Water Absorption and Solubility of Fluoride-Based Restorative Materials Exposed to Ionizing Radiation
Abstract
1. Introduction
Study Aim
2. Materials and Methods
2.1. Sample Size Determination
2.2. Sample Preparation
2.3. Water Absorption and Solubility
2.4. Statistical Analysis
3. Results
4. Discussion
Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Pereira, I.F.; Firmino, R.T.; Meira, H.C.; Do Egito Vasconcelos, B.C.; De Souza Noronha, V.R.A.; Santos, V.R. Radiation-induced oral mucositis in Brazilian patients: Prevalence and associated factors. In Vivo 2019, 33, 605–609. [Google Scholar] [CrossRef]
- de Amoêdo Campos Velo, M.M.; Farha, A.L.H.; da Silva Santos, P.S.; Shiota, A.; Sansavino, S.Z.; Souza, A.T.; Honório, H.M.; Wang, L. Radiotherapy alters the composition, structural and mechanical properties of root dentin in vitro. Clin. Oral Investig. 2018, 22, 2871–2878. [Google Scholar] [CrossRef]
- Rodrigues, R.B.; Soares, C.J.; Junior, P.C.S.; Lara, V.C.; Arana-Chavez, V.E.; Novais, V.R. Influence of radiotherapy on the dentin properties and bond strength. Clin. Oral Investig. 2018, 22, 875–883. [Google Scholar] [CrossRef]
- Krol, D.M.; Whelan, K.; The Section on Oral Health. Maintaining and improving the oral health of young children. Pediatrics 2023, 151, e2022060417. [Google Scholar] [CrossRef] [PubMed]
- Elad, S.; Cheng, K.K.F.; Lalla, R.V.; Yarom, N.; Hong, C.; Logan, R.M.; Bowen, J.; Gibson, R.; Saunders, D.P.; Zadik, Y.; et al. MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer 2020, 126, 4423–4431. [Google Scholar] [CrossRef] [PubMed]
- Mercadante, V.; Jensen, S.B.; Smith, D.K.; Bohlke, K.; Bauman, J.; Brennan, M.T.; Coppes, R.P.; Jessen, N.; Malhotra, N.K.; Murphy, B.; et al. Salivary gland hypofunction and/or xerostomia induced by nonsurgical cancer therapies: ISOO/MASCC/ASCO guideline. J. Clin. Oncol. 2021, 39, 2825–2843. [Google Scholar] [CrossRef]
- Douchy, L.; Gauthier, R.; Abouelleil-Sayed, H.; Colon, P.; Grosgogeat, B.; Bosco, J. The effect of therapeutic radiation on dental enamel and dentin: A systematic review. Dent Mater 2022, 38, e181–e201. [Google Scholar] [CrossRef] [PubMed]
- Argiris, A.; Karamouzis, M.V.; Raben, D.; Ferris, R.L. Head and neck cancer. Lancet 2008, 371, 1695–1709. [Google Scholar] [CrossRef]
- Lieshout, H.F.J.; Bots, C.P. The effect of radiotherapy on dental hard tissue—A systematic review. Clin. Oral Investig. 2014, 18, 17–24. [Google Scholar] [CrossRef]
- Palmier, N.R.; Madrid Troconis, C.C.; Normando, A.G.C.; Guerra, E.N.S.; Araújo, A.L.D.; Arboleda, L.P.A.; Fonsêca, J.M.; Paglioni, M.d.P.; Gomes-Silva, W.; Filho, A.J.V.; et al. Impact of head and neck radiotherapy on the longevity of dental adhesive restorations: A systematic review and meta-analysis. J. Prosthet. Dent. 2021, 128, 886–896. [Google Scholar] [CrossRef]
- Muñoz, M.A.; Garín-Correa, C.; González-Arriagada, W.; Quintela Davila, X.; Häberle, P.; Bedran-Russo, A.; Luque-Martínez, I. The adverse effects of radiotherapy on the structure of dental hard tissues and longevity of dental restoration. Int. J. Radiat. Biol. 2020, 96, 910–918. [Google Scholar] [CrossRef]
- Arrifin, A.; Heidari, E.; Burke, M.; Fenlon, M.; Banerjee, A. The effect of radiotherapy for treatment of head and neck cancer on oral flora and saliva. Oral Health Prev. Dent. 2018, 16, 425–429. [Google Scholar]
- Catelan, A.; Padilha, A.C.S.; Salzedas, L.M.P.; Coclete, G.A.; dos Santos, P.H. Effect of radiotherapy on the radiopacity and flexural strength of a composite resin. Acta Odontol. Latinoam. 2008, 21, 159–162. [Google Scholar] [PubMed]
- Curtis, P.M.; Farman, A.G.; von Fraunhofer, J.A. Effects of gamma radiation on the in vitro wear of composite restorative materials. J. Dent. 1991, 19, 241–246. [Google Scholar] [CrossRef]
- Hegde, M.N.; Hegde, N.D.; Kumari, S.N.; Sanjeev, G.; Priya. Effect of ionising radiation on micro hardness property of restorative materials. J. Health Allied Sci. NU 2016, 6, 57–61. [Google Scholar] [CrossRef]
- Brandeburski, S.B.N.; Della Bona, A. Effect of ionizing radiation on properties of restorative materials. Dent. Mater. 2018, 34, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Taher, R.M.; Moharam, L.M.; Amin, A.E.; Zaazou, M.H.; El-Askary, F.S.; Ibrahim, M.N. The effect of radiation exposure and storage time on the degree of conversion and flexural strength of different resin composites. Bull. Natl. Res. Cent. 2021, 45, 146. [Google Scholar] [CrossRef]
- Itohamy, S.; Kandil, M.; El Refai, D. Head and neck cancer radiotherapy effect on some properties of different dental restorative materials: An in vitro study. J. Res. Med. Dent. Sci. 2020, 8, 153–161. [Google Scholar]
- Viero, F.L.; Boscolo, F.N.; Demarco, F.F.; Faot, F. Effect of radiotherapy on the hardness and surface roughness of two composite resins. Gen. Dent. 2011, 59, e168–e172. [Google Scholar] [PubMed]
- Dursun, E.; Güncü, G.N.; Dursun, C.K.; Kiremitçi, A.; Karabulut, E.; Akalın, F.A. Nanofilled and conventional resin-modified glass ionomer fillings combined with connective tissue grafts for treatment of gingival recessions with non-carious cervical lesions. J. Oral Sci. 2018, 60, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, S.K.; Nicholson, J.W. A review of glass-ionomer cements for clinical dentistry. J. Funct. Biomater. 2016, 7, 16. [Google Scholar] [CrossRef]
- Giannini, M.; Sauro, S. “Bioactivity” in restorative dentistry: Standing for the use of innovative materials to improve the longevity of restorations in routine dental practice. J. Adhes. Dent. 2021, 23, 176–178. [Google Scholar]
- Jefferies, S.R. Bioactive and biomimetic restorative materials: A comprehensive review. Part I. J. Esthet. Restor. Dent. 2014, 26, 14–26. [Google Scholar] [CrossRef]
- Hashem, D.F.; Foxton, R.; Manoharan, A.; Watson, T.F.; Banerjee, A. The physical characteristics of resin composite–calcium silicate interface as part of a layered/laminate adhesive restoration. Dent. Mater. 2014, 30, 343–349. [Google Scholar] [CrossRef]
- Kunert, M.; Piwonski, I.; Hardan, L.; Bourgi, R.; Sauro, S.; Inchingolo, F.; Lukomska-Szymanska, M. Dentine remineralisation induced by bioactive materials through mineral deposition: An in vitro study. Nanomaterials 2024, 14, 274. [Google Scholar] [CrossRef]
- Ivoclar; Vivadent, A.G.; Cention, N. Scientific Documentation. 2016. Available online: http://www.ivoclarvivadent.in/en-in/p/all/cention-n (accessed on 3 September 2019).
- Banic Vidal, L.S.; Veček, N.N.; Šalinović, I.; Miletić, I.; Klarić, E.; Jukić Krmek, S. Short-term fluoride release from ion-releasing dental materials. Acta Stomatol. Croat. 2023, 57, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Rifai, H.; Qasim, S.; Mahdi, S.; Lambert, M.J.; Zarazir, R.; Amenta, F.; Naim, S.; Mehanna, C. In vitro evaluation of the shear bond strength and fluoride release of a new bioactive dental composite material. J. Clin. Exp. Dent. 2022, 14, e55–e63. [Google Scholar] [CrossRef]
- Hokii, Y.; Carey, C.; Heiss, M.; Joshi, G. Fluoride ion release/recharge behavior of ion-releasing restorative materials. Dent. Mater. 2019, 35, e17–e18. [Google Scholar] [CrossRef]
- Arab, A.; Derakhshani, R.; Sayadi, M.H. Approaches for the Efficient Removal of Fluoride from Groundwater: A Comprehensive Review. Toxics. 2024, 12, 306. [Google Scholar] [CrossRef] [PubMed]
- Wanderley, E.; Lima, R.B.W.E.; De Vasconcelos, L.C.; Pontual, M.L.; Meireles, S.S.; Andrade, A.K.M.; Duarte, R.M. Effect of ionizing radiation on the properties of restorative materials. Indian J. Dent. Res. 2019, 30, 408–413. [Google Scholar] [CrossRef]
- ISO 4049:2019; Dentistry—Polymer-based restorative materials. International Organization for Standardization: Geneva, Switzerland, 2019.
- Turjanski, S.; Par, M.; Bergman, L.; Soče, M.; Grego, T.; Klarić Sever, E. Influence of ionizing radiation on fluoride-releasing dental restorative materials. Polymers 2023, 15, 632. [Google Scholar] [CrossRef]
- Amorim, D.M.G.; Veríssimo, A.H.; Ribeiro, A.K.C.; Souza, R.O.A.; Assunção, I.V.; Caldas, M.R.G.; Borges, B.C.D. Effects of ionizing radiation on surface properties of current restorative dental materials. J. Mater. Sci. Mater. Med. 2021, 32, 69. [Google Scholar] [CrossRef]
- Silva, E.M.; Amaral, C.M.; Jardim, R.N.; Barbosa, M.P.; Rabello, T.B. Influence of specimen dimension, water immersion protocol, and surface roughness on water sorption and solubility of resin-based restorative materials. Materials 2024, 17, 984. [Google Scholar] [CrossRef]
- Čekalović Agović, S.; Klarić, E.; Ivanišević, A.; Soče, M.; Grego, T.; Radin Nujić, I. The influence of ionizing radiation on fluoride release from restorative dental materials: A comparative in vitro study. Appl. Sci. 2024, 14, 9701. [Google Scholar] [CrossRef]
- Musanje, L.; Shu, M.; Darvell, B.W. Water sorption and mechanical behavior of cosmetic direct restorative materials in artificial saliva. Dent. Mater. 2001, 17, 394–401. [Google Scholar] [CrossRef]
- Braden, M.; Clarke, R.L. Water absorption characteristics of dental microfine composite filling materials. I. Proprietary materials. Biomaterials 1984, 5, 369–372. [Google Scholar] [CrossRef]
- Braden, M.; Causton, E.E.; Clarke, R.L. Diffusion of water in composite filling materials. J. Dent. Res. 1976, 55, 730–732. [Google Scholar] [CrossRef] [PubMed]
- Alshali, R.Z.; Salim, N.A.; Satterthwaite, J.D.; Silikas, N. Long-term sorption and solubility of bulk-fill and conventional resin-composites in water and artificial saliva. J. Dent. 2015, 43, 1511–1518. [Google Scholar] [CrossRef] [PubMed]
- Gonulol, N.; Ozer, S.; Sen Tunc, E. Water sorption, solubility, and color stability of giomer restoratives. J. Esthet. Restor. Dent. 2015, 27, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, J.W. Maturation processes in glass-ionomer dental cements. Acta Biomater. Odontol. Scand. 2018, 4, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Ilie, N. Maturation of restorative glass ionomers with simplified application procedure. J. Dent. 2018, 79, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Savas, S.; Colgecen, O.; Yasa, B.; Kucukyilmaz, E. Color stability, roughness, and water sorption/solubility of glass ionomer-based restorative materials. Niger. J. Clin. Pract. 2019, 22, 824–832. [Google Scholar]
- Jih, M.G.; Cho, H.J.; Cha, E.J.; Park, T.Y. Comparative analysis of water absorption and water solubility of alkasite-based restorative material. J. Korean Dent. Sci. 2023, 16, 74–79. [Google Scholar]
| Material | Manufacturer (Country) | Classification/Type | Main Ingredients | Supplier/Purchase Link | LOT |
|---|---|---|---|---|---|
| Equia Forte HT | GC Corporation (Tokyo, Japan) | Resin-coated high-viscosity glass ionomer restorative | Powder: fluoroaluminosilicate glass; Liquid: polyacrylic acid solution; Protective light-cured resin coat | https://www.gc.dental | 2303108 |
| Cention Forte | Ivoclar Vivadent (Schaan, Liechtenstein) | Alkasite restorative material | Resin matrix (UDMA, DCP, aromatic aliphatic UDMA); alkaline glass fillers (fluoride, calcium, hydroxide release) | https://www.ivoclar.com | ZL08SP |
| Fuji IX GP Extra | GC Corporation (Tokyo, Japan) | Conventional high-viscosity GIC | Powder: fluoroaluminosilicate glass; Liquid: polyacrylic acid solution | https://www.gc.dental | 230105A |
| Fuji Triage | GC Corporation (Tokyo, Japan) | Glass ionomer-based fissure sealant/protective coating | Powder: fluoroaluminosilicate glass, pigments; Liquid: polyacrylic acid solution | https://www.gc.dental | 2206031 |
| Activa Presto | Pulpdent Corporation (Watertown, MA, USA) | Bioactive resin-modified restorative | Ionic resin system; bioactive glass fillers releasing fluoride, calcium, phosphate | https://www.pulpdent.com | 220419 |
| Luminos | Unodent (Witham, UK) | Resin-based fluoride-releasing restorative | Methacrylate-based resin matrix; fluoride-containing fillers | https://www.presidentdental.de | 20250225 |
| Beautifil II | Shofu Inc. (Kyoto, Japan) | Giomer composite | Resin matrix (Bis-GMA, TEGDMA); surface prereacted glass-ionomer (PRG) fillers | https://www.shofu.com | 032266 |
| Absorption Day 1 | Absorption Day 7 | Absorption Day 14 | Absorption Day 21 | Absorption Day 35 | Solubility 1 | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Material | Type | Median | IQR | p Value | Median | IQR | p Value | Median | IQR | p Value | Median | IQR | p Value | Median | IQR | p Value | Median | IQR | p Value |
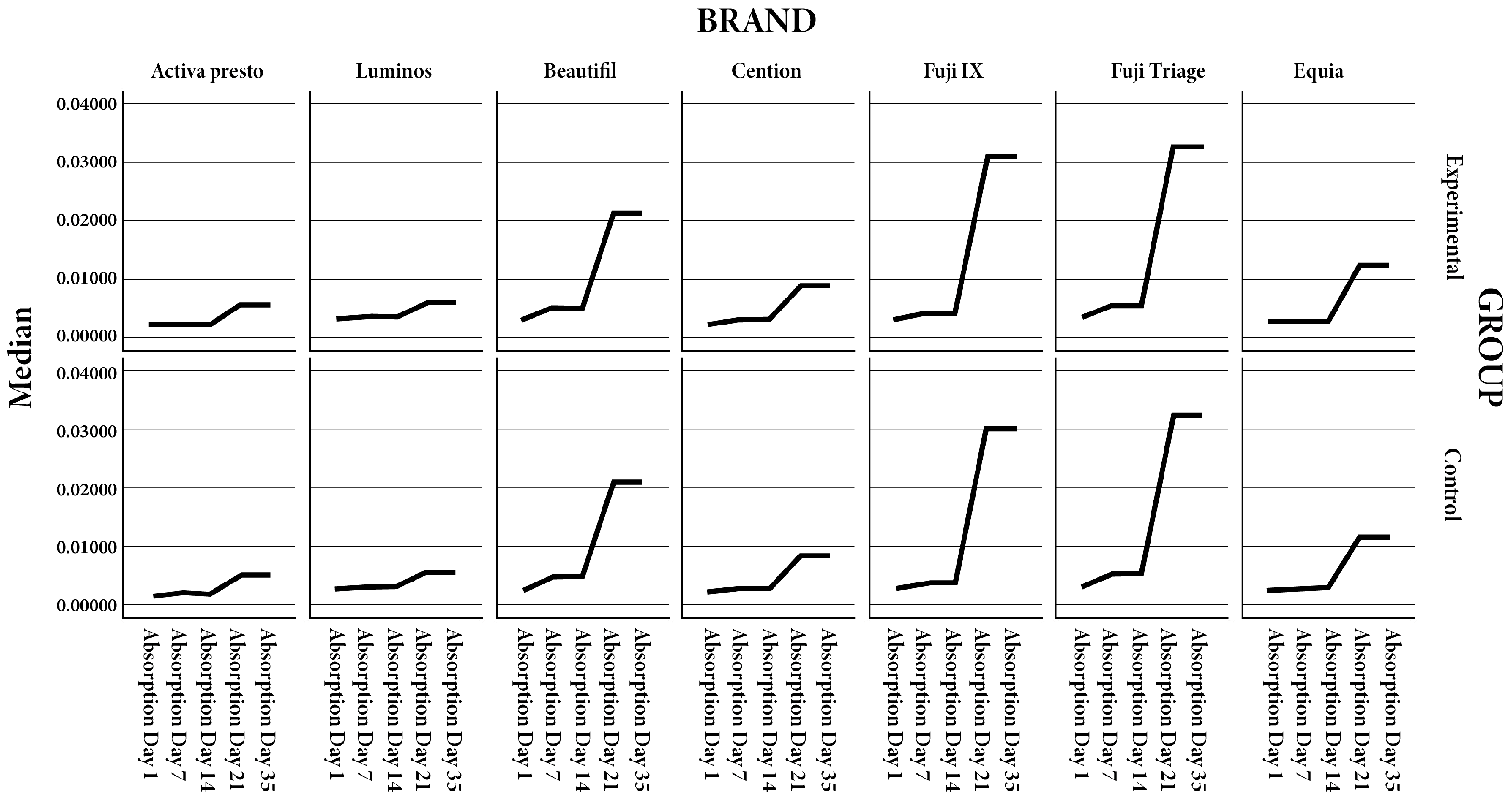
| Activa presto | E | 0.0017 | 0.0015–0.0018 | 0.0021 | 0.0019–0.0025 | 0.00205 | 0.002–0.0024 | 0.00525 | 0.0043–0.0058 | 0.00505 | 0.0042–0.0059 | 0.0099 | 0.0098–0.016 | ||||||
| Activa presto | C | 0.0016 | 0.0014–0.0018 | 0.529 | 0.00205 | 0.0019–0.0023 | 0.481 | 0.00205 | 0.0019–0.0023 | 0.436 | 0.00505 | 0.0043–0.0058 | 0.796 | 0.00485 | 0.004–0.0058 | 0.739 | 0.0098 | 0.0095–0.014 | 0.481 |
| Luminos | E | 0.0027 | 0.0025–0.0028 | 0.0035 | 0.003–0.0038 | 0.0032 | 0.003–0.0034 | 0.0057 | 0.005–0.0062 | 0.0057 | 0.0052–0.006 | 0.00875 | 0.0085–0.0114 | ||||||
| Luminos | C | 0.00255 | 0.0025–0.0026 | 0.315 | 0.003 | 0.003–0.0034 | 0.28 | 0.003 | 0.003–0.0032 | 0.353 | 0.0056 | 0.005–0.0062 | 0.739 | 0.0056 | 0.005–0.0062 | 0.631 | 0.0085 | 0.0083–0.0112 | 0.481 |
| Beautifil | E | 0.0025 | 0.002–0.003 | 0.0047 | 0.0042–0.0052 | 0.0048 | 0.004–0.005 | 0.02115 | 0.017–0.0234 | 0.02105 | 0.017–0.0231 | 0.01175 | 0.0013–0.0126 | ||||||
| Beautifil | C | 0.00215 | 0.002–0.0029 | 0.28 | 0.0046 | 0.0042–0.0052 | 0.579 | 0.00465 | 0.004–0.005 | 0.739 | 0.02105 | 0.017–0.0234 | 0.684 | 0.02105 | 0.017–0.023 | 0.796 | 0.01175 | 0.0012–0.0126 | 0.579 |
| Cention | E | 0.00195 | 0.0018–0.0024 | 0.00275 | 0.0025–0.003 | 0.0029 | 0.0027–0.0035 | 0.00855 | 0.007–0.0096 | 0.0087 | 0.007–0.0098 | 0.00825 | 0.007–0.0094 | ||||||
| Cention | C | 0.0019 | 0.0018–0.0022 | 0.684 | 0.0027 | 0.0025–0.0028 | 0.481 | 0.0028 | 0.0026–0.0033 | 0.631 | 0.0085 | 0.0069–0.0092 | 0.579 | 0.0085 | 0.0069–0.0092 | 0.631 | 0.0086 | 0.0092–0.664 | 0.664 |
| Fuji IX | E | 0.00295 | 0.0024–0.0032 | 0.00385 | 0.0035–0.0042 | 0.004 | 0.0039–0.0046 | 0.03075 | 0.0299–0.0317 | 0.03075 | 0.0299–0.0317 | 0.02185 | 0.0199–0.0235 | ||||||
| Fuji IX | C | 0.00285 | 0.0022–0.003 | 0.739 | 0.0037 | 0.0034–0.004 | 0.579 | 0.004 | 0.0037–0.0045 | 0.796 | 0.0303 | 0.0299–0.0311 | 0.631 | 0.0303 | 0.0299–0.0311 | 0.353 | 0.0206 | 0.0218–0.028 | 0.28 |
| Fuji Triage | E | 0.0033 | 0.0028–0.0037 | 0.0054 | 0.005–0.0057 | 0.0051 | 0.0049–0.0054 | 0.03255 | 0.032–0.0329 | 0.03265 | 0.032–0.0329 | 0.0221 | 0.0219–0.0235 | ||||||
| Fuji Triage | C | 0.003 | 0.0027–0.0032 | 0.579 | 0.00525 | 0.005–0.0055 | 0.315 | 0.00505 | 0.0046–0.0052 | 0.684 | 0.03235 | 0.032–0.0329 | 0.739 | 0.03235 | 0.032–0.0329 | 0.739 | 0.022 | 0.0214–0.0232 | 0.436 |
| Equia | E | 0.00235 | 0.0018–0.0028 | 0.0027 | 0.0022–0.0029 | 0.00285 | 0.0026–0.003 | 0.01185 | 0.0112–0.0123 | 0.01185 | 0.0112–0.0123 | 0.01245 | 0.0118–0.0129 | ||||||
| Equia | C | 0.0022 | 0.0019–0.0027 | 0.529 | 0.0026 | 0.0024–0.0028 | 0.796 | 0.0028 | 0.0024–0.0032 | 0.739 | 0.01165 | 0.0111–0.0134 | 0.684 | 0.01165 | 0.0111–0.0134 | 0.684 | 0.0127 | 0.012–0.0129 | 0.684 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Čekalović Agović, S.; Klarić, E.; Ivanišević, A.; Soče, M.; Grego, T.; Radin Nujić, I. Water Absorption and Solubility of Fluoride-Based Restorative Materials Exposed to Ionizing Radiation. Polymers 2025, 17, 2736. https://doi.org/10.3390/polym17202736
Čekalović Agović S, Klarić E, Ivanišević A, Soče M, Grego T, Radin Nujić I. Water Absorption and Solubility of Fluoride-Based Restorative Materials Exposed to Ionizing Radiation. Polymers. 2025; 17(20):2736. https://doi.org/10.3390/polym17202736
Chicago/Turabian StyleČekalović Agović, Sara, Eva Klarić, Ana Ivanišević, Majana Soče, Timor Grego, and Irena Radin Nujić. 2025. "Water Absorption and Solubility of Fluoride-Based Restorative Materials Exposed to Ionizing Radiation" Polymers 17, no. 20: 2736. https://doi.org/10.3390/polym17202736
APA StyleČekalović Agović, S., Klarić, E., Ivanišević, A., Soče, M., Grego, T., & Radin Nujić, I. (2025). Water Absorption and Solubility of Fluoride-Based Restorative Materials Exposed to Ionizing Radiation. Polymers, 17(20), 2736. https://doi.org/10.3390/polym17202736






