Insights into the Development of Corrosion Protection Coatings
Abstract
1. Introduction
1.1. Corrosion
1.2. Classification of Corrosion
- (a)
- Uniform (or almost uniform), in which there is corrosion of all areas of the metal at the same (or a similar) rate, for example, oxidation and tarnishing.
- (b)
- Localized corrosion, where there is higher rate of corrosion in some areas of the metal surface because of the presence of ‘heterogeneities’ in the metal, the environment, or the geometry of the structure as a whole. Examples include crevice corrosion, filiform corrosion, and deposit attack.
- (c)
- Pitting, where there is a highly localized attack at specific areas causing the formation of small pits that penetrate the metal and might lead to perforation, for example, pitting of passive metals such as aluminium alloys, etc.
- (d)
- Selective dissolution, where in an alloy, usually, the most active component is selectively removed from an alloy. Examples include, de-aluminification, graphitization, etc.
- (e)
- Conjoint action, where corrosion and a mechanical factor act together, for example, a localized attack or fracture due to the combined action of a mechanical factor and corrosion [1].
1.3. Factors Affecting Corrosion
| Nature of metal | Nature of corroding environment |
| This further depends upon the following: | This further depends upon the following: |
| (a) Position in galvanic series; | (a) Temperature; |
| (b) Purity of metal; | (b) Humidity of air; |
| (c) Nature of surface film; | (c) Effect of pH. |
| (d) Nature of corrosive product. |
2. Corrosion Control by Use of Coatings
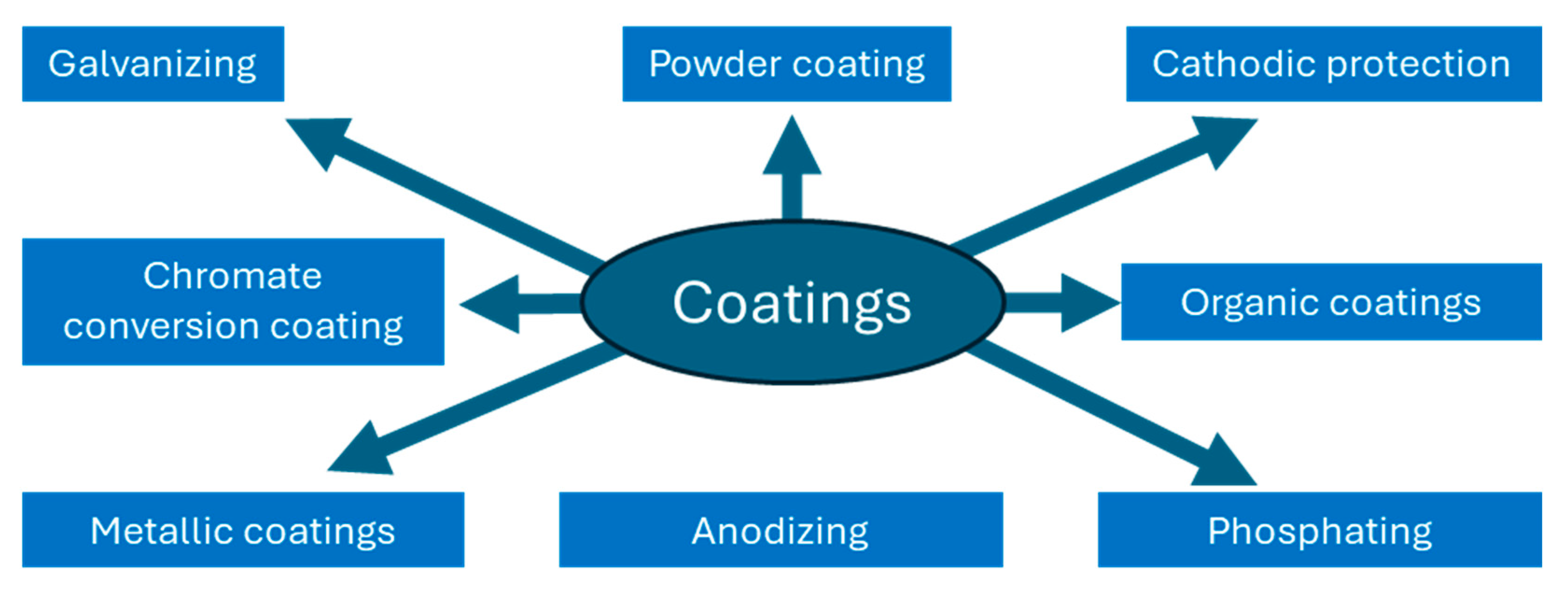
2.1. Coatings
2.2. Polymer Coatings
2.3. Classification of Polymer Coatings
2.4. Corrosion Testing Methods
3. Protective Mechanism of Anti-Corrosive Coating
4. Development in Nanostructured Anti-Corrosive Polymer Coating
4.1. Epoxy-Based Nanocomposite Polymer Coatings
4.1.1. Epoxy/Polyaniline
4.1.2. Epoxy/Polypyrrole
4.1.3. Epoxy/Polyurethane
4.1.4. Epoxy/Poly(vinyl alcohol)
4.1.5. Epoxy/Polyester
4.1.6. Epoxy/Polyamide
4.1.7. Epoxy/Poly(dimethylsiloxane)
4.1.8. Epoxy/Polythiophene
4.2. Nanocomposite Polymer Coatings for Anti-Corrosion Other Than Epoxy-Based
4.2.1. Polyaniline (PANi)-Based Nanocomposite
4.2.2. Graphene Oxide (GO)-Based Coating
4.2.3. Zeolitic Imidazole Framework Nanoparticles
4.2.4. Bioactive Polymer Nanocomposite
4.2.5. Epoxy-Based Nanocomposite
4.2.6. Polymethyl Methacrylate (PMMA) Hybrid Nanoparticles
4.2.7. Superhydrophobic Zinc Based Coating
4.2.8. Chitosan-Based Nanocomposite Coatings
4.2.9. Polyurethane-Based Nanocomposite
4.2.10. Nanostructured Carbon-Based Coatings
4.2.11. Nanostructured Al2O3-13TiO2 Coating for Corrosion Protection
4.2.12. Acrylic Nanocomposite
5. Future Perspectives
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| APS | Ammonium peroxydisulfate |
| AEAPS | 3-(2-aminoethylamino) propyl dimethoxy methylsilane |
| BPA | Bisphenol A |
| BET | Brunauer–Emmett–Teller |
| BDDE | 1,4-butanediol diglycidyl ether |
| CNO | Carbon nano-onion |
| CD | Cathodic disbondment test |
| Ce–La | Cerium–lanthanum conversion coating |
| CPs | Conducting polymers |
| CSA | Camphorsulfonic acid |
| CS | Chitosan |
| CEPD | Cathodic electrophoretic deposition |
| CLSM | Confocal laser scanning microscope |
| TCS | Chitosan modified TiO2 nanotubes |
| CNSL | Cashew nutshell liquid |
| CNC | Conductive carbon nanocomposite coating |
| CPCC | Chromate/phosphate conversion coated |
| DBSA | Dodecylbenzene sulfonic acid |
| DDA | Dodecylamine |
| DMSO | Dimethyl sulfoxide |
| DSC | Differential scanning calorimetry |
| DND | Dodecyl amine modified OND |
| EIS | Electrochemical impedance spectroscopy |
| EPA | Epoxy blend with polyaniline |
| EPM | Epoxy blend with polyaniline/MMT |
| EDS | Energy dispersive X-ray spectroscopy |
| EPE | Epoxy ester |
| EPZ | Epoxy/PANI-ZnO hybrid nanocomposite |
| epH | Epoxy-phenolic |
| FESEM | Field emission scanning electron microscopy |
| FTIR | Fourier transform infrared |
| FHA | Fluoro hydroxyapatite |
| GO | Graphene oxide |
| GPC | Gel permeation chromatography |
| PGHEP | Hydroxyl epoxy phosphate monomer |
| HA | Hydroxyapatite |
| HBP | Hyperbranched polyester-amide polymer |
| HNPs | Hybrid nanoparticles |
| HRTEM | High-resolution transmission electron microscopy |
| ICP | Intrinsically conducting polymers |
| ICP-OES | Inductively coupled plasma optical emission spectroscopy |
| ICR | Interface contact resistance |
| IPNs | Interpenetrating polymer networks |
| FT-IR | Infrared spectroscopy |
| LDH | Layered double hydroxides |
| MMT | Montmorillonite |
| MG | Modified GO |
| MNC | Modified NC |
| MIO | Micaceous iron oxide |
| MBI | 2-mercaptobenzimidazole |
| KH-570 | Methacryloxy propyl trimethoxyl silane |
| MSNs | Mesoporous silica nanoparticles |
| MF | Melamine formaldehyde |
| MD | Molecular dynamics |
| MC | Monte Carlo |
| MCPC | Melamine-cured polyester coating |
| NPs | Nanocomposite |
| NC | Nanoclay |
| OCP | Open circuit potential |
| OMMT | Organo-modified montmorillonite |
| OND | Oxidized nanodiamond |
| PEDOT | poly (3,4-ethylenedioxythiophene) |
| PANI | Polyaniline |
| PMHS | poly(methylhydrogen)siloxane |
| Pani-LGS | Polyaniline lignosulfonate-doped polyaniline |
| PCAIPs | Percent and coated alumina inhibitor particles |
| PMMA | Poly methyl methacrylate |
| PU | Polyurethane |
| PAniC | Polyaniline clay |
| PACN | Polyaniline clay nanocomposite |
| PEF | Protection efficiency |
| PPy | Polypyrrole |
| PDAP | PPy-deposited alumina particles |
| Ppy–MMT | Polypyrrole-montmorillonite |
| PCC | PPy/chitosan composites |
| PVA | Poly(vinyl) alcohol |
| PDA | poly-dopamine |
| PDMS | Poly(dimethylsiloxane) |
| PTh | polythiophene |
| PS | Polystyrene |
| PI | Polyimide |
| PBI | polybenzimidazole |
| PDP | potentiodynamic polarization |
| PEDOT | poly(3,4-ethylene dioxythiophene) |
| QM | Quantum mechanical |
| RM | rheometric mechanical spectroscopy |
| rGO | reduced graphene oxide |
| SST | Salt spray test |
| SAXS | Small angle X-ray scattering |
| SNF | Silicon nanofilaments |
| SD | Sodium dodecyl sulfate |
| SPANi | Sulfonated polyaniline |
| SEM | Scanning electron microscope |
| TGA | Thermogravimetric analysis |
| TA | Tannic acid |
| TNZ | Ti-Nb-Zr |
| TEM | Transmission electron microscopy |
| TNT | TiO2 nanotubes |
| TG/DTA | Thermogravimetry differential thermal analysis |
| UV–Vis | UV–vis absorption spectra |
| UF | Urea formaldehyde |
| VOCs | Volatile organic compounds |
| WAV | Water-based alkyd varnish |
| WTM | Wet transfer method |
| WVT | Water vapor transmission |
| WPU | Waterborne polyurethanes |
| XPS | X-ray photoelectron spectroscopy |
| XRD | X-ray diffraction |
| ZRP | Zinc-rich epoxy primer’s |
| ZRP | Zinc-rich paints |
| ZAPP | Zinc aluminum polyphosphate |
| ZIF | Zeolitic imidazole framework |
References
- Shreir, L.L. Basic Concepts of Corrosion; Elsevier B.V.: Amsterdam, The Netherlands, 2010; pp. 1:3–1:15. [Google Scholar]
- Gonçalves, M.C.; Margarido, F. Materials for Construction and Civil Engineering; Springer: Cham, Switzerland, 2015. [Google Scholar]
- Revie, R.W. Uhlig’s Corrosion Handbook; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Jadhav, N.; Vetter, C.A.; Gelling, V.J. The effect of polymer morphology on the performance of a corrosion inhibiting polypyrrole/aluminum flake composite pigment. Electrochim. Acta 2013, 28, 28–43. [Google Scholar] [CrossRef]
- Quraishi, M.A.; Chauhan, D.S.; Saji, V.S. Heterocyclic Organic Corrosion; Elsevier Inc.: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Sastri, V.S. Corrosion Inhibitors: Principles and Applications; John Wiley & Sons: Hoboken, NJ, USA, 1998. [Google Scholar]
- Sastri, V.S. Green Corrosion Inhibitors: Theory and Practice; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Shreir, L.L. Basic Concepts of Corrosion. Shreir’s Corrosion; Elsevier: Oxford, UK, 2010; pp. 89–100. [Google Scholar]
- Mazumder, M.A.J.; Sheardown, H.; Al-Ahmed, A. Functional Polymers; Springer International Publishing: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Harsimran, S.; Santosh, K.; Rakesh, K. Overview of corrosion and its control: A critical review. Proc. Eng. Sci. 2021, 3, 13–24. [Google Scholar] [CrossRef]
- Berradja, A. Electrochemical techniques for corrosion and tribocorrosion monitoring: Fundamentals of electrolytic corrosion. In Corrosion Inhibitors; IntechOpen: London, UK, 2019. [Google Scholar]
- Sorensen, P.A.; Kill, S.; Dam-Johansen, S.; Weinell, C.F. Anticorrosive coatings: A review. J. Coat. Technol. Res. 2009, 6, 135–176. [Google Scholar] [CrossRef]
- Shchukin, D.G.; Zheludkevich, M.; Yasakau, K.; Lamaka, S.; Ferreira, M.G.H.; Möhwald, H. Layer-by-Layer Assembled Nanocontainers for Self-Healing Corrosion Protection. Adv. Mater. 2006, 18, 1672–1678. [Google Scholar] [CrossRef]
- Gomez, H.; Ram, M.K.; Alvi, F.; Stefanakos, E.; Kumar, A. Novel synthesis, characterization, and corrosion inhibition properties of nanodiamond−polyaniline films. J. Phys. Chem. C 2010, 114, 18797–18804. [Google Scholar] [CrossRef]
- Shahid, M. Corrosion protection with eco-friendly inhibitors. Adv. Nat. Sci. Nanosci. Nanotechnol. 2011, 2, 043001. [Google Scholar] [CrossRef]
- Pillay, C.; Lin, J. The Impact of Additional Nitrates in Mild Steel Corrosion in a Seawater/Sediment System. Corros. Sci. 2014, 80, 416–426. [Google Scholar] [CrossRef]
- Al-Sabagh, A.M.; Migahed, M.A.; Sadeek, S.A.; El Basiony, N.M. Inhibition of Mild Steel Corrosion and Calcium Sulfate Formation in Highly Saline Synthetic Water by a Newlyn Synthesized Anionic Carboxylated Surfactant. Egypt. J. Pet. 2018, 27, 811–821. [Google Scholar] [CrossRef]
- Aljibori, H.S.; Alamiery, A.; Kadhum, A.A.H. Advances in corrosion protection coatings: A comprehensive review. J. Corros. Scale Inhib. 2023, 12, 1476–1520. [Google Scholar]
- Le Pen, C.; Lacabanne, C.; Pébère, N. Structure of waterborne coatings by electrochemical impedance spectroscopy and a thermostimulated current method: Influence of fillers. Prog Org Coat. 2000, 39, 167–175. [Google Scholar] [CrossRef]
- Golru, S.S.; Attar, M.M.; Ramezanzadeh, B. Studying the Influence of nano-Al2O3 Particles on the Corrosion Performance and Hydrolytic Degradation Resistance of an Epoxy/polyamide Coating on AA-1050. Prog. Org. Coat. 2014, 77, 1391–1399. [Google Scholar] [CrossRef]
- Blinova, N.V.; Stejskal, J.; Trchová, M.; Prokeš, J.; Omastová, M. Polyaniline and polypyrrole: A comparative study of the preparation. Eur. Polym. J. 2007, 43, 2331–2341. [Google Scholar] [CrossRef]
- Yan, M.; Vetter, C.A.; Gelling, V.J. Corrosion inhibition performance of polypyrrole Al flake composite coatings for Al alloys. Corros. Sci. 2013, 70, 37–45. [Google Scholar] [CrossRef]
- Annibaldi, V.; Rooney, A.D.; Breslin, C.B. Corrosion protection of copper using polypyrrole electrosynthesised from a salicylate solution. Corros. Sci. 2012, 59, 179–185. [Google Scholar] [CrossRef]
- Guimard, N.K.; Gomez, N.; Schmidt, C.E. Conducting polymers in biomedical engineering. Prog. Polym. Sci. 2007, 32, 876–921. [Google Scholar] [CrossRef]
- Palraj, S.; Selvaraj, M.; Vidhya, M.; Rajagopal, G. Synthesis and characterization of epoxy–silicone–polythiophene interpenetrating polymer network for corrosion protection of steel. Prog. Org. Coat. 2012, 75, 356–363. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, J.; Zheng, Y.; Huang, Z.; Feng, L.; Jiang, L. Stable, superhydrophobic, and conductive polyaniline/polystyrene films for corrosive environments. Adv. Funct. Mater. 2006, 16, 568–574. [Google Scholar] [CrossRef]
- Lee, K.; Cho, S.; Park, S.H.; Heeger, A.J.; Lee, C.W.; Lee, S. Metallic transport in polyaniline. Nature 2006, 441, 65–68. [Google Scholar] [CrossRef]
- Guo, X.; Small, J.P.; Klare, J.E.; Wang, Y.; Purewal, M.S.; Tam, I.W.; Hong, B.H.; Caldwell, R.; Huang, L.; O’Brien, S.; et al. Covalently bridging gaps in single-walled carbon nanotubes with conducting molecules. Science 2006, 311, 356–359. [Google Scholar] [CrossRef]
- Wu, G.; More, K.L.; Johnston, C.M.; Zelenay, P. High-performance electrocatalysts for oxygen reduction derived from polyaniline, iron, and cobalt. Science 2011, 332, 443–447. [Google Scholar] [CrossRef]
- Deshpande, P.P.; Jadhav, N.G.; Gelling, V.J.; Sazou, D. Conducting polymers for corrosion protection: A review. J. Coat. Technol. Res. 2014, 11, 473–494. [Google Scholar] [CrossRef]
- Ates, A. A review on conducting polymer coatings for corrosion protection. J. Adhes. Sci. Technol. 2016, 30, 1510–1536. [Google Scholar] [CrossRef]
- Rashid, A.B.; Haque, M.; Islam, S.M.M.; Labib, K.M.R.U. Nanotechnology-enhanced fiber-reinforced polymer composites: Recent advancements on processing techniques and applications. Heliyon 2024, 30, e24692. [Google Scholar] [CrossRef]
- Krishnaswamy, S.; Marchante, V.; Abhyankar, H.; Huang, Z.; Brighton, J. Non-isothermal Cure Kinetics of Aerogel/epoxy Composites Using Differential Scanning Calorimetry. Polym. Plast. Technol. Eng. 2019, 58, 1757–1765. [Google Scholar] [CrossRef]
- Mustapha, R.; Rahmat, A.R.; Abdul Majid, R.; Mustapha, S.N.H. Vegetable Oil-based Epoxy Resins and Their Composites with Bio-based Hardener: A Short Review. Polym. Plast. Technol. Eng. 2019, 58, 1311–1326. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhao, J.; Li, S.; Tan, Z.; Zhang, H. Contribution of Ungrafted Segments in Core-Shell Impact Modifier in the Toughening of PBT Resins by Epoxy Functionalized Poly (butadiene-graft-styrene). Polym. Plast. Technol. Eng. 2018, 57, 1697–1705. [Google Scholar] [CrossRef]
- Singh, K.; Panda, B.P.; Mohanty, S.; Nayak, S.K.; Gupta, M.K. Recent Developments on Epoxy-Based Thermally Conductive Adhesives (TCA): A Review. Polym. Plast. Technol. Eng. 2018, 57, 903–934. [Google Scholar] [CrossRef]
- Weiss, K.D. Paint and coatings: A mature industry in transition. Prog. Polym. Sci. 1997, 22, 203–245. [Google Scholar] [CrossRef]
- Jin, F.L.; Li, X.; Park, S.J. Synthesis and application of epoxy resins: A review. J. Ind. Eng. Chem. 2015, 29, 1–11. [Google Scholar] [CrossRef]
- Hosseini, M.G.; Jafari, M.; Najjar, R. Effect of Polyaniline–Montmorillonite Nanocomposite Powders Addition on Corrosion Performance of Epoxy Coatings on Al 5000. Surf. Coat. Technol. 2011, 206, 280–286. [Google Scholar] [CrossRef]
- Gupta, G.; Birbilis, N.; Cook, A.B.; Khanna, A.S. Polyaniline-lignosulfonate/epoxy Coating for Corrosion Protection of AA2024-T3. Corros. Sci. 2013, 67, 256–267. [Google Scholar] [CrossRef]
- Mostafaei, A.; Nasirpour, F. Epoxy/polyaniline–ZnO nanorods hybrid nanocomposite coatings: Synthesis, characterization and corrosion protection performance of conducting paints. Prog. Org. Coat. 2014, 77, 146–159. [Google Scholar] [CrossRef]
- Navarchian, A.H.; Joulazadeh, M.; Karimi, F. Investigation of corrosion protection performance of epoxy coatings modified by polyaniline/clay nanocomposites on steel surfaces. Prog. Org. Coat. 2014, 77, 347–353. [Google Scholar] [CrossRef]
- Arefinia, R.; Shariatpanahi, H.; Neshati, J. Anticorrosion properties of smart coating based on polyaniline nanoparticles/epoxy-ester system. Prog. Org. Coat. 2012, 75, 502–508. [Google Scholar] [CrossRef]
- Akbarinezhad, E.; Ebrahimi, M.; Sharif, M.F.; Ghanbarzadeh, A. Evaluating protection performance of zinc-rich epoxy paints modified with polyaniline and polyaniline-clay nanocomposite. Prog. Org. Coat. 2014, 77, 1299–1308. [Google Scholar] [CrossRef]
- Baniasadi, H.; Ramazani, S.A.A.; Mashayekhan, S.; Ghaderinezhad, F. Preparation of conductive polyaniline/graphene nanocomposites via in situ emulsion polymerization and product characterization. Synth. Met. 2014, 196, 199–205. [Google Scholar] [CrossRef]
- Jafari, Y.; Ghoreishi, S.M.; Shabani-Nooshabadi, M. Polyaniline/Graphene nanocomposite coatings on copper: Electropolymerization, characterization, and evaluation of corrosion protection performance. Synth. Met. 2016, 217, 220. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, M.; Qu, H.; Zhang, X.; Wei, H.; Luo, Z.; Colorado, H.A.; Wei, S.; Guo, Z. Interfacial polymerized polyaniline/graphite oxide nanocomposites toward electrochemical energy storage. Polymer 2012, 53, 5953–5964. [Google Scholar] [CrossRef]
- Pecher, J.; Mecking, S. Nanoparticles of Conjugated Polymers. Chem. Rev. 2010, 110, 6260–6279. [Google Scholar] [CrossRef]
- Flamini, D.O.; Saidman, S.B. Electrodeposition of Polypyrrole onto NiTi and the Corrosion Behaviour of the Coated Alloy. Corros. Sci. 2010, 52, 229–234. [Google Scholar] [CrossRef]
- Qi, K.; Qiu, Y.; Chen, Z.; Guo, X. Corrosion of Conductive Polypyrrole: Effects of Continuous Cathodic and Anodic Polarisation. Corros. Sci. 2013, 69, 376–388. [Google Scholar] [CrossRef]
- Gergely, A.; Pfeifer, E.; Bertóti, I.; Török, T.I.; Kálmán, E. Corrosion Protection of Cold-rolled Steel by Zinc-rich Epoxy Paint Coatings Loaded with Nano-size Alumina Supported Polypyrrole. Corros. Sci. 2011, 53, 3486–3499. [Google Scholar] [CrossRef]
- Gergely, A.; Bertóti, I.; Török, T.I.; Pfeifer, E.; Kálmán, E. Corrosion Protection with Zinc-rich Epoxy Paint Coatings Embedded with Various Amounts of Highly Dispersed Polypyrrole-deposited Alumina Monohydrate Particles. Prog. Org. Coat. 2013, 76, 17–32. [Google Scholar] [CrossRef]
- Mollahosseini, E.; Noroozian, A. Electrodeposition of a Highly Adherent and Thermally Stable Polypyrrole Coating on Steel from Aqueous Polyphosphate Solution. Synth. Met. 2009, 159, 1247–1254. [Google Scholar] [CrossRef]
- Merisalu, M.; Kahro, T.; Kozlova, J.; Niilisk, A.; Nikolajev, A.; Marandi, M.; Floren, A.; Alles, H.; Sammelselg, V. Graphene-polypyrrole Thin Hybrid Corrosion Resistant Coatings for Copper. Synth. Met. 2015, 200, 16–23. [Google Scholar] [CrossRef]
- Hosseini, M.G.; Raghibi-Boroujeni, M.; Ahadzadeh, I.; Najjar, R.; Dorraji, M.S. Effect of Polypyrrole–Montmorillonite Nanocomposites Powder Addition on Corrosion Performance of Epoxy Coatings on Al 5000. Prog. Org. Coat. 2009, 66, 321–327. [Google Scholar] [CrossRef]
- Ramôa, S.D.; Barra, G.M.; Merlini, C.; Schreiner, W.H.; Livi, S.; Soares, B.G. Production of Montmorillonite/polypyrrole Nanocomposites through in Situ Oxidative Polymerization of Pyrrole: Effect of Anionic and Cationic Surfactants on Structure and Properties. Appl. Clay Sci. 2015, 104, 160–167. [Google Scholar] [CrossRef]
- Kumar, M.; Sureshb, B.; Das, S.; Obot, S.I.B.; Adesina, S. Ramakrishna. Promising bio-composites of polypyrrole and chitosan: Surface protective and in vitro biocompatibility performance on 316L SS implants. Carbohydr. Polym. 2017, 173, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, M.; Fotouhi, L.; Ehsani, A.; Naseri, M. Enhancement of corrosion resistance of polypyrrole using metal oxide nanoparticles: Potentiodynamic and electrochemical impedance spectroscopy study. J. Colloid Interface Sci. 2017, 505, 213–219. [Google Scholar] [CrossRef]
- Potvin, E.; Brossard, L.; Larochelle, G. Corrosion Protective Performances of Commercial low-VOC Epoxy/urethane Coatings on Hot-rolled 1010 Mild Steel. Prog. Org. Coat. 1997, 31, 363–373. [Google Scholar] [CrossRef]
- Samimi, A.; Zarinabadi, S. Application Polyurethane as Coating in Oil and Gas Pipelines. Int. J. Sci. Investig. 2012, 1, 43–45. [Google Scholar]
- Agavriloaie, L.; Oprea, S.; Barbuta, M.; Luca, F. Characterisation of Polymer Concrete with Epoxy Polyurethane Acryl Matrix. Constr. Build. Mater. 2012, 37, 190–196. [Google Scholar] [CrossRef]
- Moradi, M.; Yeganeh, H.; Pazokifard, S. Synthesis and assessment of novel anticorrosive PU coatings containing an amine-functionalized nanoclay additive prepared by the cathodic electrophoretic deposition method. RSC. Adv. 2016, 6, 28089–28102. [Google Scholar] [CrossRef]
- Sharmin, E.; Ashraf, S.M.; Ahmad, S. Synthesis, Characterization, Antibacterial and Corrosion Protective Properties of Epoxies, Epoxy-polyols and Epoxy-polyurethane Coatings from Linseed and Pongamia Glabra Seed Oils. Int. J. Bio. Macromol. 2007, 40, 407–422. [Google Scholar] [CrossRef]
- Mohammadi, A.; Barikani, M.; Doctorsafaei, A.H.; Isfahani, A.P.; Shams, E.; Ghalei, B. Aqueous dispersion of polyurethane nanocomposites based on calix[4]arenes modified graphene oxide nanosheets: Preparation, characterization, and anti-corrosion properties. J. Chem. Eng. 2018, 349, 466–480. [Google Scholar] [CrossRef]
- Diniz, F.B.; De Andrade, G.F.; Martins, C.R.; De Azevedo, W.M. A Comparative Study of Epoxy and Polyurethane Based Coatings Containing polyaniline-DBSA Pigments for Corrosion Protection on Mild Steel. Prog. Org. Coat. 2013, 76, 912–916. [Google Scholar] [CrossRef]
- Salunkhe, A.B.; Khot, V.M.; Thorat, N.D.; Phadatare, M.R.; Sathish, C.I.; Dhawale, D.S.; Pawar, S.H. Polyvinyl Alcohol Functionalized Cobalt Ferrite Nanoparticles for Biomedical Applications. Appl. Surf. Sci. 2013, 264, 598–604. [Google Scholar] [CrossRef]
- Elessawy, N.A.; Gouda, M.H.; Elnouby, M.; Taha, N.A.; Youssef, M.E.; Santos, D.M. Polyvinyl alcohol/polyaniline/carboxylated graphene oxide nanocomposites for coating protection of cast iron in simulated seawater. Polymers 2022, 14, 1791. [Google Scholar] [CrossRef]
- Hikku, G.S.; Jeyasubramanian, K.; Venugopal, A.; Ghosh, R. Corrosion resistance behaviour of graphene/polyvinyl alcohol nanocomposite coating for aluminium-2219 alloy. J. Alloys Compd. 2017, 716, 259–269. [Google Scholar] [CrossRef]
- Paul, A.; Shamsheera, K.O.; Prasad, A.R.; Joseph, A. Electroanalytical and surface studies on the protective action of a coating of PVA@3WGO on mild steel in acidic and saline environments. Results Surf. Interfaces 2021, 4, 100018. [Google Scholar] [CrossRef]
- Ding, J.H.; Rahman, O.U.; Peng, W.; Dou, H.M.; Yu, H.B. A novel hydroxyl epoxy phosphate monomer enhancing the anticorrosive performance of waterborne Graphene/Epoxy coatings. Appl. Surf. Sci. 2018, 427, 981–991. [Google Scholar] [CrossRef]
- Ramezanzadeh, B.; Vakili, H.; Amini, R. The effects of addition of Poly (vinyl) alcohol (PVA) as a green corrosion inhibitor to the phosphate conversion coating on the anticorrosion and adhesion properties of the epoxy coating on the steel substrate. Appl. Surf. Sci. 2015, 327, 174–181. [Google Scholar] [CrossRef]
- Song, W.; Zhao, X.; Jin, Z.; Fan, L.; Ji, X.; Deng, J.; Duan, J. Poly(vinyl alcohol) for multi-functionalized corrosion protection of metals: A review. J. Clean. Prod. 2023, 394, 136390. [Google Scholar] [CrossRef]
- Naidu, K.S.; Palaniappan, S. Formation of PANI-PVA salt via H-bonding between PVA and PANI: Aqueous coating for electrostatic discharge, sensor and corrosion applications. Sens. Int. 2020, 1, 100006. [Google Scholar] [CrossRef]
- Sarkar, N.; Sahoo, G.; Das, R.; Prusty, G.; Sahu, D.; Swain, S.K. Anticorrosion performance of three-dimensional hierarchical PANI@BN nanohybrids. Ind. Eng. Chem. Res. 2016, 55, 2921–2931. [Google Scholar] [CrossRef]
- Xu, C.; Shi, L.; Guo, L.; Wang, X.; Wang, X.; Lian, H. Fabrication and characteristics of graphene oxide/nanocellulose fiber/poly(vinyl alcohol) film. J. Appl. Polym. Sci. 2017, 134, 45345. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, Y.; Wu, H.; Geng, S.; Wang, F. Corrosion protection properties of an environmentally friendly polyvinyl alcohol coating reinforced by a heating treatment and lignin nanocellulose. Prog. Org. Coat. 2021, 155, 106224. [Google Scholar] [CrossRef]
- Miao, X.; Meng, Y.; Li, X. A Novel All-purpose Epoxy-terminated Hyperbranched Polyether Sulphone Toughener for an Epoxy/amine System. Polymer 2015, 60, 88–95. [Google Scholar] [CrossRef]
- Mirabedini, S.M.; Moradian, S.; Scantlebury, J.D.; Thompson, G.E. Characterization and Corrosion Performance of Powder Coated Aluminium Alloy. Iran. Polym. J. 2003, 12, 261–269. [Google Scholar]
- Ligon-Auer, S.C.; Schwentenwein, M.; Gorsche, C.; Stampfl, J.; Liska, R. Toughening of Photo-curable Polymer Networks: A Review. Polym. Chem. 2016, 7, 257–286. [Google Scholar] [CrossRef]
- Sari, M.G.; Shamshiri, M.; Ramezanzadeh, B. Fabricating an Epoxy Composite Coating with Enhanced Corrosion Resistance through Impregnation of Functionalized Graphene Oxide-co-montmorillonite Nanoplatelet. Corros. Sci. 2017, 129, 38–53. [Google Scholar] [CrossRef]
- Sari, M.G.; Ramezanzadeh, B.; Shahbazi, M.; Pakdel, A.S. Influence of Nanoclay Particles Modification by Polyester-amide Hyperbranched Polymer on the Corrosion Protective Performance of the Epoxy Nanocomposite. Corros. Sci. 2015, 92, 162–172. [Google Scholar] [CrossRef]
- Nikravesh, B.; Ramezanzadeh, B.; Sarabi, A.A.; Kasiriha, S.M. Evaluation of the Corrosion Resistance of an Epoxy-polyamide Coating Containing Different Ratios of Micaceous Iron oxide/Al Pigments. Corros. Sci. 2011, 53, 1592–1603. [Google Scholar] [CrossRef]
- Ramezanzadeh, B.; Attar, M.M.; Farzam, M. Study on the Anticorrosion Performance of the Epoxy–Polyamide Nanocomposites Containing ZnO Nanoparticles. Prog. Org. Coat. 2011, 72, 410–422. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, J.; Liu, X.; Wang, Z.; Wang, S. High Performance Self-healing Epoxy/polyamide Protective Coating Containing Epoxy Microcapsules and Polyaniline Nanofibers for Mild Carbon Steel. Indus. Engineer. Chem. Res. 2013, 52, 10172–10180. [Google Scholar] [CrossRef]
- Mirzakhanzadeh, Z.; Kosari, A.; Moayed, M.H.; Naderi, R.; Taheri, P.; Mol, J.M.C. Enhanced Corrosion Protection of Mild Steel by the Synergetic Effect of Zinc Aluminum Polyphosphate and 2-mercaptobenzimidazole Inhibitors Incorporated in Epoxy-polyamide Coatings. Corros. Sci. 2018, 138, 372–379. [Google Scholar] [CrossRef]
- Zhou, T.; Wang, X.; Cheng, P.; Wang, T.; Xiong, D.; Wang, X. Improving the Thermal Conductivity of Epoxy Resin by the Addition of a Mixture of Graphite Nanoplatelets and Silicon Carbide Microparticles. Exp. Polym. Lett. 2013, 7, 585–594. [Google Scholar] [CrossRef]
- Ammar, S.; Ramesh, K.; Vengadaesvaran, B.; Ramesh, S.; Arof, A.K. Amelioration of Anticorrosion and Hydrophobic Properties of epoxy/ PDMS Composite Coatings Containing Nano ZnO Particles. Prog. Org. Coat. 2016, 92, 54–65. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, M.; Zhang, J.; Shao, Q.; Li, J.; Li, H.; Lin, B.; Yu, M.; Chen, S.; Guo, Z. Excellent Corrosion Protection Performance of Epoxy Composite Coatings Filled with Silane Functionalized Silicon Nitride. J. Polym. Res. 2018, 25, 130. [Google Scholar] [CrossRef]
- Zhang, D.; Qian, H.; Wang, L.; Li, X. Comparison of Barrier Properties for a Superhydrophobic Epoxy Coating under Different Simulated Corrosion Environments. Corros. Sci. 2016, 103, 230–241. [Google Scholar] [CrossRef]
- Zhang, M.; Jing, X.; Zhao, S.; Shao, P.; Zhang, Y.; Yuan, S.; Li, Y.; Gu, C.; Wang, X.; Ye, Y.; et al. Electropolymerization of Molecular-sieving Polythiophene Membranes for H2 Separation. Angew. Chem. 2019, 58, 8768–8772. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, D.P.; Awasthi, G.P.; Maharjan, B.; Lee, J.; Kim, B.S.; Park, C.H.; Kim, C.S. Synthesis of Polythiophene Nanoparticles by Surfactant-free Chemical Oxidative Polymerization Method: Characterization, in Vitro Biomineralization, and Cytotoxicity Evaluation. J. Indus. Eng. Chem. 2019, 77, 243–252. [Google Scholar] [CrossRef]
- Stankiewicz, A.; Szczygieł, I.; Szczygieł, B. Self-healing coatings in anti-corrosion applications. J. Mater. Sci. 2013, 48, 8041–8051. [Google Scholar] [CrossRef]
- Kausar, A. Corrosion prevention prospects of polymeric nanocomposites: A review. J. Plast. Film. Sheeting 2019, 35, 181–202. [Google Scholar] [CrossRef]
- Pour-Ali, S.; Dehghanian, C.; Kosari, A. In situ synthesis of polyaniline–camphorsulfonate particles in an epoxy matrix for corrosion protection of mild steel in NaCl solution. Corros. Sci. 2014, 85, 204–214. [Google Scholar] [CrossRef]
- Perrin, F.X.; Phan, T.A.; Nguyen, D.L. Synthesis and characterization of polyaniline nanoparticles in phosphonic acid amphiphile aqueous micellar solutions for waterborne corrosion protection coatings. J. Polym. Sci. Part A Polym. Chem. 2015, 53, 1606–1616. [Google Scholar] [CrossRef]
- Thomas, K.A.; Nair, S.; Rajeswari, R.; Natarajan, V.; Mukundan, T.; John, R. Electrochemical behaviour of PANi/polyurethane antifouling coating in salt water studied by electrochemical impedance spectroscopy. Prog. Org. Coat. 2015, 89, 267–270. [Google Scholar] [CrossRef]
- Xing, C.; Zhang, Z.; Yu, L.; Waterhouse, G.I.; Zhang, L. Anti-corrosion performance of nanostructured poly (aniline-co-metanilic acid) on carbon steel. Prog. Org. Coat. 2014, 77, 354–360. [Google Scholar] [CrossRef]
- Tian, Z.; Yu, H.; Wang, L.; Saleem, M.; Ren, F.; Ren, P.; Chen, Y.; Sun, R.; Sun, Y.; Huang, L. Recent progress in the preparation of polyaniline nanostructures and their applications in anticorrosive coatings. RSC Adv. 2014, 4, 28195–28208. [Google Scholar] [CrossRef]
- Motlatle, A.M.; Ray, S.S.; Scriba, M. Polyaniline-clay composite-containing epoxy coating with enhanced corrosion protection and mechanical properties. Synth. Met. 2018, 245, 102–110. [Google Scholar] [CrossRef]
- Qiu, S.; Chen, C.; Cui, M.; Li, W.; Zhao, H.; Wang, L. Corrosion protection performance of waterborne epoxy coatings containing self-doped polyaniline nanofiber. Appl. Surf. Sci. 2017, 407, 213–222. [Google Scholar] [CrossRef]
- Zhao, Y.; Xing, C.; Zhang, Z.; Yu, L. Superhydrophobic polyaniline/polystyrene micro/nanostructures as anticorrosion coatings. React. Funct. Polym. 2017, 119, 95–104. [Google Scholar] [CrossRef]
- Makhlouf, A.S.H. Handbook of Smart Coatings for Materials Protection; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Hu, R.G.; Zhang, S.; Bu, J.F.; Lin, C.J.; Song, G.L. Recent progress in corrosion protection of magnesium alloys by organic coatings. Prog. Org. Coat. 2012, 73, 129–141. [Google Scholar] [CrossRef]
- Kim, B.H.; Jung, J.H.; Hong, S.H.; Joo, J.; Epstein, A.J.; Mizoguchi, K.; Kim, J.W.; Choi, H.J. Nanocomposite of polyaniline and Na+− montmorillonite clay. Macromolecules 2002, 35, 1419–1423. [Google Scholar] [CrossRef]
- Kausar, A. Polyimide, polybenzimidazole-in situ-polyaniline nanoparticle and carbon nano-onion-based nanocomposite designed for corrosion protection. Int. J. Polym. Anal. Charact. 2017, 22, 557–567. [Google Scholar] [CrossRef]
- Khan, M.I.; Amari, A.; Mustafa, A.; Shoukry, H.; Ali, I.H.; Umoren, S.A.; Kumar, A.M. Synthesis, characterization and application of ferrochrome slag/polyaniline nanocomposite as corrosion protection coatings for carbon steel. Int. J. Electrochem. Sci. 2018, 13, 7385–7396. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, S.; Wang, P.; Chen, S.; Lu, Z.; Li, W. Electropolymerization and corrosion protection performance of the Nb: TiO2 nanofibers/polyaniline composite coating. J. Taiwan Inst. Chem. Eng. 2019, 103, 190–198. [Google Scholar] [CrossRef]
- Lei, Y.; Qiu, Z.; Tan, N.; Du, H.; Li, D.; Liu, J.; Liu, T.; Zhang, W.; Chang, X. Polyaniline/CeO2 nanocomposites as corrosion inhibitors for improving the corrosive performance of epoxy coating on carbon steel in 3.5% NaCl solution. Prog. Org. Coat. 2020, 139, 105430. [Google Scholar] [CrossRef]
- Abd El-Lateef, H.M.; Khalaf, M.M. Novel dispersed Tl2O3-SiO2/polyaniline nanocomposites: In-situ polymerization, characterization and enforcement as a corrosion protective layer for carbon-steel in acidic chloride medium. Colloids Surf. A Physicochem. Eng. Asp. 2019, 573, 95–111. [Google Scholar] [CrossRef]
- Yang, N.; Yang, T.; Wang, W.; Chen, H.; Li, W. Polydopamine modified polyaniline-graphene oxide composite for enhancement of corrosion resistance. J. Hazard. Mater. 2019, 377, 142–151. [Google Scholar] [CrossRef]
- Haddadi, S.A.; Mehmandar, E.; Jabari, H.; SA, A.R.; Mohammadkhani, R.; Yan, N.; Arjmand, M. Zinc-doped silica/polyaniline core/shell nanoparticles towards corrosion protection epoxy nanocomposite coatings. Compos. Part B Eng. 2021, 212, 108713. [Google Scholar] [CrossRef]
- Sutter, E.; Albrecht, P.; Camino, F.E.; Sutter, P. Monolayer graphene as ultimate chemical passivation layer for arbitrarily shaped metal surfaces. Carbon 2010, 48, 4414–4420. [Google Scholar] [CrossRef]
- Wang, P.; Yao, T.; Sun, B.; Fan, X.; Dong, S.; Bai, Y.; Shi, Y. A cost-effective method for preparing mechanically stable anti-corrosive superhydrophobic coating based on electrochemically exfoliated graphene. Colloids Surf. A Physicochem. Eng. Asp. 2017, 513, 396–401. [Google Scholar] [CrossRef]
- Alam, R.; Mobin, M.; Aslam, J. Polypyrrole/graphene nanosheets/rare earth ions/dodecyl benzene sulfonic acid nanocomposite as a highly effective anticorrosive coating. Surf. Coat. Technol. 2016, 307, 382–391. [Google Scholar] [CrossRef]
- Maeztu, J.D.; Rivero, P.J.; Berlanga, C.; Bastidas, D.M.; Palacio, J.F.; Rodriguez, R. Effect of graphene oxide and fluorinated polymeric chains incorporated in a multilayered sol-gel nanocoating for the design of corrosion resistant and hydrophobic surfaces. Appl. Surf. Sci. 2017, 419, 138–149. [Google Scholar] [CrossRef]
- Hayatgheib, Y.; Ramezanzadeh, B.; Kardar, P.; Mahdavian, M. A comparative study on fabrication of a highly effective corrosion protective system based on graphene oxide-polyaniline nanofibers/epoxy composite. Corros. Sci. 2018, 133, 358–373. [Google Scholar] [CrossRef]
- Olajire, A.A. Corrosion inhibition of offshore oil and gas production facilities using organic compound inhibitors-A review. J. Mol. Liq. 2017, 248, 775–808. [Google Scholar] [CrossRef]
- Sharifi, Z.; Pakshir, M.; Amini, A.; Rafiei, R. Hybrid graphene oxide decoration and water-based polymers for mild steel surface protection in saline environment. J. Ind. Eng. Chem. 2019, 74, 41–54. [Google Scholar] [CrossRef]
- Sari, M.G.; Abdolmaleki, M.; Rostami, M.; Ramezanzadeh, B. Nanoclay dispersion and colloidal stability improvement in phenol novolac epoxy composite via graphene oxide for the achievement of superior corrosion protection performance. Corros. Sci. 2020, 173, 108799. [Google Scholar] [CrossRef]
- Haddadi, S.A.; Najmi, P.; Keshmiri, N.; Tanguy, N.; van der Kuur, C.; Yan, N.; Mekonnen, T.; Arjmand, M. Cerium-doped tannic acid-reduced graphene oxide nanoplatform/epoxy nanocomposite coatings with enhanced mechanical and Bi-functional corrosion protection properties. Compos. B Eng. 2022, 239, 109969. [Google Scholar] [CrossRef]
- Bahlakeh, G.; Ramezanzadeh, B.; Ramezanzadeh, M. Corrosion protective and adhesion properties of a melamine-cured polyester coating applied on steel substrate treated by a nanostructure cerium–lanthanum film. J. Taiwan Inst. Chem. Eng. 2017, 81, 419–434. [Google Scholar] [CrossRef]
- Hu, J.; Gan, M.; Ma, L.; Zhang, J.; Xie, S.; Xu, F.; Shen, J.Z.X.; Yin, H. Preparation and enhanced properties of polyaniline/grafted intercalated ZnAl-LDH nanocomposites. Appl. Surf. Sci. 2015, 328, 325–334. [Google Scholar] [CrossRef]
- Gao, Z.; Wang, J.; Li, Z.; Yang, W.; Wang, B.; Hou, M.; He, Y.; Liu, Q.; Mann, T.; Yang, P.; et al. Graphene nanosheet/Ni2+/Al3+ layered double-hydroxide composite as a novel electrode for a supercapacitor. Chem. Mat. 2011, 23, 3509–3516. [Google Scholar] [CrossRef]
- Luo, X.; Yuan, S.; Pan, X.; Zhang, C.; Du, S.; Liu, Y. Synthesis and enhanced corrosion protection performance of reduced graphene oxide nanosheet/ZnAl layered double hydroxide composite films by hydrothermal continuous flow method. ACS Appl. Mater. Interfaces 2017, 9, 18263–18275. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Wen, S.; Yang, J.; Wang, J.; Chen, Y.; Luo, J.; Wu, Y. RGO modified ZnAl-LDH as epoxy nanostructure filler: A novel synthetic approach to anticorrosive waterborne coating. Surf. Coat. Technol. 2017, 326, 207–215. [Google Scholar] [CrossRef]
- Yang, S.; Wang, J.; Mao, W.; Zhang, D.; Guo, Y.; Song, Y.; Wang, J.P.; Qi, T.; Li, G.L. pH-Responsive zeolitic imidazole framework nanoparticles with high active inhibitor content for self-healing anticorrosion coatings. Colloids Surf. A Physicochem. Eng. 2018, 555, 18–26. [Google Scholar] [CrossRef]
- Ong, J.L.; Chan, D.C. Hydroxyapatite and their use as coatings in dental implants: A review. Crit. Rev. Biomed. Eng. 2000, 28, 667–707. [Google Scholar] [CrossRef] [PubMed]
- Jugowiec, D.; Łukaszczyk, A.; Cieniek, Ł.; Kowalski, K.; Rumian, Ł.; Pietryga, K.; Kot, M.; Pamuła, E.; Moskalewicz, T. Influence of the electrophoretic deposition route on the microstructure and properties of nano-hydroxyapatite/chitosan coatings on the Ti-13Nb-13Zr alloy. Surf. Coat. Technol. 2017, 324, 64–79. [Google Scholar] [CrossRef]
- Luo, S.C.; Mohamed Ali, E.; Tansil, N.C.; Yu, H.H.; Gao, S.; Kantchev, E.A.; Ying, J.Y. Poly (3, 4-ethylenedioxythiophene)(PEDOT) nanobiointerfaces: Thin, ultrasmooth, and functionalized PEDOT films with in vitro and in vivo biocompatibility. Langmuir 2008, 24, 8071–8077. [Google Scholar] [CrossRef]
- Kumar, A.M.; Adesina, A.Y.; Hussein, M.A.; Ramakrishna, S.; Al-Aqeeli, N.; Akhtar, S.; Saravanan, S. PEDOT/FHA nanocomposite coatings on newly developed Ti-Nb-Zr implants: Biocompatibility and surface protection against corrosion and bacterial infections. Mater. Sci. Eng. C 2019, 98, 482–495. [Google Scholar] [CrossRef]
- Jana, D.; Sun, C.L.; Chen, L.C.; Chen, K.H. Effect of chemical doping of boron and nitrogen on the electronic, optical, and electrochemical properties of carbon nanotubes. Prog. Mater. Sci. 2013, 58, 565–635. [Google Scholar] [CrossRef]
- Rawat, N.K.; Ahmad, S.; Panda, P.K. Influence of boron incorporation on poly (phenyldiammine) nanostructures: Novel, well-defined and highly conducting nanospheres dispersed smart corrosion protective epoxy coatings. Compos. Commun. 2018, 9, 81–85. [Google Scholar] [CrossRef]
- Pourhashem, S.; Duan, J.; Guan, F.; Wang, N.; Gao, Y. New effects of TiO2 nanotube/g-C3N4 hybrids on the corrosion protection performance of epoxy coatings. J. Mol. Liq. 2020, 317, 114214. [Google Scholar] [CrossRef]
- Xavier, J.R. Novel multilayer epoxy nanocomposite coatings for superior hydrophobic, mechanical and corrosion protection properties of steel. Diam. Relat. Mater. 2022, 123, 108882. [Google Scholar] [CrossRef]
- Rahmani, P.; Shojaei, A.; Tavandashti, N.P. Nanodiamond loaded with corrosion inhibitor as efficient nanocarrier to improve anticorrosion behavior of epoxy coating. J. Ind. Eng. Chem. 2020, 83, 153–163. [Google Scholar] [CrossRef]
- Bahremand, F.; Shahrabi, T.; Ramezanzadeh, B. Epoxy coating anti-corrosion properties enhancement via the steel surface treatment by nanostructured samarium oxide-poly-dopamine film. J. Hazard. Mater. 2021, 403, 123722. [Google Scholar] [CrossRef]
- Sun, J.; Ji, J.; Chen, Z.; Liu, S.; Zhao, J. Epoxy resin composites with commercially available graphene: Toward high toughness and rigidity. RSC Adv. 2019, 9, 33147–33154. [Google Scholar] [CrossRef]
- Dev, B.; Nipu, S.A.; Rahman, M.A.; Mahmud, K.R.; Riyad, M.R.; Rahman, M.Z. Multiscale Glass Fiber/Epoxy Nanocomposites Incorporated with Graphene and Zinc Oxide Nanoparticles: Enhanced Mechanical Properties. Macromol. Mater. Eng. 2025, 310, 2400245. [Google Scholar] [CrossRef]
- Abakah, R.R.; Huang, F.; Hu, Q.; Wang, Y.; Liu, J. Comparative study of corrosion properties of different graphene nanoplate/epoxy composite coatings for enhanced surface barrier protection. Coating 2021, 11, 285. [Google Scholar] [CrossRef]
- Rau, S.R.; Vengadaesvaran, B.; Ramesh, K.; Arof, A.K. Studies on the adhesion and corrosion performance of an acrylic-epoxy hybrid coating. J. Adhes. 2012, 88, 282–293. [Google Scholar] [CrossRef]
- Potdar, S.B.; Praveen, B.V.S.; Sonawane, S.H. Sonochemical approach for synthesis of zinc oxide-poly methyl methacrylate hybrid nanoparticles and its application in corrosion inhibition. Ultrason. Sonochem. 2020, 68, 105200. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, F.C.; Pulcinelli, S.H.; Santilli, C.V.; Hammer, P. Protective PMMA-silica coatings for aluminum alloys: Nanostructural control of elevated thermal stability and anticorrosive performance. Prog. Org. Coat. 2021, 152, 106129. [Google Scholar] [CrossRef]
- Lv, X.S.; Qin, Y.; Liang, H.; Zhao, B.; He, Y.; Cui, X. A facile method for constructing a superhydrophobic zinc coating on a steel surface with anti-corrosion and drag-reduction properties. Appl. Surf. Sci. 2021, 562, 150192. [Google Scholar] [CrossRef]
- Li, L.T.; Gao, B.; Tong, Z.; Yang, Y.; Li, Y. Chitosan and graphene oxide nanocomposites as coatings for controlled-release fertilizer. Water Air Soil Pollut. 2019, 230, 146. [Google Scholar] [CrossRef]
- Liu, Y.; Zou, C.; Yan, X.; Xiao, R.; Wang, T.; Li, M. β-Cyclodextrin modified natural chitosan as a green inhibitor for carbon steel in acid solutions. Ind. Eng. Chem. Res. 2015, 54, 5664–5672. [Google Scholar] [CrossRef]
- Ma, I.W.; Sh, A.; Ramesh, K.; Vengadaesvaran, B.; Ramesh, S.; Arof, A.K. Anticorrosion properties of epoxy-nanochitosan nanocomposite coating. Prog. Org. Coat. 2017, 113, 74–81. [Google Scholar]
- Yang, S.; Lei, P.; Shan, Y.; Zhang, D. Preparation and characterization of antibacterial electrospun chitosan/poly (vinyl alcohol)/graphene oxide composite nanofibrous membrane. Appl Surf Sci. 2018, 435, 832–840. [Google Scholar] [CrossRef]
- Pourhashem, S.; Duan, J.; Zhou, Z.; Ji, X.; Sun, J.; Dong, X.; Wang, L.; Guan, F.; Hou, B. Investigating the effects of chitosan solution and chitosan modified TiO2 nanotubes on the corrosion protection performance of epoxy coatings. Mater. Chem. Phys. 2021, 270, 124751. [Google Scholar] [CrossRef]
- Ghosh, T.; Karak, N. Cashew nut shell liquid terminated self-healable polyurethane as an effective anticorrosive coating with biodegradable attribute. Prog. Org. Coat. 2020, 139, 105472. [Google Scholar] [CrossRef]
- Khan, S.; Ghosal, A.; Bhat, S.A.; Zafar, F.; Azam, M.; Alam, M.; Shahid, M.; Haq, Q.M.R.; Nishat, N. Phenolic lipid derived coordination polymer nanocomposites: Synthesis, characterization and surface protective coating applications. Appl. Surf. Sci. Adv. 2022, 11, 100290. [Google Scholar] [CrossRef]
- Arianpouya, N.; Shishesaz, M.; Arianpouya, M.; Nematollahi, M. Evaluation of synergistic effect of nanozinc/nanoclay additives on the corrosion performance of zinc-rich polyurethane nanocomposite coatings using electrochemical properties and salt spray testing. Surf. Coat. Technol. 2013, 216, 199–206. [Google Scholar] [CrossRef]
- Arianpouya, N.; Shishesaz, M.; Ashrafi, A. Analysis of synergistic effect of nanozinc/nanoclay additives on the corrosion performance of zinc-rich polyurethane nanocomposite coatings. Polym. Compos. 2012, 33, 1395–1402. [Google Scholar] [CrossRef]
- Pukha, V.E.; Glukhov, A.A.; Belmesov, A.A.; Kabachkov, E.N.; Khodos, I.I.; Khadem, M.; Kim, D.E.; Karaseov, P.A. Corrosion-resistant nanostructured carbon-based coatings for applications in fuel cells based on bipolar plates. Vacuum 2023, 218, 112643. [Google Scholar] [CrossRef]
- Zhu, J.; Wu, J.; Zhang, Q.; Yang, Y.; Miao, X.; Hao, T.; Shi, D.; Jiang, T.; Li, R.K.; Zhang, Q.; et al. Rational fabrication of high-durability superhydrophobic coatings based on the excellent compatibility between nanoparticles and polymers. Prog. Org. Coat. 2023, 175, 107358. [Google Scholar] [CrossRef]
- Wang, K.; Wei, Z.; Wei, Z.; Ying, G.; Hong, S. Enhanced corrosion resistance of subsonic plasma sprayed nanostructured Al2O3-13TiO2 coating by ultrasound-assisted sealing. Ceram. Int. 2023, 49, 13852–13859. [Google Scholar] [CrossRef]
- Harb, S.V.; Trentin, A.; Torrico, R.F.O.; Pulcinelli, S.H.; Santilli, C.V.; Hammer, P. Organic-inorganic hybrid coatings for corrosion protection of metallic surfaces. In New Technologies in Protective Coatings; Giudice, G.C.C., Ed.; Intech: Rijeka, Yugoslavia, 2017; pp. 19–51. [Google Scholar]
- Eduok, U.; Szpunar, J.; Ebenso, E. Synthesis and characterization of anticorrosion zirconia/acrylic nanocomposite resin coatings for steel. Prog. Org. Coat. 2019, 137, 105337. [Google Scholar] [CrossRef]
- Bozorg, M.; Ramezani, A. Characterization and protective performance of acrylic-based nanocomposite coating reinforced with silica nanoparticles. Mater. Corros. 2017, 68, 725–730. [Google Scholar] [CrossRef]
- Nguyen-Tri, P.; Nguyen, T.A.; Carriere, P.; Ngo Xuan, C. Nanocomposite coatings: Preparation, characterization, properties, and applications. Int. J. Corros. 2018, 2018, 4749501. [Google Scholar] [CrossRef]
- Figueira, R.B.; Silva, C.J.R.; Pereira, E.V. Organic-inorganic hybrid sol-gel coatings for metal corrosion protection: A review of recent progress. J. Coat. Technol. Res. 2015, 12, 1–35. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saikia, M.; Dutta, T.; Jadhav, N.; Kalita, D.J. Insights into the Development of Corrosion Protection Coatings. Polymers 2025, 17, 1548. https://doi.org/10.3390/polym17111548
Saikia M, Dutta T, Jadhav N, Kalita DJ. Insights into the Development of Corrosion Protection Coatings. Polymers. 2025; 17(11):1548. https://doi.org/10.3390/polym17111548
Chicago/Turabian StyleSaikia, Monmi, Trisha Dutta, Niteen Jadhav, and Deep J. Kalita. 2025. "Insights into the Development of Corrosion Protection Coatings" Polymers 17, no. 11: 1548. https://doi.org/10.3390/polym17111548
APA StyleSaikia, M., Dutta, T., Jadhav, N., & Kalita, D. J. (2025). Insights into the Development of Corrosion Protection Coatings. Polymers, 17(11), 1548. https://doi.org/10.3390/polym17111548







