Abstract
Pesticides are commonly used to control weeds, diseases, and pests in soybean crops, although natural enemies are also key components of integrated pest management programs protecting soybean from pests. The predatory mite Neoseiulus idaeus Denmark & Muma is commonly found in soybeans associated with tetranychid mites. We determined the compatibility of some pesticides used in Brazilian soybean fields with N. idaeus on the basis of their lethal and sub-lethal effects. We assessed the acute toxicity of pesticides that are considered harmless to females of N. idaeus and evaluated the effects on the mite offspring. Acephate as well as imidacloprid and bifenthrin were most toxic to adult females and were classified as harmful. Chlorfenapyr, indoxacarb, and the neonicotinoid and pyrethroid mixtures were classified as slightly to moderately harmful. Immatures of N. idaeus were able to develop and reach the adult stage when treated with chlorantraniliprole, flubendiamide, glyphosate, and teflubenzuron, but immature survival was reduced to 42 and 64% for offspring of females treated with chlorantraniliprole or teflubenzuron, respectively. Reproduction was most affected in females exposed to chlorantraniliprole and teflubenzuron; these females had daily oviposition rates of 0.5 and 0.4 egg/female/day, respectively, and mean fecundities of 2.7 and 5.8 eggs/female, respectively. On the basis of sublethal effects and life table parameters, chlorantraniliprole was classified as moderately harmful, while the other pesticides tested were classified as harmless. In considering the use of pesticides while targeting the conservation of N. idaeus as a predator of important pests in soybean crops, one must prioritize pesticides that will most likely have less harmful effects on this predator in the field.
1. Introduction
Soybean (Glycine max (L.) Merrill) is one of the main foods consumed in the world [1,2,3] due to its highly nutritious composition, including edible proteins used in human and animal diets [4]. Soybean also has important social and economic roles, generating employment and income though production chains. World soybean production in 2021–2022 was 352.74 million metric tons for 130.43 million hectares planted [5]. In Brazil, the largest global soybean producer, the 2021–2022 harvest corresponded to 126.00 million metric tons per hectare of planted area, with a productivity of 3.07 metric tons per hectare [6].
Soybean production can be affected by weeds, diseases, and pests throughout the production cycle. Major lepidopteran soybean pests are Anticarsia gemmatalis Hübner (Erebidae), Chrysodeixis includens (Walker), Helicoverpa armigera (Hübner), and the Spodoptera complex (Noctuidae) [7,8,9,10,11,12,13]. Stink bugs represent another group of important soybean pests, primarily Nezara viridula (Linnaeus), Piezodorus guildinii (West.), and Euschistus heros (Fabricius) (Hemiptera: Pentatomidae) [14,15,16,17]. Moreover, many species of tetranychid mites occasionally occur in soybean fields [18,19,20,21,22,23,24]. For example, Mononychellus planki (McGregor) and Tetranychus urticae Koch (Acari: Tetranychidae) have been reported to damage soybean and other crops [25,26,27,28].
Indiscriminate use of pesticides causes ecological problems, including contamination of soil and water, adverse effects on non-target organisms, and pest resistance to insecticides [29,30]. Integrated pest management aims to rationalize the use of control tactics, especially to reduce the application of broad-spectrum insecticides and to promote the integration of different pest-control tactics, mainly through the use of culture methods and biological-control agents [31,32,33]. Finding ecofriendly methods to control pests is vital. For this reason, biological control of pests using pathogens, parasitoids, and predators has received considerable attention in recent years.
Among predators, phytoseiid mites have been considered for their potential as control agents for phytophagous mites as well as certain insects, including whiteflies and thrips [34,35,36,37]. The family Phytoseiidae contains approximately 2557 valid species [38,39] and is one of the best-studied groups among Acari. Neoseiulus idaeus Denmark & Muma (Acari: Phytoseiidae) is a type III generalist predator [36], which feeds on different types of prey but develops and reproduces better on a diet of tetranychid mites. This predatory mite has a broad distribution in Brazil [38,39] and has been reported on several crop plants, including soybean [23,40].
Laboratory studies have shown that N. idaeus develops better when fed with Tetranuchus ludeni Zacher (Acari: Tetranychidae) and T. urticae, two important spider mites in soybean [41,42]. Reichert et al. [41,42] discussed the importance of N. idaeus as a control agent for tetranychids. In addition, the potential of N. idaeus to control T. urticae has been reported in laboratory and semi-field conditions [43,44,45,46].
In this study, we tested the compatibility of the main pesticides used in Brazilian soybean fields with the predator N. idaeus under laboratory conditions. In addition to the acute toxicity to the predatory mites, we evaluated the sublethal effects of five pesticides that caused very low mortality. For this, we assessed the development and reproductive parameters with a life table and fertility study, using the offspring of a pesticide-treated parental generation.
2. Materials and Methods
The experimental bioassays were conducted at the Integrated Pest Management Laboratory of the Department of Entomology and Acarology at Luiz de Queiroz College of Agriculture, University of São Paulo (ESALQ/USP), Piracicaba, São Paulo, Brazil.
For all bioassays, the experimental units consisted of a cylindrical acrylic container (2.5 × 1.5 cm) with a base of carrageenan, where a disc of soybean leaf, variety “BMX Power RR” (2.0 cm in diameter) sprayed with a pesticide or water (negative control), was placed. The top of the cylinder was sealed with cling film (Magipack®, MP Magipack Embalagens, Criciuma, SC, Brazil) to prevent the mites from escaping. The experimental design of the bioassays was completely randomized. All bioassays were conducted in a climate-controlled chamber at 25 ± 2 °C, 75 ± 10% RH, and 12 h photoperiod.
2.1. Mites
The predatory mites were initially collected in February 2021 from soybean plants in the experimental area of the Department of Entomology and Acarology, ESALQ/USP. The mites were kept in rearing units like those described by McMurtry and Scriven [47], which consisted of a rectangular plastic container with a hydrophilic sponge saturated with water. A leaf of Canavalia ensiformis (Linnaeus) DC. (Fabaceae) was placed on the sponge, and a layer of hydrophilic cotton on the leaf’s edges prevented the mites from escaping and maintained leaf turgidity. The mites were kept in a room (22–27 °C, 75 ± 10% RH, in darkness), and were continually fed with two-spotted spider mites (60% eggs and 40% immature stages). The initial colony of spider mites was obtained by Promip Manejo Integrado LTDA, Engenheiro Coelho, SP, Brazil. These mites were reared on C. ensiformis plants in a climate-controlled chamber at 25 ± 3 °C, 75 ± 10% RH, and 14 h photoperiod. Every seven days, a new rearing unit was assembled, and the predatory mites were transferred to it. Identification of the T. urticae and N. idaeus specimens was confirmed by Geovanny Barroso.
2.2. Pesticides
The pesticides selected for the bioassays were chosen on the basis of the Brazilian Phytosanitary Agrochemicals System (Agrofit) list [48]. They were tested at the maximum dosage recommended by the manufacturer for their biological target (Table 1).

Table 1.
Common pesticides used to control weeds and pests in Brazilian soybean fields.
2.3. Acute Toxicity to Adult Females
Each pesticide was sprayed (1.5–2.0 mg solution cm–2) onto a disc of soybean leaf using a calibrated Potter tower (Burkard Scientific, Uxbridge, UK) with the pressure adjusted to 0.7 kg cm–2, corresponding to a deposit of 1.8 ± 0.1 mg cm–2 of leaf area, as recommended by the International Organization for Biological and Integrated Control of Noxious Animals and Plants (IOBC) [49,50]. A few minutes after the spraying, the leaf disc was placed in an experimental unit and dried for 2 h in a climate-controlled room in the dark. Then, a single adult female of N. idaeus (mated and up to 48 h after emergence) was transferred to each unit. A leaf fragment with all life stages of spider mites (ad libitum) was added as the food source for the predator 4 h after the female N. idaeus was transferred to ensure that this predator was in contact with the previously sprayed disc during that time. The numbers of live and dead mites were evaluated 24 and 48 h after the application of the treatments. Mites were considered dead when they did not react to being touched with a fine brush. In total, the experiment had 12 treatments (pesticides) and a negative control (distilled water), with each treatment repeated 20 times, and each replicate contained one predator (adult female).
2.4. Sublethal Effects on Offspring
The sublethal effect was evaluated for the pesticides that caused corrected mortality (Cm) of up to 25% in adult females and were, therefore, classified as harmless according to the IOBC [51]. For this, a new adult female (48 h old) was transferred to a pesticide-treatment experimental unit as in the previous bioassay. Each egg laid by that female was carefully transferred to a new, pesticide-free experimental unit. Initially, each treatment consisted of approximately 50 repetitions. In this case, each repetition contained one 12-hour-old egg. Survival and duration of immature stages (larvae, protonymphs, and deutonymphs) were evaluated every 12 h until the adults emerged. The newly emerged adult females and males were placed in new experimental units to determine the pre-oviposition and oviposition periods, fecundity (number of eggs laid by females), egg viability (number of hatched larvae), and longevity of females. As the number of males in the treatments was insufficient to form all the couples, males of indeterminate age were collected in the rearing colonies and confined with the remaining females. The mites were fed daily ad libitum with a mixture of all spider mite stages (75% eggs and immatures).
The viability of eggs from the adult females treated with pesticides (parental generation, F0) was determined on the basis of the number of hatched larvae in each experimental unit. Likewise, the first 50 eggs laid by adult females of the offspring (maternal generation, F1) were evaluated. Eggs that did not progress to larvae within seven days were considered non-viable.
2.5. Data Analysis
The data for mortality and viability of eggs laid by exposed adult females (F0), and viability of the eggs laid by their offspring (F1) were analyzed with a generalized linear model with a binomial distribution. The goodness of fit was confirmed using the “hnp” package [52]. The comparisons were performed by contrasts from a binomial generalized linear model using the glht function from the multcomp package [53]. The R software 4.1.2 [54] was used for these analyses.
The sublethal effects on mites assessed for each pesticide were expressed as: E% = 100 − (100 − Cm) × Er, where Er is the coefficient of reduction or toxicity, determined by: Er = (Ftreatment/Fcontrol) × (Vtreatment/Vcontrol), where F is the ratio of the total number of eggs laid per female in the specified treatment (Ftreatment) to the total number in the control (Fcontrol); V is the ratio of the total number of larvae that hatched from eggs in this treatment (Vtreatment) to those in the control (Vcontrol) at the end of the test; and Cm is the percentage mortality calculated according to Abbott [55]. The data were analyzed considering the recommendations of the IOBC-WPRS [50,51], and toxicity classes were assigned on the basis of overall effects (E) to classify each pesticide as follows: Class 1 = harmless (E < 25%); Class 2 = slightly harmful (25 ≤ E ≤ 50%); Class 3 = moderately harmful (50% ≤ E ≤ 75%); and Class 4 = harmful (E > 75%).
The effects of the pesticides on the offspring (F1) were estimated from life table data [56], using the Age–Stage method developed by Chi [57] with the TWOSEX-MSChart software [57,58]. The means and standard errors of the biological parameters were obtained using a paired bootstrap test, as proposed by Huang and Chi [59], with 100,000 bootstraps. The mean and standard errors were estimated for Dunn’s test with Bonferroni correction (p < 0.05) when this was not possible using the Age–Stage method.
3. Results
3.1. Acute Toxicity to Adult Females
Mortality rates differed among the pesticides and were highest for acephate and for imidacloprid and bifenthrin (Table 2). These insecticides were toxic over the short term and caused 100% mortality within 48 h. Mortality rates higher than 25% were observed for the pesticides formulated with a ready-mix of neonicotinoid and pyrethroid. In contrast, chlorantraniliprole, glyphosate, and teflubenzuron caused low mortality to the mite and, therefore, were classified as harmless.

Table 2.
Mortality rate ± standard error (SE) and confidence intervals (CI) of Neoseiulus idaeus females 24 and 48 h after contact with pesticide residues on soybean leaves, corrected mortality (Cm) after 48 h, and IOBC-WPRS classification of pesticides.
3.2. Sublethal Effects on Offspring
3.2.1. Development Time and Survival Rates of Immature Stages
Offspring of N. idaeus could develop and completed their life cycle when the progenitor females were exposed to chlorantraniliprole residue. Nevertheless, this pesticide reduced the egg viability most, achieving 69% survival (Table 3). The offspring of females exposed to flubendiamide had a shorter egg development time, significantly different from all treatments (χ2 = 67.5; df = 4; p < 0.001). Likewise, the larvae that emerged from viable eggs had a shorter mean duration in the teflubenzuron, which differed from chlorantraniliprole and flubendiamide but was equal to glyphosate and control treatments (χ2 = 33.4; df = 4; p < 0.001). Significant differences in protonymph duration were detected (χ2 = 22.9; df = 4; p < 0.001); however, post hoc tests revealed the only difference observed was for individuals in the teflubenzuron treatment (χ2 = 22.9; df = 4; p < 0.001). In contrast, deutonymphs in the glyphosate treatment lasted longer than all but those whose mothers were treated with chlorantraniliprole (χ2 = 28.6; df = 4; p <0.001). Despite the statistical differences observed in the duration of each immature stage, the total development time (egg–adult) was similar for all treatments (χ2 = 8.1, df = 4; p = 0.09).

Table 3.
Mean duration of the different developmental stages ± SE (% survival) of Neoseiulus idaeus after contact with residues of pesticides used on soybean crops, at 25 ± 1 °C, 75 ± 10% RH, and 12 h photoperiod.
3.2.2. Reproductive Parameters and Adult Longevities
The pre-oviposition times were relatively longer for the offspring of females treated with chlorantraniliprole and teflubenzuron, though not significantly different than the glyphosate and control treatments (Table 4). The oviposition period was shorter for chlorantraniliprole, which was statistically different from the control but similar to flubendiamide, glyphosate, and teflubenzuron (χ2 = 14.9; df = 4; p = 0.004). However, the post-oviposition time had no difference among the treatments (χ2 = 6.1; df = 4; p = 0.188).

Table 4.
Mean (±SE) of pre-oviposition, oviposition, and post-oviposition periods (days), fecundity (eggs per female), eggs/female/day, and longevity of Neoseiulus idaeus after contact with residues of pesticides used on soybean crops, at 25 ± 1 °C, 75 ± 10% RH, and 12 h photoperiod.
The mean fecundity differed in females exposed to chlorantraniliprole, teflubenzuron, and glyphosate from those exposed to flubendiamide and control (χ2 = 31.9; df = 4, p = < 0.001). However, no significant differences among treatments were observed in daily oviposition (χ2 = 6.5; df = 4; p = 0.164) (Table 4).
Newly emerged and mated females showed oviposition peaks between Days 3 and 12 after emergence. Higher oviposition peaks were observed in the control and flubendiamide treatments (Figure 1). In the control treatment, mortality of adult females occurred beginning with Day 8 and increased progressively until the last adult died on Day 20. Females in the other treatments showed gradual mortality between the second and third days after emergence. In the control treatment, the mean daily oviposition increased progressively from the second day onward, reaching the highest peak on the sixth day after emergence. The mean longevity of adult females did not differ statistically among treatments (χ2 = 5.7; df = 4; p = 0.220). In contrast, adult males had longer lifespans in the glyphosate treatment than the control, which did not statistically differ from chlorantraniliprole, flubendiamide, and teflubenzuron (χ2 = 12.4; df = 4; p = 0.014).
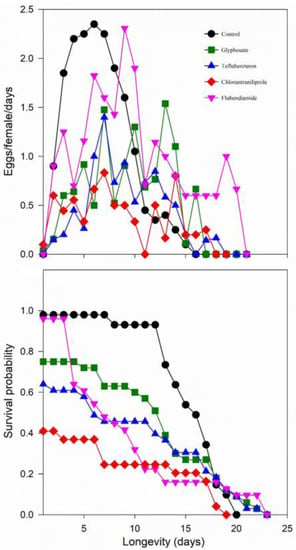
Figure 1.
Mean of eggs/female/day and survival probability of females of Neoseiulus idaeus after contact with residues of pesticides used on soybean crops, at 25 ± 1 °C, 75 ± 10% RH, and 12 h photoperiod.
3.2.3. Population Growth Parameters
The net reproductive rate (R0) of N. idaeus was considerably higher in the flubendiamide and control treatments, with a projected increase of 8–9 times in each generation, respectively (Table 5). The intrinsic growth rate (rm), which represents the population growth rate based on the daily number of females generated by females, was affected by chlorantraniliprole, glyphosate, and teflubenzuron. The finite increase rate (λ) indicates the ratio of population growth of N. idaeus, which in this case did not differ statistically among treatments. Finally, the mean generation time (T) was longer in the chlorantraniliprole, glyphosate, and teflubenzuron treatments, which were statistically different from flubendiamide.

Table 5.
Life table parameters ± SE of the parental generation of Neoseiulus idaeus after contact with residues of pesticides used on soybean crops, at 25 ± 1 °C, 75 ± 10% RH, and 12 h photoperiod.
3.2.4. Age–Stage Survival Rate
The probability of emergence/hatching and survivability of each of the stages of N. idaeus after contact with pesticides was estimated by the age–stage survival rate (Sxj), which represents the probability of a newborn individual surviving to age x at stage j (Figure 2).
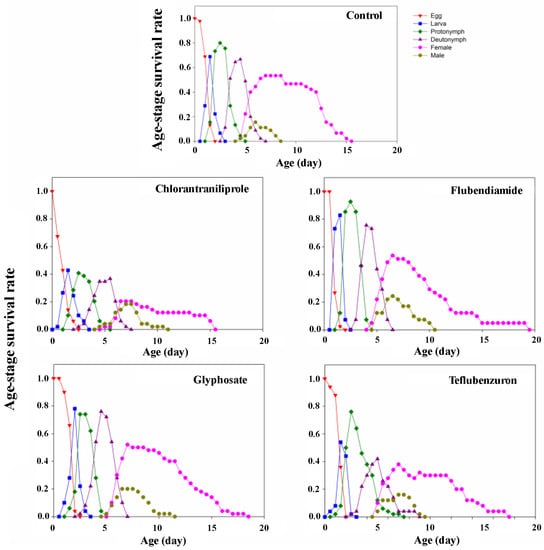
Figure 2.
Age–stage-specific survival rate (Sxj) of Neoseiulus idaeus after contact with residues of pesticides used on soybean crops, at 25 ± 1 °C, 75 ± 10% RH, and 12 h photoperiod.
Consistent with results of the survival of the different mite stages (Section 3.2.1), the estimated projection in the graph Sxj showed that the survival curves also indicated a lower survival rate in the egg and deutonymph stages of the offspring of the females treated with chlorantraniliprole and teflubenzuron, respectively. In all treatments, the overlapping stages indicated that hatching/emergence rates varied among individuals at each stage of development.
3.3. Egg Viability
The viability of eggs produced in the parental generation (F0) by females treated with chlorantraniliprole was 42%, representing a significant decrease compared with other treatment groups (χ2 = 105.63; p < 0.001). However, for all treatments, the eggs generated by the maternal (F1) generation had high viability (Table 6), with no statistical differences among treatments (χ2 = 9.09; p = 0.059).

Table 6.
Mean egg viability ± SE and (CI) of the parental generation (F0) and offspring (F1) of Neoseiulus idaeus after contact with residues of pesticides used on soybean crops, at 25 ± 1 °C, 75 ± 10% RH, and 12 h photoperiod.
3.4. IOBC Sublethal Toxicity Classification
The IOBC-WPRS determines the physiological selectivity of the pesticides on the basis of lethal and sublethal effects of bioassays under laboratory conditions. The results of these studies are then used to determine application rates in production settings. In this case, the pesticides flubendiamide, glyphosate, and teflubenzuron were classified as harmless (Class 1: E < 25%; Table 7). Only chlorantraniliprole was classified as moderately harmful (Class 3: 25% ≤ E ≤ 50%).

Table 7.
Sublethal effects on the parental generation (F0) of Neoseiulus idaeus after contact with residues of pesticides used on soybean crops, at 25 ± 1 °C, 75 ± 10% RH, and 12 h photoperiod.
3.5. Morphological Characteristics of the Stages
Although the morphological characteristics of N. idaeus were not compared statistically, we provide information on morphology to describe the development cycle and biology of the predator. Eggs of N. idaeus are elliptical, with a smooth translucent chorion (Figure 3A). Larvae are opaque white and move slowly (Figure 3B). In all treatments, the larvae did not feed on any of the immature stages of T. urticae. The protonymphs are more agile and constantly seek their prey, maintaining the coloration of the larval stages (Figure 3C). The pale-yellow deutonymphs also search actively for their prey (Figure 3D). Adult males and females are pale yellow (Figure 3E,F); females are larger and have a pyriform body, while males are smaller and have a flatter body.

Figure 3.
Life stages of immatures and adults of the predatory mite Neoseiulus idaeus. (A) eggs; (B) larva; (C) protonymph; (D) deutonymph; (E) adult female; and (F) adult male.
4. Discussion
Several studies have determined the lethal and sublethal effects of pesticides on predatory mites to evaluate their compatibility for inclusion in integrated pest management programs. Such studies reduce ecological and environmental risks and the consequences to agricultural systems. Here, we observed that the acute toxicity of pesticides to N. idaeus was highest for acephate and for imidacloprid and bifenthrin, causing mortality of 100%, and for thiamethoxam and lambda-cyhalothrin, causing mortality of 75%. On the basis of the highly toxic effects on the predator, we do not recommend their use in the field without studies in semi-field and field conditions.
The toxic effects of acephate on natural enemies of pests are well known. Studies by Mochizuki [60] on Amblyseius womersleyi Schicha, and by Ferla and Moraes [61] on Euseius concordis (Chant) and Neoseiulus anonymus Denmark & Muma (all Phytoseiidae) showed that this insecticide is harmful. Silva et al. [62] described a similar effect on two Brazilian populations of E. concordis. In addition to phytoseiid predators, acephate was also classified as harmful to Cosmolaelaps sabelisi (Laelapidae) [63]. Mixtures of neonicotinoids and pyrethroids may have a higher risk of toxicity to natural enemies because of the possible synergistic effect [64,65]. This phenomenon occurs when combining modes of action, by additive or potentiation effects [66,67] because, while pyrethroids act by blocking sodium ions in the presynaptic membrane [68,69,70], neonicotinoids act as antagonists of acetylcholine receptors on the postsynaptic neuron [71,72,73]. Both types of compounds cause neuron overexcitation, muscle exhaustion, paralysis, and death in exposed individuals. Therefore, on the basis of the mortality of N. idaeus females, the ready-mix pesticides were classified, using IOBC toxicity categories, from highest to lowest as: acetamiprid and fenpropathrin > acetamiprid and alpha-cypermethrin = thiamethoxam and lambda-cyhalothrin > imidacloprid and bifenthrin.
When females of N. idaeus were treated with a methoxyfenozide and spinetoram mixture, the effects on mortality were less significant than with the other mixtures. The effects of diacylhydrazine in mixtures with spinosyn on predatory mites have not been studied. However, the two compounds act specifically in combination. Methoxyfenozide accelerates molting, binding to the ecdysone receptor [74]. Therefore, the action of diacylhydrazine is expected to be more evident in immature stages than in adults, as suggested by several studies that affirmed its innocuity [75,76,77,78,79,80,81,82]. In contrast, the spinosyn-based insecticides can also be neurotoxic, acting by contact or ingestion [83], and harmful effects on natural enemies are more evident when they are ingested [84]. For example, higher mortality rates were observed in females of C. sabelisi (Laelapidae) when they ingested a mixture of Rabidtella axey (Nematoda) contaminated with spinosyn residues at a dose recommended for controlling thrips [63]. Driesche et al. [85] found similar effects on Neoseiulus cucumeris (Oudemans) and Iphiseius degenerans (Berlese) (Phytoseiidae). After contact with three different doses of spinosad, a population of Typhlodromus (Typhlodromus) pyri Scheuten (Phytoseiidae) was significantly affected, with high total mortality rates [86].
In this study, chlorfenapyr caused 53% mortality and was classified as moderately harmful. Although this insecticide caused significant mortality in females of N. idaeus, other species of Phytoseiidae have shown higher tolerance. The acute toxicity of any insecticide to non-target organisms will depend on the dose and detoxification capacity of an individual. For example, Cloyd et al. [87] tested three different chlorfenapyr doses on females of Phytoseiulus persimilis Athias-Henriot (Phytoseiidae) and observed that mortality rates reached 100% on the seventh day of evaluation, while females of Neoseiulus californicus McGregor (Phytoseiidae), G. aculeifer, and Stratiolaelaps miles (Berlese) (Laelapidae) treated under similar conditions were less affected. Indoxacarb caused 29% mortality and was considered moderately harmful. Indoxacarb is a neurotoxin and a sodium channel blocker; in most studies, contact-exposed predatory mites have shown little sensitivity. For example, Bostanian et al. [88] reported that females of Neoseiulus fallacis (Garman) (Phytoseiidae) exposed to 0.05 g A.I. L−1 of indoxacarb showed high survival after 72 hours. Likewise, Bostanian et al. [89] found that three doses of indoxacarb (0.05, 0.114, and 0.228 g of a.i. L–1) were harmless to N. fallacis and P. persimilis under laboratory conditions.
Although chlorantraniliprole did not cause significant mortality in females exposed to residues, the progeny showed sublethal effects, mainly a 56% reduction in survival of immatures. This high mortality of immature stages affected the sex ratio, resulting in similar numbers of males and females generated. Although there was a strong trend, the effect on the pre-oviposition period of newly emerged females and the considerable reductions in fecundity and daily oviposition were not statistically significant. Thus, we suggest that effects on reproductive parameters are due to infertility of females and males. In addition to our study, Khan et al. [90] reported that the development and reproduction of Paederus fuscipes (Staphylinidae: Coleoptera) were affected after the beetles were exposed to chlorantraniliprole. Nawaz et al. [91] found that the second and fourth-instar larval and pupal stages of Harmonia axyridis (Pallas) (Coleoptera: Coccinellidae) were prolonged when treated with LC10 (2.42 mg of a.i. L−1) and LC30 (12.06 mg of a.i. L−1) doses of chlorantraniliprole, and the fecundity and longevity of adult females were also reduced. However, many other studies have concluded that this insecticide is safe for other natural enemies, including predatory arthropods [81,92,93,94,95,96,97].
Females treated with teflubenzuron showed a 34% reduction in survival of immatures, although this was not statistically significant. The mean fecundity and daily oviposition rate (eggs/female/day) were both statistically lower. Souza et al. [98] found that the reduction in larval hatching and adult emergence of the predator Chrysoperla externa (Hagen) (Neuroptera: Chrysopidae) was one of the most evident sublethal effects caused by teflubenzuron. Another interesting sublethal effect was reported by Pérez-Aguilar et al. [99]: the offspring of females of Engytatus varians (Heteroptera: Miridae) derived from nymphs treated with teflubenzuron were significantly smaller than the offspring from the control treatment. These authors also reported higher survival rates of nymphs from the offspring of adult females that had been treated with this insecticide. In addition to these effects, teflubenzuron reduced the feeding activity of the predator Orius insidiosus (Say) (Hemiptera: Anthocoridae) [100], as well as the predation rate of Orius laevigatus (Fieber) (Hemiptera: Anthocoridae) [101]. Teflubenzuron is an insect growth regulator of the benzoylurea group, which inhibits chitin synthesis and is used mainly to control lepidopteran larvae [102]. In this case, ingestion and contact with particles of this insecticide by predatory insects and mites can cause problems related to the effects on immature stages. For that reason, the mortality rate for N. idaeus was significantly higher in the offspring than in the adult females.
Flubendiamide did not cause significant lethal effects on exposed adult females. However, the eggs generated by these females hatched more quickly than eggs from the control females. In this case, even though there was a statistical difference in the duration of the egg phase, the observed effects can be ascribed to chance, particularly as such effects have not been reported for other arthropods exposed to flubendiamide under the conditions used in our study. Furthermore, the fecundity of the progeny was lower compared with the control. These effects are a consequence of oocyte maturation, regulated by the juvenile hormone and the molting hormone, both of which also play essential roles in the reproduction of mites and, in this case, may have had negative effects on reproduction. Similar effects were observed in the progeny of females treated with glyphosate, but in this case, the reason for the effects on predatory mites is unknown. However, evidence indicates that glyphosate induces oxidative stress through lipid peroxidation of antioxidant defenses, reducing immunity in many organisms, including arthropods [103,104,105]. Here, this oxidative stress may be related to the reduction in the offspring of females treated with glyphosate, an hypothesis that needs further investigation.
Life tables summarize the population characteristics of a species whose development, fecundity, survival rate, and population growth respond to environmental or biotic factors. In this case, two-sex life tables provide the growth trend of the N. idaeus population on the basis of the sublethal effects observed during its development and reproduction. The life table parameters indicated better growth and stability projections for the offspring of mites exposed to flubendiamide, similar to the control treatment. In this case, the net reproductive rate (R0) calculated for offspring (F1) females treated with flubendiamide indicated that each female of N. idaeus produced 7.90 females generated by females every 16.45 days (T), with daily population growth (λ) of 1.13% for a daily production of 0.127 females/female/day (rm). In the chlorantraniliprole and teflubenzuron treatments, the instantaneous population growth rates were very low, indicating that the populations in these treatments were very close to the rate of decline, due to the lower density of predators produced because of the negative reproductive effects.
As with chlorantraniliprole, teflubenzuron appears to have lower toxicity to a range of non-target organisms compared with the broad-spectrum pesticides reported here. Chlorantraniliprole and flubendiamine are anthranilic and phthalic diamides, respectively, and function by activating ryanodine receptors, leading to the uncontrolled release of calcium from intracellular stores [106]. Although these compounds act in a similar way in organisms that come into contact with or ingest their molecules, their sublethal effects on predatory mites differed. While chlorantraniliprole was classified as moderately harmful, flubendiamine was classified as harmless. The sublethal effects of chlorantraniliprole were driven by the high mortality in the immature stage, which most likely occurred because this systemic insecticide may have persisted longer than flubendiamide on the treated leaf discs, thus increasing the exposure time of the mites.
5. Conclusions
Based on all the effects evaluated under laboratory conditions, the pesticides compatible with the predatory mite were flubendiamide, glyphosate, and teflubenzuron. Studies in semi-field and field conditions are encouraged to evaluate the impact of insecticides that were not classified as compatible here since their interaction with abiotic factors can result in weaker responses than those observed under laboratory conditions. In addition, compatible pesticides for N. idaeus must be evaluated regarding their risk to the many other natural enemies and pollinators associated with soybean crops in Brazil.
Author Contributions
G.B., P.T.Y. and L.L.G. conceived and designed the research; G.B., L.L.G., M.Y. and E.D.R.S. conducted experiments and collected the data; G.B., F.H.I.F., J.d.B.P. and L.V.d.Q.O. analyzed the data; G.B. and L.L.G. wrote the manuscript; G.B., L.L.G., F.H.I.F., M.Y., E.D.R.S., J.d.B.P., L.V.d.Q.O. and P.T.Y. contributed to the interpretation of results and overall improvement of the manuscript. All authors commented on previous versions of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
Funding was provided by the Fundação de Amparo à Pesquisa do Estado de São Paulo for L.L.G. (2020/15134-6), F.H.I.F. (2019/26145-1), and J.dB.P. (2019/26099-0) and by the São Paulo Advanced Research Center for Biological Control/ FAPESP and Koppert Biological Systems for P.T.Y. (2018/02317-5).
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We would like to thank Hsin Chi from National Chung Hsing University in Taiwan for help with the Age–Stage, two-sex life table Software. We thank Janet W. Reid for help with manuscript editing and proofreading. We thank José Bruno Malaquias from the Entomology Laboratory, Center for Agrarian Sciences, Federal University of Paraíba, Areia, Brazil, for valuable suggestions on statistical analyses. Thanks to Marcelo Poletti, C.O. of the Promip Manejo Integrado de Pragas, for help with the initial colony of Tetranychus urticae. Thanks to the reviewers for their valuable suggestions.
Conflicts of Interest
The authors declare that they have no competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Hartman, G.L.; West, E.D.; Herman, T.K. Soybean: Worldwide production, use, and constraints caused by pathogens and pests. Food Sec. 2011, 3, 5–17. [Google Scholar] [CrossRef]
- Voora, V.; Larrea, C.; Bermudez, S. Global Market Report: Soybeans; International Institute for Sustainable Development (IISD): Winnipeg, MB, Canada, 2020; Available online: https://www.jstor.org/stable/resrep26554 (accessed on 5 February 2023).
- Gaonkar, V.; Rosentrater, K.A. Chapter 4—Soybean. In Integrated Processing Technologies for Food and Agricultural By-Products; Pan, Z., Zhang, R., Zicari, S., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 73–104. [Google Scholar]
- Thrane, M.; Paulsen, P.V.; Orcutt, M.W.; Krieger, T.M. Soy protein: Impacts, production, and applications (Ch. 2). In Sustainable Protein Sources; Nadathur, S.R., Wanasundara, J.P.D., Scanlin, L., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 23–45. [Google Scholar]
- USDA, United States Department of Agriculture. Foreign Agricultural Service, World Agricultural Production. Circular Series WAP 7–22 July 2022; Available online: https://apps.fas.usda.gov/psdonline/circulars/production.pdf (accessed on 7 July 2022).
- CONAB, Companhia Nacional de Abastecimento. Acompanhamento da safra Brasileira de Grãos. Safra 2021/22, 10° Levantamento; Available online: https://www.conab.gov.br/info-agro/safras/graos/boletim-da-safra-de-graos (accessed on 23 July 2022).
- Hoffmann-Campo, C.B.; Moscadi, F.; Corrêa-Ferreira, B.S.; Sosa-Gómez, D.R.; Panizzi, A.R.; Corso, I.C.; Gazzoni, D.L.; Oliveira, E.B. Pragas da Soja no Brasil e seu Manejo Integrado; Embrapa Soja: Londrina, Brazil, 2000; 70p. [Google Scholar]
- Bueno, R.C.O.F.; Bueno, A.F.; Moscardi, F.; Parra, J.R.P.; Hoffmann-Campo, C.B. Lepidopteran larva consumption of soybean foliage: Basis for developing multiple-species economic thresholds for pest management decisions. Pest Manag. Sci. 2011, 67, 170–174. [Google Scholar] [CrossRef]
- Bernardi, O.; Malvestiti, G.S.; Dourado, P.M.; Oliveira, W.S.; Martinelli, S.; Berger, G.U.; Head, G.P.; Omoto, C. Assessment of the high dose concept and level of control provided by MON 87701 × MON 89788 soybean against Anticarsia gemmatalis and Pseudoplusia includens (Lepidoptera: Noctuidae) in Brazil. Pest Manag. Sci. 2012, 68, 1083–1091. [Google Scholar] [CrossRef]
- Moscardi, F.; Bueno, A.F.; Sosa-Gómez, D.R.; Roggia, S.; Hoffmann-Campo, C.B.; Aline Pomari, F.; Corso, I.C.; Yano, S.A.C. Artrópodes que atacam as folhas da soja. In Soja: Manejo Integrado de Insetos e Outros Artrópodes-Praga; Hoffman-Campo, C.B., Côrrea-Ferreira, B.S., Moscardi, F., Eds.; Embrapa: Brasília, Brazil, 2012; pp. 213–334. [Google Scholar]
- Czepak, C.; Albernaz, K.C.; Vivan, L.M.; Guimarães, H.O.; Carvalhais, T. Primeiro registro de ocorrência de Helicoverpa armigera (Hubner) (Lepidoptera: Noctuidae) no Brasil. Pesqui. Agropecu. Bras. 2013, 43, 110–113. [Google Scholar] [CrossRef]
- Justiniano, W.; Fernandes, M.G.; Viana, C.L.T.P. Diversity, composition and population dynamics of arthropods in the genetically modified soybeans Roundup Ready® RR1 (GT 40-3-2) and Intacta RR2 PRO® (MON87701 × 606 MON89788). J. Agric. Sci. 2014, 6, 33–44. [Google Scholar] [CrossRef]
- Stacke, R.F.; Arnemann, J.A.; Rogers, J.; Stacke, R.S.; Strahl, T.T.; Perini, C.R.; Dossin, M.F.; Pozebon, H.; Cavallin, L.A.; Guedes, J.V.C. Damage assessment of Helicoverpa armigera (Lepidoptera: Noctuidae) in soybean reproductive stages. Crop. Prot. 2018, 112, 10–17. [Google Scholar] [CrossRef]
- Panizzi, A.R.; McPherson, J.E.; James, D.G.; Javahery, M.; McPherson, R.M. Stink bugs (Pentatomidae). In Heteroptera of Economic Importance; Schaefer, C.W., Panizzi, A.R., Eds.; CRC Press: Boca Raton, FL, USA, 2000; pp. 421–474. [Google Scholar]
- Silva, F.A.C.; Da Silva, J.J.; Depieri, R.A.; Panizzi, A.R. Feeding Activity, Salivary Amylase Activity, and Superficial Damage to Soybean Seed by Adult Edessa meditabunda (F.) and Euschistus heros (F.) (Hemiptera: Pentatomidae). Neotrop. Entomol. 2012, 41, 386–390. [Google Scholar] [CrossRef]
- Smaniotto, L.F.; Panizzi, A.R. Interactions of Selected Species of Stink Bugs (Hemiptera: Heteroptera: Pentatomidae) from Leguminous Crops with Plants in the Neotropics. Fla. Entomol. 2015, 98, 7–17. [Google Scholar] [CrossRef]
- Sosa-Gómez, D.R.; Corrêa-Ferreira, B.S.; Kraemer, B.; Pasini, A.; Husch, P.E.; Vieira, C.E.D.; Martinez, C.B.R.; Lopes, I.O.N. Prevalence, damage, management and insecticide resistance of stink bug populations (Hemiptera: Pentatomidae) in commodity crops. Agric. For. Entomol. 2019, 22, 99–118. [Google Scholar] [CrossRef]
- Flechtmann, C.H.W. Ácaros de Importância Agrícola; Nobel: São Paulo, Brazil, 1972; 150p. [Google Scholar]
- Bolland, H.H.R.; Gutierrez, J.; Flechtmann, C.H.W. World Catalogue of the Spider Mite Family (Acari: Tetranychidae); Brill: Leiden, The Netherlands, 1998; 392p. [Google Scholar]
- Navia, D.; Flechtmann, C.H.W. Rediscovery and redescription of Tetranychus gigas (Acari, Prostigmata, Tetranychidae). Zootaxa 2004, 547, 1–8. [Google Scholar] [CrossRef][Green Version]
- Guedes, J.V.C.; Navia, D.; Lofego, A.C.; Dequech, S.T.B. Ácaros associados à cultura da soja no Rio Grande do Sul, Brasil. Neotrop. Entomol. 2007, 36, 288–293. [Google Scholar] [CrossRef]
- Roggia, S.; Guedes, J.V.C.; Kuss, R.C.R.; Arnemann, J.A.; Navia, D. Spider mites associated to soybean in Rio Grande do Sul, Brazil. Pesqui. Agropecu. Bras. 2008, 43, 295–301. [Google Scholar] [CrossRef][Green Version]
- Rezende, J.M.; Lofego, A.C.; Návia, D.; Roggia, S. Mites (Acari: Mesostigmata, Sarcoptiformes and Trombidiformes) associated to soybean in Brazil, including new records from the cerrado areas. Fla. Entomol. 2012, 95, 683–693. [Google Scholar] [CrossRef]
- Cavalcante, A.C.C.; Demite, P.R.; Carneiro, M.E.S.; Nunes, L.S.; Pereira, A.I.A. Mites (Acari) associated with soybean culture (Glycine max), in the southeast region of Goiás state. Magistra 2018, 29, 266–272. [Google Scholar]
- Suekane, R.; Degrande, P.E.; de Melo, E.P.; Bertoncello, T.F.; Lima Junior, I.S.; Kodama, C. Damage level of the two-spotted spider mite Tetranychus urticae Koch (Acari: Tetranychidae) in soybeans. Rev. Ceres. 2018, 59, 77–81. [Google Scholar] [CrossRef]
- Arnemann, J.A.; Fiorin, R.A.; Perini, C.R.; Storck, L.; Curioletti, L.E.; Nachman, G.; Guedes, J.V. Density and growth rates of spider mites in relation to phonological stages of soybean cultivars in Brazil. Exp. Appl. Acarol. 2015, 67, 423–440. [Google Scholar] [CrossRef]
- Arnemann, J.A.; Fiorini, R.A.; Guedes, J.V.C.; Pozebon, H.; Marques, R.F.; Perini, R.C.; Storck, L. Assessment of damage caused by the spider mite Mononychellus planki (McGregor) on soybean cultivars in South America. Aust. J. Crop Sci. 2018, 12, 1989–1996. [Google Scholar] [CrossRef]
- Padilha, G.; Fiorini, R.A.; Cargnelutti Filho, A.; Pozebon, H.; Rogers, J.; Marques, R.P.; Castilhos, L.B.; Donatti, A.; Stefanelo, L.; Burtet, L.M.; et al. Damage assessment and economic injury level of the two-spotted spider mite Tetranychus urticae in soybean. Pesqui. Agropecu. Bras. 2020, 55, e01836. [Google Scholar] [CrossRef]
- Talebi, K.; Hosseininaveh, V.; Ghadamyari, M. Ecological impacts of pesticides in agricultural ecosystem. In Pesticides in the Modern World—Risks and Benefits; Stoytcheva, M., Ed.; IntechOpen: London, UK, 2011; pp. 8–169. Available online: https://www.intechopen.com/books/431 (accessed on 13 December 2022).
- Guedes, R.N.; Smagghe, G.; Stark, J.D.; Desneux, D. Pesticide induced stress in arthropod pests for optimized integrated pest management programs. Annu. Rev. Entomol. 2016, 61, 43–62. [Google Scholar] [CrossRef]
- Kogan, M. Integrated Pest Management: Historical Perspectives and Contemporary Developments. Annu. Rev. Entomol. 1998, 43, 243–270. [Google Scholar] [CrossRef]
- Ehler, L.E. Perspective Integrated pest management (IPM): Definition, historical development and implementation, and the other IPM. Pest Manag. Sci. 2006, 62, 787–789. [Google Scholar] [CrossRef] [PubMed]
- Panizzi, A.R. History and Contemporary Perspectives of the Integrated Pest Management of Soybean in Brazil. Neotrop. Entomol. 2013, 42, 119–127. [Google Scholar] [CrossRef] [PubMed]
- McMurtry, J.A.; Croft, B.A. Life-styles of phytoseiid mites and their roles in biological control. Annu. Rev. Entomol. 1997, 42, 291–321. [Google Scholar] [CrossRef] [PubMed]
- Gerson, U.; Smiley, R.L.; Ochoa, R. Mites (Acari) for Pest Control; Blackwell Science: Oxford, UK, 2003; 539p. [Google Scholar]
- McMurtry, J.A.; de Moraes, G.J.; Sourasso, N.F. Revision of the lifestyles of phytoseiid mites (Acari: Phytoseiidae) and implications for biological control strategies. Syst. Appl. Acarol. 2013, 18, 297–320. [Google Scholar] [CrossRef]
- McMurtry, J.A.; Sourassou, N.F.; Demite, P. The Phytoseiidae (Acari: Mesostigmata) as biological control agents. In Prospects for Biological Control of Plant Feeding Mites and Other Harmful Organisms; Carrillo, D., de Moraes, G.J., Peña, J., Eds.; Springer: Cham, UK, 2015; pp. 133–149. [Google Scholar]
- Demite, P.R.; McMurtry, J.A.; de Moraes, G.J. Phytoseiidae Database: A website for taxonomic and distributional information on phytoseiid mites (Acari). Zootaxa 2014, 3795, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Demite, P.R.; de Moraes, G.J.; McMurtry, J.A.; Denmark, H.A.; Castilho, R.C. Phytoseiidae Database. Available online: www.lea.esalq.usp.br/phytoseiidae (accessed on 21 July 2022).
- Reichert, M.B.; da Silva, G.L.; Rocha, M.D.S.; Johan, L.; Ferla, N.J. Mite fauna (Acari) in soybean agroecosystem in the northwestern region of Rio Grande do Sul State, Brazil. Syst. Appl. Acarol. 2014, 19, 123–136. [Google Scholar] [CrossRef]
- Reichert, M.B.; Toldi, M.; Ferla, N.J. Feeding preference and predation rate of Neoseiulus idaeus (Acari: Phytotseiidae) feeding on different preys. Syst. Appl. Acarol. 2016, 21, 1631–1640. [Google Scholar] [CrossRef]
- Reichert, M.B.; Toldi, M.; Rode, P.A.; Ferla, J.J.; Ferla, N.J. Biological performance of the predatory mite Neoseiulus idaeus (Phytoseiidae): A candidate for the control of tetranychid mites in Brazilian soybean crops. Braz. J. Biol. 2016, 77, 361–366. [Google Scholar] [CrossRef]
- Collier, K.F.S.; Albuquerque, G.S.; de Lima, J.O.G.; Pallini, A.; Molina-Rugama, A. Neoseiulus idaeus (Acari: Phytoseiidae) as a potential biocontrol agent of the two-spotted spider mite, Tetranychus urticae (Acari: Tetranychidae) in papaya: Performance on different prey stage–host plant combinations. Exp. Appl. Acarol. 2007, 41, 27–36. [Google Scholar] [CrossRef]
- Sousa Neto, E.P.; Filgueiras, R.M.C.; Mendes, J.A.; Melo, J.W.S. Functional and numerical responses of Neoseiulus idaeus and Neoseiulus californicus to eggs of Tetranychus urticae. Int. J. Acarol. 2019, 45, 1–4. [Google Scholar]
- Pantaleão, A.A.S.S.; Moreira, J.O.T.; Sato, M.E.; Pionório, J.A.A. Population growth of Tetranychus urticae Koch (Acari: Tetranychidae) and predation rate of the pest mite by Neoseiulus idaeus Denmark & Muma (Acari: Phytoseiidae) in two grape cultivars. Arq. Inst. Biol. 2019, 88, 1–11. [Google Scholar]
- Sousa Neto, E.P.; Filgueiras, R.M.C.; Mendes, J.A.; Monteiro, N.V.; Lima, D.B.; Pallini, A.; Melo, J.W.S. A drought-tolerant Neoseiulus idaeus (Acari: Phytoseiidae) strain as a potential control agent of two-spotted spider mite, Tetranychus urticae (Acari: Tetranychidae). Biol. Control 2021, 159, 104624. [Google Scholar] [CrossRef]
- McMurtry, J.A.; Scriven, G.T. Insectary Production of Phytoseiid Mites. J. Econ. Entomol. 1965, 58, 282–284. [Google Scholar] [CrossRef]
- Brasil, Ministério da Agricultura, Pecuária e Abastecimento—Agrofit. Available online: http://agrofit.agricultura.gov.br/agrofit_cons/principal_agrofit_cons (accessed on 6 December 2021).
- Hassan, S.A.; Bigler, F.; Bogenschutz, H.; Boller, E.; Brun, J.; Calis, J.N.M.; Coremans Pelseneer, J.; Duso, C.; Grove, A.; Heimbach, U.; et al. Results of the sixth joint pesticide testing programme of the IOBC/WPRS working group ‘pesticides and beneficial organisms’. Entomophaga 1994, 39, 107–119. [Google Scholar] [CrossRef]
- van de Veire, M.; Smagghe, G.; Degheele, D. A laboratory test method to evaluate the effect of 31 pesticides on the predatory bug, Orius laevigatus (Het: Anthocoridae). Entomophaga 1996, 41, 235–243. [Google Scholar] [CrossRef]
- Hassan, S. International Organization for Biological and Integrated Control of Noxious Animals and Plants/Working Group “Pesticides and Beneficial Organisms” 1992. Guidelines for testing the effects of pesticides on beneficial organisms: Description of test methods. IOBC-WPRS Bull. 1992, 15, 3. [Google Scholar]
- Moral, R.A.; Hinde, J.; Demétrio, C.G.B. Half-Normal Plots and Overdispersed Models in R: The hnp Package. J. Stat. Softw. 2017, 81, 1–23. [Google Scholar] [CrossRef]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous Inference in General Parametric Models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing, Version 4.1.2; R Foundation for Statistical Computing: Vienna, Austria, 2020.
- Abbott, W.S. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Chi, H. Life-table analysis incorporating both sexes and variable development rates among individuals. Environ. Entomol. 1988, 17, 26–34. [Google Scholar] [CrossRef]
- Chi, H. TWOSEX-MSChart: A Computer Program for the Age-Stage, Two-Sex Life Table Analysis. 2014. Available online: http://140.120.197.173/Ecology/.%20 (accessed on 5 April 2022).
- Chi, H.; Liu, H. Two new methods for the study of insect population ecology. Bull. Inst. Zool. Acad. Sin. 1985, 24, 225–240. [Google Scholar]
- Huang, Y.B.; Chi, H. Age-stage, two-sex life tables of Bactrocera cucurbitae (Coquillett) (Diptera: Tephritidae) with a discussion on the problem of applying female age-specific life tables to insect populations. Insect Sci. 2012, 19, 263–273. [Google Scholar] [CrossRef]
- Mochizuki, M. Effectiveness and pesticide susceptibility of the pyrethroid resistant predatory mite Amblyseius womersleyi in the integrated pest management of tea pests. BioControl 2003, 48, 207–221. [Google Scholar] [CrossRef]
- Ferla, N.J.; de Moraes, G.J. Seletividade de acaricidas e inseticidas a ácaros predadores (Acari: Phytoseiidae) encontrados em seringueira no centro-oeste do Brasil. Cienc. Rural 2006, 36, 357–362. [Google Scholar] [CrossRef]
- Silva, M.Z.; Sato, M.E.; de Oliveira, C.A.L.; Rais, D.S. Toxicidade diferencial de agrotóxicos utilizados em citros para Neoseiulus californicus, Euseius concordis e Brevipalpus phoenicis. Bragantia 2011, 70, 87–95. [Google Scholar] [CrossRef]
- Barroso, G.; Pazini, J.B.; Iost Filho, F.H.; Barbosa, D.P.L.; de Paiva, A.C.R.; Matioli, T.F.; Yamamoto, P.T. Are Pesticides Used to Control Thrips Harmonious with Soil-Dwelling Predatory Mite Cosmolaelaps sabelis (Mesostigmata: Laelapidae)? J. Econ. Entomol. 2022, 115, 151–159. [Google Scholar] [CrossRef]
- Corbel, V.; Stankiewicz, M.; Bonnet, G.F.; Hougard, J.M.; Lapied, B. Synergism between insecticides permethrin and propoxur occurs through activation of presynaptic muscarinic negative feedback of acetylcholine release in the insect central nervous system. NeuroToxicology 2006, 27, 508–519. [Google Scholar] [CrossRef]
- Cedergreen, N. Quantifying synergy: A systematic review of mixture toxicity studies within environmental toxicology. PLoS ONE 2014, 9, e96580. [Google Scholar] [CrossRef]
- Kostromytska, O.S.; Buss, E.A.; Scharf, M.E. Toxicity and neurophysiological 615 effects of selected insecticides on the mole cricket, Scapteriscus vicinus (Orthoptera: Gryllotalpidae). Pestic. Biochem. Phys. 2011, 100, 27–34. [Google Scholar] [CrossRef]
- Zhao, G.-P.; Yang, F.-W.; Li, J.-W.; Xing, H.-Z.; Ren, F.-Z.; Pang, G.-F.; Li, Y.-X. Toxicities of Neonicotinoid-Containing Pesticide Mixtures on Nontarget Organisms. Environ. Toxicol. Chem. 2020, 39, 1884–1893. [Google Scholar] [CrossRef]
- Khambay, B.P.S.; Jewess, P.J. Pyrethroids. In Comprehensive Molecular Insect Science; Gilbert, L.I., Ed.; Elsevier: Amsterdam, The Netherlands, 2005; pp. 1–29. [Google Scholar]
- Wakeling, E.N.; Neal, A.P.; Atchison, W.D. Pyrethroids and Their Effects on Ion Channels. In Pesticides: Advances in Chemical and Botanical Pesticides; Soundararajan, R.P., Ed.; InTech: Rijeka, Croatia, 2012; pp. 39–66. [Google Scholar]
- Soderlund, D.M. Neurotoxicology of pyrethroid insecticides. In Advances in Neurotoxicology, 4th ed.; Aschner, M., Costa, L.G., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 113–165. [Google Scholar]
- Nauen, R.; Ebbinghaus-Kintscher, U.; Elbert, A.; Jeschke, P.; Tietjen, K. Acetylcholine Receptors as Sites for Developing Neonicotinoid Insecticides. In Biochemical Sites of Insecticide Action and Resistance; Ishaaya, I., Ed.; Springer: Berlin/Heidelberg, Germany, 2001; pp. 77–105. [Google Scholar]
- Matsuda, K.; Buckingham, S.D.; Kleier, D.; Rauh, J.J.; Grauso, M.; Sattelle, D.B. Neonicotinoids: Insecticides acting on insect nicotinic acetylcholine receptors. Trends Pharmacol. Sci. 2001, 22, 573–580. [Google Scholar] [CrossRef]
- Anadón, A.; Ares, I.; Martínez, M.; Martínez-Larrañaga, M.-R.; Martínez, M.-A. Neurotoxicity of Neonicotinoids. In Advances in Neurotoxicology; Aschner, M., Costa, L.G., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 167–207. [Google Scholar]
- Carlson, G.R.; Dhadialla, T.S.; Hunter, R.; Jansson, R.K.; Jany, C.S.; Lidert, Z.; Slawecki, R.A. The chemical and biological properties of methoxyfenozide, a new insecticidal ecdysteroid agonist. Pest Manag. Sci. 2001, 57, 115–119. [Google Scholar] [CrossRef]
- Kim, D.-S.; Brooks, D.J.; Riedl, H. Lethal and sublethal effects of abamectin, spinosad, methoxyfenozide and acetamiprid on the predaceous plant bug Deraeocoris brevis in the laboratory. BioControl 2006, 51, 465–484. [Google Scholar] [CrossRef]
- Stavrinides, M.C.; Mills, N.J. Demographic effects of pesticides on biological control of pacific spider mite (Tetranychus pacificus) by the western predatory mite (Galendromus occidentalis). Biol. Control 2009, 48, 267–273. [Google Scholar] [CrossRef]
- Colomer, I.; Aguado, P.; Medina, P.; Heredia, R.M.; Fereres, A.; Belda, J.E.; Viñuela, E. Field trial measuring the compatibility of methoxyfenozide and flonicamid with Orius laevigatus Fieber (Hemiptera: Anthocoridae) and Amblyseius swirskii (Athias-Henriot) (Acari: Phytoseiidae) in a commercial pepper greenhouse. Pest Manag. Sci. 2011, 67, 1237–1244. [Google Scholar] [CrossRef] [PubMed]
- Tirello, P.; Pozzebon, A.; Duso, C. The effect of insecticides on the non-target predatory mite Kampimodromus aberrans: Laboratory studies. Chemosphere 2013, 93, 1139–1144. [Google Scholar] [CrossRef] [PubMed]
- Döker, I.; Pappas, M.L.; Samaras, K.; Triantafyllou, A.; Kazak, C.; Broufs, G.D. Compatibility of reduced-risk insecticides with the non-target predatory mite Iphiseius degenerans (Acari: Phytoseiidae). Pest Manag. Sci. 2014, 71, 1267–1273. [Google Scholar] [CrossRef]
- Fountain, M.T.; Medd, N. Integrating pesticides and predatory mites in soft fruit crops. Phytoparasitica 2015, 43, 657–667. [Google Scholar] [CrossRef]
- Passos, L.C.; Soares, M.A.; Collares, L.J.; Malagoli, I.; Desneux, N.; Carvalho, G.A. Lethal, sublethal and transgenerational effects of insecticides on Macrolophus basicornis, predator of Tuta absoluta. Entomol. Gen. 2018, 38, 127–143. [Google Scholar] [CrossRef]
- Silva, T.G.; Souza, J.R.; Moreira, L.B.; Lima, L.L.R.; Carvalho, G.A. Survival, development and reproduction of Harmonia axyridis (Pallas, 1773) (Coleoptera: Coccinellidae) under effects of insecticides used in tomato plants. Ecotoxicology 2021, 30, 863–872. [Google Scholar] [CrossRef]
- Bacci, L.; Lupi, D.; Salvodelli, S.; Rossaro, B. A review of Spinosyns, a derivative of biological acting substances as a class of insecticides with a broad range of action against many insect pests. J. Entomol. Acarol. Res. 2016, 48, 5653. [Google Scholar] [CrossRef]
- Biondi, A.; Mommaerts, V.; Smagghe, G.; Viñuela, E.; Zappalà, L.; Desneux, L. The non-target impact of spinosyns on beneficial arthropods. Pest Manag. Sci. 2012, 68, 1523–1536. [Google Scholar] [CrossRef]
- Van Driesche, R.G.; Lyon, S.; Nunn, C. Compatibility of spinosad with predacious mites (Acari: Phytoseiidae) used to control western flower thrips (Thysanoptera: Thripidae) in greenhouse crops. Fla. Entomol. 2006, 89, 396–401. [Google Scholar] [CrossRef]
- Miles, M.; Dutton, R. Testing the effects of spinosad to predatory mites in laboratory, extended laboratory, semi-field and field studies. Pestic. Benef. Org. IOBC/WPRS Bull. 2003, 26, 9–20. [Google Scholar]
- Cloyd, R.A.; Galle, C.L.; Keith, S.R. Compatibility of Three Miticides with the Predatory Mites Neoseiulus californicus McGregor and Phytoseiulus persimilis Athias-Henriot (Acari: Phytoseiidae). HortScience 2006, 41, 707–710. [Google Scholar] [CrossRef]
- Bostanian, N.J.; Vincent, C.; Hardman, J.M.; Larocque, N. Toxicity of indoxacarb to two species of predacious mites and a predacious mirid. Pest Manag. Sci. 2004, 60, 483–486. [Google Scholar] [CrossRef] [PubMed]
- Bostanian, N.J.; Hardman, J.M.; Racette, G.; Franklin, J.L.; Lasnier, J. Inventory of predacious mites in Quebec commercial apple orchards where integrated pest management programs are implemented. Ann. Entomol. Soc. Am. 2006, 99, 536–544. [Google Scholar] [CrossRef][Green Version]
- Khan, M.M.; Hafeez, M.; Elgizawy, K.; Wang, H.; Zhao, J.; Cai, W.; Ma, W.; Hua, H. Sublethal effects of chlorantraniliprole on Paederus fuscipes (Staphylinidae: Coleoptera), a general predator in paddle field. Environ. Pollut. 2021, 291, 118171. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, M.; Cai, W.; Jing, Z.; Zhou, X.; Mabubu, J.I.; Hua, H. Toxicity and sublethal effects of chlorantraniliprole on the development and fecundity of a non-specific predator, the multicolored Asian lady beetle, Harmonia axyridis (Pallas). Chemosphere 2017, 178, 496–503. [Google Scholar] [CrossRef]
- de Castro, A.A.; Corrêa, A.S.; Legaspi, J.C.; Guedes, R.N.C.; Serrão, J.E.; Zanuncio, J.C. Survival and behavior of the insecticide-exposed predators Podisus nigrispinus and Supputius cincticeps (Heteroptera: Pentatomidae). Chemosphere 2013, 93, 1043–1050. [Google Scholar] [CrossRef]
- Gontijo, P.C.; Moscardini, V.F.; Michaud, J.P.; Carvalho, G.A. Non-target effects of chlorantraniliprole and thiamethoxam on Chrysoperla carnea when employed as sunflower seed treatments. J. Pest Sci. 2014, 87, 711–719. [Google Scholar] [CrossRef]
- Gontijo, P.C.; Moscardini, V.F.; Michaud, J.P.; Carvalho, G.A. Non-target effects of two sunflower seed treatments on Orius insidiosus (Hemiptera: Anthocoridae). Pest Manag. Sci. 2015, 71, 515–522. [Google Scholar] [CrossRef]
- Gontijo, P.C.; Abbade Neto, D.O.; Oliveira, R.L.; Michaud, J.P.; Carvalho, G.A. Non-target impacts of soybean insecticidal seed treatments on the life history and behavior of Podisus nigrispinus, a predator of fall armyworm. Chemosphere 2018, 191, 342–349. [Google Scholar] [CrossRef]
- Martinou, A.F.; Seraphides, N.; Stavrinides, M.C. Lethal and behavioral effects of pesticides on the insect predator Macrolophus pygmaeus. Chemosphere 2014, 96, 167–173. [Google Scholar] [CrossRef]
- You, Y.; Lin, T.; Wei, H.; Zeng, Z.; Fu, J.; Liu, X.; Lin, R.; Zhang, Y. Laboratory evaluation of the sublethal effects of four selective pesticides on the predatory mite Neoseiulus cucumeris (Oudemans) (Acari: Phytoseiidae). Syst. Appl. Acarol. 2016, 21, 1506–1514. [Google Scholar] [CrossRef]
- Souza, K.R.; Moreira, L.B.; Lima, L.L.R.; Silva, T.G.; Braga, P.P.M.; Carvalho, G.A. Susceptibility of Chrysoperla externa (Hagen, 1861) (Neuroptera: Chrysopidae) to insecticides used in coffee crops. Ecotoxicology 2021, 29, 1306–1314. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Aguilar, D.A.; Martínez, A.M.; Viñuela, E.; Figueroa, J.I.; Gómez, B.; Morales, S.I.; Tapia, A.; Pineda, S. Impact of the zoophytophagous predator Engytatus varians (Hemiptera: Miridae) on Bactericera cockerelli (Hemiptera: Triozidae) control. Biol. Control 2019, 132, 29–35. [Google Scholar] [CrossRef]
- Gorri, J.E.R.; Pereira, R.C.; Alves, F.M.; Fernandes, F.L.; da Silva, Í.W.; Fernandes, M.E.S. Toxicity Effect of Three Insecticides on Important Pests and Predators in Tomato Plants. Agric. Sci. 2015, 3, 1–12. [Google Scholar] [CrossRef]
- Brown, A.S.S.; Simmonds, M.S.J.; Blaney, W.M. Influence of a short exposure to teflubenzuron residues on the predation of thrips by Iphiseius degenerans (Acari: Phytoseiidae) and Orius laevigatus (Hemiptera: Anthocoridae). Pest Manag. Sci. 2003, 59, 1255–1259. [Google Scholar] [CrossRef]
- Doucet, D.; Retnakaran, A. Insect chitin: Metabolism, genomics and pest management. In Advances in Insect Physiology: Insect Growth Disruptors; Dhadialla, T.S., Ed.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 437–511. [Google Scholar]
- El-Shenawy, N.S. Oxidative stress responses of rats exposed to Roundup and its active ingredient glyphosate. Environ. Toxicol. Pharmacol. 2009, 28, 279–385. [Google Scholar] [CrossRef] [PubMed]
- de Aguiar, L.M.; Figueira, F.H.; Gottschalk, M.S.; de Rosa, C.E. Glyphosate-based herbicide exposure causes antioxidant defence responses in the fruit fly Drosophila melanogaster. Comp. Biochem. Physiol. Part C: Toxicol. Pharmacol. 2016, 185–186, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Strilbytska, O.M.; Semaniuk, U.V.; Strutynska, R.T.; Burdyliuk, N.I.; Tsiumpala, S.; Bubalo, V.; Lushchak, O. Herbicide Roundup shows toxic effects in nontarget organism Drosophila. Arch. Insect Biochem. Physiol. 2022, 110, e21893. [Google Scholar] [CrossRef] [PubMed]
- Lahm, G.P.; Cordova, D.; Barry, J.D. New and selective ryanodine receptor activators for insect control. Bioorg. Med. Chem. 2009, 17, 4127–4133. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).