Study of Polyethylene Oxide-b-Poly(ε-caprolactone-ran-δ-valerolactone) Amphiphilic Architectures and Their Effects on Self-Assembly as a Drug Carrier
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Lines
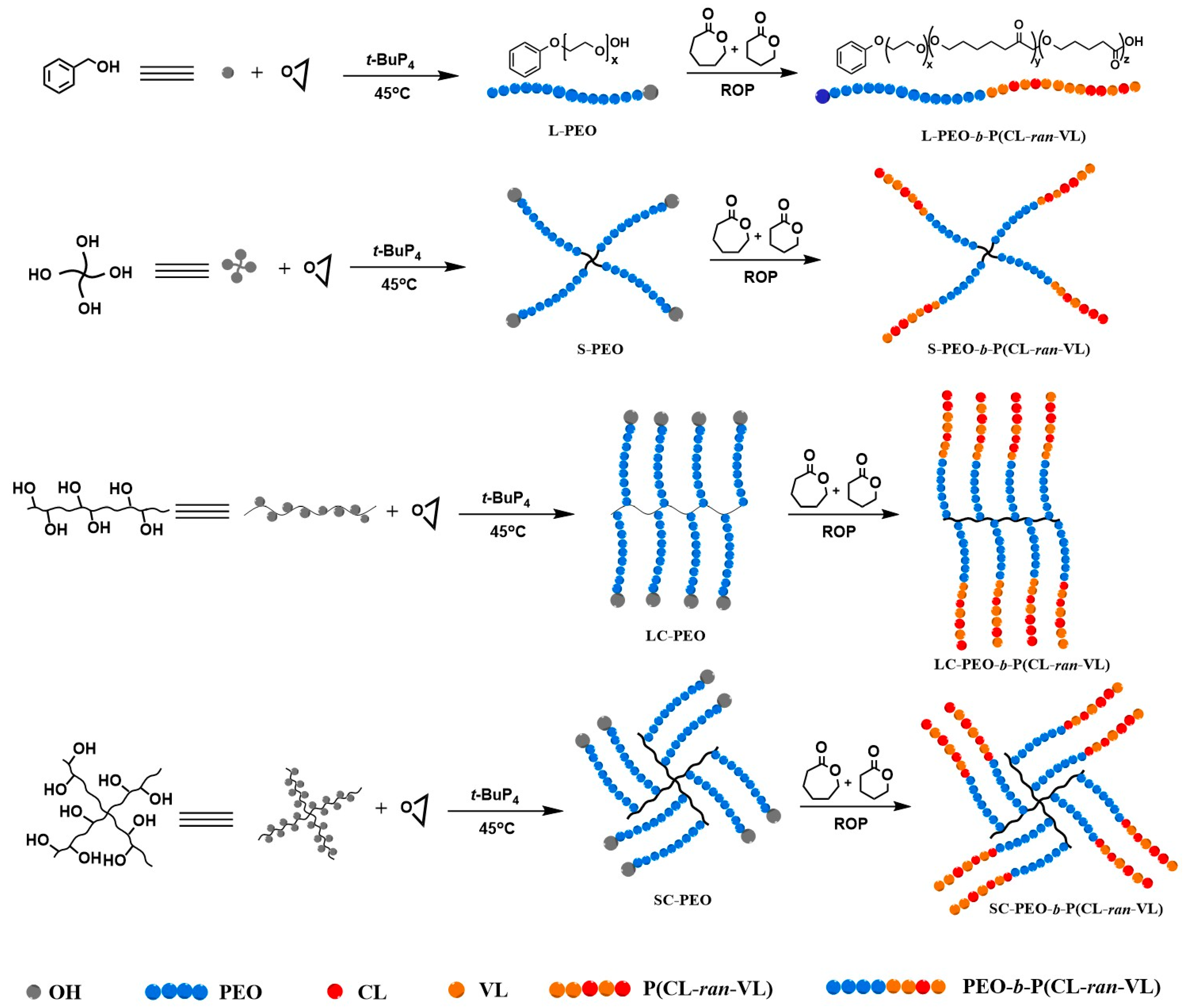
2.3. Synthesis of PEO-b-P(CL-ran-VL)
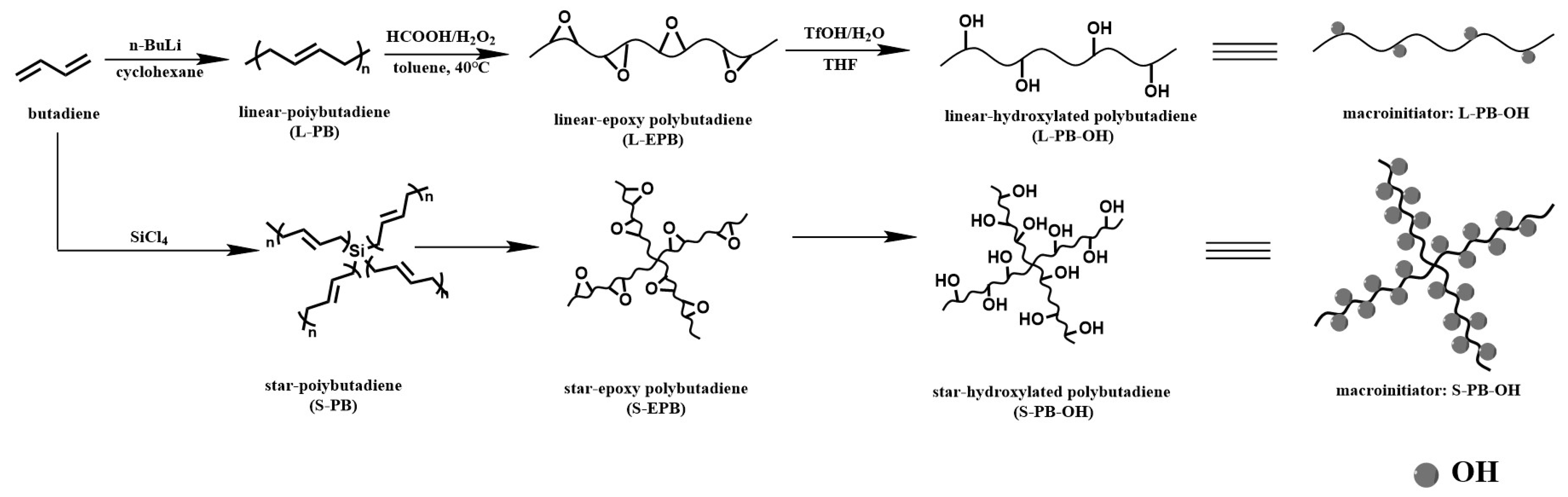
2.3.1. Synthesis of Linear and Star Hydroxylated Polybutadiene
2.3.2. Synthesis of Linear–Comb and Star–Comb Polyethylene Oxide (LC-/SC-PEO)
2.3.3. Synthesis of Linear–Comb and Star–Comb Polyethylene Oxide-b-Poly(ε-caprolactone-ran-δ-valerolactone) (LC-/SC-PEO-b-P(CL-ran-VL))
2.3.4. Synthesis of Linear and Star Polyethylene Oxide-b-Poly(ε-caprolactone-ran-δ-valerolactone) (L-/S-PEO-b-P(CL-ran-VL))
2.4. Preparation of Polymer Micelles
2.5. Characterization
2.5.1. Polymer Characterization
2.5.2. The Shape and Size of Micelles
2.5.3. Critical Micelle Concentration Determination (CMC)
2.6. Drug Loading and Encapsulation Efficiency
2.7. In Vitro Release
2.8. Cytotoxicity Study
3. Results and Discussion
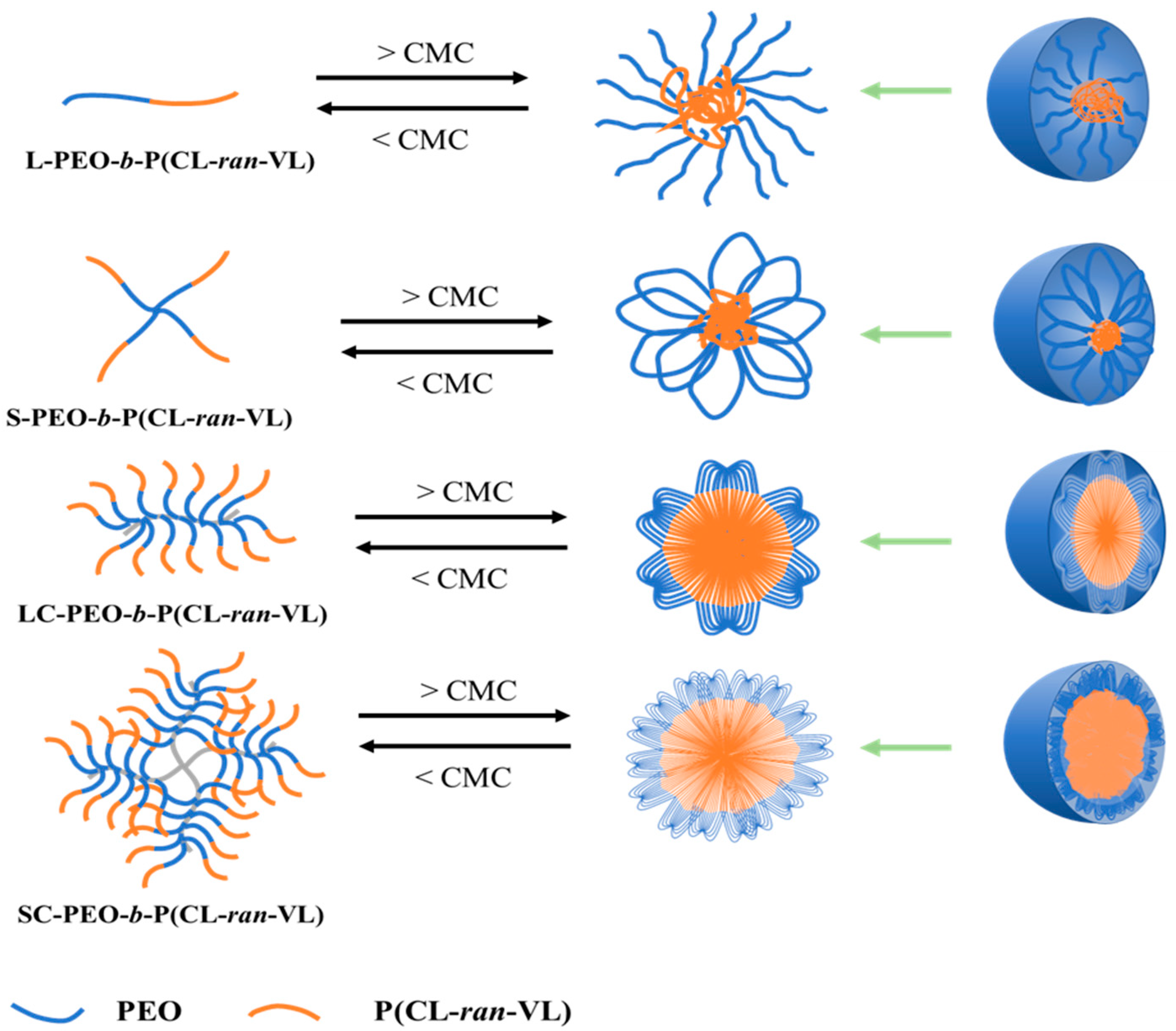
3.1. Polymer Design
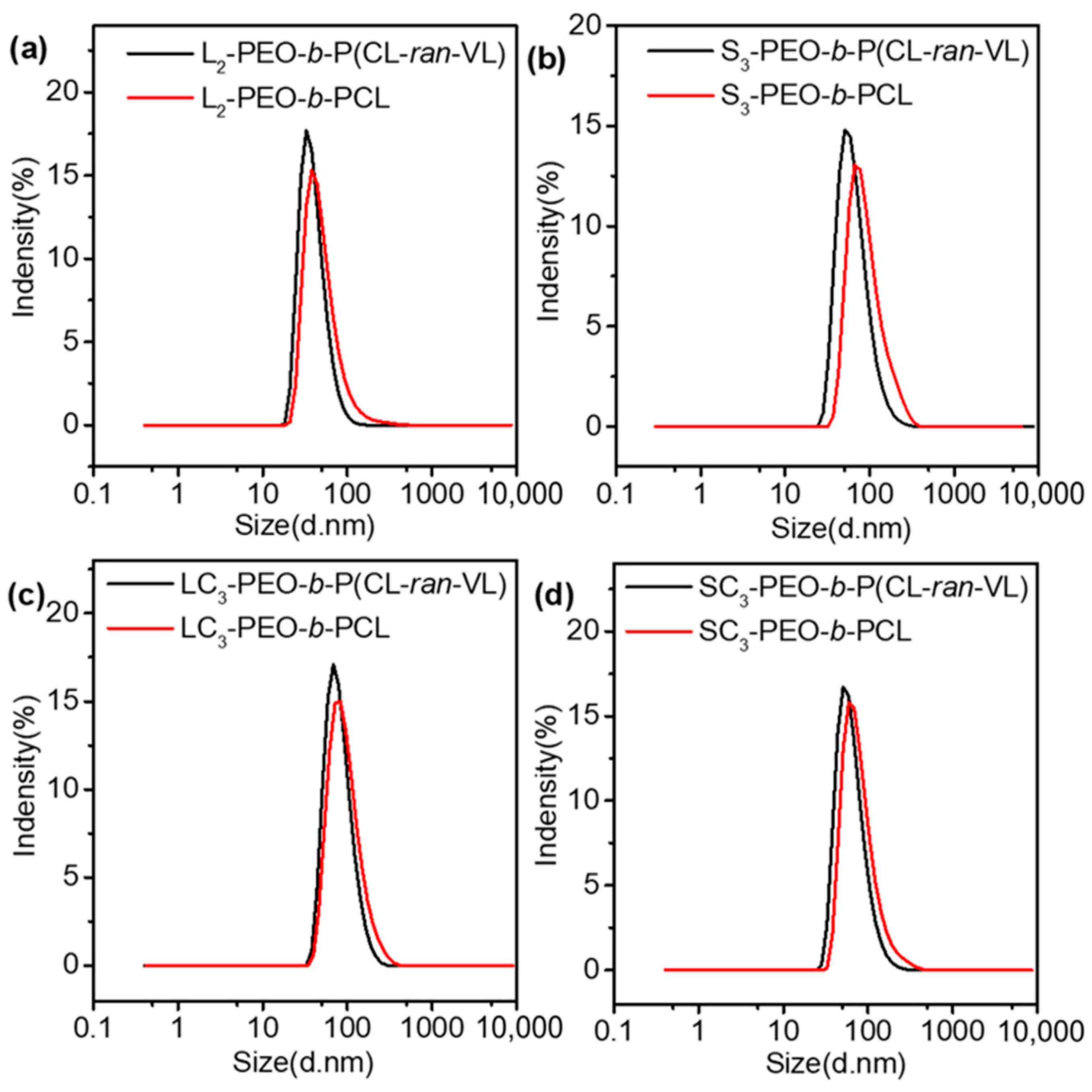
3.2. Nanoparticle Preparation—DLS Analysis
3.3. Self-Assembly (CMC Determination)
3.4. Stability and Drug Encapsulation
3.5. In Vitro Cytotoxicity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Verma, G.; Hassan, P.A. Self assembled materials: Design strategies and drug delivery perspectives. Phys. Chem. Chem. Phys. R. Soc. Chem. 2013, 15, 17016–17028. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Yue, Z.; Xie, J.; Wang, W.; Zhu, H.; Zhang, E.; Cao, Z. Micelles with ultralow critical micelle concentration as carriers for drug delivery. Nat. Biomed. Eng. Nat. Publ. Group 2018, 2, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Rösler, A.; Vandermeulen, G.W.M.; Klok, H.-A. Advanced drug delivery devices via self-assembly of amphiphilic block copolymers. Adv. Drug Deliv. Rev. 2012, 64, 270–279. [Google Scholar] [CrossRef]
- Ozawa, N.; Nishimura, T. Macromolecular architectural effects on solution self-assembly of amphiphilic AB-type block copolymers. Polym. Chem. R. Soc. Chem. 2024, 15, 349–370. [Google Scholar] [CrossRef]
- Kang, G.-Y.; Ma, W.; Liu, M.-Z.; Luo, H.-X.; Yu, C.-Y.; Wei, H. Expanding Cyclic Topology-Based Biomedical Polymer Panel: Universal Synthesis of Hetero-“8”-Shaped Copolymers and Topological Modulation of Polymer Degradation. Macromol. Rapid Commun. 2021, 42, 2100298. [Google Scholar] [CrossRef]
- Kang, G.; Liu, Y.; Li, L.; Sun, L.; Ma, W.; Meng, C.; Ma, L.; Zheng, G.; Chang, C.; Wei, H. Modulation of cyclic topology toward enhanced drug delivery, from linear and tadpole-like to dumbbell-shaped copolymers. Chem. Commun. R. Soc. Chem. 2020, 56, 3003–3006. [Google Scholar] [CrossRef]
- Ree, B.J.; Satoh, T.; Yamamoto, T. Micelle Structure Details and Stabilities of Cyclic Block Copolymer Amphiphile and Its Linear Analogues. Polym. Multidiscip. Digit. Publ. Inst. 2019, 11, 163. [Google Scholar] [CrossRef]
- Zamani, S.; Khoee, S. Preparation of core–shell chitosan/PCL-PEG triblock copolymer nanoparticles with ABA and BAB morphologies: Effect of intraparticle interactions on physicochemical properties. Polymer 2012, 53, 5723–5736. [Google Scholar] [CrossRef]
- Ma, W.; Kang, G.-Y.; Sun, L.; Meng, C.; Liu, Y.; Zheng, Z.; Jiang, M.-C.; Wang, D.; Pun, S.H.; Yu, C.-Y.; et al. Multicyclic topology-enhanced anticancer drug delivery. J. Control. Release 2022, 345, 278–291. [Google Scholar] [CrossRef]
- Williams, R.J.; Pitto-Barry, A.; Kirby, N.; Dove, A.P.; O’Reilly, R.K. Cyclic Graft Copolymer Unimolecular Micelles: Effects of Cyclization on Particle Morphology and Thermoresponsive Behavior. Macromol. Am. Chem. Soc. 2016, 49, 2802–2813. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, H.; Feng, N.; Xin, X.; Xu, Y.; Huo, P.; Wang, X.; Zhang, N. Construction and antitumor effects of antitumor micelles with cyclic RGD-modified anlotinib. Nanomed. Nanotechnol. Biol. Med. 2020, 28, 102224. [Google Scholar]
- Chen, C.; Weil, T. Cyclic polymers: Synthesis, characteristics, and emerging applications. Nanoscale Horiz. R. Soc. Chem. 2022, 7, 1121–1135. [Google Scholar]
- Wang, R.; Yin, C.; Liu, C.; Sun, Y.; Xiao, P.; Li, J.; Yang, S.; Wu, W.; Jiang, X. Phenylboronic Acid Modification Augments the Lysosome Escape and Antitumor Efficacy of a Cylindrical Polymer Brush-Based Prodrug. J. Am. Chem. Soc. Am. Chem. Soc. 2021, 143, 20927–20938. [Google Scholar]
- Hyun Kim, K.; Nam, J.; Choi, J.; Seo, M.; Bang, J. From macromonomers to bottlebrush copolymers with sequence control: Synthesis, properties, and applications. Polym. Chem. R. Soc. Chem. 2022, 13, 2224–2261. [Google Scholar]
- Sivokhin, A.; Orekhov, D.; Kazantsev, O.; Otopkova, K.; Sivokhina, O.; Chuzhaykin, I.; Spitsina, E.; Barinov, D. Anionic Oligo(ethylene glycol)-Based Molecular Brushes: Thermo- and pH-Responsive Properties. Polym. Multidiscip. Digit. Publ. Inst. 2024, 16, 3493. [Google Scholar]
- Zhao, S.; Yang, H.; Zuo, C.; Sun, L.; Ma, L.; Wei, H. pH-sensitive drug release of star-shaped micelles with OEG brush corona. RSC Adv. R. Soc. Chem. 2016, 6, 111217–111225. [Google Scholar]
- Lim, H.J.; Lee, H.; Kim, K.H.; Huh, J.; Ahn, C.-H.; Kim, W.J. Effect of molecular architecture on micellization, drug loading and releasing of multi-armed poly(ethylene glycol)-b-poly(ε-caprolactone) star polymers. Colloid Polym. Sci. 2013, 291, 1817–1827. [Google Scholar]
- Ma, G.; Zhang, C.; Zhang, L.; Sun, H.; Song, C.; Wang, C.; Kong, D. Doxorubicin-loaded micelles based on multiarm star-shaped PLGA–PEG block copolymers: Influence of arm numbers on drug delivery. J. Mater. Sci. Mater. Med. 2015, 27, 17. [Google Scholar]
- Celentano, W.; Ordanini, S.; Bruni, R.; Marocco, L.; Medaglia, P.; Rossi, A.; Buzzaccaro, S.; Cellesi, F. Complex poly(ε-caprolactone)/poly(ethylene glycol) copolymer architectures and their effects on nanoparticle self-assembly and drug nanoencapsulation. Eur. Polym. J. 2021, 144, 110226. [Google Scholar]
- Celentano, W.; Pizzocri, M.; Moncalvo, F.; Pessina, P.; Matteoli, M.; Cellesi, F.; Passoni, L. Functional Poly(ε-caprolactone)/Poly(ethylene glycol) Copolymers with Complex Topologies for Doxorubicin Delivery to a Proteinase-Rich Tumor Environment. ACS Appl. Polym. Mater. Am. Chem. Soc. 2022, 4, 8043–8056. [Google Scholar]
- Dong, R.; Zhou, Y.; Zhu, X. Supramolecular Dendritic Polymers: From Synthesis to Applications. Acc. Chem. Res. Am. Chem. Soc. 2014, 47, 2006–2016. [Google Scholar] [CrossRef]
- Wang, C.; He, W.; Wang, F.; Yong, H.; Bo, T.; Yao, D.; Zhao, Y.; Pan, C.; Cao, Q.; Zhang, S.; et al. Recent progress of non-linear topological structure polymers: Synthesis, and gene delivery. J. Nanobiotechnol. 2024, 22, 40. [Google Scholar]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. Nat. Publ. Group 2021, 20, 101–124. [Google Scholar]
- Kuperkar, K.; Patel, D.; Atanase, L.I.; Bahadur, P. Amphiphilic Block Copolymers: Their Structures, and Self-Assembly to Polymeric Micelles and Polymersomes as Drug Delivery Vehicles. Polym. Multidiscip. Digit. Publ. Inst. 2022, 14, 4702. [Google Scholar]
- Sinsinbar, G.; Bindra, A.K.; Liu, S.; Chia, T.W.; Eng, E.C.Y.; Loo, S.Y.; Lam, J.H.; Schultheis, K.; Nallani, M. Amphiphilic Block Copolymer Nanostructures as a Tunable Delivery Platform: Perspective and Framework for the Future Drug Product Development. Biomacromol. Am. Chem. Soc. 2024, 25, 541–563. [Google Scholar] [CrossRef] [PubMed]
- Altay, E.; Rzayev, J. Synthesis of star-brush polymer architectures from end-reactive molecular bottlebrushes. Polymer 2016, 98, 487–494. [Google Scholar]
- Mielanczyk, A.; Kupczak, M.; Burek, M.; Mielanczyk, l.; Klymenko, O.; Wandzik, I.; Neugebauer, D. Functional (mikto)stars and star-comb copolymers from d-gluconolactone derivative: An efficient route for tuning the architecture and responsiveness to stimuli. Polym. Elsevier 2018, 146, 331–343. [Google Scholar]
- Schramm, O.G.; Meier, M.A.; Hoogenboom, R.; Erp, H.P.; Gohyac, J.F.; Schubert, U. Polymeric nanocontainers with high loading capacity of hydrophobic drugs. Soft Matter R. Soc. Chem. 2009, 5, 1662–1667. [Google Scholar] [CrossRef]
- Zhu, X.; Fryd, M.; Tran, B.D.; Ilies, M.A.; Wayland, B. Modifying the Hydrophilic–Hydrophobic Interface of PEG-b-PCL To Increase Micelle Stability: Preparation of PEG-b-PBO-b-PCL Triblock Copolymers, Micelle Formation, and Hydrolysis Kinetics. Macromol. Am. Chem. Soc. 2012, 45, 660–665. [Google Scholar] [CrossRef]
- Kheiri Manjili, H.; Ghasemi, P.; Malvandi, H.; Mousav, S.; Attari, E.; Danafar, H. Pharmacokinetics and in vivo delivery of curcumin by copolymeric mPEG-PCL micelles. Eur. J. Pharm. Biopharm. 2017, 116, 17–30. [Google Scholar] [CrossRef]
- El Yousfi, R.; Achalhi, N.; Brahmi, M.; Youssef El, O.; Tahani, A.; Soufian El, B.; Abderahmane, E. Controlled synthesis of linear and multi ARM amphiphilic copolymers consisting of P4VP and PCL for tailored nano-aggregate formation. J. Mol. Liq. 2024, 394, 123774. [Google Scholar] [CrossRef]
- Li, Z.; Tan, B.H. Towards the development of polycaprolactone based amphiphilic block copolymers: Molecular design, self-assembly and biomedical applications. Mater. Sci. Eng. C 2014, 45, 620–634. [Google Scholar] [CrossRef] [PubMed]
- Faÿ, F.; Renard, E.; Langlois, V.; Linossier, I.; Vallée-Rehel, K. Development of poly(ε-caprolactone-co-l-lactide) and poly(ε-caprolactone-co-δ-valerolactone) as new degradable binder used for antifouling paint. Eur. Polym. J. 2007, 43, 4800–4813. [Google Scholar] [CrossRef]
- Coudane, J.; Nottelet, B.; Mouton, J.; Garric, X.; Berghe, H. Poly(ε-caprolactone)-Based Graft Copolymers: Synthesis Methods and Applications in the Biomedical Field: A Review. Molecules 2022, 27, 7339. [Google Scholar] [CrossRef]
- Hardouin Duparc, V.; Shakaroun, R.M.; Slawinski, M.; Carpentier, J.-F.; Guillaume, S.M. Ring-opening (co)polymerization of six-membered substituted δ-valerolactones with alkali metal alkoxides. Eur. Polym. J. 2020, 134, 109858. [Google Scholar] [CrossRef]
- Wu, T.; Leng, X.; Wang, Y.; Wei, Z.; Li, Y. Linear- and star-brush poly(ethylene glycol)s: Synthesis and architecture-dependent crystallization behavior. Polymer 2020, 202, 122661. [Google Scholar] [CrossRef]
- Ren, Y.; Wei, Z.; Leng, X.; Bian, Y.; Li, Y. Relationships between Architectures and Properties of Highly Branched Polymers: The Cases of Amorphous Poly(trimethylene carbonate) and Crystalline Poly(ε-caprolactone). J. Phys. Chem. B Am. Chem. Soc. 2016, 120, 4078–4090. [Google Scholar] [CrossRef]
- Bian, Y.; Leng, X.; Wei, Z.; Wang, Z.; Tu, Z.; Wang, Y.; Li, Y. End-Chain Fluorescent Highly Branched Poly(l-lactide)s: Synthesis, Architecture-Dependence, and Fluorescent Visible Paclitaxel-Loaded Microspheres. Biomacromol. Am. Chem. Soc. 2019, 20, 3952–3968. [Google Scholar] [CrossRef]
- Wu, T.; Wei, Z.; Ren, Y.; Yu, Y.; Leng, X.; Li, Y. Highly branched linear-comb random copolyesters of ε-caprolactone and δ-valerolactone: Isodimorphism, mechanical properties and enzymatic degradation behavior. Polym. Degrad. Stab. 2018, 155, 173–182. [Google Scholar] [CrossRef]
- Ren, Y.; Gao, Q.; Zhou, C.; Wei, Z.; Zhang, Y.; Li, Y. Facile synthesis of well-defined linear-comb highly branched poly(ε-caprolactone) using hydroxylated polybutadiene and organocatalyst. RSC Adv. R. Soc. Chem. 2015, 5, 27421–27430. [Google Scholar] [CrossRef]
- Yan, J.; Ye, Z.; Chen, M.; Liu, Z.; Xiao, Y.; Zhang, Y.; Zhou, Y.; Tan, W.; Lang, M. Fine Tuning Micellar Core-Forming Block of Poly(ethylene glycol)-block-poly(ε-caprolactone) Amphiphilic Copolymers Based on Chemical Modification for the Solubilization and Delivery of Doxorubicin. Biomacromol. Am. Chem. Soc. 2011, 12, 2562–2572. [Google Scholar] [CrossRef]
- Buwalda, S.; Al Samad, A.; El Jundi, A.; Bethry, A.; Bakkour, Y.; Coudane, J.; Nottelet, B. Stabilization of poly(ethylene glycol)-poly(ε-caprolactone) star block copolymer micelles via aromatic groups for improved drug delivery properties. J. Colloid Interface Sci. 2018, 514, 468–478. [Google Scholar] [CrossRef] [PubMed]
- Chai, T.; Zhang, M.; Wang, S.; Feng, J.; Feng, X.; Shao, S.; Lu, C.; Jin, G. Based on sodium alginate coatings and dendritic copolymeric modification of curcumin delivery system: pH-sensitive nanospheres and strong tumor cytotoxicity. Int. J. Biol. Macromol. 2025, 284, 137962. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, A.E.; Gilbert, R.J. Curcumin Release from Biomaterials for Enhanced Tissue Regeneration Following Injury or Disease. Bioeng. Multidiscip. Digit. Publ. Inst. 2023, 10, 262. [Google Scholar] [CrossRef]
- Tian, L.; Pei, R.; Zhong, L.; Ji, Y.; Zhou, D.; Zhou, S. Enhanced targeting of 3D pancreatic cancer spheroids by aptamer-conjugated polymeric micelles with deep tumor penetration. Eur. J. Pharmacol. 2021, 894, 173814. [Google Scholar] [CrossRef]
- Xie, Q.; Han, L.; Shan, G.; Bao, Y.; Pan, P. Polymorphic Crystalline Structure and Crystal Morphology of Enantiomeric Poly(lactic acid) Blends Tailored by a Self-Assemblable Aryl Amide Nucleator. ACS Sustain. Chem. Eng. Am. Chem. Soc. 2016, 4, 2680–2688. [Google Scholar] [CrossRef]
- Li, H.; Yu, Z.; Wang, S.; Long, X.; Zhang, L.-M.; Zhu, Z.; Yang, L. Photosensitizer-encapsulated amphiphilic chitosan derivative micelles: Photoactivity and enhancement of phototoxicity against human pancreatic cancer cells. J. Photochem. Photobiol. BBiol. 2015, 142, 212–219. [Google Scholar] [CrossRef]
- Wang, J.; Ma, W.; Tu, P. The mechanism of self-assembled mixed micelles in improving curcumin oral absorption: In vitro and in vivo. Colloids Surf. B Biointerfaces 2015, 133, 108–119. [Google Scholar] [CrossRef]
- Ghezzi, M.; Pescina, S.; Padula, C.; Santi, P.; Favero, E.D.; Cantùb, L.; Nicoli, S. Polymeric micelles in drug delivery: An insight of the techniques for their characterization and assessment in biorelevant conditions. J. Control. Release 2021, 332, 312–336. [Google Scholar] [CrossRef]
- Yamada, S.; Sasaki, E.; Ohno, H.; Hanaoka, K. Heat-guided drug delivery via thermally induced crosslinking of polymeric micelles. Commun. Chem. Nat. Publ. Group 2024, 7, 287. [Google Scholar] [CrossRef]
- Karayianni, M.; Pispas, S. Block copolymer solution self-assembly: Recent advances, emerging trends, and applications. J. Polym. Sci. 2021, 59, 1874–1898. [Google Scholar] [CrossRef]
- Honda, S.; Yamamoto, T.; Tezuka, Y. Topology-Directed Control on Thermal Stability: Micelles Formed from Linear and Cyclized Amphiphilic Block Copolymers. J. Am. Chem. Soc. Am. Chem. Soc. 2010, 132, 10251–10253. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Hong, D.; Liu, Z.; Jia, F.; Zhou, Y.; Leng, C. Controllable preparation and characterization of the thermosensitive block polymers. J. Polym. Res. 2013, 20, 235. [Google Scholar] [CrossRef]
- Tominaga, Y.; Mizuse, M.; Hashidzume, A.; Morishima, Y.; Sato, T. Flower Micelle of Amphiphilic Random Copolymers in Aqueous Media. J. Phys. Chem. B Am. Chem. Soc. 2010, 114, 11403–11408. [Google Scholar] [CrossRef]
- Owen, S.C.; Chan DP, Y.; Shoichet, M.S. Polymeric micelle stability. Nano Today 2012, 7, 53–65. [Google Scholar] [CrossRef]
- Huang, X.; Brazel, C.S. On the importance and mechanisms of burst release in matrix-controlled drug delivery systems. J. Control. Release 2001, 73, 121–136. [Google Scholar] [CrossRef]

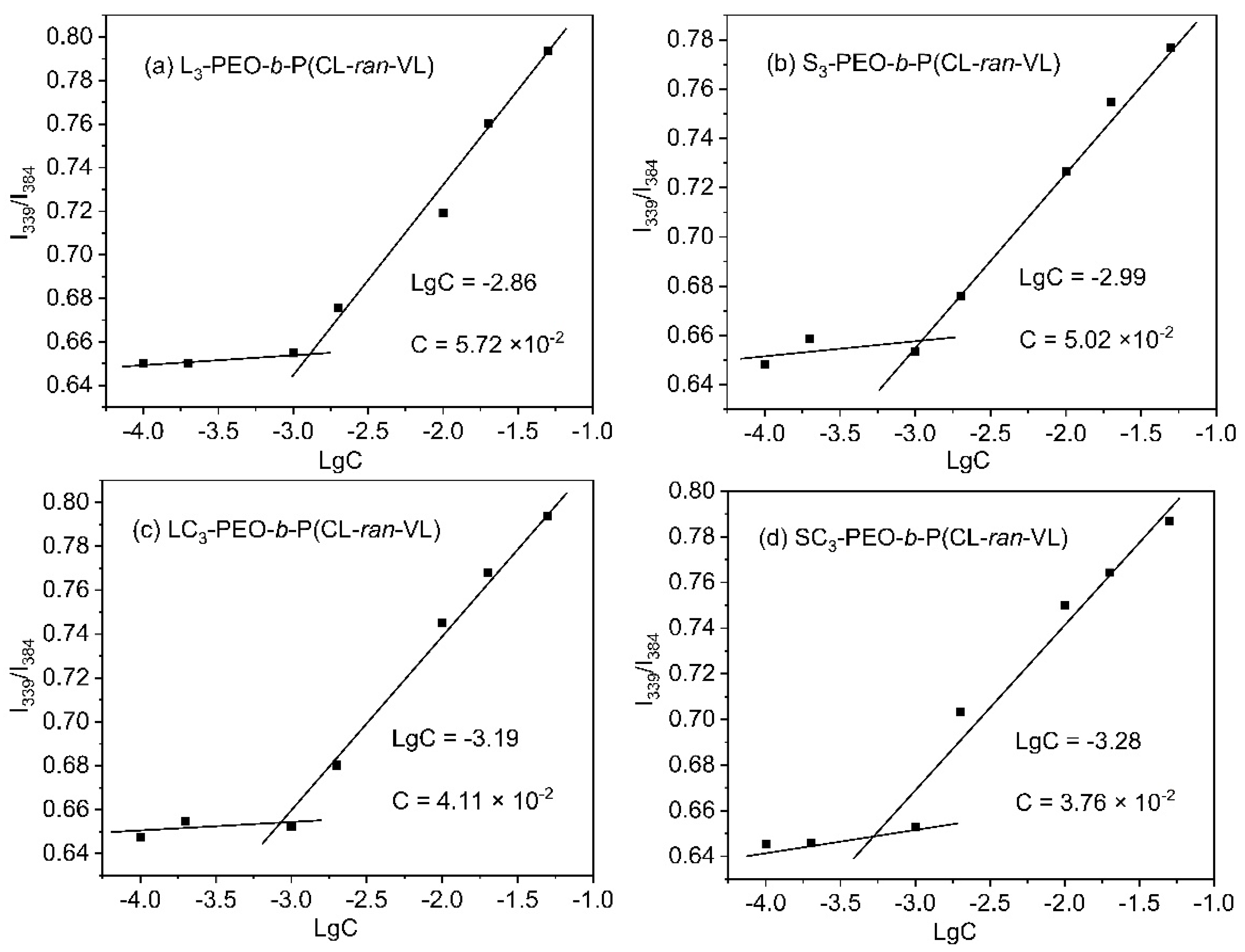
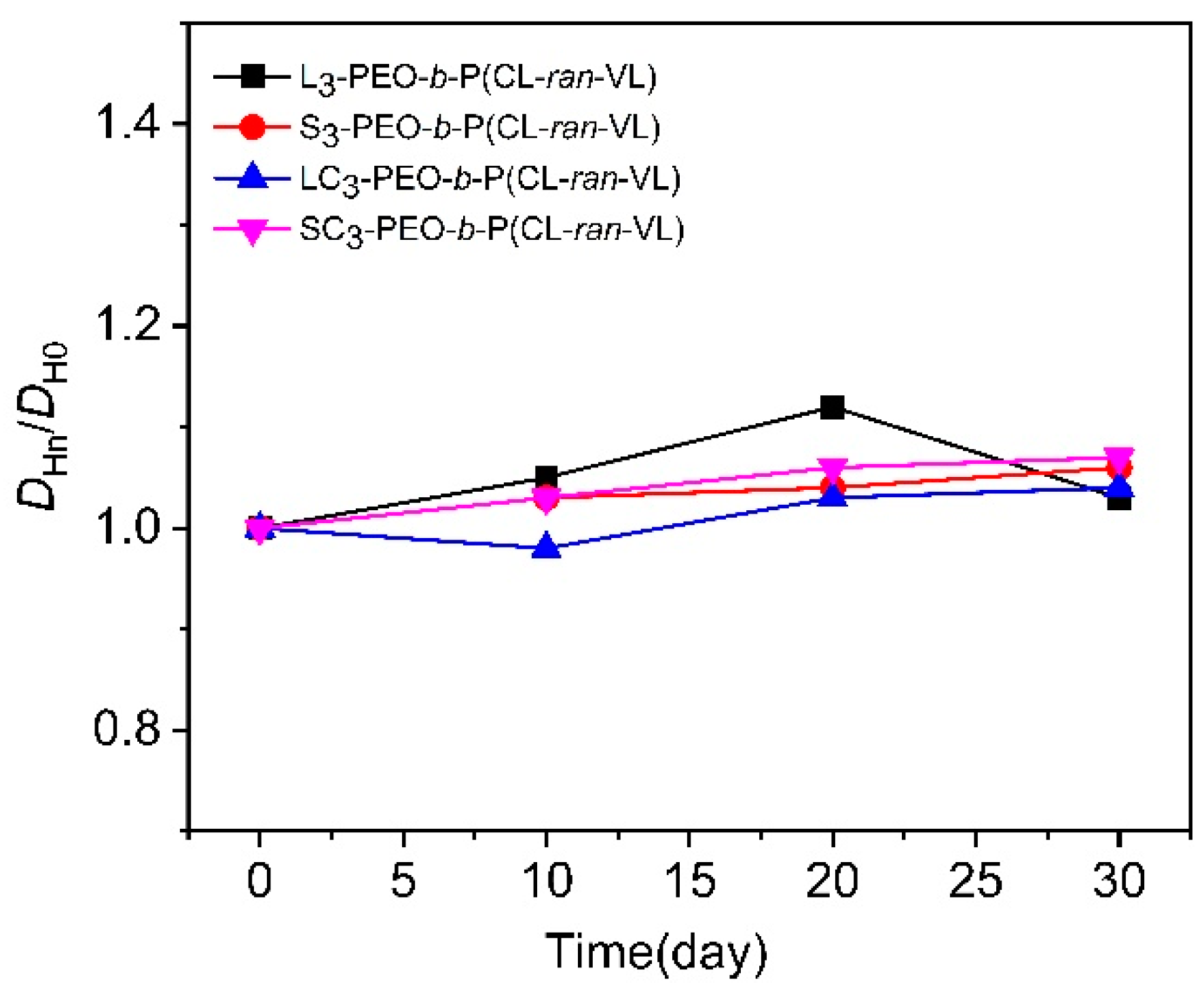
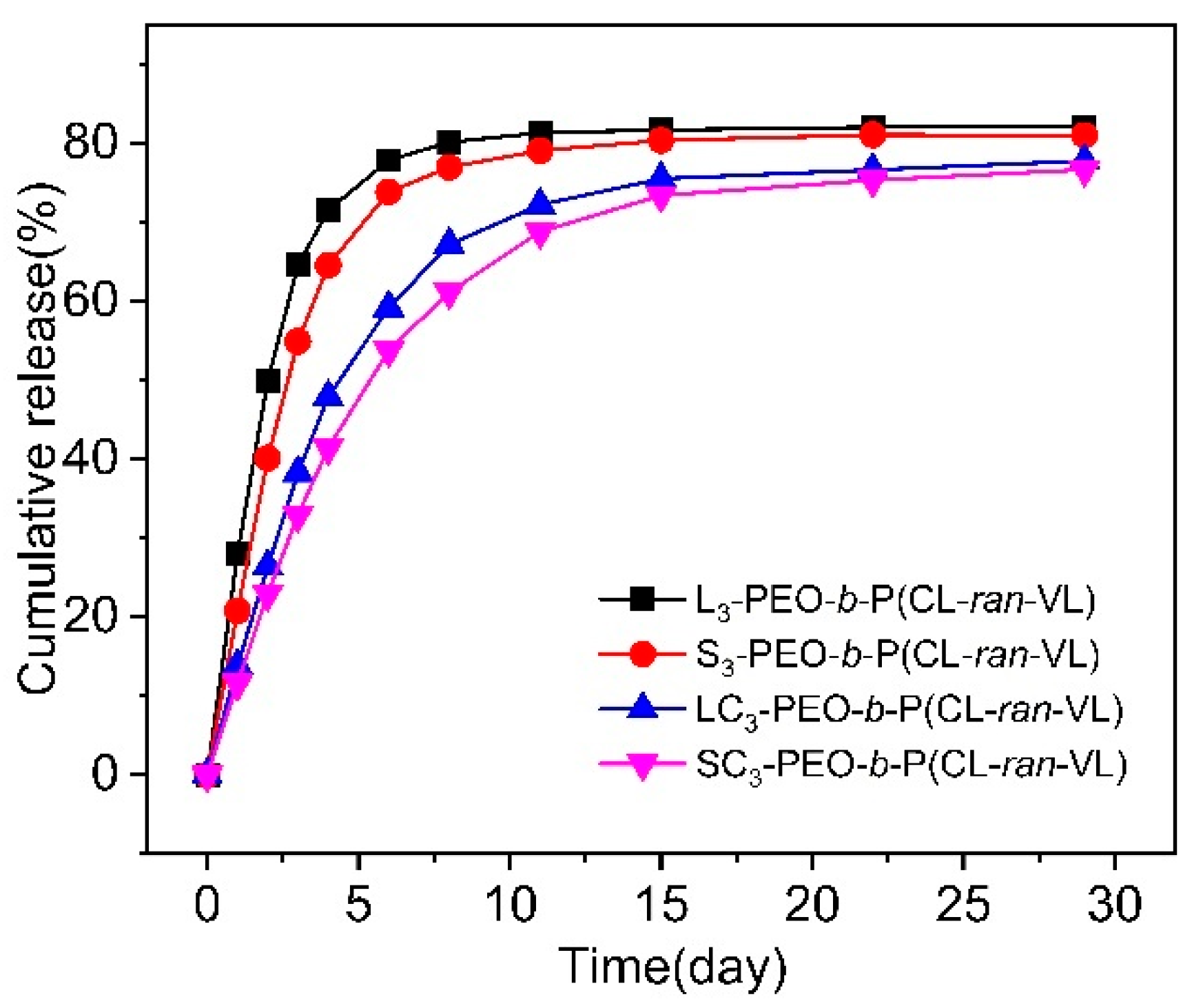
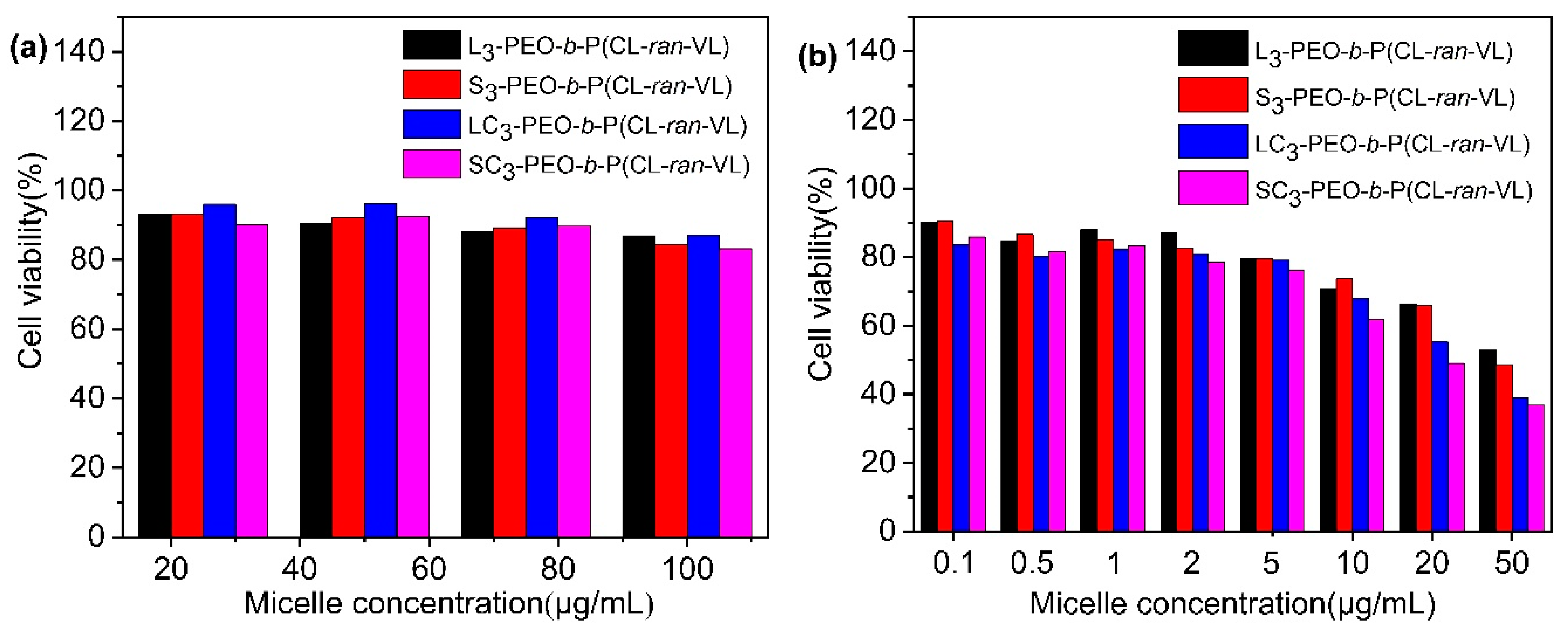
| Sample | [OH] a | [EO]/[OH] b | PEO Block | Total | PEO/PET i | PEO/PET j | Yield k (%) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mn c (kg/mol) | Mn d (kg/mol) | PDI e | Mn f (kg/mol) | Mn g (kg/mol) | PDI h | ||||||
| L1-PEO-b-P(CL-ran-VL) | - | 40 | 0.9 | - | 1.07 | 1.8 | - | 1.18 | 1.00 | 0.87 | 74 |
| L2-PEO-b-P(CL-ran-VL) | - | 80 | 1.9 | - | 1.09 | 3.7 | - | 1.18 | 1.05 | 0.87 | 80 |
| L3-PEO-b-P(CL-ran-VL) | - | 150 | 4.0 | - | 1.10 | 8.1 | - | 1.09 | 0.98 | 0.92 | 85 |
| S1-PEO-b-P(CL-ran-VL) | - | 30 | 3.7 | 0.94 | 1.05 | 6.4 | 1.36 | 1.21 | 1.37 | 0.92 | 94 |
| S2-PEO-b-P(CL-ran-VL) | - | 50 | 5.4 | 1.35 | 1.08 | 9.6 | 2.42 | 1.16 | 1.29 | 0.89 | 93 |
| S3-PEO-b-P(CL-ran-VL) | - | 70 | 7.4 | 1.85 | 1.11 | 13.4 | 3.36 | 1.21 | 1.35 | 0.84 | 89 |
| LC1-PEO-b-P(CL-ran-VL) | 23 | 40 | 19.6 | 0.85 | 1.14 | 34.3 | 1.49 | 1.16 | 1.33 | 0.79 | 85 |
| LC2-PEO-b-P(CL-ran-VL) | 23 | 50 | 30.4 | 1.32 | 1.12 | 51.4 | 2.24 | 1.13 | 1.44 | 0.92 | 87 |
| LC3-PEO-b-P(CL-ran-VL) | 23 | 60 | 43.2 | 1.88 | 1.06 | 75.7 | 3.29 | 1.21 | 1.32 | 0.94 | 83 |
| SC1-PEO-b-P(CL-ran-VL) | 23 | 40 | 22.3 | 1.01 | 1.14 | 40.1 | 1.82 | 1.13 | 1.25 | 0.83 | 77 |
| SC2-PEO-b-P(CL-ran-VL) | 23 | 50 | 34.7 | 1.58 | 1.15 | 58.1 | 2.64 | 1.25 | 1.48 | 0.84 | 81 |
| SC3-PEO-b-P(CL-ran-VL) | 23 | 60 | 41.6 | 1.89 | 1.10 | 72.8 | 3.31 | 1.21 | 1.33 | 0.98 | 79 |
| Sample | DH (1) (nm) | PDI (1) | DH-Cur (2) (nm) | PDI (2) |
|---|---|---|---|---|
| L1-PEO-b-(CL-ran-VL) | 42.0 ± 1 | 0.12 ± 0.04 | 41.7 ± 1 | 0.13 ± 0.02 |
| L2-PEO-b-(CL-ran-VL) | 54.6 ± 1 | 0.23 ± 0.01 | 50.2 ± 1 | 0.24 ± 0.01 |
| L3-PEO-b-(CL-ran-VL) | 87.3 ± 1 | 0.13 ± 0.01 | 76.4 ± 1 | 0.16 ± 0.03 |
| S1-PEO-b-(CL-ran-VL) | 128.8 ± 1 | 0.10 ± 0.01 | 106.3 ± 1 | 0.13 ± 0.01 |
| S2-PEO-b-(CL-ran-VL) | 117.4 ± 1 | 0.19 ± 0.02 | 94.2 ± 1 | 0.21 ± 0.01 |
| S3-PEO-b-(CL-ran-VL) | 110.6 ± 1 | 0.16 ± 0.01 | 93.2 ± 1 | 0.17 ± 0.02 |
| LC1-PEO-b-(CL-ran-VL) | 128.6 ± 1 | 0.23 ± 0.03 | 107.2 ± 1 | 0.26 ± 0.01 |
| LC2-PEO-b-(CL-ran-VL) | 117.0 ± 1 | 0.18 ± 0.01 | 87.7 ± 1 | 0.19 ± 0.01 |
| LC3-PEO-b-(CL-ran-VL) | 102.8 ± 1 | 0.11 ± 0.01 | 92.4 ± 1 | 0.14 ± 0.01 |
| SC1-PEO-b-(CL-ran-VL) | 143.5 ± 1 | 0.12 ± 0.01 | 125.3 ± 1 | 0.16 ± 0.01 |
| SC2-PEO-b-(CL-ran-VL) | 118.7 ± 1 | 0.12 ± 0.03 | 118.2 ± 1 | 0.13 ± 0.01 |
| SC3-PEO-b-(CL-ran-VL) | 96.8 ± 1 | 0.17 ± 0.01 | 100.2 ± 1 | 0.19 ± 0.01 |
| Sample | DL (%) | EE (%) |
|---|---|---|
| L1-PEO-b-(CL-ran-VL) | 4.5 ± 0.5 | 22.3 ± 1 |
| L2-PEO-b-(CL-ran-VL) | 5.5 ± 0.2 | 27.5 ± 1 |
| L3-PEO-b-(CL-ran-VL) | 7.0 ± 1 | 34.8 ± 2 |
| S1-PEO-b-(CL-ran-VL) | 4.9 ± 1 | 24.3 ± 1 |
| S2-PEO-b-(CL-ran-VL) | 5.7 ± 0.5 | 28.6 ± 1 |
| S3-PEO-b-(CL-ran-VL) | 6.5 ± 1 | 32.4 ± 2 |
| LC1-PEO-b-(CL-ran-VL) | 5.0 ± 1 | 25.1 ± 1 |
| LC2-PEO-b-(CL-ran-VL) | 6.1 ± 1 | 30.4 ± 1 |
| LC3-PEO-b-(CL-ran-VL) | 7.7 ± 1 | 38.5 ± 1 |
| SC1-PEO-b-(CL-ran-VL) | 5.5 ± 1 | 27.3 ± 1 |
| SC2-PEO-b-(CL-ran-VL) | 6.6 ± 1 | 33.1 ± 3 |
| SC3-PEO-b-(CL-ran-VL) | 8.2 ± 1 | 39.8 ± 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Wu, T.; Li, Y.; Liu, J.; Wang, Y.; Wang, K.; Li, Y.; Leng, X. Study of Polyethylene Oxide-b-Poly(ε-caprolactone-ran-δ-valerolactone) Amphiphilic Architectures and Their Effects on Self-Assembly as a Drug Carrier. Polymers 2025, 17, 1030. https://doi.org/10.3390/polym17081030
Wang C, Wu T, Li Y, Liu J, Wang Y, Wang K, Li Y, Leng X. Study of Polyethylene Oxide-b-Poly(ε-caprolactone-ran-δ-valerolactone) Amphiphilic Architectures and Their Effects on Self-Assembly as a Drug Carrier. Polymers. 2025; 17(8):1030. https://doi.org/10.3390/polym17081030
Chicago/Turabian StyleWang, Chaoqun, Tong Wu, Yidi Li, Jie Liu, Yanshai Wang, Kefeng Wang, Yang Li, and Xuefei Leng. 2025. "Study of Polyethylene Oxide-b-Poly(ε-caprolactone-ran-δ-valerolactone) Amphiphilic Architectures and Their Effects on Self-Assembly as a Drug Carrier" Polymers 17, no. 8: 1030. https://doi.org/10.3390/polym17081030
APA StyleWang, C., Wu, T., Li, Y., Liu, J., Wang, Y., Wang, K., Li, Y., & Leng, X. (2025). Study of Polyethylene Oxide-b-Poly(ε-caprolactone-ran-δ-valerolactone) Amphiphilic Architectures and Their Effects on Self-Assembly as a Drug Carrier. Polymers, 17(8), 1030. https://doi.org/10.3390/polym17081030








