Abstract
The population of Croatian autochthonous cultivars has a high degree of infection with economically important viruses, so it is necessary to carry out the elimination of the viruses in some cultivars to obtain healthy planting material. In this research, we tested in vitro meristem culture establishment on 18 autochthonous cultivars with different viral infections and the possibility of GLRaV-3 elimination through in vitro meristem culture. Plant material was sampled in a vineyard in two phenological stages, 10 days before flowering and 10 days after flowering of the grapevine. Apical meristem explants (1 mm) were placed into the MS culture medium supplemented with 0.5 mg/L benzyl adenine (BA) and 0.05 mg/L indol-3-acetic acid (IAA), and their survival, regeneration, and rooting were monitored. The results showed that the cultivar and the growth phase have a significant impact on the success of in vitro culture. In all cultivars studied higher success of in vitro culture establishment parameters (survival, regeneration, and rooting) was obtained in the case of explants sampled after flowering, with the exception of one cultivar for explants survival. Contrary to expectations, genotypes infected with three viruses (GLRaV-1, GLRaV-3, and GFLV) showed better results than genotypes infected with one or two viruses. The results showed successful in vitro establishment of Croatian autochthonous cultivar and GRLaV-3 elimination in one cultivar. However, due to the significant effect of cultivar, for routine application of this in vitro protocol on more than 100 autochthonous cultivars in need of sanitation, further studies should be conducted.
1. Introduction
Grapevine represents a significant crop in agricultural production in Croatia. It is assumed that the grapevine is one of the first crops cultivated in this area and its cultivation left behind a great impact on the tradition of winemaking [1].
Despite genetic erosion of autochthonous cultivars which started after the phylloxera infestation at the beginning of the 20th century and was followed by the introduction of international cultivars, Croatia still has more than 130 autochthonous cultivars [2]. The biggest obstacle to the revitalization of the indigenous assortment is the lack of certified and virus-free plant material. Previous researchers have shown that in the population of Croatian autochthonous cultivars there is a high level of infection with economically important viruses [3,4,5,6,7]. Grapevine fanleaf virus (GFLV), arabis mosaic virus (ArMV), and grapevine leafroll-associated virus 1 and 3 (GLRaV-1, GLRaV-3) are considered to be economically important grapevine viruses with worldwide distribution and significant impact on yields; therefore, they should not be present in propagation material. In some cases, healthy plant material of autochthonous cultivars is not available, so it is necessary to carry out the sanitation process to eliminate viruses. The authors [3] emphasize that GLRaV-3 virus causes the most infections on the costal part in the population of Croatian autochthonous cultivars; thus, there is a current need for sanitation programs targeting it.
Apical meristem culture is a successful method of virus elimination [8]. Sanitation is performed by isolating the apical meristem in the appropriate conditions, placing it in a tube with medium, and exposing it to tissue culture conditions [9]. The size of the apical meristem can significantly affect the success of virus elimination, according to several authors [9,10,11].
Fayek et al. [11] were testing the size of meristems and concentration of benzyladenin cytokine growth regulator on regeneration cultivar explant scion on the regeneration of ‘Flame Seedless’ cultivar explants and efficiency of GLRaV-3 and GFLV virus elimination. They showed that a meristem size of 0.5 mm is optimal for the elimination of both viruses (90–95%), while the plants developed from explants with a size of 1 mm had a much lower range of virus elimination (55–60%). Roubelakis-Angelakis [10] claims that there are significant differences in the requirements of meristem culture protocols in vitro between genotypes.
Grapevine has great genetic variability, so maintenance of its genotypes requires large investments. The maintenance of field collections, presented as gene banks for grapevine genetic resources, requires high costs and is time-consuming [12]. In addition, genotypes in field collections are exposed to harmful abiotic and biotic factors, such as frost, hail, and various pathogens [13]. Doroshenko and Puzirnova [14] state that by using biotechnological methods based on in vitro cultivation, with traditional in situ and ex situ methods, it is possible to obtain sustainable management of the genetic resources of the grapevine. They consider that the most important biotechnological method for the long-term preservation of important cultivars is in vitro collections. The advantages of in vitro collections are the safe preservation of healthy cultivars and rapid micropropagation of important genetic material at significantly lower costs than in vivo collections.
Previous research indicates that meristem culture is efficient in virus elimination [8,9,11], but there is not enough information available about the effects of different phenological stages on the success of the establishment of tissue culture in vitro. Also, there is limited information available on the differences in performance of numerous Croatian autochthonous cultivars in tissue culture conditions. This study aimed to evaluate the influence of the cultivar and two phenological stages of the grapevine, in which meristematic shoot tips were taken in the vineyard, on the growth and development of the meristem tip culture in vitro, and the efficiency of the meristem culture for GLRaV-3 elimination.
2. Materials and Methods
2.1. Chemicals and Reagents
Sodium hypochlorite solution (NaOCl) EMPLURA and ethanol gradient grade for liquid chromatography LiChrosolv® were purchased from Merck KGaA (Darmstadt, Germany). Murashige and Skoog medium (MS), plant preservative mixture (PPM), and agar were obtained from Duchefa Biochemie (Haarlem, The Netherlands). TWEEN 20 Cell Culture Tested, 6-Benzylaminopurine hydrochloride (BA) suitable for plant cell culture, indol-3-acetic acid (IAA) BioReagent, suitable for plant cell culture, sucrose for molecular biology, indole-3-butyric acid (IBA) suitable for plant cell culture, sodium carbonate (Na2CO3) BioXtra, sodium bicarbonate (NaHCO3) BioXtra, polyvinylpyrrolidone molecular weight 40,000 (PVP40), bovine serum albumin (BSA), BioReagent, suitable for cell culture, acetic acid glacial, ACS reagent, glycine, BioUltra, for molecular biology, sodium chloride (NaCl), BioXtra, ethylenediaminetetraacetic acid (EDTA), BioUltra, Triton X, BioXtra, and β-mercaptoethanol, BioUltra, for molecular biology were purchased from Sigma-Aldrich (St. Louis, MO, USA).
2.2. Plant Material and Virus Incidence
Plant material was taken from the National Collection of autochthonous grapevine cultivars, located at experimental station Jazbina of the Department for Viticulture and Enology, Faculty of Agriculture, University of Zagreb. The research was conducted on 18 autochthonous cultivars: ‘Glavinuša’, ‘Plavac mali sivi’, ‘Mladenka’, ‘Bilan bijeli’, ‘Debit’, ‘Ljutun’, ‘Žumić’, ‘Zlatarica vrgorska’, ‘Divjaka’, ‘Babica’, ‘Lasina’, ‘Malvasija dubrovačka’, ‘Ninčuša’, ‘Dobričić’, ‘Cetinka’, ‘Mejsko belo’, ‘Rudežuša’ i ‘Privlačka belina’. Some of the cultivars selected already have significant local importance for wine production, while others, due to their high qualitative potential, are in the process of being revitalized in certain wine-growing areas. Virus incidence (i.e., sanitary status) of cultivars in research was determined by enzyme-linked immunosorbent assay (DAS-ELISA) at the Department of Plant Pathology (University of Zagreb Faculty of Agriculture), using kits provided by Agritest (Valenzano, Italy). ELISA was performed in February 2019 using dormant cuttings from two or three adult vines per cultivar, in the winter period prior to the season of introduction into in vitro meristem culture.
According to the ELISA results, which included the detection of ArMV, GFLV, GLRaV-1 and GLRaV-3, most cultivars showed mixed virus infections with the dominance of GLRaV-3 and GLRaV-1, and were divided into five categories: I—infection with GLRaV-3, II—infection with GLRaV-1 + GLRaV-3, III—infection with GLRaV-1 + GLRaV-3 + GFLV, IV—infection with GFLV, and V—infection with GLRaV-3 + GFLV (Table 1).

Table 1.
Sanitary status of selected vines from vineyards selected for the establishment of in vitro meristem culture determined by ELISA.
2.3. Sampling of Material
A sampling of the apical part of the shoots with three apical leaves from previously analyzed vines (Table 1) was conducted during 2019 in two phenological stages, characterized by their intensive growth (elongation): (1) approximately 10 days before flowering, and (2) 10 days after the end of flowering, corresponding to BBCH [15] phenological stages 57 and 71. Approximately half of the shoots available on vines were taken in each of the two phenological stages. The number of explants available per vine in each phenological stage was in a range from 7 to 20, depending on the cultivar and individual vine vigor. Immediately after sampling, plant material was put in plastic bags and transferred to the tissue culture laboratory where it was put in water until sterilization.
2.4. Culture Conditions
To initiate in vitro culture, apical, 2 cm long fragments from collected shoots were surface sterilized in a laminar flow hood using the following protocol: first, they were rinsed with tap water for 30 s followed by dipping in 70% ethanol for 30 s. After ethanol was removed, plant material was immersed in a 5% NaOCl solution with two drops of Tween20 for 15 min and then rinsed three times in sterile water. The excision of meristems 1 mm in size was made under binocular and five explants were inoculated in a 90 mm Petri plate. The culture medium employed for initiation of growth was MS medium [16] supplemented with 0.5 mg/L BA, 0.05 mg/L IAA, 0.5 mg/L PPM, and 30 g/L sucrose, solidified with 8 g/L agar, and pH was adjusted to 5.8 before autoclaving at 121 °C for 20 min. Cultures were kept in growth chambers (Aralab FitoClima PL 1200) at a constant 26 ± 0.5 °C under a 12/12 h light/dark regime provided by a white LED plant growth tube with a light intensity of 100 µmol/m2/s. After initial culture, individual explants were transferred further to a fresh culture medium every four weeks in culture tubes. After 8–9 weeks in culture conditions, formed plants were transferred to rooting medium (MS supplemented with 0.6 mg/L IBA) without multiplication. In the case of necrosis or traces of hyperhydricity, plants were transferred to hormone-free medium. Explants with contamination in the initial culture were immediately excluded and not calculated in the final percentage of success. Observations of the growth and plant development were regularly made. Four weeks after inoculation, survival was recorded as a percentage from the number of explants inoculated to the initial culture, counting only green explants with 1–2 leaves (%of survival = (N survival/N inoculated to initial culture) × 100). Eight weeks after inoculation, regeneration was recorded as a percentage from the number of explants inoculated to the initial culture, counting developed explants with 2–3 leaves developed in plants (%of regrowth = (N regrowth/N inoculated to initial culture) × 100). Thirteen weeks after inoculation, the number of rooted plants was recorded, and the percentage calculated from the number of explants inoculated to the initial culture (% of rooting = (N rooting/N inoculated to initial culture) × 100).
2.5. GLRaV-3 Elimination Success
The efficiency of virus elimination was tested by analyzing the leaf tissue of in vitro plants maintained under tissue culture conditions for eight months. Altogether, 11 well-developed in vitro plants, obtained from two sampled mother vines in phenological stage 2 infected only with GLRaV- 3, were selected for this purpose: Zlatarica vrgorska (6 in vitro plantlets) and Glavinuša 3 (5 in vitro plantlets). The presence of viruses was verified by real-time reverse transcription polymerase chain reaction (RT-qPCR) on a Thermo Fisher Scientific 7500 real-time PCR system platform (Applied Biosystems, Waltham, MA, USA). For this purpose, total RNA was isolated from 0.1 g petioles using liquid nitrogen and extracted with GGB buffer (0.015 M Na2CO3, 0.035 M NaHCO3, 0.0005 M PVP 40, 1 g/500 mL BSA, 0.25 g/500 mL Tween 20, pH 9.6 with acetic acid). Subsequently, 8 µL of the extract was transferred to a PCR tube containing 100 µL of the GES master mix (0.1 M glycine, 0.05 M NaCl, 0.001 M EDTA, 0.5% Triton X, 1% β-mercaptoethanol, pH 9.0 with NaOH), followed by denaturation in a thermocycler for 10 min at 95 °C. Purity and integrity of RNA was measured spectrophotometrically (A260/A280 and A260/A230) using a NanoPhotometer P330 spectrophotometer (Implen, Essen, Germany). Detection of viruses by quantitative polymerase chain reaction was performed using primers and probes described by Diaz-Lara et al. [17] for GLRaV-3 and Osman et al. [18] for GLRaV-1 and 18S rRNA [19] as an internal control; a positive and negative controls for each virus was included. The RT-qPCR reaction was prepared in a 20 µL reaction mixture. The master mix consisted of: 0.4 µM of each primer (Macrogen, Seoul, South Korea), 0.150 µM of each probe (Macrogen, South Korea), 5 µL of TaqMan™ Fast Virus 1- Step Master Mix (ThermoFisher Scientific, Waltham, MA, USA), 10.6 µL of ultrapure water, and 2 µL of total RNA (concentration ranged from 5 to 23, depending on the sample). Reaction conditions were: initial denaturation at 95 °C for 10 min, 40 cycles of denaturation at 94 °C for 15 s, and elongation at 60 °C for 1 min.
2.6. Statistical Analysis
Percentages were calculated based on the initial number inoculated to the medium in each category. All measured 159 parameters were analyzed using ANOVA with three replicates. Effects of cultivar, sanitary status (category), and the phenological stage were tested separately as well as their interaction for survival, regeneration, and rooting rates of the in vitro explants. Differences between given means were evaluated by Duncan’s multiple range test at a confidence level of 95% (p < 0.05). Statistical analysis was carried out using SAS 9.3 software (SAS Institute Inc., Cary, NC, USA).
3. Results
3.1. Effect of the Cultivar on the Growth and Development of In Vitro Meristem Culture
Statistical analysis revealed significant differences in regeneration between cultivars, whereas differences in survival and rooting were not significant (Table 2). The highest rate of explants survival was recorded in the case of Plavac mali sivi cv. (67.5%), while the lowest was recorded in the case of Debit cv. (29.28%). The difference among other cultivars was in a range from 38.64% to 52.8%.

Table 2.
Effect of the cultivar on the growth and development of in vitro meristem culture.
The highest range of regenerated plants was detected for cultivars Plavac mali sivi, Mejsko belo, and Dobričić (47.23–53.22%); cultivar Mladenka had the lowest regeneration (6.82%), whereas cultivars Divjaka, Babica, and Debit did not regenerate any plants.
Cultivar Plavac mali sivi also had the highest rate of rooting (39.64%), while the lowest rate was detected in the case of Mladenka.
3.2. Effect of the Phenological Stage on the Growth and Development of In Vitro Meristem Culture
The effect of the phenological stage in which meristems were collected from vines on the survival and regeneration of meristems and rooting of plants has been evaluated (Table 3). The phenological stage 1 presents the period just before the flowering of shoots and growth phase 2 presents the period just after the flowering of the shoots.

Table 3.
Effect of the phenological stage on the growth and development of in vitro meristem culture.
Significant effects of the phenological stage on all three parameters of meristematic tissue culture development (survival, regeneration, and rooting) were detected. Significantly better results were obtained for all of them in phenological stage 2 (meristems collected from vines 10 days after flowering). The most visible difference is in the survival of the initial culture, which was higher than 70%, whereas in stage 1 it was only 21.34% (Table 3).
In Figure 1, comparison of survival of meristematic explants from specific cultivars in two phenological stages reveals that the majority of cultivars gave a higher percentage of survival in the case of explants collected after flowering, except for cv. Malvasija dubrovačka which had the same results in both phenological stages and Zlatarica Vrgorska which is the only cultivar that displayed better results in survival of the explants collected from vines before flowering.
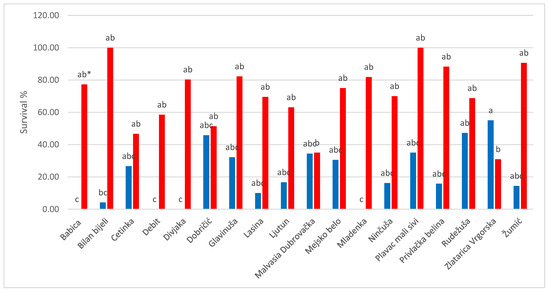
Figure 1.
The survival of cultivars in two growth phases (before and after flowering). Blue columns represent the growth phase before flowering, and the red columns represent the growth phase after flowering. * Different letters represent significantly different values among cultivars within growth phase according to Duncan’s test, p ≤ 0.05.
3.3. Effect of the Sanitary Status on the Growth and Development of In Vitro Meristem Culture
The sanitary status between cultivars has been divided into categories (Table 1). Category III (GLRaV-1, GLRaV-3 + GFLV) of sanitary status reached the highest results in all phases of growth (survival, regeneration, and rooting of plants), although this gradually declined (Figure 2). Survival and regeneration revealed that category III is significantly different from category I (GLRaV-3) and II (GLRaV-1 + GLRaV-3). In rooting of plants, category III is significantly different from other categories of sanitary status.
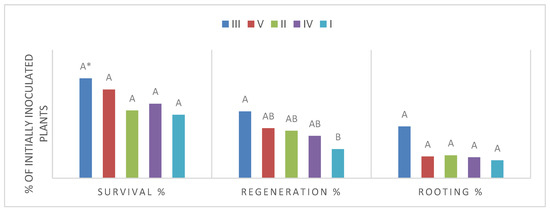
Figure 2.
Effect of sanitary status on the survival, regeneration and rooting. Category of virus infection: I—GLRaV-3; II—GLRaV-1 + GLRaV-3; III—GLRaV-1 + GLRaV-3 + GFLV; IV—GFLV; V—GLRaV-3 + GFLV. * Different letters represent significantly different values according to Duncan’s test, p ≤ 0.05.
3.4. Efficiency of GLRaV-3 Elimination
The GLRaV-3 was successfully eliminated in 5 out of 11 in vitro plants tested and all of the plants with successful elimination belonged to Glavinuša. Elimination was achieved in plants from the cultivar Glavinuša I vine, while in the plantlets from cultivar Zlatarica vrgorska this virus remained. Based on this we can conclude that in the case of Glavinuša I we obtained an elimination rate of 23.8% from initially inoculated explants.
4. Discussion
Based on the results of the influence of cultivar and phenological stage of grapevine on the success of in vitro meristem culture, a significant influence of cultivar has been confirmed. In initial culture, four weeks after inoculation in survival and in rooting of plants these differences were not so clear. From 18 autochthonous cultivars in research, the most successful survival, regeneration and rooting have been confirmed within cultivar Plavac mali sivi. The lowest survival rate was recorded within cultivars Babica and Debit, where regeneration and rooting of plants has not been achieved at all, as well as within cultivar Divjaka. This fact has been confirmed earlier, when Marković et al. [20] revealed the clear differences in survival, regrowth, and growth parameters among eight Croatian autochthonous cultivars. Also, Hančević et al. [21] confirmed a great influence of virus-infected genotypes within one cultivar (Plavac mali) in the success of virus elimination in tissue culture conditions.
The introduction of shoot meristems sampled in the phenological stage after flowering (BBCH 71) resulted in a significantly higher percentage of survival four weeks after inoculation, regenerated plants eight weeks after flowering, and rooted plants compared to the sampling in the growth phase before flowering. Apical meristems were bigger than in the first phenological stage, so the meristem excision was easier. Also, Bota et al. [22] took the samples in July, their maximum growth period, for testing thermotherapy in the field compared to chamber thermotherapy. The authors emphasize that better results of in vitro population were obtained with shoot tips than meristems [22]. Therefore, for further research and multiplication of in vitro meristem culture, it can be recommended to choose a plant material sampled from vineyards at the stage of intensive growth of shoots after flowering. Botti et al. [23] also confirmed that the date and the type of explants are important for in vitro proliferation, but the period of sampling material in their research was in winter. Preliminary results showing that the sampling period after flowering (BBCH 71) could be most suitable for tissue culture establishment with limited samples were obtained one year before this research in our laboratory (unpublished data).
Virus elimination by meristem culture taken in the growth phase after flowering resulted in successful elimination of GLRaV-3 in 50% of tested plants, but with the evident influence of cultivar. As previously reported for the Croatian cultivar Plavac mali, the success of virus elimination by in vitro growth might depend on the initial virus composition [21]. Results in this research imply that meristem culture could be combined with thermotherapy for better success of virus elimination. As this study was conducted on a limited number of samples/varieties, the results are useful for planning future large-scale activities for Croatian autochthonous grapevine varieties to obtain virus-free planting material that could be offered to nurseries and vine producers as part of a revitalization program.
5. Conclusions
We can confirm the influence of cultivar and phenological stage when the plant material was taken for the establishment of in vitro populations of Croatian autochthonous cultivars. Better results were achieved in the phenological stage after flowering and partial elimination of GLRaV-3 was achieved in one autochthonous cultivar. Thus, future research activities should be dedicated to the choice of the in vitro protocols and culture medium for meristem culture, as well as combining virus elimination techniques.
Author Contributions
Conceptualization, I.T., D.P. and J.K.K.; methodology, I.Š., P.Š. and Z.M.; formal analysis, A.Z. and D.V.; writing—original draft preparation, Z.M.; writing—review and editing, D.P., I.T. and J.K.K.; funding acquisition, E.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the project KK.01.1.1.01.0005 Biodiversity and Molecular PlantBreeding, Centre of Excellence for Biodiversity and Molecular Plant Breeding (CoE CroP-BioDiv), Zagreb, Croatia.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mirošević, N.; Karoglan Kontić, J. Vinogradarstvo; Globus: Zagreb, Croatia, 2008. [Google Scholar]
- Maletic, E.; Preiner, D.; Karoglan Kontić, J.; Šimon, S.; Pejić, I. Istraživanja unutarsortne varijabilnosti vinove loze u Hrvatskoj i klonska selekcija. Rad. Zavoda Za Znan. I Umjetnički Rad U Požegi 2016, 5, 1–11. [Google Scholar] [CrossRef][Green Version]
- Karoglan Kontić, J.; Preiner, D.; Šimon, S.; Zdunic, G.; Poljuha, D.; Maletic, E. Sanitary Status of Croatian Native Grapevine Varieties. Agric. Conspec. Sci. 2009, 74, 99–103. [Google Scholar]
- Poljuha, D.; Sladonja, B.; Bubola, M. Incidence of viruses infecting grapevine varieties in Istria (Croatia). J. Food Agric. Environ. 2010, 8, 166–169. [Google Scholar]
- Voncina, D.; Al Rwahnih, M.; Rowhani, A.; Gouran, M.; Almeida, R.P.P. Viral Diversity in Autochthonous Croatian Grapevine Cultivars. Plant Dis. 2017, 101, 1230–1235. [Google Scholar] [CrossRef] [PubMed]
- Voncina, D.; Almeida, R.P.P. Screening of some Croatian autochthonous grapevine varieties reveals a multitude of viruses, including novel ones. Arch. Virol. 2018, 163, 2239–2243. [Google Scholar] [CrossRef] [PubMed]
- Voncina, D.; Preiner, D.; Simon, S.; Cvjetkovic, B.; Maletic, E.; Pejic, I.; Karoglan Kontic, J. Distribution of nine viruses in Croatian autochthonous grapevine (Vitis vinifera L.) cultivars from Dalmatian region included in clonal selection. J. Cent. Eur. Agric. 2019, 20, 262–273. [Google Scholar] [CrossRef]
- Barlass, M.; Skene, K.G.M. In vitro plantlet formation from Citrus species and hybrids. Sci. Hortic. 1982, 17, 333–341. [Google Scholar] [CrossRef]
- Panattoni, A.; Luvisi, A.; Triolo, E. Elimination of viruses in plants: Twenty years of progress. Span. J. Agric. Res. 2013, 11, 173–188. [Google Scholar] [CrossRef]
- Roubelakis-Angelakis, K. Molecular Biology and Biotechnology of the Grapevine; Kluwer Academic Publishers: Boston, MA, USA, 2001. [Google Scholar]
- Fayek, M.A.; Jomaa, A.H.; Shalbay, A.-B.A. Meristem Tip Culture for In Vitro Eradication of Grapevine Leaf Roll-associated Virus-1 (GLRaV-1) and Grapevine Fan Leaf Virus (GFLV) from Infected Flame Seedless Grapevine Plantlets. Iniciación A La Investig. 2009, 4, 1–11. [Google Scholar]
- Dal Bosco, D.; Sinski, I.; Comachio, V.; Maia, J.D.G.; Ritschel, P.S.; Quecini, V. In Vitro Techniques for Grapevine Germplasm Conservation. In XI International Conference on Grapevine Breeding and Genetics; Li, S.H., Archbold, D., London, J., Eds.; 2015; Volume 1082, pp. 201–205. Available online: https://www.ishs.org/ishs-article/1082_27 (accessed on 1 July 2021).
- Maletic, E.; Karoglan Kontić, J.; Pejić, I.; Preiner, D.; Zdunic, G.; Bubola, M.; Stupic, D.; Andabaka, Z.; Markovic, Z.; Šimon, S.; et al. Zelena Knjiga: Hrvatske Izvorne Sorte Vinove Loze; Državni Zavod Za Zaštitu Prirode: Zagreb, Croatia, 2015. [Google Scholar]
- Doroshenko, N.; Puzirnova, V. Biotechnological methods of preservation of the grape gene pool in the in vitro collection. In Proceedings of the International Scientific Online-Conference Bioengineering in the Organization of Processes Concerning Breeding and Reproduction of Perennial Crops 2020, Krasnodar, Russia, 6–8 October 2020; Volume 25. [Google Scholar]
- Lorenz, D.; Eichhorn, K.; Bleiholder, H.; Klose, R.; Meier, U.; Weber, E. Growth Stages of the Grapevine: Phenological growth stages of the grapevine (Vitis vinifera L. ssp. vinifera)—Codes and descriptions according to the extended BBCH scale. Aust. J. Grape Wine Res. 1995, 1, 100–103. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tabacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Diaz-Lara, A.; Klaassen, V.; Stevens, K.; Sudarshana, M.R.; Rowhani, A.; Maree, H.J.; Chooi, K.M.; Blouin, A.G.; Habili, N.; Song, Y.S.; et al. Characterization of grapevine leafroll-associated virus 3 genetic variants and application towards RT-qPCR assay design. PLoS ONE 2018, 13, e208862. [Google Scholar] [CrossRef] [PubMed]
- Osman, F.; Leutenegger, C.; Golino, D.; Rowhani, A. Real-time RT-PCR (TaqMan (R)) assays for the detection of Grapevine Leafroll associated viruses 1-5 and 9. J. Virol. Methods 2007, 141, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Osman, F.; Rowhani, A. Application of a spotting sample preparation technique for the detection of pathogens in woody plants by RT-PCR and real-time PCR (TaqMan). J. Virol. Methods 2006, 133, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Markovic, Z.; Preiner, D.; Bosnjak, A.M.; Safner, T.; Stupic, D.; Andabaka, Z.; Maletic, E.; Chatelet, P.; Engelmann, F.; Kontic, J.K. In vitro introduction of healthy and virus-infected genotypes of native Croatian grapevine cultivars. Cent. Eur. J. Biol. 2014, 9, 1087–1098. [Google Scholar] [CrossRef][Green Version]
- Hancevic, K.; Zdunic, G.; Voncina, D.; Radic, T. Virus composition influences virus elimination success and in vitro growth characteristics of the grapevine cv. plavac mali. J. Plant Pathol. 2015, 97, 199–202. [Google Scholar]
- Bota, J.; Cretazzo, E.; Montero, R.; Rossello, J.; Cifre, J. Grapevine fleck virus (GFKV) elimination in a selected clone of Vitis vinifera L. cv. manto negro and its effects on photosynthesis. J. Int. Des Sci. De La Vigne Et Du Vin 2014, 48, 11–19. [Google Scholar] [CrossRef]
- Botti, C.; Garay, L.; Reginato, G. The influence of culture dates, genotype and size and type of shoot apices on in-vitro shoot proliferation of vitis-vinifera cvs thompson seedless, ribier and black seedless. Vitis 1993, 32, 125–126. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).