Abstract
Cultivation of the peanut (Arachis hypogaea L.) on the same land contributes to the accumulation of root exudates, leading to increased soil pathogens and decreased yield. Trichoderma harzianum is a naturally occurring endophytic biocontrol fungus that can enhance plant growth, nutrient uptake, and tolerance to biotic and abiotic stresses. Separately, Bradyrhizobium spp. is a biological nitrogen-fixing (BNF) bacterium favoring nodule formation in peanut roots which promotes nitrogen fixation. The dynamics of the symbiotic association between these two organisms were evaluated in the laboratory and greenhouse conditions. Peanuts were cultivated in pots inoculated with either Bradyrhizobium or Trichoderma or both to evaluate growth, development, and yield. The in vitro study results showed that seeds treated with Trichoderma had better germination and seedling biomass (p = 0.0008) compared to the other treatments. On the other hand, the results of greenhouse studies showed that seeds inoculated with both microbes, and those inoculated with Bradyrhizobium alone had higher dry biomass (p < 0.0001) as well as higher chlorophyll content (p < 0.0001) compared to the other treatments. Understanding of the interactive effects of fungal endophytes and rhizobial bacteria on plant growth and development will help in both the nutrient and disease management of Arachis hypogaea L.
1. Introduction
Legumes have a prominent role in sustaining agricultural productivity [1,2]. Pulses, legumes, and grasslands are grown because of their ability to interact with nitrogen-fixing bacteria [3], and they can also be used in rotation systems to manage pests, diseases, and weeds [4]. Small quantities of arable land and intensive agro-industrialization have led to the cultivation of crops on the same land without crop rotation. Such exhaustive peanut production is particularly widespread in the subtropical regions of Asia. The consecutive monoculture may, however, have caused a steady decline in productivity and performance, and increased disease susceptibility [5].
Castro et al. [6] reported increased yield when peanut crops were inoculated with rhizobium, but Walker et al. [7] and Huang et al. [8] noted that continuous cropping of peanut on the same piece of land resulted in reduced yield even when there was evidence of rhizobium inoculation. Daimon et al. [9] showed that fertilization with nitrate prevents root hair infection and initiation, development, and formation of nodules. Molecular analysis of peanut nodule formation suggests that suppression of root nodules is related to feedback regulation (autoregulation) by nitrates [10,11]. Moreover, peanut crop roots secrete a variety of primary metabolites of carbon, such as sugars, amino acids or phenolic acids, and more complex secondary compounds involved in plant defense and stimulatory or inhibitory interactions with other soil microorganisms [12,13,14].
The efficient development of peanut depends on its association with rhizobia which nodulate it, to enhance its nitrogen-fixing ability. Bradyrhizobium spp. is an agronomically significant gram-negative bacterium capable of forming root nodules on peanut roots and fixing atmospheric nitrogen [15]. Biological nitrogen-fixing (BNF) bacteria, such as Rhizobium and Bradyrhizobium species, produce auxins, cytokinins, abscisic acid, vitamins, riboflavin, lipochitooligosaccharides, and lumichrome that stimulate plant growth and nitrogen-fixation in legumes [16,17]. Bacteria that fix soil nitrogen are important contributors to primary productivity and their population is significantly affected by agrochemical applications. They fix large quantities of nitrogen biologically converting approximately 200 million tons of nitrogen to ammonia globally every year [18]. However, since the introduction of seed treatments for the prevention of fungal diseases, inoculation of peanuts with beneficial microorganisms has not been feasible especially in maintaining the viability of seeds and inoculants [19].
Plants use endophytic fungal products to overcome various abiotic and biotic stress conditions [20]. Endophytes colonizing and residing in internal plant parts are efficient in suppressing a broad variety of diseases in plants and they also promote plant growth [21]. Trichoderma species in several crops, including peanut [22], are efficient in controlling soil- and seed-borne fungal diseases [23]. The potential of T. harzianum as biocontrol agent in peanuts has been widely studied for reducing aflatoxin contamination by Aspergillus flavus [24], crown rot by A. niger [25], stem rot by Sclerotium rolfsii and Rhizoctonia solani [26], and brown root rot by Fusarium solani [27]. Trichoderma’s existence within the roots of treated crops has rendered it a mycorrhizal organism [28]. Specializing factors for the biocontrol behavior of Trichoderma include space and nutrient competitiveness, and the development of diffusible and/or reactive antibiotics and hydrolytic enzymes such as chitinase and β-1,3-glucanase [23]. Several mechanisms adopted by Trichoderma, such as the solubility of insoluble minor soil nutrients [29], synthesis of growth hormones [30], and increased ingestion and translocation of less available soil minerals [31,32,33], have been suggested to influence the development of plants. Some studies also reported the antagonistic properties of these secondary metabolites which act as growth promoting factors. Horace et al. [34] documented the isolation, recognition, and biological activity of 6-pentyl-apyrone—a secondary metabolite produced by T. harzianum that has been involved as regulators of plant development. It is believed that secondary metabolites of Trichoderma can serve as auxin-like compounds that usually have the optimum activity between 10−5 and 10−6 M, but can have inhibitory effect at higher concentrations [35,36,37].
The survival and role of soil microorganisms are important to the soil ecosystems because they participate in key processes, such as the cycling of minerals, organic matter breakdown, soil structure formation, and toxins elimination [38,39]. Li et al. [40] noted that the continuous secretion of exudates by peanut roots affected microbial diversity in the soil but did not contribute to the accumulation of phenolic acids levels which could be phytotoxic. Continuously secreted substances from peanut roots into the soil can stimulate the population of fungal pathogens and reduce the population of beneficial fungi, which could lead to aggravated disease of monocultural peanuts [41]. The use of nitrogen-fixing bacteria as biofertilizer should, therefore, be embraced to leverage various positive effects on land [42] whereas endophytes living and colonizing in inner plant habitats have been shown to be efficient in stimulating plant growth and depressing disease in a wide range of plants. This greenhouse study to understand the compatibility of Bradyrhizobium and Trichoderma on growth, development and yield of Arachis hypogaea L. was therefore based on the concept of enhancing agro-ecosystem with minimal dependence on agrochemical and energy inputs, in which synergetic microbial interactions provide the system with the mechanism to support soil fertility and crop production functions.
2. Materials and Methods
2.1. Peanut Cultivar and Microbial Strains Used
Peanut (Arachis hypogaea) seeds of variety Valencia C were obtained from the College of Agricultural, Consumer and Environmental Sciences, New Mexico State University. Commercially available endophytic fungal spores of “Trichoderma harzianum” ExceedHSD (1 × 108 spores/mL) was obtained from Vision Biologicals. The potting mixture, 2:2:1 topsoil: perlite: peat moss, was inoculated with 20 mL of 1% of the endophyte inoculum diluted with deionized water per pot. The potting mixture was subjected to a temperature of 100 °C under tarp cover for three weeks to ensure a complete elimination of soil borne pathogens. Treatment with the endophyte inoculum was performed after the potting medium conditioned to the normal greenhouse temperature of 25 °C.
Commercially available nitrogen-fixing rhizobium species Bradyrhizobium spp. (America’s Best Inoculant®) was obtained from Advanced Biological marketing. The peanut seeds were inoculated with 10% of the inoculum diluted with deionized water.
2.2. Viability of Inoculated Seeds In Vitro
The ability of the microorganisms to exhibit any kind of antagonistic properties during germination was evaluated in vitro by inoculating the sterilized peanut seeds with the above-specified concentrations of the inoculum. This included four different treatments in two trials with 10 seeds in each treatment and allowed to germinate in moist towel paper. The untreated seeds were considered the control (S), while the other treatments were seeds treated with Bradyrhizobium only (S + B), seeds with Trichoderma only (S + T), and seeds treated with both Bradyrhizobium and Trichoderma (S + B + T). To study the effects of these treatments on the germination of peanuts 10 days after inoculation (DAI), all the seeds with emerged radicle and open cotyledons were considered as germinated and their fresh biomass was recorded.
2.3. Greenhouse Studies to Assess the Microorganism Effect on Growth and Development
Peanut seeds were planted in 28 cm diameter by 23 cm height pots, filled with the soil mixture specified above. All the treatments were arranged in randomized complete block design (RCBD) to compensate and/or account for light gradient within the greenhouse as the pots were placed on one side of the greenhouse only. The greenhouse was maintained under natural light conditions and 60–70% RH. The temperatures varied from 28–35 °C during the day and 20–24°C in the night. There were four treatments with 36 pots per treatment in three replications for a total of 432 pots, with three seeds sown in each pot. Each individual treatment was placed in nine rows of pots with four pots per row. The four treatments included: seed only (S); seeds inoculated with Bradyrhizobium and Trichoderma (S + B + T); seeds inoculated with Bradyrhizobium only (S + B); seeds inoculated with Trichoderma only (S + T).
Bradyrhizobium inoculum was sprayed on the seeds and then sowed immediately after drying, whereas Trichoderma inoculum was added directly to the potting mixture after sowing. Plants were irrigated with deionized water so that no extraneous nutrients were added to the growing medium. The crops were harvested after 125 days of sowing and the dry biomass of plant parts along with number of survived plants and mature pods were recorded.
2.4. Data Analysis
The following parameters were measured for statistical analyses.
- Germination percentage (%).
- Seedling and plant heights (cm).
- Chlorophyll concentration using SPAD-502 Plus chlorophyll meter manufactured by Konica Minolta Inc., Japan (SPAD Units).
- Dry biomass of the harvested plant parts (g).
- Number of matured pods per pot.
- Number of harvested plants per pot.
- Seed biomass per treatment and 100-seed weight per treatment.
In addition, data collected from these parameters were used to calculate the following indices:
Harvest index (HI) = {Yield (seed or pod weight)/Biological yield (total dry weight)} × 100
Shelling percentage = (weight of seeds per treatment/weight of pods in the same treatment) × 100
Seedling vigor index = (germination percentage × mean shoot length) × 100 Relative growth rate
The data were analyzed by Analysis of Variance (ANOVA) in SAS version 9.4, and treatment means were compared using the Student–Newman–Keuls (SNK) method at 5% significance level.
3. Results
3.1. In Vitro Study of Germination of Seeds Inoculated with Bradyrhizobium and Trichoderma Assay 10 Days after Inoculation (DAI)
Statistical analysis indicated that the seeds inoculated with Trichoderma had a significantly higher germination rate (Table 1) and the seedling fresh biomass (Figure 1) (p = 0.0008) at 10 DAI.

Table 1.
Effect of inoculants on germination percentage and seedling biomass 10 days after inoculation (DAI) at in-vitro conditions.

Figure 1.
Results from in vitro germination and growth tests. Treatments include seed only as control, treatment inoculated with nitrogen fixing Bradyrhizobium bacteria, treatment co-inoculated with Bradyrhizobium and Trichoderma, and treatment inoculated with plant growth promoting endophytic fungi Trichoderma.
3.2. Greenhouse Studies
3.2.1. Germination and Seedling Vigor
Unlike in vitro results, inoculated treatments did not have any effect on both germination and seedling vigor (Table 2) on the 10th day after sowing (DAS). However, 20 DAS, treatments inoculated with Trichoderma showed an increase in germination (p = 0.0506). The study also concluded that seedling vigor index, 20 DAS, was not affected by the inoculation of Bradyrhizobium, Trichoderma or their co-inoculation. In fact, Table 6 shows that co-inoculation of Bradyrhizobium and Trichoderma could be antagonistic to the enhancement effect of Trichoderma on peanut seed germination.

Table 2.
Effect of inoculations on the germination and seedling vigor index.
3.2.2. Relative Growth Rate (RGR)
Plant height, calculated as a mean of five plants, was recorded every two weeks. The co-inoculated treatment and treatment inoculated with Bradyrhizobium had higher relative plant heights compared to the control and treatment inoculated with Trichoderma alone (p = 0.0008, Table 3).

Table 3.
Effect of individual and co-inoculation of Bradyrhizobium and Trichoderma on relative height growth and chlorophyll content.
3.2.3. Chlorophyll Content
Minolta developed the Soil Plant Analysis Development (SPAD) values as the proportional amount of chlorophyll present in the leaves [43]. Chlorophyll content analysis indicates that inoculation of peanut seedlings with Bradyrhizobium increased chlorophyll content (p < 0.0001, Table 3) and the difference was visible 10 weeks after sowing, as the leaves in the control and Trichoderma inoculated treatments started to turn yellow while the co-inoculated and Bradyrhizobium treatments were green. The SPAD readings were at peak 30 DAS; thereafter, they declined gradually in Bradyrhizobium inoculated treatments, but the decline was abrupt in the control and Trichoderma inoculated treatments (Figure 2).
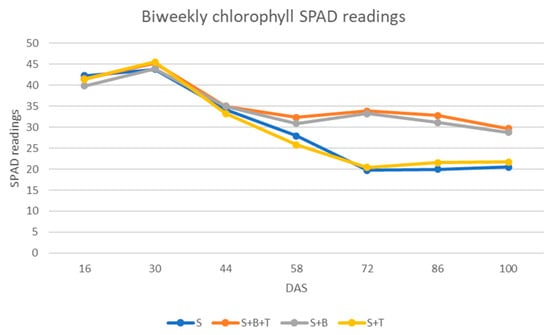
Figure 2.
Comparison of biweekly Soil Plant Analysis Development (SPAD) chlorophyll readings of uninoculated control, co-inoculated with Bradyrhizobium and Trichoderma, inoculated with Bradyrhizobium, and inoculated with Trichoderma. S—Seed only; S + B—Seed inoculated with Bradyrhizobium; S + B + T—Seed co-inoculated with Bradyrhizobium and Trichoderma; S + T—Seed inoculated with Trichoderma
3.2.4. Effect of Co-Inoculated Microorganisms on Dry Matter and Yield of Peanut Crop
Table 4 shows the treatment effects on the biomass of peanut plant parts (p < 0.0001) along with the number of plants that survived in each pot (p = 0.0488) at the time of harvest. Co-inoculation with Bradyrhizobium and Trichoderma has the highest survival rate, shoot and root biomass, followed by the treatment inoculated with Bradyrhizobium alone. These findings agree with Badawi et al. (2011) conclusions that, co-inoculations resulted to significant difference in the biomass of plants compared to the control. The number of pods, dry weight of pods, seeds and shells were higher in the treatment inoculated with Bradyrhizobium and treatment co-inoculated with Bradyrhizobium and Trichoderma (Table 4). Crops co-inoculated with Bradyrhizobium and Trichoderma, and those inoculated with Bradyrhizobium alone significantly increased biomass and yield of the species. Table 5 and Table 6 show the interactive effects of these microbes on peanut growth, development, and yield.

Table 4.
Effect of individual and co-inoculation of peanut with Bradyrhizobium and Trichoderma on plant survival, growth, development, and yield.

Table 5.
Effect of individual and co-inoculation of Bradyrhizobium and Trichoderma on peanut yield parameters.

Table 6.
Interactive effects of Bradyrhizobium and Trichoderma on growth, development, and yield of peanut.
3.2.5. Other Yield Traits: 100 Seed Weight, Shelling Percentage, Seed and Pod Harvest Index (HI)
In peanuts, kernel size analysis, computed with 100 seed weight [44], and the number of seeds per pod are used to determine the qualitative commercial value of the crop [45]. The weight of 100 peanut seeds was recorded after drying and the inoculations did not enhance the kernel mass (p = 0.0898). Moreover, shelling percentage was not enhanced either (p = 0.0770), whereas analysis of pod and seed HI data showed significant difference (p < 0.0001). Seed HI and pod HI, measures of yield efficiency in peanut, were assessed as a fraction of economic biomass to total crop biomass. Results of seed and pod harvest indices (Table 5) indicate that inoculation with the microorganisms separately and co-inoculation had no statistically significant change in the computed commercial attributes of peanut except the treatment with Trichoderma alone for pod and seed HI, and treatment with Bradyrhizobium alone for seed HI. However, the computed interaction effects of these microbes show the synergistic effect of Bradyrhizobium and Trichoderma in improving the commercial attributes of peanut (Table 6).
3.2.6. Interaction of Bradyrhizobium and Trichoderma in Peanut Growth, Development, and Yield
Colby [46] published an easily adoptable mathematical method to decipher synergism or antagonism due to the interaction of two or three combined chemicals in a cultural practice. He worked with a combination of herbicides, but the mathematical model is applicable to a variety of conditions. We adopted his equation to understand the interactive effects of these microorganisms on growth, development, and yield of peanut in greenhouse-controlled environment. Table 6 summarizes the effects of the microbes on the growth, development, and yield of peanut.
4. Discussion
Legume plants are closely linked to microbes that affect the plant features related to the provision of nutrients, plant growth, defenses, and abiotic stress [47]. It has been suggested that the thorough use of soil microbial potential is essential for better nutrient management [4]. The current research evaluated the effect of co-inoculations of nitrogen-fixing rhizobia and plant growth-promoting (PGP) endophyte in the enhancement of peanut germination, growth, development and yield under greenhouse conditions. The data show that the co-inoculation of Bradyrhizobium and Trichoderma had enhanced effect on growth, development, and yield of peanut.
The results from the in vitro germination tests were consistent with the observations of Yedidia et al. [48] on cucumber. They observed that Trichoderma enhanced seedling emergence and dry weight of cucumber five days after inoculation. The effect of Trichoderma on peanut germination was statistically significant from the control and, in fact, the co-inoculation of Trichoderma and Bradyrhizobium may be antagonistic to peanut germination (Table 6). This result makes sense since seed germination requires primarily abiotic conditions of conducive temperature for the species, moisture and oxygen. These conditions also favor the growth of some microbes which could explain the germination enhancement observed in this study.
From the greenhouse studies the trends in relative plant height (RPH) synchronized with the physiological maturity of the crop and the highest RPH was recorded 76 DAS and thereafter, height growth declined gradually indicating crop reproductive maturity (Figure 3). The efficiency of nitrogen fixation in peanuts resulted in the accumulation of nitrogen in plant tissues which in turn reflected the synthesis of chlorophyll [49]. SPAD readings for the Bradyrhizobium inoculated treatments are higher compared to others and this might be because of the Bradyrhizobium’s fundamental function of biological nitrogen fixation [50] (Table 3). Treatments co-inoculated with Bradyrhizobium enhanced the synthesis of chlorophyll and height growth, resulting in significant increases in plant dry weight and yield [51]. Although the co-inoculated treatment had similar statistical effects as the treatment with Bradyrhizobium alone in all measured growth and development parameters of peanut except seed HI, but the interactive test showed that co-inoculation had an advantageous effect on plant biomass which can be correlated to the PGP- characteristics of Trichoderma such as stimulating substantial and vigorous plant roots and synthesis of plant hormones (31,32) along with biological nitrogen fixation by Bradyrhizobium (see Table 6). This postulation supports the highest mean biomass of the shoot, root, pod, seed, and shell in the co-inoculated treatment compared to the other treatments. Although the results of the 100 seed weight, shelling percentage, seed HI and pod HI showed no statistical difference between the treatments, the interactive effects of co-inoculation with Bradyrhizobium and Trichoderma had qualitative value in peanut. This is an indication of added market value to yield when peanut cropping adopts co-inoculation with these microbes in cultural practices. Furthermore, seed HI was significantly higher in the co-inoculation treatment than the treatment with Bradyrhizobium alone. It is important to note the sensitivity of Colby’s [46] mathematical interactive method on the qualitative value of the crop. Statistical analysis showed no significant difference on the qualitatively computed information about the species but the slight differences between the treatments were discernable by Colby [46].
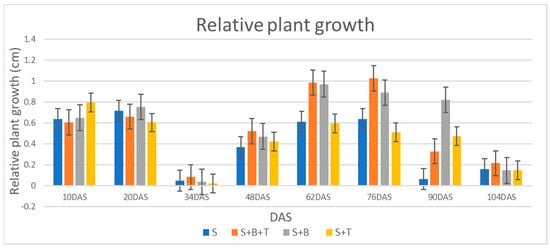
Figure 3.
Comparison of relative plant heights of uninoculated control, treatment co-inoculated Bradyrhizobium and Trichoderma, treatment inoculated with Bradyrhizobium only, and treatment inoculated with Trichoderma alone. RPH in centimeter was analyzed at 14-day interval.
Although Badawi et al. [51] concluded that co-inoculation resulted to increased peanut growth and development in field conditions, this study, performed in controlled pathogen free conditions, confirms their results. Additionally, this study revealed that plant growth promotion is not a common function of Trichoderma [52].
Nieto-Jacobo et al. [53] demonstrated that the production of IAA was dependent on Trichoderma strains as influenced by different external stimuli. In addition, various Trichoderma species have been shown to affect plant growth and the growth of other pathogens in conducive soil environments [53,54,55,56,57]. However, a range of effects by Trichoderma secondary metabolites have been documented [54]. These effects range from no response to sensitive response by plants.
The positive interaction of Bradyrhizobium and Trichoderma increased the various yield attributes when compared to the control, as indicated by the interactive effects of these microorganisms (Table 6). Zhang et al. [58] noted that Trichoderma inoculation increased the secretion of flavonoids by peanut roots and this induced rhizobial nod genes leading to improved Bradyrhizobial colonization. Crops co-inoculated with Bradyrhizobium and Trichoderma, and those inoculated with Bradyrhizobium alone increased the biomass and yield of the species. Increase in biomass and yield demonstrate the compatibility between microorganisms and the synergism among them to promote and enhance crop growth, development, and yield.
5. Conclusions
This study assessed the growth promoting properties of co-inoculation of microorganisms in peanut crop production. While co-inoculations are not a usual practice, this work confirms that co-inoculation with Bradyrhizobium and Trichoderma enhanced growth, development, and yield in Arachis hypogaea. The inoculations improved growth attributes of the species, such as chlorophyll content, relative height growth, survival rate, biomass, and yield, as well as parameters such as 100 seed weight, shelling percentage, seed HI and pod HI. Moreover, the results of the study showed that the beneficial effects of co-inoculation occurred at seedling growth stage, leading us to conclude that the timing for co-inoculation of peanut with Bradyrhizobium and Trichoderma should be performed at seedling emergence. The findings of this study gave a better understanding of the effects of trilateral symbiotic association between plant–rhizobia–endophyte in leguminous crops. These associations will help in developing agro-ecosystems with minimal dependence on agrochemical inputs, and they simulate natural ecological interactions between crop plants and soil organisms.
Author Contributions
A.O.A. and R.T.K.R.N. conceived, designed, and performed the experiment. R.T.K.R.N. collected the data and analyzed it with A.O.A. They also wrote the manuscript. S.N. contributed lab materials, supervised research progress and edited the final manuscript. He also provided lab space for the research. All authors have read and agreed to the published version of the manuscript.
Funding
Funding for this research was provided by the Welhausen Family Student Scholarship fund. Ravi Teja Kumar Reddy Neelipally’s study at the College of Agriculture, Natural Resources & Human Sciences, Texas A&M University-Kingsville was supported by this fund.
Acknowledgments
The authors acknowledge the support of Greta Schuster for her help to procure the peanut seeds used for the study.
Conflicts of Interest
The authors declare no conflict of interest of any kind. The funding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
References
- Giller, K.E.; Wilson, K.J. Nitrogen Fixation in Tropical Cropping Systems; CAB International: Wallingford, UK, 1991; pp. 167–237. [Google Scholar]
- Graham, P.H.; Vance, C.P. Nitrogen Fixation in Perspective: An Overview of Research and Extension Needs. Field Crops Res. 2000, 65, 93–106. [Google Scholar] [CrossRef]
- Vance, C.P. Enhanced Agricultural Sustainability through Biological Nitrogen Fixation. In Biological Fixation of Nitrogen for Ecology and Sustainable Agriculture; Legocki, A., Bothe, H., Puhler, A., Eds.; Springer: Berlin, Germany, 1997; pp. 179–186. [Google Scholar]
- Howieson, J.G.; O’Hara, G.W.; Carr, S.J. Changing Roles for Legumes in Mediterranean Agriculture: Developments from an Australian Perspective. Field Crops Res. 2000, 65, 107–122. [Google Scholar] [CrossRef]
- Li, P.D.; Dai, C.C.; Wang, X.X.; Zhang, T.L.; Chen, Y. Variation of Soil Enzyme Activities and Microbial Community Structure in Peanut Monocropping System in Subtropical China. African J. Agric. Res. 2012, 7, 1870–1879. [Google Scholar]
- Castro, S.; Permigiani, M.; Vinocur, M.G.; Fabra, A. Nodulation in peanut (Arachis hypogaea L.) roots in the presence of native and inoculated rhizobia strains. Appl. Soil Ecology. 1999, 13, 39–44. [Google Scholar] [CrossRef]
- Walker, M.E.; Minton, N.A.; Dowler, C.C. Effects of herbicides, a nematicide and Rhizobium inoculant on yield, chemical composition, and nodulation of Starr peanuts (Arachis hypogaea L.). Peanut Sci. 1976, 3, 49–51. [Google Scholar] [CrossRef][Green Version]
- Huang, Y.Q.; Han, X.R.; Yang, J.F.; Liang, C.H.; Zhan, X.M. Autotoxicity of peanut and identification of phytotoxic substances in rhizosphere soil. Allelopath. J. 2013, 31, 297–308. [Google Scholar]
- Daimon, H.; Hori, K.; Shimizu, A.; Nakagawa, M. Nitrate-Induced Inhibition of Root Nodule Formation and Nitrogenase Activity in the Peanut (Arachis hypogaea L.). Plant Prod. Sci. 1999, 2, 81–86. [Google Scholar] [CrossRef][Green Version]
- Daimon, H.; Yoshioka, M. Responses of root nodule formation and nitrogen fixation activity to nitrate in a spit-root system in Peanut (Arachis hypogaea L.). J. Agron. Crop. Sci. 2001, 187, 89–95. [Google Scholar] [CrossRef]
- Ferguson, B.J.; Indrasumunar, A.; Hayashi, S.; Lin, M.; Lin, Y.; Reid, D.E.; Gresshoff, P.M. Molecular analysis of legume nodule development and autoregulation. J. Integr. Plant Biol. 2010, 52, 61–76. [Google Scholar] [CrossRef]
- Bertin, C.; Yang, X.; Weston, L. The role of root exudates and allelochemicals in the rhizosphere. Plant. Soil. 2003, 256, 67–83. [Google Scholar] [CrossRef]
- Jones, D.L.; Hodge, A.; Kuzyakov, Y. Plant and mycorrhizal regulation of rhizodeposition. New Phytol. 2004, 163, 459–480. [Google Scholar] [CrossRef]
- Bais, H.P.; Weir, T.L.; Perry, L.G.; Gilroy, S.; Vivanco, J.M. The role of root exudates in rhizosphere interactions with plants and other organisms. Ann. Rev. Plant. Biol. 2006, 57, 233–266. [Google Scholar] [CrossRef] [PubMed]
- Isawa, T.; Sameshima, R.; Mitsui, H.; Minamisawa, K. IS1631 occurrence in Bradyrhizobium japonicum highly reiterated sequence-possessing strains with high copy numbers of repeated sequences RSα and RSβ. Appl. Environ. Microbiol. 1999, 65, 3493–3501. [Google Scholar] [CrossRef] [PubMed]
- Hardarson, G. Methods for enhancing symbiotic nitrogen fixation. Plant. Soil. 1993, 152, 1–17. [Google Scholar] [CrossRef]
- Herridge, D.F.; Marcellos, H.; Felton, W.L.; Turner, G.L.; Peoples, M.B. Legume N2 Fixation an Efficient Source of N for Cereal Production, Nuclear Methods in Soil-Plant Aspects of Sustainable Agriculture (Proc. Sem. Colombo, 1993); IAEA: Vienna, Austria, 1993. [Google Scholar]
- Glazer, N.; Nikaido, H. Fundamentals of applied microbiology. In Microbial Biotechnology, 2nd ed.; Barton, L., Hamilton, A., Eds.; Cambridge University Press: Cambridge, UK, 2007; p. 29. [Google Scholar]
- O’Callaghan, M. Microbial inoculation of seed for improved crop performance: Issues and opportunities. Appl. Microbiol. Biotechnol. 2016, 100, 5729–5746. [Google Scholar] [CrossRef]
- Rodriguez, R.; Redman, R. More than 400 million years of evolution and some plants still can’t make it on their own: Plant stress tolerance via fungal symbiosis. J. Exp. Bot. 2008, 59, 1109–1114. [Google Scholar] [CrossRef]
- Manjula, K.; Singh, S.D.; Kishore, G.K. Role of endophytic bacteria in biological control of plant diseases. Annu. Rev. Plant. Pathol. 2002, 1, 231–252. [Google Scholar]
- Podile, A.R.; Kishore, G.K. Biological control of peanut disease. In Biological Control of Crop Diseases; Gnanamanickam, S.S., Ed.; Marcel Dekker, Inc.: New York, NY, USA, 2002; pp. 131–160. [Google Scholar]
- Kubicek, C.P.; Mach, R.L.; Peterbauer, C.K.; Lorito, M. Trichoderma: From genes to biocontrol. J. Plant. Pathol. 2001, 83, 11–23. [Google Scholar]
- Emma, W.G.; Simeon, O.K. The Use of Trichoderma harzianum and T. viride as Potential Biocontrol Agents against Peanut Microflora and Their Effectiveness in Reducing Aflatoxin Contamination of Infected Kernels. Biotechnology 2008, 7, 439–447. [Google Scholar]
- Kishore, G.K.; Pande, S.; Rao, J.N.; Podile, A.R. Biological Control of Crown Rot of Groundnut by Trichoderma Harzianum and T. viride. Int. Arachis Newslett. 2001, 21, 39–40. [Google Scholar]
- Eald, Y.; Barak, R.; Chet, I. Parasitism of sclerotia of Sclerotium rolfsii by Trichoderma harzianum. Soil Biol. Biochem. 1984, 16, 381–386. [Google Scholar] [CrossRef]
- Federico, G.; Rojo, M.M.; Reynoso, M.F.; Sofía, N.; Chulze, A.M.T. Biological control by Trichoderma species of Fusarium solani causing peanut brown root rot under field conditions. Crop Prot. 2007, 26, 549–555. [Google Scholar]
- Neumann, B.; Laing, M. Trichoderma: An ally in the quest for soil system sustainability. In Biological Approaches to Sustainable Soil Systems; Uphoff, N., Ball, A.S., Fernandes, E., Herren, H., Husson, O., Laing, M., Palm, C., Pretty, J., Sanchez, P., Sanginga, N., et al., Eds.; CRC Press, Taylor & Farncis Group: Boca Raton, FL, USA; London, UK; New York, NY, USA, 2006; pp. 491–500. [Google Scholar]
- Altomare, C.; Norvell, W.A.; Bjorkman, T.; Harman, G.E. Solubilization of phosphate and micronutrients by the plant-growth-promoting and biocontrol fungus Trichoderma harzianum Rifai 1295–22. Appl. Environ. Microbiol. 1999, 65, 2926–2933. [Google Scholar] [CrossRef] [PubMed]
- Windham, M.T.; Elad, Y.; Baker, R. A mechanism for increased plant growth induced by Trichoderma spp. Phytopathology 1986, 76, 518–521. [Google Scholar] [CrossRef]
- Baker, R. Improved Trichoderma spp. for promoting crop productivity. Trends Biotechnol. 1989, 7, 34–38. [Google Scholar] [CrossRef]
- Kleifeld, O.; Chet, I. Trichoderma—Plant interaction and its effect on increased growth response. Plant. Soil 1992, 144, 267–272. [Google Scholar] [CrossRef]
- Inbar, J.; Abramsky, M.; Chet, I. Plant growth enhancement and disease control by Trichoderma harzianum in vegetable seedlings under commercial conditions. Eur. J. Plant. Pathol. 1994, 100, 337–346. [Google Scholar] [CrossRef]
- Horace, G.; Cutler, R.H.; Cox, F.G.; Crumley, P.D.C. 6-Pentyl-α-pyrone from Trichoderma harzianum: Its Plant Growth Inhibitory and Antimicrobial Properties. Agric. Biol. Chem. 1986, 50, 2943–2945. [Google Scholar]
- Thimann, K.V. On the Nature of Inhibitions Caused by Auxin. Am. J. Bot. 1937, 7, 407–412. [Google Scholar] [CrossRef]
- Cleland, R. The dosage-response curve for auxin-induced cell elongation: A reevaluation. Planta 1972, 104, 1–9. [Google Scholar] [CrossRef]
- Brenner, M.L. Modern methods for plant growth substance analysis. Ann. Rev. Plant. Physiol. 1981, 32, 511–538. [Google Scholar] [CrossRef]
- Brussaard, L.; Ruiter, P.C.; Brown, G.G. Soil biodiversity for agricultural sustainability. Agric. Ecosys. Environ. 2007, 121, 233–244. [Google Scholar] [CrossRef]
- Van Elsas, J.D.; Jansson, J.K.; Trevors, J.T. Modern Soil Microbiology, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2007; pp. 177–210. [Google Scholar]
- Li, X.G.; Ding, C.F.; Hua, K.; Zhang, T.L.; Zhang, Y.N.; Zhao, L.; Yang, Y.R.; Liu, J.G.; Wang, X.X. Soil sickness of peanuts is attributable to modifications in soil microbes induced by peanut root exudates rather than to direct allelopathy. Soil Biol. Biochem. 2014, 78, 149–159. [Google Scholar] [CrossRef]
- Li, C.; Li, X.; Kong, W.; Wu, Y.; Wang, J. Effect of monoculture soybean on soil microbial community in the Northeast China. Plant. Soil. 2010, 330, 423–433. [Google Scholar] [CrossRef]
- Berg, G. Plant-microbe interactions promoting plant growth and health: Perspectives for controlled use of microorganisms in agriculture. Appl. Microbiol. Biotechnol. 2009, 84, 11–18. [Google Scholar] [CrossRef]
- Shenker, M.; Oliver, I.; Helmann, M.; Hadar, Y.; Chen, Y. Utilization by tomatoes of iron mediated by a siderophore produced by Rhizopus arrhizus. J. Plant. Nutr. 1992, 15, 2173–2182. [Google Scholar] [CrossRef]
- Zamurrad, M.; Tariq, M.; Shah, F.H.; Subhani, A.; Ijaz, M.; Iqbal, M.S.; Koukab, M. Performance based evaluation of groundnut genotypes under medium rainfall conditions of chakwal. J. Agric. Food Appl. Sci. 2013, 1, 9–12. [Google Scholar]
- Jeyaramraja, P.R.; Woldesenbet, F. Characterization of yield components in certain groundnut (Arachis hypogaea L.) varieties of Ethiopia. J. Exp. Biol. Agric. Sci. 2014, 2, 592–596. [Google Scholar]
- Colby, S. Calculating Synergistic and Antagonistic Responses of Herbicide Combinations. Weeds 1967, 15, 20–22. [Google Scholar] [CrossRef]
- Bulgarelli, D.; Rott, M.; Schlaeppi, K.; Themaat, E.V.L.; Ahmadinejad, N.; Assenza, F. Revealing structure and assembly cues for Arabidopsis root-inhabiting bacterial microbiota. Nature 2012, 488, 91–95. [Google Scholar] [CrossRef]
- Yedidia, I.; Benhamou, N.; Chet, I. Induction of defense response in cucumber plants (Cucumis sativus L.) by the biocontrol agent Trichoderma harzianum. Appl. Environ. Microbiol. 1999, 65, 1061–1070. [Google Scholar] [CrossRef] [PubMed]
- Nageswara, R.R.C.; Talwar, H.S.; Wright, G.C. Rapid assessment of specific leaf area and leaf nitrogen in peanut (Arachis hypogaea L.) using a chlorophyll meter. J. Agron. Crop. Sci. 2001, 186, 175–182. [Google Scholar] [CrossRef]
- Mekhemar, G.A.A.; Shaaban, M.; Ragab, A.A.; Biomy, A.M.M. Response of faba bean to inoculation with Rhizobium leguminosarum bv. Viceae and plant growth promoting rhizobacteria under newly reclaimed soils. J. Appl. Sci. 2005, 20, 126–144. [Google Scholar]
- Badawi, F.S.F.; Biomy, A.M.M.; Desoky, A.H. Peanut plant growth and yield as influenced by co-inoculation with Bradyrhizobium and some rhizo-microorganisms under sandy loam soil conditions. Ann. Agric. Sci. 2011, 56, 17–25. [Google Scholar] [CrossRef]
- Lee, S.; Yap, M.; Behringer, G.; Hung, R.; Bennett, J.W. Volatile organic compounds emitted by Trichoderma species mediate plant growth. Fungal Biol. Biotechnol. 2016, 3, 7. [Google Scholar] [CrossRef]
- Nieto-Jacobo, M.F.; Steyaert, J.M.; Salazar-Badillo, F.B.; Nguyen, D.V.; Rostas, M.; Braithwaite, M.; De Souza, J.T.; Jimenez-Bremont, J.F.; Ohkura, M.; Stewart, A.; et al. Environmental Growth Conditions of Trichoderma spp. Affects Indole Acetic Acid Derivatives, Volatile Organic Compounds, and Plant Growth Promotion. Front. Plant. Sci. 2017, 8, 102. [Google Scholar] [CrossRef]
- McFadden, A.G.; Sutton, J.C. Relationships of populations of Trichoderma spp. in soil to disease in maize. Can. J. Plant. Sci. 1975, 55, 579–586. [Google Scholar] [CrossRef]
- Yang, H.; Powell, N.T.; Baker, K.R. The influence of Trichoderma harzianum on root-knot Fusarium wilt complex in cotton. J. Nematol. 1976, 8, 81–86. [Google Scholar]
- Komon-Zelazowska, M.; Bissett, J.; Zafari, D.; Hatvani, L.; Manczinger, L.; Woo, S.; Lorito, M.; Kredics, L.; Kubicek, C.P.; Druzhinina, I.S. Genetically closely related but phenotypically divergent Trichoderma species cause green mold disease in oyster mushroom farms worldwide. Appl. Environ. Microbiol. 2007, 73, 7415–7426. [Google Scholar] [CrossRef]
- Aly, A.A.; Hussein, E.M.; Allam, A.D.A.; Amein, A.M.; El-Samawaty, A.M.A. Use of Trichoderma spp., Aspergillus spp., and Penicillium spp. to suppress damping-off of cotton seedlings. J. Agri. Sci. Mansoura Univ. 2000, 25, 7611–7619. [Google Scholar]
- Zhang, W.; Wang, H.W.; Wang, X.X.; Xie, X.G.; Siddikee, M.A.; Xu, R.S.; Dai, C.C. Enhanced nodulation of peanut when co-inoculated with fungal endophyte Phomopsis liquidambari and bradyrhizobium. Plant Phys. Biochem. 2016, 98, 1–11. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).