Recyclable Palladium-Polysiloxane Catalyst with Ultra-Low Metal Leaching for Drug Synthesis
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
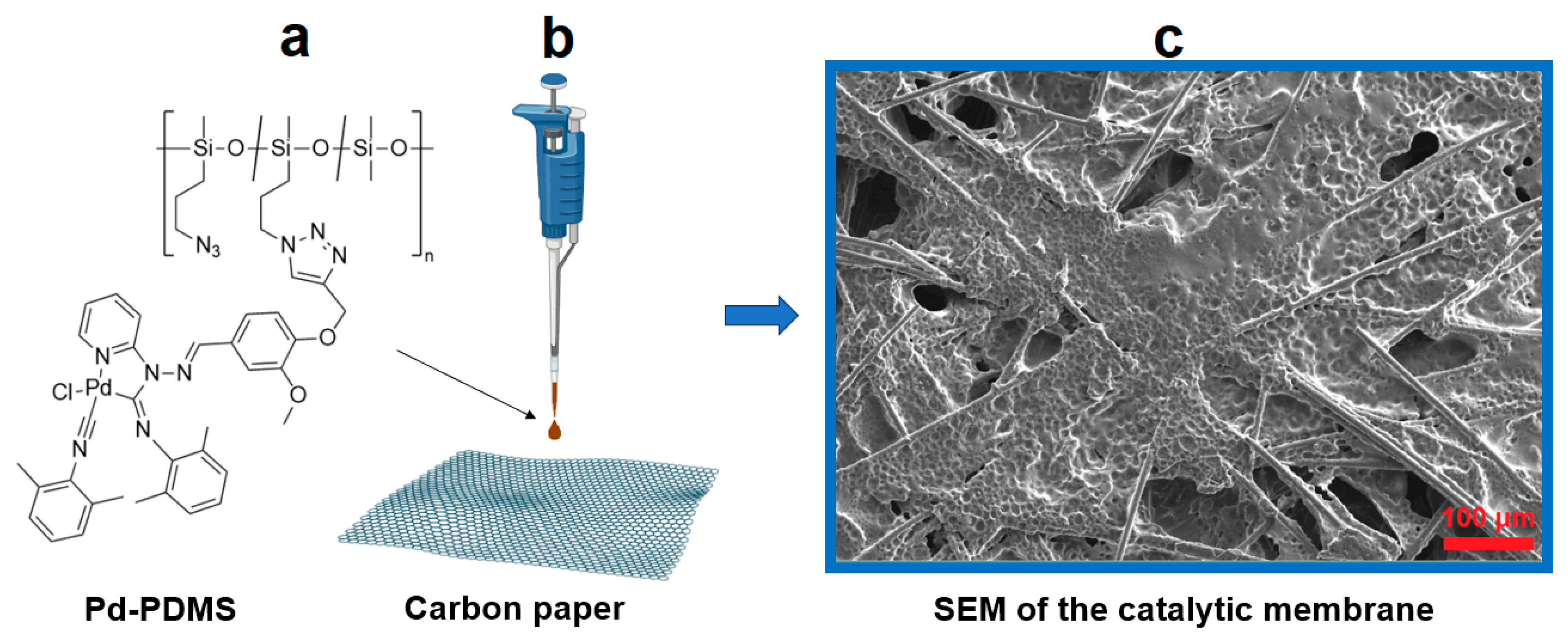
2.3. Pd-PDMS Application on the Carbon Support
2.4. Catalytic Reactions
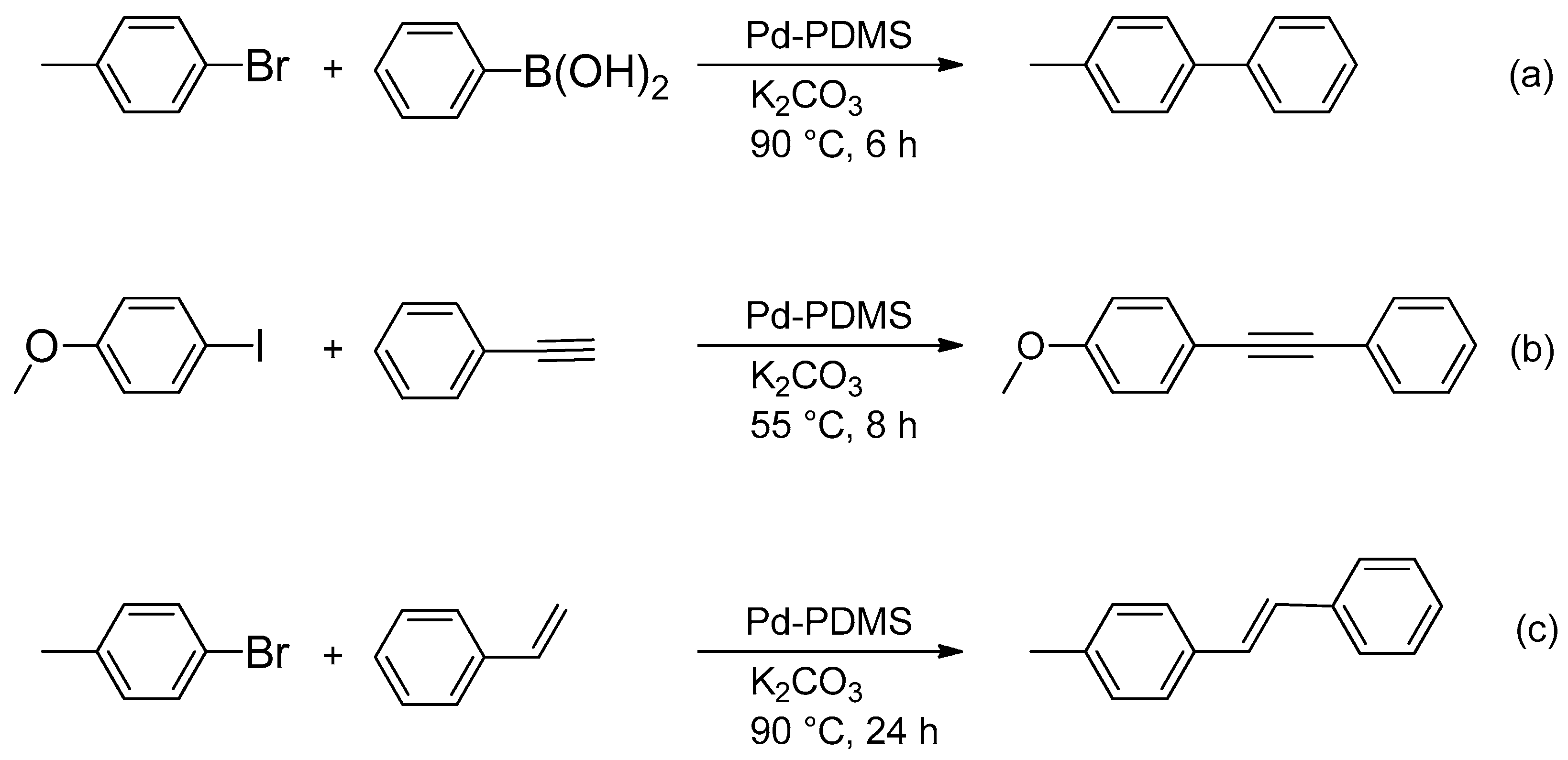
2.4.1. Sonogashira Reaction with Pd-PDMS Catalytic Membrane
2.4.2. Control of the Stability of 4-Iodoanisole in the Sonogashira Conditions Without Phenylacetylene
2.4.3. Heck Reaction with Pd-PDMS Catalytic Membrane
2.4.4. Control Experiment with Styrene in the Heck Reaction Conditions (Checking Styrene Polymerization)
3. Results and Discussion
3.1. Catalytic Membrane Fabrication and Characterisation by SEM and XPS
3.2. Catalytic Performance
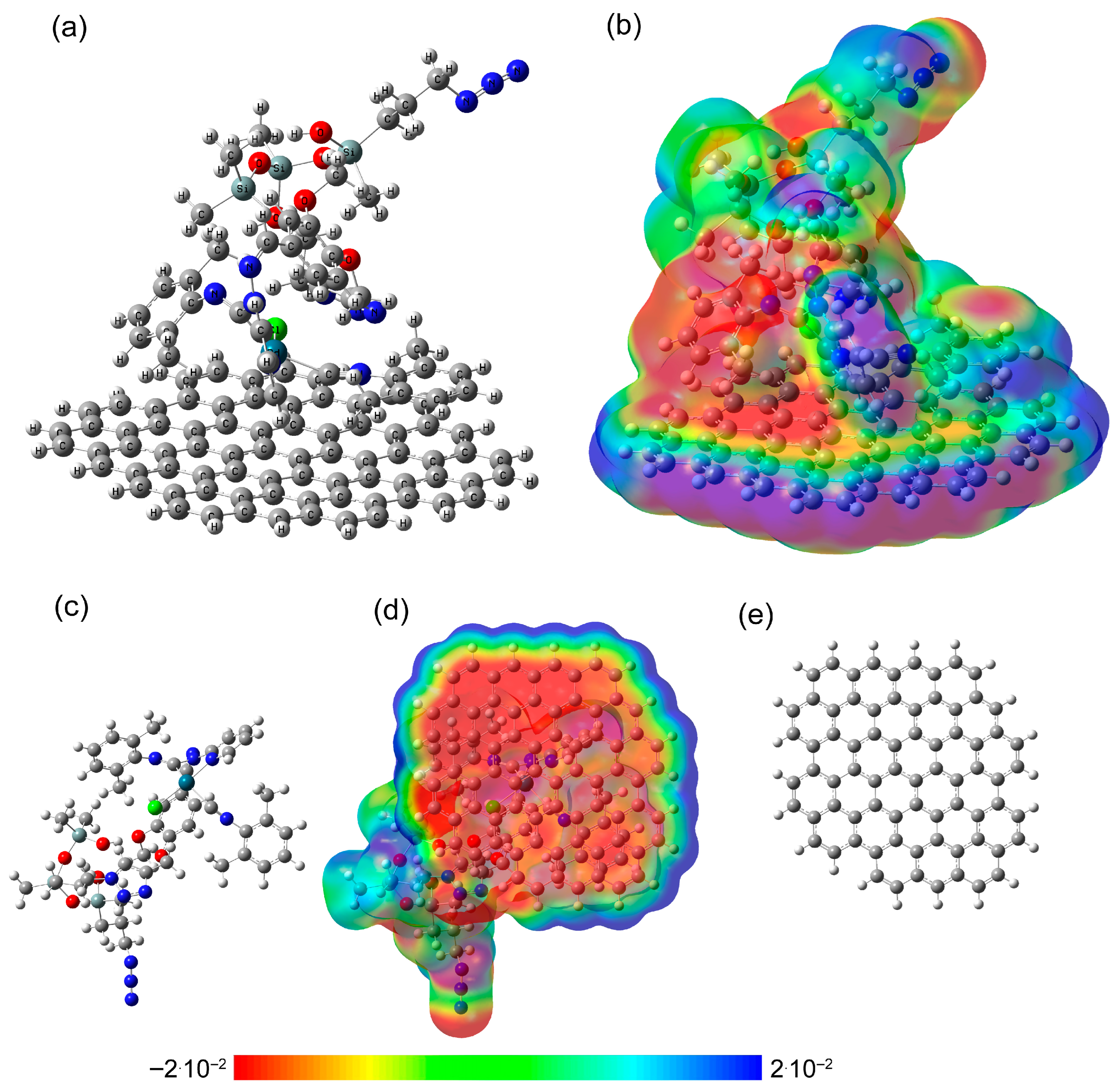
3.3. DFT Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chauhan, B.P.S.; Rathore, J.S.; Bandoo, T. “Polysiloxane-Pd” Nanocomposites as Recyclable Chemoselective Hydrogenation Catalysts. J. Am. Chem. Soc. 2004, 126, 8493–8500. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Guo, Z.; Wang, L.; Zeng, Y.; Liang, X.; Dong, F.; Zhu, P.; Liu, H.; Wang, D.; Li, Y. Atomically Dispersed Palladium Catalyst for Chemoselective Hydrogenation of Quinolines. Nano Lett. 2024, 24, 12666–12675. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Yang, Z.; Liu, J.; Gui, Q.; Chen, X.; Tan, Z.; Shi, J.-C. Pd-Catalyzed Reduction of Aldehydes to Alcohols Using Formic Acid as the Hydrogen Donor. Synth. Commun. 2014, 44, 280–288. [Google Scholar] [CrossRef]
- Pootheri, N.; Lee, S. Palladium-Catalyzed One-Pot Synthesis of N-Formylaniline Derivatives Using Oxalic Acid as a Dual Carbon Monoxide and Hydrogen Donor. Org. Lett. 2024, 26, 9407–9412. [Google Scholar] [CrossRef]
- Mao, Y.; Liu, Y.; Hu, Y.; Wang, L.; Zhang, S.; Wang, W. Pd-Catalyzed Debenzylation and Deallylation of Ethers and Esters with Sodium Hydride. ACS Catal. 2018, 8, 3016–3020. [Google Scholar] [CrossRef]
- Buskes, M.J.; Blanco, M.-J. Impact of Cross-Coupling Reactions in Drug Discovery and Development. Molecules 2020, 25, 3493. [Google Scholar] [CrossRef]
- Rayadurgam, J.; Sana, S.; Sasikumar, M.; Gu, Q. Palladium Catalyzed C–C and C–N Bond Forming Reactions: An Update on the Synthesis of Pharmaceuticals from 2015–2020. Org. Chem. Front. 2021, 8, 384–414. [Google Scholar] [CrossRef]
- Takale, B.S.; Kong, F.-Y.; Thakore, R.R. Recent Applications of Pd-Catalyzed Suzuki–Miyaura and Buchwald–Hartwig Couplings in Pharmaceutical Process Chemistry. Organics 2022, 3, 1–21. [Google Scholar] [CrossRef]
- Zhang, X.; Han, Y.; Zhang, Y.; Liang, Y. Transition-Metal-Catalyzed Transformations Involving the Heck Reaction. Adv. Synth. Catal. 2023, 365, 2436–2466. [Google Scholar] [CrossRef]
- Yan, F.; Zhang, X.; Li, D.; Zhu, N.; Bao, H. Recent Applications of the Sonogashira Reaction in the Synthesis of Drugs and Their Derivatives: A Review. Appl. Organomet. Chem. 2025, 39, e7932. [Google Scholar] [CrossRef]
- Farhang, M.; Akbarzadeh, A.R.; Rabbani, M.; Ghadiri, A.M. A Retrospective-Prospective Review of Suzuki–Miyaura Reaction: From Cross-Coupling Reaction to Pharmaceutical Industry Applications. Polyhedron 2022, 227, 116124. [Google Scholar] [CrossRef]
- Yang, S.; Yin, Q.; Liu, X.; Bi, Y.; Fang, X. Total Synthesis of (−)-Steganacin via Stereocontrolled Atroposelective Intramolecular Mizoroki-Heck Reaction and Evaluation of Biological Activity. Org. Lett. 2025, 27, 8997–9001. [Google Scholar] [CrossRef] [PubMed]
- Palladino, C.; Fantoni, T.; Ferrazzano, L.; Muzzi, B.; Ricci, A.; Tolomelli, A.; Cabri, W. New Mechanistic Insights into the Copper-Free Heck–Cassar–Sonogashira Cross-Coupling Reaction. ACS Catal. 2023, 13, 12048–12061. [Google Scholar] [CrossRef]
- Gazvoda, M.; Virant, M.; Pinter, B.; Košmrlj, J. Mechanism of Copper-Free Sonogashira Reaction Operates through Palladium-Palladium Transmetallation. Nat. Commun. 2018, 9, 4814. [Google Scholar] [CrossRef] [PubMed]
- Martek, B.A.; Gazvoda, M.; Urankar, D.; Košmrlj, J. Designing Homogeneous Copper-Free Sonogashira Reaction through a Prism of Pd–Pd Transmetalation. Org. Lett. 2020, 22, 4938–4943. [Google Scholar] [CrossRef] [PubMed]
- Rossino, G.; Marrubini, G.; Brindisi, M.; Granje, M.; Linciano, P.; Rossi, D.; Collina, S. A Green Heck Reaction Protocol towards Trisubstituted Alkenes, Versatile Pharmaceutical Intermediates. Front. Chem. 2024, 12, 1431382. [Google Scholar] [CrossRef]
- Economidou, M.; Mistry, N.; Wheelhouse, K.M.P.; Lindsay, D.M. Palladium Extraction Following Metal-Catalyzed Reactions: Recent Advances and Applications in the Pharmaceutical Industry. Org. Process Res. Dev. 2023, 27, 1585–1615. [Google Scholar] [CrossRef]
- Horbaczewskyj, C.S.; Fairlamb, I.J.S. Pd-Catalyzed Cross-Couplings: On the Importance of the Catalyst Quantity Descriptors, Mol % and Ppm. Org. Process Res. Dev. 2022, 26, 2240–2269. [Google Scholar] [CrossRef]
- Garrett, C.E.; Prasad, K. The Art of Meeting Palladium Specifications in Active Pharmaceutical Ingredients Produced by Pd-Catalyzed Reactions. Adv. Synth. Catal. 2004, 346, 889–900. [Google Scholar] [CrossRef]
- Golovenko, E.A.; Kocheva, A.N.; Semenov, A.V.; Baykova, S.O.; Deriabin, K.V.; Baykov, S.V.; Boyarskiy, V.P.; Islamova, R.M. Palladium-Functionalized Polysiloxane Drop-Casted on Carbon Paper as a Heterogeneous Catalyst for the Suzuki–Miyaura Reaction. Polymers 2024, 16, 2826. [Google Scholar] [CrossRef]
- Lu, J.; Toy, P.H. Organic Polymer Supports for Synthesis and for Reagent and Catalyst Immobilization. Chem. Rev. 2009, 109, 815–838. [Google Scholar] [CrossRef]
- Visan, A.I.; Popescu-Pelin, G.; Socol, G. Degradation Behavior of Polymers Used as Coating Materials for Drug Delivery—A Basic Review. Polymers 2021, 13, 1272. [Google Scholar] [CrossRef] [PubMed]
- Tian, R.; Li, K.; Lin, Y.; Lu, C.; Duan, X. Characterization Techniques of Polymer Aging: From Beginning to End. Chem. Rev. 2023, 123, 3007–3088. [Google Scholar] [CrossRef] [PubMed]
- Astruc, D.; Lu, F.; Aranzaes, J.R. Nanoparticles as Recyclable Catalysts: The Frontier between Homogeneous and Heterogeneous Catalysis. Angew. Chem. Int. Ed. 2005, 44, 7852–7872. [Google Scholar] [CrossRef] [PubMed]
- Ayogu, J.I.; Elahi, N.; Zeinalipour-Yazdi, C.D. Emerging Trends in Palladium Nanoparticles: Sustainable Approaches for Enhanced Cross-Coupling Catalysis. Catalysts 2025, 15, 181. [Google Scholar] [CrossRef]
- De Vries, J.G.; Jackson, S.D. Homogeneous and Heterogeneous Catalysis in Industry. Catal. Sci. Technol. 2012, 2, 2009. [Google Scholar] [CrossRef]
- Mastalir, Á.; Molnár, Á. Palladium Nanoparticles Supported on Porous Silica Materials as Heterogeneous Catalysts of C−C Coupling and Cross-Coupling Reactions. ChemCatChem 2023, 15, e202300643. [Google Scholar] [CrossRef]
- Campanati, M.; Fornasari, G.; Vaccari, A. Fundamentals in the Preparation of Heterogeneous Catalysts. Catal. Today 2003, 77, 299–314. [Google Scholar] [CrossRef]
- Zaera, F. Designing Sites in Heterogeneous Catalysis: Are We Reaching Selectivities Competitive With Those of Homogeneous Catalysts? Chem. Rev. 2022, 122, 8594–8757. [Google Scholar] [CrossRef]
- Stochmal, E.; Strzezik, J.; Krowiak, A. Physicochemical and Catalytic Properties of Polysiloxane Network–Pt Systems. RSC Adv. 2017, 7, 26342–26360. [Google Scholar] [CrossRef]
- Zhou, M.; Li, T.; Xu, B. Easy-Handling and Low-Leaching Heterogeneous Palladium and Platinum Catalysts via Coating with a Silicone Elastomer. Tetrahedron Lett. 2019, 60, 948–952. [Google Scholar] [CrossRef]
- Struwe, J.; Ackermann, L.; Gallou, F. Recent Progress in Copper-Free Sonogashira-Hagihara Cross-Couplings in Water. Chem. Catal. 2023, 3, 100485. [Google Scholar] [CrossRef]
- Baranovskii, E.M.; Khistiaeva, V.V.; Deriabin, K.V.; Petrovskii, S.K.; Koshevoy, I.O.; Kolesnikov, I.E.; Grachova, E.V.; Islamova, R.M. Re(I) Complexes as Backbone Substituents and Cross-Linking Agents for Hybrid Luminescent Polysiloxanes and Silicone Rubbers. Molecules 2021, 26, 6866. [Google Scholar] [CrossRef] [PubMed]
- Deriabin, K.V.; Golovenko, E.A.; Antonov, N.S.; Baykov, S.V.; Boyarskiy, V.P.; Islamova, R.M. Platinum Macrocatalyst for Heterogeneous Si–O Dehydrocoupling. Dalton Trans. 2023, 52, 5854–5858. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.G.A. Gaussian 09, Revision C.01; ScienceOpen, Inc.: Berlin, Germany, 2010. [Google Scholar]
- Chai, J.-D.; Head-Gordon, M. Long-Range Corrected Hybrid Density Functionals with Damped Atom–Atom Dispersion Corrections. Phys. Chem. Chem. Phys. 2008, 10, 6615. [Google Scholar] [CrossRef]
- Binkley, J.S.; Pople, J.A.; Hehre, W.J. Self-Consistent Molecular Orbital Methods. 21. Small Split-Valence Basis Sets for First-Row Elements. J. Am. Chem. Soc. 1980, 102, 939–947. [Google Scholar] [CrossRef]
- Gordon, M.S.; Binkley, J.S.; Pople, J.A.; Pietro, W.J.; Hehre, W.J. Self-Consistent Molecular-Orbital Methods. 22. Small Split-Valence Basis Sets for Second-Row Elements. J. Am. Chem. Soc. 1982, 104, 2797–2803. [Google Scholar] [CrossRef]
- Zhao, L.-H.; Weng, J.-Y.; Zhao, W.; Ruan, W.-J.; Xin, F.; Zhang, Y.-H. Density Functional Theory Study on the Influence of Pyrrolidine Substituent of C60 Bisadduct on Its Supramolecular Interaction with Porphine. Chem. Phys. 2013, 423, 43–48. [Google Scholar] [CrossRef]
- Ye, D.; Lan, Q.; Liao, Q.; Yang, Y.; Chen, R.; Wang, S.; Liu, Z.; Zhu, X. Role of Defects and Oxygen-Functional Groups in Carbon Paper Cathode for High-Performance Direct Liquid Fuel Cells. Carbon 2022, 192, 170–178. [Google Scholar] [CrossRef]
- Golovenko, E.A.; Pankin, D.V.; Deriabin, K.V.; Kirichenko, S.O.; Perevyazko, I.; Koroleva, A.V.; Islamova, R.M. Modified with Ferrocenyl-Containing Oligo- and Polysiloxanes Multi-Walled Carbon Nanotubes for Soft Conductive Silicone Composites. Mater. Today Commun. 2024, 41, 110429. [Google Scholar] [CrossRef]
- Bai, B.; Chen, Q.; Zhao, X.; Zhuo, D.; Xu, Z.; Wang, Z.; Wu, M.; Tan, H.; Peng, S.; Guo, G. Enhancing Electroreduction of CO2 to Formate of Pd Catalysts Loaded on TiO2 Nanotubes Arrays by N, B-Support Modification. ChemistrySelect 2019, 4, 8626–8633. [Google Scholar] [CrossRef]
- Kumar, N.; Haviar, S.; Zeman, P. Three-Layer PdO/CuWO4/CuO System for Hydrogen Gas Sensing with Reduced Humidity Interference. Nanomaterials 2021, 11, 3456. [Google Scholar] [CrossRef]
- Golovenko, E.A.; Pankin, D.V.; Kirichenko, S.O.; Islamova, R.M. Influence of Ferrocenyl Groups Content in Polysiloxanes on Carbon Nanotubes Performance. J. Mater. Sci Mater. Electron. 2025, 36, 1536. [Google Scholar] [CrossRef]
- Kanwal, I.; Mujahid, A.; Rasool, N.; Rizwan, K.; Malik, A.; Ahmad, G.; Shah, S.A.A.; Rashid, U.; Nasir, N.M. Palladium and Copper Catalyzed Sonogashira Cross Coupling an Excellent Methodology for C-C Bond Formation over 17 Years: A Review. Catalysts 2020, 10, 443. [Google Scholar] [CrossRef]
- Mohajer, F.; Heravi, M.M.; Zadsirjan, V.; Poormohammad, N. Copper-Free Sonogashira Cross-Coupling Reactions: An Overview. RSC Adv. 2021, 11, 6885–6925. [Google Scholar] [CrossRef]
- Islam, S.M.; Mondal, P.; Roy, A.S.; Mondal, S.; Hossain, D. Heterogeneous Suzuki and Copper-Free Sonogashira Cross-Coupling Reactions Catalyzed by a Reusable Palladium(II) Complex in Water Medium. Tetrahedron Lett. 2010, 51, 2067–2070. [Google Scholar] [CrossRef]
- Hui, A.W.; Hamielec, A.E. Thermal Polymerization of Styrene at High Conversions and Temperatures. An Experimental Study. J. Appl. Polym. Sci. 1972, 16, 749–769. [Google Scholar] [CrossRef]
- Khuong, K.S.; Jones, W.H.; Pryor, W.A.; Houk, K.N. The Mechanism of the Self-Initiated Thermal Polymerization of Styrene. Theoretical Solution of a Classic Problem. J. Am. Chem. Soc. 2005, 127, 1265–1277. [Google Scholar] [CrossRef]
- Lamblin, M.; Nassar-Hardy, L.; Hierso, J.; Fouquet, E.; Felpin, F. Recyclable Heterogeneous Palladium Catalysts in Pure Water: Sustainable Developments in Suzuki, Heck, Sonogashira and Tsuji–Trost Reactions. Adv. Synth. Catal. 2010, 352, 33–79. [Google Scholar] [CrossRef]
- Veerakumar, P.; Thanasekaran, P.; Lu, K.-L.; Liu, S.-B.; Rajagopal, S. Functionalized Silica Matrices and Palladium: A Versatile Heterogeneous Catalyst for Suzuki, Heck, and Sonogashira Reactions. ACS Sustain. Chem. Eng. 2017, 5, 6357–6376. [Google Scholar] [CrossRef]
- Yadav, A.; Mishra, P.C. Dimers and Trimers of Polycyclic Aromatic Hydrocarbons as Models of Graphene Bilayers and Trilayers: Enhanced Electron Density at the Edges. Mol. Phys. 2014, 112, 88–96. [Google Scholar] [CrossRef]
- Golovenko, E.A.; Pankin, D.V.; Deriabin, K.V.; Volkov, A.I.; Kirichenko, S.O.; Levin, O.V.; Islamova, R.M. Ligand Exchange Reaction between Ferrocene and Multiwalled Carbon Nanotubes: A Contemporary Approach. Langmuir 2024, 40, 6909–6917. [Google Scholar] [CrossRef] [PubMed]
- Adnan, M.; Pinnick, R.A.; Tang, Z.; Taylor, L.W.; Pamulapati, S.S.; Carfagni, G.R.; Pasquali, M. Bending Behavior of CNT Fibers and Their Scaling Laws. Soft Matter 2018, 14, 8284–8292. [Google Scholar] [CrossRef] [PubMed]
- Malenov, D.P.; Zarić, S.D. Stacking Interactions of Aromatic Ligands in Transition Metal Complexes. Coord. Chem. Rev. 2020, 419, 213338. [Google Scholar] [CrossRef]
- Cai, D.; Neyer, A.; Kuckuk, R.; Heise, H.M. Raman, Mid-Infrared, near-Infrared and Ultraviolet–Visible Spectroscopy of PDMS Silicone Rubber for Characterization of Polymer Optical Waveguide Materials. J. Mol. Struct. 2010, 976, 274–281. [Google Scholar] [CrossRef]
- Talianov, P.M.; Rzhevskii, S.S.; Pankin, D.V.; Deriabin, K.V.; Islamova, R.M.; Manshina, A.A. Structural Features of Functional Polysiloxanes Radical and Ionic Photo-Curing for Laser Printing Applications. J. Polym. Res. 2021, 28, 37. [Google Scholar] [CrossRef]
- Durig, J.R.; Layton, R.; Sink, D.W.; Mitchell, B.R. Far Infrared Spectra of Palladium Compounds—I. The Influence of Ligands upon the Palladium Chloride Stretching Frequency. Spectrochim. Acta 1965, 21, 1367–1378. [Google Scholar] [CrossRef]
- Mieczyńska, E.; Borkowski, T.; Cypryk, M.; Pospiech, P.; Trzeciak, A.M. Palladium Supported on Triazolyl-Functionalized Polysiloxane as Recyclable Catalyst for Suzuki–Miyaura Cross-Coupling. Appl. Catal. Gen. 2014, 470, 24–30. [Google Scholar] [CrossRef]
- Borkowski, T.; Zawartka, W.; Pospiech, P.; Mizerska, U.; Trzeciak, A.M.; Cypryk, M.; Tylus, W. Reusable Functionalized Polysiloxane-Supported Palladium Catalyst for Suzuki–Miyaura Cross-Coupling. J. Catal. 2011, 282, 270–277. [Google Scholar] [CrossRef]
- Zawartka, W.; Pośpiech, P.; Cypryk, M.; Trzeciak, A.M. Palladium Supported on Aminopropyl-Functionalized Polymethylsiloxane Microspheres: Simple and Effective Catalyst for the Suzuki–Miyaura C–C Coupling. J. Mol. Catal. Chem. 2015, 407, 230–235. [Google Scholar] [CrossRef]


| Medicine | Pharmacological Activity or Disease | Reaction |
|---|---|---|
| Selpercatinib | Lung and thyroid cancer | Suzuki reaction |
| Darolutamide | Prostate cancer | Suzuki reaction |
| Siponimod | Multiple sclerosis | Suzuki reaction |
| Tezacaftor | Cystic fibrosis | Sonogashira reaction |
| Ribociclib | Breast cancer | Sonogashira reaction |
| Letermovir | Antiviral drug | Heck reaction |
| Palbociclib | Anticancer activity | Heck reaction |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golovenko, E.A.; Petrova, P.P.; Pankin, D.V.; Baykov, S.V.; Kukushkin, V.Y.; Boyarskiy, V.P.; Islamova, R.M. Recyclable Palladium-Polysiloxane Catalyst with Ultra-Low Metal Leaching for Drug Synthesis. Polymers 2025, 17, 3066. https://doi.org/10.3390/polym17223066
Golovenko EA, Petrova PP, Pankin DV, Baykov SV, Kukushkin VY, Boyarskiy VP, Islamova RM. Recyclable Palladium-Polysiloxane Catalyst with Ultra-Low Metal Leaching for Drug Synthesis. Polymers. 2025; 17(22):3066. https://doi.org/10.3390/polym17223066
Chicago/Turabian StyleGolovenko, Ekaterina A., Polina P. Petrova, Dmitrii V. Pankin, Sergey V. Baykov, Vadim Yu. Kukushkin, Vadim P. Boyarskiy, and Regina M. Islamova. 2025. "Recyclable Palladium-Polysiloxane Catalyst with Ultra-Low Metal Leaching for Drug Synthesis" Polymers 17, no. 22: 3066. https://doi.org/10.3390/polym17223066
APA StyleGolovenko, E. A., Petrova, P. P., Pankin, D. V., Baykov, S. V., Kukushkin, V. Y., Boyarskiy, V. P., & Islamova, R. M. (2025). Recyclable Palladium-Polysiloxane Catalyst with Ultra-Low Metal Leaching for Drug Synthesis. Polymers, 17(22), 3066. https://doi.org/10.3390/polym17223066








