PEGylation Enhances Colloidal Stability and Promotes Ligand-Mediated Targeting of LAF–Xenopeptide mRNA Complexes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Synthesis of LAF-XP Carriers
2.2.2. Copper-Free Click Reaction for DSPE-PEG-GE11 Conjugate Formation
2.2.3. Unmodified and PEGylated LAF-XP Polyplex Formation
2.2.4. Zetasizer Measurements
2.2.5. Cell Culture
2.2.6. Transfections
2.2.7. Luciferase Gene Expression Assay
2.2.8. Cellular Uptake Study-Flow Cytometry
2.2.9. Steric Stabilization of LAF-XP mRNA Polyplexes Against Salt-Induced Aggregation Through PEGylation
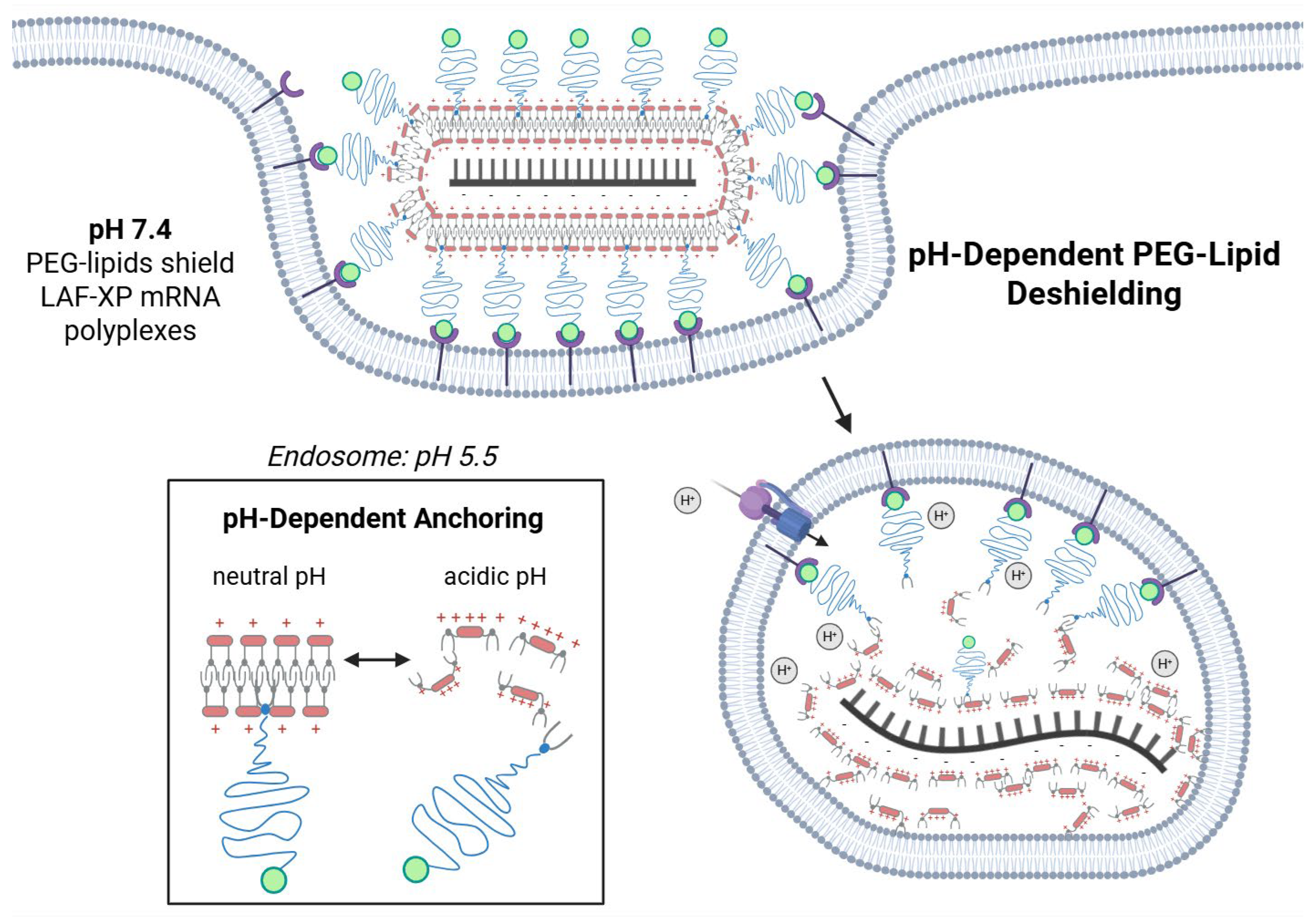
2.2.10. pH-Triggered Deshielding and Destabilization of LAF-XP mRNA Polyplexes
2.2.11. Steric Stabilization of LAF-XP mRNA Polyplexes Against Protein-Induced Aggregation Through PEGylation
2.2.12. Serum Assay–Preparation of Serum-Incubated LAF-XP mRNA Polyplexes
2.2.13. DLS Measurements of Serum-Incubated LAF-XP mRNA Polyplexes
2.2.14. Nanoparticle Tracking Analysis of Serum-Incubated LAF-Xp mRNA Polyplexes
2.2.15. Transfection Efficiency Assessment of Serum-Incubated LAF-XP mRNA Polyplexes
2.2.16. Isolation and Purification of Protein-Corona-Coated LAF-XP mRNA Polyplexes
2.2.17. Protein Corona Determination via Mass Spectrometry (MS) Analysis of Serum-Coated mRNA LAF-XP Polyplexes
2.2.18. SDS-PAGE and Silver Staining of Protein-Corona-Coated mRNA LAF-XP Polyplexes
2.2.19. Statistical Analysis
3. Results and Discussion
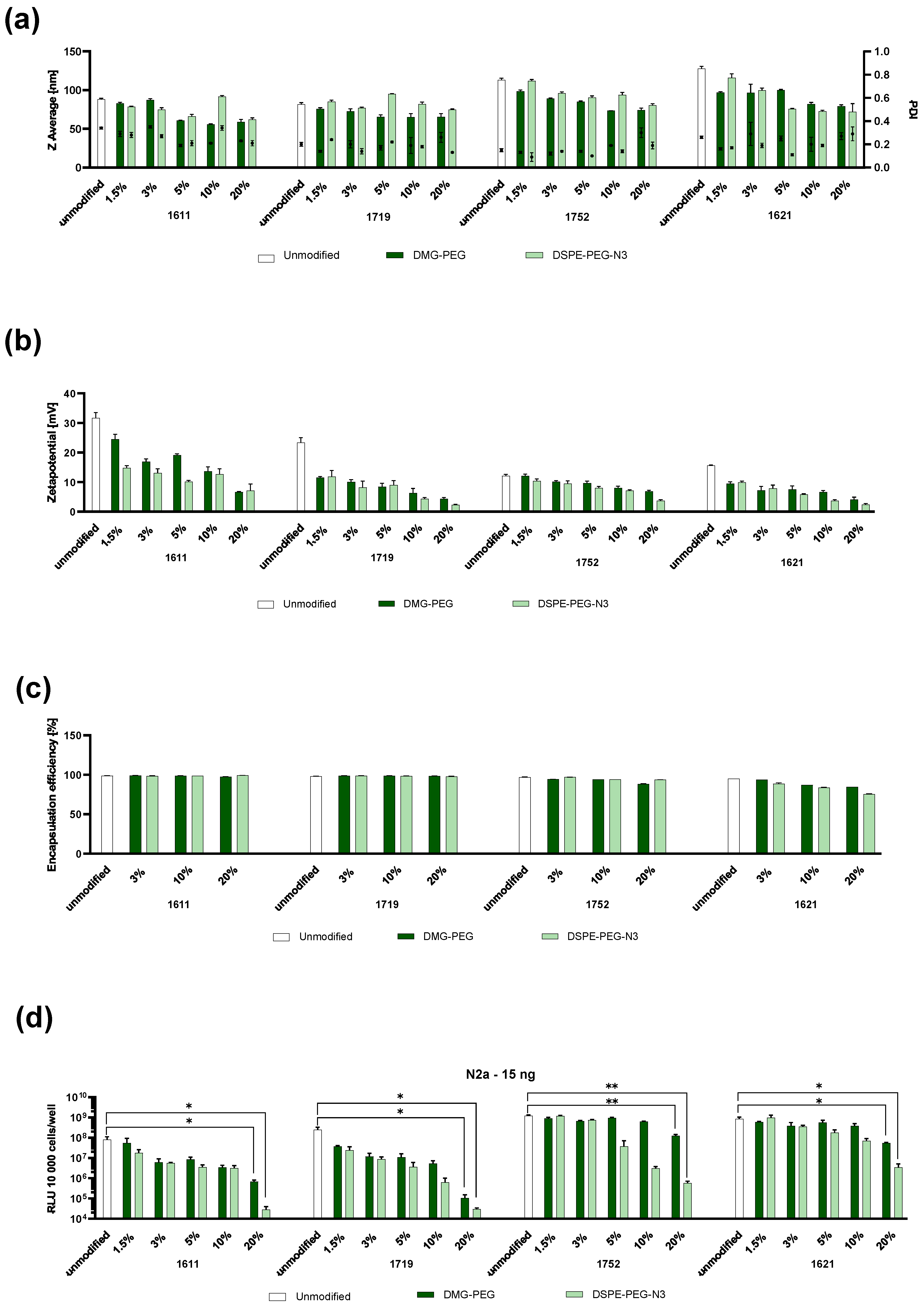
3.1. Formulation and Physicochemical and In Vitro Assessment of PEGylated LAF-XP mRNA Polyplexes
3.2. Targeting of LAF-XP mRNA Polyplexes with GE11
3.3. PEGylation and GE11 Targeting of LAF-XP pDNA Polyplexes
3.4. Colloidal Stability
3.4.1. Steric Stabilization of LAF-XP mRNA Polyplexes Against Salt-Induced Aggregation Through PEGylation
3.4.2. Reduction in the N/P Ratio Through PEGylation
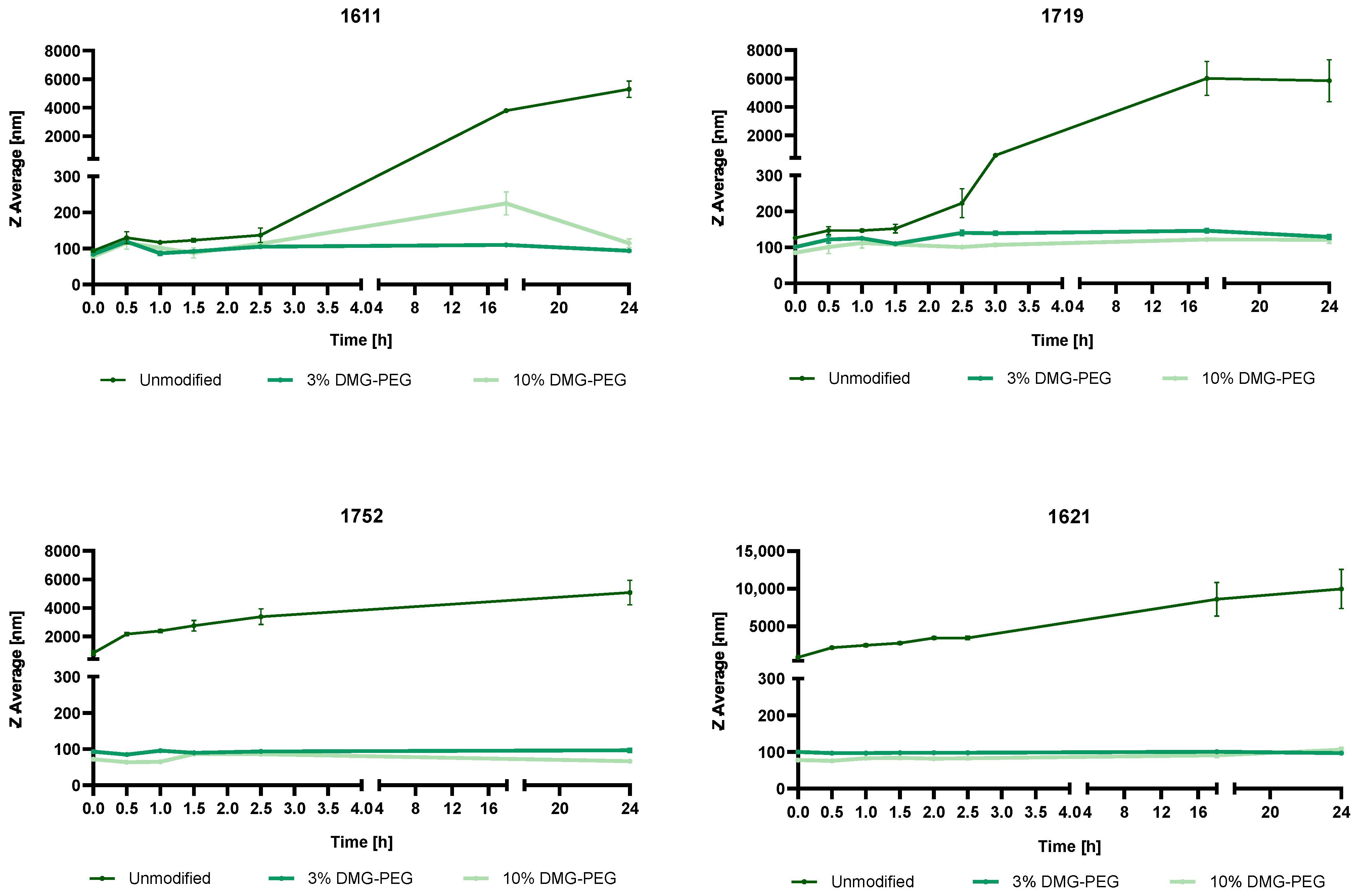
3.4.3. Overcoming mRNA LAF-XP mRNA Polyplex Instability Through PEGylation
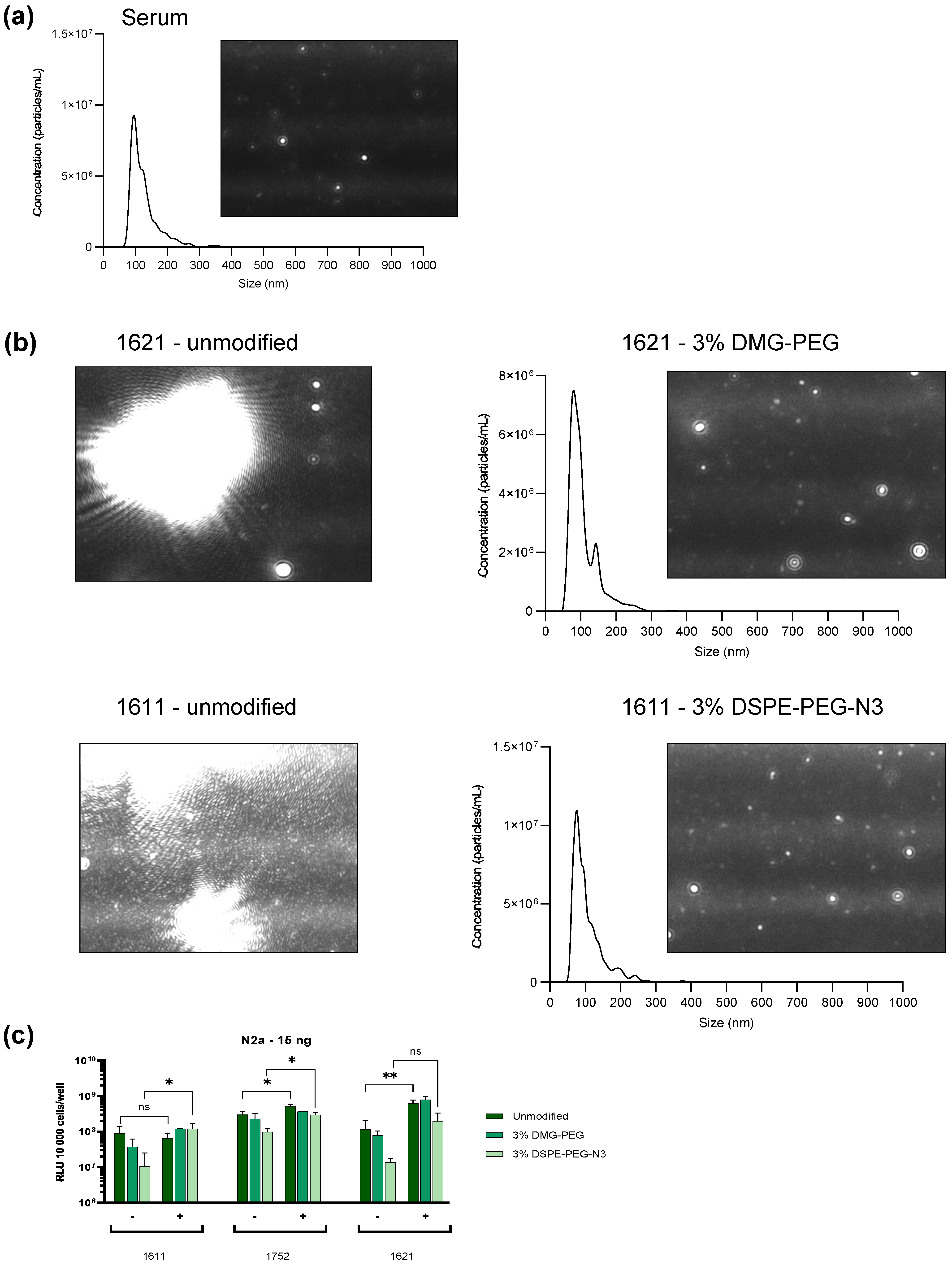
3.5. Protein Corona
3.6. Steric Stabilization of LAF-XP mRNA Polyplexes Against Protein- and Serum-Induced Aggregation Through PEGylation
3.7. In Vivo Dose Study: 1621 and 1752
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| mRNA | Messenger ribonucleic acid |
| pDNA | Plasmid deoxyribonucleic acid |
| siRNA | Small interfering ribonucleic acid |
| DMG-PEG | 1,2-Dimyristoyl-rac-glycero-3-methoxypolyethylene glycol-2k |
| DSPE-PEG-N3 | 1,2-Distearoyl-sn-glycero-3-phosphoethanolamine-N [azido (polyethylene glycol)-2k] |
| (SPAAC) | Strain-promoted azide-alkyne cycloaddition |
| DBCO | Dibenzocyclooctyne |
| LNP | Lipid nanoparticle |
| FDA | Food and Drug Administration |
| COVID-19 | Coronavirus disease 2019 |
| pHPMA | Poly(N-(2-hydroxypropyl)methacrylamide) |
| pOx | Poly(2-oxazoline) |
| apoE | Apolipoprotein E |
| LAF | Lipo-amino fatty acid |
| LAF-XP | Lipo-amino fatty acid–xenopeptide |
| SPPS | Solid-phase-assisted peptide synthesis |
| Stp | Succinoyl tetraethylene pentamine |
| EGFR | Epidermal growth factor receptor |
| HEPES | 4-(2-Hydroxyethyl)-1-piperazineethansulfonic acid |
| HBG | HEPES-buffered glucose |
| PBS | Phosphate-buffered saline |
| DLS | Dynamic light scattering |
| N2A | Neuro-2a cell line |
| HEPG2 | Human hepatocellular carcinoma cell line |
| HUH7 | Human hepatoma cell line |
| PDI | Polydispersity index |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| HPLC | High-performance liquid chromatography |
| MALDI-TOF-MS | Matrix-assisted laser desorption/ionization–time of flight–mass spectrometry |
| EtBr | Ethidium bromide |
| FGG | Fibrinogen |
| Csf1r | Colony stimulating factor 1 receptor |
| PEI | Polyethylenimine |
| NTA | Nanoparticle tracking analysis |
References
- Bulcha, J.T.; Wang, Y.; Ma, H.; Tai, P.W.L.; Gao, G. Viral vector platforms within the gene therapy landscape. Signal Transduct. Target. Ther. 2021, 6, 53. [Google Scholar] [CrossRef]
- Wang, J.H.; Gessler, D.J.; Zhan, W.; Gallagher, T.L.; Gao, G. Adeno-associated virus as a delivery vector for gene therapy of human diseases. Signal Transduct. Target. Ther. 2024, 9, 78. [Google Scholar] [CrossRef]
- Felgner, P.L.; Barenholz, Y.; Behr, J.P.; Cheng, S.H.; Cullis, P.; Huang, L.; Jessee, J.A.; Seymour, L.; Szoka, F.; Thierry, A.R.; et al. Nomenclature for synthetic gene delivery systems. Hum. Gene Ther. 1997, 8, 511–512. [Google Scholar] [CrossRef]
- van der Meel, R.; Wender, P.A.; Merkel, O.M.; Lostalé-Seijo, I.; Montenegro, J.; Miserez, A.; Laurent, Q.; Sleiman, H.; Luciani, P. Next-generation materials for nucleic acid delivery. Nat. Rev. Mater. 2025, 10, 490–499. [Google Scholar] [CrossRef]
- Witten, J.; Hu, Y.; Langer, R.; Anderson, D.G. Recent advances in nanoparticulate RNA delivery systems. Proc. Natl. Acad. Sci. USA 2024, 121, e2307798120. [Google Scholar] [CrossRef]
- Berger, S.; Lachelt, U.; Wagner, E. Dynamic carriers for therapeutic RNA delivery. Proc. Natl. Acad. Sci. USA 2024, 121, e2307799120. [Google Scholar] [CrossRef]
- Freitag, F.; Wagner, E. Optimizing synthetic nucleic acid and protein nanocarriers: The chemical evolution approach. Adv. Drug Deliv. Rev. 2021, 168, 30–54. [Google Scholar] [CrossRef]
- Luo, T.; Liang, H.; Jin, R.; Nie, Y. Virus inspired and mimetic designs in nonviral gene delivery. J. Gene Med. 2019, 21, e3090. [Google Scholar] [CrossRef]
- Nabel, G.J.; Nabel, E.G.; Yang, Z.Y.; Fox, B.A.; Plautz, G.E.; Gao, X.; Huang, L.; Shu, S.; Gordon, D.; Chang, A.E. Direct gene transfer with DNA-liposome complexes in melanoma: Expression, biologic activity, and lack of toxicity in humans. Proc. Natl. Acad. Sci. USA 1993, 90, 11307–11311. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, Y.; Hatakeyama, H.; Sato, Y.; Hyodo, M.; Akita, H.; Harashima, H. Gene silencing via RNAi and siRNA quantification in tumor tissue using mend, a liposomal siRNA delivery system. Mol. Ther. 2013, 21, 1195–1203. [Google Scholar] [CrossRef]
- Anderson, D.G.; Lynn, D.M.; Langer, R. Semi-automated synthesis and screening of a large library of degradable cationic polymers for gene delivery. Angew. Chem. Int. Ed. Engl. 2003, 42, 3153–3158. [Google Scholar] [CrossRef] [PubMed]
- Itaka, K.; Harada, A.; Yamasaki, Y.; Nakamura, K.; Kawaguchi, H.; Kataoka, K. In situ single cell observation by fluorescence resonance energy transfer reveals fast intra-cytoplasmic delivery and easy release of plasmid DNA complexed with linear polyethylenimine. J. Gene Med. 2004, 6, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Pack, D.W.; Hoffman, A.S.; Pun, S.; Stayton, P.S. Design and development of polymers for gene delivery. Nat. Rev. Drug Discov. 2005, 4, 581–593. [Google Scholar] [CrossRef] [PubMed]
- Miyata, K.; Nishiyama, N.; Kataoka, K. Rational design of smart supramolecular assemblies for gene delivery: Chemical challenges in the creation of artificial viruses. Chem. Soc. Rev. 2012, 41, 2562–2574. [Google Scholar] [CrossRef]
- Lachelt, U.; Wagner, E. Nucleic acid therapeutics using polyplexes: A journey of 50 years (and beyond). Chem. Rev. 2015, 115, 11043–11078. [Google Scholar] [CrossRef]
- Kumar, R.; Chalarca, C.F.S.; Bockman, M.R.; Bruggen, C.V.; Grimme, C.J.; Dalal, R.J.; Hanson, M.G.; Hexum, J.K.; Reineke, T.M. Polymeric delivery of therapeutic nucleic acids. Chem. Rev. 2021, 121, 11527–11652. [Google Scholar] [CrossRef]
- Dirisala, A.; Uchida, S.; Li, J.; Van Guyse, J.F.R.; Hayashi, K.; Vummaleti, S.V.C.; Kaur, S.; Mochida, Y.; Fukushima, S.; Kataoka, K. Effective mrna protection by poly(l-ornithine) synergizes with endosomal escape functionality of a charge-conversion polymer toward maximizing mrna introduction efficiency. Macromol. Rapid Commun. 2022, 43, e2100754. [Google Scholar] [CrossRef] [PubMed]
- Cullis, P.R.; Felgner, P.L. The 60-year evolution of lipid nanoparticles for nucleic acid delivery. Nat. Rev. Drug Discov. 2024, 23, 709–722. [Google Scholar] [CrossRef]
- Lin, Y.; Li, M.; Luo, Z.; Meng, Y.; Zong, Y.; Ren, H.; Yu, X.; Tan, X.; Liu, F.; Wei, T.; et al. Tissue-specific mRNA delivery and prime editing with peptide-ionizable lipid nanoparticles. Nat. Mater. 2025. [Google Scholar] [CrossRef]
- Adams, D.; Gonzalez-Duarte, A.; O’Riordan, W.D.; Yang, C.C.; Ueda, M.; Kristen, A.V.; Tournev, I.; Schmidt, H.H.; Coelho, T.; Berk, J.L.; et al. Patisiran, an RNAi therapeutic, for hereditary transthyretin amyloidosis. N. Engl. J. Med. 2018, 379, 11–21. [Google Scholar] [CrossRef]
- Verbeke, R.; Lentacker, I.; De Smedt, S.C.; Dewitte, H. The dawn of mRNA vaccines: The COVID-19 case. J. Control. Release 2021, 333, 511–520. [Google Scholar] [CrossRef]
- Schoenmaker, L.; Witzigmann, D.; Kulkarni, J.A.; Verbeke, R.; Kersten, G.; Jiskoot, W.; Crommelin, D.J.A. mRNA-lipid nanoparticle COVID-19 vaccines: Structure and stability. Int. J. Pharm. 2021, 601, 120586. [Google Scholar] [CrossRef]
- Gillmore, J.D.; Gane, E.; Taubel, J.; Pilebro, B.; Echaniz-Laguna, A.; Kao, J.; Litchy, W.; Shahda, S.; Haagensen, A.; Walsh, L.; et al. Nexiguran ziclumeran gene editing in hereditary ATTR with polyneuropathy. N. Engl. J. Med. 2025, 393, 1375–1386. [Google Scholar] [CrossRef]
- Chollet, P.; Favrot, M.C.; Hurbin, A.; Coll, J.L. Side-effects of a systemic injection of linear polyethylenimine-DNA complexes. J. Gene Med. 2002, 4, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Plank, C.; Mechtler, K.; Szoka, F.C., Jr.; Wagner, E. Activation of the complement system by synthetic DNA complexes: A potential barrier for intravenous gene delivery. Hum. Gene Ther. 1996, 7, 1437–1446. [Google Scholar] [CrossRef]
- Merkel, O.M.; Urbanics, R.; Bedocs, P.; Rozsnyay, Z.; Rosivall, L.; Toth, M.; Kissel, T.; Szebeni, J. In vitro and in vivo complement activation and related anaphylactic effects associated with polyethylenimine and polyethylenimine-graft-poly(ethylene glycol) block copolymers. Biomaterials 2011, 32, 4936–4942. [Google Scholar] [CrossRef]
- Mosqueira, V.C.F.; Legrand, P.; Gulik, A.; Bourdon, O.; Gref, R.; Labarre, D.; Barratt, G. Relationship between complement activation, cellular uptake and surface physicochemical aspects of novel peg-modified nanocapsules. Biomaterials 2001, 22, 2967–2979. [Google Scholar] [CrossRef] [PubMed]
- Wen, P.; Ke, W.; Dirisala, A.; Toh, K.; Tanaka, M.; Li, J. Stealth and pseudo-stealth nanocarriers. Adv. Drug Deliv. Rev. 2023, 198, 114895. [Google Scholar] [CrossRef]
- Hong, K.; Zheng, W.; Baker, A.; Papahadjopoulos, D. Stabilization of cationic liposome-plasmid DNA complexes by polyamines and poly(ethylene glycol)-phospholipid conjugates for efficient in vivo gene delivery. FEBS Lett. 1997, 400, 233–237. [Google Scholar] [CrossRef]
- Wheeler, J.J.; Palmer, L.; Ossanlou, M.; MacLachlan, I.; Graham, R.W.; Zhang, Y.P.; Hope, M.J.; Scherrer, P.; Cullis, P.R. Stabilized plasmid-lipid particles: Construction and characterization. Gene Ther. 1999, 6, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Katayose, S.; Kataoka, K. Water-soluble polyion complex associates of DNA and poly(ethylene glycol)-poly(l-lysine) block copolymer. Bioconjug Chem. 1997, 8, 702–707. [Google Scholar] [CrossRef] [PubMed]
- Ogris, M.; Brunner, S.; Schuller, S.; Kircheis, R.; Wagner, E. Pegylated DNA/transferrin-PEI complexes: Reduced interaction with blood components, extended circulation in blood and potential for systemic gene delivery. Gene Ther. 1999, 6, 595–605. [Google Scholar] [CrossRef]
- Itaka, K.; Yamauchi, K.; Harada, A.; Nakamura, K.; Kawaguchi, H.; Kataoka, K. Polyion complex micelles from plasmid DNA and poly(ethylene glycol)-poly(l-lysine) block copolymer as serum-tolerable polyplex system: Physicochemical properties of micelles relevant to gene transfection efficiency. Biomaterials 2003, 24, 4495–4506. [Google Scholar] [CrossRef]
- Tockary, T.A.; Osada, K.; Chen, Q.; Machitani, K.; Dirisala, A.; Uchida, S.; Nomoto, T.; Toh, K.; Matsumoto, Y.; Itaka, K.; et al. Tethered peg crowdedness determining shape and blood circulation profile of polyplex micelle gene carriers. Macromolecules 2013, 46, 6585–6592. [Google Scholar] [CrossRef]
- Roy, P.; Kreofsky, N.W.; Chalarca, C.F.S.; Reineke, T.M. Binary copolymer blending enhances pDNA delivery performance and colloidal shelf stability of quinine-based polyplexes. Bioconjug. Chem. 2025, 36, 770–781. [Google Scholar] [CrossRef]
- Katayose, S.; Kataoka, K. Remarkable increase in nuclease resistance of plasmid DNA through supramolecular assembly with poly(ethylene glycol)-poly(l-lysine) block copolymer. J. Pharm. Sci. 1998, 87, 160–163. [Google Scholar] [CrossRef]
- Erbacher, P.; Bettinger, T.; Belguise-Valladier, P.; Zou, S.; Coll, J.L.; Behr, J.P.; Remy, J.S. Transfection and physical properties of various saccharide, poly(ethylene glycol), and antibody-derivatized polyethylenimines (PEI). J. Gene Med. 1999, 1, 210–222. [Google Scholar] [CrossRef] [PubMed]
- Merdan, T.; Kunath, K.; Petersen, H.; Bakowsky, U.; Voigt, K.H.; Kopecek, J.; Kissel, T. Pegylation of poly(ethylene imine) affects stability of complexes with plasmid DNA under in vivo conditions in a dose-dependent manner after intravenous injection into mice. Bioconjug. Chem. 2005, 16, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Dash, P.R.; Read, M.L.; Fisher, K.D.; Howard, K.A.; Wolfert, M.; Oupicky, D.; Subr, V.; Strohalm, J.; Ulbrich, K.; Seymour, L.W. Decreased binding to proteins and cells of polymeric gene delivery vectors surface modified with a multivalent hydrophilic polymer and retargeting through attachment of transferrin. J. Biol. Chem. 2000, 275, 3793–3802. [Google Scholar] [CrossRef] [PubMed]
- Burke, R.S.; Pun, S.H. Synthesis and characterization of biodegradable hpma-oligolysine copolymers for improved gene delivery. Bioconjug. Chem. 2010, 21, 140–150. [Google Scholar] [CrossRef][Green Version]
- Gaspar, V.M.; Goncalves, C.; de Melo-Diogo, D.; Costa, E.C.; Queiroz, J.A.; Pichon, C.; Sousa, F.; Correia, I.J. Poly(2-ethyl-2-oxazoline)-PLA-g-PEI amphiphilic triblock micelles for co-delivery of minicircle DNA and chemotherapeutics. J. Control. Release 2014, 189, 90–104. [Google Scholar] [CrossRef]
- Yamaleyeva, D.N.; Makita, N.; Hwang, D.; Haney, M.J.; Jordan, R.; Kabanov, A.V. Poly(2-oxazoline)-based polyplexes as a peg-free plasmid DNA delivery platform. Macromol. Biosci. 2023, 23, e2300177. [Google Scholar] [CrossRef]
- Heller, P.; Birke, A.; Huesmann, D.; Weber, B.; Fischer, K.; Reske-Kunz, A.; Bros, M.; Barz, M. Introducing peptoplexes: Polylysine-block-polysarcosine based polyplexes for transfection of HEK 293T cells. Macromol. Biosci. 2014, 14, 1380–1395. [Google Scholar] [CrossRef]
- Klein, P.M.; Klinker, K.; Zhang, W.; Kern, S.; Kessel, E.; Wagner, E.; Barz, M. Efficient shielding of polyplexes using heterotelechelic polysarcosines. Polymers 2018, 10, 689. [Google Scholar] [CrossRef]
- Bayraktutan, H.; Kopiasz, R.J.; Elsherbeny, A.; Espuga, M.M.; Gumus, N.; Oz, U.C.; Polra, K.; McKay, P.F.; Shattock, R.J.; Ordóñez-Morán, P.; et al. Polysarcosine functionalised cationic polyesters efficiently deliver self-amplifying mRNA. Polym. Chem. 2024, 15, 1862–1876. [Google Scholar] [CrossRef]
- Hou, X.; Zaks, T.; Langer, R.; Dong, Y. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 2021, 6, 1078–1094. [Google Scholar] [CrossRef]
- Kim, J.; Eygeris, Y.; Gupta, M.; Sahay, G. Self-assembled mRNA vaccines. Adv. Drug Deliv. Rev. 2021, 170, 83–112. [Google Scholar] [CrossRef]
- Lokugamage, M.P.; Vanover, D.; Beyersdorf, J.; Hatit, M.Z.C.; Rotolo, L.; Echeverri, E.S.; Peck, H.E.; Ni, H.; Yoon, J.K.; Kim, Y.; et al. Optimization of lipid nanoparticles for the delivery of nebulized therapeutic mRNA to the lungs. Nat. Biomed. Eng. 2021, 5, 1059–1068. [Google Scholar] [CrossRef]
- Hald Albertsen, C.; Kulkarni, J.A.; Witzigmann, D.; Lind, M.; Petersson, K.; Simonsen, J.B. The role of lipid components in lipid nanoparticles for vaccines and gene therapy. Adv. Drug Deliv. Rev. 2022, 188, 114416. [Google Scholar] [CrossRef]
- Klibanov, A.L.; Maruyama, K.; Torchilin, V.P.; Huang, L. Amphipathic polyethyleneglycols effectively prolong the circulation time of liposomes. FEBS Lett. 1990, 268, 235–237. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.M.; Hansen, C.; Martin, F.; Redemann, C.; Yau-Young, A. Liposomes containing synthetic lipid derivatives of poly(ethylene glycol) show prolonged circulation half-lives in vivo. Biochim. Biophys. Acta 1991, 1066, 29–36. [Google Scholar] [CrossRef]
- Lasic, D.D.; Martin, F.J.; Gabizon, A.; Huang, S.K.; Papahadjopoulos, D. Sterically stabilized liposomes: A hypothesis on the molecular origin of the extended circulation times. Biochim. Biophys. Acta 1991, 1070, 187–192. [Google Scholar] [CrossRef]
- Papahadjopoulos, D.; Allen, T.M.; Gabizon, A.; Mayhew, E.; Matthay, K.; Huang, S.K.; Lee, K.D.; Woodle, M.C.; Lasic, D.D.; Redemann, C.; et al. Sterically stabilized liposomes: Improvements in pharmacokinetics and antitumor therapeutic efficacy. Proc. Natl. Acad. Sci. USA 1991, 88, 11460–11464. [Google Scholar] [CrossRef]
- Monck, M.A.; Mori, A.; Lee, D.; Tam, P.; Wheeler, J.J.; Cullis, P.R.; Scherrer, P. Stabilized plasmid-lipid particles: Pharmacokinetics and plasmid delivery to distal tumors following intravenous injection. J. Drug Target. 2000, 7, 439–452. [Google Scholar] [CrossRef] [PubMed]
- Tranchant, I.; Thompson, B.; Nicolazzi, C.; Mignet, N.; Scherman, D. Physicochemical optimisation of plasmid delivery by cationic lipids. J. Gene Med. 2004, 6 (Suppl. 1), S24–S35. [Google Scholar] [CrossRef]
- Gabizon, A.; Catane, R.; Uziely, B.; Kaufman, B.; Safra, T.; Cohen, R.; Martin, F.; Huang, A.; Barenholz, Y. Prolonged circulation time and enhanced accumulation in malignant exudates of doxorubicin encapsulated in polyethylene-glycol coated liposomes. Cancer Res. 1994, 54, 987–992. [Google Scholar]
- Meyer, O.; Kirpotin, D.; Hong, K.; Sternberg, B.; Park, J.W.; Woodle, M.C.; Papahadjopoulos, D. Cationic liposomes coated with polyethylene glycol as carriers for oligonucleotides. J. Biol. Chem. 1998, 273, 15621–15627. [Google Scholar] [CrossRef]
- Akinc, A.; Querbes, W.; De, S.; Qin, J.; Frank-Kamenetsky, M.; Jayaprakash, K.N.; Jayaraman, M.; Rajeev, K.G.; Cantley, W.L.; Dorkin, J.R.; et al. Targeted delivery of RNAi therapeutics with endogenous and exogenous ligand-based mechanisms. Mol. Ther. 2010, 18, 1357–1364. [Google Scholar] [CrossRef]
- Chen, S.; Tam, Y.Y.C.; Lin, P.J.C.; Sung, M.M.H.; Tam, Y.K.; Cullis, P.R. Influence of particle size on the in vivo potency of lipid nanoparticle formulations of siRNA. J. Control. Release 2016, 235, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Harvie, P.; Wong, F.M.; Bally, M.B. Use of poly(ethylene glycol)-lipid conjugates to regulate the surface attributes and transfection activity of lipid-DNA particles. J. Pharm. Sci. 2000, 89, 652–663. [Google Scholar] [CrossRef]
- Mui, B.L.; Tam, Y.K.; Jayaraman, M.; Ansell, S.M.; Du, X.; Tam, Y.Y.; Lin, P.J.; Chen, S.; Narayanannair, J.K.; Rajeev, K.G.; et al. Influence of polyethylene glycol lipid desorption rates on pharmacokinetics and pharmacodynamics of siRNA lipid nanoparticles. Mol. Ther. Nucleic Acids 2013, 2, e139. [Google Scholar] [CrossRef]
- Waggoner, L.E.; Miyasaki, K.F.; Kwon, E.J. Analysis of PEG-lipid anchor length on lipid nanoparticle pharmacokinetics and activity in a mouse model of traumatic brain injury. Biomater. Sci. 2023, 11, 4238–4253. [Google Scholar] [CrossRef]
- Suzuki, T.; Suzuki, Y.; Hihara, T.; Kubara, K.; Kondo, K.; Hyodo, K.; Yamazaki, K.; Ishida, T.; Ishihara, H. PEG shedding-rate-dependent blood clearance of PEGylated lipid nanoparticles in mice: Faster PEG shedding attenuates anti-PEG IgM production. Int. J. Pharm. 2020, 588, 119792. [Google Scholar] [CrossRef]
- Katakowski, J.A.; Mukherjee, G.; Wilner, S.E.; Maier, K.E.; Harrison, M.T.; DiLorenzo, T.P.; Levy, M.; Palliser, D. Delivery of sirnas to dendritic cells using DEC205-targeted lipid nanoparticles to inhibit immune responses. Mol. Ther. 2016, 24, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Parhiz, H.; Shuvaev, V.V.; Pardi, N.; Khoshnejad, M.; Kiseleva, R.Y.; Brenner, J.S.; Uhler, T.; Tuyishime, S.; Mui, B.L.; Tam, Y.K.; et al. Pecam-1 directed re-targeting of exogenous mRNA providing two orders of magnitude enhancement of vascular delivery and expression in lungs independent of apolipoprotein e-mediated uptake. J. Control. Release 2018, 291, 106–115. [Google Scholar] [CrossRef]
- Veiga, N.; Goldsmith, M.; Diesendruck, Y.; Ramishetti, S.; Rosenblum, D.; Elinav, E.; Behlke, M.A.; Benhar, I.; Peer, D. Leukocyte-specific siRNA delivery revealing IRF8 as a potential anti-inflammatory target. J. Control. Release 2019, 313, 33–41. [Google Scholar] [CrossRef]
- Rosenblum, D.; Gutkin, A.; Kedmi, R.; Ramishetti, S.; Veiga, N.; Jacobi, A.M.; Schubert, M.S.; Friedmann-Morvinski, D.; Cohen, Z.R.; Behlke, M.A.; et al. CRISPR-Cas9 genome editing using targeted lipid nanoparticles for cancer therapy. Sci. Adv. 2020, 6, eabc9450. [Google Scholar] [CrossRef]
- Tombácz, I.; Laczkó, D.; Shahnawaz, H.; Muramatsu, H.; Natesan, A.; Yadegari, A.; Papp, T.E.; Alameh, M.G.; Shuvaev, V.; Mui, B.L.; et al. Highly efficient CD4+ T cell targeting and genetic recombination using engineered CD4+ cell-homing mRNA-LNPs. Mol. Ther. 2021, 29, 3293–3304. [Google Scholar] [CrossRef]
- Escudé Martinez de Castilla, P.; Verdi, V.; de Voogt, W.; Sentí, M.E.; Koekman, A.C.; Rietveld, J.; van Kempen, S.; Yang, Q.; van Merris, J.; Jenster, G.; et al. Nanobody-decorated lipid nanoparticles for enhanced mRNA delivery to tumors in vivo. Adv. Healthc. Mater. 2025, 14, e2500605. [Google Scholar] [CrossRef]
- Papp, T.E.; Zeng, J.; Shahnawaz, H.; Akyianu, A.; Breda, L.; Yadegari, A.; Steward, J.; Shi, R.; Li, Q.; Mui, B.L.; et al. CD47 peptide-cloaked lipid nanoparticles promote cell-specific mRNA delivery. Mol. Ther. 2025, 33, 3195–3208. [Google Scholar] [CrossRef]
- Thalmayr, S.; Grau, M.; Peng, L.; Pöhmerer, J.; Wilk, U.; Folda, P.; Yazdi, M.; Weidinger, E.; Burghardt, T.; Höhn, M.; et al. Molecular chameleon carriers for nucleic acid delivery: The sweet spot between lipoplexes and polyplexes. Adv. Mater. 2023, 35, e2211105. [Google Scholar] [CrossRef]
- Germer, J.; Lessl, A.L.; Pöhmerer, J.; Grau, M.; Weidinger, E.; Höhn, M.; Yazdi, M.; Cappelluti, M.A.; Lombardo, A.; Lächelt, U.; et al. Lipo-xenopeptide polyplexes for CRISPR/Cas9 based gene editing at ultra-low dose. J. Control. Release 2024, 370, 239–255. [Google Scholar] [CrossRef] [PubMed]
- Yazdi, M.; Pohmerer, J.; Kafshgari, M.H.; Seidl, J.; Grau, M.; Hohn, M.; Vetter, V.; Hoch, C.C.; Wollenberg, B.; Multhoff, G.; et al. In vivo endothelial cell gene silencing by siRNA-LNPs tuned with lipoamino bundle chemical and ligand targeting. Small 2024, 20, e2400643. [Google Scholar] [CrossRef]
- Haase, F.; Pöhmerer, J.; Yazdi, M.; Grau, M.; Zeyn, Y.; Wilk, U.; Burghardt, T.; Höhn, M.; Hieber, C.; Bros, M.; et al. Lipoamino bundle LNPs for efficient mRNA transfection of dendritic cells and macrophages show high spleen selectivity. Eur. J. Pharm. Biopharm. 2024, 194, 95–109. [Google Scholar] [CrossRef]
- Philipp, J.; Dabkowska, A.; Reiser, A.; Frank, K.; Krzyszton, R.; Brummer, C.; Nickel, B.; Blanchet, C.E.; Sudarsan, A.; Ibrahim, M.; et al. pH-dependent structural transitions in cationic ionizable lipid mesophases are critical for lipid nanoparticle function. Proc. Natl. Acad. Sci. USA 2023, 120, e2310491120. [Google Scholar] [CrossRef]
- Yanez Arteta, M.; Kjellman, T.; Bartesaghi, S.; Wallin, S.; Wu, X.; Kvist, A.J.; Dabkowska, A.; Szekely, N.; Radulescu, A.; Bergenholtz, J.; et al. Successful reprogramming of cellular protein production through mRNA delivered by functionalized lipid nanoparticles. Proc. Natl. Acad. Sci. USA 2018, 115, E3351–E3360. [Google Scholar] [CrossRef]
- Rodl, W.; Schaffert, D.; Wagner, E.; Ogris, M. Synthesis of polyethylenimine-based nanocarriers for systemic tumor targeting of nucleic acids. Methods Mol. Biol. 2013, 948, 105–120. [Google Scholar] [CrossRef]
- Steffens, R.C.; Thalmayr, S.; Weidinger, E.; Seidl, J.; Folda, P.; Hohn, M.; Wagner, E. Modulating efficacy and cytotoxicity of lipoamino fatty acid nucleic acid carriers using disulfide or hydrophobic spacers. Nanoscale 2024, 16, 13988–14005. [Google Scholar] [CrossRef]
- Morys, S.; Levacic, A.K.; Urnauer, S.; Kempter, S.; Kern, S.; Radler, J.O.; Spitzweg, C.; Lachelt, U.; Wagner, E. Influence of defined hydrophilic blocks within oligoaminoamide copolymers: Compaction versus shielding of pdna nanoparticles. Polymers 2017, 9, 142. [Google Scholar] [CrossRef]
- Smith, R.J.; Beck, R.W.; Prevette, L.E. Impact of molecular weight and degree of conjugation on the thermodynamics of DNA complexation and stability of polyethylenimine-graft-poly(ethylene glycol) copolymers. Biophys. Chem. 2015, 203–204, 12–21. [Google Scholar] [CrossRef]
- Koning, G.A.; Morselt, H.W.; Kamps, J.A.; Scherphof, G.L. Uptake and intracellular processing of peg-liposomes and peg-immunoliposomes by kupffer cells in vitro. J. Liposome Res. 2001, 11, 195–209. [Google Scholar] [CrossRef]
- Mishra, S.; Webster, P.; Davis, M.E. Pegylation significantly affects cellular uptake and intracellular trafficking of non-viral gene delivery particles. Eur.J Cell Biol. 2004, 83, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Walker, G.F.; Fella, C.; Pelisek, J.; Fahrmeir, J.; Boeckle, S.; Ogris, M.; Wagner, E. Toward synthetic viruses: Endosomal pH-triggered deshielding of targeted polyplexes greatly enhances gene transfer in vitro and in vivo. Mol. Ther. 2005, 11, 418–425. [Google Scholar] [CrossRef]
- Wang, T.; Upponi, J.R.; Torchilin, V.P. Design of multifunctional non-viral gene vectors to overcome physiological barriers: Dilemmas and strategies. Int. J. Pharm. 2012, 427, 3–20. [Google Scholar] [CrossRef]
- Lai, T.C.; Kataoka, K.; Kwon, G.S. Pluronic-based cationic block copolymer for forming pDNA polyplexes with enhanced cellular uptake and improved transfection efficiency. Biomaterials 2011, 32, 4594–4603. [Google Scholar] [CrossRef]
- Vetter, V.C.; Wagner, E. Targeting nucleic acid-based therapeutics to tumors: Challenges and strategies for polyplexes. J. Control. Release 2022, 346, 110–135. [Google Scholar] [CrossRef]
- Chen, J.; Gamou, S.; Takayanagi, A.; Shimizu, N. A novel gene delivery system using EGF receptor-mediated endocytosis. FEBS Lett. 1994, 338, 167–169. [Google Scholar] [CrossRef]
- Kircheis, R.; Ostermann, E.; Wolschek, M.F.; Lichtenberger, C.; Magin-Lachmann, C.; Wightman, L.; Kursa, M.; Wagner, E. Tumor-targeted gene delivery of tumor necrosis factor-alpha induces tumor necrosis and tumor regression without systemic toxicity. Cancer. Gene Ther. 2002, 9, 673–680. [Google Scholar] [CrossRef][Green Version]
- Bellocq, N.C.; Pun, S.H.; Jensen, G.S.; Davis, M.E. Transferrin-containing, cyclodextrin polymer-based particles for tumor-targeted gene delivery. Bioconjug. Chem. 2003, 14, 1122–1132. [Google Scholar] [CrossRef] [PubMed]
- Merdan, T.; Callahan, J.; Petersen, H.; Kunath, K.; Bakowsky, U.; Kopeckova, P.; Kissel, T.; Kopecek, J. Pegylated polyethylenimine-fab’ antibody fragment conjugates for targeted gene delivery to human ovarian carcinoma cells. Bioconjug. Chem. 2003, 14, 989–996. [Google Scholar] [CrossRef] [PubMed]
- Oba, M.; Fukushima, S.; Kanayama, N.; Aoyagi, K.; Nishiyama, N.; Koyama, H.; Kataoka, K. Cyclic RGD peptide-conjugated polyplex micelles as a targetable gene delivery system directed to cells possessing αvβ3 and αvβ5 integrins. Bioconjug. Chem. 2007, 18, 1415–1423. [Google Scholar] [CrossRef] [PubMed]
- Oba, M.; Aoyagi, K.; Miyata, K.; Matsumoto, Y.; Itaka, K.; Nishiyama, N.; Yamasaki, Y.; Koyama, H.; Kataoka, K. Polyplex micelles with cyclic RGD peptide ligands and disulfide cross-links directing to the enhanced transfection via controlled intracellular trafficking. Mol. Pharm. 2008, 5, 1080–1092. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zheng, M.; Librizzi, D.; Renette, T.; Merkel, O.M.; Kissel, T. Efficient and tumor targeted sirna delivery by polyethylenimine-graft-polycaprolactone-block-poly(ethylene glycol)-folate (pei-pcl-peg-fol). Mol. Pharm. 2016, 13, 134–143. [Google Scholar] [CrossRef]
- Kim, M.W.; Jeong, H.Y.; Kang, S.J.; Choi, M.J.; You, Y.M.; Im, C.S.; Lee, T.S.; Song, I.H.; Lee, C.G.; Rhee, K.J.; et al. Cancer-targeted nucleic acid delivery and quantum dot imaging using EGF receptor aptamer-conjugated lipid nanoparticles. Sci. Rep. 2017, 7, 9474. [Google Scholar] [CrossRef]
- Mickler, F.M.; Mockl, L.; Ruthardt, N.; Ogris, M.; Wagner, E.; Brauchle, C. Tuning nanoparticle uptake: Live-cell imaging reveals two distinct endocytosis mechanisms mediated by natural and artificial EGFR targeting ligand. Nano Lett. 2012, 12, 3417–3423. [Google Scholar] [CrossRef]
- Ren, H.; Zhou, L.; Liu, M.; Lu, W.; Gao, C. Peptide GE11–polyethylene glycol–polyethylenimine for targeted gene delivery in laryngeal cancer. Med. Oncol. 2015, 32, 185. [Google Scholar] [CrossRef]
- Lee, D.; Lee, Y.M.; Kim, J.; Lee, M.K.; Kim, W.J. Enhanced tumor-targeted gene delivery by bioreducible polyethylenimine tethering EGFR divalent ligands. Biomater. Sci. 2015, 3, 1096–1104. [Google Scholar] [CrossRef]
- Schmohl, K.A.; Gupta, A.; Grunwald, G.K.; Trajkovic-Arsic, M.; Klutz, K.; Braren, R.; Schwaiger, M.; Nelson, P.J.; Ogris, M.; Wagner, E.; et al. Imaging and targeted therapy of pancreatic ductal adenocarcinoma using the theranostic sodium iodide symporter (NIS) gene. Oncotarget 2017, 8, 33393–33404. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, J.; Truebenbach, I.; Reinhard, S.; Klein, P.M.; Höhn, M.; Kern, S.; Morys, S.; Loy, D.M.; Wagner, E.; et al. Double click-functionalized siRNA polyplexes for gene silencing in epidermal growth factor receptor-positive tumor cells. ACS Biomater. Sci. Eng. 2020, 6, 1074–1089. [Google Scholar] [CrossRef]
- Yu, L.; Wang, Q.; Wong, R.C.H.; Zhao, S.; Ng, D.K.P.; Lo, P.-C. Synthesis and biological evaluation of phthalocyanine-peptide conjugate for EGFR-targeted photodynamic therapy and bioimaging. Dye. Pigment. 2019, 163, 197–203. [Google Scholar] [CrossRef]
- Kursa, M.; Walker, G.F.; Roessler, V.; Ogris, M.; Roedl, W.; Kircheis, R.; Wagner, E. Novel shielded transferrin-polyethylene glycol-polyethylenimine/DNA complexes for systemic tumor-targeted gene transfer. Bioconjug. Chem. 2003, 14, 222–231. [Google Scholar] [CrossRef]
- Maurstad, G.; Stokke, B.T.; Varum, K.M.; Strand, S.P. Pegylated chitosan complexes DNA while improving polyplex colloidal stability and gene transfection efficiency. Carbohydr. Polym. 2013, 94, 436–443. [Google Scholar] [CrossRef]
- Tang, M.X.; Szoka, F.C. The influence of polymer structure on the interactions of cationic polymers with DNA and morphology of the resulting complexes. Gene Ther. 1997, 4, 823–832. [Google Scholar] [CrossRef] [PubMed]
- Needham, D.; McIntosh, T.J.; Lasic, D.D. Repulsive interactions and mechanical stability of polymer-grafted lipid membranes. Biochim. Biophys. Acta 1992, 1108, 40–48. [Google Scholar] [CrossRef]
- Oupicky, D.; Ogris, M.; Howard, K.A.; Dash, P.R.; Ulbrich, K.; Seymour, L.W. Importance of lateral and steric stabilization of polyelectrolyte gene delivery vectors for extended systemic circulation. Mol. Ther. 2002, 5, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Pun, S.H.; Davis, M.E. Development of a nonviral gene delivery vehicle for systemic application. Bioconjugate Chem. 2002, 13, 630–639. [Google Scholar] [CrossRef] [PubMed]
- Vader, P.; van der Aa, L.J.; Engbersen, J.F.; Storm, G.; Schiffelers, R.M. Physicochemical and biological evaluation of siRNA polyplexes based on pegylated poly(amido amine)s. Pharm. Res. 2012, 29, 352–361. [Google Scholar] [CrossRef]
- Adolph, E.J.; Nelson, C.E.; Werfel, T.A.; Guo, R.; Davidson, J.M.; Guelcher, S.A.; Duvall, C.L. Enhanced performance of plasmid DNA polyplexes stabilized by a combination of core hydrophobicity and surface pegylation. J. Mater. Chem. B 2014, 2, 8154–8164. [Google Scholar] [CrossRef]
- Wu, T.; Wang, L.; Ding, S.; You, Y. Fluorinated PEG-polypeptide polyplex micelles have good serum-resistance and low cytotoxicity for gene delivery. Macromol. Biosci. 2017, 17, 1700114. [Google Scholar] [CrossRef]
- Paunovska, K.; Sago, C.D.; Monaco, C.M.; Hudson, W.H.; Castro, M.G.; Rudoltz, T.G.; Kalathoor, S.; Vanover, D.A.; Santangelo, P.J.; Ahmed, R.; et al. A direct comparison of in vitro and in vivo nucleic acid delivery mediated by hundreds of nanoparticles reveals a weak correlation. Nano Lett. 2018, 18, 2148–2157. [Google Scholar] [CrossRef]
- Paunovska, K.; Loughrey, D.; Sago, C.D.; Langer, R.; Dahlman, J.E. Using large datasets to understand nanotechnology. Adv. Mater. 2019, 31, e1902798. [Google Scholar] [CrossRef]
- Huayamares, S.G.; Lokugamage, M.P.; Rab, R.; Da Silva Sanchez, A.J.; Kim, H.; Radmand, A.; Loughrey, D.; Lian, L.; Hou, Y.; Achyut, B.R.; et al. High-throughput screens identify a lipid nanoparticle that preferentially delivers mRNA to human tumors in vivo. J. Control. Release 2023, 357, 394–403. [Google Scholar] [CrossRef]
- Berger, S.; Berger, M.; Bantz, C.; Maskos, M.; Wagner, E. Performance of nanoparticles for biomedical applications: The in vitro/in vivo discrepancy. Biophys. Rev. 2022, 3, 011303. [Google Scholar] [CrossRef]
- Dilliard, S.A.; Sun, Y.; Brown, M.O.; Sung, Y.C.; Chatterjee, S.; Farbiak, L.; Vaidya, A.; Lian, X.; Wang, X.; Lemoff, A.; et al. The interplay of quaternary ammonium lipid structure and protein corona on lung-specific mRNA delivery by selective organ targeting (sort) nanoparticles. J. Control. Release 2023, 361, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Alberg, I.; Kramer, S.; Schinnerer, M.; Hu, Q.; Seidl, C.; Leps, C.; Drude, N.; Möckel, D.; Rijcken, C.; Lammers, T.; et al. Polymeric nanoparticles with neglectable protein corona. Small 2020, 16, e1907574. [Google Scholar] [CrossRef]
- Zhang, X.; Si, S.; Lieberwirth, I.; Landfester, K.; Mailänder, V. Engineered protein corona sustains stealth functionality of nanocarriers in plasma. J. Nano Biotechnol. 2025, 23, 512. [Google Scholar] [CrossRef]
- Voke, E.; Arral, M.; Squire, H.J.; Lin, T.J.; Coreas, R.; Lui, A.; Iavarone, A.T.; Pinals, R.L.; Whitehead, K.A.; Landry, M. Protein corona formed on lipid nanoparticles compromises delivery efficiency of mRNA cargo. Nat. Commun. 2025, 16, 8699. [Google Scholar] [CrossRef]
- Rademacker, S.; Carneiro, S.P.; Molbay, M.; Catapano, F.; Forné, I.; Imhof, A.; Wibel, R.; Heidecke, C.; Hölig, P.; Merkel, O.M. The impact of lipid compositions on siRNA and mRNA lipid nanoparticle performance for pulmonary delivery. Eur. J. Pharm. Sci. 2025, 212, 107182. [Google Scholar] [CrossRef]
- He, X.; Wang, R.; Cao, Y.; Ding, Y.; Chang, Y.; Dong, H.; Xie, R.; Zhong, G.; Yang, H.; Li, J. Lung-specific mrna delivery by ionizable lipids with defined structure-function relationship and unique protein corona feature. Adv. Sci. 2025, 12, e2416525. [Google Scholar] [CrossRef]
- van Straten, D.; Sork, H.; van de Schepop, L.; Frunt, R.; Ezzat, K.; Schiffelers, R.M. Biofluid specific protein coronas affect lipid nanoparticle behavior in vitro. J. Control. Release 2024, 373, 481–492. [Google Scholar] [CrossRef]
- Lundqvist, M.; Stigler, J.; Elia, G.; Lynch, I.; Cedervall, T.; Dawson, K.A. Nanoparticle size and surface properties determine the protein corona with possible implications for biological impacts. Proc. Natl. Acad. Sci. USA 2008, 105, 14265–14270. [Google Scholar] [CrossRef]
- Lundqvist, M.; Stigler, J.; Cedervall, T.; Berggard, T.; Flanagan, M.B.; Lynch, I.; Elia, G.; Dawson, K. The evolution of the protein corona around nanoparticles: A test study. ACS Nano 2011, 5, 7503–7509. [Google Scholar] [CrossRef] [PubMed]
- Cedervall, T.; Lynch, I.; Lindman, S.; Berggard, T.; Thulin, E.; Nilsson, H.; Dawson, K.A.; Linse, S. Understanding the nanoparticle-protein corona using methods to quantify exchange rates and affinities of proteins for nanoparticles. Proc. Natl. Acad. Sci. USA 2007, 104, 2050–2055. [Google Scholar] [CrossRef]
- Vilanova, O.; Mittag, J.J.; Kelly, P.M.; Milani, S.; Dawson, K.A.; Radler, J.O.; Franzese, G. Understanding the kinetics of protein-nanoparticle corona formation. ACS Nano 2016, 10, 10842–10850. [Google Scholar] [CrossRef]
- Milani, S.; Bombelli, F.B.; Pitek, A.S.; Dawson, K.A.; Radler, J. Reversible versus irreversible binding of transferrin to polystyrene nanoparticles: Soft and hard corona. ACS Nano 2012, 6, 2532–2541. [Google Scholar] [CrossRef]
- Zelphati, O.; Uyechi, L.S.; Barron, L.G.; Szoka, F.C., Jr. Effect of serum components on the physico-chemical properties of cationic lipid/oligonucleotide complexes and on their interactions with cells. Biochim. Biophys. Acta 1998, 1390, 119–133. [Google Scholar] [CrossRef] [PubMed]
- Senior, J.H.; Trimble, K.R.; Maskiewicz, R. Interaction of positively-charged liposomes with blood: Implications for their application in vivo. Biochim. Biophys. Acta 1991, 1070, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Casper, J.; Schenk, S.H.; Parhizkar, E.; Detampel, P.; Dehshahri, A.; Huwyler, J. Polyethylenimine (PEI) in gene therapy: Current status and clinical applications. J. Control. Release 2023, 362, 667–691. [Google Scholar] [CrossRef]
- Takeda, K.M.; Yamasaki, Y.; Dirisala, A.; Ikeda, S.; Tockary, T.A.; Toh, K.; Osada, K.; Kataoka, K. Effect of shear stress on structure and function of polyplex micelles from poly(ethylene glycol)-poly(l-lysine) block copolymers as systemic gene delivery carrier. Biomaterials 2017, 126, 31–38. [Google Scholar] [CrossRef]
- Yin, D.; Wen, H.; Wu, G.; Li, S.; Liu, C.; Lu, H.; Liang, D. Pegylated gene carriers in serum under shear flow. Soft Matter 2020, 16, 2301–2310. [Google Scholar] [CrossRef]
- Wen, H.; Yu, Q.; Yin, Y.; Pan, W.; Yang, S.; Liang, D. Shear effects on stability of DNA complexes in the presence of serum. Biomacromolecules 2017, 18, 3252–3259. [Google Scholar] [CrossRef]
- Hatakeyama, H.; Akita, H.; Harashima, H. A multifunctional envelope type nano device (MEND) for gene delivery to tumours based on the EPR effect: A strategy for overcoming the PEG dilemma. Adv. Drug Deliv. Rev. 2011, 63, 152–160. [Google Scholar] [CrossRef]
- Guo, X.; MacKay, J.A.; Szoka, F.C., Jr. Mechanism of pH-triggered collapse of phosphatidylethanolamine liposomes stabilized by an ortho ester polyethyleneglycol lipid. Biophys. J. 2003, 84, 1784–1795. [Google Scholar] [CrossRef]
- Murthy, N.; Campbell, J.; Fausto, N.; Hoffman, A.S.; Stayton, P.S. Design and synthesis of pH-responsive polymeric carriers that target uptake and enhance the intracellular delivery of oligonucleotides. J. Control. Release 2003, 89, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Fella, C.; Walker, G.F.; Ogris, M.; Wagner, E. Amine-reactive pyridylhydrazone-based PEG reagents for pH-reversible PEI polyplex shielding. Eur. J. Pharm. Sci. 2008, 34, 309–320. [Google Scholar] [CrossRef]
- Nie, Y.; Günther, M.; Gu, Z.; Wagner, E. Pyridylhydrazone-based PEGylation for pH-reversible lipopolyplex shielding. Biomaterials 2011, 32, 858–869. [Google Scholar] [CrossRef] [PubMed]
- Takae, S.; Miyata, K.; Oba, M.; Ishii, T.; Nishiyama, N.; Itaka, K.; Yamasaki, Y.; Koyama, H.; Kataoka, K. PEG-detachable polyplex micelles based on disulfide-linked block catiomers as bioresponsive nonviral gene vectors. J. Am. Chem. Soc. 2008, 130, 6001–6009. [Google Scholar] [CrossRef] [PubMed]
- Hatakeyama, H.; Akita, H.; Kogure, K.; Oishi, M.; Nagasaki, Y.; Kihira, Y.; Ueno, M.; Kobayashi, H.; Kikuchi, H.; Harashima, H. Development of a novel systemic gene delivery system for cancer therapy with a tumor-specific cleavable PEG-lipid. Gene Ther. 2007, 14, 68–77. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Folda, P.; Weidinger, E.; Seidl, J.; Yazdi, M.; Pöhmerer, J.; Grau, M.; Minde, D.P.; Ali, M.; Kimna, C.; Wagner, E. PEGylation Enhances Colloidal Stability and Promotes Ligand-Mediated Targeting of LAF–Xenopeptide mRNA Complexes. Polymers 2025, 17, 2979. https://doi.org/10.3390/polym17222979
Folda P, Weidinger E, Seidl J, Yazdi M, Pöhmerer J, Grau M, Minde DP, Ali M, Kimna C, Wagner E. PEGylation Enhances Colloidal Stability and Promotes Ligand-Mediated Targeting of LAF–Xenopeptide mRNA Complexes. Polymers. 2025; 17(22):2979. https://doi.org/10.3390/polym17222979
Chicago/Turabian StyleFolda, Paul, Eric Weidinger, Johanna Seidl, Mina Yazdi, Jana Pöhmerer, Melina Grau, David P. Minde, Mayar Ali, Ceren Kimna, and Ernst Wagner. 2025. "PEGylation Enhances Colloidal Stability and Promotes Ligand-Mediated Targeting of LAF–Xenopeptide mRNA Complexes" Polymers 17, no. 22: 2979. https://doi.org/10.3390/polym17222979
APA StyleFolda, P., Weidinger, E., Seidl, J., Yazdi, M., Pöhmerer, J., Grau, M., Minde, D. P., Ali, M., Kimna, C., & Wagner, E. (2025). PEGylation Enhances Colloidal Stability and Promotes Ligand-Mediated Targeting of LAF–Xenopeptide mRNA Complexes. Polymers, 17(22), 2979. https://doi.org/10.3390/polym17222979







