Abstract
Particulate matters (PMs) such as PM10 and PM2.5 were collected at a bus stop and were analyzed using pyrolysis-gas chromatography/mass spectrometry to identify organic polymeric materials in them. The major pyrolysis products of the PM samples were isoprene, toluene, styrene, dipentene, and 1-alkenes. The pyrolysis products generated from the PM samples were identified using reference polymeric samples such as common rubbers (natural rubber, butadiene rubber, and styrene-butadiene rubber), common plastics (polyethylene, polypropylene, polystyrene, and poly(ethylene terephthalate)), plant-related components (bark, wood, and leaf), and bitumen. The major sources of the principal polymeric materials in the PM samples were found to be the abrasion of the tire tread and asphalt pavement, plant-related components, and lint from polyester fabric. The particles produced by the abrasion of the tire tread and asphalt pavement on the road were non-exhaustive sources, while the plant-related components and lint from polyester fabric were inflowed from the outside.
1. Introduction
Particulate matter (PM) is caused by emitting directly into the atmosphere and by converting from gaseous substances to particles by reaction with other substances in the air [1]. Primary emission sources can be divided into natural sources and anthropogenic sources. PM is generated mainly by anthropogenic factors such as vehicle emissions, industrial fuel combustion, domestic fuel burning, cooking, and biomass combustion [1,2]. PM2.5 and PM10 refer to particles with an aerodynamic diameter smaller than 2.5 and 10 μm, respectively.
According to a study on PM2.5 sources produced for 1990–2014, on average globally, 25% was transportation, 15% was industrial activity, 20% was combustion of household fuel, 22% was unspecified sources of human origin, and 18% was natural dust and sea salt [3]. Research on PM sources from 15 different sampling regions in the Republic of Korea for 2000–2017 reported that motor vehicles, secondary aerosol, combustion and industry, natural source, soil dust, biomass and field burning, and other PM sources were at 28, 34, 14, 2, 7, 11, and 4%, respectively [4].
It is known that PM in the atmosphere is composed of three major components, which are water-soluble ions (SO42−, NO3−, NH4+, and alkalis and alkaline earth metal cations), carbon (organic carbon and elemental carbon), and heavy metals [1,5]. The water-soluble ions are analyzed by mainly using ion exchange chromatography (IEC) [6,7,8,9]. The carbon components can be divided into organic carbon (OC) and elemental carbon (EC), and thermal methods are used to estimate OC and EC [10,11,12]. Trace metals have been analyzed using X-ray fluorescence, inductively coupled plasma-atomic emission spectroscopy, and inductively coupled plasma-mass spectrometry [5,12,13,14].
Besides the ionic species, OC/EC, and trace metals, PM can also contain various organic components. There can be organic materials such as polyaromatic hydrocarbons (PAHs) and microplastics in PM. PAHs have been analyzed using liquid chromatography and gas chromatography [9,15]. The analysis of microplastics in PM has been primarily focused on classification by color or shape using stereomicroscopy [16,17,18,19,20]. There are various suspended atmospheric microplastics: poly(ethylene terephthalate) (PET), polyethylene (PE), polyamide (PA), polystyrene (PS), polypropylene (PP), and so on. PET- and PA-type plastics are widely used in the textile industry, while PP can be derived from industrial products such as textiles, car fenders, and bottle caps. Plastic products can decompose when they are exposed to sunlight, and their tiny particles may be released into the air.
Tire tread wear particles (TWPs) are generated by friction between the tire tread and road surface. In general, TWPs exist as tire-road wear particles (TRWPs) in the form of TWP encrusted with various mineral particles [2]. Pyrolysis-gas chromatography/mass spectrometry (Py-GC/MS) has been widely used for the analysis of TRWPs [2,21,22,23]. The contribution levels of TRWPs to PM10 collected in France, USA, and Japan were assessed using Py-GC/MS, and it was reported that there were TRWPs of 0.14–2.80% in the PM10 samples [22]. PM2.5 samples collected near houses, parks, schools, and businesses in Europe (London), Japan (Tokyo), and US (Los Angeles) were also analyzed using Py-GC/MS, and it was reported that TRWP contents in the PM2.5 samples were 0.11–0.49%, 0.10–0.33%, and 0.10–0.68% in London, Tokyo, and Los Angeles, respectively [2].
Component analysis via pyrolytic technique has been primarily focused on microplastic analysis in environmental samples such as sediment and soil rather than atmospheric samples [24,25,26,27,28]. In this study, PM samples were collected at a bus stop, the organic polymeric components in them were analyzed using Py-GC/MS, and the kinds of polymeric components were determined using the pyrolysis products. Various polymer reference samples such as rubbers, plastics, plant matter, and bitumen were employed to identify the polymeric components in the PM samples. The kinds of polymeric materials in the PM samples were determined by comparing their principal pyrolysis products with those of the references. The sources of the polymeric components in the PM samples were investigated. Py-GC/MS is a useful method for the identification of polymeric materials via the interpretation of the pyrolysis products [29,30], and it is suitable for the analysis of trace organic compound analysis because it can be conducted with a very small sample size.
2. Materials and Methods
2.1. Materials
2.1.1. Environmental Sample
PM10 and PM2.5 were collected at a bus stop near Sejong University, Republic of Korea (37°32′58.8″ N 127°04′32.3″ E). The PM10 sample was collected in October 2020 (for 12 h), and the PM2.5 samples were collected in November 2020 (PM2.5(1), for 14 h), February 2021 (PM2.5(2), for 24 h), May 2021 (PM2.5(3), for 24 h), and August 2021 (PM2.5(4), for 24 h). The PM sampling was carried out using a low volume particulate sampler of KMS-4200 (Kemik Co., Seoul, Korea) and a filter with a diameter of 47 mm.
Road dust that accumulated between the curb and road at the bus stop was collected. It was collected by sweeping with a broom and was separated by size using a sieve shaker from Octagon 200 (Endecotts Co., London, UK). TRWP and asphalt wear particles (AWP) were selected using an image analyzer (EGVM 35B, EG Tech. Co., Seoul, Korea).
2.1.2. Reference Samples
SMR CV60, BR01, and SBR1502 were used for NR, BR, and SBR as the reference rubber samples, respectively. Oak tree components of bark, wood, and leaf were employed as the reference plant-related component (PRC) samples. The PRC samples were obtained in Achasan, located in Gwangjin-gu (Korea), where there is no contamination by anthropogenic factors. PE, PP, PS, and PET were used as the reference plastic samples, and they were purchased from Sigma-Aldrich Co. (St. Louis, MI, USA). Bitumen, used as a binder of asphalt pavement, was also employed as the reference sample.
2.2. Pyrolysis-Gas Chromatography/Mass Spectrometry (Py-GC/MS)
Py-GC/MS analysis was carried out using a JCI-55 Curie-point pyrolyzer (Japan Analytical Industry Co., Tokyo, Japan) coupled to an Agilent 6890 gas chromatograph equipped with a 5973 mass spectrometer (Agilent Techonology Inc., Santa Clara, CA, USA). All samples were pyrolyzed using a pyrofoil of 590 °C Curie temperature for 10 s under Helium (He) atmosphere. The pyrolysis products were separated through GC, and each separated peak was identified by interpreting its mass spectrum. A DB-5MS capillary column (30 m × 0.32 mm, 0.25 μm film thickness, Agilent Technology Inc., Santa Clara, CA, USA) was used. The injector temperature was 250 °C. Two GC oven temperature programs were used as follows: (1) 30 °C (held for 3 min) to 160 °C (held for 1 min) at 8 °C/min, then to 250 °C (held for 3 min) at 10 °C/min; and (2) 30 °C (held for 3 min) to 50 °C (held for 3 min) at 10 °C/min, then to 180 °C (held for 1 min) at 10 °C/min and raised up to 250 °C (held for 3 min) at 10 °C/min again. The interface temperature of GC to MS was 250 °C. The electron ionization (70 eV) was used to ionize the pyrolysis products. The MS source temperature was 230 °C.
3. Results and Discussion
3.1. Py-GC/MS Analysis of the PM Samples
Figure 1 and Figure 2 show Py-GC/MS chromatograms of the PM10 and PM2.5 samples, respectively. Their major pyrolysis products are summarized in Table 1 and Table 2, respectively. The common pyrolysis products detected from the five PM samples were isoprene, toluene, styrene, dipentene, and 1-alkenes. Compared to the PM10 sample, there were some different pyrolysis products such as furfural, acetophenone, 4-methylphenol, and benzoic acid in the PM2.5 sample. The kinds and abundances of pyrolysis products of the four PM2.5 samples were different from each other. The major pyrolysis products of the PM2.5(1) sample were nearly the same as those of the PM10 sample, whereas the other PM2.5 samples showed some different pyrolysis products, as listed in Table 2. Furfural was detected in the PM2.5(2) and PM2.5(3) samples. Acetophenone and benzoic acid were not observed in the PM2.5(1) sample. The composition of the PM sample should be dependent on the sampling time and weather. The kinds of polymeric materials to generate the principal pyrolysis products were analyzed in comparison with the reference samples of rubbers, plastics, PRCs, and bitumen.
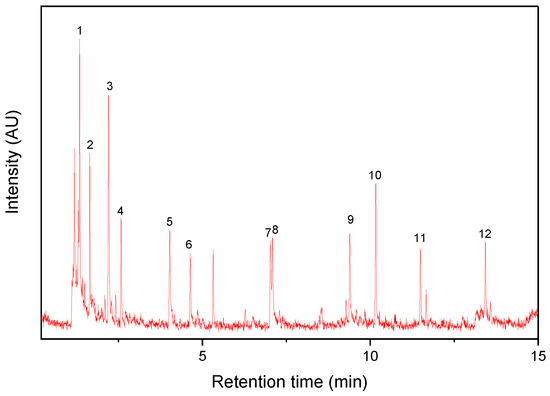
Figure 1.
Py-GC/MS chromatogram of the PM10 sample.
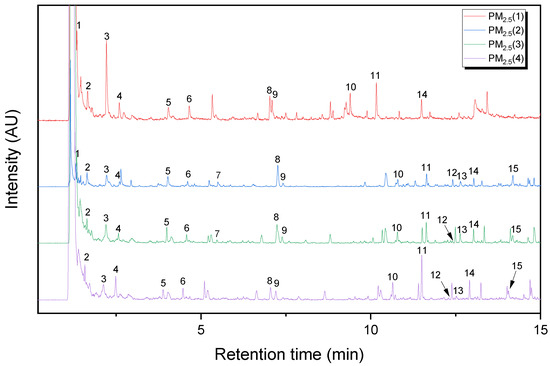
Figure 2.
Py-GC/MS chromatograms of the PM2.5(1), PM2.5(2), PM2.5(3), and PM2.5(4) samples.

Table 1.
Peak areas and relative intensity ratios (the reference: 1-undecene) of the major pyrolysis products detected in the PM10 sample.

Table 2.
Peak areas and relative intensity ratios (the reference: 1-undecene) of the major pyrolysis products detected in the PM2.5 samples.
3.2. Py-GC/MS Analysis of the Reference Samples
In order to identify the kinds of polymeric materials in the PM samples, principal pyrolysis products of the reference samples such as NR, BR, SBR, PE, PP, PS, PET, bitumen, and PRCs (bark, wood, and leaf) were analyzed. They were pyrolyzed under the same conditions as the PM samples, and their major pyrolysis products were assigned. The principal pyrolysis products of the rubbers (NR, BR, and SBR), plastics (PE, PP, PS, and PET), and bitumen are listed in Table 3, while those of the PRCs are summarized in Table 4.

Table 3.
Principal pyrolysis products of the reference organic polymeric samples.

Table 4.
Relative intensity ratios (the reference: 2-methoxy-4-methylphenol) of principal pyrolysis products produced from the oak tree components.
The principal pyrolysis products of NR were isoprene and dipentene, corresponding to the monomer and dimer of the repeat unit (isoprene), respectively, because NR is a polymer of isoprene, and the key pyrolysis products of isoprene and dipentene are the monomer and dimer of the repeat unit [31,32,33]. The isoprene and dipentene were detected as main pyrolysis products in the PM samples. This implies that there was an NR component in the PM samples. NR should originate from tire tread wear particles. In general, bus tire treads are mainly made of NR [34,35,36,37,38]. Hence, the source of the NR should be bus tire tread wear. Abrasion of tire tread occurs mainly during the start and stop of a vehicle rather than during stable driving. The principal pyrolysis products of BR are 1,3-butadiene and 4-vinylcyclohexene (VCH, dimer of 1,3-butadiene), but there was no VCH in the PM samples. This indicates that there was no BR component in the PM samples. There are various principal pyrolysis products of SBR, such as 1,3-butadiene, VCH, styrene, 2-phenylpropene (2-PP), 3-phenylcyclopentene (3-PCP), and 4-phenylcyclohexene (4-PCH) [39,40,41,42]. If there were SBR components in the PM samples, some of them must have been detected. Styrene was clearly observed in the Py-GC/MS chromatograms of the PM samples, but most of the others were not detected. This implies that there was no SBR component in the PM samples and that the source of the styrene was not SBR.
It is well known that the principal pyrolysis products of PE are alkanes, 1-alkenes, and alkadienes [26]. In Py-GC/MS chromatograms of the PM samples, 1-alkenes were clearly detected, but alkanes and alkadienes were rarely observed. Hence, the source of 1-alkenes detected in the PM samples was not PE. 2-Methyl-1-pentene, 2,4-dimethyl-1-heptene, and 2,4,6-trimethyl-1-nonene are typical pyrolysis products of PP, but they were not detected in the PM samples. This indicates that there was no PP component in the PM samples. Styrene is one of the major pyrolysis products of PS, and it was observed in the PM samples, but 2-PP and biphenyl, as the major pyrolysis products of PS, were not detected in the PM samples. Thus, PS was not the source of styrene in the PM samples. Acetophenone and benzoic acid were observed in the PM2.5(2) and PM2.5(3) samples, and they are principal pyrolysis products of PET [43,44]. This implies that there was a PET component in the PM2.5(2) and PM2.5(3) samples.
Bitumen is widely used as a binder of asphalt pavement. The vomponents of bitumen vary widely, and they are different according to the suppliers. The principal pyrolysis products of bitumen were 1,3-butadiene, styrene, phenol, 1-ethenyl-4-methylbenzene, indene, 1-undecene, 1-dodecene, and 4-(1-methylethyl)-phenol, as listed in Table 3. Among them, the pyrolysis products that matched with the PM samples were styrene and 1-alkenes such as 1-undecene and 1-dodecene. Hence, it can be concluded that one of the styrene sources should be bitumen and that one of the 1-alkene sources should also be bitumen.
The principal pyrolysis products of the PRCs varied according to the kinds of components, such as bark, wood, and leaf, as listed in Table 4. The kinds of principal pyrolysis products of the two leaf samples were nearly the same, but the relative abundances were different from each other. One of the key pyrolysis products of PRCs is furfural produced from cellulose [45,46]. Furfural was abundantly observed in all the PRC samples. 1-Alkenes such as 1-undecene and 1-dodecene were also detected in the PRC samples. 1-Alkenes should be pyrolysis products of waxes, and it is known that there is plant epicuticular wax in the surface layer [47,48]. 1-Alkenes were observed in all the Py-GC/MS chromatograms of the PM samples, and furfural was detected in those of the PM2.5(2) and PM2.5(3) ones. Since furfural is the pyrolysis product of the wood component, the analytical results indicate that the PM2.5(2) and PM2.5(3) samples contained the wood component. The PM samples might contain some bark or leaf component because plant epicuticular wax generally exists in the surface layer of bark and leaf.
3.3. Py-GC/MS Analysis of the TRWP and AWP Collected near the Bus Stop
Single TRWP and AWP collected near the bus stop were pyrolyzed, and their Py-GC/MS chromatograms are shown in Figure 3 and Figure 4, respectively. The TRWP chromatogram clearly showed the key pyrolysis products of NR (isoprene and dipentene), and styrene was also observed, but VCH was not detected. If the TRWP was a debris of an SBR or NR/BR compound, the chromatogram must show VCH as one of the major pyrolysis products because VCH is the key pyrolysis product of BR and SBR. Hence, the TRWP was a particle of an NR compound, and it should come from the abrasion of bus tire tread composed of NR. Thus, it can be concluded that the isoprene and dipentene detected in the PM samples originated from abrasion of bus tire tread.
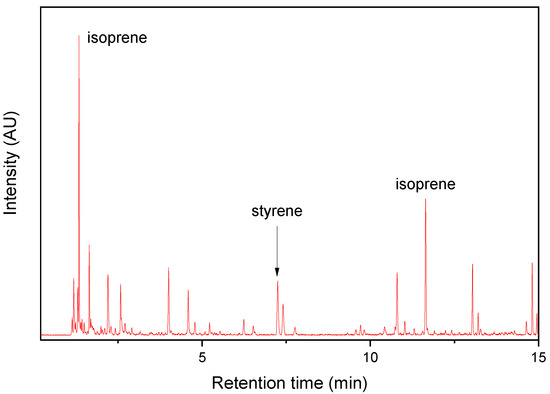
Figure 3.
Py-GC/MS chromatogram of the TRWP collected near the PM sampling site.
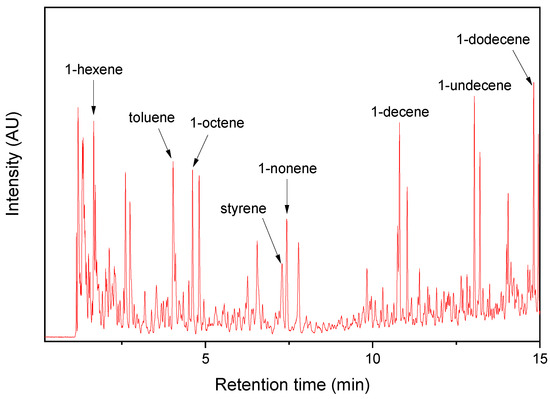
Figure 4.
Py-GC/MS chromatogram of the AWP collected near the PM sampling site.
The Py-GC/MS chromatogram of the AWP showed various pyrolysis products (Figure 4). The major pyrolysis products were 1-alkenes, toluene, and styrene. The 1-alkenes detected in the PM samples should come from the AWPs. Since styrene was also one of the major pyrolysis products of the AWP, its detection in the TRWP should come from the AWP. During the abrasion process of the bus tire tread, friction between the tire tread and asphalt pavement occurs, and a few small AWPs can be attached to the TWP surface.
3.4. Sources of the Organic Polymeric Components in the PM Samples
The sources of the organic polymeric components in the PM10 sample are summarized in Figure 5. Isoprene and dipentene are the key pyrolysis products of NR, which is the main component of bus tire tread compounds. Hence, isoprene and dipentene originated from TWPs of buses. PRCs could also produce isoprene and dipentene as pyrolysis products, but their abundances were very small, so the contribution level of the PRCs in producing isoprene and dipentene could be negligible. Styrene was one of the major pyrolysis products formed from AWP, and it was also one of the main pyrolysis products of PS and SBR. However, styrene dimer and trimer and VCH were not detected in the PM samples. Thus, styrene should originate from AWP. 1-Alkenes are key pyrolysis products of wax and one of the major pyrolysis products of bitumen. PRCs also have epicuticular wax. Hence, the sources of 1-alkenes should be PRCs and AWP.
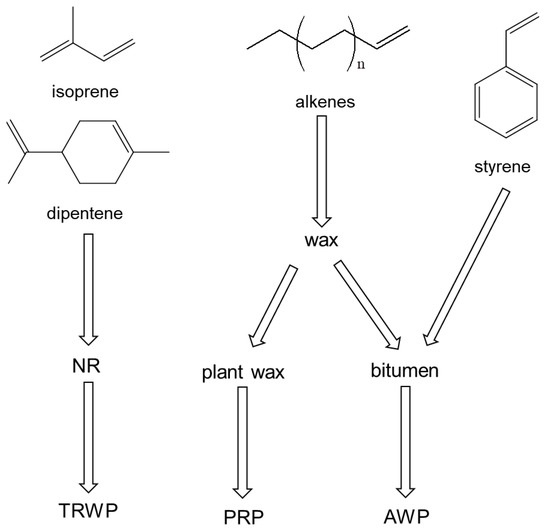
Figure 5.
Sources of the principal pyrolysis products obtained from the PM10 sample.
The sources of the organic polymeric components in the PM2.5 samples are described in Figure 6. The PM2.5 samples also showed isoprene, dipentene, styrene, and 1-alkenes as the pyrolysis products. Hence, like the PM10 sample, the PM2.5 samples contained TWPs, AWPs, and PRCs. Additionally, furfural, acetophenone, and benzoic acid were observed in the PM2.5 samples. Acetophenone and benzoic acid are major pyrolysis products of PET, which can originate from the lint of polyester (PET) fabric/clothes. The detection of furfural can provide evidence that the sample contains a wood component.
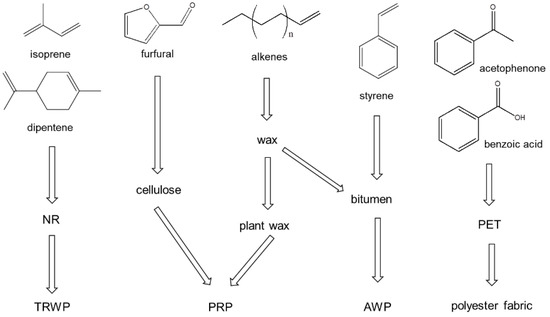
Figure 6.
Sources of the principal pyrolysis products obtained from the PM2.5 samples.
Figure 7 shows the kinds of organic polymeric materials in the PM samples collected at the bus stop, and the key pyrolysis products for identifying the kinds of polymeric materials by using Py-GC/MS. In summary, there are TRWPs, AWPs, PRCs, and polyester fabric (lint of clothes) as PM near bus stops on asphalt pavement roads. The kinds and abundances of polymeric components in the PM samples can be dependent on the sampling site, time, road conditions, and traffic volumes.
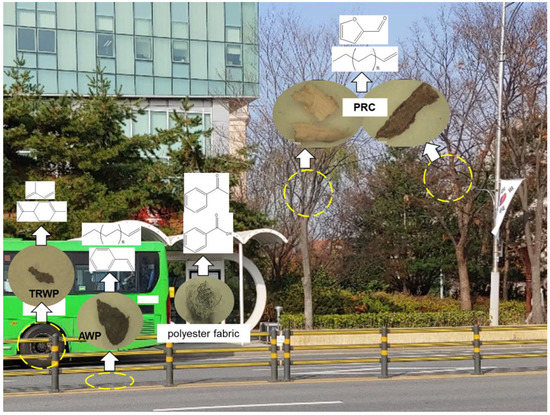
Figure 7.
PM sources produced near the bus stop, and the key pyrolysis products for identifying polymeric materials using Py-GC/MS. The yellow circles indicate potential PM sources.
4. Conclusions
The kinds of polymeric components in PM samples were identified by interpreting their pyrolysis products. In order to clearly identify the kinds of polymeric materials in the PM samples, reference samples such as NR, BR, SBR, PE, PP, PS, PET, bitumen, and PRCs (bark, wood, and leaf) were employed, and their pyrolysis products were analyzed. The major pyrolysis products generated from the PM samples were isoprene, toluene, styrene, dipentene, and 1-alkenes. The main polymeric components found in the PM samples were wear particles of tire tread and asphalt pavement, plant-related components, and the lint of polyester fabric. Compared to the PM10 sample, there were some different pyrolysis products such as furfural, acetophenone, 4-methylphenol, and benzoic acid in the PM2.5 samples. Furfural is one of the major pyrolysis products of plant-related components, while acetophenone and benzoic acid are the principal pyrolysis products of PET. The kinds and abundances of polymeric components in PM samples can be dependent on the sampling site, time, road conditions, and traffic volumes. The analysis of various polymeric components in PM samples can be useful for understanding various sources of PM.
Author Contributions
E.C.: sample preparation, formal analysis, data curation, visualization, writing—first draft. S.-S.C.: resources, supervision, writing—original draft & editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Technology Innovation Program funded by the Ministry of Trade, Industry and Energy, Republic of Korea (Project Number 20003587).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhang, R.; Wang, G.; Guo, S.; Zamora, M.L.; Ying, Q.; King, Y.; Wang, W.; Hu, M.; Wang, Y. Formation of urban fine particulate matter. Chem. Rev. 2015, 115, 3803–3855. [Google Scholar] [CrossRef] [PubMed]
- Panko, J.M.; Hitchcock, K.M.; Fuller, G.W.; Green, D. Evaluation of tire wear contribution to PM2.5 in urban environment. Atmosphere 2019, 10, 99. [Google Scholar] [CrossRef]
- Karagulian, F.; Belis, C.A.; Dora, C.F.C.; Prüss-Ustün, A.M.; Bonjour, S.; Adair-Rohani, H.; Amann, M. Contributions to cities’ ambient particulate matter (PM): A systematic review of local source contributions at global level. Atmos. Environ. 2015, 120, 475–483. [Google Scholar] [CrossRef]
- Ryou, H.G.; Heo, J.; Kim, S.-Y. Source apportionment of PM10 and PM2.5 air pollution, and possible impacts of study characteristics in South Korea. Environ. Pollut. 2018, 240, 963–972. [Google Scholar] [CrossRef]
- Park, S.-M.; Song, I.-H.; Park, J.S.; Oh, J.; Moon, K.J.; Shin, H.J.; Ahn, J.Y.; Lee, M.-D.; Kim, J.; Lee, G. Variation of PM2.5 chemical compositions and their contributions to light extinction in Seoul. Aerosol Air Qual. Res. 2018, 18, 2220–2229. [Google Scholar] [CrossRef]
- Sun., J.; Qin, D.; Mayeswski, P.A.; Dibb, J.E.; Whitlow, S.; Li, Z.; Yang, Q. Soluble species in aerosol and snow and their relationship at Glacier 1, Tien Shan, China. J. Geophys. Res. Atmos. 1998, 103, 28021–28028. [Google Scholar] [CrossRef]
- Zhao., S.; Li, Z.; Zhou, P. Ion chemistry and individual particle analysis of atmospheric aerosols over Mt. Bogda of eastern Tianshan Mountains, Central Asia. Environ. Monit. Assess. 2011, 180, 409–426. [Google Scholar] [CrossRef]
- Cheng, Y.; Lee, S.; Gu, Z.; Ho, K.; Zhang, Y.; Huang, Y.; Chow, J.C.; Watson, J.G.; Cao, J.; Chang, R. PM2.5 and PM10-2.5 chemical composition and source apportionment near a Hong Kong roadway. Particuology 2015, 18, 96–104. [Google Scholar] [CrossRef]
- Jancsek-Turóczi, B.; Hoffer, A.; Nyírő-Kósa, I.; Gelencsér, A. Sampling and characterization of resuspended and respirable. J. Aerosol Sci. 2013, 65, 69–76. [Google Scholar] [CrossRef][Green Version]
- Li, W.; Bai, Z. Characteristics of organic and elemental carbon in atmospheric fine particles in Tianjin, China. Particuology 2009, 7, 432–437. [Google Scholar] [CrossRef]
- Watson, J.G.; Chow, J.C.; Antony Chen, L.-W. Summary of organic and elemental carbon/black carbon analysis methods and intercomparisons. Aerosol Air Qual. Res. 2005, 5, 65–102. [Google Scholar] [CrossRef]
- Schaap, M.; Weijers, E.P.; Mooibroek, D.; Nguyen, L.; Hoogerbrugge, R. Composition and Origin of Particulate Matter in the Netherlands; Netherlands Environmental Assessment Agency: The Hague, The Netherlands, 2010. [Google Scholar]
- Querol, X.; Alastuey, A.; Rodriguez, S.; Plana, F.; Mantilla, E.; Ruiz, C.R. Monitoring of PM10 and PM2.5 around primary particulate anthropogenic emission sources. Atmos. Environ. 2001, 35, 845–858. [Google Scholar] [CrossRef]
- Zhou, X.; Li, Z.; Zhang, T.; Wang, F.; Tao, Y.; Zhang, X.; Wang, F.; Huang, J.; Cheng, T.; Jiang, H.; et al. Chemical nature and predominant sources of PM10 and PM2.5 from multiple sites on the Silk Road, Northwest China. Atmos. Pollut. Res. 2021, 12, 425–436. [Google Scholar] [CrossRef]
- Martellini, T.; Giannoni, M.; Lepri, L.; Katsoyiannis, A.; Cincinelli, A. One year intensive PM2.5 bound polycyclic aromatic hydrocarbons monitoring in the area of Tuscany, Italy. Concentrations, source understanding and implications. Environ. Pollut. 2012, 164, 252–258. [Google Scholar] [CrossRef]
- Akhbarizadeh, R.; Dobaradaran, S.; Torkmahalleh, M.A.; Saeedi, R.; Aibaghi, R.; Ghasemi, F.F. Suspended fine particulate matter (PM2.5), microplastics (MPs), and polycyclic aromatic hydrocarbons (PAHs) in air: Their possible relationships and health implication. Environ. Res. 2021, 192, 110339–110351. [Google Scholar] [CrossRef] [PubMed]
- Narmadha, V.V.; Jose, J.; Patil, S.; Farooqui, M.O.; Srimuruganandam, B.; Saravanadevi, S.; Krishnamurthi, K. Assessment of microplastics in roadside suspended dust from urban and rural environment of Nagpur, India. Int. J. Environ. Res. 2020, 14, 629–640. [Google Scholar] [CrossRef]
- Gaston, E.; Woo, M.; Steele, C.; Sukumaran, S.; Anderson, S. Microplastics differ between indoor and outdoor air masses: Insights from multiple microscopy methodologies. Appl. Spectrosc. 2020, 74, 1079–1098. [Google Scholar] [CrossRef]
- Liu, K.; Wang, X.; Fang, T.; Xu, P.; Zhu, L.; Li, D. Source and potential risk assessment of suspended atmospheric microplastics in Shanghai. Sci. Total Environ. 2019, 675, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, S.; Keshavarzi, B.; Moore, F.; Turner, A.; Kelly, F.J.; Dominguez, A.O.; Jaafarzadeh, N. Distribution and potential health impacts of microplastics and microrubbers in air and street dusts from Asaluyeh Country, Iran. Environ. Pollut. 2019, 244, 153–164. [Google Scholar] [CrossRef]
- Unice, K.M.; Kreider, M.L.; Panko, J.M. Use of deuterated internal standard with pyrolysis-GC/MS dimeric marker analysis to quantify tire tread particles in the environment. Int. J. Environ. Res. Public Health 2012, 9, 4033–4055. [Google Scholar] [CrossRef]
- Panko, J.M.; Chu, J.; Kreider, M.L.; Unice, K.M. Measurement of airborne concentrations of tire and road wear particles in urban and rural areas of France, Japan, and the United States. Atmos. Environ. 2013, 72, 192–199. [Google Scholar] [CrossRef]
- ISO/TS 20593; Ambient Air-Determination of the Mass Concentration of Tire and Road Wear Particles (TRWP)-Pyrolysis-GC-MS Method.
- Dierkes, G.; Lauschke, T.; Becher, S.; Schumacher, H.; Földi, C.; Ternes, T. Quantification of microplastics in environmental samples via pressurized liquid extraction and pyrolysis-gas chromatography. Anal. Bioanal. Chem. 2019, 411, 6959–6968. [Google Scholar] [CrossRef] [PubMed]
- Eisentraut, P.; Dümichen, E.; Ruhl, A.S.; Jekel, M.; Albrecht, M.; Gehde, M.; Braun, U. Two birds with one stone-fast and simultaneous analysis of microplastics: Microparticles derived from thermoplastics and tire wear. Environ. Sci. Technol. Lett. 2018, 5, 608–613. [Google Scholar] [CrossRef]
- Steinmetz, Z.; Kintzi, A.; Muñoz, K.; Schaumann, G.E. A simple method for the selective quantification of polyethylene, polypropylene, and polystyrene plastic debris in soil by pyrolysis-gas chromatography/mass spectrometry. J. Anal. Appl. Pyrolysis 2020, 147, 104803. [Google Scholar] [CrossRef]
- David, J.; Steinmetz, Z.; Kučerik, J.; Schaumann, G.E. Quantitative analysis of poly (ethylene terephthalate) microplastics in soil via thermogravimetry-mass spectrometry. Anal. Chem. 2018, 90, 8793–8799. [Google Scholar] [CrossRef]
- Fischer, M.; Scholz-Bottcher, B.M. Microplastics analysis in environmental samples-recent pyrolysis-gas chromatography-mass spectrometry method improvements to increase the reliability of mass-related data. Anal. Methods 2019, 11, 2489–2497. [Google Scholar] [CrossRef]
- Bower, N.W.; Blanchet, C.J.K. Analytical pyrolysis-chromatography: Something old, something new. J. Chem. Educ. 2010, 87, 467–469. [Google Scholar] [CrossRef]
- Buco, S.; Moragues, M.; Doumenq, P.; Noor, A.; Mille, G. Analysis of polycyclic aromatic hydrocarbons in contaminated soil by Curie point pyrolysis coupled to gas chromatography-mass spectrometry, an alternative to conventional methods. J. Chromatogr. A 2004, 1026, 223–229. [Google Scholar] [CrossRef]
- Choi, S.-S.; Ko, J.-E. Influence of thermal aging on pyrolysis pattern of carbon black-filled NR composite. Macromol. Res. 2007, 15, 482–485. [Google Scholar] [CrossRef]
- Choi, S.-S. Correlation of crosslink density with pyrolysis pattern of natural rubber vulcanizates with efficient vulcanizing cure system. J. Anal. Appl. Pyrolysis 1999, 52, 105–112. [Google Scholar] [CrossRef]
- Januszewicz, K.; Kazimierski, P.; Suchocki, T.; Kardas, D.; Lewandowski, W.; Klugmann-Radziemska, E.; Łuczak, J. Waste rubber pyrolysis: Product yields and limonene concentration. Materials 2020, 13, 4435. [Google Scholar] [CrossRef] [PubMed]
- Son, C.E.; Yang, S.R.; Choi, S.-S. Abrasion behaviors of NR/BR compounds using laboratory abrasion tester. Elast. Compos. 2021, 56, 12–19. [Google Scholar]
- Chae, E.; Jung, U.; Choi, S.-S. Quantification of tire tread wear particles in microparticles produced on the road using oleamide as a novel marker. Environ. Pollut. 2021, 288, 117811. [Google Scholar] [CrossRef]
- Choi, S.-S.; Yang, S.R.; Chae, E.; Son, C.E. Influence of carbon black contents and rubber compositions on formation of wear debris of rubber vulcanizates. Elast. Compos. 2020, 55, 108–113. [Google Scholar]
- Miyazaki, T. Rubber Composition. European Patent EP3459996, 27 March 2019. [Google Scholar]
- Ghoreishy, M.H.R.; Alimardani, M.; Mehrabian, R.Z.; Gangali, S.T. Modeling the hyperviscoelastic behavior of a tire tread compound reinforced by silica and carbon black. J. Appl. Polym. Sci. 2013, 128, 1725–1731. [Google Scholar]
- Yang, Q.; Yu, S.; Zhong, H.; Liu, T.; Yao, E.; Zhang, Y.; Zou, H.; Du, W. Gas products generation mechanism during co-pyrolysis of styrene-butadiene rubber and natural rubber. J. Hazard. Mater. 2021, 401, 12330. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-S.; Kwon, H.-M. Analytical method for determination of microstructures of solution styrene-butadiene copolymers using 2-phenylpropene/styrene ratio of pyrolysis products. Polym. Test. 2015, 44, 153–159. [Google Scholar] [CrossRef]
- Choi, S.-S.; Kwon, H.-M. Characterization of pyrolysis products formed from styrene-1,2-unit heterosequence of styrene-butadiene copolymer. J. Anal. Appl. Pyrolysis 2013, 99, 1–8. [Google Scholar] [CrossRef]
- Ghebremeskel, G.N.; Sekinger, J.K.; Hoffpaiir, J.L.; Hendrix, C. A study of the thermal degradation products of styrene-butadiene type rubber by pyrolysis/GC/MS. Rubber Chem. Technol. 1996, 69, 874–884. [Google Scholar] [CrossRef]
- Dhahak, A.; Hild, G.; Rouaud, M.; Mauviel, G.; Burkle-Vitzthum, V. Slow pyrolysis of polyethylene terephthalate: Online monitoring of gas production and quantitative analysis of waxy products. J. Anal. Appl. Pyrolysis 2019, 142, 104664. [Google Scholar] [CrossRef]
- Yoshioka, T.; Grause, G.; Eger, C.; Kaminsky, W.; Okuwaki, A. Pyrolysis of poly(ethylene terephthalate) in a fluidised bed plant. Polym. Deg. Stab. 2004, 86, 499–504. [Google Scholar] [CrossRef]
- Choi, S.-S.; Kim, M.-C.; Kim, Y.-K. Influence of silica on formation of levoglucosan from carbohydrates by pyrolysis. J. Anal. Appl. Pyrolysis 2011, 90, 56–62. [Google Scholar] [CrossRef]
- Choi, S.-S.; Kim, M.-C.; Kim, Y.-K. Formation of methoxybenzenes from cellulose in the presence of tetramethylammonium hydroxide by pyrolysis. Bull. Korean Chem. Soc. 2013, 34, 649–652. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, P.; Xu, Z.-X.; He, Z.-X.; Wang, Q. Bio-fuel oil characteristic of rice bran wax pyrolysis. Renew. Energy 2018, 119, 193–202. [Google Scholar] [CrossRef]
- Weber, J.; Schwark, L. Epicuticular wax lipid composition of endemic European Betula species in a simulated ontogenetic/diagenetic continuum and its application to chemotaxonomy and paleobotany. Sci. Total Environ. 2020, 730, 138324. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).