth in Pisum sativum. J. Plant Physiol. 2010, 167, 805–811. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, B.; Xu, Z.; Shi, Z.; Chen, S.; Huang, X.; Chen, J.; Wang, X. Involvement of reactive oxygen species in endosperm cap weakening and embryo elongation growth during lettuce seed germination. J. Exp. Bot. 2014, 65, 3189–3200. [Google Scholar] [CrossRef] [PubMed]
- Kubala, S.; Wojtyla, Ł.; Quinet, M.; Lechowska, K.; Lutts, S.; Garnczarska, M. Enhanced expression of the proline synthesis gene P5CSA in relations to seed osmopriming improvement of Brassica napus germination under salinity stress. J. Plant Physiol. 2015, 183, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Katsuya-Gaviria, K.; Caro, E.; Carrillo-Barral, N.; Iglesias-Fernández, R. Reactive oxygen species (ROS) and nucleic acid modifications during seed dormancy. Plants 2020, 9, 679. [Google Scholar] [CrossRef]
- Hąc-Wydro, K.; Flasiński, M. The studies on the toxicity mechanism of environmentally hazardous natural (IAA) and synthetic (NAA) auxin–the experiments on model Arabidopsis thaliana and rat liver plasma membranes. Colloids Surf. B Biointerfaces 2015, 130, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Fotouo-M, H.; Vorster, J.; Du Toit, E.S.; Robbertse, P.J. The effect of natural long-term packaging methods on antioxidant components and malondialdehyde content and seed viability Moringa oleifera oilseed. S. Afr. J. Bot. 2020, 129, 17–24. [Google Scholar] [CrossRef]
- Ebone, L.A.; Caverzan, A.; Chavarria, G. Physiologic alterations in orthodox seeds due to deterioration processes. Plant Physiol. Biochem. 2019, 145, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Hnilickova, H.; Kraus, K.; Vachova, P.; Hnilicka, F. Salinity stress affects photosynthesis, malondialdehyde formation, and proline content in Portulaca oleracea L. Plants 2021, 10, 845. [Google Scholar] [CrossRef]
- Salinas, R.; Sánchez, E.; Ruíz, J.M.; Lao, M.T.; Romero, L. Proline, betaine, and choline responses to different phosphorus levels in green bean. Commun. Soil Sci. Plant Anal. 2013, 44, 465–472. [Google Scholar] [CrossRef]
- Jabeen, S.; Kusar, S.; Akram, M.A.; Haroon, A.; Ahmad, M.S.A.; Iqbal, A.; Latif, M.U. Proline induced changes in redox balance and photosynthetic activity of wheat (Triticum aestivum L.) under saline conditions. Plant Prot. 2022, 6, 57–73. [Google Scholar] [CrossRef]
- Moghaddam, M.; Farhadi, N.; Panjtandoust, M.; Ghanati, F. Seed germination, antioxidant enzymes activity and proline content in medicinal plant Tagetes minuta under salinity stress. Plant Biosyst.-Int. J. Deal. All Asp. Plant Biol. 2020, 154, 835–842. [Google Scholar] [CrossRef]
- Signorelli, S. The fermentation analogy: A point of view for understanding the intriguing role of proline accumulation in stressed plants. Front. Plant Sci. 2016, 7, 1339. [Google Scholar] [CrossRef] [PubMed]
- El Moukhtari, A.; Cabassa-Hourton, C.; Farissi, M.; Savouré, A. How does proline treatment promote salt stress tolerance during crop plant development? Front. Plant Sci. 2020, 11, 1127. [Google Scholar] [CrossRef]
- Kavi Kishor, P.B.; Sreenivasulu, N. Is proline accumulation per se correlated with stress tolerance or is proline homeostasis a more critical issue? Plant Cell Environ. 2014, 37, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Hosseinifard, M.; Stefaniak, S.; Ghorbani Javid, M.; Soltani, E.; Wojtyla, Ł.; Garnczarska, M. Contribution of exogenous proline to abiotic stresses tolerance in plants: A review. Int. J. Mol. Sci. 2022, 23, 5186. [Google Scholar] [CrossRef] [PubMed]
- Hare, P.D.; Cress, W.A.; Van Staden, J. A regulatory role for proline metabolism in stimulating Arabidopsis thaliana seed germination. Plant Growth Regul. 2003, 39, 41–50. [Google Scholar] [CrossRef]
- Jabeen, M.; Jabeen, A.; Ahmad, M. Role of compatible solutes in alleviating effect of abiotic stress in plants. Int. J. Educ. Res. 2022, 3, 141–153. [Google Scholar] [CrossRef]
- Abrantes, F.D.L.; Ribas, A.F.; Vieira, L.G.E.; Machado-Neto, N.B.; Custódio, C.C. Seed germination and seedling vigour of transgenic tobacco (Nicotiana tabacum L.) with increased proline accumulation under osmotic stress. J. Hortic. Sci. Biotechnol. 2019, 94, 220–228. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Bratovcic, A. Antioxidant enzymes and their role in preventing cell damage. Act. Sci. Nutr. Health, 2020, 4, 132–138. [Google Scholar] [CrossRef]
- Asgher, M.; Ahmed, S.; Sehar, Z.; Gautam, H.; Gandhi, S.G.; Khan, N.A. Hydrogen peroxide modulates activity and expression of antioxidant enzymes and protects photosynthetic activity from arsenic damage in rice (Oryza sativa L.). J. Hazard. Mater. 2021, 401, 123365. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).