Abstract
The industrial hemp market is rapidly expanding, and best crop practices need to be assessed. This study aimed to determine the effect of fertilization and weed management on hemp crops and weed flora during the 2019 and 2020 growing seasons. Field experiments were laid out in a split–split plot design with three replicates: two hemp cultivars as main plots, three fertilization treatments as sub-plots, and three sub-sub-plots (weedy, weed-free, and herbicide application). For the computation of crop traits, 10 plants were randomly selected by each plot. Weed species were collected by quadrate for density and biomass weight determination. Increased fertilization rate positively affected plant height and dry biomass, while no impact was recorded on yield and yield components. The density and biomass of nitrophilous weeds were enhanced by fertilization, negatively affecting hemp growth. Marked differences were recorded on the competitive ability of cultivars since “Fedora 17” decreased the weed studied traits better than “Uso 31”. Herbicide application decreased the biomass of annual and perennial weeds by 38% and 13%, respectively, while it caused a reduction in hemp growth and yield of “Uso 31” plants. Further research should be conducted to address hemp yield losses under different cultural practices and weed control systems.
1. Introduction
The cannabis plant (Cannabis sativa L.) is one of the world’s oldest domesticated crops, which has been widely cultivated due to its industrial [1], nutritional [2], medicinal, and recreational [3] potential. From regulatory and application perspectives, cannabis plants are categorized based on the level of Δ9-tetrahydrocannabinol (THC), one of the most important phytocannabinoids [4]. Plants are generally classified and regulated as industrial hemp if they contain less than 0.3% THC in the dried flower (this level varies by country) or drug type with more than this threshold [5].
In Europe, hemp was a major fiber crop for many centuries and has been cultivated for the great strength and high quality of its bast fibers [6]. Nowadays, hemp is grown in more than 47 countries around the world for its fibers, stem, leaves, seed, and inflorescences [7,8].
As hemp is a polyvalent, multi-use, and multi-functional crop of great economic significance, there is a rising interest in its cultivation [8]. The hemp market is anticipated to grow swiftly in the coming years, while its demand has increased [7]. In Europe, hemp continues to be used in the textile industry as a green substitute for synthetic fibers, and it is also used as a construction material, biofuel, and cosmetic [9,10]. In addition, innovative applications of hemp in the food and pharmaceutical industries are an area of intensive research globally as well as in Greece [11,12]. In the food sector, hemp can be consumed in the form of edible seeds, crude oil, and protein cake. Hemp seed is rich in protein and fatty acids (ω–3, ω–6), while its seed oil is endowed with minerals and vitamins, making hemp ideal for a healthy diet [13,14,15].
In the pharmaceutical industry, hemp gains more and more ground due to the new consumer trend for medicines produced from natural compounds [3]. Its medical properties derive from the composition of secondary metabolites such as terpenes, flavonoids, and cannabinoids [16]. Among these metabolites, the best known are the cannabinoids and, more specifically, cannabidiol (CBD), with its medical properties, and Δ9-tetrahydrocannabinol (THC), the dominant psychoactive chemical compound [5]. The ratio of CBD to THC determines the suitability of hemp as a medical compound in the pharmaceutical industry [7].
Even though hemp is widely used in over 25,000 products in the global market [16], there is a lack of science-based information concerning the agronomical management of the crop [17]. Legal restrictions regarding its cultivation have impeded scientific work since the end of the 20th century because of the association of hemp with the presence of THC [18]. Thanks to the recent legalization that has reauthorized the cultivation and exploitation of hemp, several European Union (EU) countries now permit the cultivation of cultivars with THC content lower than 0.2% declared in European Commission (EC) regulation [19,20].
Modern breeding is utilized to keep the THC content of hemp within acceptable limits and maximize the hemp yield [21]. Although hemp is naturally a dioecious plant with separate male and female members, breeders have managed to create monoecious cultivars with low content of THC and increased CBD content [22,23]. In several European countries, monoecious dual-purpose cultivars are preferred to the dioecious [24]. This preference for improved cultivars is also justified by their capacity for joint fiber and seed production with higher inflorescence yield and seed yield as well [25]. For recently recultivated crops such as hemp, the adaptability of cultivars to various environments influences the agronomical and quality features and, consequently, requires investigation to a greater extent [21].
In addition to the genotype choice and the agroecological conditions, hemp productivity is also affected by cultivation practices [26]. Hemp has been classed as a high-yielding crop due to its low requirements in cultivation techniques [6]. Nevertheless, several studies demonstrate that hemp calls for special attention to nutritional elements, particularly nitrogen [27]. Nitrogen (N) has been proven as a major macronutrient that plays a crucial role in crop nutrition and yield [28]. Several studies verify the effect of nitrogen on significant plant functions such as growth development, photosynthesis, flowering, and senescence [29,30]. Regarding the agronomic features, the plant height and dry biomass of hemp are positively affected by the additional supply of nitrogen, while it negatively impacts the yield and quality of fiber [31]; as for inflorescence indices, high nitrogen rates of N increase the length and weight of hemp inflorescences [25,32]. In addition, seed oil content increases with additional nitrogen in contrast to the protein content of the seed [33]. It is also remarkable that N uptake influences the secondary metabolites, as has been recorded in aromatic plants [34]. A recent study demonstrates that the additional supply of nitrogen leads to a rise in CBD content [33].
Given that the effect of nitrogen on hemp productivity and quality is clear, the supply of nitrogen fertilizer constitutes the main cultivation practice [35]. However, nutrients such as nitrogen are not only crucial elements for crops since several weed species also present high responsiveness to N [36]. In these cases, the addition of N fertilizer can increase the competitiveness of weeds in a certain environment [37], impacting their establishment, emergence, density, and development [38,39]. Thus, weed competition for nutrients can decrease the yield and quality of crops as well as their harvest ability [40]. To manage crop losses, weed control and cultivation practices are significant in agricultural systems [41]. Volunteer plants are common in the fields where hemp has been cultivated [42]. It is worth to be noted that the seeds of many weeds and volunteer hemp plants are difficult to remove properly from hemp seed and even less in certified seed for propagation [43]. Management methods, such as hand pulling for small infestations and repeated herbicide applications, must be applied to minimize invasion risk, especially in natural areas [44].
In the case of hemp, weed control was frequently considered nonessential for fiber production, particularly under high crop densities [6,26,45]. This advantage of hemp over weeds is mostly attributed to its great competitiveness for nutrients, water, and light [46]. Thanks to the rapid growth rate and quick soil surface coverage after the initial growth phase, a vigorous hemp crop can efficiently suppress weed growth [6,47]. The weeding role of the crop is further enhanced by its ability to release allelochemicals such as terpenoids and cannabinoids with stimulatory effects [47]. These compounds act as natural herbicides, impeding seed germination and the growth of several weed species [48]. Given these weed suppression effects, hemp fits well into crop rotations, reducing the weed infestation for the subsequent crop and constituting a sustainable approach [49].
Even though the crop has been grown in Europe without the application of herbicides [6], weed management practices such as planting date and density are considered advantageous for hemp productivity [26,45]. More specifically, early planting and increased seeding rate are recommended to decrease weed density and biomass [6]. However, recent studies indicate that the weed-suppressive ability of hemp is inadequate at low plant densities [50]. In these cases, weed infestation during the initial growth phase can result in significant yield losses of hemp [50,51,52]. As a result, several researchers have concluded that weed control is necessary at a low seeding rate, especially when hemp is grown for seed and/or inflorescences [50,51,53].
While the hemp market is expanding and the demand for its seed and secondary metabolites is rising, there is a call for intensification of research into hemp cultivation practices. To the best of our knowledge, the design of a weed management program for the crop is still in its infancy, as little information exists. It is also known that, even in hemp-producing countries, adequate research has not been conducted about the tolerance of hemp to herbicides [6,50,53], while there are no labeled herbicides for use in Europe.
Therefore, this study aimed to shed light on the effect of fertilization and weed management on two monoecious dual-purpose cultivars and their weed flora. The experiment was designed to assess the null hypothesis that nitrogen fertilization would result positively not only in the growth and yield of hemp but also in several weed species. Regarding the hemp crop, growth parameters and seed yield were evaluated after different weed management, hand hoeing, and herbicide applications for both improved cultivars. Furthermore, hemp tolerance to pre-emergence herbicide was tested in the field under semi-arid Mediterranean conditions.
2. Materials and Methods
2.1. Experimental Site and Weather Conditions
A field experiment was carried out at the Agricultural University farm located in Athens (southern Greece: latitude 37°58′ N, longitude 23°32′ E, altitude 30 m above sea level) during the 2019 and 2020 growing seasons. The soil was clay loam (29.3% clay, 33.8% silt, and 36.9% sand) with pH 7.17; available phosphorus (P) 13.2 mg kg−1 soil, available potassium (K) 201 mg kg−1 soil, and 1.17% organic matter. Weather data (average air temperature and rainfall) were recorded daily and are reported as mean monthly data for the periods in which this study was conducted (Figure 1). The weather conditions during the growing season in 2019 and 2020 were quite different. Average air temperatures during the growing periods did not vary considerably from the long-run averages; however, during June and August 2019, the average temperatures were higher than in the second year (2020). The long-term mean annual rainfall at the site is 435.9 mm, but temporal variability is high. In 2019, there was significantly lower rainfall during the growing season (5.8 mm) than in 2020 (64 mm). These climatic conditions are typical for the Mediterranean basin, where the summers are long and warm to hot, and full irrigation is essential for growing crops.
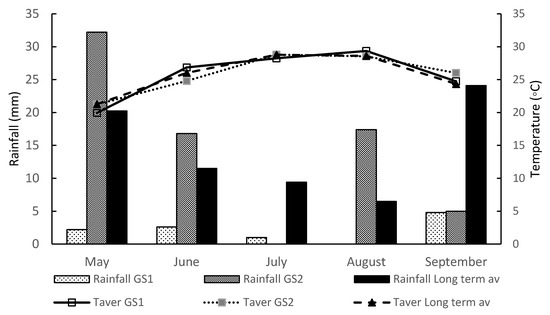
Figure 1.
Monthly means of the mean (Taver) air temperatures (°C) and total rainfall (mm) for 2019 (GS1), 2020 (GS2) growing seasons, and the long-term 30-year average (1989–2018) at the experimental site.
2.2. Experimental Design and Plant Material
The experimental design was a split–split plot with two European hemp cultivars as the main plots, fertilization treatments as the sub-plots, and weed management as the sub-sub-plots in a randomized complete block design replicated three times. The monoecious hemp cultivars with different earliness, named “Uso 31” (early) and “Fedora 17” (medium maturing cultivar), widely grown in European environments, including Greece, were used for the experiments. Seeds of industrial hemp cultivars were obtained from iHempFarms EAD (Veliko Tarnovo, Bulgaria). The fertilizer treatments consisted of three different rates distributed as organic fertilizer 7-4-7 (Biogen, Phytothreptiki S.A., Athens, Greece) (control, F1 (70 kg N, 40 kg P2O5, 70 kg K2O) and F2 (140 kg N, 80 kg P2O5, 140 kg K2O) per hectare). Fertilizers were broadcast and incorporated into the soil using a cultivator before sowing. In the 2019 growing season, two different practices of weed management were applied (weed-free (WF) and weedy (W)), while in 2020, herbicide application (HT) was also performed. The crop was kept free of weeds in the hand-hoeing plots (WF), while the rest did not receive any weed management (W). Immediately after sowing, the pre-emergence herbicide pendimethalin (Stomp, Aqua 455 CS) was applied at a rate of 1.6 kg ai ha−1 in the HT plots. Applications were performed with a hand-held pressurized sprayer (Venus 2, Viopsec Kalimeris S.A., Athens, Greece) equipped with a ceramic hollow cone nozzle (HCI 80, Albuz, Évreux, France). Spraying was performed at a pressure of 300 kPa; the sprayer was calibrated to deliver the equivalent of 200 L ha−1. The first irrigation in all plots occurred the following day and was carefully conducted to allow for complete infiltration without pooling on the soil surface. The experimental plots were 5 m × 2.4 m and consisted of 8 rows 0.30 apart. The plot size of each fertilization treatment was 10 m × 7.2 m in the first growing season (2019) and 15 m × 7.2 m in the second one (2020). Hemp seeds were sown by hand at 3 cm depth on 31 May 2019 and 22 May 2020 to achieve the planned crop density of 650,000–700,000 plants ha−1 with wheat (Triticum turgidum subsp. durum L.) as the preceding crop. A drip irrigation system was installed in the experimental field and scheduled to water when it was necessary.
2.3. Measurements and Calculations
For the determination of plant height and above-ground biomass dry weight, 10 hemp plants were randomly selected from the middle rows of the experimental plots at 51 days after sowing (DAS) during the vegetative growth of the hemp plants in both growing seasons. Plant height was measured from the base of the plant to the growing tip. Biomass dry weight was determined after drying for 72 h at 80 °C until their weight remained constant and then weighed. At the end of August 2019, a serious cotton bollworm attack (Helicoverpa armigera (Hübner), Lepidoptera: Noctuidae) damaged most of the plants preventing harvest. In the second growing season (2020), the crop was harvested on 27 September. At harvest time, all plants in an area of 6 m2 from the middle rows were cut above soil level, and their seeds were air-dried, threshed, cleaned, and weighed for seed yield and 1000-seed weight determination, after removing the empty seeds. Among the harvested plants, samples from 10 plants were taken randomly, separated into stems, leaves, inflorescences, and seeds, weighed, and the length of main and secondary inflorescences and seed numbers were recorded.
Data on weed population and dry matter were also recorded at 51 DAS, when most of the weeds had already emerged. Weeds were sampled from three 0.5 m−2 quadrates placed on the diagonal of each experimental plot. Above-ground weed biomass was collected, identified, and counted, then oven-dried at 70 °C for 48 h to a constant weight to record weed dry weight. Values obtained (means of three quadrates) were transformed per area unit for density (numbers m−2) and biomass (g m−2). Different weed diversity indices were also calculated based on the data of the weed present in the experimental plots [54]. The Shannon–Weiner diversity index (H′) [55] provides an overall assessment of weed species diversity [H′ = −∑ pi ln pi]. The uniformity of a weed community can be represented by the Evenness index (E), determined as the Shannon–Weiner diversity index (H′) divided by H′max equal to ln S [56]. Simpson index (λ, modified by Berger and Parker [57]) measures the probability that two individuals randomly selected from a sampling site will belong to the different species [λ = 1 − ∑ pi2]. Margalef’s index [56]) was used to evaluate species richness [DMg = (S − 1) (ln N)−1]. Where S is the total number of species, pi represents the relative abundance of a specific species and is calculated as pi = ni/N, N is the total number of individuals in each plot, and n is the number of individuals per species present in each plot.
The relative response index (RRI) was adopted to assess the effect of fertilization on hemp crop (RRIH) or weed (RRIW) fitness [58]. The RRI is calculated as follows:
where CB represents the hemp or weed above-ground biomass in the control plots, and TB represents hemp or weed response in the fertilization treatments. RRI values < 0 mean that crop (or weed) biomass is promoted by a given treatment than the control. The response comparison index (RCI) was used to quantify whether the addition of fertilization treatments has a more positive influence on hemp crops or hemp weeds [59]. The RCI index is calculated as follows:
RRI = (CB − TB)/(CB + TB),
RCI = RRIw − RRIH,
RCI values higher, equal to, or lower than zero indicate that the positive effect of the fertilization treatments on hemp crops is higher, equal, or lower than the positive influence on the hemp weeds.
2.4. Statistical Analysis
Analysis of variance (ANOVA) was conducted for all data using the SigmaPlot 12 statistical software (Systat Software Inc., San Jose, CA, USA). Before analysis, the weed data (density and above-ground biomass weight) were transformed as square roots to homogenize the variance [60]. However, the non-transformed means are presented in the tables. Differences between treatments and their interactions were compared by using Fisher’s least significant difference (LSD) test, where probabilities are equal to or less than 0.05 (a = 5%).
3. Results
3.1. Agronomic Traits, Yield, and Yield Contributing Characteristics of Hemp
Based on the combined analysis of variance, significant effects of fertilization (F), cultivar (C), and weed management (WF) were observed on studied plant growth traits during the first growing season (p < 0.001). Fertilization demonstrated a significant impact on plant growth, recording the highest plant height and biomass values at the 140 kg ha−1 rate for both cultivars regardless of weed management practices. Lower height and biomass were obtained from the early flowering cultivar “Uso 31”, while the highest values were observed in the cultivar “Fedora 17” under all fertilizer rates and weed management practices. Regarding the presence of weeds, the crop competed only weakly with weeds for nutrients, water, sunlight, and space, and thus plant growth was reduced in the weedy plots. Plant height and biomass decreased by 27% and 49%, respectively, under the presence of weeds regardless of fertilization rates and cultivars. All three factors also showed a large interaction for above-ground biomass (p < 0.001), while they were of similar magnitude in the case of plant height (Table 1).

Table 1.
The effects of the experimental factors on the average values and the analysis of the height and above-ground biomass of hemp crop across the two growing seasons, 2019 and 2020. WM: weed management treatments (WF: weed-free; W: weedy; HT: herbicide-treated); C: cultivars; F: fertilization treatments (Control: without fertilization; F1: 70 kg ha−1; F2: 140 kg ha−1).
Even though the cultivar, fertilization, and weed management practices showed a significant effect on plant height and biomass during the second experimental season, shorter plants with higher biomass were recorded for both cultivars in comparison to the first season. Among the cultivars, “Fedora 17” demonstrated that the tallest plants and the highest values of biomass ranged between 1.14 m and 1.48 m, and 279.9 g m−2 and 1081.2 g m−2, respectively. Both plant height and biomass were boosted by the maximum application rate of fertilization of 140 kg ha−1 in almost all studied cases for both cultivars. Concerning weed control, the hand-hoeing method showed the highest efficacy against weeds, resulting in plants of increased height and biomass by 16% and 62%, respectively. Selecting the herbicide treatment caused an 8% reduction in the plant height, whereas the biomass was positively affected by this treatment, showing a 7% increase as compared to plants in weedy plots. All three factors showed no interaction for the studied plant growth traits (Table 1).
Regarding the yield parameters of hemp, the cultivar demonstrated a significant effect on inflorescence length and seed weight per inflorescence (p < 0.05), inflorescence weight, seed number per inflorescence, and seed yield (p < 0.01). The longest and heaviest inflorescences seen in the medium maturing cultivar “Fedora 17” range between 18.2 cm and 35.3 cm, and between 2.69 g and 11.1 g, respectively. As for the seed number and seed weight per inflorescence, “Fedora 17” produced 28% more and 22% heavier seeds per inflorescence than “Uso 31”. Moreover, lower seed yield was poorly performed on average from the early flowering cultivar “Uso 31”, while the highest values were more than duplicated in the cultivar “Fedora 17”, producing 12,537.3 t ha−1 and 2924.6 t ha−1, respectively (Table 2).

Table 2.
The effects of the experimental factors on the average values and the analysis of yield and yield components of hemp in the 2020 growing season. WM: weed management treatments (WF: weed-free; W: weedy; HT: herbicide treatment); C: cultivars; F: fertilization treatments (Control: without fertilization; F1: 70 kg ha−1; F2: 140 kg ha−1).
The combined analysis of variance showed insignificant differences between fertilization treatments, whereas weed management practices demonstrated a significant effect on all yield traits (p < 0.001). Concerning weed control, the hand-hoeing method resulted in a significant increase in inflorescence length and weight (25% and 46%, respectively), seed number and weight per inflorescence (45% and 29%), and seed yield (33%) compared to plants in weedy plots for both cultivars (p < 0.001). All yield components showed an interaction between cultivar and weed management. In the case of the cultivar “Fedora 17”, the hand-hoeing method followed by the herbicide treatment was characterized by superior efficacy of all yield components as compared to weedy plots. On the other hand, the herbicide treatment that was applied to plants of “Uso 31” did not favor the plant yield, causing even lower yields than those of plants in weedy plots (Table 2).
All yield components also showed an interaction between fertilization and weed management. In the case of the cultivar “Uso 31”, all studied yield traits showed an increase after the medium rate of fertilization of 70 kg ha−1 in both weed-free and weedy plots, whereas no addition of fertilizer favored yield in plots treated with herbicide. As for “Fedora 17”, the highest values of yield traits were recorded in plants of weed-free and herbicide-treated plots after the maximum application rate of 140 kg ha−1 and in plants of weedy plots after the medium rate of fertilizer (Table 2).
3.2. Weed Species Composition, Weed Density, and Above-Ground Biomass
Weeds were classified according to their biological cycle. A total of 10 weed species were identified during the two experimental seasons, including five annual and five perennial species. In the first growing season, the dominant annual species were Portulaca oleracea L., Amaranthus retroflexus L., and Echinochloa crus-galli (L.) P.Beauv, constituting 20.3% of the total weeds (Figure 2a). Among the perennial weeds, the most frequent species was Solanum elaeagnifolium Cav., followed by Malva sylvestris L. and Convolvulus arvensis L., representing 99.8% of perennials and 78.6% of the total weed species (Figure 2b). The dominant annual and perennial species remained the same during the two growing seasons, whereas there were differences in their density. In the second growing season, the percentage of major short-lived species became higher and the percentage of perennial species lower, 44.7% and 28.7% of the total weed species, respectively. Differences were also recorded at the nitrophilous weed species, where the density of A. retroflexus, E. crus-galli, Portulaca oleracea, and M. sylvestris increased by 25.95% in the first season to 45.6% in the subsequent cropping season. Weed control treatments, including herbicide treatment, were least efficacious in the second season, since the total density of A. retroflexus, E. crus-galli, Tribulus terrestris L., and Chenopodium album L. was increased by 25% (Figure 2a).
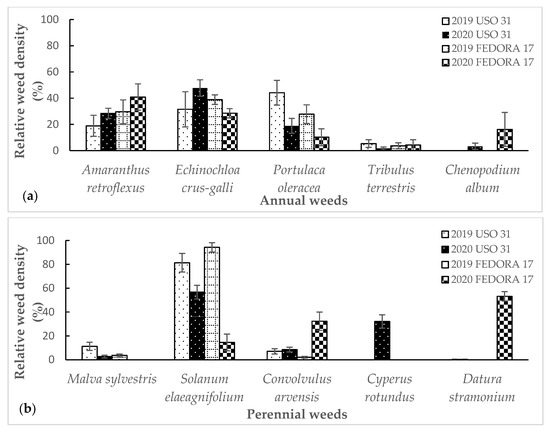
Figure 2.
Relative weed density of annual (a) and perennial weed species (b) of hemp crop across the growing seasons 2019 and 2020. Vertical bars indicate the standard errors of the means.
According to the combined analysis of variance, the effects of fertilization, cultivar, and weed management on the densities of most dominant annual and perennial weeds were significant across the 2019 growing season. Fertilizing the early cultivar “Uso 31” provoked an increase in weed density, recording the highest values at the 140 kg ha−1 rate for the nitrophilous A. retroflexus, E. crus-galli, P. oleracea, and M. sylvestris. The highest mean values were recorded in the annual P. oleracea and the perennial S. elaeagnifolium, recording 32 and 168 plants m−2, respectively. Weed species of the cultivar “Fedora 17” and especially S. elaeagnifolium were also positively affected by fertilization, reaching 174 plants m−2, whereas the density of major weeds in total decreased by 21% compared to “Uso 31”. All three factors showed a large interaction for weed density of almost all major species (p < 0.001), while they were of similar magnitude in the case of E. crus-galli and S. elaeagnifolium (Table 3).

Table 3.
The effects of the experimental factors on the average values and the analysis of weed density and weed above-ground dry biomass of weedy plots in the 2019 growing season. AMRE, A. retroflexus, ECCR, E. crus-galli, POOL, P. oleracea, MASY, M. sylvestris, SOEL, S. elaeagnifolium, COAR, C. arvensis. WM: weed management treatments (WF: weed-free; W: weedy; HT: herbicide-treated); C: cultivars; F: fertilization treatments (Control: without fertilization; F1: 70 kg ha−1; F2: 140 kg ha−1).
Results revealed that the dry biomass of the six dominant weeds was significantly affected by the fertilization treatments, whereas the impact of the cultivar was insignificant on most of these weeds. The interaction terms between cultivars by fertilization and fertilization by weed management were also significant. The average values of weed biomass were comparatively highest, mainly in the case of the cultivar “Uso 31”, recording 175.3 g m−2. Among cultivars, “Fedora 17” proved to compete better with weeds despite the addition of fertilizer, presenting lower weed biomass by 38% in the first experimental season (Table 3).
This study revealed that fertilization, cultivar, and their interaction significantly influenced the weed density of the most dominant species during the second growing season. The maximum number of total weeds was recorded at 140 kg ha−1 and was higher by 15% compared to 70 kg ha−1 for both cultivars, regardless of weed management practices. Among cultivars, “Uso 31” was of lower competitiveness against the weeds, since it was infected by twice the total number of major weeds compared with “Fedora 17”. Concerning weed management, plots subjected to herbicide treatment showed a significantly decreased weed density in both cultivars (p < 0.001). The implementation of herbicide resulted in a 61% reduction in annual dominant weeds and a 19% reduction in perennials in cultivar “Uso 31”. Similar effects were recorded in plots of “Fedora 17” under herbicide treatment, where the number of major annual and perennial weeds decreased by 50% and 32%, respectively (Table 4).

Table 4.
The effects of the experimental factors on the average values and the analysis of weed density and weed above-ground dry biomass of weedy plots in the 2020 growing season. AMRE, A. retroflexus, ECCR, E. crus-galli, POOL, P. oleracea, MASY, M. sylvestris, SOEL, S. elaeagnifolium, COAR, C. arvensis. WM: weed management treatments (WF: weed-free; W: weedy; HT: herbicide-treated); C: cultivars; F: fertilization treatments (Control: without fertilization; F1: 70 kg ha−1; F2: 140 kg ha−1).
Based on the combined analysis of variance, significant effects of fertilization and weed management were observed on dry weed biomass, while the impact of the cultivar was insignificant during the second growing season. Among the cultivars, “Uso 31” demonstrated the highest values of weed biomass for most of the dominant weed species except for nitrophilous A. retroflexus and the perennial C. arvensis. Results demonstrated that biomass was enhanced by the maximum applicated fertilizer rate of 140 kg ha−1 in almost all studied scenarios, especially in the case of nitrophilous weed species A. retroflexus, E. crus-galli, and P. oleracea. Regarding weed control, the herbicide application showed great efficacy against weeds, decreasing the total biomass of annuals and perennials by 38% and 13%, respectively. The highest mean values were seen in the annual A. retroflexus and the perennial S. elaeagnifolium, recording 175.5 g m−2 and 224.7 g m−2, respectively, for both cultivars (Table 4).
3.3. Weed Diversity Indices
Shannon index values, a measure of weed diversity, revealed that the significant differences across cultivars are a function of fertilization treatments during the first growing season (p < 0.05). Among cultivars, “Fedora 17” demonstrated the highest values in the plots subjected to no fertilization, and the lowest values of weed diversity in the fertilized plots. In contrast, the addition of fertilizer favored the weed diversity in the “Uso 31” plots, resulting in a greater number of weed species under the treatment of 140 kg ha−1, followed by the 70 kg ha−1 in the early cultivar “Uso 31” (Figure 3a).
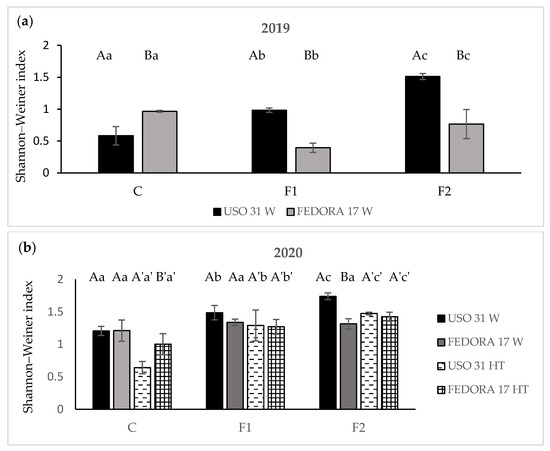
Figure 3.
Shannon–Weiner index of weeds in hemp crop during the growing season 2019 (a) and during the growing season 2020 (b). Vertical bars indicate the standard errors of the means. Values without a common letter are statistically significant according to LSD (0.05).
Concerning the second growing season, weed diversity was significantly affected by fertilization across cultivars and weed management treatments (p < 0.05). The early cultivar “Uso 31” was influenced more by the addition of fertilizer, recording the highest Shannon index values at 140 kg ha−1 compared to “Fedora 17” under all weed management practices. With respect to weed management, plots of “Uso 31” subjected to herbicide treatment showed a significantly decreased weed diversity in all studied cases (p < 0.05). Similar effects of herbicide implementation were recorded in non-fertilized plots of “Fedora 17”, whereas the effectiveness of herbicide was not significant in the index of fertilized plots of cultivars compared to weedy plots (Figure 3b).
The results revealed that the Simpson index (D) was affected by the fertilization in both cultivars during the first growing season. In terms of “Uso 31”, the fertilization resulted in a significant increase in the weed diversity, recording the highest index values after the addition of 140 kg ha−1 fertilizer (p < 0.05). On the contrary, “Fedora 17” presented greater values of the Simpson index in the unfertilized plots than in the fertilized ones. Among cultivars, the highest values were recorded in the plots of the cultivar “Uso 31” regardless of fertilization treatments (Figure 4a).
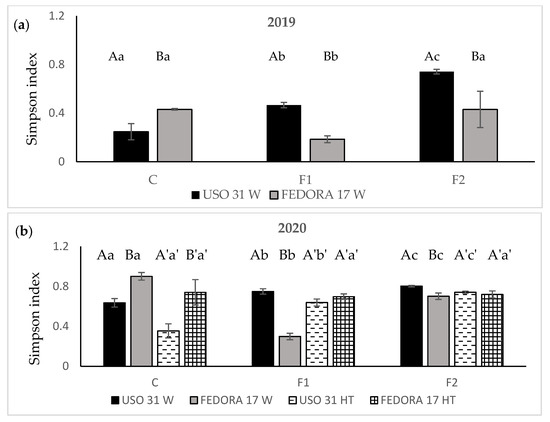
Figure 4.
Simpson index of weeds in hemp crop during the growing season 2019 (a) and the growing season 2020 (b). Vertical bars indicate the standard errors of the means. Values without a common letter are statistically significant according to LSD (0.05).
For the second growing season, weed diversity was significantly affected by fertilization in most of the studied cases (p < 0.05). Simpson index value in cultivar “Uso 31” increased in both weedy and herbicide-subjected plots as the rate of fertilizer became greater. Even though herbicide treatment decreased the values in some studied cases, the Simpson index values remained close to 1 and were classified as high. Results indicated that no difference in weed dominance is influenced by using the herbicide (Figure 4b).
Results revealed that the Evenness index was significantly affected by fertilization across cultivars (p < 0.05) during the first growing season. The uniformity of weed species in plots of “Uso 31” was enhanced sequentially by increasing the rate of fertilizer and recorded the highest values at the rate of 140 kg ha−1. Similar results were noted in cultivar “Fedora 17”, with the difference that the addition of a lower rate of fertilizer caused a decrease in weed uniformity. These results showed that the Pielou index was generally affected by fertilization treatments (Figure 5a).


Figure 5.
Evenness index of weeds in hemp crop during the growing season 2019 (a) and the growing season 2020 (b). Vertical bars indicate the standard errors of the means. Values without a common letter are statistically significant according to LSD (0.05).
Regarding the second growing season, the Evenness index values exceeded 0.8 in most of the cases. These findings revealed that the fertilization combined with the herbicide application resulted in the uniformity of weed species in both cultivars. In terms of weedy plots, fertilization enhanced the abundance of weed species in plots of “Uso 31”, whereas it provoked the reduction in weed diversity in plots of “Fedora 17” (Figure 5b).
Concerning the first growing season, Richness index values were not significantly affected by the addition of fertilizer in the plots of the cultivar “Uso 31”. Moreover, the total number of weed species per sample included in plots of “Fedora 17” decreased by the fertilization treatments showing statistically significant differences compared with unfertilized plots (p < 0.05). Among cultivars, the values of the richness index were, in general, higher in the cultivar “Uso 31” (Figure 6a).
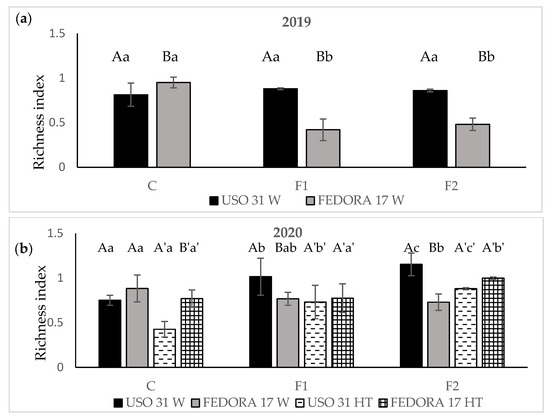
Figure 6.
Richness index of weeds in hemp crop during the growing season 2019 (a) and during the growing season 2020 (b). Vertical bars indicate the standard errors of the means. Values without a common letter are statistically significant according to LSD (0.05).
This study revealed that fertilization and herbicide application significantly influenced the Richness index in plots of “Uso 31” during the second growing season (p < 0.05). In particular, the total number of weed species per sample was enhanced sequentially by increasing the rate of fertilizer and recorded the highest values at the rate of 140 kg ha−1 in both weedy and herbicide-subjected plots. Regarding “Fedora 17”, a significantly decreasing trend in the weedy plots and an increasing trend in the herbicide-subjected plots was observed in the total number of weed species after the addition of fertilization (Figure 6b).
3.4. Hemp/Weed Competition
The robustness of the hemp was significantly affected by all the fertilization and weed management treatments during the first growing season (p < 0.005). Moreover, there was an interaction between cultivar × fertilization × weed management on the relative response index of hemp (RRIH) (Table 5). The RRIH ranged from −0.29 to 0.009 in weed-free and weedy plots, respectively, regardless of cultivar. In weed-free plots, the hemp biomass of both cultivars was enhanced by fertilization, mostly at the rate of 140 kg ha−1. Among cultivars, the index was lower in “Fedora 17” than “Uso 31” in weed-free plots recording average values of −0.29 and −0.20, respectively, under the addition of the maximum rate of fertilizer. In the weedy plots, the application of 70 kg ha−1 of fertilizer boosted only the growth of “Fedora 17” plants, whereas the maximum rate of fertilizer positively affected both cultivars and especially “Uso 31”.

Table 5.
The effect of the interaction of cultivar × fertilization × weed management on the relative response index of hemp crop across the two growing seasons 2019 and 2020. WM: weed management treatments (weed-free; weedy; HT: herbicide-treated); C: cultivars (Uso 31; Fedora 17); F: fertilization treatments (F1: 70 kg ha−1; F2: 140 kg ha−1).
With respect to the second growing season, the effect of the interaction of cultivar × fertilization × weed management on the RRIH was insignificant (p < 0.005). In weed-free plots, hemp was enhanced by the addition of fertilizer without any significant differences between cultivars and fertilization treatments, recording lower values compared to those of the first growing season. Furthermore, the presence of weeds impacted negatively on the plant growth of “Uso 31” in the medium-fertilized weedy plots. Similar results were recorded on “Fedora 17” plants in weedy plots subjected to fertilization of 140 kg ha−1. Herbicide implementation promoted the biomass of hemp across the cultivars and fertilization treatments. In particular, “Uso 31” plants fertilized at 140 kg ha−1 were favored the most after the herbicide application recording the lowest RRIH of any other weed management treatment across cultivars, fertilization treatments, and growing seasons.
The relative response index of weeds (RRIW), which was indicative of weed biomass, was insignificantly affected in most cases by the interaction of cultivar × fertilization during the first growing season (Table 6). The effect of fertilization on the RRIW of nitrophilous weeds was always higher compared with the non-nitrophilous. Among cultivars, the lowest values of RRIW were in the nitrophilous weeds of “Uso 31”, recording average values of −0.50 at 140 kg ha−1.

Table 6.
The effect of interaction of cultivar × fertilization × weed management on the relative response index of nitrophilous and non-nitrophilous weeds across the two growing seasons 2019 and 2020. WM: weed management treatments (weed-free; weedy; HT: herbicide-treated); C: cultivars (Uso 31; Fedora 17); F: fertilization treatments (F1: 70 kg ha−1; F2: 140 kg ha−1).
Regarding the second growing season, similar results revealed that all fertilization treatments enhanced the biomass of nitrophilous weeds recording the lowest value of −0.68 in weedy plots of the cultivar “Uso 31” at 140 kg ha−1 (Table 6). Furthermore, and contrary to the results of the first growing season, the RRIW of non-nitrophilous weeds was also reduced by the addition of 70 and 140 kg ha−1 as well, except the value of weeds in “Fedora 17” at the medium rate of fertilizer. The herbicide implementation decreased the RRIW of both nitrophilous and non-nitrophilous weeds across cultivars. The lowest values in plots subjected to herbicide application were shown in nitrophilous weeds of “Uso 31” and non-nitrophilous weeds of “Fedora 17” at 140 kg ha−1 (−0.73 and −0.16, respectively).
Agronomic traits of hemp, yield, yield contributing characteristics, and relative response indices of hemp and weeds were evaluated to determine their association across different cultivars and weed management practices using correlation analysis. The relative response index of weeds (RRIW) positively correlated with the weight of inflorescence (Infl W), number of seeds (Seed N), the weight of seeds (Seed W), and yield in the weedy plots of the cultivar “Uso 31” in the second growing season (Table 7). On the contrary, RRIW was negatively related to plant height in the plots of the cultivar “Uso 31”, which were subjected to herbicide application. Moreover, positive associations were found between the relative response index of nitrophilous weeds (RRINW) and length of inflorescence (Infl L), Infl W, Seed N, and Seed W in weedy plots of “Uso 31”. Correlation analysis revealed a positive relationship between the relative response index of hemp (RRIH) and yield in the weedy plots of the cultivar “Fedora 17”. In addition, both RRIW and RRINW showed negative correlations with Infl W, Seed W, and yield in the herbicide-subjected plots of the cultivar ’Fedora 17” (Table 7).

Table 7.
Pearson correlation coefficients and significance levels between relative response indices (RRI of hemp (RRIH), RRI of weeds (RRIW), RRI of nitrophilous weeds (RRINW), and RRI of non-nitrophilous weeds (RRINNW)) and agronomic traits (Inflorescence length (Infl L) and weight (Infl W), Seed number (Seed N) and weight (Seed W) per inflorescence, seed yield) of two hemp cultivars (Uso 31; Fedora 17) for weedy and herbicide-treated (HT) in 2020 growing season.
The difference in plant biomass between hemp and weeds, expressed as response comparison index (RCI), was affected by an interaction of cultivar × fertilization × weed management across the two growing seasons, 2019 and 2020 (Figure 7a–c). With respect to the first growing season of 2019, RCI values of non-nitrophilous weeds were higher than zero in weedy plots of both cultivars, recording the highest values of 0.51 in “Uso 31” and 0.64 in “Fedora 17” at the 140 kg ha−1. In addition, negative values of RCI were found for nitrophilous weeds in “Fedora 17” under both fertilization treatments, and only at the 140 kg ha−1 in “Uso 31”, while the opposite trend was observed at the 70 kg ha−1 (Figure 7a).
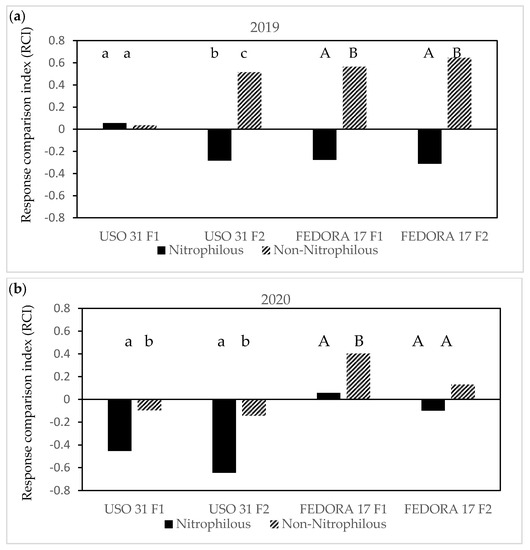
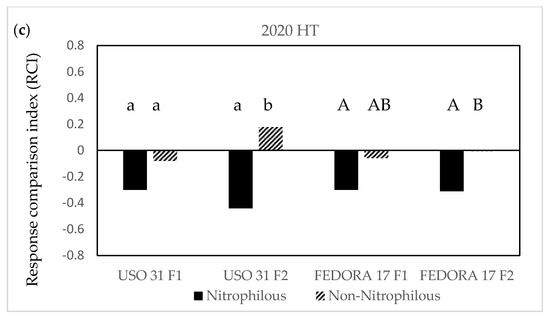
Figure 7.
The effect of the interaction of the cultivar × fertilization × weed management on the response comparison index (RCI) in hemp crops during the 2019 growing season (a) and during the 2020 growing season (b,c). Values without a common letter are statistically significant according to LSD (0.05).
In weedy conditions, the RCI varied between the growing seasons, and it was lower in “Uso 31” for both nitrophilous and non-nitrophilous weeds under the two fertilization treatments. Among the weeds and fertilization, the lowest values were observed at the nitrophilous weeds of weedy “Uso 31” at 140 kg ha−1. Regarding the cultivar of “Fedora 17”, RCI values were higher than zero in weedy plots recording the highest values of 0.40 in non-nitrophilous weeds at 70 kg ha−1, whereas there was an opposite trend of nitrophilous weeds at the 140 kg ha−1 (Figure 7b). Herbicide application reduced the RCI values of nitrophilous and non-nitrophilous below zero in all cultivars and fertilization treatments except for the non-nitrophilous weeds in plots of “Uso 31” at 140 kg ha−1. Among cultivars, the lowest RCI values were recorded in the nitrophilous weeds at the maximum rate of fertilizer (Figure 7c).
4. Discussion
While the interest in the innovative applications of hemp is growing and the demand for its products is rising globally [7,9,11], cultivation practices are needed to enhance crop productivity. The literature review demonstrated that the genotype choice of monoecious dual-purpose cultivars [24], the addition of nutritional elements such as nitrogen [25,33], and the weed control of crops [50,53] all have an impact on the agronomic and yield features of hemp.
4.1. Agronomic Traits of Hemp
The two monoecious dual-purpose cultivars which were studied in our research differed significantly in plant height and dry above-ground biomass in both growing seasons. The literature justifies that result by mentioning that the early flowering cultivar can have higher plant growth due to the longer available vegetative growth and the exposure to a high amount of radiation [61]. However, the result of our study is exactly the opposite since the medium maturity cultivar “Fedora 17” presented significantly taller plants with higher biomass than the early flowering “Uso 31”. That finding of our study could be justified by the fact that the postponed sowing date from May to August reduced the length of the vegetative phase, as well as the hemp biomass in the early cultivar. Comparable results were reported in the low latitudes of Southern Italy by Tang et al. [62].
Concerning fertilization, the most required macronutrients for hemp are nitrogen (N), phosphorus (P), and potassium (K) [63]. Among them, N has been proven to play a crucial role in plant functions, positively affecting the hemp height and biomass [25,31,32]. Only a couple of studies revealed that P and K fertilization had a very limited impact on hemp growth [64,65]. Even though knowledge concerning the mixed effect of NPK is limited, some studies reported that both P and K fertilization could boost the positive effect of N fertilization when they were added together [66]. These findings are well depicted in our experiments after the use of NPK fertilizer. More specifically, NPK fertilization enhanced plant growth in both cultivars, even after the addition of the medium rate of fertilizer (70 kg N, 40 kg P2O5, 70 kg K2O ha−1) across the two growing seasons. However, the medium fertilizer rate did not meet the demand for hemp crops, while the double rate (140 kg N, 80 kg P2O5, and 140 kg K2O ha−1) resulted in the tallest plants with the highest biomass regardless of weed management practices. The positive response of plant height to N fertilization observed in our study is in full accordance with previous studies under Greek conditions [33,67]. Similar beneficial results were recorded in plant biomass, where the greatest effect on hemp above-ground biomass was recorded at the highest nitrogen level [25,68].
Significant differences were also observed in the plant growth of hemp under the presence of weeds in both cultivars and growing seasons. Plant height and biomass decreased by 27% and 49%, respectively, during the 2019 growing season and by 14% and 38%, respectively, during 2020, regardless of fertilization rates and cultivars. These findings bear out our original assumption that the weed-suppressive ability of hemp is inadequate at lower plant densities for the cultivation of dual-purpose cultivars that are also focused on seed production. Previous studies verify the significant yield losses of hemp caused by weed infestation during the first growth phase [50,51].
Among the weed management practices, the hand-hoeing method boosted crop growth, reducing competition with weeds for nutrients, water, sunlight, and space in both growing seasons. In contrast to hand hoeing, the pre-emergence application of pendimethalin in plots of the cultivar “Uso 31” led to shorter plants with lower biomass than the plants of weedy plots. That result also is well depicted by the negative correlation between RRIW and plant height. Moreover, herbicide implementation in the plots of “Fedora 17” caused higher plant length and biomass, but still lower than in the plants of plots subjected to hand hoeing. The negative impact of pendimethalin on plant growth could be attributed to potential phytotoxic effects that can be provoked in hemp plants of both cultivars. The literature verifies that the application of pendimethalin and several pre-emergence herbicides of group 2 are relative to transient injuries [53]. Moreover, a recent study reported an insignificant negative impact of pendimethalin on hemp biomass [69], while other research reports a reduction of 54–77% in the biomass of other hemp cultivars [70].
4.2. Yield and Yield Contributing Characteristics of Hemp
Concerning the yield components, the medium-maturity cultivar “Fedora 17” presented the longest and heaviest inflorescences with the highest seed number and weight per inflorescence, and twice the seed yield compared with “Uso 31”. Previous studies identified the superiority of “Fedora 17” in crop productivity against “Uso 31” [71,72]. Seed yield was comparable to the range of 0.3 to 1.10 t ha−1 reported in other surveys performed in various environments of Europe [73,74,75].
The yield components of cultivars responded differently to the addition of fertilizer. As for inflorescence indices of both cultivars, they were favored by the addition of fertilizer, recording the highest values at the medium and maximum rate of fertilizer in “Uso 31” and “Fedora 17”, respectively. Previous studies verify the beneficial effect of N on inflorescence length and weight [25,26,37]. Furthermore, seed yield tended to be higher at 70 kg ha−1 of fertilizer in plants of the cultivar “Uso 31” and at 70 kg ha−1 in plants of the cultivar “Fedora 17”. Previous studies presented the positive effect of N fertilization on seed yield compared with unfertilized plants [25,26,35,37,76,77]. However, findings support that nitrogen availability has little or no significant effect on seed weight [37,78,79].
All weed management practices resulted in a significant impact on the yield components of hemp. The weed appearance of hemp in weedy plots negatively affected all the studied yield components of both cultivars. Weed infestation reduced almost by half the seed number and weight per inflorescence of “Uso 31” and the inflorescence weight of “Fedora 17”. Previous research conducted on hemp has claimed that weed management practices are necessary in order to ensure sufficient yields [8,51,80].
Among weed management practices, the hand-hoeing method was the most efficacious in both cultivars, resulting in a significant increase in all yield components. The effect of herbicide application on yield components differed between cultivars. Regarding the early cultivar “Uso 31”, pendimethalin did not favor the hemp yield, causing even lower values than those of plants in weedy plots. On the other hand, the productivity of the cultivar “Fedora 17” was positively affected by the herbicide treatment of all yield components as compared to weedy plots. The reduction in weed biomass of nitrophilous weeds was correlated with inflorescence weight, seed weight, and seed yield, leading to higher values (RRINW). Previous studies demonstrated that no seed differences were observed due to pre-emergence herbicides, including pendimethalin, relative to untreated plots [53,70]. The results of our study are partially in accordance with these findings only in the case of “Fedora 17”. The difference in herbicide effect in seed yield between cultivars may be attributed to the fact that “Uso 31” probably presented greater sensitivity to pendimethalin. A recent study confirms that hemp is highly susceptible to pendimethalin and many other pre-emergence herbicides [70]. Even though the herbicide was not overly harmful to “Uso 31”, there were possibly some injuries to the hemp. Therefore, extra space was created between plants in the row compared with the weed-free plots, and the light penetration increased. Thus, it can be assumed that lateral growth was promoted to the detriment of seed production. At any rate, the high sensitivity of some cultivars to pesticides should also be considered.
4.3. Weed Species Composition, Weed Density, and Above-Ground Biomass
The weed flora of the hemp crop consisted of five annuals (P. oleracea, A. retroflexus, E. crus-galli, T. terrestris, and C. album) and five perennial weeds (S. elaeagnifolium, M. sylvestris, C. arvensis, Cyperus rotundus L., and Datura stramonium L.) during both growing seasons. Marked differences were recorded in the relative weed densities between the two seasons since the total density of several annual weed species increased during 2020. This finding could be attributed to a higher level of rainfall that probably favored the weed seed bank, promoting the emergence and development of these species.
Among cultivars, “Fedora 17” competed better with weeds since the lowest density and above-ground biomass of weeds were recorded in these plots. These results can also be attributed to the short plants of “Uso 31” with limited above-ground biomass that are not capable of forming an adequate canopy closure. Similar results were reported by other studies, which found the highest weed density and biomass in the plots of the early cultivar “Uso 31” [26,50,52].
Fertilization increased the weed density, especially after the application of 140 kg ha−1 compared with unfertilized plots. In particular, the highest mean values of weed density were recorded for the nitrophilous species A. retroflexus, P. oleracea, E. crus-galli, and M. sylvestris. The opposite response to fertilization was recorded in the perennial weeds S. elaeagnifolium and C. arvensis. The findings of our study are in line with our original assumption that nutrients such as nitrogen are not only crucial elements for crops, since several weed species show high responsiveness to N [39]. Fertilization increased the competitiveness of weeds, impacting their establishment, emergence, density, and development. Comparable results were mentioned by Kousta et al. [52] after the addition of a high N dose.
Regarding weed management, plots subjected to herbicide treatment demonstrated a significantly decreased weed density in both cultivars. The implementation of herbicide resulted in a 61% reduction in annual dominant weeds and a 19% reduction in perennial density in cultivar “Uso 31”. Similar effects were recorded in plots of “Fedora 17” under herbicide treatment, where the number of major annual and perennial weeds decreased by 50% and 32%, respectively. Moreover, the herbicide application showed great efficacy against weeds, decreasing the total biomass of annuals and perennials by 38% and 13%, respectively. A previous study verified the efficacy of pendimethalin against weeds and found that the herbicide reduced the weed biomass by 46% compared with weedy plots [69]. On the contrary, C. arvensis showed a different response, recording higher biomass after the implementation of herbicide than in weedy plots. Similar results indicate the ineffectiveness of pendimethalin against the broadleaf weed Convolvulus arvensis in sunflowers [81].
During the growing seasons, the density of annual species progressively increased, while one of the perennial species was reduced. The literature verifies that perennial weeds such as S. elaeagnifolium are highly invasive and aggressive as it is acclimatized to various environments [82,83]. Therefore, they show increased resistance to abiotic and abiotic stress, decreasing the agricultural tools available for their control. In our study, the reduction in perennial weeds, the most noxious and competitive, change weed flora and has a positive impact on the next crop as well.
4.4. Weed Diversity Indices
Even though weed diversity is important for increased yield stability and agricultural sustainability [84,85], the adverse effects of weed competition against the hemp crop should be lessened. Compilation of diversity values in both growing seasons provides important information for the design of the appropriate weed management plan. Based on the results, the weed diversity was described by the Shannon, Simpson, Evenness, and Richness indices.
Among cultivars, the weed diversity was greater in plots of “Uso 31” than in “Fedora 17” ones, regardless of fertilization and weed management treatments. That fact could be attributed to the very low weed competitive ability of “Uso 31”, which boosted the emergence and development of different weed species. Furthermore, weed diversity was favored by fertilization in the “Uso 31” plots, especially at the maximum rate of fertilizer 140 kg ha−1. This increase in weed diversity could be assigned to nutrient effects since nitrogen may mostly impact the diversity and abundance of several weeds [52]. Opposite effects were recorded in the case of “Fedora 17”, where the fertilized plots showed lower diversity of weed species than the unfertilized ones. This finding of our study is in accordance with the results of [85,86], which claimed that high rates of fertilization decreased biodiversity. The different effects of fertilization on “Fedora 17” plots could be justified by the fact that nitrogen enhanced the nitrophilous weeds, resulting in their relative dominance compared with other non-nitrophilous weeds.
Regarding weed management, plots of “Uso 31” subjected to herbicide treatment showed decreased weed diversity indices, and similar effects were recorded in unfertilized plots of “Fedora 17”. Even though herbicide application insignificantly decreased the values in some studied cases, the weed diversity values remained high. A previous study found that pre-emergence herbicides led to great species diversity and low species dominance [87]. In general, the estimation of indices signifies that there was abundance and uniformity of weed species, while no weed dominance was recorded.
4.5. Hemp/Weed Competition
A greater soil nitrogen availability augmented the biomass and the relative fitness of hemp (RRIH < 0) in both cultivars regardless of weed presence. Among cultivars, the highest biomass was recorded in tall plants of “Fedora 17” after the addition of 140 kg ha−1. Weed presence impacted negatively on the plant growth of fertilized plots of “Uso 31” and “Fedora 17”, resulting in higher RRIH values compared with weed-free plots.
Even though fertilization led to an increase in the hemp biomass (RRIH < 0), it also caused an increase in the biomass of the nitrophilous and non-nitrophilous weeds. The addition of nitrogen resulted in a weaker suppressive effect of the crop on weed emergence and growth, especially at the vegetative stage of the crop. Thus, weeds took advantage of the available soil nitrogen (RRIW < 0). Similar results were provoked by the combination of herbicide application and fertilization, where the biomass of both nitrophilous and non-nitrophilous weeds was positively affected across cultivars. Their positive effect on nitrophilous weeds can be attributed only to the fertilization treatment, while the weed density of nitrophilous weeds was higher in the plots subjected to the herbicide. In that case, the major nitrophilous weeds such as A. retroflexus, E. crus-galli, P. oleracea, and C. album were not susceptible to pendimethalin. A previous study referred to the inefficacy of pendimethalin against A. retroflexus and C. album in potato crops [88]. Concerning the non-nitrophilous weeds, the high weed biomass cannot be ascribed to herbicide treatment since none of these weeds belongs to the species which are within the range of pendimethalin activity. Therefore, the increase in RRINW could be caused by fertilization treatments, as is also well depicted in the RCI after the herbicide application. In particular, the response comparison index (RCI) indicated that despite the variability between the growing seasons, herbicide application reduced the growth of many weeds and, consequently, their response to fertilization. Furthermore, our results revealed the notable competitive ability of “Fedora 17” against weeds under both fertilization treatments.
5. Conclusions
Weed flora is considered harmful to crop growth and yield, but it is fundamental for preserving biodiversity in agroecosystems. Fertilization alters soil nutrient levels, which affects not only crop growth, but also the diversity and growth of weeds. Competition between crops and weeds for nutrients, and for other factors, such as light, water, and space at different nutrient levels, are complex interactions that depend on many factors. As a result, actions that alter nutrient availability will affect the weed community and the crop’s competitive ability. Currently, the aim of weed management should be to reduce the impact of weeds on crop yield by maintaining a diverse community of relatively controllable weed species in a way that any weed species that is difficult to control does not become dominant. The present study confirmed the dose–response effect of fertilization on plant height and dry biomass of hemp, while no impact was recorded on yield and yield components. The density and biomass of nitrophilous weeds were enhanced by fertilization, negatively affecting hemp growth. “Fedora 17” was the cultivar with significantly higher competitive ability against weeds compared to “Uso 31”. Our findings also revealed that herbicide application decreased the biomass of annual and perennial weeds by 38% and 13%, respectively, while it caused a reduction in hemp growth and yield of “Uso 31” plants. Therefore, selectivity studies ought to be conducted in a wide range of cultivars and under different pedoclimatic conditions. To sum up, further investigation is needed to implement integrated management practices and to reveal more competitive cultivars in order to achieve the maximum benefit for the crop and the most efficient control of weeds.
Author Contributions
Methodology, I.K., A.K. and A.M.; data curation, I.K., A.K., P.P. and A.M.; writing—original draft, I.K. and A.M.; writing—review and editing, I.K., I.T. and P.P.; supervision, P.P. The authors contributed equally to this work. All authors have read and agreed to the published version of the manuscript.
Funding
This research was co-financed by the European Regional Development Fund of the European Union and Greek national funds through the Operational Program Competitiveness, Entrepreneurship, and Innovation, under the call RESEARCH-CREATE-INNOVATE (project code: T1EDK-04301).
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy restrictions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Karche, T.; Singh, M.R. The application of hemp (Cannabis sativa L.) for a green economy: A review. Turk. J. Bot. 2019, 43, 710–723. [Google Scholar] [CrossRef]
- Krüger, M.; van Eeden, T.; Beswa, D. Cannabis sativa cannabinoids as functional ingredients in snack foods–Historical and developmental aspects. Plants 2022, 11, 3330. [Google Scholar] [CrossRef] [PubMed]
- Hesami, M.; Pepe, M.; Baiton, A.; Jones, A.M.P. Current status and future prospects in cannabinoid production through in vitro culture and synthetic biology. Biotechnol. Adv. 2023, 62, 108074. [Google Scholar] [CrossRef] [PubMed]
- Kovalchuk, I.; Pellino, M.; Rigault, P.; van Velzen, R.; Ebersbach, J.; Ashnest, J.R.; Mau, M.; Schranz, M.E.; Alcorn, J.; Laprairie, R.B.; et al. The genomics of Cannabis and its close relatives. Annu. Rev. Plant Biol. 2020, 71, 713–739. [Google Scholar] [CrossRef] [PubMed]
- Hesami, M.; Pepe, M.; Alizadeh, M.; Rakei, A.; Baiton, A.; Jones, A.M.P. Recent advances in cannabis biotechnology. Ind. Crops Prod. 2020, 158, 213026. [Google Scholar] [CrossRef]
- Amaducci, S.; Scordia, D.; Liu, F.H.; Zhang, Q.; Guo, H.; Testa, G.; Cosentino, S.L. Key cultivation techniques for hemp in Europe and China. Ind. Crops Prod. 2015, 68, 2–16. [Google Scholar] [CrossRef]
- Johnson, R. Hemp as an Agricultural Commodity; Congressional Research Service: Washington, DC, USA, 2014. [Google Scholar]
- Schluttenhofer, C.; Yuan, L. Challenges towards Revitalizing Hemp: A Multifaceted Crop. Trends Plant Sci. 2017, 22, 917–929. [Google Scholar] [CrossRef] [PubMed]
- Andre, C.M.; Hausman, J.F.; Guerriero, G. Cannabis sativa: The plant of the thousand and one molecules. Front Plant Sci. 2016, 7, 19. [Google Scholar] [CrossRef]
- Tsaliki, E.; Kalivas, A.; Jankauskiene, Z.; Irakli, M.; Cook, C.; Grigoriadis, I.; Panoras, I.; Vasilakoglou, I.; Dhima, K. Fibre and Seed Productivity of Industrial Hemp (Cannabis sativa L.) varieties under Mediterranean Conditions. Agronomy 2021, 11, 171. [Google Scholar] [CrossRef]
- Bilalis, D.; Karidogianni, S.; Roussis, I.; Kouneli, V.; Kakabouki, I.; Folina, A. Cannabis sativa L.: A new promising crop for medical and industrial use. Bull. Univ. Agric. Sci. Vet. Med. 2019, 76, 145–150. [Google Scholar] [CrossRef]
- Alexander, S.P.H. Therapeutic potential of cannabis-related drugs. Prog. Neuropsychopharmacol. Biol. Psychiatry 2016, 64, 157–166. [Google Scholar] [CrossRef]
- Rodriguez-Leyva, D.; Pierce, G.N. The Cardiac and Haemostatic Effects of Dietary Hempseed. Nutr. Metab. 2010, 7, 32. [Google Scholar] [CrossRef] [PubMed]
- Lukin, A.; Bitiutskikh, K. Investigation on the use of hemp flour in cookie production. Bulg. J. Agric. Sci. 2017, 23, 664–667. [Google Scholar]
- Gorelick, J.; Bernstein, N. Chemical and physical elicitation for enhanced cannabinoid production in cannabis. In Cannabis sativa L.—Botany and Biotechnology; Chandra, S., Lata, H., ElSohly, M.A., Eds.; Springer International Publishing: Basel, Switzerland, 2017; pp. 439–456. [Google Scholar]
- Salentijn, E.M.J.; Zhang, Q.; Amaducci, S.; Yang, M.; Trindade, L.M. New developments in fiber hemp (Cannabis sativa L.) breeding. Ind. Crops Prod. 2015, 68, 32–41. [Google Scholar] [CrossRef]
- Zatta, A.; Monti, A.; Venturi, G. Eighty years of studies on industrial hemp in the Po valley (1930–2010). J. Nat. Fibers 2012, 9, 80–196. [Google Scholar] [CrossRef]
- European Commission. Common Catalogue of Varieties of Agricultural Plant Species. Available online: https://op.europa.eu/ (accessed on 10 December 2022).
- European Commission. Delegated Regulation (EU) No 639/2014. Available online: https://eur-lex.europa.eu/ (accessed on 10 December 2022).
- Ascrizzi, R.; Ceccarini, L.; Tavarini, S.; Flamini, G.; Angelini, L.G. Valorisation of hemp inflorescence after seed harvest: Cultivation site and harvest time influence agronomic characteristics and essential oil yield and composition. Ind. Crops Prod. 2019, 139, 111541. [Google Scholar] [CrossRef]
- Sebastian, J.S.V.; Dong, X.; Trostle, C.; Pham, H.; Joshi, M.V.; Jessup, R.W.; Burow, M.D.; Provin, T.L. Hemp agronomy: Current advances, questions, challenges, and opportunities. Agronomy 2023, 13, 475. [Google Scholar] [CrossRef]
- Welling, M.T.; Liu, L.; Shapter, T.; Raymond, C.A.; King, G.J. Characterization of cannabinoid composition in a diverse Cannabis sativa L. germplasm collection. Euphytica 2017, 208, 463–475. [Google Scholar] [CrossRef]
- Calzolari, D.; Magagnini, G.; Lucini, L.; Grassi, G.; Appendino, G.B.; Amaducci, S. High added-value compounds from Cannabis threshing residues. Ind. Crops Prod. 2017, 108, 558–563. [Google Scholar] [CrossRef]
- Baldini, M.; Ferfuia, C.; Piani, B.; Sepulcri, A.; Dorigo, G.; Zuliani, F.; Danuso, F.; Cativello, C. The performance and potentiality of monoecius hemp (Cannabis sativa L.) cultivars as a multipurpose crop. Agronomy 2018, 8, 162. [Google Scholar] [CrossRef]
- Papastylianou, P.; Kakabouki, I.; Travlos, I. Effect of nitrogen fertilization on growth and yield of industrial hemp (Cannabis sativa L.). Not. Bot. Horti Agrobot. 2017, 46, 197–201. [Google Scholar] [CrossRef]
- Campiglia, E.; Radicetti, E.; Mancinelli, R. Plant density and nitrogen fertilization affect agronomic performance of industrial hemp (Cannabis sativa L.) in Mediterranean environment. Ind. Crops Prod. 2017, 100, 246–254. [Google Scholar] [CrossRef]
- Adesina, I.; Bhowmik, A.; Sharma, H.; Shahbazi, A. A review of the current state of knowledge of growing conditions, agronomic soil health practices and utilities of hemp in the United States. Agriculture 2020, 10, 129. [Google Scholar] [CrossRef]
- Masclaux-Daubresse, C.; Daniel-Vedele, F.; Dechorgnat, J.; Chardon, F.; Gaufichon, L.; Suzuki, A. Nitrogen uptake, assimilation, and remobilization in plants: Challenges for sustainable and productive agriculture. Ann. Bot. 2010, 105, 1141–1157. [Google Scholar] [CrossRef] [PubMed]
- Curci, P.L.; Cigliano, R.A.; Zuluaga, D.L.; Janni, M.; Sanseverino, W.; Sonnante, G. Transcriptomic response of durum wheat to nitrogen starvation. Sci. Rep. 2017, 7, 1176. [Google Scholar] [CrossRef] [PubMed]
- Ogden, M.; Hoefgen, R.; Roessner, U.; Persson, S.; Khan, G.A. Feeding the walls: How does nutrient availability regulate cell wall composition? Int. J. Mol. Sci. 2018, 19, 2691. [Google Scholar] [CrossRef]
- Vera, C.L.; Malhi, S.S.; Raney, J.P.; Wang, Z.H. The effect of N and P fertilization on growth, seed yield and quality of industrial hemp in the Parkland region of Saskatchewan. Can. J. Plant Sci. 2004, 84, 939–947. [Google Scholar] [CrossRef]
- Caplan, D.; Dixon, M.; Zheng, Y. Optimal rate of organic fertilizer during the flowering stage for Cannabis grown in two coir-based substrates. HortScience 2017, 52, 1796–1803. [Google Scholar] [CrossRef]
- Kakabouki, I.; Kousta, A.; Folina, A.; Karydogianni, S.; Zisi, C.; Kouneli, V.; Papastylianou, P. Effect of Fertilization with Urea and Inhibitors on Growth, Yield and CBD Concentration of Hemp (Cannabis sativa L.). Sustainability 2021, 13, 2157. [Google Scholar] [CrossRef]
- Rioba, N.B.; Itulya, F.M.; Saidi, M.; Dudai, N.; Bernstein, N. Effects of nitrogen, phosphorus and irrigation frequency on essential oil content and composition of sage (Salvia officinalis L.). J. Appl. Res. Med. Aromat. Plants 2015, 2, 21–29. [Google Scholar] [CrossRef]
- Tang, K.; Struik, P.C.; Yin, X.; Calzolari, D.; Musio, S.; Thouminot, C.; Bjelková, M.; Stramkale, V.; Magagnini, G.; Amaducci, S. A comprehensive study of planting density and nitrogen fertilization effect on dual-purpose hemp (Cannabis sativa L.) cultivation. Ind Crops Prod. 2017, 107, 427–438. [Google Scholar] [CrossRef]
- Blackshaw, R.E.; Brandt, R.N.; Janzen, H.H.; Entz, T.; Grant, C.A.; Derksen, D.A. Differential response of weed species to added nitrogen. Weed Sci. 2003, 51, 532–539. [Google Scholar] [CrossRef]
- Dhima, K.V.; Eleftherohorinos, I.G. Influence of nitrogen on competition between winter cereals and sterile oat. Weed Sci. 2001, 49, 77–82. [Google Scholar] [CrossRef]
- Sweeney, A.E.; Renner, K.A.; Laboski, C.; Davis, A. Effect of fertilizer nitrogen on weed emergence and growth. Weed Sci. 2008, 56, 714–721. [Google Scholar] [CrossRef]
- Travlos, I.; Papastylianou, P.; Alexos, A.; Kanatas, P.; Bilalis, D.; Tsekoura, A.; Kakabouki, I.; Cheimona, N. Changes of weed flora due to nitrogen addition in sunflower. Not. Bot. Horti Agrobot. 2019, 47, 1337–1339. [Google Scholar] [CrossRef]
- Gurmani, A.R.; Khan, S.U.; Mehmood, T.; Ahmed, W.; Rafique, M. Exploring the Allelopathic Potential of Plant Extracts for Weed Suppression and Productivity in Wheat (Triticum aestivum L.). Gesunde Pflanz. 2021, 73, 29–37. [Google Scholar] [CrossRef]
- Struik, P.C.; Amaducci, S.; Bullard, M.J.; Stutterheim, N.C.; Venturi, G.; Cromack, H.T.H. Agronomy of fibre hemp (Cannabis sativa L.) in Europe. Ind. Crops Prod. 2000, 11, 107–118. [Google Scholar] [CrossRef]
- Small, E.; Pocock, T.; Cavers, P.B. The biology of Canadian weeds. 119. Cannabis sativa L. Can. J. Plant Sci. 2003, 83, 217–237. [Google Scholar] [CrossRef]
- Kostuik, J.; Williams, D.W. Hemp agronomy–Grain and Fiber Production. In Industrial Hemp as a Modern Commodity Crop; Fike, J., Riddle, T., Nelson, J., Flaherty, P., Williams, D.W., Eds.; American Society of Agronomy, Crop Science Society: Madison, WI, USA, 2019; pp. 59–72. [Google Scholar]
- Ganavan, S.; Brym, Z.T.; Brundu, G.; Dehnen-Schmutz, K.; Lieurance, D.; Petri, T.; Wadlington, W.H.; Wilson, J.R.U.; Flory, S.L. Cannabis de-domestication and invasion risk. Biol. Conserv. 2022, 274, 109709. [Google Scholar]
- Hall, J.; Bhattarai, S.P.; Midmore, D.J. Effect of industrial hemp (Cannabis sativa L.) planting density on weed suppression, crop growth, physiological responses, and fibre yield in the subtropics. Renew. Bioresour. 2014, 2, 1. [Google Scholar]
- Ameh, S.J.; Obodozie, O.O.; Inyang, U.S.; Abubakar, M.S.; Garba, M. Current phytotherapy—A perspective on the science and regulation of herbal medicine. J. Med. Plant Res. 2010, 4, 72–81. [Google Scholar]
- Pudelko, K.; Majchrzak, L.; Narozna, D. Allelopathic effect of fibre hemp (Cannabis sativa L.) on monocot and dicot plant species. Ind Crops Prod. 2014, 56, 191–199. [Google Scholar] [CrossRef]
- Deeley, M.R. Could cannabis provide an answer to climate change? J. Ind. Hemp 2002, 7, 133–138. [Google Scholar] [CrossRef]
- Robson, M.C.; Fowler, S.M.; Lampkin, N.H.; Leifert, C.; Leitch, M.; Robinson, D.; Watson, C.A.; Litterick, A.M. The Agronomic and Economic Potential of Break Crops for Ley/Arable Rotations in Temperate Organic Agriculture. Adv. Agron. 2002, 77, 369–427. [Google Scholar]
- Jankauskiené, Z.; Gruzdeviené, E.; Lazauskas, S. Potential of industrial hemp (Cannabis sativa L.) genotypes to suppress weeds. Zemdirbyste 2014, 101, 265–270. [Google Scholar] [CrossRef]
- Cole, C.; Zurbo, B. Industrial hemp– a new crop for NSW. Primefacts 2008, 801, 1–6. [Google Scholar]
- Kousta, A.; Papastylianou, P.; Cheimona, N.; Travlos, I.; Kakabouki, I.; Bilalis, D. Effect of Fertilization and Weed Management on Weed Flora of Hemp Crop. Bull. Univ. Agric. Sci. Vet. Med. 2020, 77, 45–51. [Google Scholar] [CrossRef]
- Flessner, M.; Bryd, J.; Bamber, K.; Fike, J. Evaluating herbicide tolerance of industrial hemp (Cannabis sativa L.). Crop Sci. 2020, 60, 419–427. [Google Scholar] [CrossRef]
- Nkoa, R.; Owen, M.D.K.; Swanton, C.J. Weed abundance, distribution, diversity, and community analyses. Weed Sci. 2015, 63, 64–90. [Google Scholar] [CrossRef]
- Shanon, C.E. A mathematical theory of communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Magurran, A.E. Ecological Diversity and its Measurement; Princeton University Press: Princeton, NJ, USA, 1988. [Google Scholar]
- Berger, W.; Parker, F.L. Diversity of planktonic Forminifera in deep-sea sediments. Science 1970, 168, 1345–1347. [Google Scholar] [CrossRef]
- Williams, I.I.; Mortensen, M.M.; Doran, D.A.J.W. Assessment of weed and crop fitness in cover crop residues for integrated weed management. Weed Sci. 1998, 46, 595–603. [Google Scholar] [CrossRef]
- Campiglia, E.; Radicetti, E.; Brunetti, P.; Mancinelli, R. Do cover crop species and residue management play a leading role in pepper productivity? Sci. Hortic. 2014, 166, 97–104. [Google Scholar] [CrossRef]
- Gomez, K.A.; Gomez, A.A. Statistical Procedures for Agricultural Research, 2nd ed.; John Wiley and Sons: New York, NY, USA, 1984; p. 680. [Google Scholar]
- Van der Werf, H.M.G.; Mathijsen, W.W.J.M.; Havenkort, A.J. The potential of hemp (Cannabis sativa L.) for sustainable fibre production. A crop physiological appraisal. Ann. Appl. Bot. 1996, 129, 109–123. [Google Scholar] [CrossRef]
- Tang, K.; Struik, P.C.; Yin, X.; Thouminot, C.; Bjelková, M.; Stramkale, V.; Amaducci, S. Comparing hemp (Cannabis sativa L.) cultivars for dual-purpose production under contrasting environments. Ind. Crops Prod. 2016, 87, 33–44. [Google Scholar] [CrossRef]
- Tang, K.; Wang, J.; Yang, Y.; Deng, G.; Yu, J.; Hu, W.; Guo, L.; Du, G.; Liu, F. Fiber Hemp (Cannabis sativa L.) Yield and Its Response to Fertilization and Planting Density in China. Ind. Crops Prod. 2022, 177, 114542. [Google Scholar] [CrossRef]
- Finnan, J.; Burke, B. Potassium fertilization of hemp (Cannabis sativa). Ind. Crop. Prod. 2013, 41, 419–422. [Google Scholar] [CrossRef]
- Aubin, M.; Seguin, P.; Vanasse, A.; Gaetan, F.T.; Mustafa, A.F.; Charron, J. Industrial hemp response to nitrogen, phosphorus, and potassium fertilization. Crop For. Turfgrass Manag. 2015, 1, 1–10. [Google Scholar] [CrossRef]
- Ivonyi, I.; Izsoki, Z.; van der Werf, H.M. Influence of nitrogen supply and P and K levels of the soil on dry matter and nutrient accumulation of fiber hemp. J. Int. Hemp Assoc. 1997, 4, 84–90. [Google Scholar]
- Wogiatzi, E.; Gougoulias, N.; Giannoulis, K.D.; Kamvoukou, C.-A. Effect of Irrigation and Fertilization Levels on Mineral Composition of Cannabis sativa L. Leaves. Not. Bot. Horti Agrobot. 2019, 47, 1073–1080. [Google Scholar] [CrossRef]
- Deng, G.; Du, G.; Yang, Y.; Bao, Y.; Liu, F. Planting density and fertilization evidently influence the fiber yield of hemp (Cannabis sativa L.). Agronomy 2019, 9, 368. [Google Scholar] [CrossRef]
- Maxwell, B.A. Effects of Herbicides on Industrial Hemp (Cannabis sativa) Phytotoxicity, Biomass, and Seed Yield. Master’s Thesis, Western Kentucky University, Bowling Green, KY, USA, 2016. [Google Scholar]
- Ortmeier-Clarke, H.J.; Oliveira, M.C.; Arneson, N.J.; Conley, S.P.; Werle, R. Dose–response screening of industrial hemp to herbicides commonly used in corn and soybean. Weed Technol. 2022, 36, 245–252. [Google Scholar] [CrossRef]
- Baldini, M.; Ferfuia, C.; Zuliani, F.; Danuso, F. Suitability assessment of different hemp (Cannabis sativa L.) varieties to the cultivation environment. Ind. Crops Prod. 2020, 143, 111860. [Google Scholar] [CrossRef]
- Faux, A.-M.; Draye, X.; Lambert, R.; d’Andrimont, R.; Raulier, P.; Bertin, P. The Relationship of Stem and Seed Yields to Flowering Phenology and Sex Expression in Monoecious Hemp (Cannabis sativa L.). Eur. J. Agron. 2013, 47, 11–22. [Google Scholar] [CrossRef]
- Gorchs, G.; Lloveras, J.; Serrano, L.; Cela, S. Hemp yields and its rotation effects on wheat under rainfed mediterranean conditions. Agron. J. 2017, 109, 1551–1560. [Google Scholar] [CrossRef]
- Mediavilla, V.; Jonquera, M.; Schmid-Slembrouck, I.; Soldati, A. Decimal Code for Growth Stages of Hemp (Cannabis sativa L.). J. Int. Hemp Assoc. 1998, 5, 68–74. [Google Scholar]
- Vogl, C.R.; Mölleken, H.; Lissek-Wolf, G.; Surböck, A.; Kobert, J. Hemp (Cannabis sativa L.) as a resource for green cosmetics: Yield of seed and fatty acid compositions of 20 varieties under the growing conditions of organic farming in Austria. J. Ind. Hemp 2004, 9, 51–68. [Google Scholar] [CrossRef]
- Stafecka, I.; Stramkale, V.; Stramkalis, A.; Kroica, I.; Ivanovs, S. Impact of the agro-environmental factors on the seed yield and yields components productivity of Latvian original hemp. J. Res. Appl. Agric. Eng. 2016, 61, 164–167. [Google Scholar]
- Papastylianou, P.; Kousta, A.; Kakabouki, I.; Travlos, I.; Iliadi, D. Nitrogen utilization efficiency and yield traits of dual-purpose industrial hemp cultivars in a Mediterranean environment. Arch. Agron. Soil Sci. 2021, 69, 104–118. [Google Scholar] [CrossRef]
- Maļceva, M.; Vikmane, M.; Stramkale, V. Changes of photosynthesis related parameters and productivity of Cannabis sativa under different nitrogen supply. Environ. Exp. Biol. 2011, 9, 61–69. [Google Scholar]
- Dan, A.; Duda, M.M.; Moldova, C.; Florian, T. The influence of different seeding space and organic fertilization level upon production and mass of 1000 seeds, recorded in some hemp varieties. Bull. Univ. Agric. Sci. Vet. Med. 2015, 72, 57–60. [Google Scholar] [CrossRef]
- Sandler, L.N.; Gibson, K.A. A all for weed research in industrial hemp (Cannabis sativa L.). Weed Res. 2019, 59, 255–259. [Google Scholar] [CrossRef]
- Grozi, D.; Delchev, G.D.; Stayanova, A.K. Stability of vegetation-applied herbicides and their mixtures with complex foliar fertilizer lactofol B at oil-bearing sunflower by influence of different meteorological conditions. J. Int. Sci. Publ. Agric. Food 2014, 2, 1314–8591. [Google Scholar]
- Tataridas, A.; Jabran, K.; Kanatas, P.; Oliveira, R.S.; Freitas, H.; Travlos, I. Early detection, herbicide resistance screening, and integrated management of Invasive Plant Species: A review. Pest Manag. Sci. 2022, 78, 3957–3972. [Google Scholar] [CrossRef]
- Uludag, A.; Gbehounou, G.; Kashefi, J.; Bouhache, M.; Bon, M.C.; Bell, C.; Lagopodi, A.L. Review of the current situation for Solanum elaeagnifolium in the Mediterranean Basin. EPPO Bull. 2016, 46, 139–147. [Google Scholar] [CrossRef]
- Schütte, G.; Eckerstorfer, M.; Rastelli, V.; Reichenbecher, W.; Restrepo-Vassalli, S.; Ruohonen-Lehto, M.; Saucy, W.A.G.; Mertens, M. Herbicide resistance and biodiversity: Agronomic and environmental aspects of genetically modified herbicide-resistant plants. Environ. Sci. Eur. 2017, 29, 1–12. [Google Scholar] [CrossRef]
- Pyšek, P.; Lepš, J. Response of a weed community to nitrogen fertilization: A multivariate analysis. J. Veg. Sci. 1991, 2, 237–244. [Google Scholar] [CrossRef]
- Inouye, R.; Tilman, D. Convergence and divergence of old-field vegetation after 11 year of nitrogen addition. Ecology 1995, 76, 1872–1887. [Google Scholar] [CrossRef]
- Grundy, A.C.; Mead, A.; Bond, W.; Clark, G.; Burston, S. The impact of herbicide management on long-term changes in the diversity and species composition of weed populations. Weed Res. 2010, 51, 187–200. [Google Scholar] [CrossRef]
- Alebrahim, M.T.; Majd, R.; Rashed Mohassel, M.H.; Wilkakson, S.; Baghestani, M.A.; Ghorbani, R.; Kudsk, P. Evaluating the efficacy of pre- and post-emergence herbicides for controlling Amaranthus retroflexus L. and Chenopodium album L. in potato. Crop Protect. 2012, 42, 345–350. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).