In Situ Synthesis of Non-Cytotoxic Tellurium Nanoparticle and Methacrylate Photopolymer Resin Composite with Antibacterial Activity
Abstract
1. Introduction
2. Materials and Methods
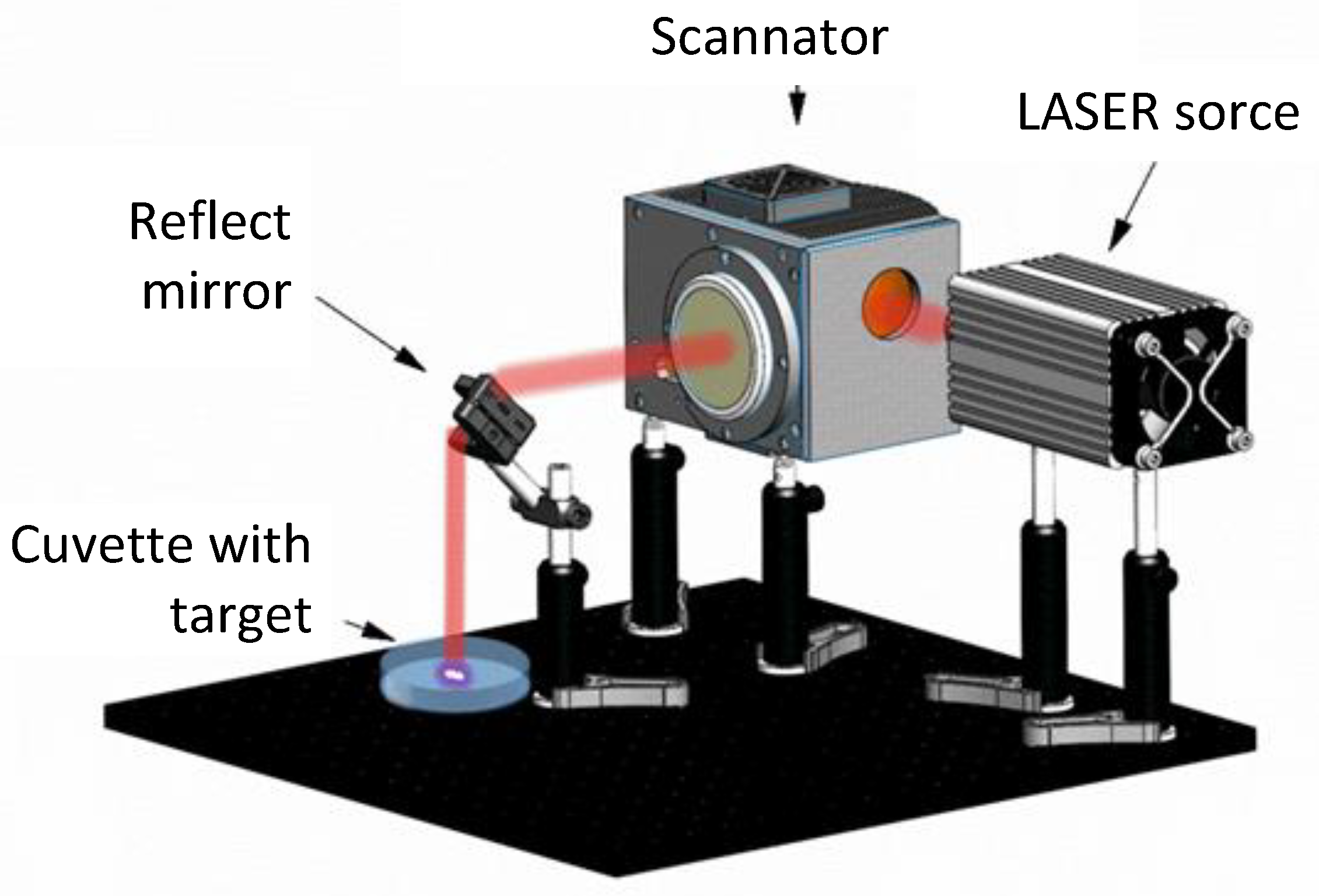
2.1. Synthesis of TeNPs
2.2. Physicochemical Characteristics of Received Nanoparticles
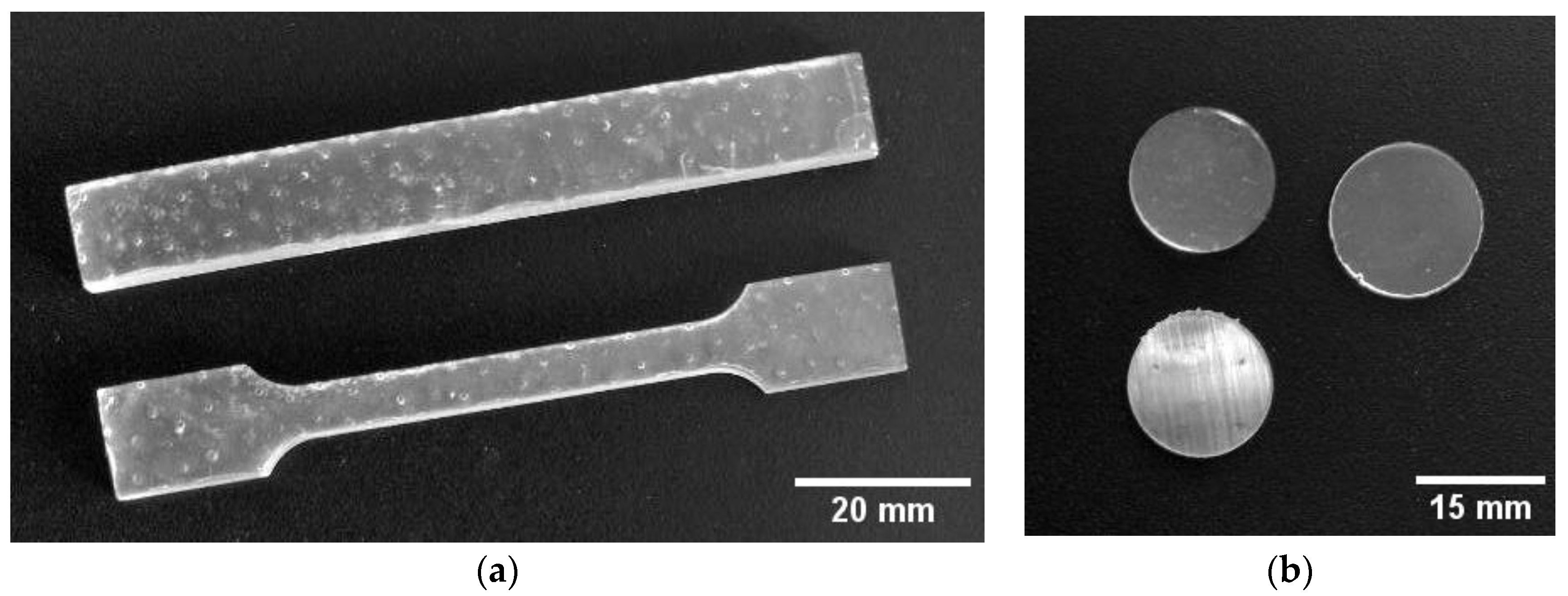
2.3. Preparation of Nanocomposite MPR+TeNP Material
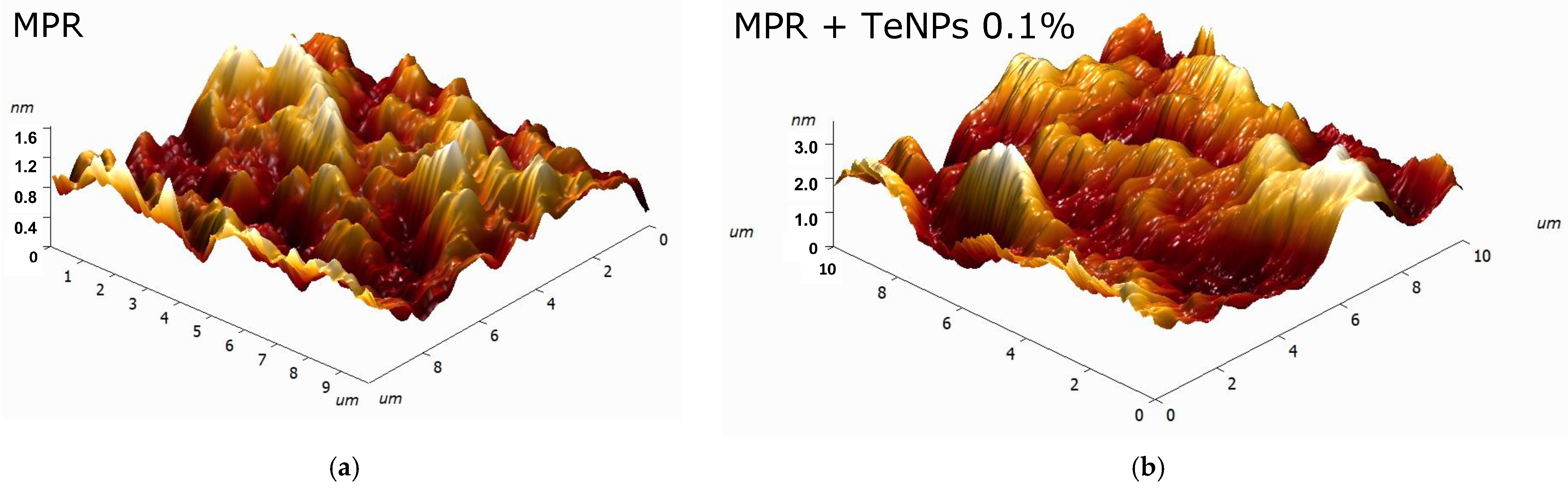
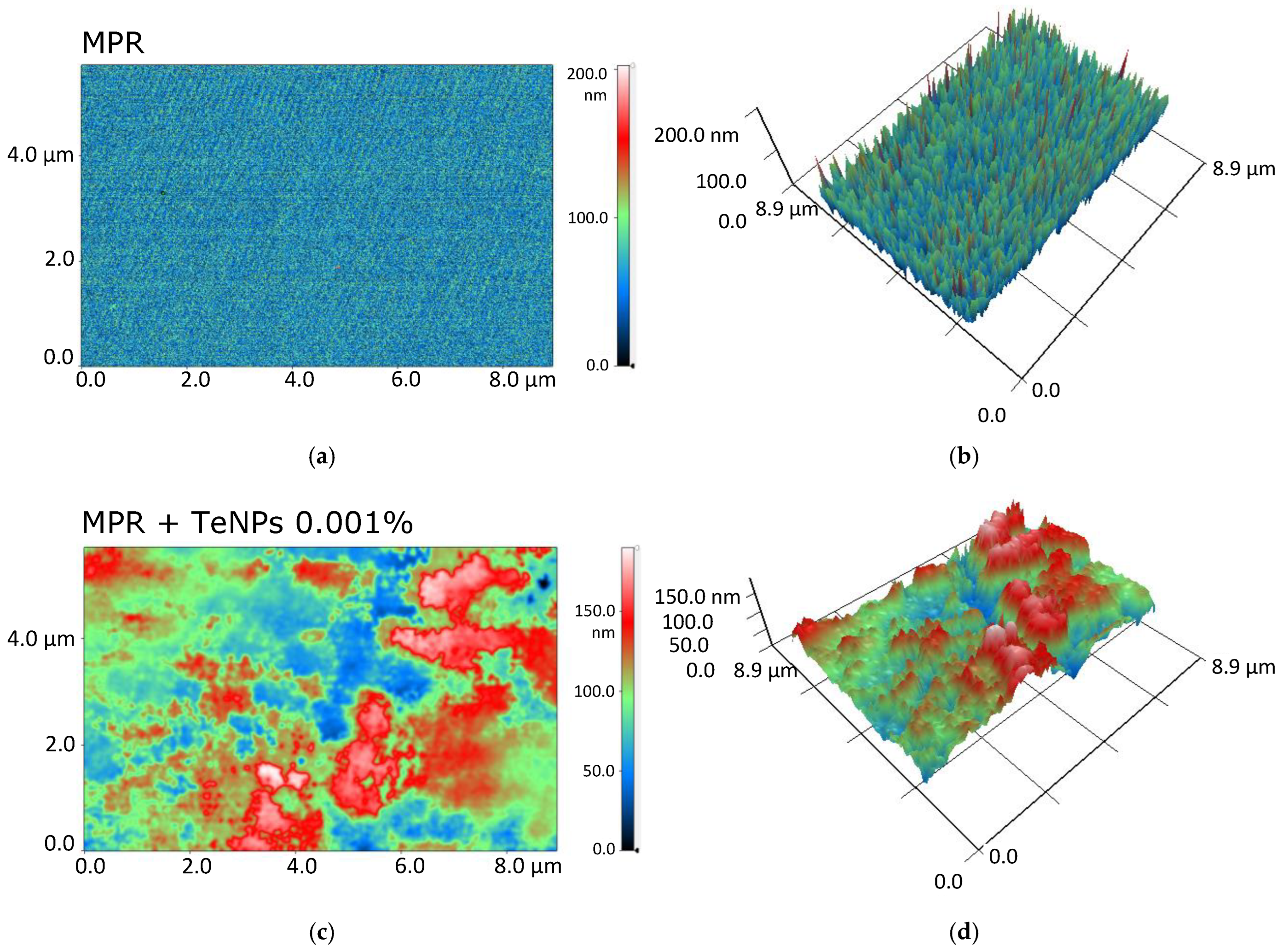
2.4. Study of the Composition and Microrelief of the Surface
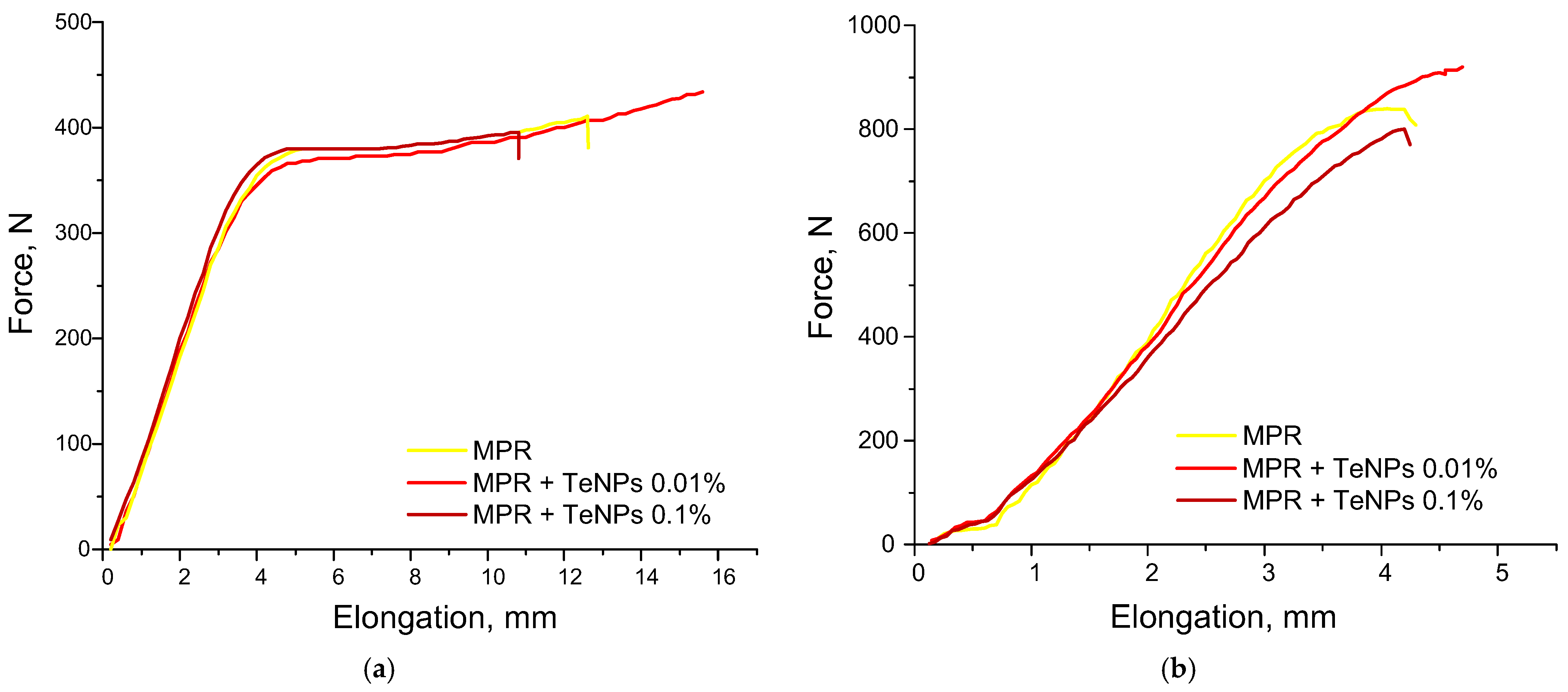
2.5. Study of Strength Characteristics
2.6. Evaluation of ROS Generation in Aqueous Solutions in the Presence of MPR+TeNPs
2.7. Quantitative Analysis of Macromolecular Damage (Generation of 8-Oxoguanine and Long-Lived Reactive Proteins Species)
2.8. Study of Antibacterial Activity
2.9. Evaluation of Cytotoxic Effect
2.10. Statistical Processing
3. Results
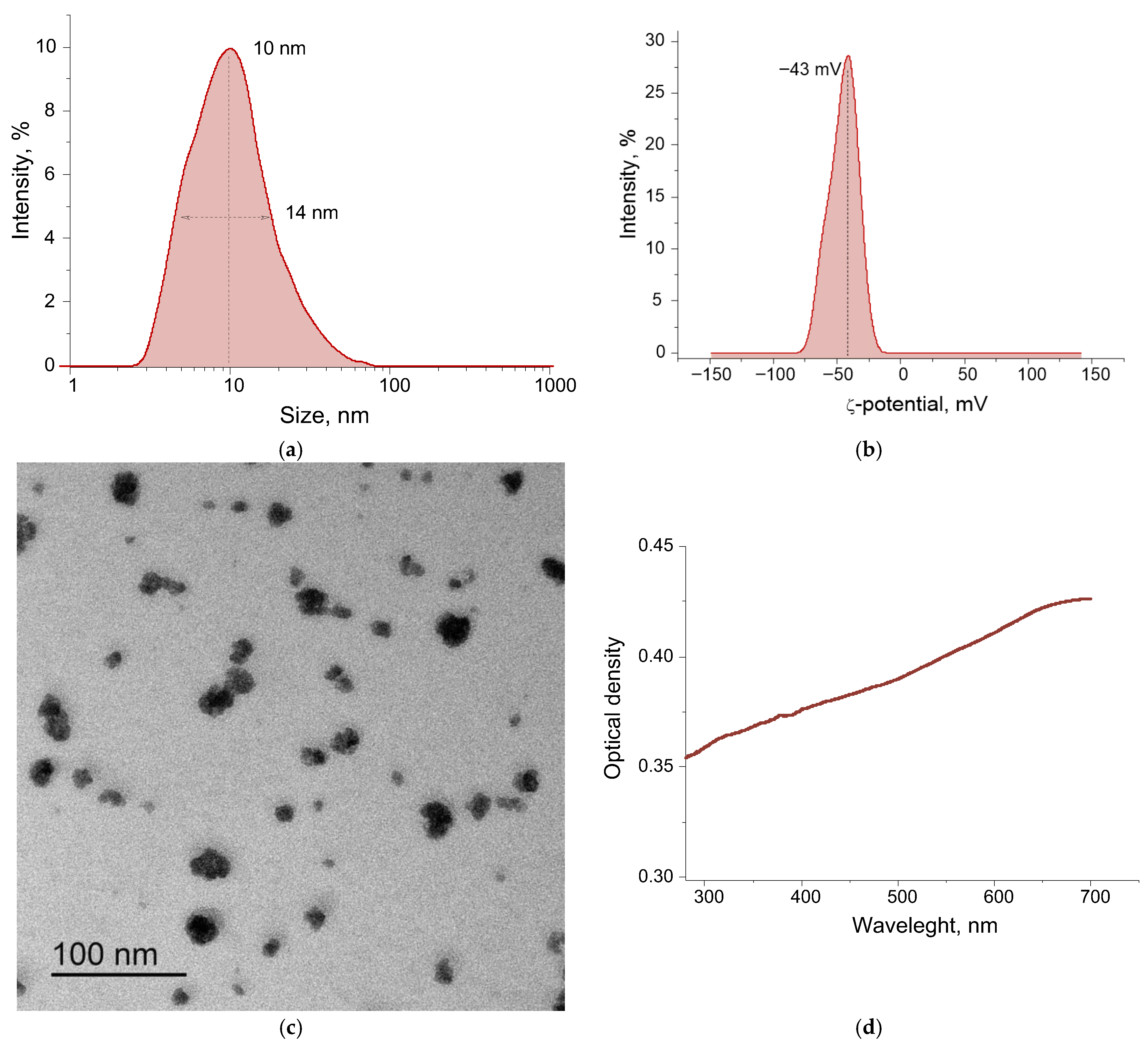
3.1. The Physicochemical Properties of the Obtained Nanoparticles
3.2. Physicochemical Properties of the Obtained MPR+TeNP Composites
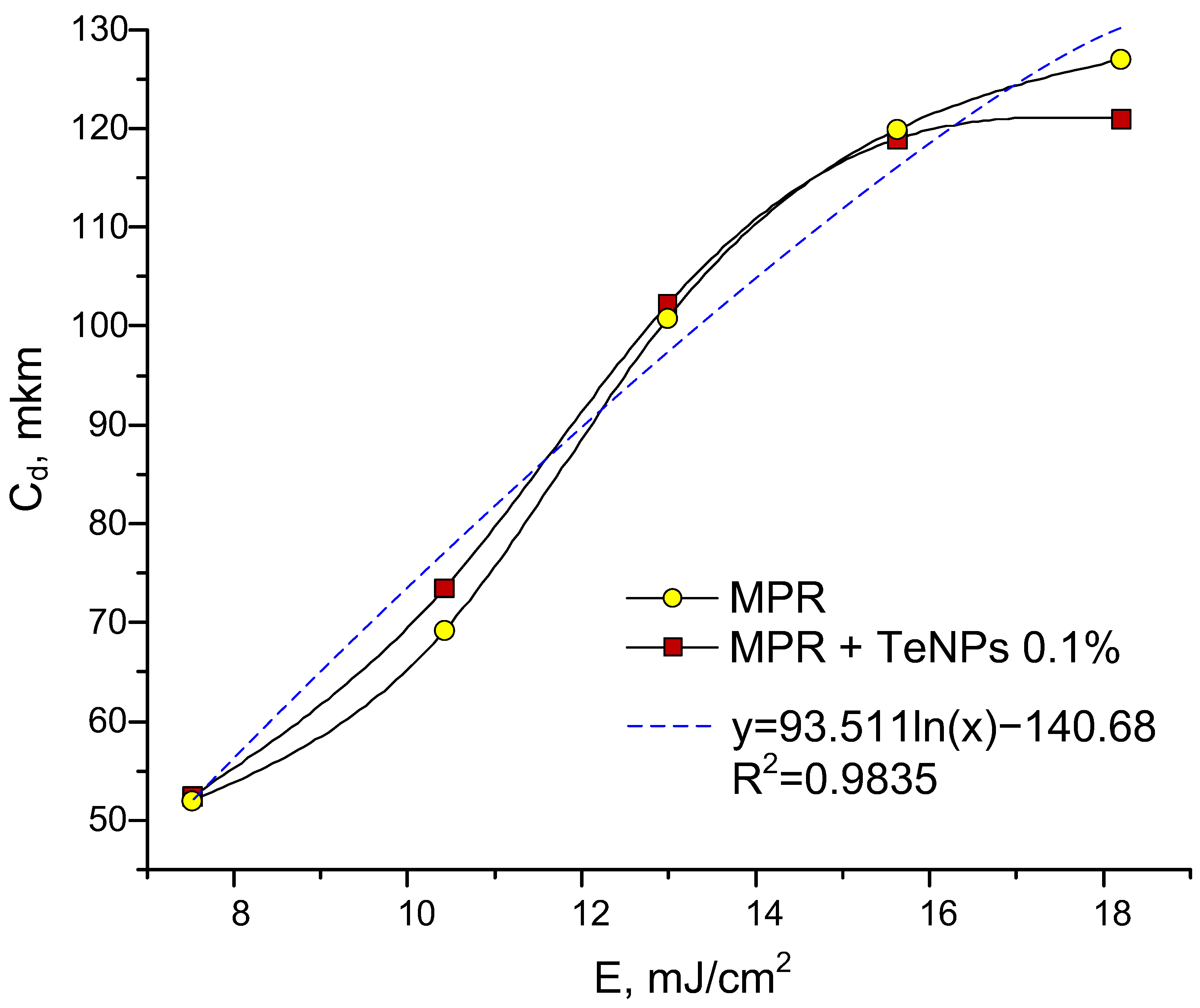
3.3. Jacobs Working Curves for Photopolymer Compositions and Study of Physical and Mechanical Properties
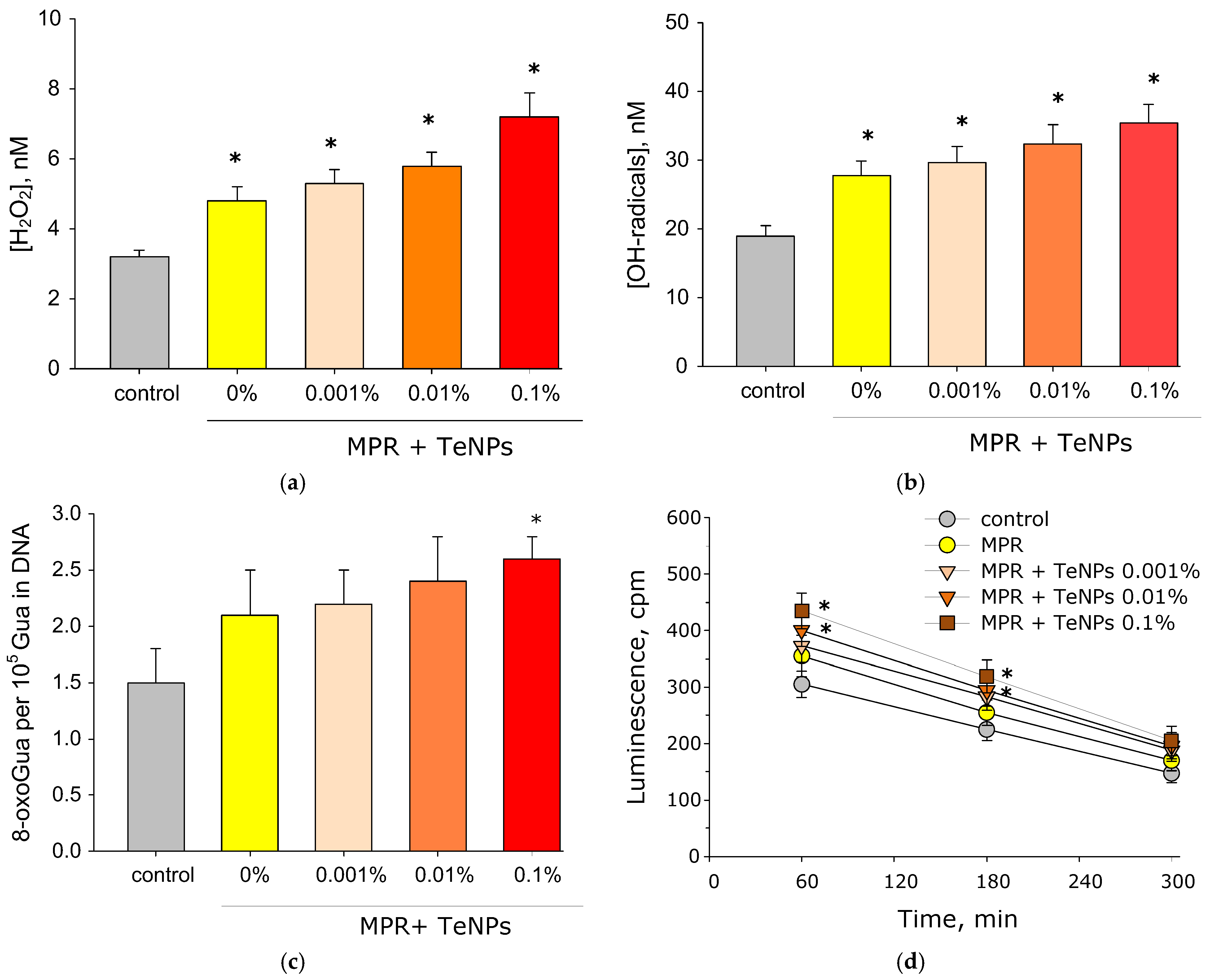
3.4. Generation of ROS, 8-Oxoguanine, and LRPSs in the Presence of MPR+TeNP Composites
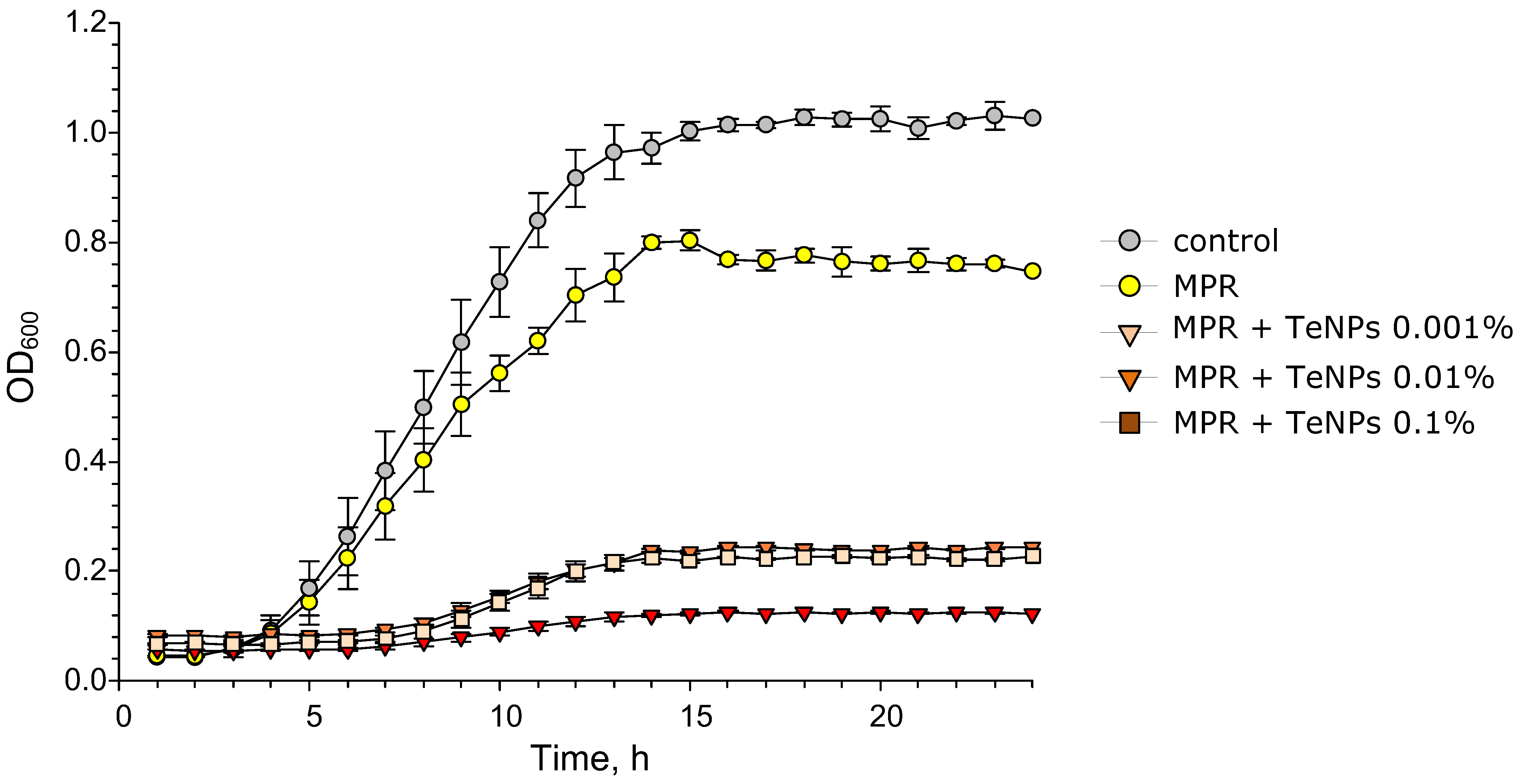
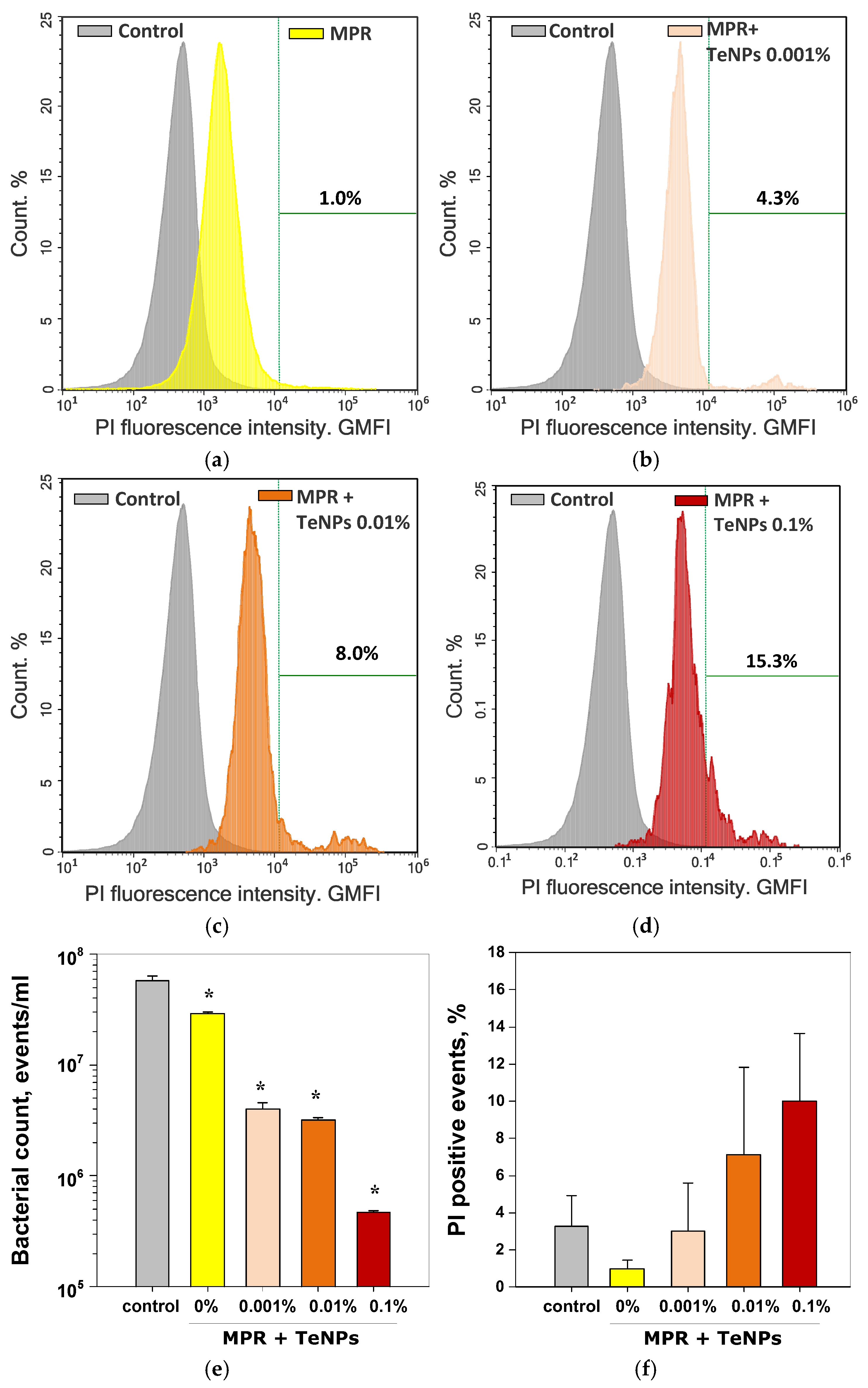
3.5. Antibacterial Activity of MPR+TeNP Composites
3.6. Cytostatic Effect of MPR+TeNP Composites
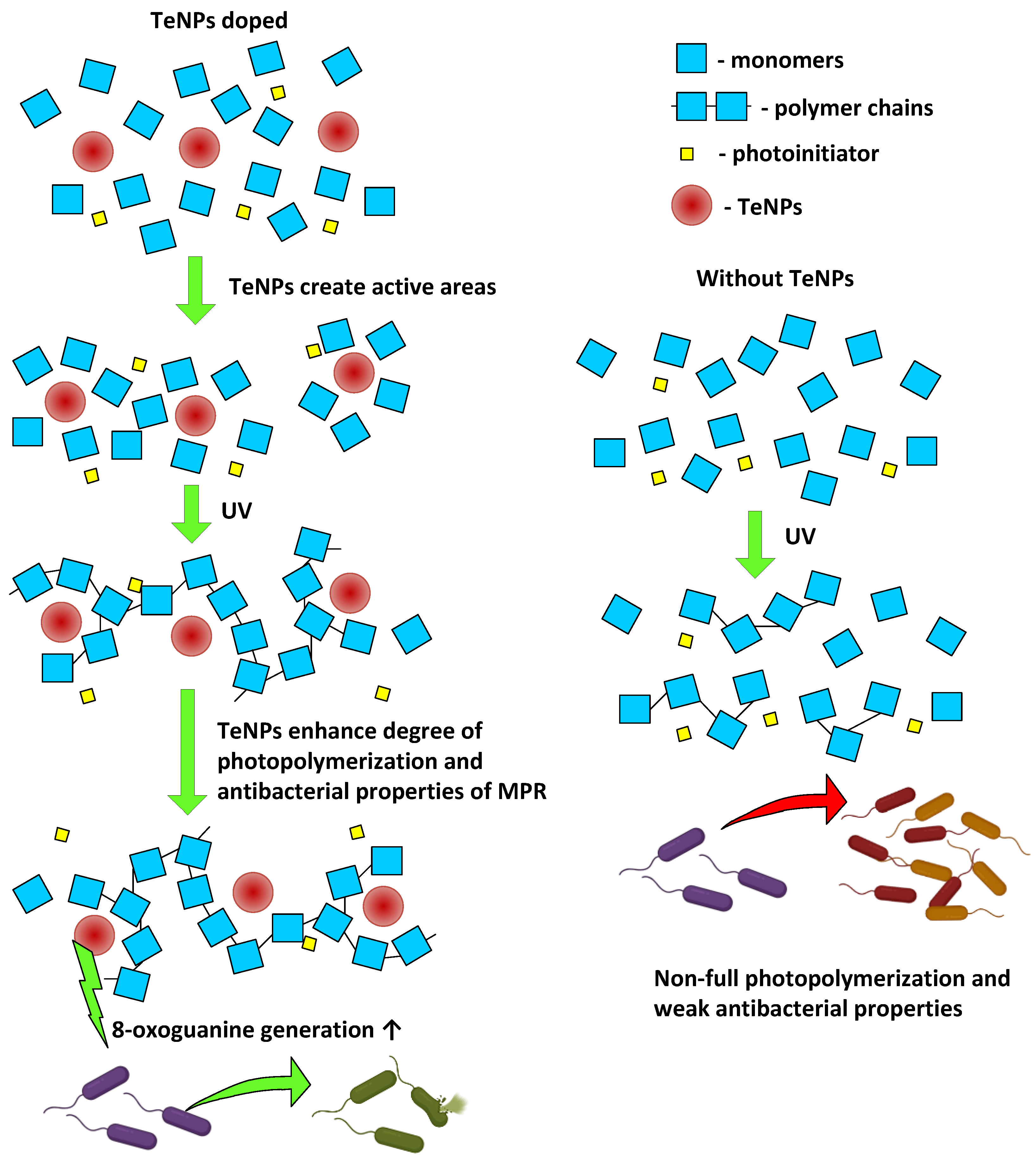
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AFM | Atomic force microscopy |
| ANOVA | Analysis of variance |
| BPP | Bath photopolymerization |
| DLS | Dynamic light scattering |
| ELISA | Enzyme-linked immunosorbent assay |
| FTIR | Fourier-transform infrared spectroscopy |
| HSF | Human spleen fibroblasts |
| LRPSs | Long-lived reactive proteins species |
| MIM | Modulation interference microscopy |
| MPR | Methacrylate photopolymer resin |
| NPs | Nanoparticles |
| PI | Propidium iodide |
| PMMA | Poly(methyl methacrylate) |
| ROS | Reactive oxygen species |
| SeNPs | Selenium nanoparticles |
| TEM | Transmission electron microscopy |
| TeNPs | Tellurium nanoparticles |
| UV | Ultraviolet |
References
- Puzatova, A.; Shakor, P.; Laghi, V.; Dmitrieva, M. Large-Scale 3D Printing for Construction Application by Means of Robotic Arm and Gantry 3D Printer: A Review. Buildings 2022, 12, 2023. [Google Scholar] [CrossRef]
- Konnikov, E.A.; Konnikova, O.A.; Rodionov, D.G. Impact of 3D-Printing Technologies on the Transformation of Industrial Production in the Arctic Zone. Resources 2019, 8, 20. [Google Scholar] [CrossRef]
- Girskas, G.; Kligys, M. 3D Concrete Printing Review: Equipment, Materials, Mix Design, and Properties. Buildings 2025, 15, 2049. [Google Scholar] [CrossRef]
- Aghajani, M.; Garshasbi, H.R.; Naghib, S.M.; Mozafari, M.R. 3D Printing of Hydrogel Polysaccharides for Biomedical Applications: A Review. Biomedicines 2025, 13, 731. [Google Scholar] [CrossRef]
- Kwon, S.; Hwang, D. Understanding and Resolving 3D Printing Challenges: A Systematic Literature Review. Processes 2025, 13, 1772. [Google Scholar] [CrossRef]
- Sun, K.; Peng, X.; Gan, Z.; Chen, W.; Li, X.; Gong, T.; Xiao, P. 3D Printing/Vat Photopolymerization of Photopolymers Activated by Novel Organic Dyes as Photoinitiators. Catalysts 2022, 12, 1272. [Google Scholar] [CrossRef]
- Timofticiuc, I.-A.; Călinescu, O.; Iftime, A.; Dragosloveanu, S.; Caruntu, A.; Scheau, A.-E.; Badarau, I.A.; Didilescu, A.C.; Caruntu, C.; Scheau, C. Biomaterials Adapted to Vat Photopolymerization in 3D Printing: Characteristics and Medical Applications. J. Funct. Biomater. 2023, 15, 7. [Google Scholar] [CrossRef]
- Andjela, L.; Abdurahmanovich, V.M.; Vladimirovna, S.N.; Mikhailovna, G.I.; Yurievich, D.D.; Alekseevna, M.Y. A review on Vat Photopolymerization 3D-printing processes for dental application. Dent. Mater. 2022, 38, e284–e296. [Google Scholar] [CrossRef]
- Dutta, A.; Rajasekaran, R.; Ray, P.G.; Seesala, V.S.; Dogra, N.; Ghorai, S.K.; Ojha, A.; Mukherjee, K.; Gupta, S.; Chattopadhyay, S.; et al. Influence of surface engineering on 3D printed Ti lattice structure towards enhanced tissue integration: An in vitro and in vivo study. Talanta Open 2023, 8, 100256. [Google Scholar] [CrossRef]
- Ghorai, S.K.; Maji, S.; Subramanian, B.; Maiti, T.K.; Chattopadhyay, S. Coining attributes of ultra-low concentration graphene oxide and spermine: An approach for high strength, anti-microbial and osteoconductive nanohybrid scaffold for bone tissue regeneration. Carbon 2019, 141, 370–389. [Google Scholar] [CrossRef]
- Hata, K.; Ikeda, H.; Nagamatsu, Y.; Masaki, C.; Hosokawa, R.; Shimizu, H. Development of Dental Poly(methyl methacrylate)-Based Resin for Stereolithography Additive Manufacturing. Polymers 2021, 13, 4435. [Google Scholar] [CrossRef]
- Khatoon, S.; Khandelwal, A.; Raj, A.; Ahmad, G. Fabrication of FFF 3D-printed surfaces for PMMA-based biomedical device employing the pre-processing optimization to eliminate the post-processing steps. Prog. Addit. Manuf. 2023, 9, 1003–1014. [Google Scholar] [CrossRef]
- Ramanathan, S.; Lin, Y.-C.; Thirumurugan, S.; Hu, C.-C.; Duann, Y.-F.; Chung, R.-J. Poly(methyl methacrylate) in Orthopedics: Strategies, Challenges, and Prospects in Bone Tissue Engineering. Polymers 2024, 16, 36. [Google Scholar] [CrossRef]
- Thirugnanasamabandam, A.; Nallamuthu, R.; Renjit, M.; Gnanasagaran, C.L. 3D-printed PLA/PMMA polymer composites: A consolidated feasible characteristic investigation for dental applications. Polym. Eng. Sci. 2024, 64, 4019–4031. [Google Scholar] [CrossRef]
- Yarikov, A.; Gorbatov, R.; Stolyarov, I.; Smirnov, I.; Fraerman, A.; Sosnin, A.; Perlmutter, O. Application of additive 3D printing technologies in traumatology/orthopedics and neurosurgery. Vrach 2021, 32, 8–15. [Google Scholar] [CrossRef]
- Aftab, M.; Ikram, S.; Ullah, M.; Khan, N.; Naeem, M.; Khan, M.A.; Bakhtiyor o’g’li, R.B.; Qizi, K.S.S.; Erkinjon Ugli, O.O.; Abdurasulovna, B.M.; et al. Recent Trends and Future Directions in 3D Printing of Biocompatible Polymers. J. Manuf. Mater. Process. 2025, 9, 129. [Google Scholar] [CrossRef]
- Filimonova, E.A.; Lozovaya, A.V.; Prosyankin, E.E.; Mustafina, A.R.; Chapala, P. Thermal treatment influence on optical properties of 3D printed objects by vat photopolymerization. Prog. Addit. Manuf. 2024, 10, 2703–2713. [Google Scholar] [CrossRef]
- Zhakeyev, A.; Zhang, L.; Xuan, J. Photoactive resin formulations and composites for optical 3D and 4D printing of functional materials and devices. In 3D and 4D Printing of Polymer Nanocomposite Materials; Elsevier: Amsterdam, The Netherlands, 2020; pp. 387–425. [Google Scholar]
- Arossi, G.A.; Abdou, N.A.; Hung, B.; Garcia, I.M.; Zimmer, R.; Melo, M.A. Safety of 3D-Printed Acrylic Resins for Prosthodontic Appliances: A Comprehensive Cytotoxicity Review. Appl. Sci. 2024, 14, 8322. [Google Scholar] [CrossRef]
- Brooks, A.K.; Yadavalli, V.K. Post-Print Processing to Minimize Cytotoxicity of 3D-Printed Photopolymer Resins for Biomedical Applications. J. Appl. Polym. Sci. 2024, 142, e56545. [Google Scholar] [CrossRef]
- Azlin, M.; Ilyas, R.; Zuhri, M.; Sapuan, S.; Harussani, M.; Sharma, S.; Nordin, A.; Nurazzi, N.; Afiqah, A. 3D Printing and Shaping Polymers, Composites, and Nanocomposites: A Review. Polymers 2022, 14, 180. [Google Scholar] [CrossRef] [PubMed]
- Darbandi, K.R.; Amin, B.K. Innovation and Evaluations of 3D Printing Resins Modified with Zirconia Nanoparticles and Silver Nanoparticle-Immobilized Halloysite Nanotubes for Dental Restoration. Coatings 2024, 14, 310. [Google Scholar] [CrossRef]
- Jain, K.; Shukla, R.; Yadav, A.; Ujjwal, R.R.; Flora, S.J.S. 3D Printing in Development of Nanomedicines. Nanomaterials 2021, 11, 420. [Google Scholar] [CrossRef]
- Luo, X.; Cheng, H.; Wu, X. Nanomaterials Reinforced Polymer Filament for Fused Deposition Modeling: A State-of-the-Art Review. Polymers 2023, 15, 2980. [Google Scholar] [CrossRef]
- Shah, M.; Ullah, A.; Azher, K.; Ur Rehman, A.; Akturk, N.; Juan, W.; Tüfekci, C.S.; Salamci, M.U. The Influence of Nanoparticle Dispersions on Mechanical and Thermal Properties of Polymer Nanocomposites Using SLA 3D Printing. Crystals 2023, 13, 285. [Google Scholar] [CrossRef]
- Zhang, N.; Wang, Z.; Zhao, Z.; Zhang, D.; Feng, J.; Yu, L.; Lin, Z.; Guo, Q.; Huang, J.; Mao, J.; et al. 3D printing of micro-nano devices and their applications. Microsyst. Nanoeng. 2025, 11, 35. [Google Scholar] [CrossRef]
- Li, P.; Yin, R.; Cheng, J.; Lin, J. Bacterial Biofilm Formation on Biomaterials and Approaches to Its Treatment and Prevention. Int. J. Mol. Sci. 2023, 24, 11680. [Google Scholar] [CrossRef]
- Halawa, E.M.; Fadel, M.; Al-Rabia, M.W.; Behairy, A.; Nouh, N.A.; Abdo, M.; Olga, R.; Fericean, L.; Atwa, A.M.; El-Nablaway, M.; et al. Antibiotic action and resistance: Updated review of mechanisms, spread, influencing factors, and alternative approaches for combating resistance. Front. Pharmacol. 2024, 14, 1305294. [Google Scholar] [CrossRef]
- Evstigneeva, S.S.; Chumakov, D.S.; Tumskiy, R.S.; Khlebtsov, B.N.; Khlebtsov, N.G. Detection and imaging of bacterial biofilms with glutathione-stabilized gold nanoclusters. Talanta 2023, 264, 124773. [Google Scholar] [CrossRef]
- Nastulyavichus, A.A.; Tolordava, E.R.; Ulturgasheva, E.V.; Shelygina, S.N.; Babina, S.P.; Saraeva, I.N.; Kudryashov, S.I. Study of Laser Transfer Regimes to Increase the Efficiency of Application of Antibacterial Silver Nanoparticles. Bull. Lebedev Phys. Inst. 2025, 52, 82–87. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Serov, D.A.; Astashev, M.E.; Semenova, A.A.; Lisitsyn, A.B. Ag2O Nanoparticles as a Candidate for Antimicrobial Compounds of the New Generation. Pharmaceuticals 2022, 15, 968. [Google Scholar] [CrossRef] [PubMed]
- Bakina, O.; Svarovskaya, N.; Ivanova, L.; Glazkova, E.; Rodkevich, N.; Evstigneev, V.; Evstigneev, M.; Mosunov, A.; Lerner, M. New PMMA-Based Hydroxyapatite/ZnFe2O4/ZnO Composite with Antibacterial Performance and Low Toxicity. Biomimetics 2023, 8, 488. [Google Scholar] [CrossRef] [PubMed]
- Badaraev, A.D.; Lerner, M.I.; Bakina, O.V.; Sidelev, D.V.; Tran, T.-H.; Krinitcyn, M.G.; Malashicheva, A.B.; Cherempey, E.G.; Slepchenko, G.B.; Kozelskaya, A.I.; et al. Antibacterial Activity and Cytocompatibility of Electrospun PLGA Scaffolds Surface-Modified by Pulsed DC Magnetron Co-Sputtering of Copper and Titanium. Pharmaceutics 2023, 15, 939. [Google Scholar] [CrossRef]
- Kozelskaya, A.I.; Verzunova, K.N.; Akimchenko, I.O.; Frueh, J.; Petrov, V.I.; Slepchenko, G.B.; Bakina, O.V.; Lerner, M.I.; Brizhan, L.K.; Davydov, D.V.; et al. Antibacterial Calcium Phosphate Coatings for Biomedical Applications Fabricated via Micro-Arc Oxidation. Biomimetics 2023, 8, 444. [Google Scholar] [CrossRef]
- Parmar, A.; Kaur, G.; Kapil, S.; Sharma, V.; Sachar, S.; Sandhir, R.; Sharma, S. Green chemistry mediated synthesis of PLGA-Silver nanocomposites for antibacterial synergy: Introspection of formulation parameters on structural and bactericidal aspects. React. Funct. Polym. 2019, 141, 68–81. [Google Scholar] [CrossRef]
- Smirnova, V.V.; Chausov, D.N.; Serov, D.A.; Kozlov, V.A.; Ivashkin, P.I.; Pishchalnikov, R.Y.; Uvarov, O.V.; Vedunova, M.V.; Semenova, A.A.; Lisitsyn, A.B.; et al. A Novel Biodegradable Composite Polymer Material Based on PLGA and Silver Oxide Nanoparticles with Unique Physicochemical Properties and Biocompatibility with Mammalian Cells. Materials 2021, 14, 6915. [Google Scholar] [CrossRef] [PubMed]
- Tolordava, E.R.; Nastulyavichus, A.A.; Ulturgasheva, E.V.; Shelygina, S.N.; Saraeva, I.N.; Kudryashov, S.I. Antibacterial Activity of Metal Nanoparticles in an Ex Vivo Wound Infection Model. Mol. Genet. Microbiol. Virol. 2025, 39, 268–274. [Google Scholar] [CrossRef]
- Khlebtsov, B.N. Functional Nanoparticles: Synthesis and Practical Applications. Colloid J. 2023, 85, 475–478. [Google Scholar] [CrossRef]
- Han, H.-W.; Patel, K.D.; Kwak, J.-H.; Jun, S.-K.; Jang, T.-S.; Lee, S.-H.; Knowles, J.C.; Kim, H.-W.; Lee, H.-H.; Lee, J.-H. Selenium Nanoparticles as Candidates for Antibacterial Substitutes and Supplements against Multidrug-Resistant Bacteria. Biomolecules 2021, 11, 1028. [Google Scholar] [CrossRef]
- Lesnichaya, M.; Perfileva, A.; Nozhkina, O.; Gazizova, A.; Graskova, I. Synthesis, toxicity evaluation and determination of possible mechanisms of antimicrobial effect of arabinogalactane-capped selenium nanoparticles. J. Trace Elem. Med. Biol. 2022, 69, 126904. [Google Scholar] [CrossRef]
- Huang, T.; Holden, J.A.; Heath, D.E.; O’Brien-Simpson, N.M.; O’Connor, A.J. Engineering highly effective antimicrobial selenium nanoparticles through control of particle size. Nanoscale 2019, 11, 14937–14951. [Google Scholar] [CrossRef]
- Alhadrami, H.A.; Shoudri, R.A.M. Titanium Oxide (TiO2) Nanoparticles for Treatment of Wound Infection. 2020; Preprints. [Google Scholar] [CrossRef]
- Modi, S.; Yadav, V.K.; Choudhary, N.; Alswieleh, A.M.; Sharma, A.K.; Bhardwaj, A.K.; Khan, S.H.; Yadav, K.K.; Cheon, J.-K.; Jeon, B.-H. Onion Peel Waste Mediated-Green Synthesis of Zinc Oxide Nanoparticles and Their Phytotoxicity on Mung Bean and Wheat Plant Growth. Materials 2022, 15, 2393. [Google Scholar] [CrossRef]
- Tang, A.; Ren, Q.; Wu, Y.; Wu, C.; Cheng, Y. Investigation into the Antibacterial Mechanism of Biogenic Tellurium Nanoparticles and Precursor Tellurite. Int. J. Mol. Sci. 2022, 23, 11697. [Google Scholar] [CrossRef] [PubMed]
- Abed, R.N.; Zainulabdeen, K.; Abdallh, M.; Yousif, E.; Rashad, A.A.; Jawad, A.H. The optical properties behavior of modify poly(methyl methacrylate) nanocomposite thin films during solar energy absorption. J. Non-Cryst. Solids 2023, 609, 122257. [Google Scholar] [CrossRef]
- Alkayal, N.S.; Al Ghamdi, M.A. Cross-Linked Poly(methyl methacrylate) Nanocomposites’ Synthesis, Characterization, and Antibacterial Effects. Polymers 2025, 17, 269. [Google Scholar] [CrossRef] [PubMed]
- El-Bashir, S.; Althumairi, N.; Alzayed, N. Durability and Mechanical Performance of PMMA/Stone Sludge Nanocomposites for Acrylic Solid Surface Applications. Polymers 2017, 9, 604. [Google Scholar] [CrossRef]
- Galant, K.; Turosz, N.; Chęcińska, K.; Chęciński, M.; Cholewa-Kowalska, K.; Karwan, S.; Chlubek, D.; Sikora, M. Silver Nanoparticles (AgNPs) Incorporation into Polymethyl Methacrylate (PMMA) for Dental Appliance Fabrication: A Systematic Review and Meta-Analysis of Mechanical Properties. Int. J. Mol. Sci. 2024, 25, 12645. [Google Scholar] [CrossRef]
- Gao, Y.; Chen, X.; Zhang, Y.; Dong, X.-H.; Yu, Q.; Wang, L. Moisture-Resistant, High-Performance Polarizing Films via Aligned PMMA/CNT Composite Fibers: A Scalable Electrospinning Approach. Molecules 2025, 30, 2169. [Google Scholar] [CrossRef]
- Komeijani, M.; Bahri-Laleh, N.; Mirjafary, Z.; D’Alterio, M.C.; Rouhani, M.; Sakhaeinia, H.; Moghaddam, A.H.; Mirmohammadi, S.A.; Poater, A. PLA/PMMA Reactive Blending in the Presence of MgO as an Exchange Reaction Catalyst. Polymers 2025, 17, 845. [Google Scholar] [CrossRef]
- Uçar, E.; Dogu, M.; Demirhan, E.; Krause, B. PMMA/SWCNT Composites with Very Low Electrical Percolation Threshold by Direct Incorporation and Masterbatch Dilution and Characterization of Electrical and Thermoelectrical Properties. Nanomaterials 2023, 13, 1431. [Google Scholar] [CrossRef]
- Ao, B.; Jiang, H.; Cai, X.; Liu, D.; Tu, J.; Shi, X.; Wang, Y.; He, F.; Lv, J.; Li, J.; et al. Synthesis of Tellurium Nanoparticles Using Moringa oleifera Extract, and Their Antibacterial and Antibiofilm Effects against Bacterial Pathogens. Microorganisms 2024, 12, 1847. [Google Scholar] [CrossRef]
- Sári, D.; Ferroudj, A.; Semsey, D.; El-Ramady, H.; Brevik, E.C.; Prokisch, J. Tellurium and Nano-Tellurium: Medicine or Poison? Nanomaterials 2024, 14, 670. [Google Scholar] [CrossRef] [PubMed]
- Vahidi, H.; Kobarfard, F.; Alizadeh, A.; Saravanan, M.; Barabadi, H. Green nanotechnology-based tellurium nanoparticles: Exploration of their antioxidant, antibacterial, antifungal and cytotoxic potentials against cancerous and normal cells compared to potassium tellurite. Inorg. Chem. Commun. 2021, 124, 108385. [Google Scholar] [CrossRef]
- Zhu, H.; Fan, L.; Wang, K.; Liu, H.; Zhang, J.; Yan, S. Progress in the Synthesis and Application of Tellurium Nanomaterials. Nanomaterials 2023, 13, 2057. [Google Scholar] [CrossRef]
- Palomba, M.; Binetti, S.; Le Donne, A.; Coscia, U.; Ambrosone, G.; Carotenuto, G. Tellurium-based nanocomposites for plastic electronic applications. In AIP Conference Proceedings, Proceedings of the VIII International Conference on “Times of Polymers and Composites”: From Aerospace to Nanotechnology, Naples, Italy, 19–23 June 2016; AIP Publishing LLC: Melville, NY, USA, 2016. [Google Scholar]
- Wang, L.; Cao, W.; Xu, H. Tellurium-Containing Polymers: Towards Biomaterials and Optoelectronic Materials. ChemNanoMat 2016, 2, 479–488. [Google Scholar] [CrossRef]
- Yadav, A.K.; Mohammad, N.; Khanna, P.K. Novel synthesis of polyaniline/tellurium (PANI/Te) nanocomposite and its EMI shielding behavior. Mater. Adv. 2023, 4, 4409–4416. [Google Scholar] [CrossRef]
- Chen, Y.; Sathiyaseelan, A.; Zhang, X.; Jin, Y.; Wang, M.-H. Preparation of antibacterial tellurium nanorod-incorporated thermosensitive pluronic F-127 hydrogels for wound healing applications. J. Drug Deliv. Sci. Technol. 2025, 111, 107107. [Google Scholar] [CrossRef]
- Hu, J.; Ran, S.; Huang, Z.; Liu, Y.; Hu, H.; Zhou, Y.; Ding, X.; Yin, J.; Zhang, Y. Antibacterial tellurium-containing polycarbonate drug carriers to eliminate intratumor bacteria for synergetic chemotherapy against colorectal cancer. Acta Biomater. 2024, 185, 323–335. [Google Scholar] [CrossRef]
- Matharu, R.K.; Charani, Z.; Ciric, L.; Illangakoon, U.E.; Edirisinghe, M. Antimicrobial activity of tellurium-loaded polymeric fiber meshes. J. Appl. Polym. Sci. 2018, 135, 46368. [Google Scholar] [CrossRef]
- Bergoglio, M.; Najmi, Z.; Ferla, F.; Scalia, A.C.; Cochis, A.; Rimondini, L.; Vernè, E.; Sangermano, M.; Miola, M. Tellurium-Doped Silanised Bioactive Glass–Chitosan Hydrogels: A Dual Action for Antimicrobial and Osteoconductive Platforms. Polymers 2025, 17, 1651. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-C.; Zeng, Y.-J.; Lin, Y.-W.; Tai, H.-C.; Don, T.-M. In Situ Encapsulation of Camptothecin by Self-Assembly of Poly(acrylic acid)-b-Poly(N-Isopropylacrylamide) and Chitosan for Controlled Drug Delivery. Polymers 2023, 15, 2463. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Liu, C.; Tong, H.; Sun, Y.; Huang, M.; Ren, G.; Xie, H. Encapsulation of EGCG by Zein-Gum Arabic Complex Nanoparticles and In Vitro Simulated Digestion of Complex Nanoparticles. Foods 2022, 11, 2131. [Google Scholar] [CrossRef]
- Megahed, S.; Wutke, N.; Liu, Y.; Klapper, M.; Schulz, F.; Feliu, N.; Parak, W.J. Encapsulation of Nanoparticles with Statistical Copolymers with Different Surface Charges and Analysis of Their Interactions with Proteins and Cells. Int. J. Mol. Sci. 2024, 25, 5539. [Google Scholar] [CrossRef]
- Sánchez, A.; Mejía, S.P.; Orozco, J. Recent Advances in Polymeric Nanoparticle-Encapsulated Drugs against Intracellular Infections. Molecules 2020, 25, 3760. [Google Scholar] [CrossRef]
- Varlamova, E.G.; Gudkov, S.V.; Turovsky, E.A. Opposite Effects of Small and Large Diameter Selenium Nanoparticles on the Redox-Status and Survival of Cortical Cells in Toxic Models In Vitro. Biol. Trace Elem. Res. 2025, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Turovsky, E.A.; Varlamova, E.G.; Rogachev, V.V.; Gudkov, S.V. Tellurium Nanoparticles Produced by Laser Ablation Induce Selective Anticancer Effects via ROS-Mediated Apoptosis and Calcium Signaling Pathways: In Vitro Screening. Biochem. Biophys. Res. Commun. 2025, 782, 152555. [Google Scholar] [CrossRef]
- ISO 10993-18:2020, I; Biological Evaluation of Medical Devices. Part 18: Chemical Characterization of Medical Device Materials Within a Risk Management Process. ISO: Geneva, Switzerland, 2020. Available online: https://www.iso.org/standard/64750.html (accessed on 1 January 2020).
- ISO 179-1:2023, I; Plastics—Determination of Charpy Impact Properties. Part 1: Non-Instrumented Impact Test. ISO: Geneva, Switzerland, 2023. Available online: https://www.iso.org/standard/84393.html (accessed on 1 June 2023).
- ISO 527-2:2025, I; Plastics—Determination of Tensile Properties. Part 2: Test Conditions for Moulding and Extrusion Plastics. ISO: Geneva, Switzerland, 2025. Available online: https://www.iso.org/standard/527-2 (accessed on 1 June 2025).
- Simakin, A.V.; Burmistrov, D.E.; Baimler, I.V.; Gritsaeva, A.V.; Serov, D.A.; Astashev, M.E.; Chapala, P.; Validov, S.Z.; Yanbaev, F.M.; Gudkov, S.V. TiO2 Nanoparticles Obtained by Laser Sintering When Added to Methacrylate Photopolymer Resin Improve Its Physicochemical Characteristics and Impart Antibacterial Properties. Inorganics 2025, 13, 233. [Google Scholar] [CrossRef]
- Burmistrov, D.E.; Serov, D.A.; Baimler, I.V.; Gritsaeva, A.V.; Chapala, P.; Simakin, A.V.; Astashev, M.E.; Karmanova, E.E.; Dubinin, M.V.; Nizameeva, G.R.; et al. Polymethyl Methacrylate-like Photopolymer Resin with Titanium Metal Nanoparticles Is a Promising Material for Biomedical Applications. Polymers 2025, 17, 1830. [Google Scholar] [CrossRef] [PubMed]
- D638-22, A; Standard Test Method for Tensile Properties of Plastics. ASTM International: West Conshohocken, PA, USA, 2022. Available online: https://store.astm.org/d0638-22.html (accessed on 21 July 2022).
- Sevostyanov, M.A.; Kolmakov, A.G.; Sergiyenko, K.V.; Kaplan, M.A.; Baikin, A.S.; Gudkov, S.V. Mechanical, physical–chemical and biological properties of the new Ti–30Nb–13Ta–5Zr alloy. J. Mater. Sci. 2020, 55, 14516–14529. [Google Scholar] [CrossRef]
- Chernikov, A.V.; Gudkov, S.V.; Shtarkman, I.N.; Bruskov, V.I. Oxygen effect in heat-induced DNA damage. Biophysics 2007, 52, 185–190. [Google Scholar] [CrossRef]
- Ivanov, V.E.; Usacheva, A.M.; Chernikov, A.V.; Bruskov, V.I.; Gudkov, S.V. Formation of long-lived reactive species of blood serum proteins induced by low-intensity irradiation of helium-neon laser and their involvement in the generation of reactive oxygen species. J. Photochem. Photobiol. B Biol. 2017, 176, 36–43. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Shtarkman, I.N.; Chernikov, A.V.; Usacheva, A.M.; Bruskov, V.I. Guanosine and inosine (riboxin) eliminate the long-lived protein radicals induced X-ray radiation. Dokl. Biochem. Biophys. 2007, 413, 50–53. [Google Scholar] [CrossRef] [PubMed]
- Sarimov, R.M.; Nagaev, E.I.; Matveyeva, T.A.; Binhi, V.N.; Burmistrov, D.E.; Serov, D.A.; Astashev, M.E.; Simakin, A.V.; Uvarov, O.V.; Khabatova, V.V.; et al. Investigation of Aggregation and Disaggregation of Self-Assembling Nano-Sized Clusters Consisting of Individual Iron Oxide Nanoparticles upon Interaction with HEWL Protein Molecules. Nanomaterials 2022, 12, 3960. [Google Scholar] [CrossRef] [PubMed]
- Rudenko, Y.; Kozlov, V.; Burmistrov, D.; Fedyakova, N.; Bermeshev, M.; Chapala, P. Comprehensive investigation of phosphine oxide photoinitiators for vat photopolymerization. Prog. Addit. Manuf. 2025, 10, 8405–8418. [Google Scholar] [CrossRef]
- Pochapski, D.J.; Carvalho dos Santos, C.; Leite, G.W.; Pulcinelli, S.H.; Santilli, C.V. Zeta Potential and Colloidal Stability Predictions for Inorganic Nanoparticle Dispersions: Effects of Experimental Conditions and Electrokinetic Models on the Interpretation of Results. Langmuir 2021, 37, 13379–13389. [Google Scholar] [CrossRef]
- Wang, H.; Zou, H.; Wang, C.; Lv, S.; Jin, Y.; Hu, H.; Wang, X.; Chi, Y.; Yang, X. Controllable Synthesis, Formation Mechanism, and Photocatalytic Activity of Tellurium with Various Nanostructures. Micromachines 2023, 15, 1. [Google Scholar] [CrossRef]
- Tsai, H.-W.; Yaghoubi, A.; Chan, T.-C.; Wang, C.-C.; Liu, W.-T.; Liao, C.-N.; Lu, S.-Y.; Chen, L.-J.; Chueh, Y.-L. Electrochemical synthesis of ultrafast and gram-scale surfactant-free tellurium nanowires by gas–solid transformation and their applications as supercapacitor electrodes for p-doping of graphene transistors. Nanoscale 2015, 7, 7535–7539. [Google Scholar] [CrossRef]
- Grasser, M.A.; Pietsch, T.; Blasius, J.; Hollóczki, O.; Brunner, E.; Doert, T.; Ruck, M. Coexistence of Tellurium Cations and Anions in Phosphonium-Based Ionic Liquids. Chem. A Eur. J. 2021, 28, e202103770. [Google Scholar] [CrossRef]
- Rudenko, Y.; Prosyankin, E.; Mustafina, A.; Fuki, M.; Borisov, R.; Fedyakova, N.; Bermeshev, M.; Chapala, P. Investigation of the Light Intensity and Temperature Influences on Double Bond Conversion in Resins for Vat Photopolymerization via Fourier Transform Infrared Spectroscopy. Macromol. Chem. Phys. 2025, 226, 2400398. [Google Scholar] [CrossRef]
- Rudenko, Y.; Lozovaya, A.; Asanova, L.; Fedyakova, N.; Chapala, P. Light intensity influence on critical energy and penetration depth for vat photopolymerization technology. Prog. Addit. Manuf. 2023, 9, 553–561. [Google Scholar] [CrossRef]
- Dzhardimalieva, G.; Bondarenko, L.; Illés, E.; Tombácz, E.; Tropskaya, N.; Magomedov, I.; Orekhov, A.; Kydralieva, K. Colloidal Stability of Silica-Modified Magnetite Nanoparticles: Comparison of Various Dispersion Techniques. Nanomaterials 2021, 11, 3295. [Google Scholar] [CrossRef] [PubMed]
- Oberdisse, J. Aggregation of colloidal nanoparticles in polymer matrices. Soft Matter 2006, 2, 29–36. [Google Scholar] [CrossRef]
- Sharifzadeh, E.; Karami, M.; Ader, F. Formation of nanoparticle aggregates and agglomerates in polymer nanocomposites and their distinct impacts on the mechanical properties. Polym. Eng. Sci. 2023, 63, 1303–1313. [Google Scholar] [CrossRef]
- Zare, Y. Study of nanoparticles aggregation/agglomeration in polymer particulate nanocomposites by mechanical properties. Compos. Part A: Appl. Sci. Manuf. 2016, 84, 158–164. [Google Scholar] [CrossRef]
- Deshpande, P.A.; Delgado, A.H.S.; Young, A.M. Methacrylate peak determination and selection recommendations using ATR-FTIR to investigate polymerisation of dental methacrylate mixtures. PLoS ONE 2021, 16, e0252999. [Google Scholar] [CrossRef]
- Leggat, P.A.; Kedjarune, U. Toxicity of methyl methacrylate in dentistry. Int. Dent. J. 2003, 53, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Nicholas, C.A.; Lawrence, W.H.; Autian, J. Embryotoxicity and fetotoxicity from maternal inhalation of methyl methacrylate monomer in rats. Toxicol. Appl. Pharmacol. 1979, 50, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Pemberton, M.A.; Lohmann, B.S. Risk Assessment of residual monomer migrating from acrylic polymers and causing Allergic Contact Dermatitis during normal handling and use. Regul. Toxicol. Pharmacol. 2014, 69, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Pituru, S.M.; Greabu, M.; Totan, A.; Imre, M.; Pantea, M.; Spinu, T.; Tancu, A.M.C.; Popoviciu, N.O.; Stanescu, I.-I.; Ionescu, E. A Review on the Biocompatibility of PMMA-Based Dental Materials for Interim Prosthetic Restorations with a Glimpse into Their Modern Manufacturing Techniques. Materials 2020, 13, 2894. [Google Scholar] [CrossRef]
- Ahamad Said, M.N.; Hasbullah, N.A.; Rosdi, M.R.H.; Musa, M.S.; Rusli, A.; Ariffin, A.; Shafiq, M.D. Polymerization and Applications of Poly(methyl methacrylate)–Graphene Oxide Nanocomposites: A Review. ACS Omega 2022, 7, 47490–47503. [Google Scholar] [CrossRef]
- Glazkova, E.; Bakina, O.; Rodkevich, N.; Mosunov, A.; Evstigneev, M.; Evstigneev, V.; Klimenko, V.; Lerner, M. Antibacterial Properties of PMMA Functionalized with CuFe2O4/Cu2O/CuO Nanoparticles. Coatings 2022, 12, 957. [Google Scholar] [CrossRef]
- Siddiqui, M.N.; Redhwi, H.H.; Vakalopoulou, E.; Tsagkalias, I.; Ioannidou, M.D.; Achilias, D.S. Synthesis, characterization and reaction kinetics of PMMA/silver nanocomposites prepared via in situ radical polymerization. Eur. Polym. J. 2015, 72, 256–269. [Google Scholar] [CrossRef]
- Jabeen, S.; Gul, S.; Kausar, A.; Muhammad, B.; Farooq, M. An Innovative Approach to the Synthesis of PMMA/PEG/Nanobifiller Filled Nanocomposites with Enhanced Mechanical and Thermal Properties. Polym.-Plast. Technol. Mater. 2018, 58, 427–442. [Google Scholar] [CrossRef]
- Guryanova, S.V. Regulation of Immune Homeostasis via Muramyl Peptides-Low Molecular Weight Bioregulators of Bacterial Origin. Microorganisms 2022, 10, 1526. [Google Scholar] [CrossRef]
- Guryanova, S.V.; Gigani, O.B.; Gudima, G.O.; Kataeva, A.M.; Kolesnikova, N.V. Dual Effect of Low-Molecular-Weight Bioregulators of Bacterial Origin in Experimental Model of Asthma. Life 2022, 12, 192. [Google Scholar] [CrossRef]
- Arjmand, B.; Alavi-Moghadam, S.; Sarvari, M.; Rezaei-Tavirani, M.; Rezazadeh- Mafi, A.; Arjmand, R.; Nikandish, M.; Nasli-Esfahani, E.; Larijani, B. Critical roles of cytokine storm and bacterial infection in patients with COVID-19: Therapeutic potential of mesenchymal stem cells. Inflammopharmacology 2023, 31, 171–206. [Google Scholar] [CrossRef]
- Eloseily, E.M.; Cron, R.Q. Bacteria-Associated Cytokine Storm Syndrome. In Cytokine Storm Syndrome; Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2024; pp. 275–283. [Google Scholar]
- Nie, J.; Zhou, L.; Tian, W.; Liu, X.; Yang, L.; Yang, X.; Zhang, Y.; Wei, S.; Wang, D.W.; Wei, J. Deep insight into cytokine storm: From pathogenesis to treatment. Signal Transduct. Target. Ther. 2025, 10, 112. [Google Scholar] [CrossRef]
- Alfei, S.; Schito, G.C.; Schito, A.M.; Zuccari, G. Reactive Oxygen Species (ROS)-Mediated Antibacterial Oxidative Therapies: Available Methods to Generate ROS and a Novel Option Proposal. Int. J. Mol. Sci. 2024, 25, 7182. [Google Scholar] [CrossRef]
- Elbehiry, A.; Abalkhail, A. Antimicrobial Nanoparticles Against Superbugs: Mechanistic Insights, Biomedical Applications, and Translational Frontiers. Pharmaceuticals 2025, 18, 1195. [Google Scholar] [CrossRef]
- Gao, F.; Shao, T.; Yu, Y.; Xiong, Y.; Yang, L. Surface-bound reactive oxygen species generating nanozymes for selective antibacterial action. Nat. Commun. 2021, 12, 745. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, H.; Cheng, J.; Li, H.; Wu, X.; Zhang, D.; Shi, X.; Zhang, J.; Han, N.; Chen, Y. Initiative ROS generation of Cu-doped ZIF-8 for excellent antibacterial performance. Chem. Eng. J. 2023, 466, 143201. [Google Scholar] [CrossRef]
- Zheng, G.; Tang, Z.; Peng, F. Ultrasound-activated inorganic nanomaterials to generate ROS for antibacterial applications. Biomater. Sci. 2025, 13, 2628–2641. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Lyakhov, G.A.; Pustovoy, V.I.; Shcherbakov, I.A. Influence of Mechanical Effects on the Hydrogen Peroxide Concentration in Aqueous Solutions. Phys. Wave Phenom. 2019, 27, 141–144. [Google Scholar] [CrossRef]
- Temnov, A.A.; Sklifas, A.N.; Zhalimov, V.K.; Sharapov, M.G.; Fadeev, R.S.; Kobyakova, M.I.; Kukushkin, N.I.; Rogov, K.A. Paracrine Effects of Stem Cell Conditioned Medium on Reactive Oxygen Species Production in Blood Neutrophils in Acetaminophen-Induced Liver Failure. Biophysics 2023, 68, 70–78. [Google Scholar] [CrossRef]
- Goncharov, R.G.; Filkov, G.I.; Trofimenko, A.V.; Boyarintsev, V.V.; Novoselov, V.I.; Sharapov, M.G. The Protective Effect of a Chimeric PSH Antioxidant Enzyme in Renal Ischemia–Reperfusion Injury. Biophysics 2020, 65, 303–312. [Google Scholar] [CrossRef]
- Wang, L.; Li, B.; Dionysiou, D.D.; Chen, B.; Yang, J.; Li, J. Overlooked Formation of H2O2 during the Hydroxyl Radical-Scavenging Process When Using Alcohols as Scavengers. Environ. Sci. Technol. 2022, 56, 3386–3396. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Gudkova, O.Y.; Chernikov, A.V.; Bruskov, V.I. Protection of mice against X-ray injuries by the post-irradiation administration of guanosine and inosine. Int. J. Radiat. Biol. 2009, 85, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Sharapov, M.G.; Gudkov, S.V.; Lankin, V.Z. Hydroperoxide-Reducing Enzymes in the Regulation of Free-Radical Processes. Biochemistry 2021, 86, 1256–1274. [Google Scholar] [CrossRef] [PubMed]
- Kornienko, J.S.; Smirnova, I.S.; Pugovkina, N.A.; Ivanova, J.S.; Shilina, M.A.; Grinchuk, T.M.; Shatrova, A.N.; Aksenov, N.D.; Zenin, V.V.; Nikolsky, N.N.; et al. High doses of synthetic antioxidants induce premature senescence in cultivated mesenchymal stem cells. Sci. Rep. 2019, 9, 1296. [Google Scholar] [CrossRef] [PubMed]
- Vostrikova, S.M.; Grinev, A.B.; Gogvadze, V.G. Reactive Oxygen Species and Antioxidants in Carcinogenesis and Tumor Therapy. Biochemistry 2020, 85, 1254–1266. [Google Scholar] [CrossRef]
- Zhevak, T.; Shelekhova, T.; Chesnokova, N.; Tsareva, O.; Chanturidze, A.; Litvitsky, P.; Andriutsa, N.; Samburova, N.; Budnik, I. The relationship between oxidative stress and cytogenetic abnormalities in B-cell chronic lymphocytic leukemia. Exp. Mol. Pathol. 2020, 116, 104524. [Google Scholar] [CrossRef]
- Goncharov, R.G.; Sharapov, M.G. Ischemia–Reperfusion Injury: Molecular Mechanisms of Pathogenesis and Methods of Their Correction. Mol. Biol. 2023, 57, 1143–1164. [Google Scholar] [CrossRef]
- Sies, H. Oxidative eustress: On constant alert for redox homeostasis. Redox Biol. 2021, 41, 101867. [Google Scholar] [CrossRef]
- Ravanat, J.L. Cellular background level of 8-oxo-7,8-dihydro-2′-deoxyguanosine: An isotope based method to evaluate artefactual oxidation of DNA during its extraction and subsequent work-up. Carcinogenesis 2002, 23, 1911–1918. [Google Scholar] [CrossRef]
- Chiorcea-Paquim, A.-M. 8-oxoguanine and 8-oxodeoxyguanosine Biomarkers of Oxidative DNA Damage: A Review on HPLC–ECD Determination. Molecules 2022, 27, 1620. [Google Scholar] [CrossRef] [PubMed]
- Matsui, A.; Ikeda, T.; Enomoto, K.; Hosoda, K.; Nakashima, H.; Omae, K.; Watanabe, M.; Hibi, T.; Kitajima, M. Increased formation of oxidative DNA damage, 8-hydroxy-2′-deoxyguanosine, in human breast cancer tissue and its relationship to GSTP1 and COMT genotypes. Cancer Lett. 2000, 151, 87–95. [Google Scholar] [CrossRef]
- Katerji, M.; Filippova, M.; Duerksen-Hughes, P. Approaches and Methods to Measure Oxidative Stress in Clinical Samples: Research Applications in the Cancer Field. Oxidative Med. Cell. Longev. 2019, 2019, 1–29. [Google Scholar] [CrossRef]
- Li, D.; Zhang, W.; Zhu, J.; Chang, P.; Sahin, A.; Singletary, E.; Bondy, M.; Hazra, T.; Mitra, S.; Lau, S.S.; et al. Oxidative DNA damage and 8-hydroxy-2-deoxyguanosine DNA glycosylase/apurinic lyase in human breast cancer. Mol. Carcinog. 2001, 31, 214–223. [Google Scholar] [CrossRef]
- De Rosa, M.; Johnson, S.A.; Opresko, P.L. Roles for the 8-Oxoguanine DNA Repair System in Protecting Telomeres From Oxidative Stress. Front. Cell Dev. Biol. 2021, 9, 758402. [Google Scholar] [CrossRef]
- Li, C.; Xue, Y.; Ba, X.; Wang, R. The Role of 8-oxoG Repair Systems in Tumorigenesis and Cancer Therapy. Cells 2022, 11, 3798. [Google Scholar] [CrossRef]
- Kondo, S.; Toyokuni, S.; Iwasa, Y.; Tanaka, T.; Onodera, H.; Hiai, H.; Imamura, M. Persistent oxidative stress in human colorectal carcinoma, but not in adenoma. Free. Radic. Biol. Med. 1999, 27, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Kruchinin, A.A.; Kamzeeva, P.N.; Zharkov, D.O.; Aralov, A.V.; Makarova, A.V. 8-Oxoadenine: A «New» Player of the Oxidative Stress in Mammals? Int. J. Mol. Sci. 2024, 25, 1342. [Google Scholar] [CrossRef]
- Caraceni, P.; De Maria, N.; Ryu, H.S.; Colantoni, A.; Roberts, L.; Maidt, M.L.; Pye, Q.; Bernardi, M.; Van Thiel, D.H.; Floyd, R.A. Proteins but not Nucleic Acids are Molecular Targets for the Free Radical Attack During Reoxygenation of Rat Hepatocytes. Free. Radic. Biol. Med. 1997, 23, 339–344. [Google Scholar] [CrossRef]
- Davies, M. Long-lived reactive species formed on proteins induce changes in protein and lipid turnover. Free. Radic. Biol. Med. 2014, 75, S6–S7. [Google Scholar] [CrossRef]
- Simpson, J.A.; Narita, S.; Gieseg, S.; Gebicki, S.; Gebicki, J.M.; Dean, R.T. Long-lived reactive species on free-radical-damaged proteins. Biochem. J. 1992, 282, 621–624. [Google Scholar] [CrossRef]
- Burmistrov, D.E.; Yanykin, D.V.; Paskhin, M.O.; Nagaev, E.V.; Efimov, A.D.; Kaziev, A.V.; Ageychenkov, D.G. Additive Production of a Material Based on an Acrylic Polymer with a Nanoscale Layer of Zno Nanorods Deposited Using a Direct Current Magnetron Discharge: Morphology, Photoconversion Properties, and Biosafety. Materials 2021, 14, 6586. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Garmash, S.A.; Shtarkman, I.N.; Chernikov, A.V.; Karp, O.E.; Bruskov, V.I. Long-lived protein radicals induced by X-ray irradiation are the source of reactive oxygen species in aqueous medium. Dokl Biochem. Biophys 2010, 430, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Bruskov, V.I.; Karp, O.E.; Garmash, S.A.; Shtarkman, I.N.; Chernikov, A.V.; Gudkov, S.V. Prolongation of oxidative stress by long-lived reactive protein species induced by X-ray radiation and their genotoxic action. Free. Radic. Res. 2012, 46, 1280–1290. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Novak, B.; Namendorf, C.; Steigenberger, B.; Zhang, Y.; Turck, C.W. Long-lived proteins and DNA as candidate predictive biomarkers for tissue associated diseases. iScience 2024, 27, 109642. [Google Scholar] [CrossRef]
- Jena, A.B.; Samal, R.R.; Bhol, N.K.; Duttaroy, A.K. Cellular Red-Ox system in health and disease: The latest update. Biomed. Pharmacother. 2023, 162, 114606. [Google Scholar] [CrossRef]
- Kurutas, E.B. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: Current state. Nutr. J. 2016, 15, 71. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Mestres, G.; Singh, V.; Effati, P.; Poon, J.-F.; Engman, L.; Ott, M. Selenium- and Tellurium-Based Antioxidants for Modulating Inflammation and Effects on Osteoblastic Activity. Antioxidants 2017, 6, 13. [Google Scholar] [CrossRef] [PubMed]

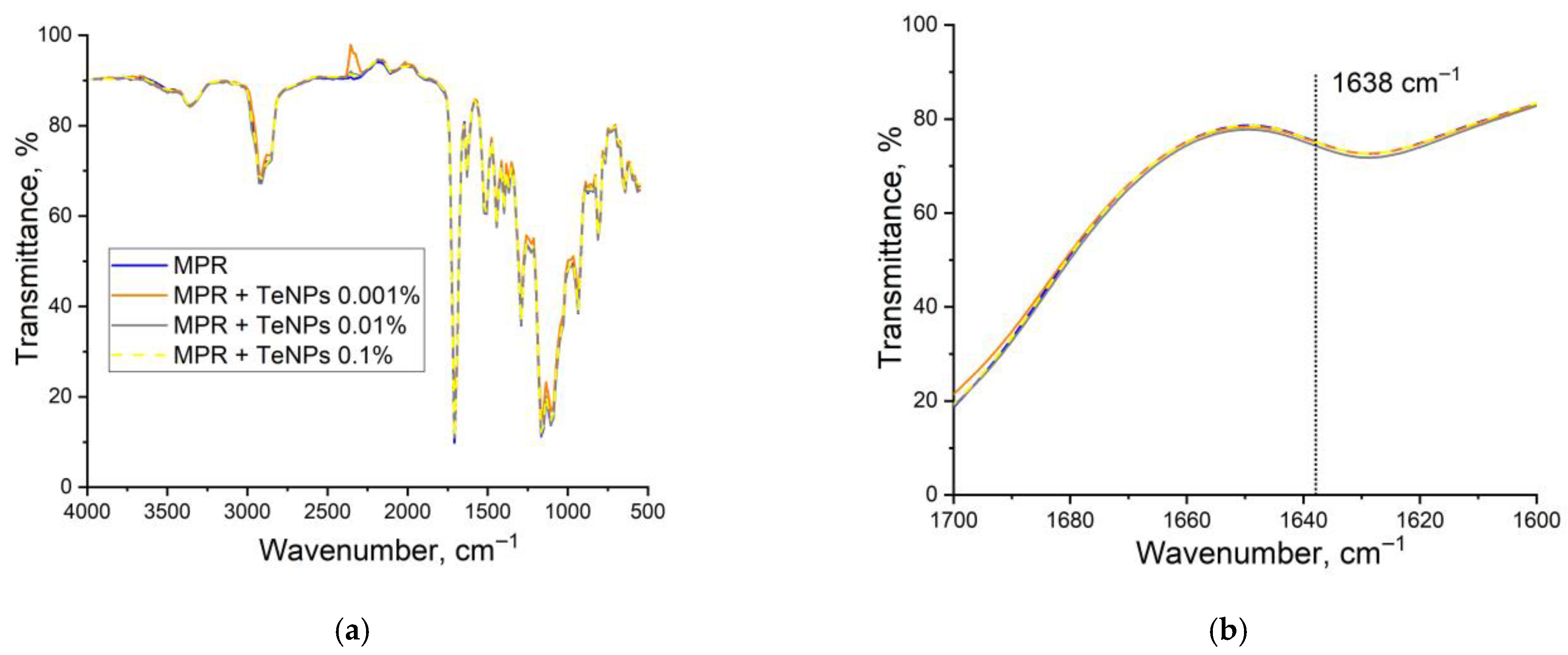



| Absorption Band, cm−1 | Assignment |
|---|---|
| 3400–3200 | O-H stretching vibrations |
| 2950–2800 | C-H stretching vibrations |
| 1713 | C=O stretching vibrations |
| 881 | =CH deformation vibrations |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serov, D.A.; Simakin, A.V.; Burmistrov, D.E.; Baimler, I.V.; Chapala, P.P.; Astashev, M.E.; Yanbaev, F.M.; Kozlov, V.A.; Gudkov, S.V. In Situ Synthesis of Non-Cytotoxic Tellurium Nanoparticle and Methacrylate Photopolymer Resin Composite with Antibacterial Activity. Polymers 2025, 17, 2735. https://doi.org/10.3390/polym17202735
Serov DA, Simakin AV, Burmistrov DE, Baimler IV, Chapala PP, Astashev ME, Yanbaev FM, Kozlov VA, Gudkov SV. In Situ Synthesis of Non-Cytotoxic Tellurium Nanoparticle and Methacrylate Photopolymer Resin Composite with Antibacterial Activity. Polymers. 2025; 17(20):2735. https://doi.org/10.3390/polym17202735
Chicago/Turabian StyleSerov, Dmitriy A., Aleksandr V. Simakin, Dmitriy E. Burmistrov, Ilya V. Baimler, Pavel P. Chapala, Maxim E. Astashev, Fatikh M. Yanbaev, Valeriy A. Kozlov, and Sergey V. Gudkov. 2025. "In Situ Synthesis of Non-Cytotoxic Tellurium Nanoparticle and Methacrylate Photopolymer Resin Composite with Antibacterial Activity" Polymers 17, no. 20: 2735. https://doi.org/10.3390/polym17202735
APA StyleSerov, D. A., Simakin, A. V., Burmistrov, D. E., Baimler, I. V., Chapala, P. P., Astashev, M. E., Yanbaev, F. M., Kozlov, V. A., & Gudkov, S. V. (2025). In Situ Synthesis of Non-Cytotoxic Tellurium Nanoparticle and Methacrylate Photopolymer Resin Composite with Antibacterial Activity. Polymers, 17(20), 2735. https://doi.org/10.3390/polym17202735











