Application of Biochar Produced from Crop Residues on Trace Elements Contaminated Soils: Effects on Soil Properties, Enzymatic Activities and Brassica rapa Growth
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soils
2.2. Biochar Production
2.3. Soil Parameters
2.4. Enzymatic Activities
2.5. Quantification of Trace Element Species in Soils
- Fraction 1 (Acid extractable): extracted with acetic acid 0.11 mol L−1.
- Fraction 2 (Reducible): extracted with hydroxylammonium chloride 0.5 mol L−1.
- Fraction 3 (Oxidizable): extracted with hydrogen peroxide 8.8 mol L−1 and ammonium acetate 1.0 mol L−1.
- Fraction 4 (Residual): extracted with aqua regia (1:3 v/v of HNO3:HCl).
2.6. Pot Trial under Greenhouse Conditions
2.6.1. Incubation Step
2.6.2. Pot Trial with Plants of Brassica rapa L. ssp. pekinensis
2.7. Statistical Analysis
3. Results and Discussion
3.1. Effect of Biochar Addition on Soil Physical Properties and Elemental Analysis
3.2. Effects of Biochar Addition on Enzymatic Activities
3.3. Effects of Biochar Addition on the Bioavailability of Trace Elements (CaCl2-Extractable Trace Elements)
- Soil: APS control soil showed the greatest concentrations of available Cd, Cu, Mn, Ni and Zn, whereas MAPS control soil displayed the highest concentration of available Ba (Table 5).
- Feedstock: The amount of bioavailable Zn was lower for soils amended with RHB than with OPB biochars.
- Biochar dose: The bioavailability of Cd, Cu and Zn was greater for control soils than for that amended with 5% of biochars, whereas it was greater for all elements except for Ni for control soils than for that amended with 10% of biochars. In addition, 10% of biochar amendment significantly reduced the contents of all bioavailable trace elements in comparison with 5% of amendment.
- Biochar pyrolysis temperature: Bioavailable contents of Cd, Cu and Zn were greater for control soils than for soils amended with biochars produced at 400 °C, whereas all contents, except for Ni, were greater than for soils amended with biochars produced at 500 °C. Moreover, soils amended with biochars produced at 500 °C showed lower bioavailable contents of Ba, Mn and Zn than biochars produced at 400 °C, evidencing the importance of the selection of pyrolysis conditions when the biochars are produced.
- Biochar pyrolysis residence time: Soils amended with biochars pyrolyzed for 4 h showed lower bioavailable contents of Mn and Zn than those heated for 1 h.
3.4. Impact of Biochar Addition on the Concentrations of Trace Element Species in Soils
3.5. Effects of Biochar Addition on the Germination and Development of Brassica rapa pekinensis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- EEA. Progress in Management of Contaminated Sites; CSI 015; European Environmental Agency: Copenhagen, Denmark, 2007.
- Hmid, A.; Chami, Z.A.; Sillen, W.; Vocht, A.D.; Vangronsveld, J. Olive mill waste biochar: A promising soil amendment for metal immobilization in contaminated soils. Environ. Sci. Pollut. Res. 2015, 22, 1444–1456. [Google Scholar] [CrossRef]
- Xian, Y.; Wang, M.; Chen, W. Quantitative assessment on soil enzyme activities of heavy metal contaminated soils with various soil properties. Chemosphere 2015, 139, 604–608. [Google Scholar] [CrossRef]
- Sungur, A.; Soylak, M.; Ozcan, H. Investigation of Heavy Metal Mobility and Availability by the BCR Sequential Extraction Procedure: Relationship between Soil Properties and Heavy Metals Availability. Chem. Speciat. Bioavailab. 2014, 26, 219–230. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.; Tiwari, S.; Hasan, A.; Saxena, V.; Pandey, L.M. Recent advances in conventional and contemporary methods for remediation of heavy metal-contaminated soils. Biotechnology 2018, 8, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Sales da Silva, I.G.; Gomes de Almeida, F.C.; Padilha da Rocha e Silva, N.M.; Casazza, A.A.; Converti, A.; Asfora Sarubbo, L. Soil Bioremediation: Overview of Technologies and Trends. Energies 2020, 13, 4664. [Google Scholar] [CrossRef]
- Madejón, P.; Domínguez, M.T.; Gil-Martínez, M.; Navarro-Fernández, C.M.; Montiel-Rozas, M.M.; Madejón, E.; Murillo, J.M.; Cabrera, F.; Marañón, T. Evaluation of amendment addition and tree planting as measures to remediate contaminated soils: The Guadiamar case study (SW Spain). Catena 2018, 166, 34–43. [Google Scholar] [CrossRef]
- Woolf, D.; Amonette, J.; Street-Perrott, F.; Street-Perrott, F.A.; Lehmann, J.; Joseph, S. Sustainable biochar to mitigate global climate change. Nat. Commun. 2010, 1, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spokas, K.A. Review of the stability of biochar in soils: Predictability of O:C molar ratios. Carbon Manag. 2010, 1, 289–303. [Google Scholar] [CrossRef] [Green Version]
- Ali, A.; Guo, D.; Zhang, Y.; Sun, X.; Jiang, S.; Guo, Z.; Huang, H.; Liang, W.; Li, R.; Zhang, Z. Using bamboo biochar with compost for the stabilization and phytotoxicity reduction of heavy metals in mine-contaminated soils of China. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.-S.; Kim, K.R.; Kim, H.-J.; Yoon, J.-H.; Yang, J.E.; Ok, Y.S.; Owens, G.; Kim, K.-H. Effect of biochar on heavy metal immobilization and uptake by lettuce (Lactuca sativa L.) in agricultural soil. Environ. Earth Sci. 2015, 74, 1249–1259. [Google Scholar] [CrossRef]
- Yu, H.; Zou, W.; Chen, J.; Chen, H.; Yu, Z.; Huang, J.; Tang, H.; Wei, X.; Gao, B. Biochar amendment improves crop production in problem soils: A review. J. Environ. Manag. 2019, 232, 8–21. [Google Scholar] [CrossRef] [PubMed]
- Shaaban, M.; Van Zwieten, L.; Bashir, S.; Younas, A.; Núñez-Delgado, A.; Chhajro, M.A.; Kubar, K.A.; Ali, U.; Rana, M.S.; Mehmood, M.A.; et al. A concise review of biochar application to agricultural soils to improve soil conditions and fight pollution. J. Environ. Manag. 2018, 228, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Conte, P.; Bertani, R.; Sgarbossa, P.; Bambina, P.; Schmidt, H.-P.; Raga, R.; Lo Papa, G.; Chillura Martino, D.F.; Lo Meo, P. Recent Developments in Understanding Biochar’s Physical–Chemistry. Agronomy 2021, 11, 615. [Google Scholar] [CrossRef]
- Li, H.; Dong, X.; da Silva, E.B.; de Oliveira, L.M.; Chen, Y.; Ma, L.Q. Mechanisms of metal sorption by biochars: Biochars characteristics and modifications. Chemosphere 2017, 178, 466–478. [Google Scholar] [CrossRef]
- Zhao, J.; Shen, X.-J.; Domene, X.; Alcañiz, J.-M.; Liao, X.; Palet, C. Comparison of biochars derived from different types of feedstock and their potential for heavy metal removal in multiple-metal solutions. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef] [Green Version]
- González, I.; Galán, E.; Romero, A. Assessing Soil Quality in Areas Affected by Sulphide Mining. Application to Soils in the Iberian Pyrite Belt (SW Spain). Minerals 2011, 1, 73–108. [Google Scholar] [CrossRef] [Green Version]
- Xiong, Z.-T.; Li, Y.-H.; Xu, B. Nutrition Influence on Copper Accumulation by Brassica pekinensis Rupr. Ecotoxicol. Environ. Saf. 2002, 53, 200–205. [Google Scholar] [CrossRef]
- Zhang, S.; Song, J.; Gao, H.; Zhang, Q.; Lv, M.-C.; Wang, S.; Liu, G.; Pan, Y.-Y.; Christie, P.; Sun, W. Improving prediction of metal uptake by Chinese cabbage (Brassica pekinensis L.) based on a soil-plant stepwise analysis. Sci. Total Environ. 2016, 569–570, 1595–1605. [Google Scholar] [CrossRef]
- Ou, J.; Li, H.; Yan, Z.; Zhou, Y.; Bai, L.; Zhang, C.; Wang, X.; Chen, G. In situ immobilisation of toxic metals in soil using Maifan stone and illite/smectite clay. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Larkcom, J. Oriental Vegetables: The Complete Guide for Garden and Kitchen; John Murray Ltd.: London, UK, 1991. [Google Scholar]
- Tang, J.; Zhang, J.; Ren, L.; Zhou, Y.; Gao, J.; Luo, L.; Yang, Y.; Peng, Q.; Huang, H.; Chen, A. Diagnosis of soil contamination using microbiological indices: A review on heavy metal pollution. J. Environ. Manag. 2019, 242, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Wolińska, A.; Stępniewska, Z. Dehydrogenase Activity in the Soil Environment; Dehydrogenases, Chapter 8; Canuto, R.A., Ed.; Intechopen Limited: London, UK, 2012. [Google Scholar] [CrossRef] [Green Version]
- Panettieri, M.; Knicker, H.; Murillo, J.M.; Madejón, E.; Hatcher, P.G. Soil organic matter degradation in an agricultural chronosequence under different tillage regimes evaluated by organic matter pools, enzymatic activities and CPMAS 13C NMR. Soil Biol. Biochem. 2014, 78, 170–181. [Google Scholar] [CrossRef]
- Acosta-Martínez, V.; Klose, S. Soil Enzymes. In Encyclopedia of Soil Science, 2nd ed.; Taylor and Francis: New York, NY, USA, 2008; pp. 1–5. [Google Scholar] [CrossRef]
- CMA. Memorias de Actuaciones. Report by the Consejería de Medio Ambiente, Government of Andalucía, Spain. 1999. Available online: http://www.cma.junta-andalucia.es/guadiamar/indguadiamar.html (accessed on 10 March 2020).
- Van Geen, A.; Adkins, J.F.; Boyle, E.A.; Nelson, C.H.; Palanques, A. A 120-yr record of widespread contamination from mining of the Iberian pyrite belt. Geology 1997, 25, 291–294. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014, Update 2015. In International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; World Soil Resources Reports, 106; FAO: Rome, Italy, 2015; pp. 1–203. [Google Scholar]
- Campos, P.; De la Rosa, J.M. Assessing the Effects of Biochar on the Immobilization of Trace Elements and Plant Development in a Naturally Contaminated Soil. Sustainability 2020, 12, 6025. [Google Scholar] [CrossRef]
- De la Rosa, J.M.; Sánchez-Martín, A.M.; Campos, P.; Miller, A.Z. Effect of pyrolysis conditions on the total contents of polycyclic aromatic hydrocarbons in biochars produced from organic residues: Assessment of their hazard potential. Sci. Total Environ. 2019, 667, 578–585. [Google Scholar] [CrossRef]
- Veihmeyer, F.J.; Hendrickson, A.H. The moisture equivalent as a measure of the field capacity of soils. Soil Sci. 1931, 32, 181–193. [Google Scholar] [CrossRef]
- Campos, P.; Miller, A.Z.; Knicker, H.; Costa-Pereira, M.F.; Merino, A.; De la Rosa, J.M. Chemical, physical and morphological properties of biochars produced from agricultural residues: Implications for their use as soil amendment. J. Waste Manag. 2020, 105, 256–267. [Google Scholar] [CrossRef] [PubMed]
- Trevors, J.T. Dehydrogenase activity in soil: A comparison between the INT and TTC assay. Soil Biol. Biochem. 1984, 16, 673–674. [Google Scholar] [CrossRef]
- Tabatabai, M.A. Soil enzymes. In Method of Soil Analysis, Part 2. Chemical and Microbiological Properties; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; American Society of Agronomy: Madison, WI, USA, 1982; pp. 903–948. [Google Scholar]
- Houba, V.J.G.; Temminghoff, E.J.M.; Gaikhorst, G.A.; van Vark, W. Soil analysis procedures using 0.01 M calcium chloride as extraction reagent. Commun. Soil Sci. Plant Anal. 2000, 31, 1299–1396. [Google Scholar] [CrossRef]
- Pueyo, M.; Rauret, G.; Lück, D.; Yli-Halla, M.; Muntau, H.; Quevauviller, P.; López-Sánchez, J.F. The certification of the extractable contents (mass fractions) of Cd, Cr, Cu, Ni, Pb and Zn in freshwater sediment following a sequential extraction procedure. J. Environ. Monit. 2001, 3, 243–250. [Google Scholar] [CrossRef]
- Joint Research Centre; European Commission. Available online: https://ec.europa.eu/jrc/en (accessed on 25 April 2020).
- Pérez de Mora, A.; Burgos, P.; Madejón, E.; Cabrera, F.; Jaeckel, P.; Schloter, M. Microbial community structure and function in a soil contaminated by heavy metals: Effects of plant growth and different amendments. Soil Biol. Biochem. 2006, 38, 327–341. [Google Scholar] [CrossRef]
- Burgos, P.; Madejón, P.; Cabrera, F.; Madejón, E. By-products as amendment to improve biochemical properties of trace element contaminated soils: Effects in time. Int. Biodeterior. Biodegrad. 2010, 64, 481–488. [Google Scholar] [CrossRef]
- Frankenberger, W.; Johanson, J. Effect of pH On Enzyme Stability in Soils. Soil Biol. Biochem. 1982, 14, 433–437. [Google Scholar] [CrossRef]
- Shuler, M.; Kargi, F. Bioprocess Engineering Basic Concepts; Prentice-Hall Incorporation: Englewood Cliffs, NY, USA, 2010. [Google Scholar]
- Campos, P.; Miller, A.Z.; Prats, S.A.; Knicker, H.; Hagemann, N.; De la Rosa, J.M. Biochar amendment increases bacterial diversity and vegetation cover in trace element-polluted soils: A long-term field experiment. Soil Biol. Biochem. 2020, 150, 1–11. [Google Scholar] [CrossRef]
- Moore, F.; González, M.-E.; Khan, N.; Curaqueo, G.; Sanchez-Monedero, M.; Rilling, J.; Morales, E.; Panichini, M.; Mutis, A.; Jorquera, M.; et al. Copper immobilization by biochar and microbial community abundance in metal-contaminated soils. Sci. Total Environ. 2018, 616–617, 960–969. [Google Scholar] [CrossRef] [PubMed]
- Furtak, K.; Gałązka, A. Enzymatic activity as a popular parameter used to determine the quality of the soil environment. Pol. J. Agron. 2019, 37, 22–30. [Google Scholar] [CrossRef]
- Pérez de Mora, A.; Ortega-Calvo, J.J.; Cabrera, F.; Madejón, E. Changes in enzyme activities and microbial biomass after ‘‘in situ’’ remediation of a heavy metal-contaminated soil. Appl. Soil Ecol. 2005, 28, 125–137. [Google Scholar] [CrossRef]
- Jain, S.; Mishra, D.; Khare, P.; Yadav, V.; Deshmukh, Y.; Meena, A. Impact of biochar amendment on enzymatic resilience properties of mine spoils. Sci. Total Environ. 2016, 544, 410–421. [Google Scholar] [CrossRef]
- Günal, E.; Erdem, H.; Demirbaş, A. Effects of three biochar types on activity of β-glucosidase enzyme in two agricultural soils of different textures. Arch. Agron. Soil Sci. 2018, 64, 1963–1974. [Google Scholar] [CrossRef]
- Netherway, P.; Gascó, G.; Méndez, A.; Surapaneni, A.; Reichman, S.; Shah, K.; Paz-Ferreiro, J. Using Phosphorus-Rich Biochars to Remediate Lead-Contaminated Soil: Influence on Soil Enzymes and Extractable P. Agronomy 2020, 10, 454. [Google Scholar] [CrossRef] [Green Version]
- Foster, E.J.; Fogle, E.J.; Cotrufo, M.F. Sorption to Biochar Impacts β-Glucosidase and Phosphatase Enzyme Activities. Agriculture 2018, 8, 158. [Google Scholar] [CrossRef] [Green Version]
- Lammirato, C.; Miltner, A.; Kaestner, M. Effects of wood char and activated carbon on the hydrolysis of cellobiose by β-glucosidase from Aspergillus niger. Soil Biol. Biochem. 2011, 43, 1936–1942. [Google Scholar] [CrossRef]
- Vangronsveld, J.; Cunningham, S.D. Introduction to the concepts. In Metal Contaminated Soils: In-situ Inactivation and Phytorestoration; Vangronsveld, J., Cunningham, S.D., Eds.; Springer: Berlin, Germany, 1998; pp. 219–225. [Google Scholar]
- Adriano, D.C. Trace Elements in Terrestrial Environments: Biogeochemistry, Bioavailability and Risks of Metals, 2nd ed.; Springer: New York, NY, USA, 2001; p. 867. [Google Scholar]
- Kabata-Pendias, A. Soil–plant transfer of heavy metals—An environmental issue. Geoderma 2004, 122, 143–149. [Google Scholar] [CrossRef]
- Mench, M.; Vangronsveld, J.; Didier, V.; Clijsters, H. Evaluation of metal mobility, plant availability and immobilization by chemical agents in a limed-silty soil. Environ. Pollut. 1994, 86, 279–286. [Google Scholar] [CrossRef]
- Beesley, L.; Moreno-Jiménez, E.; Gomez-Eyles, J.L. Effects of biochar and greenwaste compost amendments on mobility, bioavailability and toxicity of inorganic and organic contaminants in a multi-element polluted soil. Environ. Pollut. 2010, 158, 2282–2287. [Google Scholar] [CrossRef] [PubMed]
- Mašková, T.; Herben, T. Root:shoot ratio in developing seedlings: How seedlings change their allocation in response to seed mass and ambient nutrient supply. Ecol. Evol. 2018, 8, 7143–7150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paneque, M.; Knicker, H.; Kern, J.; De la Rosa, J.M. Hydrothermal Carbonization and Pyrolysis of Sewage Sludge: Effects on Lolium perenne Germination and Growth. Agronomy 2019, 9, 363. [Google Scholar] [CrossRef] [Green Version]
- Borkert, C.M.; Cox, F.R.; Tucker, M.R. Zinc and copper toxicity in peanut, soybean, rice, and corn in soil mixtures. Commun. Soil Sci. Plant Anal. 1998, 29, 2991–3005. [Google Scholar] [CrossRef]

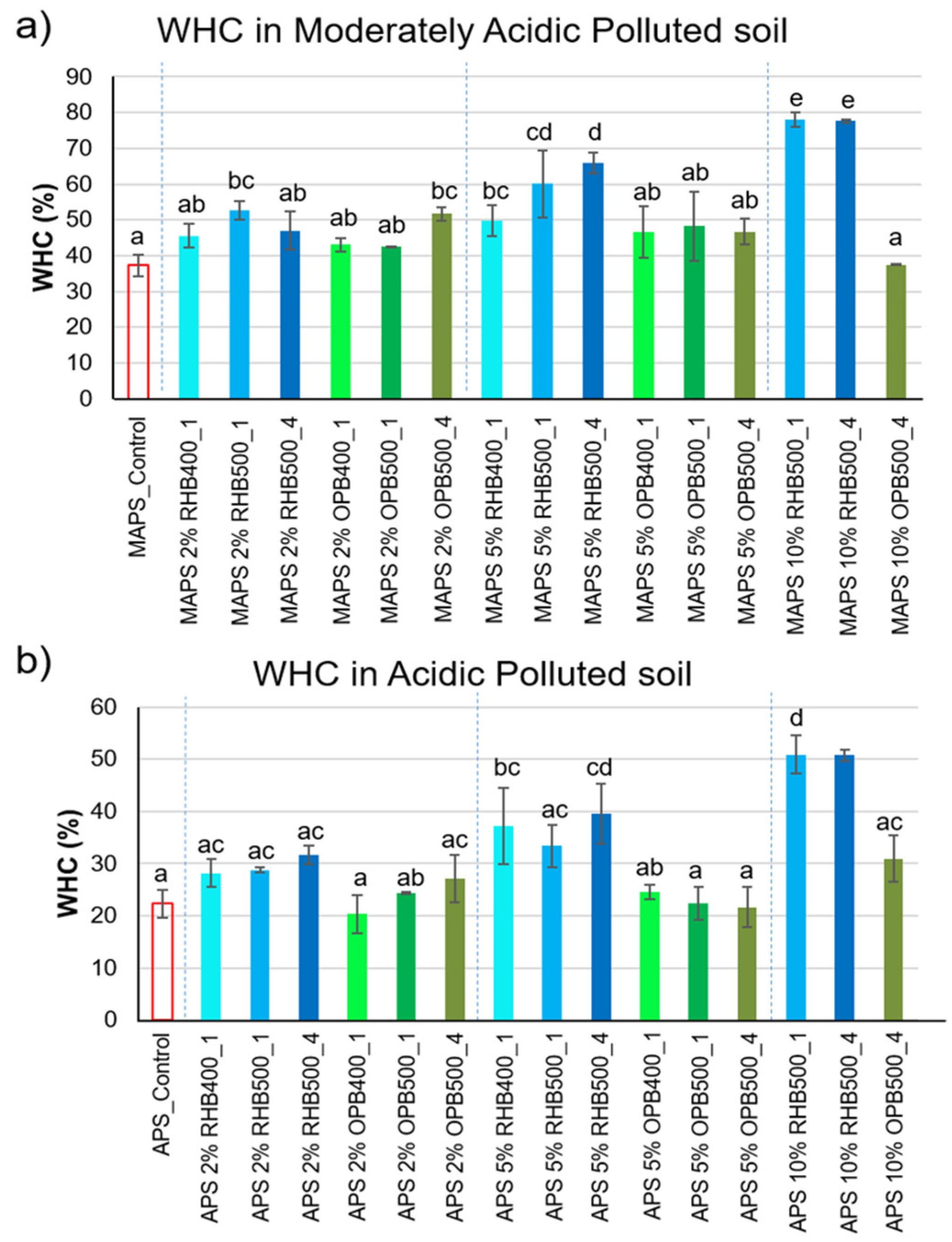
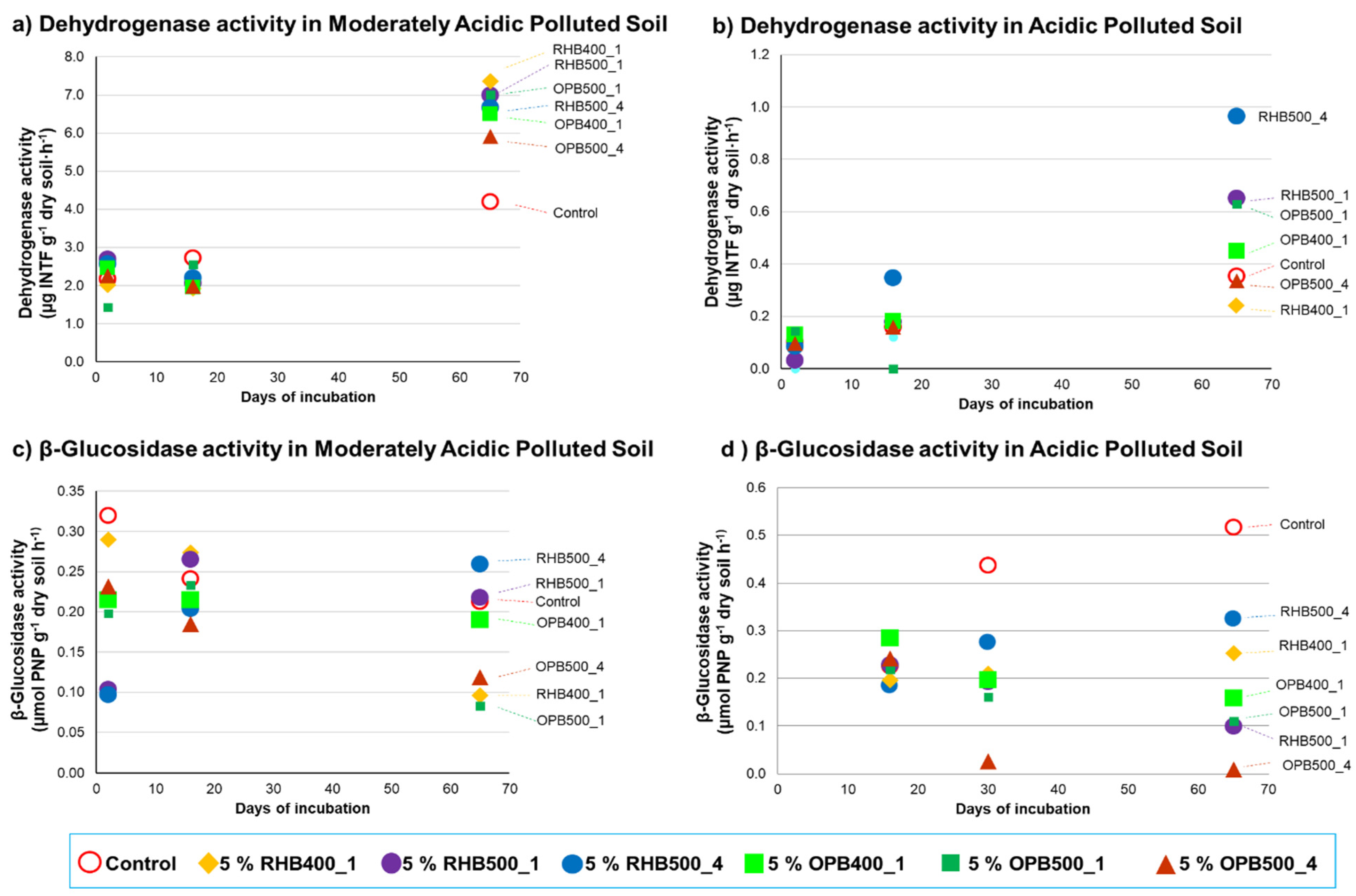
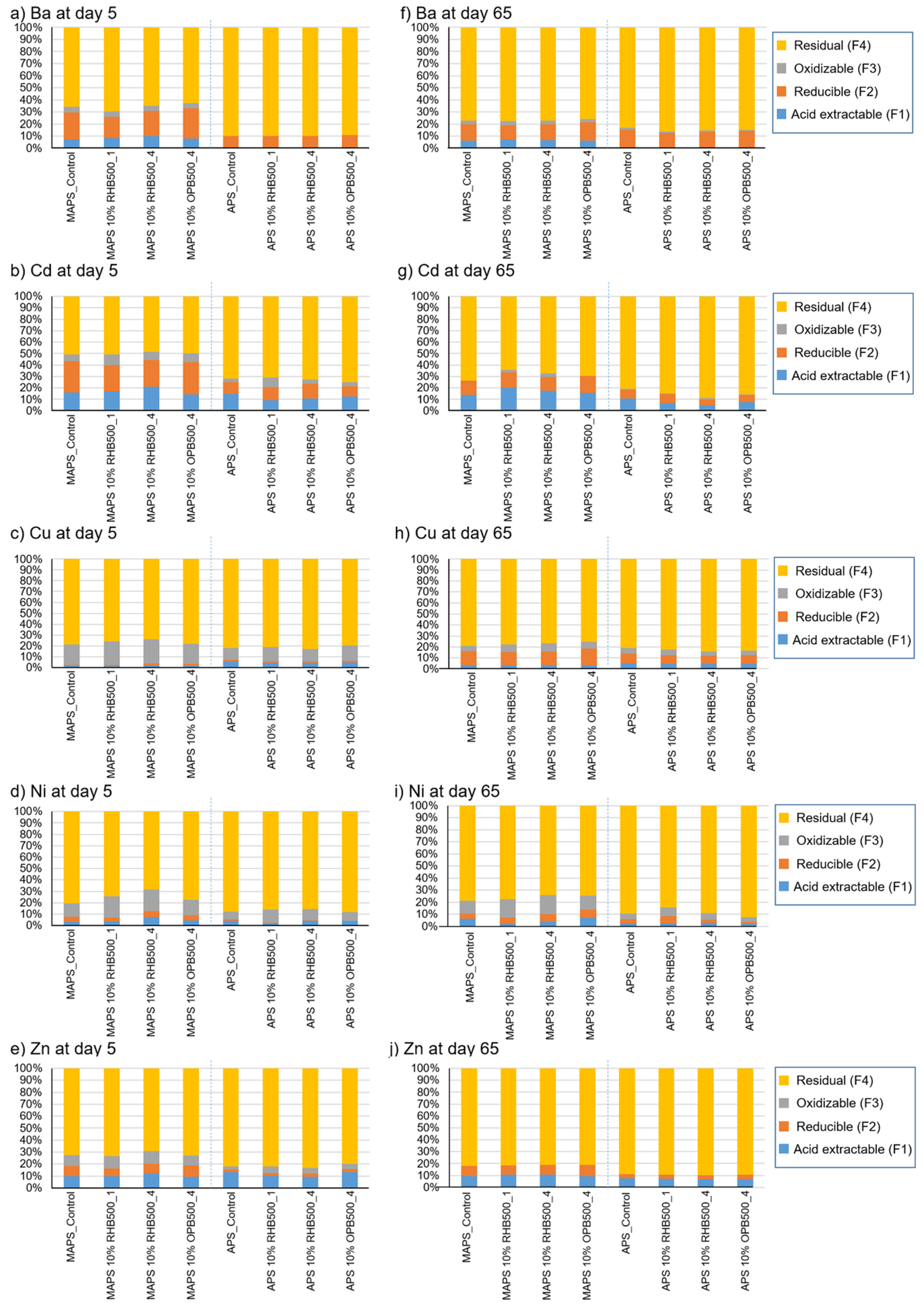

| Elemental Analysis | Total (and CaCl2-Extractable) Contents of Trace Elements (mg kg−1) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TC (g kg−1) | TN (g kg−1) | C/N | Ash (%) | pH | WHC (%) | As | Ba | Cd | Cr | Cu | Fe | Ni | Pb | Sr | Zn | |
| MAPS_o | 17 ± 1 | 2.0 ± 0.7 | 8 | 94.9 ± 0.9 | 6.5 ± 0.1 | 51.5 ± 1.2 | 115.4 (<LOQ) | 93.3 (2.6) | 1.56 (0.1) | 36.1 (<LOQ) | 215.5 (0.2) | 36,945.7 (0.6) | 15.6 (0.1) | 156.5 (0.3) | 38.6 (2.5) | 293.5 (1.3) |
| APS_o | 8 ± 0 | 0.9 ± 0.4 | 8 | 94.7 ± 0.7 | 3.6 ± 0.1 | 32.7 ± 2.4 | 367.0 (<LOQ) | 47.1 (0.1) | 1.28 (0.1) | 48.0 (<LOQ) | 240.6 (5.1) | 53,023.3 (8.9) | 15.6 (0.6) | 569.0 (<LOQ) | 53.7 (0.2) | 249.3 (20.2) |
| RHB400_1 | 501 ± 3 | 5.2 ± 0.3 | 96 | 27.9 ± 0.5 | 9.1 ± 0.0 | 121.0 ± 22.3 | 1.3 | 2.5 | <LOQ | 0.4 | 6.6 | 150.0 | <LOQ | <LOQ | 10.1 | 24.3 |
| RHB500_1 | 518 ± 1 | 6.2 ± 0.1 | 83 | 33.1 ± 0.8 | 10.5 ± 0.0 | 437.9 ± 4.4 | 7.5 | 3.4 | 0.14 | 1.5 | 9.2 | 307.4 | <LOQ | <LOQ | 14.8 | 38.9 |
| RHB500_4 | 522 ± 3 | 5.7 ± 0.1 | 91 | 35.7 ± 0.9 | 10.3 ± 0.0 | 449.8 ± 9.7 | 12.7 | 3.3 | 0.07 | 2.3 | 7.5 | 309.3 | <LOQ | 0.3 | 13.4 | 34.4 |
| OPB400_1 | 670 ± 2 | 12.9 ± 0.3 | 52 | 1.4 ± 0.2 | 7.2 ± 0.0 | 28.0 ± 2.0 | 5.0 | 0.8 | <LOQ | 0.4 | 6.2 | 40.6 | <LOQ | <LOQ | 6.6 | <LOQ |
| OPB500_1 | 605 ± 9 | 15.7 ± 0.2 | 38 | 0.8 ± 0.3 | 8.5 ± 0.1 | 30.0 ± 7.4 | 2.4 | 0.8 | 0.08 | 0.3 | 5.9 | 42.8 | <LOQ | <LOQ | 10.9 | <LOQ |
| OPB500_4 | 587 ± 2 | 13.7 ± 0.0 | 43 | 0.9 ± 0.7 | 9.1 ± 0.2 | 60.2 ± 9.2 | 6.1 | <LOQ | <LOQ | 0.5 | 3.7 | <LOQ | <LOQ | 3.2 | 3.6 | <LOQ |
| TC (g kg−1) | TN (g kg−1) | C/N | |
|---|---|---|---|
| MAPS_Control | 16.6 ± 1.3 a | 2.0 ± 0.7 a | 8 |
| MAPS 5% RHB400_1 | 34.4 ± 3.5 b | 1.1 ± 0.4 a | 32 |
| MAPS 5% RHB500_1 | 41.5 ± 2.6 c,d | 1.4 ± 0.1 a | 29 |
| MAPS 5% RHB500_4 | 34.4 ± 1.2 b | 0.6 ± 0.2 a | 53 |
| MAPS 5% OPB400_1 | 45.4 ± 1.1 d,e | 1.0 ± 0.4 a | 45 |
| MAPS 5% OPB500_1 | 49.8 ± 1.1 e | 0.9 ± 0.3 a | 54 |
| MAPS 5% OPB500_4 | 36.9 ± 0.1 b,c | 0.7 ± 0.1 a | 52 |
| MAPS 10% RHB500_1 | 50.3 ± 0.3 e | 1.8 ± 1.2 a | 28 |
| MAPS 10% RHB500_4 | 67.5 ± 2.7 f | 1.5 ± 0.1 a | 45 |
| MAPS 10% OPB500_4 | 74.0 ± 0.6 g | 1.2 ± 0.0 a | 62 |
| APS_Control | 7.8 ± 0.0 a | 0.9 ± 0.4 a | 8 |
| APS 5% RHB400_1 | 20.6 ± 0.1 c | 0.8 ± 0.0 a | 25 |
| APS 5% RHB500_1 | 17.3 ± 0.6 b | 0.5 ± 0.6 a | 37 |
| APS 5% RHB500_4 | 30.0 ± 2.6 d | 1.0 ± 0.4 a | 31 |
| APS 5% OPB400_1 | 41.1 ± 0.6 h | 0.7 ± 0.3 a | 59 |
| APS 5% OPB500_1 | 44.9 ± 0.9 g | 1.2 ± 0.1 a | 36 |
| APS 5% OPB500_4 | 34.0 ± 0.4 e | 0.8 ± 0.1 a | 41 |
| APS 10% RHB500_1 | 39.1 ± 0.2 f | 0.9 ± 0.3 a | 43 |
| APS 10% RHB500_4 | 45.4 ± 1.2 g | 0.6 ± 0.1 a | 71 |
| APS 10% OPB500_4 | 65.9 ± 0.6 i | 0.7 ± 0.2 a | 94 |
| Ba | Cd | Cu | Mn | Ni | Pb | Zn | |
|---|---|---|---|---|---|---|---|
| MAPS_Control | 2.6 ± 0.5 c | 0.05 ± 0.02 a | 0.2 ± 0.0 a | 20.5 ± 4.3 d | 0.14 ± 0.04 a,c | 0.3 ± 0.0 | 1.6 ± 0.4 a |
| MAPS 5% RHB400_1 | 2.5 ± 0.1 b,c | 0.04 ± 0.00 a,b | 0.2 ± 0.1 a | 26.9 ± 1.0 e | 0.20 ± 0.05 c | <LOQ | 1.5 ± 0.2 a |
| MAPS 5% RHB500_1 | 2.0 ± 0.2 b | 0.03 ± 0.02 a,b | 0.1 ± 0.1 a | 13.7 ± 1.6 c | 0.11 ± 0.01 a,b | <LOQ | 0.6 ± 0.0 b,c |
| MAPS 5% RHB500_4 | 2.0 ± 0.1 b | 0.02 ± 0.00 a,b | 0.2 ± 0.0 a | 10.1 ± 0.7 b,c | 0.10 ± 0.03 a,c | <LOQ | 0.3 ± 0.1 b,c,d |
| MAPS 5% OPB400_1 | 2.2 ± 0.0 b,c | 0.03 ± 0.00 a,b | 0.1 ± 0.1 a | 21.5 ± 0.5 d | 0.14 ± 0.02 a,c | <LOQ | 1.2 ± 0.2 a |
| MAPS 5% OPB500_1 | 1.5 ± 0.0 a | 0.03 ± 0.01 a,b | 0.2 ± 0.0 a | 10.4 ± 0.1 b,c | <LOQ | <LOQ | 0.5 ± 0.0 b,c,d |
| MAPS 5% OPB500_4 | 2.1 ± 0.2 b,c | 0.03 ± 0.01 a,b | 0.1 ± 0.1 a | 12.2 ± 1.3 c | 0.16 ± 0.08 b,c | <LOQ | 0.8 ± 0.1 b |
| MAPS 10% RHB500_1 | 1.2 ± 0.0 a | 0.02 ± 0.01 a,b | 0.2 ± 0.0 a | 7.2 ± 0.1 a,b | <LOQ | <LOQ | 0.2 ± 0.0 c,d |
| MAPS 10% RHB500_4 | 1.1 ± 0.0 a | 0.01 ± 0.01 b | 0.2 ± 0.0 a | 3.8 ± 0.0 a | 0.06 ± 0.01 a,c | <LOQ | 0.1 ± 0.1 d |
| MAPS 10% OPB500_4 | 1.4 ± 0.1 a | 0.01 ± 0.01 b | 0.1 ± 0.0 a | 4.0 ± 0.2 a | 0.03 ± 0.02 a,b | <LOQ | <LOQ |
| Pearson Coefficient | −0.62 | −0.79 | −0.18 | −0.59 | −0.20 | n.c. | −0.63 |
| APS_Control | 0.09 ± 0.07 a | 0.11 ± 0.03 a | 5.1 ± 0.2 b | 33.9 ± 0.1 a | 0.63 ± 0.01 a | <LOQ | 20.2 ± 0.3 g |
| APS 5% RHB400_1 | <LOQ | 0.09 ± 0.01 a | 4.1 ± 0.1 a,b | 36.2 ± 0.5 a | 0.69 ± 0.03 a | <LOQ | 19.3 ± 0.3 f,g |
| APS 5% RHB500_1 | <LOQ | 0.09 ± 0.01 a | 4.2 ± 0.0 a,b | 32.8 ± 0.2 a | 0.57 ± 0.06 a | 0.3 ± 0.1 | 17.3 ± 0.1 e |
| APS 5% RHB500_4 | <LOQ | 0.08 ± 0.01 a | 2.6 ± 0.1 a,b | 26.2 ± 0.0 a | 0.54 ± 0.01 a | 0.3 ± 0.1 | 13.3 ± 0.3 c |
| APS 5% OPB400_1 | <LOQ | 0.06 ± 0.01 a | 3.4 ± 0.1 a,b | 27.1 ± 1.2 a | 0.59 ± 0.07 a | <LOQ | 15.3 ± 0.3 d |
| APS 5% OPB500_1 | 0.06 ± 0.00 a | 0.06 ± 0.00 a | 3.0 ± 0.1 a,b | 26.6 ± 0.3 a | 0.38 ± 0.05 a | <LOQ | 15.6 ± 0.3 d |
| APS 5% OPB500_4 | <LOQ | 0.08 ± 0.02 a | 3.6 ± 0.0 a,b | 34.6 ± 0.5 a | 0.73 ± 0.06 a | <LOQ | 18.8 ± 0.3 f |
| APS 10% RHB500_1 | <LOQ | 0.08 ± 0.00 a | 1.4 ± 0.1 a | 33.5 ± 1.8 a | 0.38 ± 0.01 a | <LOQ | 12.5 ± 0.6 c |
| APS 10% RHB500_4 | 0.01 ± 0.00 a | 0.05 ± 0.00 a | 1.1 ± 0.0 a | 26.0 ± 0.6 a | 0.29 ± 0.04 a | <LOQ | 9.8 ± 0.2 a |
| APS 10% OPB500_4 | 0.04 ± 0.00 a | 0.05 ± 0.00 a | 1.1 ± 0.2 a | 21.2 ± 0.8 a | 0.29 ± 0.02 a | <LOQ | 11.4 ± 0.4 b |
| Pearson Coefficient | n.c. | −0.80 | −0.84 | −0.64 | −0.53 | n.c. | −0.82 |
| Ind. Var. | (I) Ind. Var. | (J) Ind. Var. | Mean Difference (I-J) | |||||
|---|---|---|---|---|---|---|---|---|
| Ba | Cd | Cu | Mn | Ni | Zn | |||
| Soil | MAPS | APS | 1.7896 * | −0.0488 * | −2.6025 * | −15.2353 * | −0.3997 * | −14.6951 * |
| Biochar | Control | RHB | 0.4058 * | 0.0375 * | 1.4042 * | 7.1037 * | 0.0592 | 3.2481 * |
| OPB | 0.4446 * | 0.0431 * | 1.2009 * | 7.4839 * | 0.0227 | 2.8114 * | ||
| RHB | OPB | 0.0388 | 0.0056 | −0.2033 | 0.3802 | −0.0365 | −0.4367 * | |
| Dose | 0 | 5 | 0.2640 | 0.0334 * | 0.9925 * | 5.2879 | −0.0063 | 2.0402 * |
| 10 | 0.7410 * | 0.0530 * | 1.9564 * | 11.2422 * | 0.1416 | 5.0816 * | ||
| 5 | 10 | 0.4770 * | 0.0196 * | 0.9639 * | 5.9543 * | 0.1479 * | 3.0414 * | |
| Temperature | 0 | 400 | 0.0074 | 0.0322 * | 1.1535 * | 3.1692 | −0.0100 | 1.4329 * |
| 500 | 0.5418 * | 0.0421 * | 1.3596 * | 8.4451 * | 0.0581 | 3.5172 * | ||
| 400 | 500 | 0.5344 * | 0.0099 | 0.2061 | 5.2759 * | 0.0681 | 2.0843 * | |
| Time | 0 | 1 | 0.3374 * | 0.0350 * | 1.1447 * | 5.1480 | 0.0449 | 2.3486 * |
| 4 | 0.5301 * | 0.0461 * | 1.5252 * | 9.9285 * | 0.0406 | 3.9357 * | ||
| 1 | 4 | 0.1927 | 0.0111 | 0.3805 | 4.7805 * | −0.0043 | 1.5871 * | |
| Pillai’s Trace | F | p | |
|---|---|---|---|
| Soil | 1.000 | 11823.219 | 0.000 |
| Biochar | 0.619 | 4.057 | 0.013 |
| Dose | 0.995 | 484.564 | 0.000 |
| Temperature | 0.921 | 29.270 | 0.000 |
| Time | 0.758 | 7.822 | 0.001 |
| Soil:Biochar | 0.194 | 0.603 | 0.724 |
| Soil:Dose | 0.997 | 795.507 | 0.000 |
| Soil:Temp | 0.952 | 49.618 | 0.000 |
| Soil:Time | 0.811 | 10.754 | 0.000 |
| Biochar:Dose | 0.823 | 11.656 | 0.000 |
| Biochar:Temp | 0.616 | 4.007 | 0.014 |
| Biochar:Time | 0.963 | 64.786 | 0.000 |
| Dose:Temp | 0.000 | n.c. | n.c. |
| Dose:Time | 0.904 | 23.458 | 0.000 |
| Temp:Time | 0.000 | n.c. | n.c. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campos, P.; Knicker, H.; López, R.; De la Rosa, J.M. Application of Biochar Produced from Crop Residues on Trace Elements Contaminated Soils: Effects on Soil Properties, Enzymatic Activities and Brassica rapa Growth. Agronomy 2021, 11, 1394. https://doi.org/10.3390/agronomy11071394
Campos P, Knicker H, López R, De la Rosa JM. Application of Biochar Produced from Crop Residues on Trace Elements Contaminated Soils: Effects on Soil Properties, Enzymatic Activities and Brassica rapa Growth. Agronomy. 2021; 11(7):1394. https://doi.org/10.3390/agronomy11071394
Chicago/Turabian StyleCampos, Paloma, Heike Knicker, Rafael López, and José María De la Rosa. 2021. "Application of Biochar Produced from Crop Residues on Trace Elements Contaminated Soils: Effects on Soil Properties, Enzymatic Activities and Brassica rapa Growth" Agronomy 11, no. 7: 1394. https://doi.org/10.3390/agronomy11071394
APA StyleCampos, P., Knicker, H., López, R., & De la Rosa, J. M. (2021). Application of Biochar Produced from Crop Residues on Trace Elements Contaminated Soils: Effects on Soil Properties, Enzymatic Activities and Brassica rapa Growth. Agronomy, 11(7), 1394. https://doi.org/10.3390/agronomy11071394









