Interactive Influence of Soil Erosion and Cropland Revegetation on Soil Enzyme Activities and Microbial Nutrient Limitations in the Loess Hilly-Gully Region of China
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site and Sampling
2.2. Laboratory Analysis
2.3. Calculation of Microbial Nutrient Limitation
2.4. Statistical Analysis
3. Results
3.1. Soil Physicochemical Properties
3.2. Soil Microbial C, N, P and Enzyme Activity
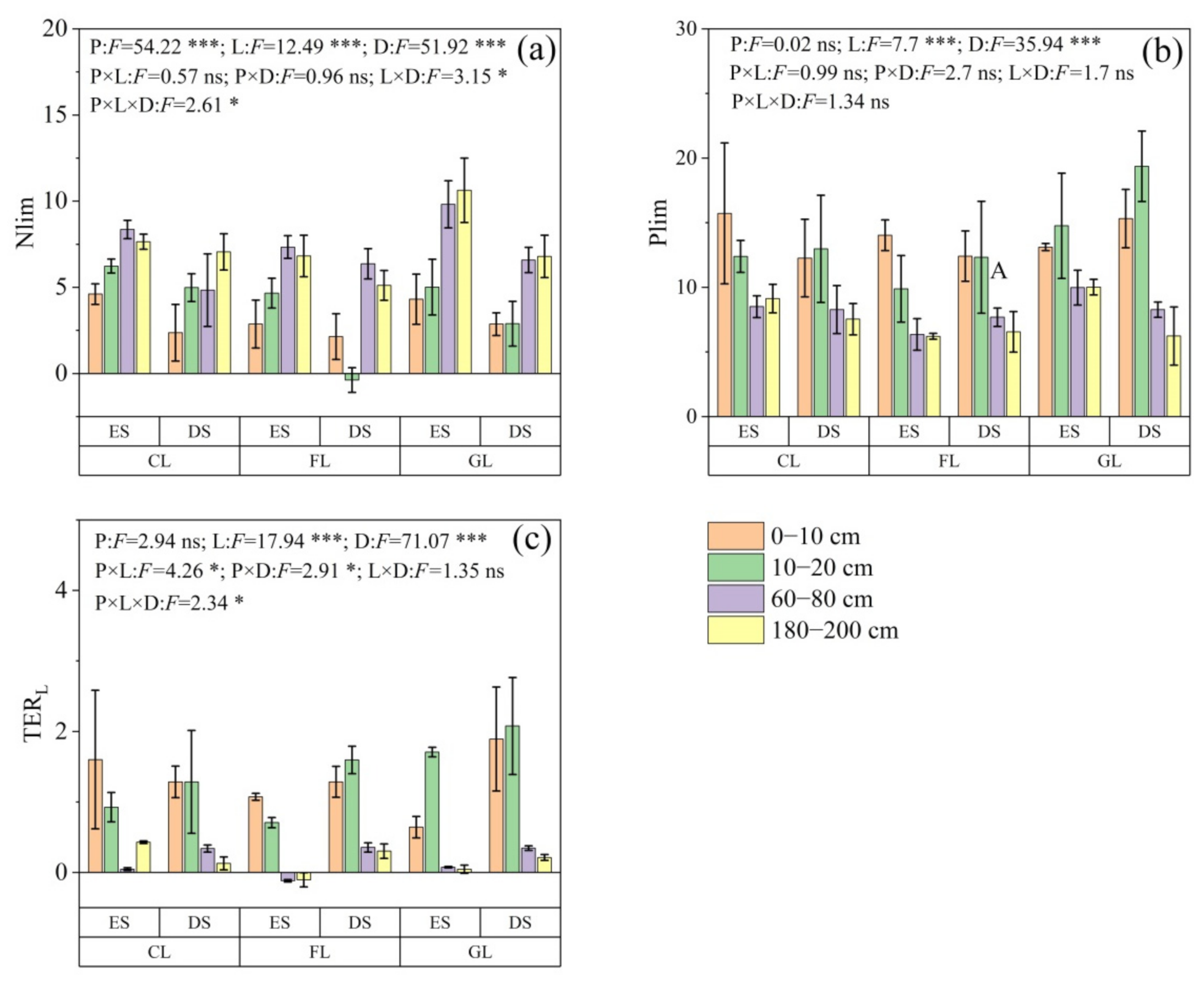
3.3. Soil Microbial Nutrient Limitation
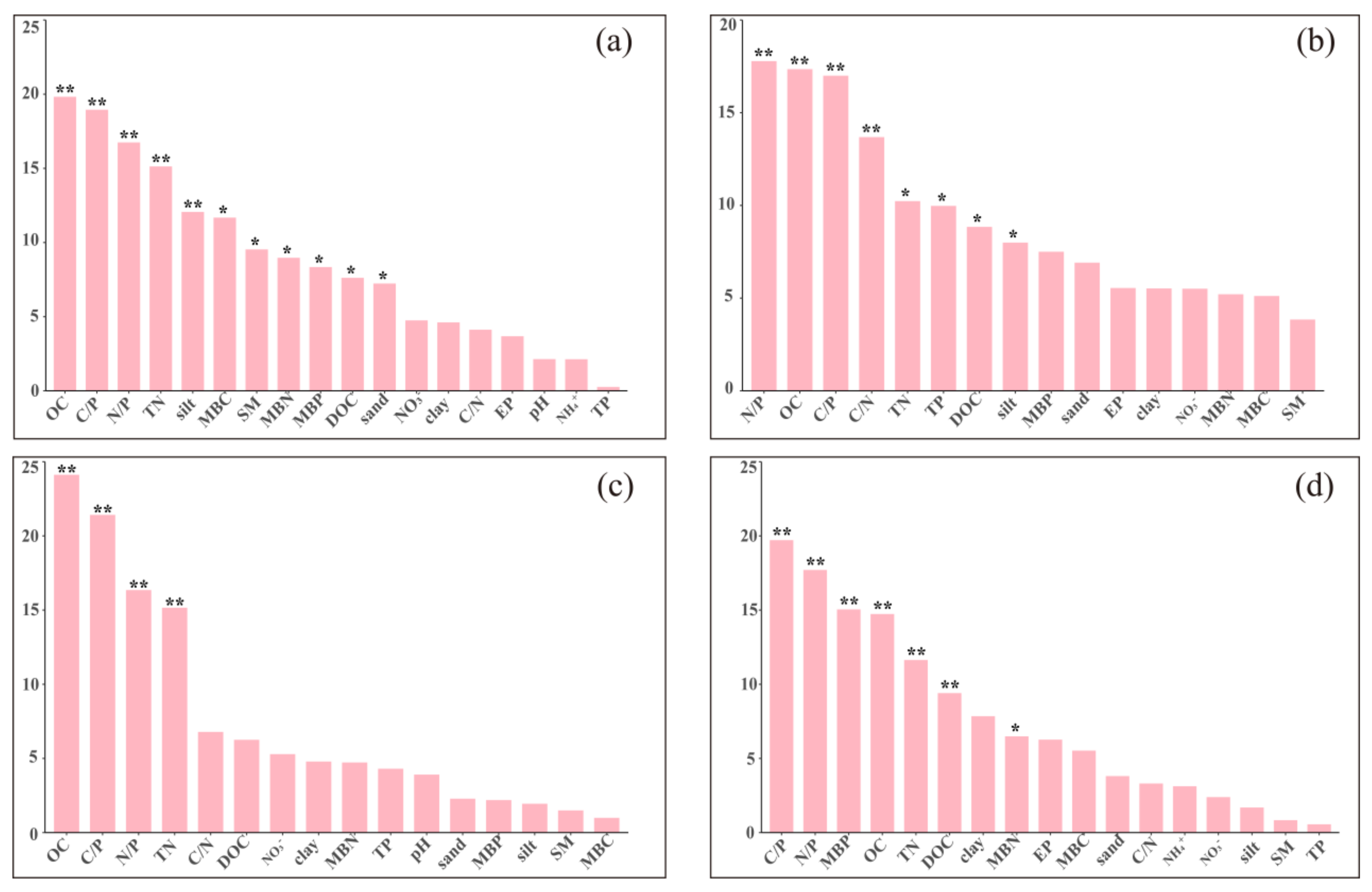
3.4. Factors Influencing Extracellular Enzyme Activities and Microbial Nutrient Limitations
4. Discussion
4.1. Variations in Soil Enzyme Activities between the Eroded and Deposited Zones and Their Dependence on Land Use and Soil Depth
4.2. Variations in Soil Microbial Nutrient Limitations between Eroded and Deposited Zones and Their Dependence on Land Use and Soil Depth
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Quinton, J.N.; Govers, G.; Van Oost, K.; Bardgett, R.D. The Impact of Agricultural Soil Erosion on Biogeochemical Cycling. Nat. Geosci. 2010, 3, 311–314. [Google Scholar] [CrossRef]
- Petito, M.; Cantalamessa, S.; Pagnani, G.; Degiorgio, F.; Parisse, B.; Pisante, M. Impact of Conservation Agriculture on Soil Erosion in the Annual Cropland of the Apulia Region (Southern Italy) Based on the RUSLE-GIS-GEE Framework. Agronomy 2022, 12, 281. [Google Scholar] [CrossRef]
- Lal, R. Accelerated Soil Erosion as a Source of Atmospheric CO2. Soil Tillage Res. 2019, 188, 35–40. [Google Scholar] [CrossRef]
- Borrelli, P.; Robinson, D.A.; Fleischer, L.R.; Lugato, E.; Ballabio, C.; Alewell, C.; Meusburger, K.; Modugno, S.; Schütt, B.; Ferro, V.; et al. An Assessment of the Global Impact of 21st Century Land Use Change on Soil Erosion. Nat. Commun. 2017, 8, 2013. [Google Scholar] [CrossRef] [PubMed]
- Lasanta, T.; Nadal-Romero, E.; Arnáez, J. Managing Abandoned Farmland to Control the Impact of Re-Vegetation on the Environment. The State of the Art in Europe. Environ. Sci. Policy 2015, 52, 99–109. [Google Scholar] [CrossRef]
- Wang, S.; Fu, B.; Piao, S.; Lü, Y.; Ciais, P.; Feng, X.; Wang, Y. Reduced Sediment Transport in the Yellow River Due to Anthropogenic Changes. Nat. Geosci. 2016, 9, 38–41. [Google Scholar] [CrossRef]
- Zhu, G.; Shangguan, Z.; Hu, X.; Deng, L. Effects of Land Use Changes on Soil Organic Carbon, Nitrogen and Their Losses in a Typical Watershed of the Loess Plateau, China. Ecol. Indic. 2021, 133, 108443. [Google Scholar] [CrossRef]
- Dungait, J.A.J.; Ghee, C.; Rowan, J.S.; McKenzie, B.M.; Hawes, C.; Dixon, E.R.; Paterson, E.; Hopkins, D.W. Microbial Responses to the Erosional Redistribution of Soil Organic Carbon in Arable Fields. Soil Biol. Biochem. 2013, 60, 195–201. [Google Scholar] [CrossRef]
- Berhe, A.A.; Barnes, R.T.; Six, J.; Marín-Spiotta, E. Role of Soil Erosion in Biogeochemical Cycling of Essential Elements: Carbon, Nitrogen, and Phosphorus. Annu. Rev. Earth Planet. Sci. 2018, 46, 521–548. [Google Scholar] [CrossRef]
- De Nijs, E.A.; Cammeraat, E.L.H. The Stability and Fate of Soil Organic Carbon during the Transport Phase of Soil Erosion. Earth-Sci. Rev. 2020, 201, 103067. [Google Scholar] [CrossRef]
- Du, L.; Wang, R.; Hu, Y.; Li, X.; Gao, S.; Wu, X.; Gao, X.; Yao, L.; Guo, S. Contrasting Responses of Soil C-Acquiring Enzyme Activities to Soil Erosion and Deposition. Catena 2021, 198, 105047. [Google Scholar] [CrossRef]
- Cui, Y.; Fang, L.; Guo, X.; Han, F.; Ju, W.; Ye, L.; Wang, X.; Tan, W.; Zhang, X. Natural Grassland as the Optimal Pattern of Vegetation Restoration in Arid and Semi-Arid Regions: Evidence from Nutrient Limitation of Soil Microbes. Sci. Total Environ. 2019, 648, 388–397. [Google Scholar] [CrossRef]
- Guan, H.; Fan, J.; Zhang, H.; Harris, W. Comparison of Drivers of Soil Microbial Communities Developed in Karst Ecosystems with Shallow and Deep Soil Depths. Agronomy 2021, 11, 173. [Google Scholar] [CrossRef]
- Xu, M.; Li, W.; Wang, J.; Zhu, Y.; Feng, Y.; Yang, G.; Zhang, W.; Han, X. Soil Ecoenzymatic Stoichiometry Reveals Microbial Phosphorus Limitation after Vegetation Restoration on the Loess Plateau, China. Sci. Total Environ. 2022, 815, 152918. [Google Scholar] [CrossRef]
- Ahmed, I.U.; Mengistie, H.K.; Godbold, D.L.; Sandén, H. Soil Moisture Integrates the Influence of Land-Use and Season on Soil Microbial Community Composition in the Ethiopian Highlands. Appl. Soil Ecol. 2019, 135, 85–90. [Google Scholar] [CrossRef]
- Hsiao, C.-J.; Sassenrath, G.F.; Zeglin, L.H.; Hettiarachchi, G.M.; Rice, C.W. Vertical Changes of Soil Microbial Properties in Claypan Soils. Soil Biol. Biochem. 2018, 121, 154–164. [Google Scholar] [CrossRef]
- Park, J.-H.; Meusburger, K.; Jang, I.; Kang, H.; Alewell, C. Erosion-Induced Changes in Soil Biogeochemical and Microbiological Properties in Swiss Alpine Grasslands. Soil Biol. Biochem. 2014, 69, 382–392. [Google Scholar] [CrossRef]
- Sarapatka, B.; Cap, L.; Bila, P. The Varying Effect of Water Erosion on Chemical and Biochemical Soil Properties in Different Parts of Chernozem Slopes. Geoderma 2018, 314, 20–26. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Lauber, C.L.; Weintraub, M.N.; Ahmed, B.; Allison, S.D.; Crenshaw, C.; Contosta, A.R.; Cusack, D.; Frey, S.; Gallo, M.E.; et al. Stoichiometry of Soil Enzyme Activity at Global Scale. Ecol. Lett. 2008, 11, 1252–1264. [Google Scholar] [CrossRef]
- Cui, Y.; Moorhead, D.L.; Guo, X.; Peng, S.; Wang, Y.; Zhang, X.; Fang, L. Stoichiometric Models of Microbial Metabolic Limitation in Soil Systems. Glob. Ecol. Biogeogr. 2021, 30, 2297–2311. [Google Scholar] [CrossRef]
- Wobeng, N.B.M.; Banfield, C.C.; Megueni, C.; Mapongmetsem, P.M.; Dippold, M.A. Impact of Legumes on Soil Microbial Activity and C Cycle Functions in Two Contrasting Cameroonian Agro-Ecological Zones. Pedobiologia 2020, 81–82, 150662. [Google Scholar] [CrossRef]
- Wang, X.; Cui, Y.; Wang, Y.; Duan, C.; Niu, Y.; Sun, R.; Shen, Y.; Guo, X.; Fang, L. Ecoenzymatic Stoichiometry Reveals Phosphorus Addition Alleviates Microbial Nutrient Limitation and Promotes Soil Carbon Sequestration in Agricultural Ecosystems. J. Soils Sediments 2022, 22, 536–546. [Google Scholar] [CrossRef]
- Xiao, L.; Liu, G.; Li, P.; Li, Q.; Xue, S. Ecoenzymatic Stoichiometry and Microbial Nutrient Limitation during Secondary Succession of Natural Grassland on the Loess Plateau, China. Soil Tillage Res. 2020, 200, 104605. [Google Scholar] [CrossRef]
- Bai, X.; Dippold, M.A.; An, S.; Wang, B.; Zhang, H.; Loeppmann, S. Extracellular Enzyme Activity and Stoichiometry: The Effect of Soil Microbial Element Limitation during Leaf Litter Decomposition. Ecol. Indic. 2021, 121, 107200. [Google Scholar] [CrossRef]
- Kanté, M.; Riah-Anglet, W.; Cliquet, J.-B.; Trinsoutrot-Gattin, I. Soil Enzyme Activity and Stoichiometry: Linking Soil Microorganism Resource Requirement and Legume Carbon Rhizodeposition. Agronomy 2021, 11, 2131. [Google Scholar] [CrossRef]
- Yan, B.; Duan, M.; Wang, R.; Li, J.; Wei, F.; Chen, J.; Wang, J.; Wu, Y.; Wang, G. Planted Forests Intensified Soil Microbial Metabolic Nitrogen and Phosphorus Limitation on the Loess Plateau, China. Catena 2022, 211, 105982. [Google Scholar] [CrossRef]
- Liu, Y.; Fu, B.; Lü, Y.; Wang, Z.; Gao, G. Hydrological Responses and Soil Erosion Potential of Abandoned Cropland in the Loess Plateau, China. Geomorphology 2012, 138, 404–414. [Google Scholar] [CrossRef]
- Kou, M.; Jiao, J.; Yin, Q.; Wang, N.; Wang, Z.; Li, Y.; Yu, W.; Wei, Y.; Yan, F.; Cao, B. Successional Trajectory Over 10 Years of Vegetation Restoration of Abandoned Slope Croplands in the Hill-Gully Region of the Loess Plateau. Land Degrad. Dev. 2016, 27, 919–932. [Google Scholar] [CrossRef]
- Jobbágy, E.G.; Jackson, R.B. The Vertical Distribution of Soil Organic Carbon and Its Relation to Climate and Vegetation. Ecol. Appl. 2000, 10, 423–436. [Google Scholar] [CrossRef]
- Koven, C.D.; Riley, W.J.; Subin, Z.M.; Tang, J.Y.; Torn, M.S.; Collins, W.D.; Bonan, G.B.; Lawrence, D.M.; Swenson, S.C. The Effect of Vertically Resolved Soil Biogeochemistry and Alternate Soil C and N Models on C Dynamics of CLM4. Biogeosciences 2013, 10, 7109–7131. [Google Scholar] [CrossRef]
- Engelhardt, I.C.; Welty, A.; Blazewicz, S.J.; Bru, D.; Rouard, N.; Breuil, M.-C.; Gessler, A.; Galiano, L.; Miranda, J.C.; Spor, A.; et al. Depth Matters: Effects of Precipitation Regime on Soil Microbial Activity upon Rewetting of a Plant-Soil System. ISME J. 2018, 12, 1061–1071. [Google Scholar] [CrossRef]
- Chen, X.; Feng, J.; Ding, Z.; Tang, M.; Zhu, B. Changes in Soil Total, Microbial and Enzymatic C-N-P Contents and Stoichiometry with Depth and Latitude in Forest Ecosystems. Sci. Total Environ. 2022, 816, 151583. [Google Scholar] [CrossRef]
- Schütz, K.; Kandeler, E.; Nagel, P.; Scheu, S.; Ruess, L. Functional Microbial Community Response to Nutrient Pulses by Artificial Groundwater Recharge Practice in Surface Soils and Subsoils. FEMS Microbiol. Ecol. 2010, 72, 445–455. [Google Scholar] [CrossRef]
- Mganga, K.Z.; Razavi, B.S.; Kuzyakov, Y. Land Use Affects Soil Biochemical Properties in Mt. Kilimanjaro Region. Catena 2016, 141, 22–29. [Google Scholar] [CrossRef]
- Kong, W.; Wei, X.; Wu, Y.; Shao, M.; Zhang, Q.; Sadowsky, M.J.; Ishii, S.; Reich, P.B.; Wei, G.; Jiao, S.; et al. Afforestation Can Lower Microbial Diversity and Functionality in Deep Soil Layers in a Semiarid Region. Glob. Chang. Biol. 2022, 28, 6086–6101. [Google Scholar] [CrossRef]
- Doetterl, S.; Berhe, A.A.; Nadeu, E.; Wang, Z.; Sommer, M.; Fiener, P. Erosion, Deposition and Soil Carbon: A Review of Process-Level Controls, Experimental Tools and Models to Address C Cycling in Dynamic Landscapes. Earth-Sci. Rev. 2016, 154, 102–122. [Google Scholar] [CrossRef]
- Gao, H.; Li, Z.; Li, P.; Jia, L.; Zhang, X. Quantitative Study on Influences of Terraced Field Construction and Check-Dam Siltation on Soil Erosion. J. Geogr. Sci. 2012, 22, 946–960. [Google Scholar] [CrossRef]
- Page, A.L. (Ed.) Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties; Agronomy Monographs; American Society of Agronomy, Soil Science Society of America: Madison, WI, USA, 1983; ISBN 978-0-89118-977-0. [Google Scholar]
- Jones, D.; Willett, V. Experimental Evaluation of Methods to Quantify Dissolved Organic Nitrogen (DON) and Dissolved Organic Carbon (DOC) in Soil. Soil Biol. Biochem. 2006, 38, 991–999. [Google Scholar] [CrossRef]
- Saiya-Cork, K.R.; Sinsabaugh, R.L.; Zak, D.R. The Effects of Long Term Nitrogen Deposition on Extracellular Enzyme Activity in an Acer Saccharum Forest Soil. Soil Biol. Biochem. 2002, 34, 1309–1315. [Google Scholar] [CrossRef]
- German, D.P.; Weintraub, M.N.; Grandy, A.S.; Lauber, C.L.; Rinkes, Z.L.; Allison, S.D. Optimization of Hydrolytic and Oxidative Enzyme Methods for Ecosystem Studies. Soil Biol. Biochem. 2011, 43, 1387–1397. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Turner, B.L.; Talbot, J.M.; Waring, B.G.; Powers, J.S.; Kuske, C.R.; Moorhead, D.L.; Follstad Shah, J.J. Stoichiometry of Microbial Carbon Use Efficiency in Soils. Ecol. Monogr. 2016, 86, 172–189. [Google Scholar] [CrossRef]
- Zeng, Y.; Fang, N.; Shi, Z. Effects of Human Activities on Soil Organic Carbon Redistribution at an Agricultural Watershed Scale on the Chinese Loess Plateau. Agric. Ecosyst. Environ. 2020, 303, 107112. [Google Scholar] [CrossRef]
- Cui, Y.; Fang, L.; Guo, X.; Wang, X.; Zhang, Y.; Li, P.; Zhang, X. Ecoenzymatic Stoichiometry and Microbial Nutrient Limitation in Rhizosphere Soil in the Arid Area of the Northern Loess Plateau, China. Soil Biol. Biochem. 2018, 116, 11–21. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, Y.; Gao, D.; Wang, X.; Liu, W.; Deng, J.; Han, X.; Yang, G.; Feng, Y.; Ren, G. Ecoenzymatic Stoichiometry and Nutrient Dynamics along a Revegetation Chronosequence in the Soils of Abandoned Land and Robinia Pseudoacacia Plantation on the Loess Plateau, China. Soil Biol. Biochem. 2019, 134, 1–14. [Google Scholar] [CrossRef]
- Lagomarsino, A.; Benedetti, A.; Marinari, S.; Pompili, L.; Moscatelli, M.C.; Roggero, P.P.; Lai, R.; Ledda, L.; Grego, S. Soil Organic C Variability and Microbial Functions in a Mediterranean Agro-Forest Ecosystem. Biol. Fertil. Soils 2011, 47, 283–291. [Google Scholar] [CrossRef]
- Raiesi, F.; Beheshti, A. Soil Specific Enzyme Activity Shows More Clearly Soil Responses to Paddy Rice Cultivation than Absolute Enzyme Activity in Primary Forests of Northwest Iran. Appl. Soil Ecol. 2014, 75, 63–70. [Google Scholar] [CrossRef]
- Kivlin, S.N.; Treseder, K.K. Soil Extracellular Enzyme Activities Correspond with Abiotic Factors More than Fungal Community Composition. Biogeochemistry 2014, 117, 23–37. [Google Scholar] [CrossRef]
- Romanowicz, K.J.; Freedman, Z.B.; Upchurch, R.A.; Argiroff, W.A.; Zak, D.R. Active Microorganisms in Forest Soils Differ from the Total Community yet Are Shaped by the Same Environmental Factors: The Influence of PH and Soil Moisture. FEMS Microbiol. Ecol. 2016, 92, fiw149. [Google Scholar] [CrossRef]
- Chen, H.; Luo, P.; Wen, L.; Yang, L.; Wang, K.; Li, D. Determinants of Soil Extracellular Enzyme Activity in a Karst Region, Southwest China. Eur. J. Soil Biol. 2017, 80, 69–76. [Google Scholar] [CrossRef]
- Henry, H.A.L. Soil Extracellular Enzyme Dynamics in a Changing Climate. Soil Biol. Biochem. 2012, 47, 53–59. [Google Scholar] [CrossRef]
- Du, E.; Terrer, C.; Pellegrini, A.F.A.; Ahlström, A.; van Lissa, C.J.; Zhao, X.; Xia, N.; Wu, X.; Jackson, R.B. Global Patterns of Terrestrial Nitrogen and Phosphorus Limitation. Nat. Geosci. 2020, 13, 221–226. [Google Scholar] [CrossRef]
- Marklein, A.R.; Houlton, B.Z. Nitrogen Inputs Accelerate Phosphorus Cycling Rates across a Wide Variety of Terrestrial Ecosystems. New Phytol. 2012, 193, 696–704. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Follstad Shah, J.J. Ecoenzymatic Stoichiometry and Ecological Theory. Annu. Rev. Ecol. Evol. Syst. 2012, 43, 313–343. [Google Scholar] [CrossRef]
- Zhang, S.; Pan, Y.; Zhou, Z.; Deng, J.; Zhao, F.; Guo, Y.; Han, X.; Yang, G.; Feng, Y.; Ren, G.; et al. Resource Limitation and Modeled Microbial Metabolism along an Elevation Gradient. Catena 2022, 209, 105807. [Google Scholar] [CrossRef]
- Vitousek, P.M.; Porder, S.; Houlton, B.Z.; Chadwick, O.A. Terrestrial Phosphorus Limitation: Mechanisms, Implications, and Nitrogen–Phosphorus Interactions. Ecol. Appl. 2010, 20, 5–15. [Google Scholar] [CrossRef]
- Lemma, B.; Kebede, F.; Mesfin, S.; Fitiwy, I.; Abraha, Z.; Norgrove, L. Quantifying Annual Soil and Nutrient Lost by Rill Erosion in Continuously Used Semiarid Farmlands, North Ethiopia. Environ. Earth Sci. 2017, 76, 190. [Google Scholar] [CrossRef]
- Jiao, F.; Wen, Z.-M.; An, S.-S.; Yuan, Z. Successional Changes in Soil Stoichiometry after Land Abandonment in Loess Plateau, China. Ecol. Eng. 2013, 58, 249–254. [Google Scholar] [CrossRef]



| Variable | Simple Term Effects | Forward Selection | ||
|---|---|---|---|---|
| Contributions % | p | Explains % | p | |
| OC | 81.8 | 0.002 | 70.2 | 0.002 |
| C/P | 79.5 | 0.002 | ||
| TN | 76.2 | 0.002 | ||
| N/P | 71.8 | 0.002 | ||
| MBN | 62.9 | 0.002 | ||
| MBP | 55.7 | 0.002 | 5.1 | 0.002 |
| MBC | 50.6 | 0.002 | 0.8 | 0.058 |
| DOC | 43.1 | 0.002 | ||
| clay | 26.7 | 0.002 | 1.3 | 0.036 |
| pH | 24.5 | 0.002 | ||
| C/N | 22.3 | 0.002 | 1 | 0.048 |
| TP | 16.4 | 0.002 | ||
| SM | 13.1 | 0.002 | 2.5 | 0.012 |
| sand | 13.0 | 0.008 | ||
| silt | 3.2 | 0.168 | ||
| NO3− | 1.0 | 0.482 | 1.8 | 0.034 |
| EP | 0.1 | 0.88 | ||
| NH4+ | <0.1 | 0.904 | ||
| Variable | Simple Term Effect | Forward Selection | ||
|---|---|---|---|---|
| Contributions % | p | Explains % | p | |
| C/P | 84.9 | 0.002 | 51.3 | 0.002 |
| OC | 82.0 | 0.002 | 1.9 | 0.006 |
| N/P | 81.2 | 0.002 | 1.3 | 0.054 |
| TN | 79.4 | 0.002 | ||
| DOC | 45.7 | 0.002 | 1 | 0.008 |
| MBN | 39.7 | 0.002 | ||
| pH | 33.2 | 0.002 | ||
| MBP | 31.9 | 0.002 | 0.9 | 0.08 |
| MBC | 29.9 | 0.002 | ||
| clay | 26.0 | 0.002 | 22.1 | 0.002 |
| C/N | 22.4 | 0.002 | 3.6 | 0.006 |
| sand | 13.4 | 0.002 | ||
| TP | 8.9 | 0.01 | ||
| NO3− | 7.7 | 0.022 | ||
| SM | 6.9 | 0.042 | ||
| NH4+ | 4.2 | 0.092 | ||
| silt | 3.8 | 0.108 | ||
| EP | 0.9 | 0.62 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, F.; Yao, Y.; Song, J.; Wang, C.; Liu, Y. Interactive Influence of Soil Erosion and Cropland Revegetation on Soil Enzyme Activities and Microbial Nutrient Limitations in the Loess Hilly-Gully Region of China. Agronomy 2022, 12, 2796. https://doi.org/10.3390/agronomy12112796
Tang F, Yao Y, Song J, Wang C, Liu Y. Interactive Influence of Soil Erosion and Cropland Revegetation on Soil Enzyme Activities and Microbial Nutrient Limitations in the Loess Hilly-Gully Region of China. Agronomy. 2022; 12(11):2796. https://doi.org/10.3390/agronomy12112796
Chicago/Turabian StyleTang, Fangwang, Yufei Yao, Jinxi Song, Chengcheng Wang, and Yu Liu. 2022. "Interactive Influence of Soil Erosion and Cropland Revegetation on Soil Enzyme Activities and Microbial Nutrient Limitations in the Loess Hilly-Gully Region of China" Agronomy 12, no. 11: 2796. https://doi.org/10.3390/agronomy12112796
APA StyleTang, F., Yao, Y., Song, J., Wang, C., & Liu, Y. (2022). Interactive Influence of Soil Erosion and Cropland Revegetation on Soil Enzyme Activities and Microbial Nutrient Limitations in the Loess Hilly-Gully Region of China. Agronomy, 12(11), 2796. https://doi.org/10.3390/agronomy12112796








