Molecular Association Between Short Linear Maltodextrin and Ferulic Acid and the Exploration of Its Applicability
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
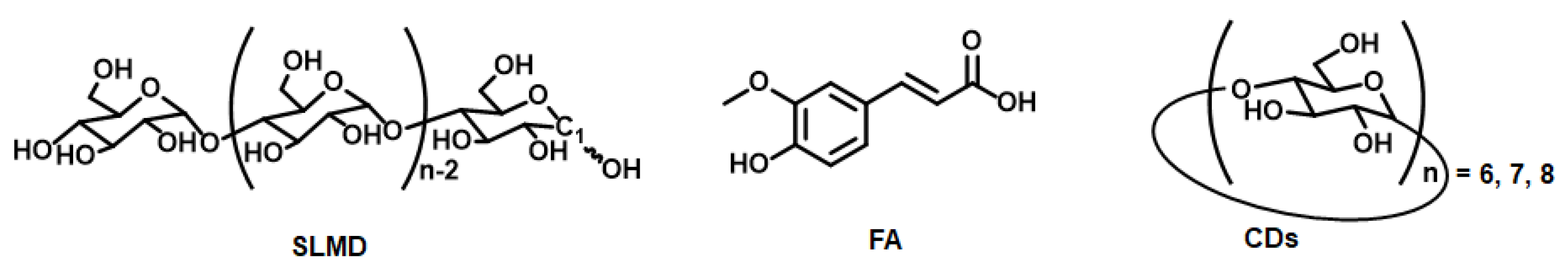
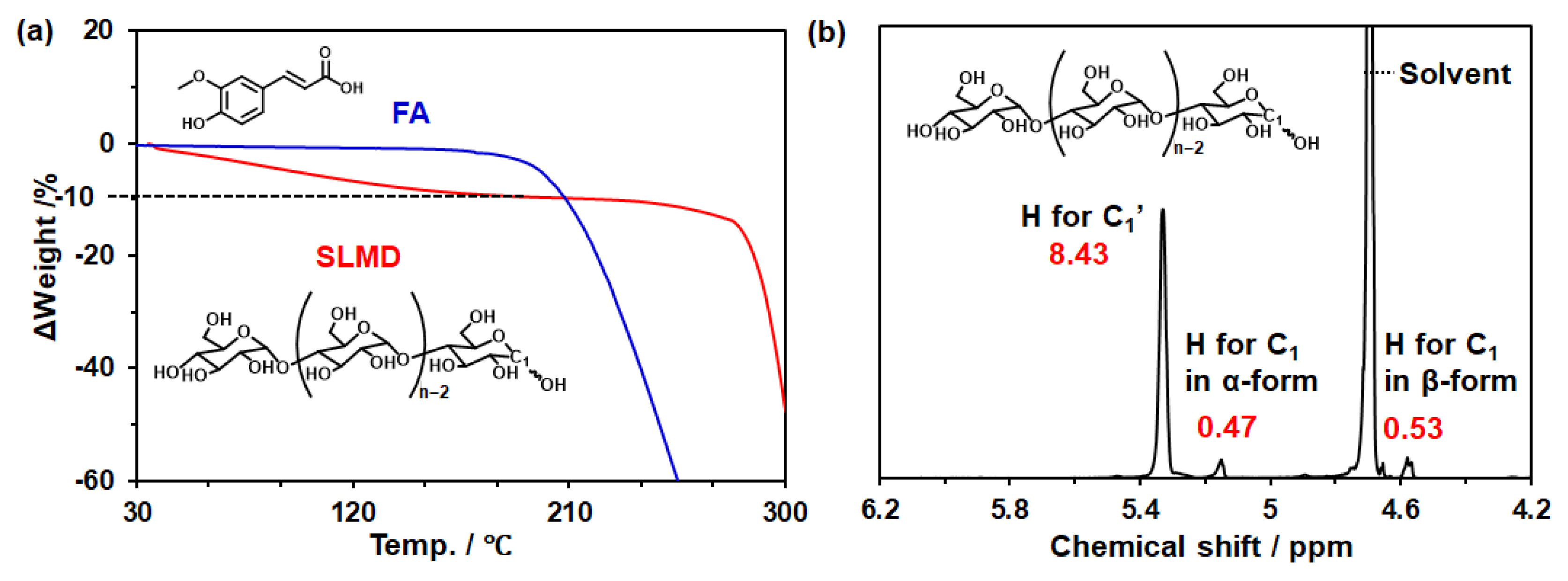
3.1. Confirmation of the State of Commercial Samples
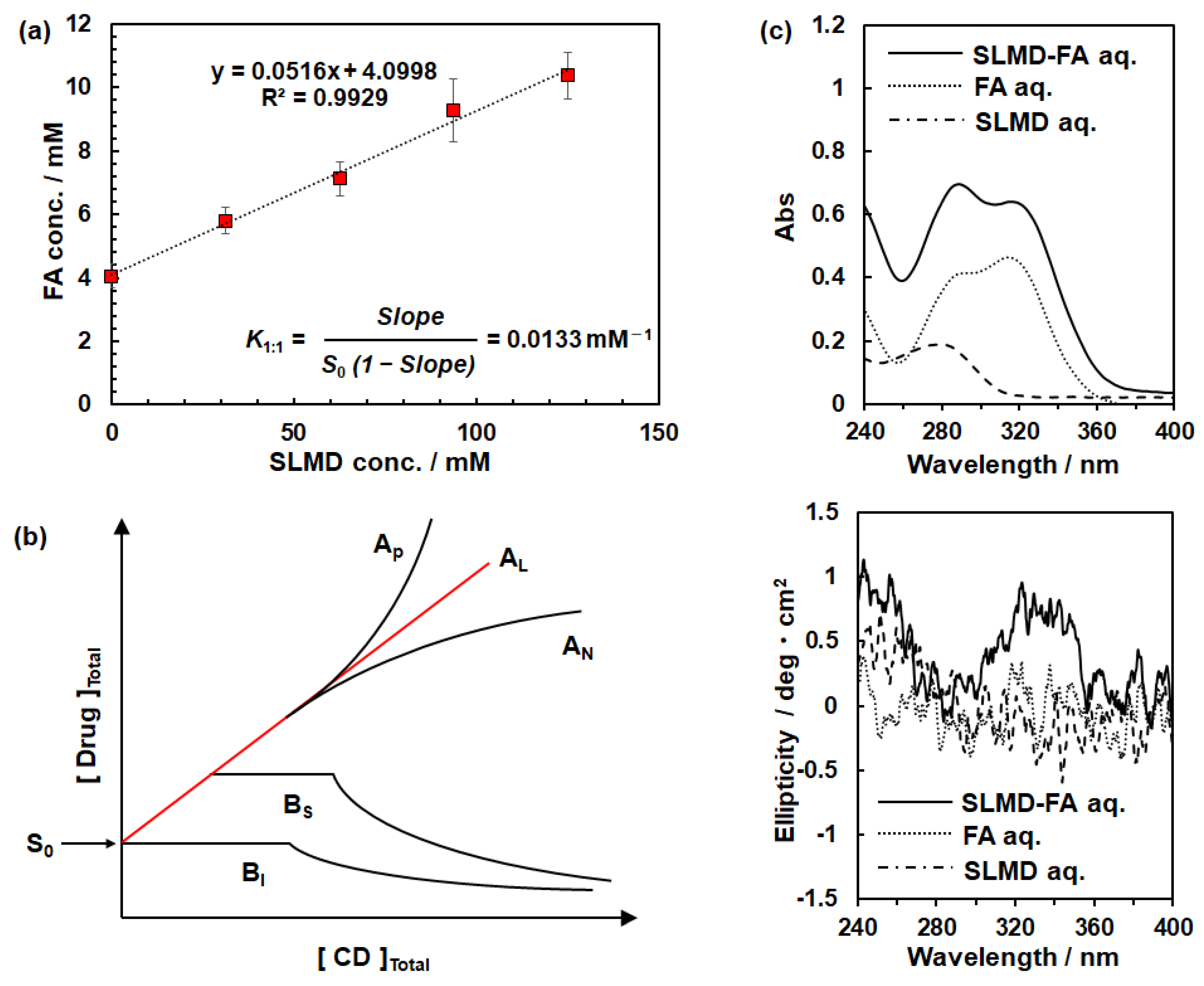
3.2. Confirmation of Complexation from Solubility Test and Circular Dichroism
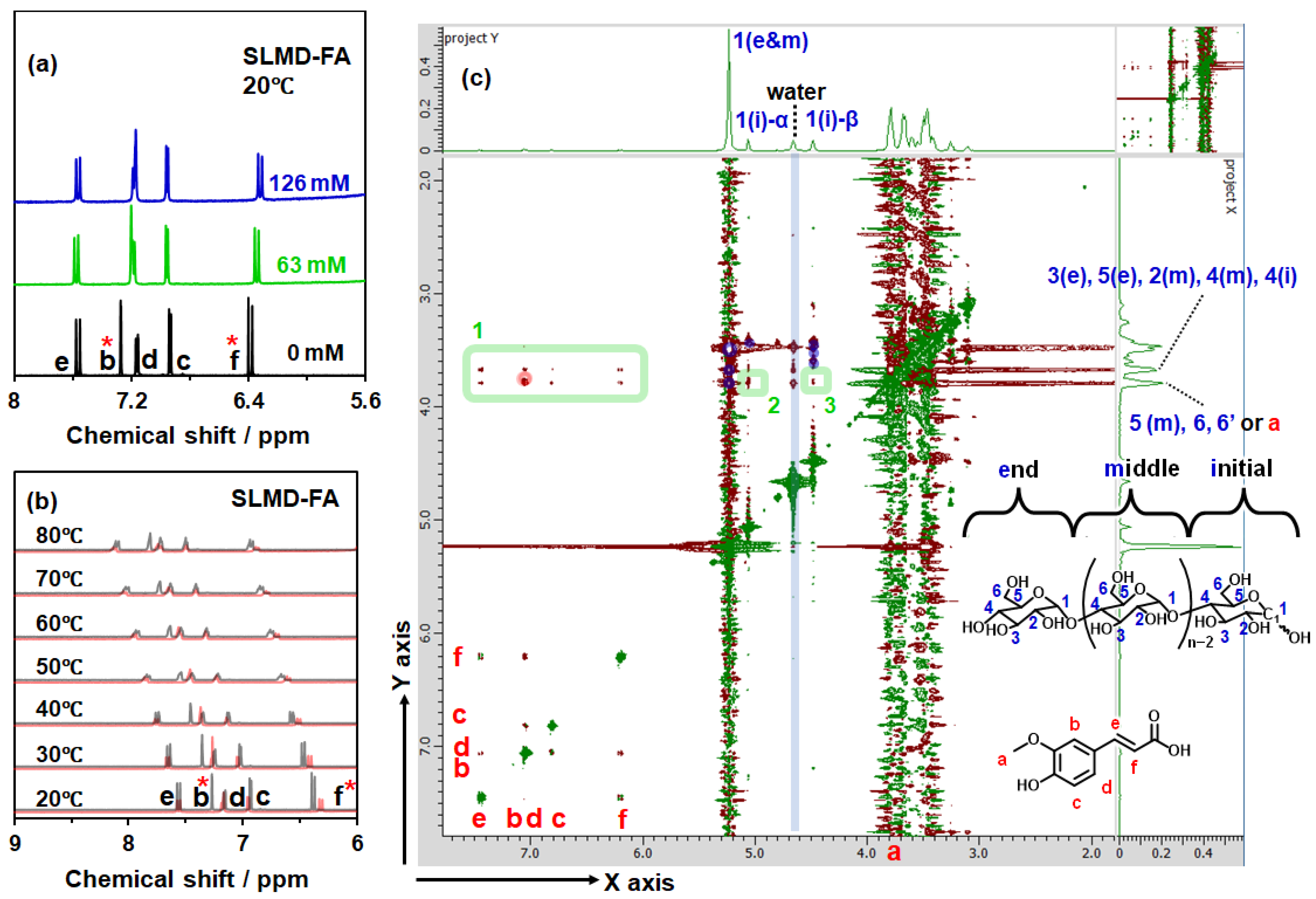
3.3. Confirmation of Complexation from Nuclear Magnetic Resonance Spectroscopy
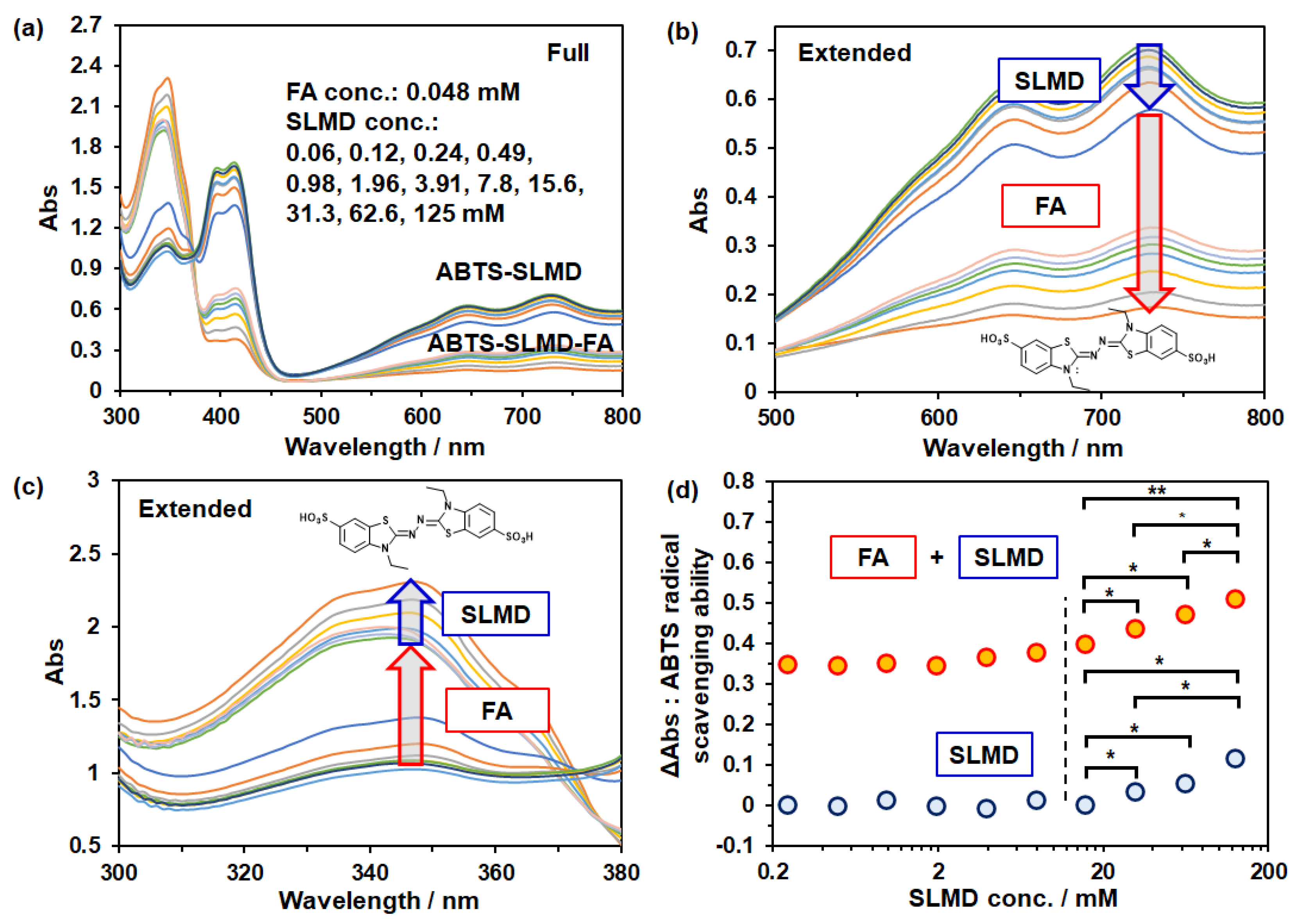
3.4. ABTS Radical Scavenging
3.5. Investigation of the Complexation Between SLMD and FA in Solid State
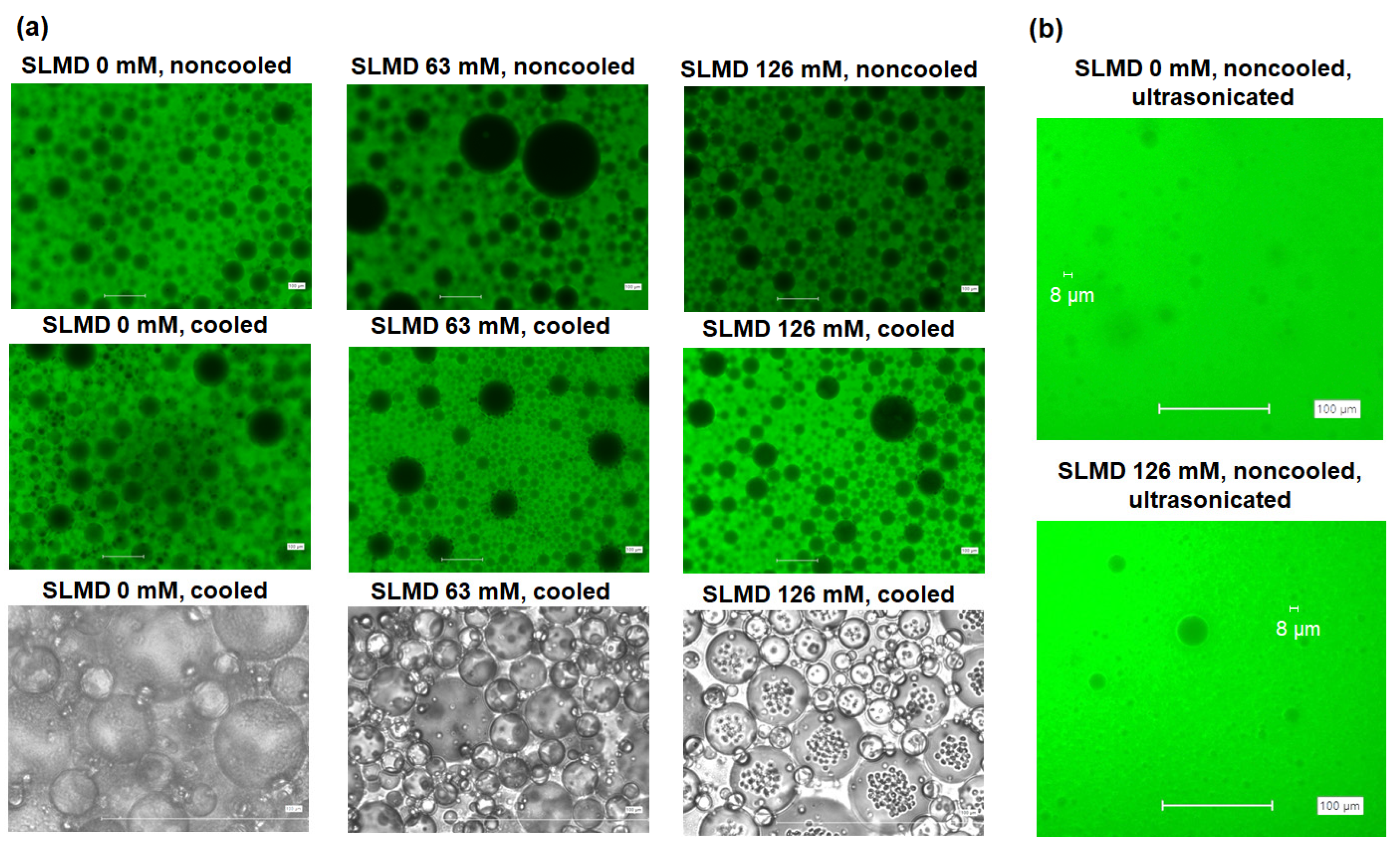
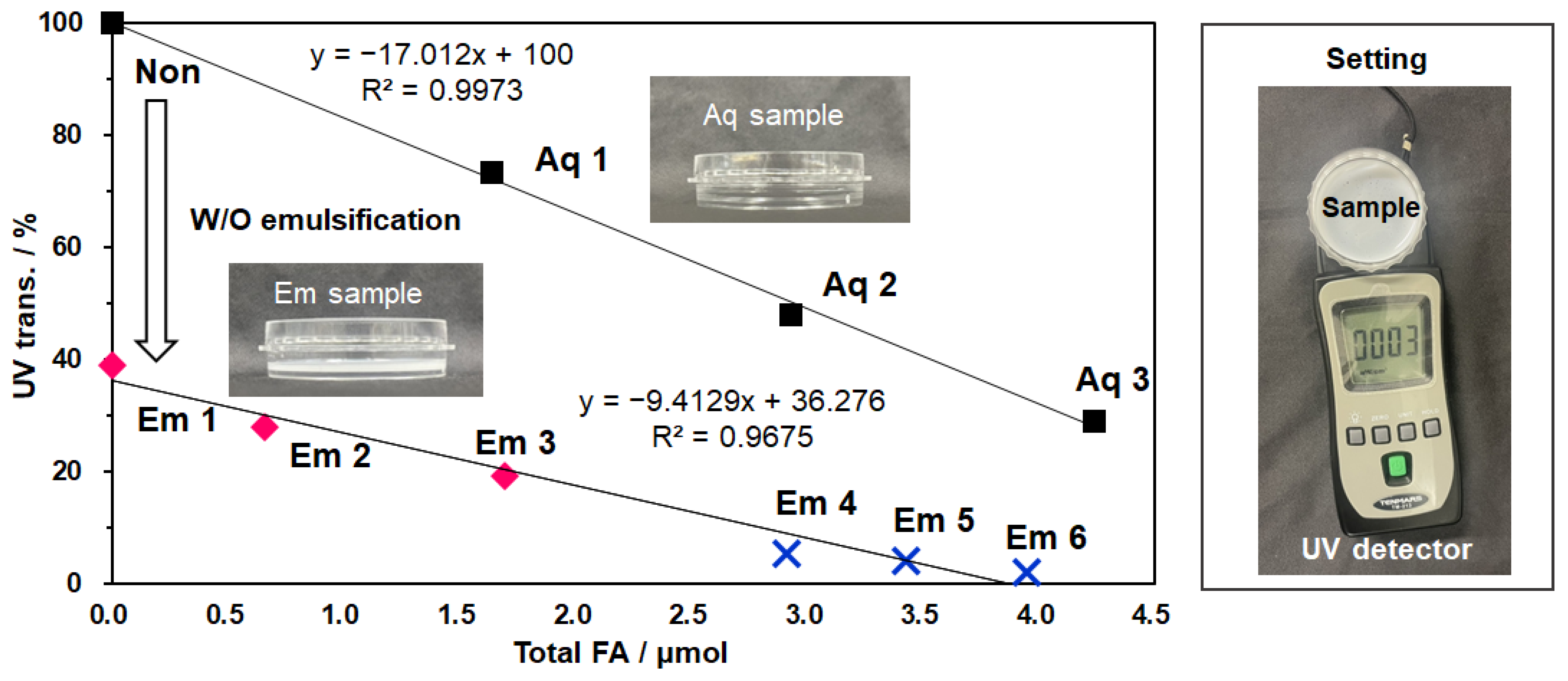
3.6. W/O Emulsion Systems
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SLMD | Short linear maltodextrin |
| FA | Ferulic acid |
| UV | ultraviolet |
| ABTS | 2,2′-Azinobis(3-ethylbenzothiazoline-6-sulfonic Acid Ammonium Salt) |
| CD | Cyclodextrin |
| TGO | Triethylhexanoin |
| NMR | Nuclear magnetic resonance |
| ROESY | Rotating-frame nuclear Overhauser effect spectroscopy |
| TG | Thermogravimetric |
| DSC | Differential scanning calorimetry |
| PXRD | Powder X-ray diffraction |
References
- Pérez, S.; Bertoft, E. The molecular structures of starch components and their contribution to the architecture of starch granules: A comprehensive review. Starch/Stärke 2010, 62, 389–420. [Google Scholar] [CrossRef]
- Ai, Y.; Jane, J.L. Gelatinization and rheological properties of starch. Starch/Stärke 2015, 67, 213–224. [Google Scholar] [CrossRef]
- Magallanes-Cruz, P.A.; Flores-Silva, P.C.; Bello-Perez, L.A. Starch structure influences its digestibility: A review. J. Food Sci. 2017, 82, 2016–2023. [Google Scholar] [CrossRef] [PubMed]
- Cereda, M.P. Starch hydrolysis: Physical, acid, and enzymatic processes. In Starch Industries: Processes and Innovative Products in Food and Non-Food Uses; Cereda, M.P., Vilpoux, O.F., Eds.; Academic Press: New York, NY, USA, 2023; Volume 3, pp. 75–113. [Google Scholar]
- Sun, L.; Miao, M. Dietary polyphenols modulate starch digestion and glycaemic level: A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 541–555. [Google Scholar] [CrossRef]
- Ren, X.; Qin, M.; Zhang, M.; Zhang, Y.; Wang, Z.; Liang, S. Highland barley polyphenol delayed the in vitro digestibility of starch and amylose by modifying their structural properties. Nutrients 2022, 14, 3743. [Google Scholar] [CrossRef]
- Fan, H.; Huang, W.; Sun, L.; Chen, Z.; Wen, Y.; Li, H.; Wang, J.; Sun, B. Modulation of starch-polyphenol complex thermal stability and antioxidant activity: The role of polyphenol structure. Int. J. Biol. Macromol. 2025, 206, 141434. [Google Scholar] [CrossRef]
- Patiño-Rodríguez, O.; Bello-Pérez, L.A. Effect of the gelatinization level of normal maize starch under extrusion on ferulic acid–starch complex formation. J. Cereal Sci. 2024, 117, 103905. [Google Scholar] [CrossRef]
- Liu, Z.; Deng, N.; Luo, S.; Liu, C.; Hu, X. Fermentation of resistant starch from the starch-ferulic acid inclusion complex compared with high-amylose corn starch. Int. J. Biol. Macromol. 2023, 246, 125647. [Google Scholar] [CrossRef] [PubMed]
- Ammala, A. Biodegradable polymers as encapsulation materials for cosmetics and personal care markets. Int. J. Cosmet. Sci. 2013, 35, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Yazid, N.S.M.; Abdullah, N.; Muhammad, N.; Matias-Peralta, H.M. Application of starch and starch-based products in food industry. J. Sci. Technol. 2018, 10, 144–174. [Google Scholar] [CrossRef]
- Garcia, M.A.V.T.; Garcia, C.F.; Faraco, A.A.G. Pharmaceutical and biomedical applications of native and modified starch: A review. Starch-Stärke 2020, 72, 1900270. [Google Scholar] [CrossRef]
- Olivato, J.B. Starch: A natural, safe, and multifunctional ingredient for cosmetic formulations. In Starch Industries: Processes and Innovative Products in Food and Non-Food Uses; Cereda, M.P., Vilpoux, O.F., Eds.; Academic Press: New York, NY, USA, 2023; Volume 3, pp. 255–269. [Google Scholar]
- Kawano, A.; Yamamoto, T.; Shinagawa, Y.; Hanashiro, I.; Yoshida, H. Enzymatic synthesis of a novel short linear maltodextrin from starch. J. Appl. Glycosci. 2025, 72, 7201101. [Google Scholar] [CrossRef]
- Surendren, A.; Mohanty, A.K.; Liu, Q.; Misra, M. A review of biodegradable thermoplastic starches, their blends and composites: Recent developments and opportunities for single-use plastic packaging alternatives. Green Chem. 2022, 24, 8606–8636. [Google Scholar] [CrossRef]
- Kawano, A.; Yamamoto, T.; Shinagawa, Y.; Hanashiro, I.; Onoue, M.; Yoshida, H. A- and B-type crystalline forms in aggregates and solidified materials prepared from short linear maltodextrin. J. Appl. Glycosci. 2025, 72, 7204202. [Google Scholar] [CrossRef]
- Hao, Z.; Han, S.; Zhao, Z.; Wu, Z.; Xu, H.; Li, C.; Zheng, M.; Zhou, Y.; Du, Y.; Yu, Z. Investigation of physicochemical properties and structure of ball milling pretreated modified starch-ferulic acid complexes. Food Chem. X 2024, 24, 101919. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Yao, X.; Chen, Z.; Ma, R.; Wen, Y.; Li, H.; Wang, J.; Sun, B. Interaction of high amylose corn starch with polyphenols: Modulating the stability of polyphenols with different structure against thermal processing. Food Chem. 2024, 437, 137708. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Chen, S.; Hu, Y.; Ye, X.; Wang, S.; Tian, J. The cooperation of maize starch and ferulic acid under different treatments and its effect on postprandial blood glucose level. Food Hydrocoll. 2024, 157, 110361. [Google Scholar] [CrossRef]
- Maibam, B.D.; Nickhil, C.; Deka, S.C. Preparation, physicochemical characterization, and in vitro starch digestibility on complex of Euryale ferox kernel starch with ferulic acid and quercetin. Int. J. Biol. Macromol. 2023, 250, 126178. [Google Scholar] [CrossRef]
- Tan, S.; Chen, H.; Huang, Y.; Liu, S.; Zheng, Z.; Guo, Z.; Xie, S. Chinese yam (Dioscorea opposita Thunb.) starch-ferulic acid complexes: Preparation, characterization, and physicochemical properties. J. Food Sci. 2025, 90, e17666. [Google Scholar] [CrossRef]
- Anselmi, C.; Centini, M.; Ricci, M.; Buonocore, A.; Granata, P.; Tsuno, T.; Facino, R.M. Analytical characterization of a ferulic acid/γ-cyclodextrin inclusion complex. J. Pharm. Biomed. Anal. 2006, 40, 875–881. [Google Scholar] [CrossRef]
- Simsek, T.; Rasulev, B.; Mayer, C.; Simsek, S. Preparation and characterization of inclusion complexes of β-cyclodextrin and phenolics from wheat bran by combination of experimental and computational techniques. Molecules 2020, 25, 4275. [Google Scholar] [CrossRef]
- Kinart, Z.; Tomaš, R. Studies of the formation of inclusion complexes derivatives of cinnamon acid with α-cyclodextrin in a wide range of temperatures using conductometric methods. Molecules 2022, 27, 4420. [Google Scholar] [CrossRef]
- Montesanti, N.; Veronese, G.; Buleon, A.; Escalier, P.C.; Kitamura, S.; Putaux, J.L. A-type crystals from dilute solutions of short amylose chains. Biomacromolecules 2010, 11, 3049–3058. [Google Scholar] [CrossRef]
- Shi, L.; Hopfer, H.; Ziegler, G.R.; Kong, L. Starch-menthol inclusion complex: Structure and release kinetics. Food Hydrocoll. 2019, 97, 105183. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, S.; Shinkawa, M.; Hirase, R.; Tsubomura, T.; Iuchi, K.; Hara, S. Development of water-insoluble vehicle comprising natural cyclodextrin—Vitamin E complex. Antioxidants 2021, 10, 490. [Google Scholar] [CrossRef] [PubMed]
- Shimba, N.; Shinagawa, M.; Hoshino, W.; Yamaguchi, H.; Yamada, N.; Suzuki, E.I. Monitoring the hydrolysis and transglycosylation activity of α-glucosidase from Aspergillus niger by nuclear magnetic resonance spectroscopy and mass spectrometry. Anal. Biochem. 2009, 393, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Saokham, P.; Muankaew, C.; Jansook, P.; Loftsson, T. Solubility of cyclodextrins and drug/cyclodextrin complexes. Molecules 2018, 23, 1161. [Google Scholar] [CrossRef]
- Kiss, E.; Szabó, V.A.; Horváth, P. Simple circular dichroism method for selection of the optimal cyclodextrin for drug complexation. J. Incl. Phenom. Macrocycl. Chem. 2019, 95, 223–233. [Google Scholar] [CrossRef]
- Zhdanov, Y.A.; Alekseev, Y.E.; Kompantseva, E.V.; Vergeichik, E.N. Induced optical activity in cyclodextrin complexes. Russ. Chem. Rev. 1992, 61, 563. [Google Scholar] [CrossRef]
- Hrabárová, E.; Valachová, K.; Rapta, P.; Šoltés, L. An alternative standard for trolox-equivalent antioxidant-capacity estimation based on thiol antioxidants. Comparative 2, 2′-azinobis [3-ethylbenzothiazoline-6-sulfonic Acid] decolorization and rotational viscometry study regarding hyaluronan degradation. Chem. Biodivers. 2010, 7, 2191–2200. [Google Scholar] [CrossRef] [PubMed]
- Zhiguang, C.; Haixia, Z.; Min, C.; Fayong, G.; Jing, L. The fine structure of starch: A review. npj Sci. Food 2025, 9, 50. [Google Scholar] [CrossRef]
- Jacobo-Velázquez, D.A. Ferulic acid: Mechanistic insights and multifaceted applications in metabolic Syndrome, food Preservation, and cosmetics. Molecules 2025, 30, 3716. [Google Scholar] [CrossRef] [PubMed]
- Monti, D.; Tampucci, S.; Chetoni, P.; Burgalassi, S.; Saino, V.; Centini, M.; Staltari, L.; Anselmi, C. Permeation and distribution of ferulic acid and its α-cyclodextrin complex from different formulations in hairless rat skin. AAPS PharmSciTech 2011, 12, 514–520. [Google Scholar] [CrossRef] [PubMed]
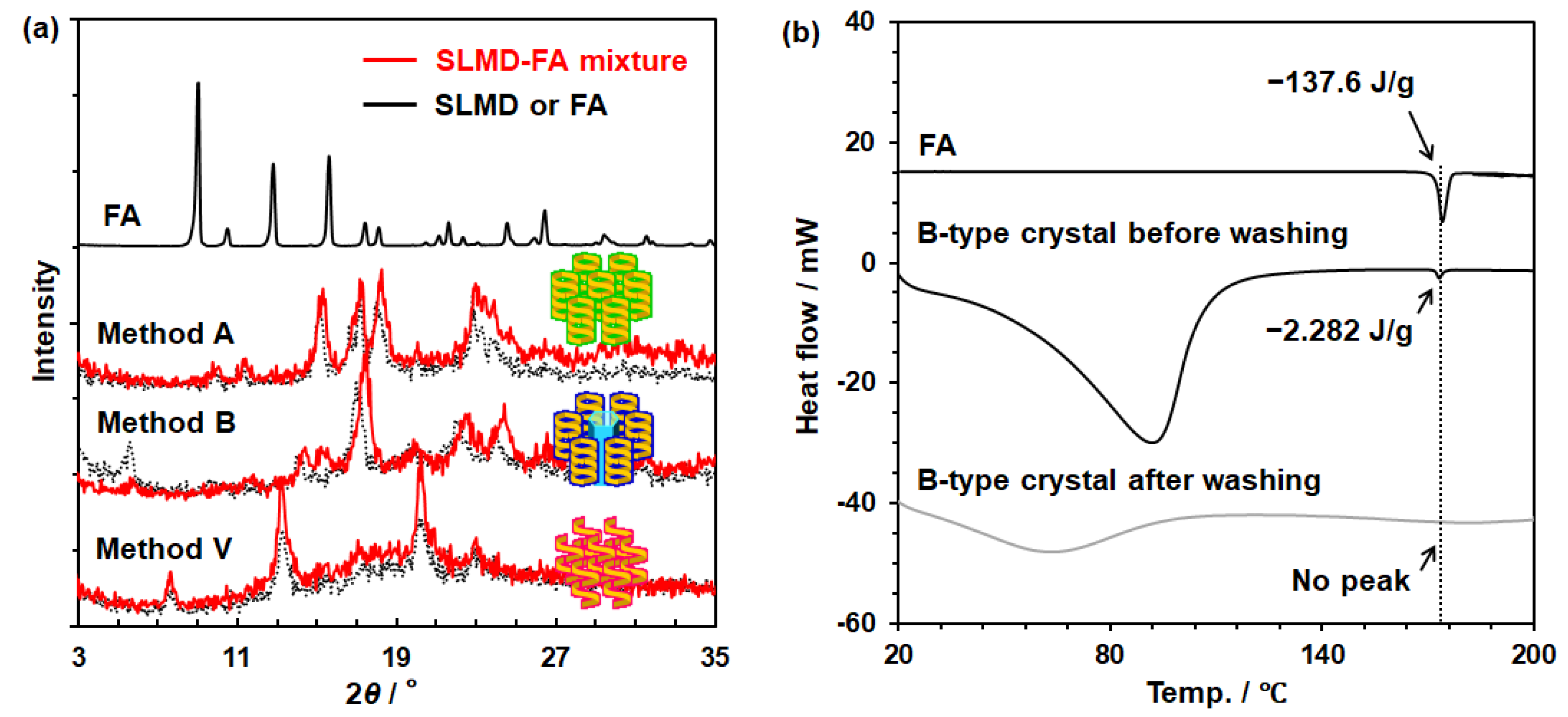
| B-Type Crystal | ||||
|---|---|---|---|---|
| Used Solvent | Before | Washed with Acetone | Washed with EtOH | Washed with IPA |
| FA/SLMD (mol/mol) | 0.43 * | 0.00 | 0.00 | 0.00 |
| 0 mM | 63 mM | 126 mM |
| 2.43 | 1.44 | 0.96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Ogawa, S.; Sugitani, D.; Matsutani, M.; Takayashiki, M.; Kawano, A. Molecular Association Between Short Linear Maltodextrin and Ferulic Acid and the Exploration of Its Applicability. Polymers 2026, 18, 166. https://doi.org/10.3390/polym18020166
Ogawa S, Sugitani D, Matsutani M, Takayashiki M, Kawano A. Molecular Association Between Short Linear Maltodextrin and Ferulic Acid and the Exploration of Its Applicability. Polymers. 2026; 18(2):166. https://doi.org/10.3390/polym18020166
Chicago/Turabian StyleOgawa, Shigesaburo, Daisuke Sugitani, Minenosuke Matsutani, Mizuho Takayashiki, and Atsushi Kawano. 2026. "Molecular Association Between Short Linear Maltodextrin and Ferulic Acid and the Exploration of Its Applicability" Polymers 18, no. 2: 166. https://doi.org/10.3390/polym18020166
APA StyleOgawa, S., Sugitani, D., Matsutani, M., Takayashiki, M., & Kawano, A. (2026). Molecular Association Between Short Linear Maltodextrin and Ferulic Acid and the Exploration of Its Applicability. Polymers, 18(2), 166. https://doi.org/10.3390/polym18020166






