Nozzle Selection and Adjuvant Impact on the Efficacy of Glyphosate and PPO-Inhibiting Herbicide Tank-Mixtures
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Establishment
2.1.1. Field Studies
2.1.2. Greenhouse Studies
2.2. Data Collection
2.2.1. Field Studies
2.2.2. Greenhouse Studies
2.3. Tank-Mixture Interaction
2.4. Statistical Analyses
3. Results
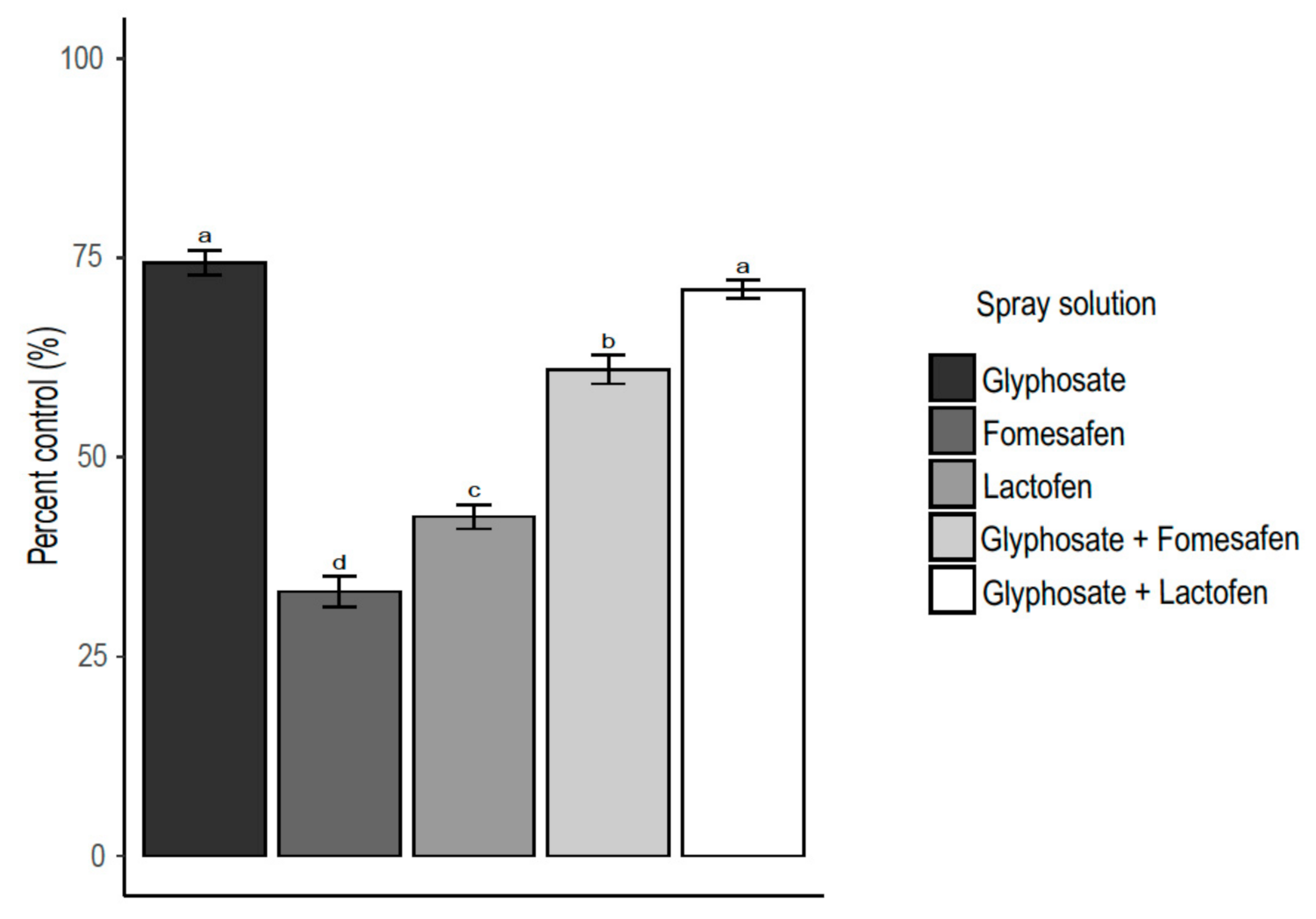
3.1. Nozzle Selection
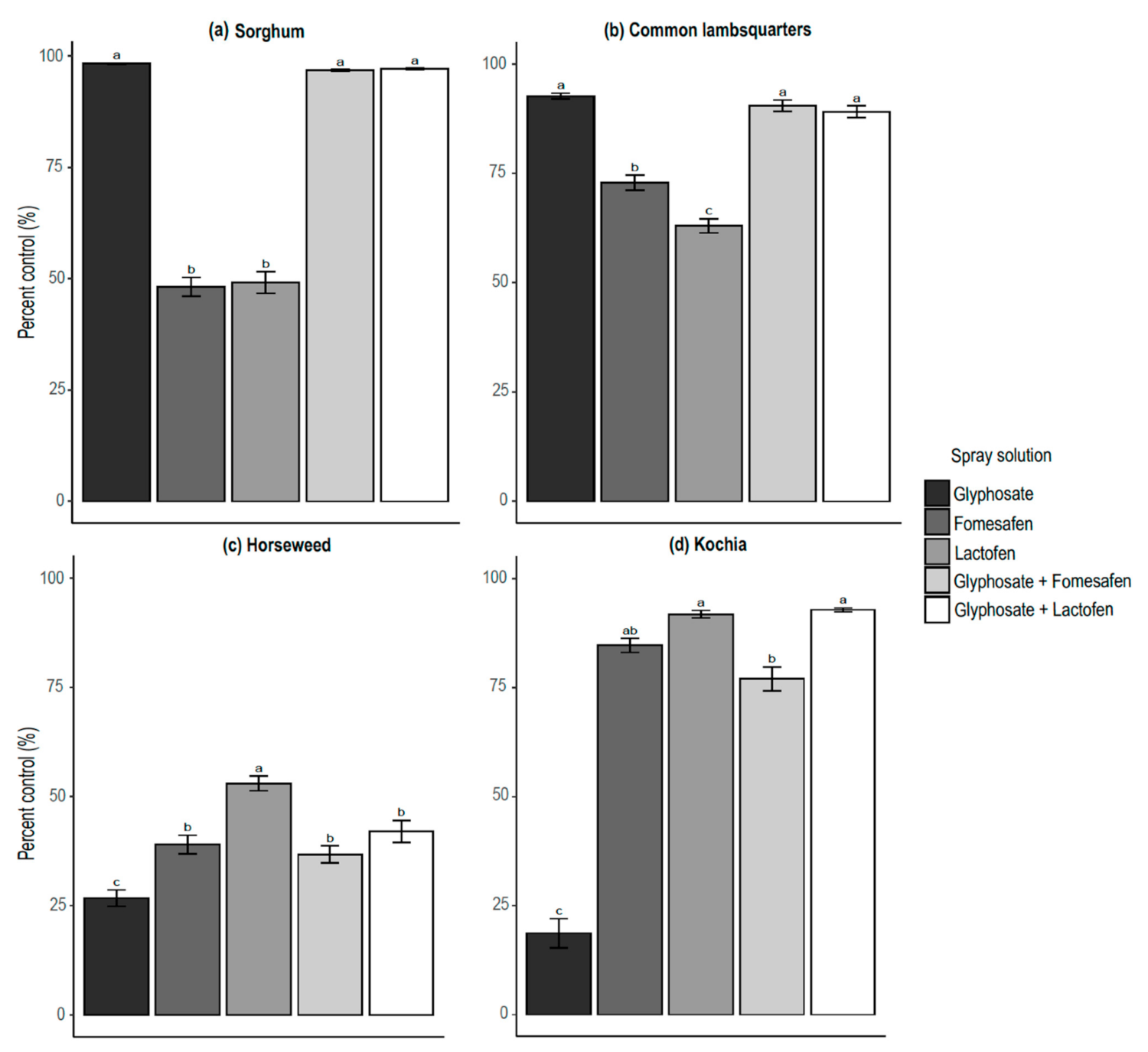
3.2. Percent of Control by Plant Species
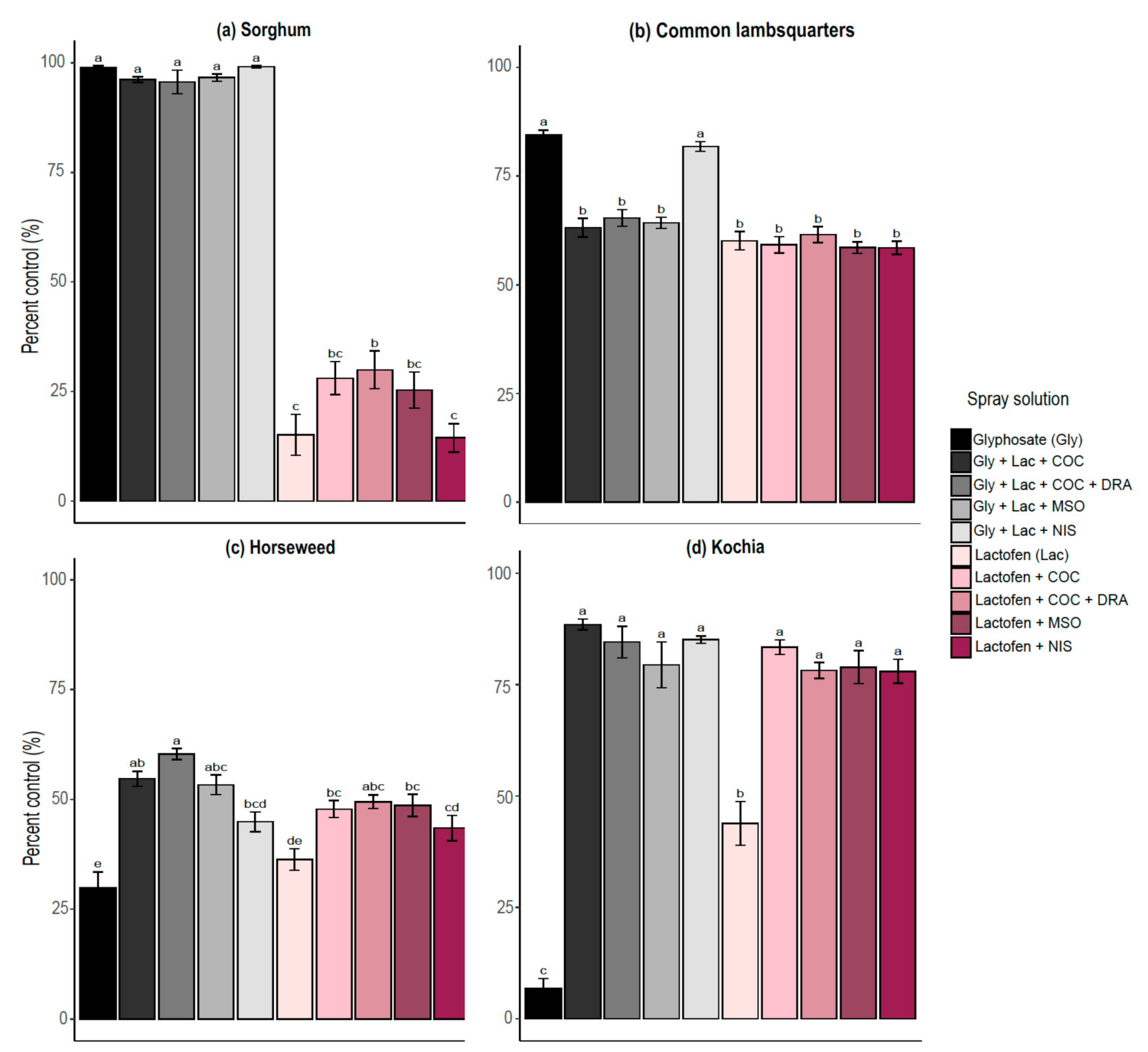
3.3. Tank Mixture Interactions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duke, S.O.; Powles, S.B. Glyphosate: A once-in-a-century herbicide. Pest Manag. Sci 2008, 64, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Knezevic, S.Z. Herbicide tolerant crops: 10 years later. Maydica 2007, 52, 245–250. [Google Scholar]
- [WSSA] Weed Science Society of America. 2019 WSSA Survey Ranks Most Common and Most Troublesome Weeds in Broadleaf Crops, Fruits and Vegetables. Available online: http://wssa.net/wssa/weed/surveys/ (accessed on 18 September 2020).
- Heap I International Survey of Herbicide-Resistant Weeds. Available online: http://www.weedscience.org (accessed on 18 September 2020).
- Husted, K. Dicamba Damage Estimate Tops 2.5 Million Acres. Harvest Public Media. Available online: https://www.harvestpublicmedia.org/post/dicamba-damage-estimate-tops-25-million-acres (accessed on 18 September 2020).
- Vieira, B.C.; Luck, J.D.; Amundsen, K.L.; Werle, R.; Gaines, T.A.; Kruger, G.R. Herbicide drift exposure leads to reduced herbicide sensitivity in Amaranthus spp. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ashworth, M.B.; Walsh, M.J.; Flower, K.C.; Powles, S.B. Recurrent selection with reduced 2, 4-D amine doses results in the rapid evolution of 2, 4-D herbicide resistance in wild radish (Raphanus raphanistrum L.). Pest Manag. Sci. 2016, 72, 2091–2098. [Google Scholar] [CrossRef]
- Tehranchian, P.; Norsworthy, J.K.; Powles, S.; Bararpour, M.T.; Bagavathiannan, M.V.; Barber, T.; Scott, R.C. Recurrent Sublethal-Dose Selection for Reduced Susceptibility of Palmer Amaranth (Amaranthus palmeri) to Dicamba. Weed Sci. 2017, 65, 206–212. [Google Scholar] [CrossRef]
- Hao, G.F.; Zuo, Y.; Yang, S.G.; Yang, G.F. Protoporphyrinogen oxidase inhibitor: An ideal target for herbicide discovery. Chim. Int. J. Chem. 2011, 65, 961–969. [Google Scholar] [CrossRef]
- Nandula, V.K.; Reddy, K.N.; Koger, C.H.; Poston, D.H.; Rimando, A.M.; Duke, S.O.; Bond, J.A.; Ribeiro, D.N. Multiple resistance to glyphosate and pyrithiobac in Palmer amaranth (Amaranthus palmeri) from Mississippi and response to flumiclorac. Weed Sci. 2012, 60, 179–188. [Google Scholar] [CrossRef]
- Nandula, V.K.; Molin, W.T.; Bond, J.A. Influence of Water Quality, Formulation, Adjuvant, Rainfastness, and Nozzle type on Efficacy of Fomesafen on Palmer amaranth (Amaranthus palmeri) Control. Am. J. Plant Sci. 2018, 9, 1660. [Google Scholar] [CrossRef]
- Starke, R.J.; Oliver, L.R. Interaction of glyphosate with chlorimuron, fomesafen, imazethapyr, and sulfentrazone. Weed Sci. 1998, 46, 652–660. [Google Scholar] [CrossRef]
- Harre, N.T.; Young, J.M.; Young, B.G. Glyphosate-induced antagonism in rapid response giant ragweed (Ambrosia trifida). Weed Technol. 2018, 32, 52–59. [Google Scholar] [CrossRef]
- Beckie, H.J.; Reboud, X. Selecting for weed resistance: Herbicide rotation and mixture. Weed Technol. 2009, 23, 363–370. [Google Scholar] [CrossRef]
- Bellinder, R.R.; Arsenovic, M.; Shah, D.A.; Rauch, B.J. Effect of weed growth stage and adjuvant on the efficacy of fomesafen and bentazon. Weed Sci. 2003, 51, 1016–1021. [Google Scholar] [CrossRef]
- Johnson, A.K.; Roeth, F.W.; Martin, A.R.; Klein, R.N. Glyphosate spray drift management with drift-reducing nozzles and adjuvants. Weed Technol. 2006, 20, 893–897. [Google Scholar] [CrossRef]
- Young, B.G.; Hart, S.E.; Wax, L.M. Interactions of sethoxydim and corn (Zea mays) postemergence broadleaf herbicides on three annual grasses. Weed Technol. 1996, 10, 914–922. Available online: www.jstor.org/stable/398793 (accessed on 18 September 2020). [CrossRef]
- Campbell, J.R.; Penner, D. Compatibility of diclofop and BAS 9052 with bentazon. Weed Sci. 1982, 30, 458–462. Available online: www.jstor.org/stable/4043741 (accessed on 18 September 2020).
- Knezevic, S.Z.; Datta, A.; Scott, J.; Klein, R.N.; Golus, J. Problem weed control in glyphosate-resistant soybean with glyphosate tank mixes and soil-applied herbicides. Weed Technol. 2009, 23, 507–512. [Google Scholar] [CrossRef]
- Penner, D. The impact of adjuvants on herbicide antagonism. Weed Technol. 1989, 3, 227–231. [Google Scholar] [CrossRef]
- Butts, T.R.; Samples, C.A.; Franca, L.X.; Dodds, D.M.; Reynolds, D.B.; Adams, J.W.; Zollinger, R.K.; Howatt, K.A.; Fritz, B.K.; Clint Hoffmann, W.; et al. Spray droplet size and carrier volume effect on dicamba and glufosinate efficacy. Pest Manag. Sci. 2018, 74, 2020–2029. [Google Scholar] [CrossRef]
- Creech, C.F.; Henry, R.S.; Fritz, B.K.; Kruger, G.R. Influence of herbicide active ingredient, nozzle type, orifice size, spray pressure, and carrier volume rate on spray droplet size characteristics. Weed Technol. 2015, 29, 298–310. [Google Scholar] [CrossRef]
- Hanks, J.E. Effect of drift retardant adjuvants on spray droplet size of water and paraffinic oil applied at ultralow volume. Weed Technol. 1995, 9, 380–384. [Google Scholar] [CrossRef]
- Knoche, M. Effect of droplet size and carrier volume on performance of foliage-applied herbicides. Crop Prot. 1994, 13, 163–178. [Google Scholar] [CrossRef]
- Berger, S.T.; Dobrow, M.H.; Ferrell, J.A.; Webster, T.M. Influence of carrier volume and nozzle selection on palmer amaranth control. Peanut Sci. 2014, 41, 120–123. [Google Scholar] [CrossRef]
- Sikkema, P.H.; Brown, L.; Shropshire, C.; Spieser, H.; Soltani, N. Flat fan and air induction nozzles affect soybean herbicide efficacy. Weed Biol. Manag. 2008, 8, 31–38. [Google Scholar] [CrossRef]
- Brown, L.; Soltani, N.; Shropshire, C.; Spieser, H.; Sikkema, P.H. Efficacy of four corn (Zea mays L.) herbicides when applied with flat fan and air induction nozzles. Weed Biol. Manag. 2007, 7, 55–61. [Google Scholar] [CrossRef]
- Creech, C.F.; Moraes, J.G.; Henry, R.S.; Luck, J.D.; Kruger, G.R. The impact of spray droplet size on the efficacy of 2,4-d, atrazine, chlorimuron-methyl, dicamba, glufosinate, and saflufenacil. Weed Technol. 2016, 30, 573–586. [Google Scholar] [CrossRef]
- Mellendorf, T.G.; Young, J.M.; Matthews, J.L.; Young, B.G. Influence of application variables on the foliar efficacy of saflufenacil on horseweed (Conyza canadensis). Weed Sci. 2015, 63, 578–586. [Google Scholar] [CrossRef]
- Ramsdale, B.K.; Messersmith, C.G. Drift-reducing nozzle effects on herbicide performance. Weed Technol. 2001, 15, 453–460. [Google Scholar] [CrossRef]
- Samuelson, S.L. Response of problematic weed populations in Nebraska to glyphosate. Master’s Thesis, Digital Commons University of Nebraska-Lincoln, Lincoln, NE, USA, 2017. [Google Scholar]
- Colby, S.R. Calculating synergistic and antagonistic responses of herbicide combinations. Weeds 1967, 15, 20–22. [Google Scholar] [CrossRef]
- Banzato, D.A.; Kronka, S.N. Experiementacao agricola 4ª, 2015, 23–26. Available online: https://scholar.google.com/scholar_lookup?title=Experimenta%C3%A7%C3%A3o%20Agr%C3%ADcola.%204a&publication_year=2015&author=D.A.%20Banzato&author=S.D.N.%20Kronka (accessed on 18 September 2020).
- Vieira, B.C.; Samuelson, S.L.; Alves, G.S.; Gaines, T.A.; Werle, R.; Kruger, G.R. Distribution of glyphosate-resistant Amaranthus spp. In Nebraska. Pest Manag. Sci. 2017, 74, 2316–2324. [Google Scholar] [CrossRef]
- Franca, L.X.; Dodds, D.M.; Butts, T.R.; Kruger, G.R.; Reynolds, D.B.; Mills, J.A.; Bond, J.A.; Catchot, A.L.; Peterson, D.G. Droplet size impact on lactofen and acifluorfen efficacy for Palmer amaranth (Amaranthus palmeri) control. Weed Technol. 2019, 34, 416–423. [Google Scholar] [CrossRef]
- Sweat, J.K.; Horak, M.J.; Peterson, D.E.; Lloyd, R.W.; Boyer, J.E. Herbicide efficacy on four Amaranthus species in soybean (Glycine max). Weed Technol. 1998, 12, 315–321. [Google Scholar] [CrossRef]
- Patzoldt, W.L.; Tranel, P.J.; Hager, A.G. Variable herbicide responses among Illinois waterhemp (Amaranthus rudis and A. turbeculatus) populations. Crop Prot. 2002, 21, 707–712. [Google Scholar] [CrossRef]
- Hager, A.G.; Wax, L.; Bollero, G.A.; Stoller, E.W. Influence of diphenylether herbicide application rate and timing on common waterhemp (Amaranthus rudis) control in soybean (Glycine max). Weed Technol. 2003, 17, 14–20. [Google Scholar] [CrossRef]
- Bond, J.A.; Oliver, L.R.; Stephenson, D.O. Response of Palmer amaranth (Amaranthus palmeri) accessions to glyphosate, fomesafen, and pyrithiobac. Weed Technol. 2006, 20, 885–892. [Google Scholar] [CrossRef]
- Whitaker, J.R.; York, A.C.; Jordan, D.L.; Culpepper, A.S. Palmer amaranth (Amaranthus palmeri) control in soybean with glyphosate and conventional herbicide systems. Weed Technol. 2010, 24, 403–410. [Google Scholar] [CrossRef]
- Gower, S.A.; Loux, M.M.; Cardina, J.; Harrison, S.K.; Sprankle, P.L.; Probst, N.J.; Bauman, T.T.; Bugg, W.; Curran, W.S.; Currie, R.S.; et al. Effect of postemergence glyphosate application timing on weed control and grain yield in glyphosate-resistant corn: Results of a 2-yr multistate study. Weed Technol. 2003, 17, 821–828. [Google Scholar] [CrossRef]
- Chahal, P.S.; Aulakh, J.S.; Jugulam, M.; Jhala, A.J. Herbicide-resistant Palmer amaranth (Amaranthus palmeri S. Wats.) in the United States: Mechanisms of resistance, impact, and management. In Herbicides, Agronomic Crops and Weed Biology; Price, A., Kelton, J., Sarunaite, L., Eds.; InTech: Rijeka, Croatia, 2015; pp. 1–29. [Google Scholar]
- Kammler, K.J.; Walters, S.A.; Young, B.G. Effects of adjuvants, halosulfuron, and grass herbicides on cucurbita spp. injury and grass Control. Weed Technol. 2010, 24, 147–152. [Google Scholar] [CrossRef]
- Merritt, L.H.; Ferguson, J.C.; Brown-Johnson, A.E.; Reynolds, D.B.; Tseng, T.M.; Lowe, J.W. Reduced herbicide antagonism of grass control through spray application technique. Agronomy 2020, 10, 1131. [Google Scholar] [CrossRef]
| Parameters | Field Experiments | |
|---|---|---|
| Site 1 | Site 2 | |
| City | Beaver City, Nebraska | Beaver City, Nebraska |
| GPS coordinates | 40.16° N, 99.91° W | 40.13° N, 99.88° W |
| Application date | 30 June 2016 | 6 July 2016 |
| Temperature (°C) | 28 | 33 |
| Relative humidity (%) | 50 | 45 |
| Weed density (plants m−2) | 50–70 | 60–80 |
| Weed height (cm) | 58 | 31 |
| Soil type | Ulysses silt loam | Holdrege silt loam |
| Experiment | Common Name | Nozzle Type a | Droplet Size Classification b,c | Manufacturer |
|---|---|---|---|---|
| Field; Greenhouse 2016/2017 | Extended Range | XR | M | Teejet Technologies, Spraying Systems Co., Wheaton, IL 62703 |
| Field; Greenhouse 2016 | Air-Induction Extended | AIXR | XC | Teejet Technologies, Spraying Systems Co., Wheaton, IL 62703 |
| Greenhouse 2016 | GuardianAIR | GA | C | Pentair Hypro, New Brighton, MN 55112 |
| Greenhouse 2016 | TurboDrop® XL | TDXL | VC | Greenleaf Technologies, Covington, LA 70434 |
| Greenhouse 2016 | Ultra Lo-Drift | ULD | UC | Pentair Hypro, New Brighton, MN 55112 |
| Field; Greenhouse 2016/2017 | Turbo Teejet® Induction | TTI | UC | Teejet Technologies, Spraying Systems Co., Wheaton, IL 62703 |
| Species | Herbicide | Observed Response | Expected Response a | t-Value | pb | Interaction |
|---|---|---|---|---|---|---|
| --------------- % -------------- | ||||||
| Sorghum | Fomesafen | 97 | 99 | 14.82 | <0.0001 | Antagonistic |
| Lactofen | 97 | 99 | 15.25 | <0.0001 | Antagonistic | |
| Common lambsquarters | Fomesafen | 90 | 98 | 5.86 | <0.0001 | Antagonistic |
| Lactofen | 89 | 97 | 6.23 | <0.0001 | Antagonistic | |
| Horseweed | Fomesafen | 37 | 55 | 9.57 | <0.0001 | Antagonistic |
| Lactofen | 42 | 66 | 9.40 | <0.0001 | Antagonistic | |
| Kochia | Fomesafen | 77 | 88 | 3.88 | <0.0001 | Antagonistic |
| Lactofen | 93 | 93 | 0.99 | <0.0001 | Additive | |
| Species | Adjuvant | Observed Response | Expected Response a | t- Value | pb | Interaction |
|---|---|---|---|---|---|---|
| --------------- % -------------- | ||||||
| Sorghum | COC | 96 | 99 | 5.03 | <0.0001 | Antagonistic |
| DRA + COC | 96 | 99 | 1.39 | 0.173 | Additive | |
| MSO | 97 | 99 | 3.30 | 0.002 | Antagonistic | |
| NIS | 99 | 99 | 0.12 | 0.898 | Additive | |
| Common lambsquarters | COC | 63 | 92 | 13.81 | <0.0001 | Antagonistic |
| DRA + COC | 65 | 94 | 15.43 | <0.0001 | Antagonistic | |
| MSO | 64 | 94 | 23.44 | <0.0001 | Antagonistic | |
| NIS | 82 | 93 | 10.57 | <0.0001 | Antagonistic | |
| Horseweed | COC | 55 | 62 | 4.63 | <0.0001 | Antagonistic |
| DRA + COC | 60 | 64 | 2.61 | 0.013 | Antagonistic | |
| MSO | 53 | 64 | 4.84 | <0.0001 | Antagonistic | |
| NIS | 45 | 60 | 7.05 | <0.0001 | Antagonistic | |
| Kochia | COC | 88 | 85 | 3.27 | 0.002 | Synergistic |
| DRA + COC | 84 | 73 | 3.22 | 0.003 | Synergistic | |
| MSO | 79 | 80 | 0.18 | 0.859 | Additive | |
| NIS | 85 | 79 | 6.94 | <0.0001 | Synergistic | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moraes, J.G.; Butts, T.R.; M. Anunciato, V.; Luck, J.D.; Hoffmann, W.C.; Antuniassi, U.R.; Kruger, G.R. Nozzle Selection and Adjuvant Impact on the Efficacy of Glyphosate and PPO-Inhibiting Herbicide Tank-Mixtures. Agronomy 2021, 11, 754. https://doi.org/10.3390/agronomy11040754
Moraes JG, Butts TR, M. Anunciato V, Luck JD, Hoffmann WC, Antuniassi UR, Kruger GR. Nozzle Selection and Adjuvant Impact on the Efficacy of Glyphosate and PPO-Inhibiting Herbicide Tank-Mixtures. Agronomy. 2021; 11(4):754. https://doi.org/10.3390/agronomy11040754
Chicago/Turabian StyleMoraes, Jesaelen G., Thomas R. Butts, Vitor M. Anunciato, Joe D. Luck, Wesley C. Hoffmann, Ulisses R. Antuniassi, and Greg R. Kruger. 2021. "Nozzle Selection and Adjuvant Impact on the Efficacy of Glyphosate and PPO-Inhibiting Herbicide Tank-Mixtures" Agronomy 11, no. 4: 754. https://doi.org/10.3390/agronomy11040754
APA StyleMoraes, J. G., Butts, T. R., M. Anunciato, V., Luck, J. D., Hoffmann, W. C., Antuniassi, U. R., & Kruger, G. R. (2021). Nozzle Selection and Adjuvant Impact on the Efficacy of Glyphosate and PPO-Inhibiting Herbicide Tank-Mixtures. Agronomy, 11(4), 754. https://doi.org/10.3390/agronomy11040754






