A Holistic Review of 3-Dimethylamino-1-Arylpropenones Based Disperse Dyes for Dyeing Polyester Fabrics: Synthesis, Characterization, and Antimicrobial Activities
Abstract
1. Introduction
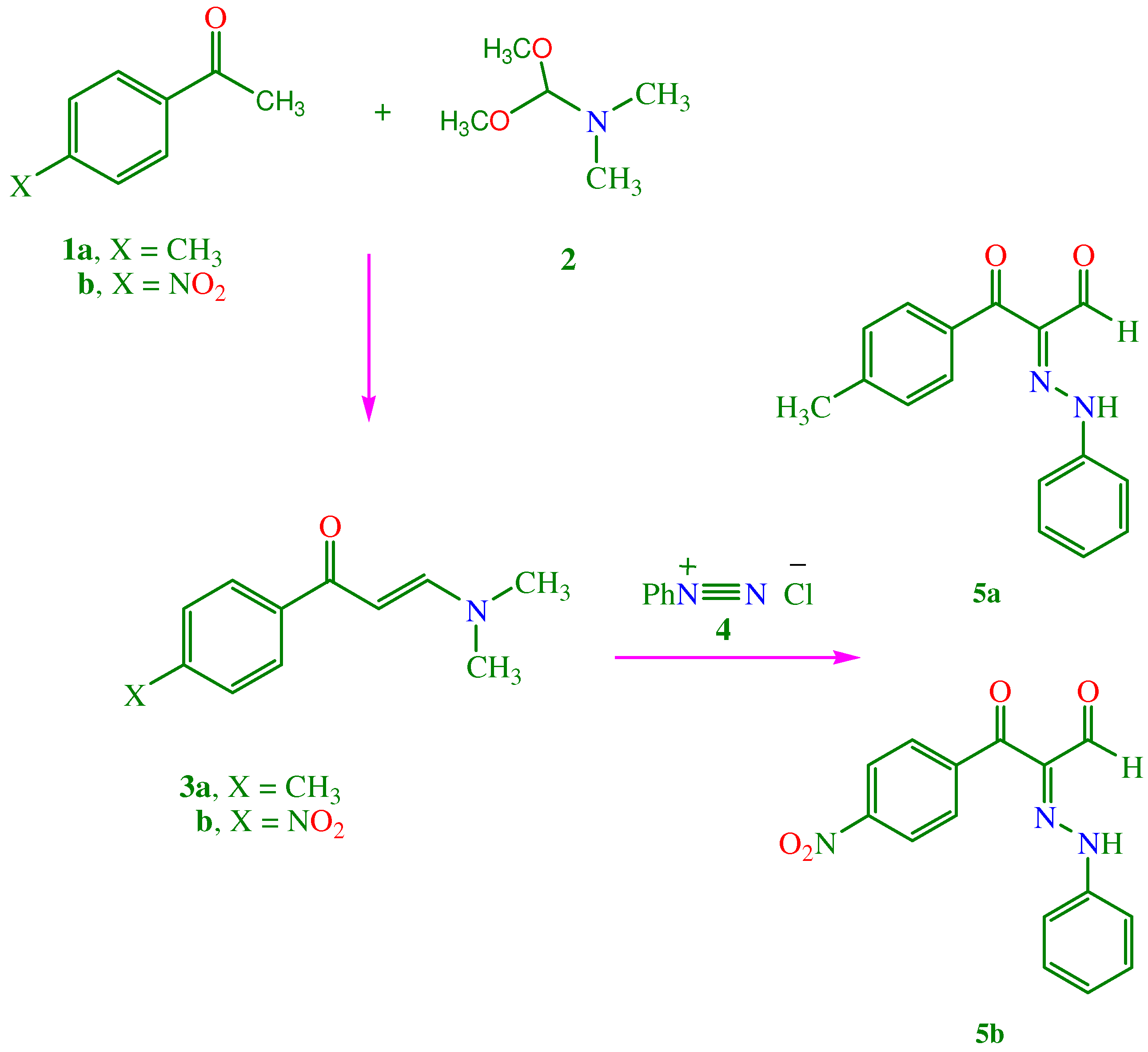
2. Chemistry
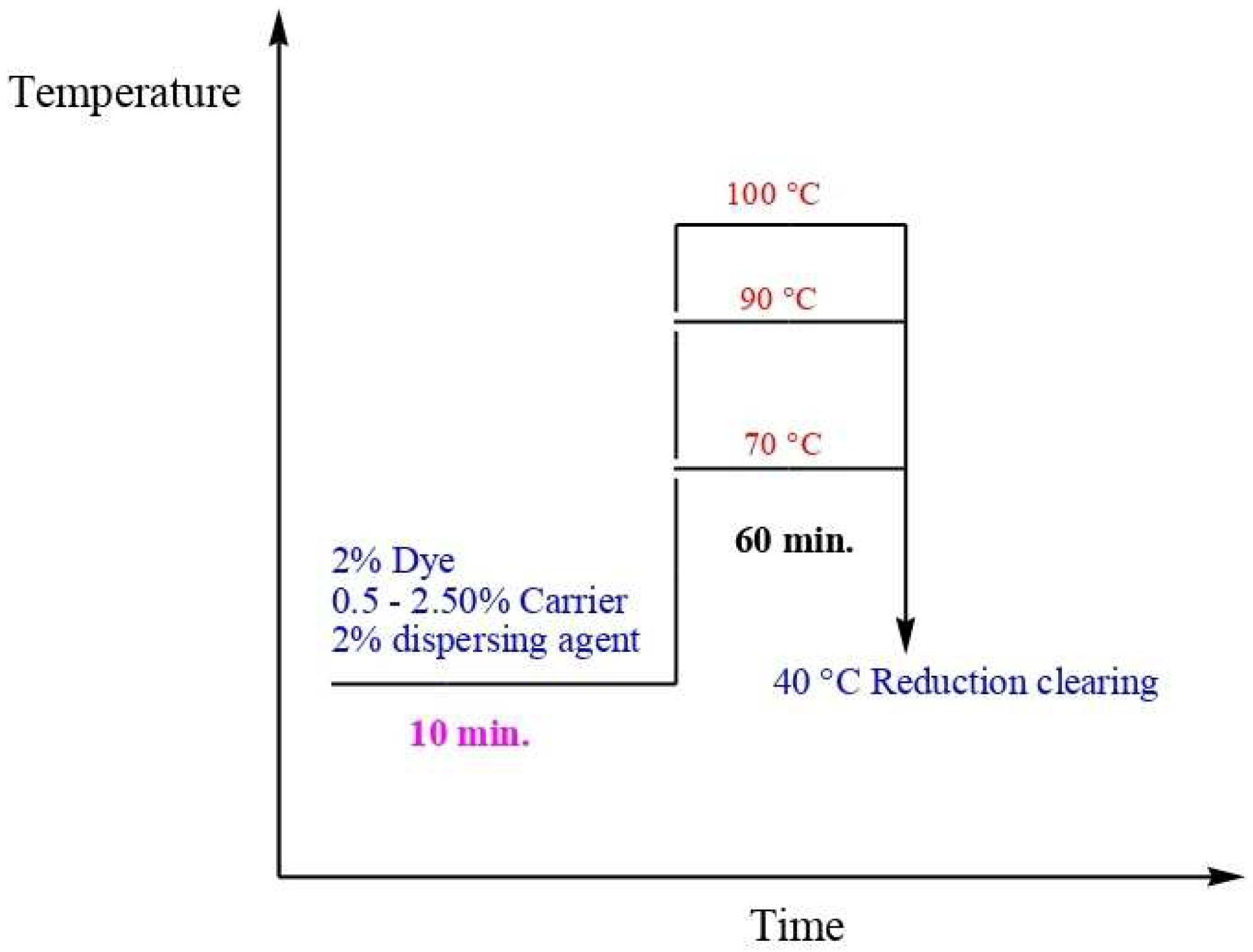
3. Dyeing at Temperatures of 70, 90, and 100 °C
3.1. Effect of Carrier Concentration
3.2. Effect of Dyeing Temperature
4. High Temperature Dyeing
5. Fastness Properties
6. Antimicrobial Activities
7. Treatment of Polyester Fabrics with ZnO NPs
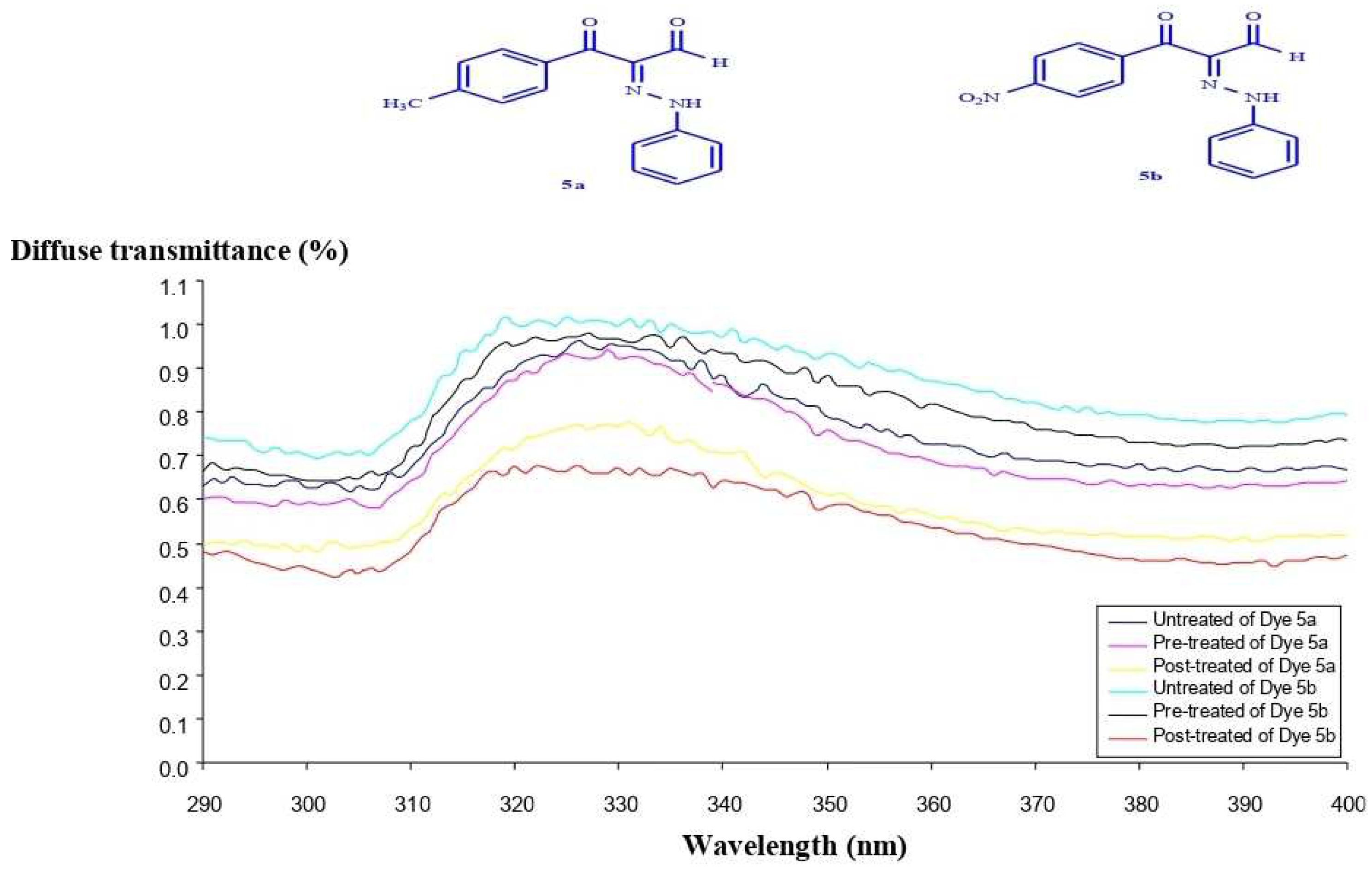
7.1. Ultraviolet Protection Factor (UPF)
7.2. Self-Cleaning Evaluations
7.3. Light Fastness Evaluations
7.4. Antimicrobial Activity Evaluation
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Al-Etaibi, A.M.; El-Apasery, M.A. Can Novel Synthetic Disperse Dyes for Polyester Fabric Dyeing Provide Added Value? Polymers 2023, 15, 1845. [Google Scholar] [CrossRef]
- Choi, T.S.; Shimizu, Y.; Shirai, H.; Hamada, K. Disperse dyeing of polyester fiber using gemini surfactants containing ammonium cations as auxiliaries. Dyes Pigments 2001, 50, 55–65. [Google Scholar] [CrossRef]
- Al-Etaibi, A.M.; El-Apasery, M.A. Facile Synthesis of Novel Disperse Dyes for Dyeing Polyester Fabrics: Demonstrating Their Potential Biological Activities. Polymers 2022, 14, 3966. [Google Scholar] [CrossRef]
- Pasquet, V.; Perwuelz, A.; Behary, N.; Isaad, J. Vanillin, a potential carrier for low temperature dyeing of polyester fabrics. J. Clean. Prod. 2013, 43, 20–26. [Google Scholar] [CrossRef]
- Alonso, D.; Gimeno, M.; Olayo, R.; Vázquez-Torres, H.; Sepúlveda-Sánchez, J.D.; Shirai, K. Cross-Linking Chitosan into UV-Irradiated Cellulose Fibers for the Preparation of Antimicrobial-Finished Textiles. Carbohydr. Polym. 2009, 77, 536–543. [Google Scholar] [CrossRef]
- El-Molla, M.M.; Ismaeil, Z.H.; Soliman, F.M.A.; Abd-El Monem, S.H. Synthesis of Several Newly Disperse Dyes and their Application in Textile Printing. J. Text. Associat. 2013, 74, 18–25. [Google Scholar]
- Elassar, A.-Z.A.; Al-Mousawi, S.M.; Helal, M.H.; Elgazzar, M.E. Synthesis of N-substituted arylhydrazones: Applying to polyester fabric as disperse dyes. Pigment Resin Technol. 2017, 46, 449–457. [Google Scholar] [CrossRef]
- Broadbent, A.D. Basic Principles of Textile Coloration, Society of Dyers and Colorists; Thanet Press Ltd.: Kent, UK, 2001; pp. 322–331. [Google Scholar]
- Lai, C.C.; Chen, K.M. Dyeing properties of modified gemini surfactants on a disperse dye-polyester system. Text. Res. J. 2008, 78, 382–389. [Google Scholar] [CrossRef]
- Salman, M.; Jabbar, A.; Farooq, S.; Bashir, I.; Rafiq, M.S.K. New heterocyclic azo-disperse dyes; their synthesis, characterization, application, photo physical properties and solvatochromic studies. J. Mol. Struct. 2023, 1287, 135664. [Google Scholar] [CrossRef]
- Ren, Z.; Qin, C.; Tanga, R.C.; Chen, G. Study on the dyeing properties of hemicyanine dyes. II. Cationic dyeable polyester. Color. Technol. 2012, 128, 147–152. [Google Scholar] [CrossRef]
- Radetic, M.; Ilic, V.; Vodnik, V.; Dimitrijevic, S.; Jovancic, P.; Saponjic, Z.; Nedeljkovic, J.M. Antibacterial Effect of Silver Nanoparticles Deposited on Corona-Treated Polyester and Polyamide Fabrics. Polym. Adv. Technol. 2008, 19, 1816–1821. [Google Scholar] [CrossRef]
- Towns, A.D. Developments in azo disperse dyes derived from heterocyclic diazo components. Dyes Pigments 1999, 42, 3–28. [Google Scholar] [CrossRef]
- Ilic, V.; Saponjic, Z.; Vodnik, V.; Lazovicic, S.; Dimitrijevic, S.; Jovancic, P.; Nedeljkovic, J.M.; Radetic, M. Bactericidal Efficiency of Silver Nanoparticles Deposited onto Radio Frequency Plasma Pretreated Polyester Fabrics. Ind. Eng. Chem. Res. 2010, 49, 7287–7293. [Google Scholar] [CrossRef]
- Mashaly, H.M.; Abdelghaffar, R.A.; Kamel, M.M.; Youssef, B.M. Dyeing of Polyester Fabric using Nano Disperse Dyes and Improving Their Light Fastness using ZnO Nano Powder. Ind. J. Sci. Technol. 2014, 7, 960–967. [Google Scholar] [CrossRef]
- Lipskikh, O. An over review on recently developed techniques, mechanisms and intermediate involved in the advanced azo dye degradation for industrial applications. J. Mol. Struct. 2021, 1224, 129195. [Google Scholar]
- Ding, M.; Ma, S.; Liu, D. Simultaneous Determination of Hydroxyanthraquinones in Rhubard and Experimental Animal Bodies by High-Performance Liquid Chromatography. Analyt. Sci. 2003, 19, 1163–1165. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Kumari, S.; Gulrajani, M. Dyeing studies with hydroxyanthraquinones extracted from Indian madder. Part 2: Dyeing of nylon and polyester with nordamncanthal. Color. Technol. 2001, 117, 333–336. [Google Scholar] [CrossRef]
- Nakpathom, M.; Somboon, B.; Narumol, N.; Mongkholrattanasit, R. High temperature dyeing of PET fabric with natural colourants extracted from annatto seeds. Pigment Resin Technol. 2019, 48, 129–136. [Google Scholar] [CrossRef]
- Torres, A.; Ruales, C.; Pulgarin, C.; Aimable, A.; Bowen, P.; Sarria, V.; Kiwi, J. Innovative High-Surface- Area CuO Pretreated Cotton Effective in Bacterial Inactivation under Visible Light. ACS Appl. Mater. Interfaces 2010, 2, 2547–2552. [Google Scholar] [CrossRef]
- Shahin, M.F.; Ahmed, R.M.; Marie, M.M. Optimizing the Dyeing Process of Alkali-Treated Polyester Fabric with Dolu Natural Dye. Int. J. Eng. Res. Appl. 2014, 4, 35–40. [Google Scholar]
- El-Adasy, A.A.A.M.; Kamel, M.K.; Saleh, M.O.; Abdel Haleem, M.; Hussein, A.M.; El-Apasery, M.A. Disperse Dyes Based on Pyrazolopyrimidinones I: Their Dyeing Applications and Antimicrobial Activities. Int. J. ChemTech Res. 2016, 9, 31–38. [Google Scholar]
- Broasca, G.; Borcia, G.; Dumitrascu, N.; Vrinceanu, N. Characterization of ZnO coated polyester fabrics for UV protection. Appl. Surf. Sci. 2013, 279, 272–278. [Google Scholar] [CrossRef]
- Tang, E.; Cheng, G.; Pang, X.; Ma, X.; Xing, F. Synthesis of nanoZnO/poly(methylmethacrylate) composite microsphere through emulsion polymerization and its UV shielding property. Colloid Polym. Sci. 2001, 284, 422–428. [Google Scholar] [CrossRef]
- Becheri, A.; Dürr, M.; LoNostro, P.; Baglioni, P. Synthesis and characterization of zinc oxide nanoparticles: Application to textiles as UV-absorbers. J. Nanopart. Res. 2008, 10, 679–689. [Google Scholar] [CrossRef]
- El-Shafei, A.; Abou-Okeil, A. ZnO/carboxymethyl chitosan bionano-composite to impart antibacterial and UV protection for cotton fabric. Carbohydr. Polym. 2011, 83, 920–925. [Google Scholar] [CrossRef]
- Yamamoto, O. Influence of particle size on the antibacterial activity of zinc oxide. Int. J. Inorg. Mater. 2001, 3, 643–646. [Google Scholar] [CrossRef]
- Selvam, S.; Sundrarajan, M. Functionalization of cotton fabric with PVP/ZnO nanoparticles for improved reactive dyeability and antibacterial activity. Carbohydr. Polym. 2012, 87, 1419–1424. [Google Scholar] [CrossRef]
- Wang, L.L.; Zhang, X.T.; Li, B.; Sun, P.P.; Yang, J.K.; Xu, H.Y.; Liu, Y.C. Superhydrophobic and ultraviolet-blocking cotton textiles. ACS Appl. Mater. Interfaces 2011, 3, 1277–1281. [Google Scholar] [CrossRef]
- El-Tahlawy, K.; El-Nagar, K.; Elhendawy, A.G. Cyclodextrin-4 Hydroxybenzophenone inclusion complex for UV protective cotton fabric. J. Text. Inst. 2007, 98, 453–462. [Google Scholar] [CrossRef]
- Khan, A.; Rahman, M.; Islam, S. Antibacterial, antifungal and cytotoxic activities of Tuberous Roots of Amorphophallus campanulatus. Turk. J. Biol. 2007, 31, 167–172. [Google Scholar]
- Isaacson, D.M.; Kirschbaum, J. Assays of antimicrobial substances. In Manual of Industrial Microbiology and Biotechnology; Demain, A.L., Solomon, N.A., Eds.; ASM: Washington, DC, USA, 1986. [Google Scholar]
- Al-Etaibi, A.; El-Apasery, M.A.; Kamel, M.M. Dyeing of polyester with disperse dyes: Part 1. Antimicrobial activity and dyeing performance of some disperse dyes. Int. J. Curr. Microbiol. Appl. Sci. 2015, 4, 923–928. [Google Scholar]
- Al-Etaibi, A.; Alnassar, H.; El-Apasery, M.A. Dyeing of Polyester with Disperse Dyes: Part 2. Synthesis and Dyeing Characteristics of Some Azo Disperse Dyes for Polyester Fabrics. Molecules 2016, 21, 855–861. [Google Scholar] [CrossRef] [PubMed]
- Al-Etaibi, A.M.; El-Apasery, M.A. Dyeing of polyester with disperse dyes: Part 3. Characterization of ZnO nanoparticles treated polyester fabrics for antibacterial, self-cleaning and UV protective. Int. J. ChemTech Res. 2016, 9, 162–169. [Google Scholar]
- Taher, F.A.; Kamel, M.M.; Mashaly, H.M.; Farahat, S.A. Functionality of Inorganic Nanostructured Materials onto Wool Fabric. Chem. Mater. Res. 2013, 3, 113–124. [Google Scholar]
- Ameen, S.; Akhtar, M.S.; Kim, Y.S.; Yang, O.B.; Shin, H.S. Synthesis and characterization of novel poly(1-naphthylamine)/zinc oxide nanocomposites: Application in catalytic degradation of methylene blue. Colloid Polym. Sci. 2010, 288, 1633–1638. [Google Scholar] [CrossRef]
- Cakır, B.A.; Budama, L.; Topel, Ö.; Hoda, N. Synthesis of ZnO nanoparticles using PS-b-PAA reverse micelle cores for UV protective, self-cleaning and antibacterial textile applications. Colloids Surf. A Physicochem. Eng. Asp. 2012, 414, 132–139. [Google Scholar] [CrossRef]
- Moafi, H.F.; Shojaie, A.F.; Zanjanchi, M.A. Photocatalytic self-cleaning properties of cellulosic fibers modified by nano-sized zinc oxide. Thin Solid Films 2011, 519, 3641–3646. [Google Scholar] [CrossRef]
- Liu, Y.; He, L.; Mustapha, A.; Li, H.; Hu, Z.Q.; Lin, M. Antibacterial activities of zinc oxide nanoparticles against Escherichia coli O157:H7. J. Appl. Microbiol. 2009, 107, 1193–1201. [Google Scholar] [CrossRef]
- Brayner, R.; Ferrari-Iliou, R.; Brivois, N.; Djediat, S.; Benedetti, M.F.; Fievet, F. Toxicological impact studies based on Escherichia coli bacteria in ultrafine ZnO nanoparticles colloidal medium. Nano Lett. 2006, 6, 866–870. [Google Scholar] [CrossRef]
- Sawai, J. Quantitative evaluation of antibacterial activities of metallic oxide powders (ZnO, MgO and CaO) by conductimetric assay. J. Microbiol. Methods 2003, 54, 177–182. [Google Scholar] [CrossRef]
- Sen, A.; Bhowal, A.; Datta, S. Comparison of dyeing of polyester fibers with natural dye and bio-mordant. Prog. Color Color. Coat. 2018, 11, 165–172. [Google Scholar]




| * Dyeing Temperature | Dye 5a | Dye 5b | % Carrier | ||
|---|---|---|---|---|---|
| ∆E | K/S | ∆E | K/S | ||
| 70 °C | 87.98 | 0.93 | 23.29 | 1.69 | 0 |
| 88.40 | 1.17 | 28.54 | 1.85 | 1 | |
| 88.19 | 1.19 | 19.33 | 1.87 | 2 | |
| 88.40 | 2.60 | 32.78 | 2.13 | 3 | |
| 89.32 | 1.68 | 31.13 | 2.04 | 4 | |
| 90.19 | 1.92 | 33.97 | 2.10 | 5 | |
| 90 °C | 94.56 | 6.53 | 46.00 | 3.34 | 0 |
| 95.60 | 7.65 | 47.55 | 3.47 | 1 | |
| 96.36 | 8.32 | 48.99 | 3.90 | 2 | |
| 97.14 | 8.90 | 50.10 | 5.15 | 3 | |
| 95.10 | 7.30 | 48.29 | 4.73 | 4 | |
| 94.54 | 6.37 | 50.40 | 5.12 | 5 | |
| 100 °C | 94.07 | 6.34 | 48.20 | 3.93 | 0 |
| 95.16 | 6.80 | 51.11 | 4.49 | 1 | |
| 96.31 | 7.72 | 56.34 | 5.85 | 2 | |
| 98.63 | 9.70 | 52.75 | 5.92 | 3 | |
| 99.58 | 12.21 | 58.04 | 8.97 | 4 | |
| 98.44 | 10.12 | 57.21 | 7.71 | 5 | |
| Dye No. | Color Shade on Polyester | L* | a* | b* | K/S | Reference |
|---|---|---|---|---|---|---|
| 5a | Greenish-yellow | 77.22 | −20.37 | 53.94 | 17.59 | [33] |
| 5b | Yellowish-orange | 67.58 | 9.29 | 61.34 | 16.69 |
| Dye No. | L* | a* | b* | K/S | Reference |
|---|---|---|---|---|---|
| Dyeing at 100 °C | |||||
| 5a | 84.81 | −14.26 | 52.16 | 12.21 | [34] |
| 5b | 78.08 | −0.21 | 51.20 | 8.97 | |
| Dyeing at 130 °C | |||||
| 5a | 77.22 | −20.37 | 53.94 | 17.59 | [33] |
| 5b | 67.58 | 9.29 | 61.34 | 16.69 | |
| Dye No. | Washing Fastness | Light Fastness | Rubbing Fastness | Perspiration Fastness | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alkaline | Acidic | |||||||||||
| Alt | SC | SW | Dry | Wet | Alt | SC | SW | Alt | SC | SW | ||
| Dyeing at 100 °C | ||||||||||||
| 5a | 5 | 5 | 5 | 3–4 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| 5b | 5 | 5 | 5 | 3–4 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| Dyeing at 130 °C | ||||||||||||
| 5a | 4–5 | 4–5 | 3–4 | 3 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| 5b | 4–5 | 4–5 | 4 | 3–4 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| Dye No. | * Inhibition Zone Diameter (Nearest mm) | Reference | |||||
|---|---|---|---|---|---|---|---|
| G-Ve Bacteria | Yeast | Fungi | G+Ve Bacteria | ||||
| E. coli | P. aeruginosa | C. albicans | A. Niger | B. cereus | S. aureus | ||
| 5a | 9 | 8 | NI | NI | 11 | 11 | [33] |
| 5b | 10 | 14 | 20 | 24 | 18 | 12 | |
| Dye No. | Treatment | UPF | ZnO % | Reference |
|---|---|---|---|---|
| Blank | 19.420 | 0 | [35] | |
| 5a | Untreated | 141.88 | 0 | |
| Pre-treated | 145.51 | 2.5 | ||
| Post-treated | 173.25 | 2.0 | ||
| 5b | Untreated | 122.37 | 0 | |
| Pre-treated | 131.55 | 2.5 | ||
| Post-treated | 190.59 | 2.5 |
| Dye No. | Treatment | ZnO% | Methyl Red Stain | Coffee Stain | Light Fastness | ||
|---|---|---|---|---|---|---|---|
| ∆E | K/S | ∆E | K/S | ||||
| untreated | 67.23 | 5.63 | 66.77 | 5.98 | 3–4 | ||
| 5a | Pre-treated | 0.5 | 61.21 | 4.36 | 57.41 | 3.45 | 5 |
| 1.0 | 53.41 | 2.84 | 52.1 | 2.78 | 2–3 | ||
| 1.5 | 53.41 | 2.73 | 57.45 | 3.45 | 2–3 | ||
| 2.0 | 51.04 | 2.37 | 54.85 | 2.82 | 3–4 | ||
| 2.5 | 53.32 | 2.72 | 58.71 | 3.61 | 3 | ||
| Post-treated | 0.5 | 60.20 | 4.03 | 54.16 | 2.88 | 3 | |
| 1.0 | 59.41 | 3.84 | 52.67 | 2.66 | 3 | ||
| 1.5 | 60.88 | 4.08 | 53.09 | 2.86 | 3 | ||
| 2.0 | 55.44 | 3.23 | 53.12 | 2.78 | 3 | ||
| 2.5 | 60.36 | 3.92 | 63.47 | 4.99 | 3 | ||
| untreated | 50.02 | 3.63 | 51.92 | 3.99 | 4 | ||
| 5b | Pre-treated | 0.5 | 52.62 | 4.15 | 54.24 | 4.27 | 5 |
| 1.0 | 54.36 | 4.37 | 58.72 | 5.05 | 5 | ||
| 1.5 | 53.29 | 4.27 | 54.85 | 4.31 | 5 | ||
| 2.0 | 52.76 | 3.76 | 54.82 | 4.19 | 5 | ||
| 2.5 | 56.23 | 3.93 | 60.71 | 4.79 | 4–5 | ||
| Post-treated | 0.5 | 54.42 | 4.31 | 51.02 | 3.96 | 5 | |
| 1.0 | 55.32 | 4.28 | 51.76 | 3.90 | 5 | ||
| 1.5 | 54.37 | 4.43 | 50.11 | 4.24 | 4 | ||
| 2.0 | 53.73 | 4.12 | 50.21 | 3.95 | 4–5 | ||
| 2.5 | 60.18 | 5.37 | 56.47 | 4.88 | 4–5 | ||
| Dye No. | ZnO% & Treatment | Inhibition Zone Diameter (Nearest mm) | Reference | ||||
|---|---|---|---|---|---|---|---|
| G+Inhibition Zone | Yeast | G-Inhibition Zone | |||||
| Bacillus subtilis | Staphylococcus aureus | Candida albicans | Escherichia coli | Klebsiellapneumoniae | |||
| 5a | Untreated | NI | NI | NI | NI | NI | [35] |
| 2.5 Pre-treatment | 11 | NI | NI | NI | NI | ||
| 2.5 Post-treatment | NI | NI | NI | NI | NI | ||
| 5b | Untreated | NI | NI | NI | NI | NI | |
| 2.5 Pre-treatment | 8 | NI | NI | NI | 10 | ||
| 2.5 Post-treatment | NI | NI | NI | NI | NI | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Etaibi, A.M.; El-Apasery, M.A. A Holistic Review of 3-Dimethylamino-1-Arylpropenones Based Disperse Dyes for Dyeing Polyester Fabrics: Synthesis, Characterization, and Antimicrobial Activities. Polymers 2024, 16, 453. https://doi.org/10.3390/polym16040453
Al-Etaibi AM, El-Apasery MA. A Holistic Review of 3-Dimethylamino-1-Arylpropenones Based Disperse Dyes for Dyeing Polyester Fabrics: Synthesis, Characterization, and Antimicrobial Activities. Polymers. 2024; 16(4):453. https://doi.org/10.3390/polym16040453
Chicago/Turabian StyleAl-Etaibi, Alya M., and Morsy Ahmed El-Apasery. 2024. "A Holistic Review of 3-Dimethylamino-1-Arylpropenones Based Disperse Dyes for Dyeing Polyester Fabrics: Synthesis, Characterization, and Antimicrobial Activities" Polymers 16, no. 4: 453. https://doi.org/10.3390/polym16040453
APA StyleAl-Etaibi, A. M., & El-Apasery, M. A. (2024). A Holistic Review of 3-Dimethylamino-1-Arylpropenones Based Disperse Dyes for Dyeing Polyester Fabrics: Synthesis, Characterization, and Antimicrobial Activities. Polymers, 16(4), 453. https://doi.org/10.3390/polym16040453






