Adsorption of Methylene Blue onto Environmentally Friendly Lignocellulosic Material Obtained from Mature Coltsfoot (Tussilago farfara) Leaves
Abstract
1. Introduction
2. Materials and Methods
2.1. Obtaining the Adsorbent Material
2.2. Adsorbent Characterization
2.3. Adsorption Experiments
2.4. Equilibrium, Kinetics, and Thermodynamics
2.5. Process Optimization
3. Results and Discussion
3.1. Adsorbent Characterization
3.2. Kinetic Study
3.3. Equilibrium Study
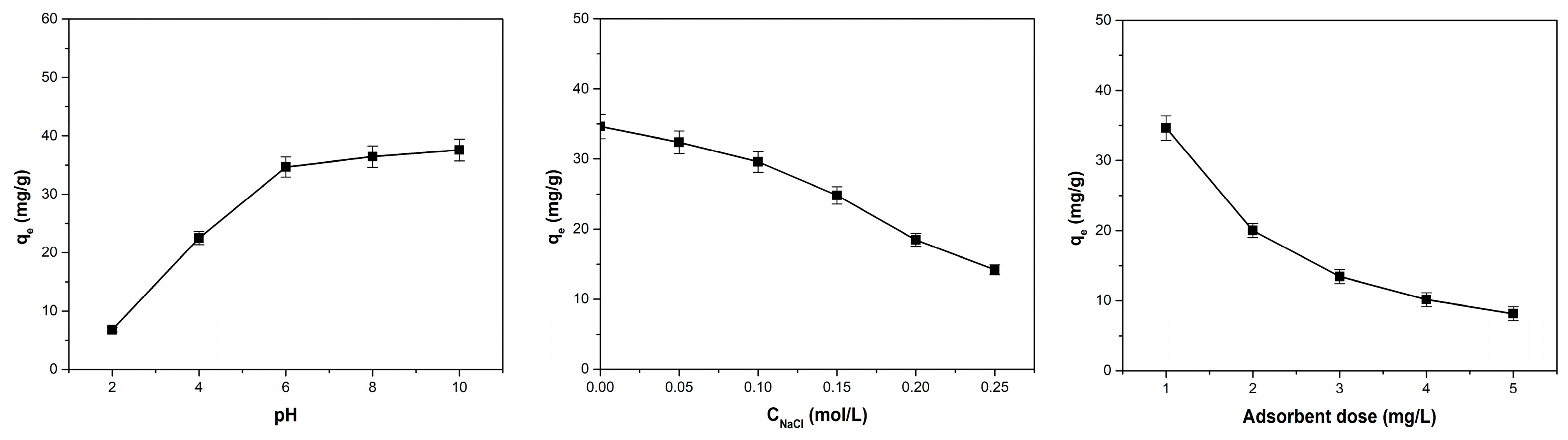
3.4. Influence of pH, Ionic Strength, and Adsorbent Dose on Adsorption Capacity
3.5. Thermodynamic Study
3.6. Adsorption Process Optimization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Lu, Y.; Wu, Q.; Meng, X.; Zhang, H.; Wang, X.; Dong, Y.; Xu, Y.; Zhang, J. Highly efficient and rapid adsorption of dyes by hollow mesoporous carbon spheres. Sep. Purif. Technol. 2025, 365, 132746. [Google Scholar] [CrossRef]
- Su, X.; Wang, X.; Ge, Z.; Bao, Z.; Lin, L.; Chen, Y.; Dai, W.; Sun, Y.; Yuan, H.; Yang, W.; et al. KOH-activated biochar and chitosan composites for efficient adsorption of industrial dye pollutants. Chem. Eng. J. 2024, 486, 150387. [Google Scholar] [CrossRef]
- Sajai, N.; Soubai, B.; El Khaider, S.; Chham, A.; Fakhreddine, R.; Mechnou, I.; Krimi, S. Valorization of calcium phosphate glasses: A sustainable and eco-friendly approach to methylene blue dye adsorption from wastewater. Results Surf. Interfaces 2025, 19, 100525. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Zekker, I.; Zhang, B.; Hendi, A.H.; Ahmad, A.; Ahmad, S.; Zada, N.; Ahmad, H.; Shah, L.A.; et al. Review on methylene blue: Its properties, uses, toxicity and photodegradation. Water 2022, 14, 242. [Google Scholar] [CrossRef]
- Kathing, C.; Saini, G. A review of various treatment methods for the removal of dyes from textile effluent. Recent Prog. Mater. 2022, 4, 1–15. [Google Scholar] [CrossRef]
- Bal, G.; Thakur, A. Distinct approaches of removal of dyes from wastewater: A review. Mater. Today Proc. 2022, 50, 1575–1579. [Google Scholar] [CrossRef]
- Hassan, M.M.; Carr, C.M. A critical review on recent advancements of the removal of reactive dyes from dyehouse effluent by ion-exchange adsorbents. Chemosphere 2018, 209, 201–219. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yang, Z.; Jiang, Y.; Shen, Z.; Zhao, P.; Meng, X. Selective catalytic activation of peroxymonocarbonate over a Co/Al2O3 catalyst. Appl. Catal. B Environ. 2025, 362, 124748. [Google Scholar] [CrossRef]
- Jian, L.; Sun, X.; Zhao, P.; Meng, X.; Dong, T. Strong interfacial interaction of α-Fe2O3/Mel-PHI mediated electron transfer for promoting rapid conversion of H2O2 to •OH during photocatalysis-self-fenton. J. Chem. Eng. 2025, 515, 163871. [Google Scholar] [CrossRef]
- Sahu, D.; Pervez, S.; Karbhal, I.; Tamrakar, A.; Mishra, A.; Verma, S.R.; Deb, M.K.; Ghosh, K.K.; Pervez, Y.F.; Shrivas, K.; et al. Applications of different adsorbent materials for the removal of organic and inorganic contaminants from water and wastewater–A review. Desalin. Water Treat. 2024, 317, 100253. [Google Scholar] [CrossRef]
- Deng, Y.; Li, Z.; Wang, R.; Shi, Y. Cellulose Nanocrystal and Polymer Composite Microspheres for Methylene Blue Adsorption. Polymers 2025, 17, 1205. [Google Scholar] [CrossRef] [PubMed]
- Mihai, S.; Bondarev, A.; Călin, C.; Sȋrbu, E.-E. Adsorbent Biomaterials Based on Natural Clays and Orange Peel Waste for the Removal of Anionic Dyes from Water. Processes 2024, 12, 1032. [Google Scholar] [CrossRef]
- Aranda-Figueroa, M.G.; Rodríguez-Torres, A.; Rodríguez, A.; Bolio-López, G.I.; Salinas-Sánchez, D.O.; Arias-Atayde, D.M.; Romero, R.J.; Valladares-Cisneros, M.G. Removal of Azo Dyes from Water Using Natural Luffa cylindrica as a Non-Conventional Adsorbent. Molecules 2024, 29, 1954. [Google Scholar] [CrossRef]
- Ceroni, L.; Benazzato, S.; Pressi, S.; Calvillo, L.; Marotta, E.; Menna, E. Enhanced Adsorption of Methylene Blue Dye on Functionalized Multi-Walled Carbon Nanotubes. Nanomaterials 2024, 14, 522. [Google Scholar] [CrossRef]
- Mosoarca, G.; Vancea, C.; Popa, S.; Dan, M.; Boran, S. Crystal Violet Adsorption on Eco-Friendly Lignocellulosic Material Obtained from Motherwort (Leonurus cardiaca L.) Biomass. Polymers 2022, 14, 3825. [Google Scholar] [CrossRef]
- Ungureanu, E.L.; Mocanu, A.L.; Stroe, C.A.; Panciu, C.M.; Berca, L.; Sionel, R.M.; Mustatea, G. Agricultural Byproducts Used as Low-Cost Adsorbents for Removal of Potentially Toxic Elements from Wastewater: A Comprehensive Review. Sustainability 2023, 15, 5999. [Google Scholar] [CrossRef]
- Yadav, M.; Singh, N.; Annu; Khan, S.A.; Raorane, C.J.; Shin, D.K. Recent Advances in Utilizing Lignocellulosic Biomass Materials as Adsorbents for Textile Dye Removal: A Comprehensive Review. Polymers 2024, 16, 2417. [Google Scholar] [CrossRef]
- Huang, Z.G.; Wang, T.; Yi, H.Y.; Li, X.B. Study on the adsorption of methylene blue from dye wastewater by Humulus japonicus leaves. In E3S Web of Conferences; EDP Sciences: Ulis, France, 2021; Volume 236, p. 03028. [Google Scholar]
- Kushwaha, A.K.; Gupta, N.; Chattopadhyaya, M.C. Removal of cationic methylene blue and malachite green dyes from aqueous solution by waste materials of Daucus carota. J. Saudi Chem. Soc. 2014, 18, 200–207. [Google Scholar] [CrossRef]
- Mosoarca, G.; Vancea, C.; Popa, S.; Dan, M.; Boran, S. A Novel High-Efficiency Natural Biosorbent Material Obtained from Sour Cherry (Prunus cerasus) Leaf Biomass for Cationic Dyes Adsorption. Materials 2023, 16, 4252. [Google Scholar] [CrossRef]
- Mosoarca, G.; Vancea, C.; Popa, S.; Dan, M.; Boran, S. The Use of Bilberry Leaves (Vaccinium myrtillus L.) as an Efficient Adsorbent for Cationic Dye Removal from Aqueous Solutions. Polymers 2022, 14, 978. [Google Scholar] [CrossRef]
- Guo, D.; Li, Y.; Cui, B.; Hu, M.; Luo, S.; Ji, B.; Liu, Y. Natural adsorption of methylene blue by waste fallen leaves of Magnoliaceae and its repeated thermal regeneration for reuse. J. Clean. Prod. 2020, 267, 121903. [Google Scholar] [CrossRef]
- Boumaza, S.; Yenounne, A.; Hachi, W.; Kaouah, F.; Bouhamidi, Y.; Trari, M. Application of Typha angustifolia (L.) Dead leaves waste as biomaterial for the removal of cationic dye from aqueous solution. Int. J. Environ. Res. 2018, 12, 561–573. [Google Scholar] [CrossRef]
- Peydayesh, M.; Rahbar-Kelishami, A. Adsorption of methylene blue onto Platanus orientalis leaf powder: Kinetic, equilibrium and thermodynamic studies. J. Ind. Eng. Chem. 2015, 21, 1014–1019. [Google Scholar] [CrossRef]
- Fiaz, R.; Hafeez, M.; Mahmood, R. Ficcus palmata leaves as a low-cost biosorbent for methylene blue: Thermodynamic and kinetic studies. Water Environ. Res. 2019, 91, 689–699. [Google Scholar] [CrossRef]
- Singh, R.; Singh, T.S.; Odiyo, J.O.; Smith, J.A.; Edokpayi, J.N. Evaluation of methylene blue sorption onto low-cost biosorbents: Equilibrium, kinetics, and thermodynamics. J. Chem. 2020, 2020, 8318049. [Google Scholar] [CrossRef]
- Khodabandehloo, A.; Rahbar-Kelishami, A.; Shayesteh, H. Methylene blue removal using Salix babylonica (Weeping willow) leaves powder as a low-cost biosorbent in batch mode: Kinetic, equilibrium, and thermodynamic studies. J. Mol. Liq. 2017, 244, 540–548. [Google Scholar] [CrossRef]
- Han, R.; Zou, W.; Yu, W.; Cheng, S.; Wang, Y.; Shi, J. Biosorption of methylene blue from aqueous solution by fallen phoenix tree’s leaves. J. Hazard. Mater. 2007, 141, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Wang, W.; Ma, X. Adsorption characteristics of methylene blue onto low cost biomass material lotus leaf. Chem. Eng. J. 2011, 171, 1–8. [Google Scholar] [CrossRef]
- Bojko, M.; Kędra, M.; Adamska, A.; Jakubowska, Z.; Tuleja, M.; Myśliwa-Kurdziel, B. Induction and Characteristics of Callus Cultures of the Medicinal Plant Tussilago farfara L. Plants 2024, 13, 3080. [Google Scholar] [CrossRef]
- Chen, S.; Dong, L.; Quan, H.; Zhou, X.; Ma, J.M.; Xia, W.; Zhou, H.; Fu, X. A review of the ethnobotanical value, phytochemistry, pharmacology, toxicity and quality control of Tussilago farfara L. (coltsfoot). J. Ethnopharmacol. 2021, 267, 113478. [Google Scholar] [CrossRef]
- Piccin, J.S.; Cadaval, T.R.S.; de Pinto, L.A.A.; Dotto, G.L. Adsorption Isotherms in Liquid Phase: Experimental, Modeling, and Interpretations. In Adsorption Processes for Water Treatment and Purification; Bonilla-Petriciolet, A., Mendoza-Castillo, D., Reynel-Avila, H., Eds.; Springer: Cham, Switzerland, 2017; pp. 19–51. [Google Scholar]
- Dotto, G.L.; Salau, N.P.G.; Piccin, J.S.; Cadaval, T.R.S.; de Pinto, L.A.A. Adsorption Kinetics in Liquid Phase: Modeling for Discontinuous and Continuous Systems. In Adsorption Processes for Water Treatment and Purification; Bonilla-Petriciolet, A., Mendoza-Castillo, D., Reynel-Avila, H., Eds.; Springer: Cham, Switzerland, 2017; pp. 53–76. [Google Scholar]
- Mosoarca, G.; Popa, S.; Vancea, C.; Dan, M.; Boran, S. Removal of Methylene Blue from Aqueous Solutions Using a New Natural Lignocellulosic Adsorbent—Raspberry (Rubus idaeus) Leaves Powder. Polymers 2022, 14, 1966. [Google Scholar] [CrossRef]
- Kokot, S.; Czarnik-Matusewicz, B.; Ozaki, Y. Two-dimensional correlation spectroscopy and principal component analysis studies of temperature-dependent IR spectra of cotton-cellulose. Biopolymers 2002, 67, 456–469. [Google Scholar] [CrossRef]
- Pang, X.; Sellaoui, L.; Franco, D.; Dotto, G.L.; Georgin, J.; Bajahzar, A.; Belmabrouk, H.; Ben Lamine, A.; Bonilla-Petriciolet, A.; Li, Z. Adsorption of crystal violet on biomasses from pecan nutshell, para chestnut husk, araucaria bark and palm cactus: Experimental study and theoretical modeling via monolayer and double layer statistical physics models. Chem. Eng. J. 2019, 378, 122101. [Google Scholar] [CrossRef]
- Pekel Bayramgil, N. Preparation of graft copolymers of cellulose derivatives and their use in recovery processes. In Cellulose-Based Graft Copolymers: Structure and Chemistry, 1st ed.; Thakur, V.K., Ed.; CRC Press: Boca Raton, FL, USA, 2015; pp. 335–364. [Google Scholar]
- Liu, X. Organic Chemistry I; KPU Pressbooks Publishing: Montreal, QC, Canada, 2021; p. 198. [Google Scholar]
- Barth, A. Infrared spectroscopy of proteins. Biochim. Biophys. Acta Bioenerg. 2007, 1767, 1073–1101. [Google Scholar] [CrossRef] [PubMed]
- Grassi, P.; Reis, C.; Drumm, F.C.; Georgin, J.; Tonato, D.; Escudero, L.B.; Kuhn, R.; Jahn, S.L.; Dotto, G.L. Biosorption of crystal violet dye using inactive biomass of the fungus Diaporthe schini. Water Sci. Technol. 2019, 79, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.T.; Yeh, T.F.; Hsu, F.L.; Kuo-Huang, L.L.; Lee, C.M.; Huang, Y.S.; Chang, S.T. Profiling the chemical composition and growth strain of giant bamboo (Dendrocalamus giganteus Munro). BioResources 2015, 10, 1260–1270. [Google Scholar] [CrossRef][Green Version]
- Sahoo, S.; Seydibeyoglu, M.O.; Mohanty, A.K.; Misra, M. Characterization of industrial lignins for their utilization in future value added applications. Biomass. Bioenergy 2011, 35, 4230–4237. [Google Scholar] [CrossRef]
- Popescu, C.M.; Popescu, M.C.; Singurel, G.; Vasile, C.; Argyropoulos, D.S.; Willfor, S. Spectral characterization of eucalyptus wood. Appl. Spectrosc. 2007, 61, 1168–1177. [Google Scholar] [CrossRef]
- Nur Zaidi, A.H.M.; Lim, L.B.L.; Usman, A. Artocarpus odoratissimus leaf-based cellulose as adsorbent for removal of methyl violet and crystal violet dyes from aqueous solution. Cellulose 2018, 25, 3037–3049. [Google Scholar] [CrossRef]
- Thinh, P.X.; Basavaraja, C.; Kim, D.G.; Huh, D.S. Characterization and electrochemical behaviors of honeycomb-patterned poly(N-vinylcarbazole)/polystyrene composite films. Polym. Bull. 2012, 69, 81–94. [Google Scholar] [CrossRef]
- Trivedi, M.K.; Tallapragada, R.M.; Branton, A.; Trivedi, D.; Nayak, G.; Mishra, R.K.; Jana, S. Characterization of physical, spectroscopic and thermal properties of biofield treated biphenyl. Am. J. Chem. Eng. 2015, 3, 58–65. [Google Scholar] [CrossRef]
- Md Salim, R.; Asik, J.; Sarjadi, M.S. Chemical functional groups of extractives, cellulose and lignin extracted from native Leucaena leucocephala bark. Wood Sci. Technol. 2021, 55, 295–313. [Google Scholar] [CrossRef]
- Shakoor, S.; Nasar, A. Removal of methylene blue dye from artificially contaminated water using citrus limetta peel waste as a very low cost adsorbent. J. Taiwan Inst. Chem. Eng. 2016, 66, 154–163. [Google Scholar] [CrossRef]
- Alghamdi, W.M.; El Mannoubi, I. Investigation of Seeds and Peels of Citrullus colocynthis as Efficient Natural Adsorbent for Methylene Blue Dye. Processes 2021, 9, 1279. [Google Scholar] [CrossRef]
- Al-Ghamdi, Y.O.; Jabli, M.; Soury, R.; Ali Khan, S. A cellulosic fruit derived from nerium oleander biomaterial: Chemical characterization and its valuable use in the biosorption of methylene blue in a batch mode. Polymers 2020, 12, 2539. [Google Scholar] [CrossRef]
- Mosoarca, G.; Popa, S.; Vancea, C.; Dan, M.; Boran, S. Modelling and Optimization of Methylene Blue Adsorption Process on Leonurus cardiaca L. Biomass Powder. Processes 2023, 11, 3385. [Google Scholar] [CrossRef]
- Hernandes, P.T.; Oliveira, M.L.S.; Georgin, J.; Franco, D.S.P.; Allasia, D.; Dotto, G.L. Adsorptive decontamination of wastewater containing methylene blue dye using golden trumpet tree bark (Handroanthus albus). Environ. Sci. Pollut. Res. 2019, 26, 31924–31933. [Google Scholar] [CrossRef] [PubMed]
- Ponnusami, V.; Vikram, S.; Srivastava, S.N. Guava (Psidium guajava) leaf powder: Novel adsorbent for removal of methylene blue from aqueous solutions. J. Hazard. Mater. 2008, 152, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Lopez, J.A.; Angosto, J.M.; Roca, M.J.; Doval Minarro, M. Taguchi design-based enhancement of heavy metals bioremoval by agroindustrial waste biomass from artichoke. Sci. Total Environ. 2019, 653, 55–63. [Google Scholar] [CrossRef]
- Zolgharnein, J.; Rastgordani, M. Optimization of simultaneous removal of binary mixture of indigo carmine and methyl orange dyes by cobalt hydroxide nano-particles through Taguchi method. J. Mol. Liq. 2018, 262, 405–414. [Google Scholar] [CrossRef]








| FTIR Bands (1/cm) | Assignment | Reference |
|---|---|---|
| 3610 | –OH stretch, free hydroxyl | [35] |
| 3300 | –OH stretching vibrations of cellulose, lignin, or hemicellulose | [36] |
| 2924 | –CH2 groups of cellulose, respectively | [37] |
| 1680 | C = C stretching | [38] |
| 1550 | amide II groups | [39,40] |
| 1422 | –C–H deformation in lignin | [41,42] |
| 1282 | –CH deformation in cellulose I and cellulose II | [43] |
| 1057 | C–O–C stretching of cellulose | [27,44] |
| 698 | aromatic out of plane C–H bending vibrations | [45,46] |
| 542 | C–H bend | [47] |
| Adsorbent | Equilibrium Time (min) | Reference |
|---|---|---|
| Humulus japonicas leaves | 20 | [18] |
| coltsfoot leaves | 20 | This study |
| Daucus carota leaves | 30 | [19] |
| sour-cherry leaves | 40 | [20] |
| bilberry leaves | 40 | [21] |
| Magnolia grandiflora leaves | 60 | [22] |
| Typha angustifolia leaves | 60 | [23] |
| Platanus orientalis leaf powder | 70 | [24] |
| Ficcus Palmata leaves | 80 | [25] |
| Ginkgo biloba leaves | 100 | [26] |
| Salix babylonica leaves | 120 | [27] |
| phoenix tree leaves | 150 | [28] |
| lotus leaf | 150 | [29] |
| Kinetic Model | Parameters | Values |
|---|---|---|
| Pseudo-first order | k1 (1/min) | 0.48 ± 0.09 |
| qe,calc (mg/g) | 34.82 ± 2.71 | |
| R2 | 0.9972 | |
| χ2 | 0.09 | |
| SSE | 2.92 | |
| ARE (%) | 14.01 | |
| Pseudo-second order | k2 (1/min) | 0.021 ± 0.006 |
| qe,calc (g/mg·min) | 37.21 ± 2.98 | |
| R2 | 0.9887 | |
| χ2 | 0.43 | |
| SSE | 12.13 | |
| ARE (%) | 3.39 | |
| Elovich | a (g/mg) | 0.23 ± 0.05 |
| b (mg/g·min) | 827 ± 141 | |
| R2 | 0.9645 | |
| χ2 | 1.42 | |
| SSE | 38.08 | |
| ARE (%) | 18.20 | |
| General order | kn (1/min) (g/mg)1/n | 8.98 ± 0.93 |
| qn (mg/g) | 19.91 ± 1.57 | |
| n | 3.34 ± 0.67 | |
| R2 | 0.9979 | |
| χ2 | 0.04 | |
| SSE | 0.69 | |
| ARE (%) | 0.94 | |
| Avrami | kAV (1/min) | 0.84 ± 0.12 |
| qAV (mg/g) | 34.82 ± 3.24 | |
| nAV | 0.57 ± 0.11 | |
| R2 | 0.9972 | |
| χ2 | 0.09 | |
| SSE | 2.92 | |
| ARE (%) | 14.01 |
| Isotherm Model | Parameters | Value |
|---|---|---|
| Langmuir | KL (L/mg) | 0.015 ± 0.002 |
| qmax (mg/g) | 344.4 ± 24.58 | |
| R2 | 0.9764 | |
| χ2 | 20.80 | |
| SSE | 2143 | |
| ARE (%) | 12.57 | |
| Freundlich | Kf (mg/g)(L/mg)1/n | 26.64 ± 3.47 |
| 1/n | 0.42 ± 0.07 | |
| R2 | 0.9134 | |
| χ2 | 56.08 | |
| SSE | 7302 | |
| ARE (%) | 20.54 | |
| Temkin | KT (L/mg) | 0.11 ± 0.03 |
| b (kJ/g) | 30.14 ± 4.9 | |
| R2 | 0.9730 | |
| χ2 | 13.85 | |
| SSE | 2228 | |
| ARE (%) | 10.89 | |
| Sips | Qsat (mg/g) | 278.1 ± 17.64 |
| KS (L/mg) | 0.0012 ± 0.0002 | |
| n | 1.78 | |
| R2 | 0.9986 | |
| χ2 | 1.22 | |
| SSE | 115 | |
| ARE (%) | 3.63 | |
| Redlich–Peterson | KRP (L/g) | 3.56 ± 0.78 |
| aRP (L/mg) | 0.0006 ± 0.0001 | |
| βRP | 1.49 ± 0.14 | |
| R2 | 0.9934 | |
| χ2 | 7.54 | |
| SSE | 602 | |
| ARE (%) | 7.41 |
| Adsorbent | Adsorption Capacity (mg/g) | Reference |
|---|---|---|
| Ficcus Palmata leaves | 6.89 | [25] |
| Ginkgo biloba leaves | 48.07 | [26] |
| Salix babylonica leaves | 60.90 | [27] |
| Daucus carota leaves | 66.50 | [19] |
| phoenix tree leaves | 80.90 | [28] |
| Typha angustifolia leaves | 106.7 | [23] |
| Platanus orientalis leaf | 114.9 | [24] |
| Humulus japonicas leaves | 145.6 | [18] |
| Magnolia grandiflora leaves | 149.2 | [22] |
| sour-cherry leaves | 168.6 | [20] |
| bilberry leaves | 200.4 | [21] |
| lotus leaf | 221.7 | [29] |
| raspberry leaves | 244.6 | [34] |
| coltsfoot leaves | 278.1 | This study |
| guava leaf | 295.0 | [53] |
| ΔG (kJ mol−1) | ΔH (kJ mol−1) | ΔS (J mol−1 K−1) | ||
|---|---|---|---|---|
| 278 K | 297 K | 311 K | ||
| −14.79 | −15.27 | −15.70 | −0.84 | 3.37 |
| Factor | Level 1 | Level 2 | Level 3 |
|---|---|---|---|
| pH | 2 | 6 | 10 |
| Contact time (min) | 5 | 20 | 40 |
| Initial dye concentration (mg/L) | 50 | 200 | 500 |
| Adsorbent dose (g/L) | 1 | 3 | 5 |
| Temperature (K) | 278 | 295 | 311 |
| Ionic strength (mol/L) | 0 | 0.15 | 0.25 |
| pH | Time (min) | Initial Dye Concentration (mg/L) | Adsorbent Dose (mg/L) | Temperature (K) | Ionic Strength (mol/L) | Dye Removal Efficiency (%) | S/N Ratio |
|---|---|---|---|---|---|---|---|
| 2 | 5 | 50 | 1 | 278 | 0 | 13.11 | 22.35 |
| 2 | 5 | 50 | 1 | 295 | 0.15 | 11.50 | 21.21 |
| 2 | 5 | 50 | 1 | 311 | 0.25 | 10.79 | 20.66 |
| 2 | 20 | 200 | 3 | 278 | 0 | 17.61 | 24.91 |
| 2 | 20 | 200 | 3 | 295 | 0.15 | 15.45 | 23.77 |
| 2 | 20 | 200 | 3 | 311 | 0.25 | 14.49 | 23.22 |
| 2 | 40 | 500 | 5 | 278 | 0 | 12.99 | 22.27 |
| 2 | 40 | 500 | 5 | 295 | 0.15 | 11.40 | 21.13 |
| 2 | 40 | 500 | 5 | 311 | 0.25 | 10.69 | 20.57 |
| 6 | 5 | 200 | 5 | 278 | 0.15 | 77.07 | 37.73 |
| 6 | 5 | 200 | 5 | 295 | 0.25 | 73.81 | 37.36 |
| 6 | 5 | 200 | 5 | 311 | 0 | 78.25 | 37.86 |
| 6 | 20 | 500 | 1 | 278 | 0.15 | 49.39 | 33.87 |
| 6 | 20 | 500 | 1 | 295 | 0.25 | 47.30 | 33.49 |
| 6 | 20 | 500 | 1 | 311 | 0 | 50.14 | 34.00 |
| 6 | 40 | 50 | 3 | 278 | 0.15 | 77.45 | 37.78 |
| 6 | 40 | 50 | 3 | 295 | 0.25 | 74.18 | 37.40 |
| 6 | 40 | 50 | 3 | 311 | 0 | 78.64 | 37.91 |
| 10 | 5 | 500 | 3 | 278 | 0.25 | 57.98 | 35.26 |
| 10 | 5 | 500 | 3 | 295 | 0 | 62.75 | 35.95 |
| 10 | 5 | 500 | 3 | 311 | 0.15 | 53.93 | 34.63 |
| 10 | 20 | 50 | 5 | 278 | 0.25 | 81.00 | 38.16 |
| 10 | 20 | 50 | 5 | 295 | 0 | 87.67 | 38.85 |
| 10 | 20 | 50 | 5 | 311 | 0.15 | 75.34 | 37.54 |
| 10 | 40 | 200 | 1 | 278 | 0.25 | 77.34 | 37.76 |
| 10 | 40 | 200 | 1 | 295 | 0 | 83.73 | 38.45 |
| 10 | 40 | 200 | 1 | 311 | 0.15 | 71.95 | 37.14 |
| Level | pH | Time | Initial Dye Concentration | Adsorbent Dose | Temperature | Ionic Strength |
|---|---|---|---|---|---|---|
| 1 | 22.24 | 31.45 | 32.43 | 31.00 | 32.24 | 32.51 |
| 2 | 36.38 | 31.98 | 33.14 | 32.32 | 31.96 | 31.65 |
| 3 | 37.09 | 32.27 | 30.14 | 32.39 | 31.51 | 31.55 |
| Delta | 14.85 | 0.82 | 3.00 | 1.40 | 0.73 | 0.96 |
| Rank | 1 | 5 | 2 | 3 | 6 | 4 |
| pH | Time | Initial Dye Concentration | Adsorbent Dose | Temperature | Ionic Strength | Errors | |
|---|---|---|---|---|---|---|---|
| Contribution percentage (%) | 87.78 | 1.19 | 7.84 | 2.20 | 0.16 | 0.52 | 0.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mosoarca, G.; Vancea, C.; Popa, S.; Radulescu-Grad, M.E.; Dan, M.; Tanasie, C.; Boran, S. Adsorption of Methylene Blue onto Environmentally Friendly Lignocellulosic Material Obtained from Mature Coltsfoot (Tussilago farfara) Leaves. Polymers 2025, 17, 1549. https://doi.org/10.3390/polym17111549
Mosoarca G, Vancea C, Popa S, Radulescu-Grad ME, Dan M, Tanasie C, Boran S. Adsorption of Methylene Blue onto Environmentally Friendly Lignocellulosic Material Obtained from Mature Coltsfoot (Tussilago farfara) Leaves. Polymers. 2025; 17(11):1549. https://doi.org/10.3390/polym17111549
Chicago/Turabian StyleMosoarca, Giannin, Cosmin Vancea, Simona Popa, Maria Elena Radulescu-Grad, Mircea Dan, Cristian Tanasie, and Sorina Boran. 2025. "Adsorption of Methylene Blue onto Environmentally Friendly Lignocellulosic Material Obtained from Mature Coltsfoot (Tussilago farfara) Leaves" Polymers 17, no. 11: 1549. https://doi.org/10.3390/polym17111549
APA StyleMosoarca, G., Vancea, C., Popa, S., Radulescu-Grad, M. E., Dan, M., Tanasie, C., & Boran, S. (2025). Adsorption of Methylene Blue onto Environmentally Friendly Lignocellulosic Material Obtained from Mature Coltsfoot (Tussilago farfara) Leaves. Polymers, 17(11), 1549. https://doi.org/10.3390/polym17111549











