Sensitized Radiation-Induced Polymerization of Indene with 1,1,2,2-Tetrachloroethane
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Equipment
2.2. Irradiation Experiments
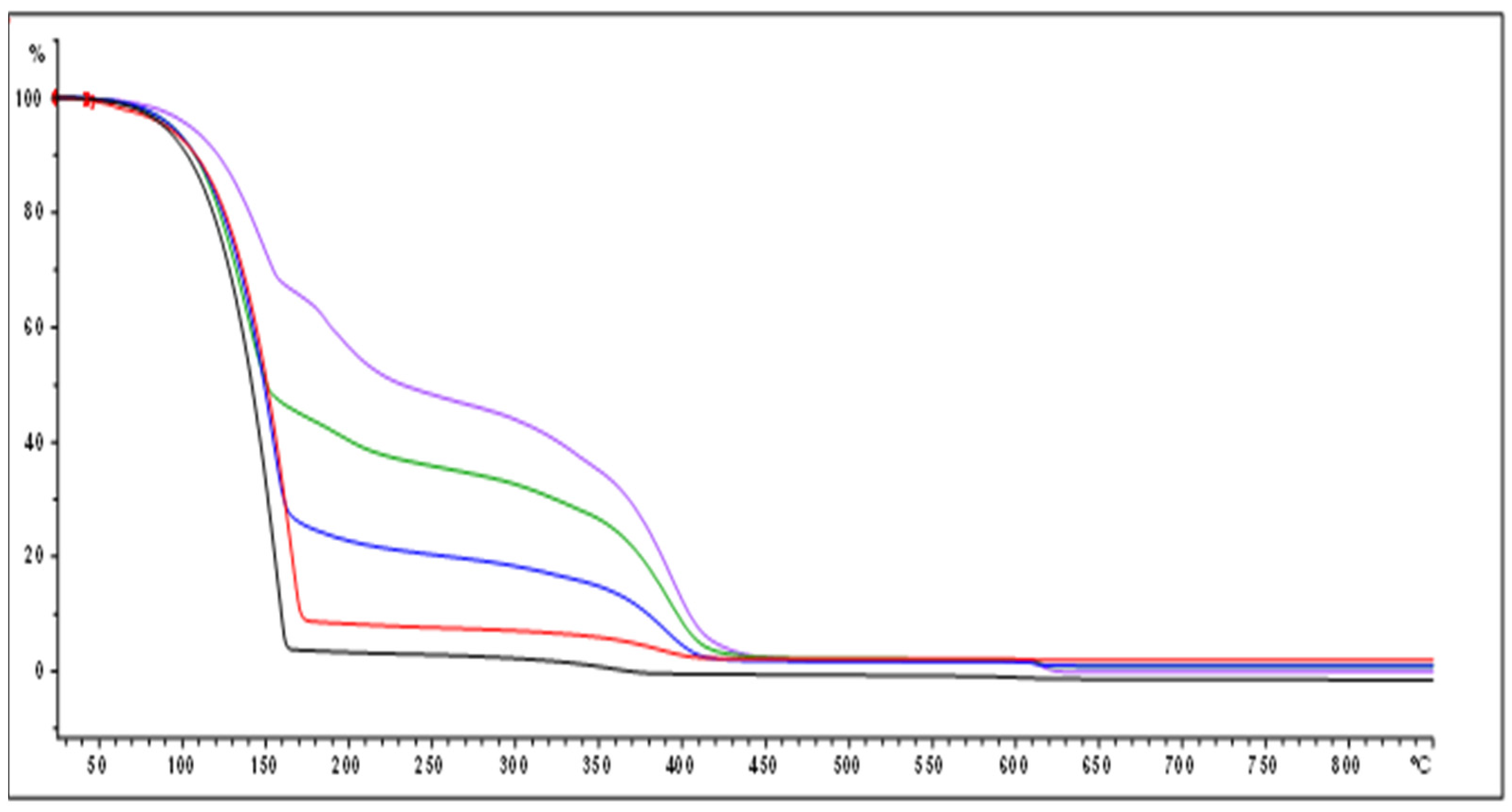
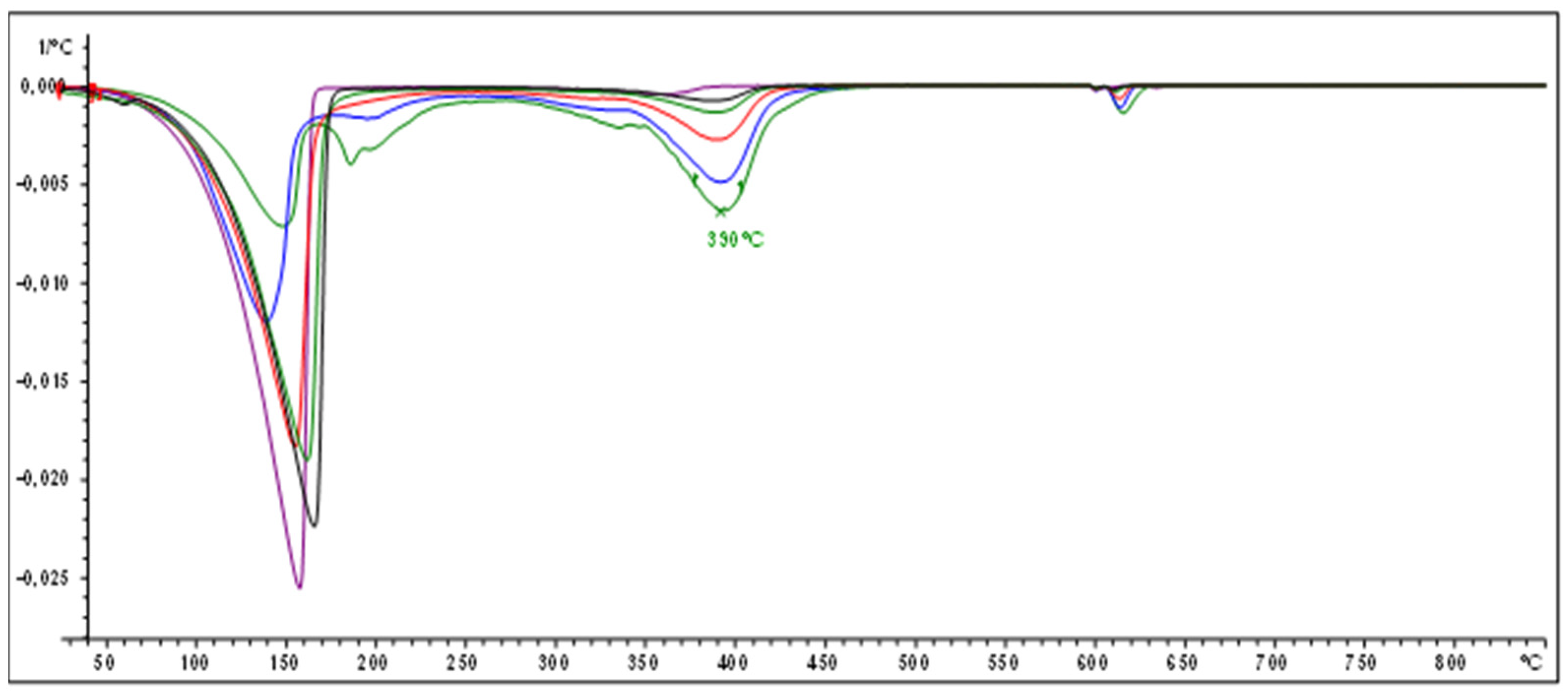
2.3. Determination of Polymer Yield by Thermogravimetric Analysis (TGA-DTG)
2.4. Determination of Polymer Yield by Polymer Precipitation and Gravimetry
3. Results and Discussion
3.1. Polymer Yield and Polymerization Kinetics
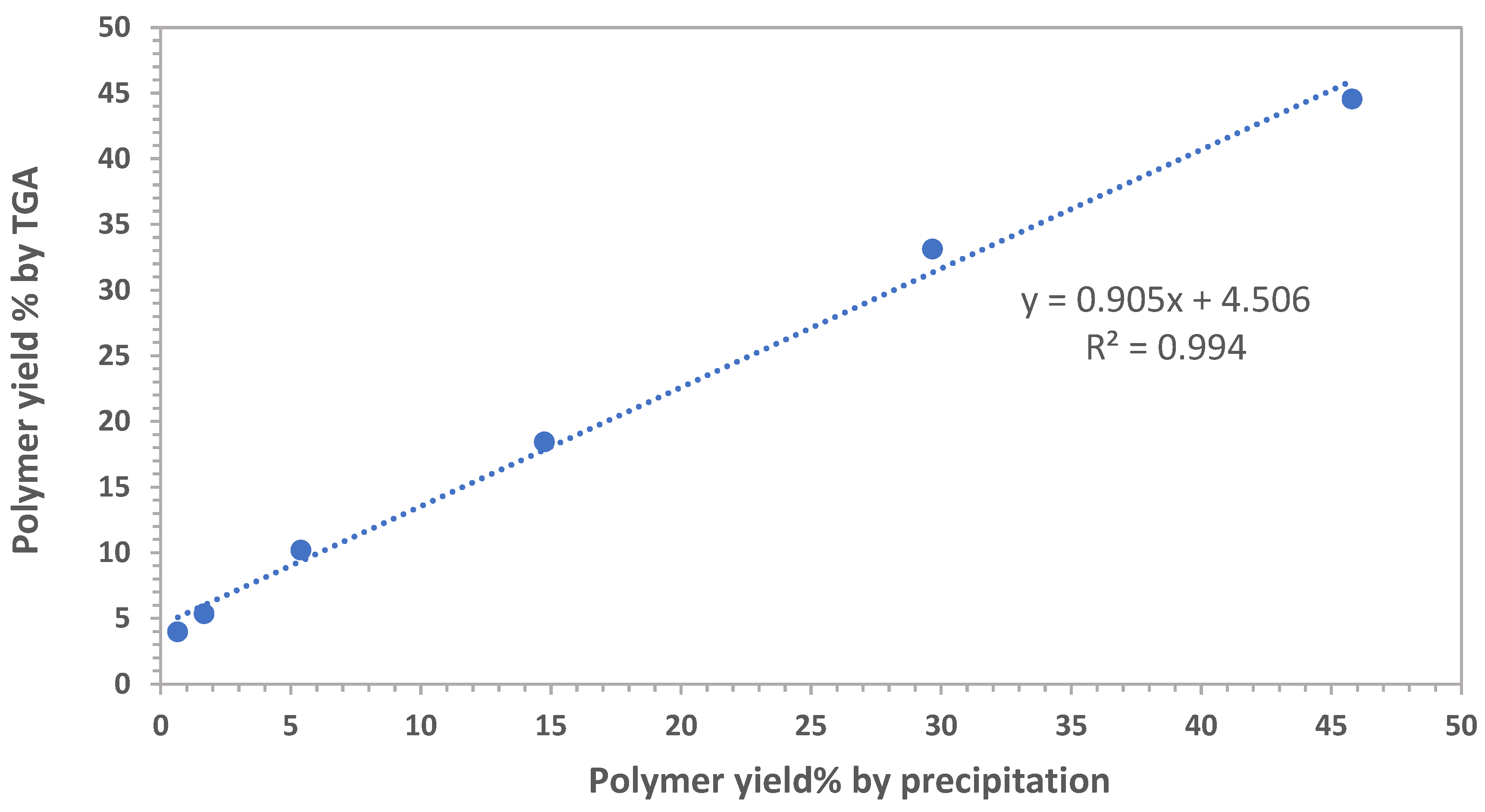
3.1.1. Polyindene Yield Determination by Thermogravimetric and Gravimetric Analysis
3.1.2. Determination of Polyindene Yield
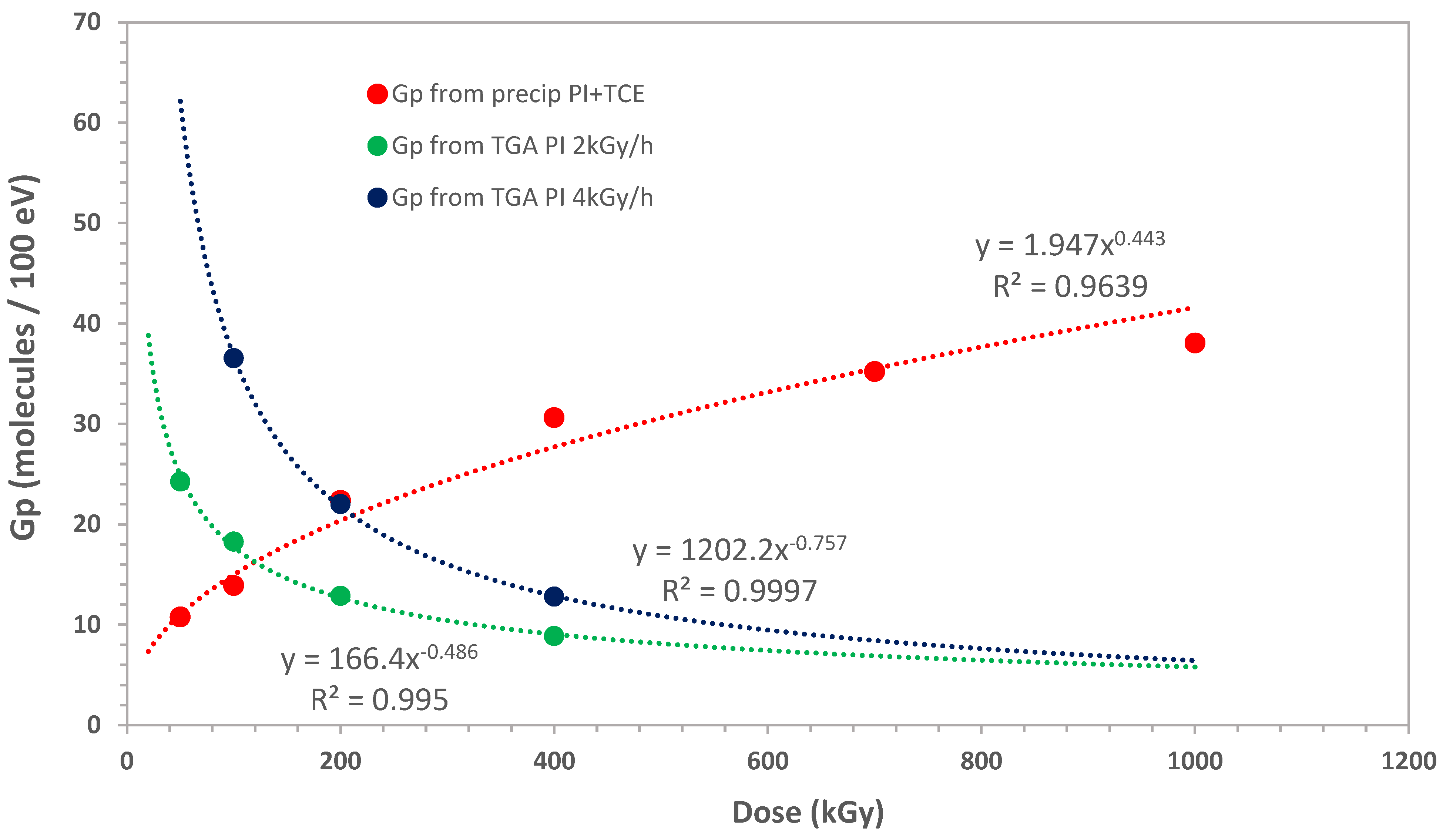
3.1.3. Kinetics of Indene Polymerization in Presence of TCE Sensitizer
3.2. Radiation Chemical Yield in Sensitized Radiation-Induced Polymerization and Polymerization Mechanisms
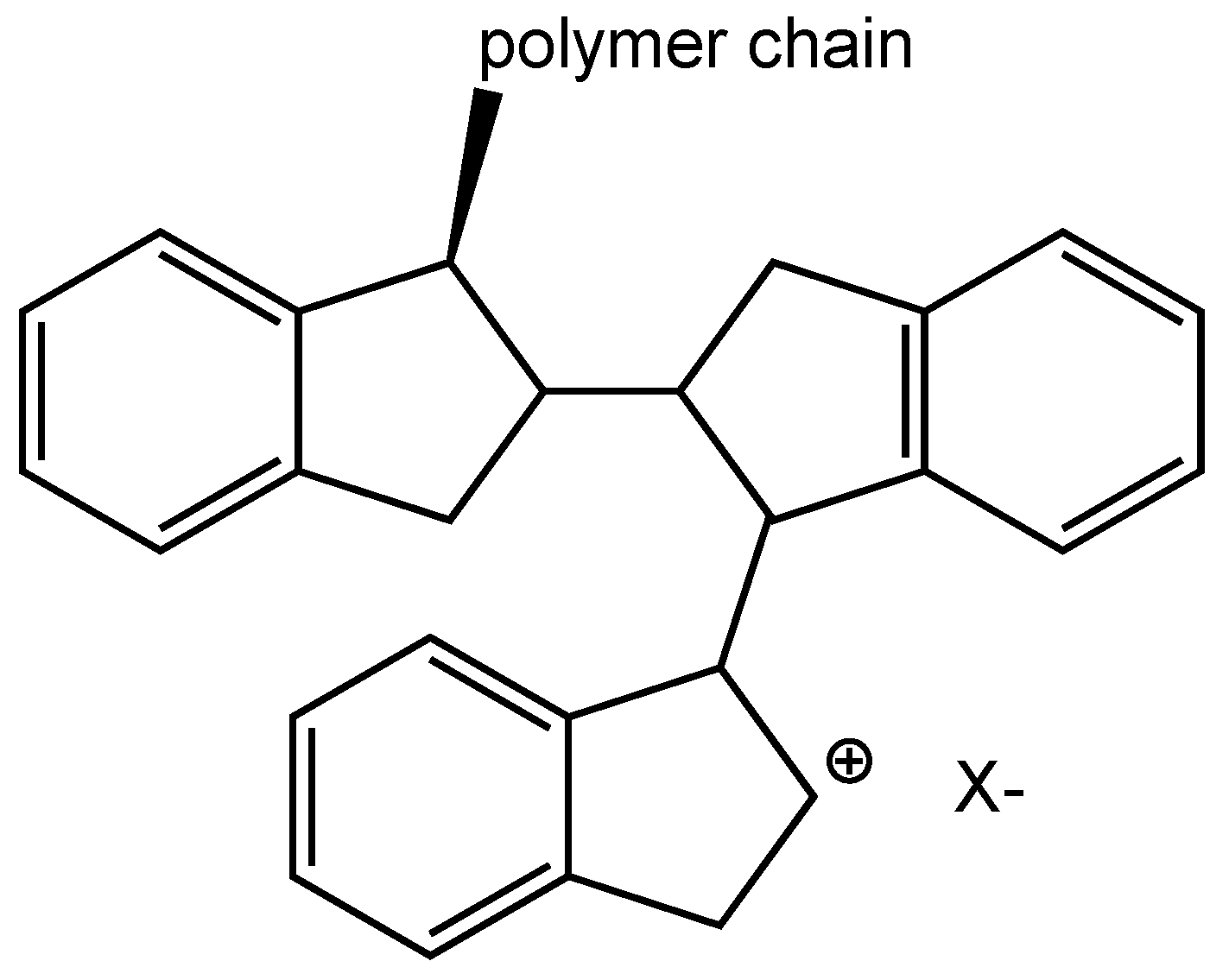
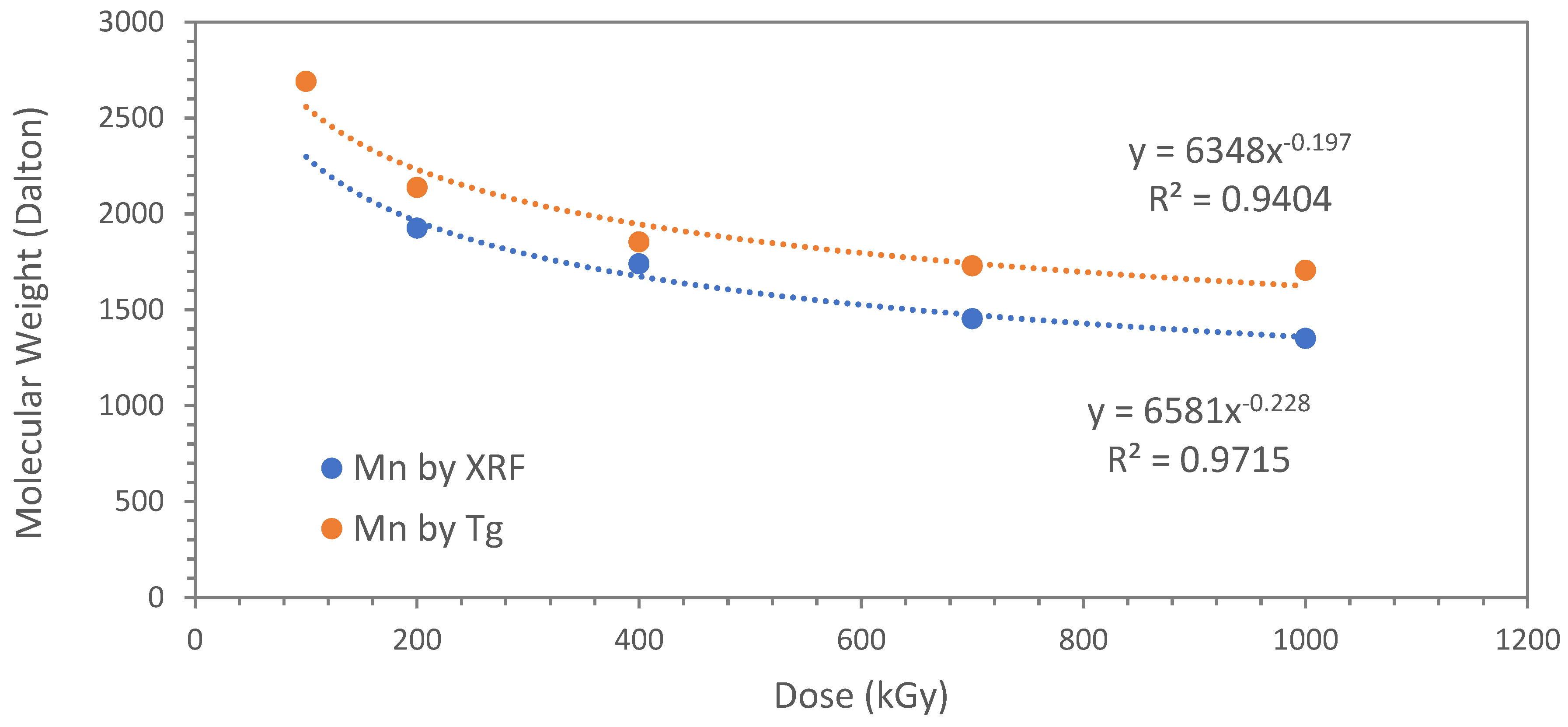
3.3. Molecular Weight and Chemical Structure of Polyindene Obtained in Sensitized Radiation-Induced Polymerization
3.4. FT–IR Spectroscopy of the Polyindenes Obtained by Sensitized Radiation-Induced Polymerization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Barzaga, R.; García-Hernández, D.A.; Manchado, A.; Di Sarcina, I.; Cemmi, A.; Cataldo, F. On the radiation-induced polymerization of indene: From laboratory study to astrochemical implications. J. Radioanal. Nucl. Chem. 2024, 333, 865–876. [Google Scholar] [CrossRef]
- Cataldo, F.; Barzaga, R.; García-Hernández, D.A.; Manchado, A. A study on poly(indene). J. Macromol. Sci. Part A 2024, 61, 454–464. [Google Scholar] [CrossRef]
- Burkhardt, A.M.; Lee, K.L.K.; Changala, P.B.; Shingledecker, C.N.; Cooke, I.R.; Loomis, R.A.; Wei, H.; Charnley, S.B.; Herbst, E.; McCarthy, B.A. Discovery of the pure polycyclic aromatic hydrocarbon indene (c-C9H8) with GOTHAM observations of TMC-1. Astrophys. J. Lett. 2021, 913, L18. [Google Scholar] [CrossRef]
- Cernicharo, J.; Agúndez, M.; Cabezas, C.; Tercero, B.; Marcelino, N.; Pardo, J.R.; De Vicente, P. Pure hydrocarbon cycles in TMC-1: Discovery of ethynyl cyclopropenylidene, cyclopentadiene, and indene. Astron. Astrophys. 2021, 649, L15. [Google Scholar] [CrossRef] [PubMed]
- Sita, M.L.; Changala, P.B.; Xue, C.; Burkhardt, A.M.; Shingledecker, C.N.; Lee, K.L.K.; Loomis, R.A.; Momjan, E.; Siebert, M.A.; McGuire, B.A. Discovery of interstellar 2-cyanoindene (2-C9H7CN) in GOTHAM observations of TMC-1. Astrophys. J. Lett. 2022, 938, L12. [Google Scholar] [CrossRef]
- García-Hernández, D.A.; Barzaga, R.; Manchado, A.; Cataldo, F. Fullerene-indene adducts (ICMA & ICBA) in an astrochemical perspective part 1: Chemical thermodynamics, stability and electronic absorption spectroscopy. Fullerenes Nanotub. Carbon Nanostruct. 2023, 31, 897–905. [Google Scholar]
- García-Hernández, D.A.; Barzaga, R.; Manchado, A.; Cataldo, F. Fullerene-indene adducts (ICMA & ICBA) in an astrochemical perspective. Part 2: FT-IR spectroscopy from− 180 °C to+ 250 °C. Fullerenes Nanotub. Carbon Nanostruct. 2023, 31, 989–998. [Google Scholar]
- Maté, B.; Tanarro, I.; Peláez, R.J.; Cernicharo, J.; Herrero, V.J. Indene energetic processing in ice mantles in the interstellar medium. Astron. Astrophys. 2024, 682, A158. [Google Scholar] [CrossRef]
- Ibragimova, R.; Kuklin, M.S.; Zarrouk, T.; Caro, M.A. Unifying the description of hydrocarbons and hydrogenated carbon materials with a chemically reactive machine learning interatomic potential. Chem. Mater. 2025, 37, 1094–1110. [Google Scholar] [CrossRef]
- Baccaro, S.; Cemmi, A.; Di Sarcina, I.; Ferrara, G. Gamma Irradiation Calliope Facility; ENEA—Casaccia Research Centre: Italy, Rome, 2019; ENEA Technical Report; RT/2019/4/ENEA. [Google Scholar]
- Ivanov, V.S. Radiation Chemistry of Polymers; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: Abingdon, UK, 2023; pp. 43–121. [Google Scholar]
- Mehnert, R. Ullmann’s Encyclopedia of Industrial Chemistry; Elvers, B., Hawkins, S., Russey, W., Schulz, G., Eds.; VCH: Weinheim, Germany, 1993; Volume A22, pp. 471–476. [Google Scholar]
- Wojnarovits, L. Handbook of Nuclear Chemistry; Vertes, A., Nagy, S., Klencsar, Z., Lovas, R.G., Rosch, F., Eds.; Springer: Berlin, Germany, 2011; Volume 3, pp. 1301–1310. [Google Scholar]
- Chapiro, A. Radiation induced polymerization. Radiat. Phys. Chem. 1979, 14, 101–116. [Google Scholar] [CrossRef]
- Milinchuk, V.K.; Tupikov, V.I. Organic Radiation Chemistry Handbook; Ellis Harwood: Chichester, UK, 1989. [Google Scholar]
- Truszkowski, S.; Shostenko, A.G. Radiolysis of chloroalkanes. High Energy Chem. 2008, 42, 89–91. [Google Scholar] [CrossRef]
- Aloni, R.; Katz, M.G.; Rajbenbach, L.A. Radiation-induced dehalogenation of 1, 1, 1, 2 tetrachloroethane and 1, 1, 2 trichloro-1 bromoethane reactions of 1, 1, 2 trichloroethyl radicals. Int. J. Chem. Kinet. 1975, 7, 699–712. [Google Scholar] [CrossRef]
- Dean, J.A. (Ed.) Lange’s Handbook of Chemistry, 15th ed.; McGraw-Hill: New York, NY, USA, 1999. [Google Scholar]
- Hahn, S.F.; Hillmyer, M.A. High glass transition temperature polyolefins obtained by the catalytic hydrogenation of polyindene. Macromolecules 2003, 36, 71–76. [Google Scholar] [CrossRef]
- Naito, K.; Nakagawa, I.; Kuratani, K.; Ichishima, I.; Mizushima, S.I. Infrared and Raman Spectra of 1, 1, 2, 2-Tetrachloroethane; Calculation of Normal Vibrations. J. Chem. Phys. 1955, 23, 1907–1910. [Google Scholar] [CrossRef]
- Chantry, G.W.; Gebbie, H.A.; Griffiths, P.R.; Lake, R.F. Far infra-red spectra and the rotational isomerism of symmetrical tetrachloro-and tetrabromo-ethane. Spectrochim. Acta 1966, 22, 125–129. [Google Scholar] [CrossRef]


| 100 kGy | 200 kGy | 400 kGy | 700 kGy | 1000 kGy | |
|---|---|---|---|---|---|
| % by TGA | 5.35 | 10.19 | 18.44 | 33.12 | 44.54 |
| % Precipitation | 1.67 | 5.39 | 14.75 | 29.67 | 45.79 |
| 200 kGy | 400 kGy | 700 kGy | 1000 kGy | |
|---|---|---|---|---|
| % Carbon | 89.83 | 89.49 | 89.78 | 88.45 |
| % Hydrogen | 6.70 | 6.67 | 6.62 | 6.60 |
| % Chlorine (*) | 3.47 | 3.84 | 4.60 | 4.95 |
| Molecular Weight (Mn Dalton) | 1925 | 1739 | 1453 | 1350 |
| kGy | Mn by XRF (Dalton) | Mn by Tg (Dalton) | Tg (°C) |
|---|---|---|---|
| 100 | n.d. | 2691 | 151.8 |
| 200 | 1925 | 2137 | 137.6 |
| 400 | 1739 | 1853 | 127.0 |
| 700 | 1453 | 1729 | 121.3 |
| 1000 | 1350 | 1705 | 120.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barzaga, R.; García-Hernández, D.A.; Manchado, A.; Di Sarcina, I.; Cemmi, A.; Cataldo, F. Sensitized Radiation-Induced Polymerization of Indene with 1,1,2,2-Tetrachloroethane. Polymers 2025, 17, 1550. https://doi.org/10.3390/polym17111550
Barzaga R, García-Hernández DA, Manchado A, Di Sarcina I, Cemmi A, Cataldo F. Sensitized Radiation-Induced Polymerization of Indene with 1,1,2,2-Tetrachloroethane. Polymers. 2025; 17(11):1550. https://doi.org/10.3390/polym17111550
Chicago/Turabian StyleBarzaga, Ransel, Domingo Aníbal García-Hernández, Arturo Manchado, Ilaria Di Sarcina, Alessia Cemmi, and Franco Cataldo. 2025. "Sensitized Radiation-Induced Polymerization of Indene with 1,1,2,2-Tetrachloroethane" Polymers 17, no. 11: 1550. https://doi.org/10.3390/polym17111550
APA StyleBarzaga, R., García-Hernández, D. A., Manchado, A., Di Sarcina, I., Cemmi, A., & Cataldo, F. (2025). Sensitized Radiation-Induced Polymerization of Indene with 1,1,2,2-Tetrachloroethane. Polymers, 17(11), 1550. https://doi.org/10.3390/polym17111550







