Heat Stability and Icing Delay on Superhydrophobic Coatings in Facile One Step
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Preparation
2.2.1. Q235 Steel Substrate Pretreatment
2.2.2. Different Amount of SiO2 and Modification Time
2.2.3. Different Amount of ZnO
2.2.4. Different Amount of SiO2 and ZnO
2.2.5. Heating Temperature
2.3. Characterization
3. Results and Discussion
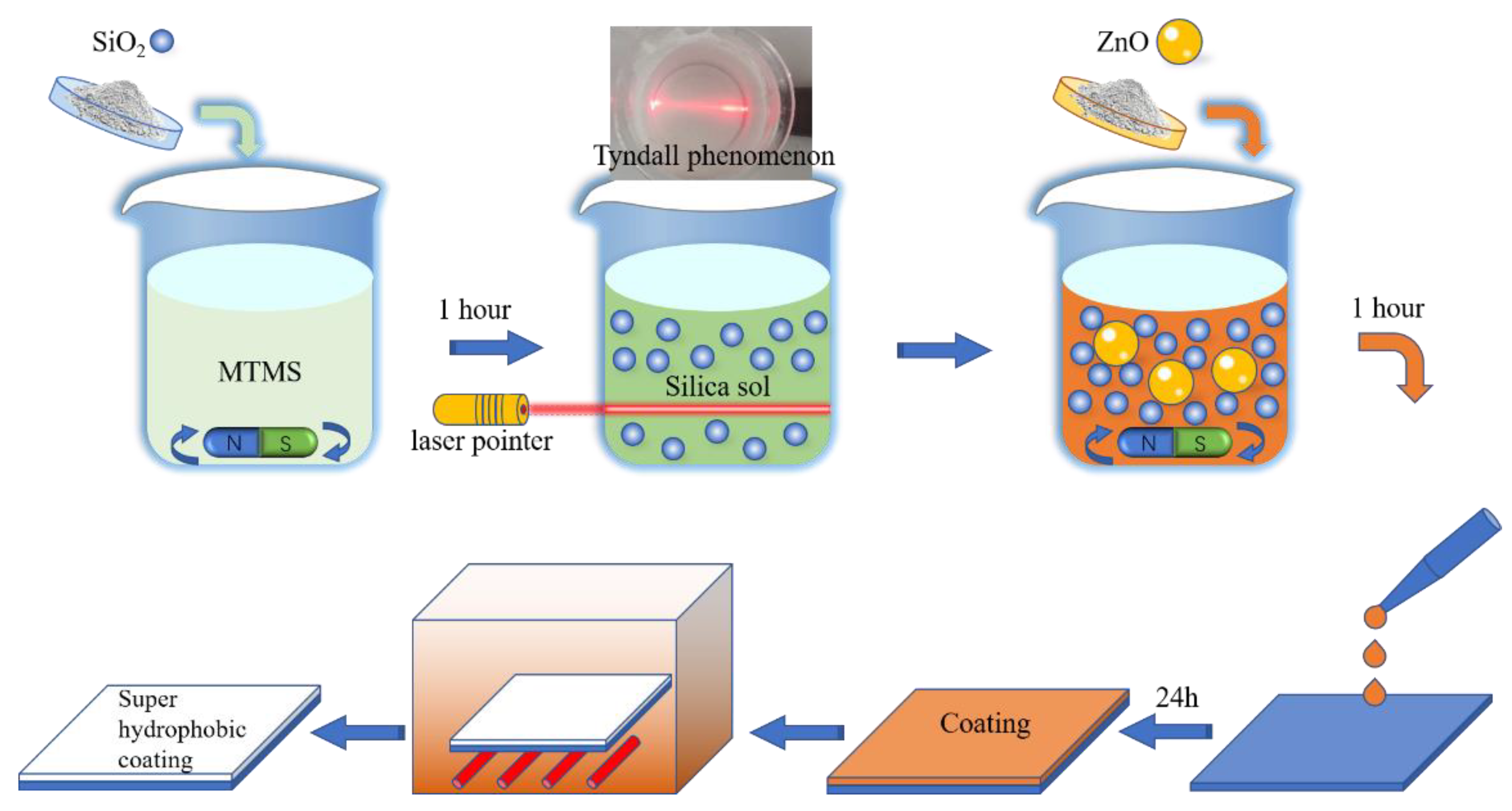
3.1. Processing
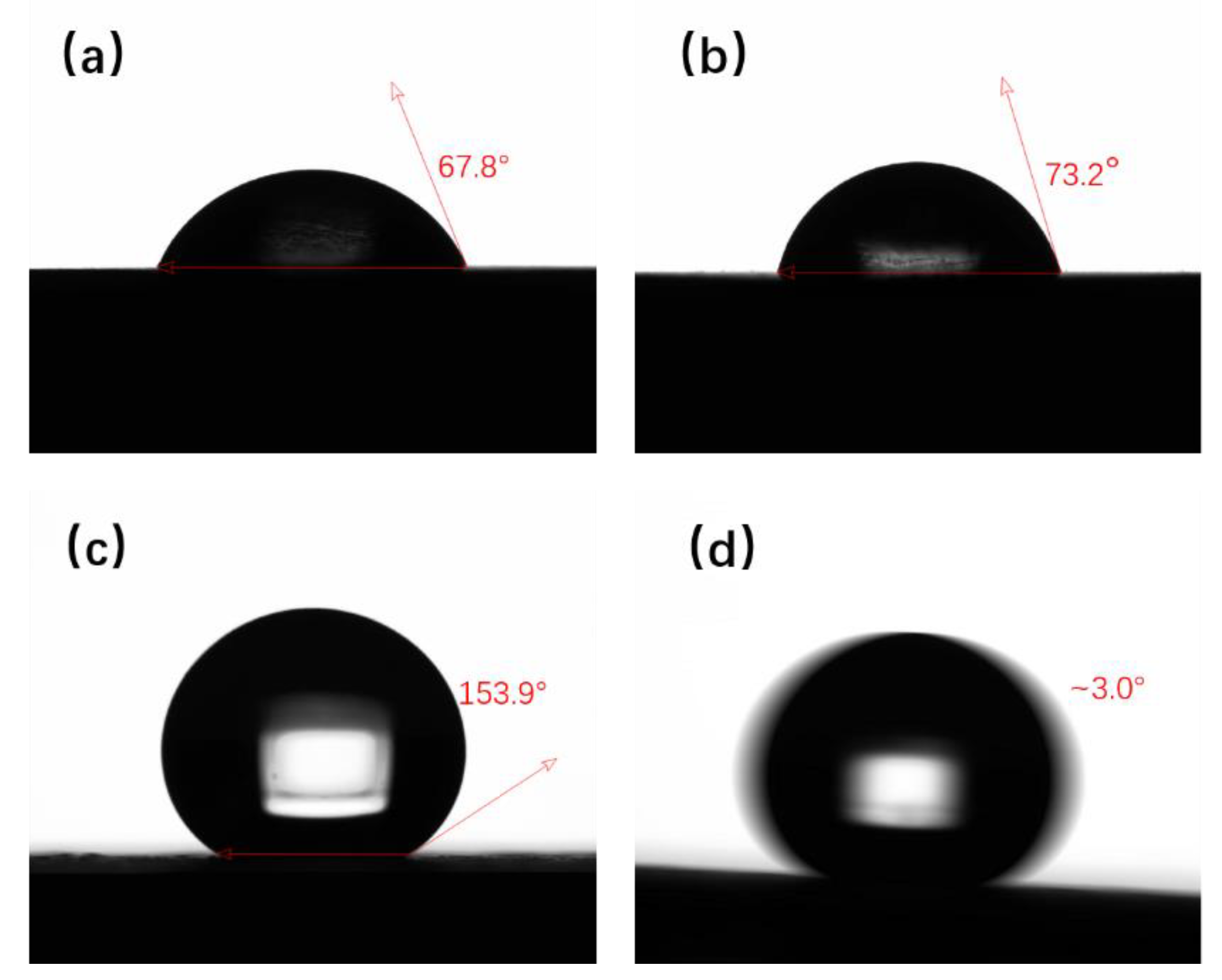
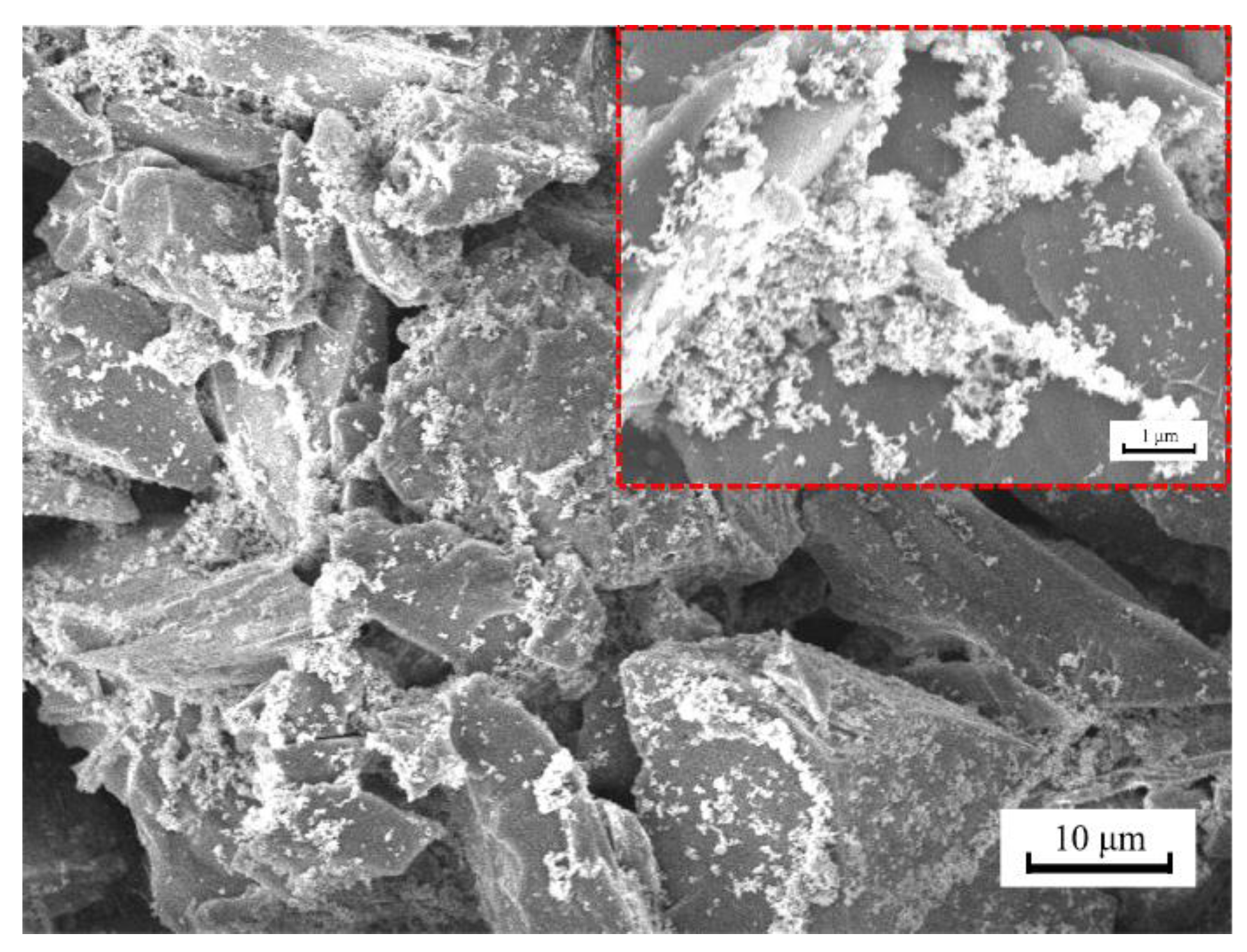
3.2. Superhydrophobicity and Morphology
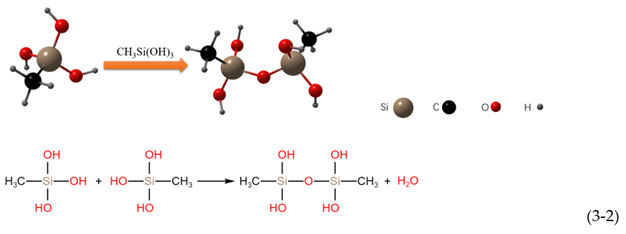
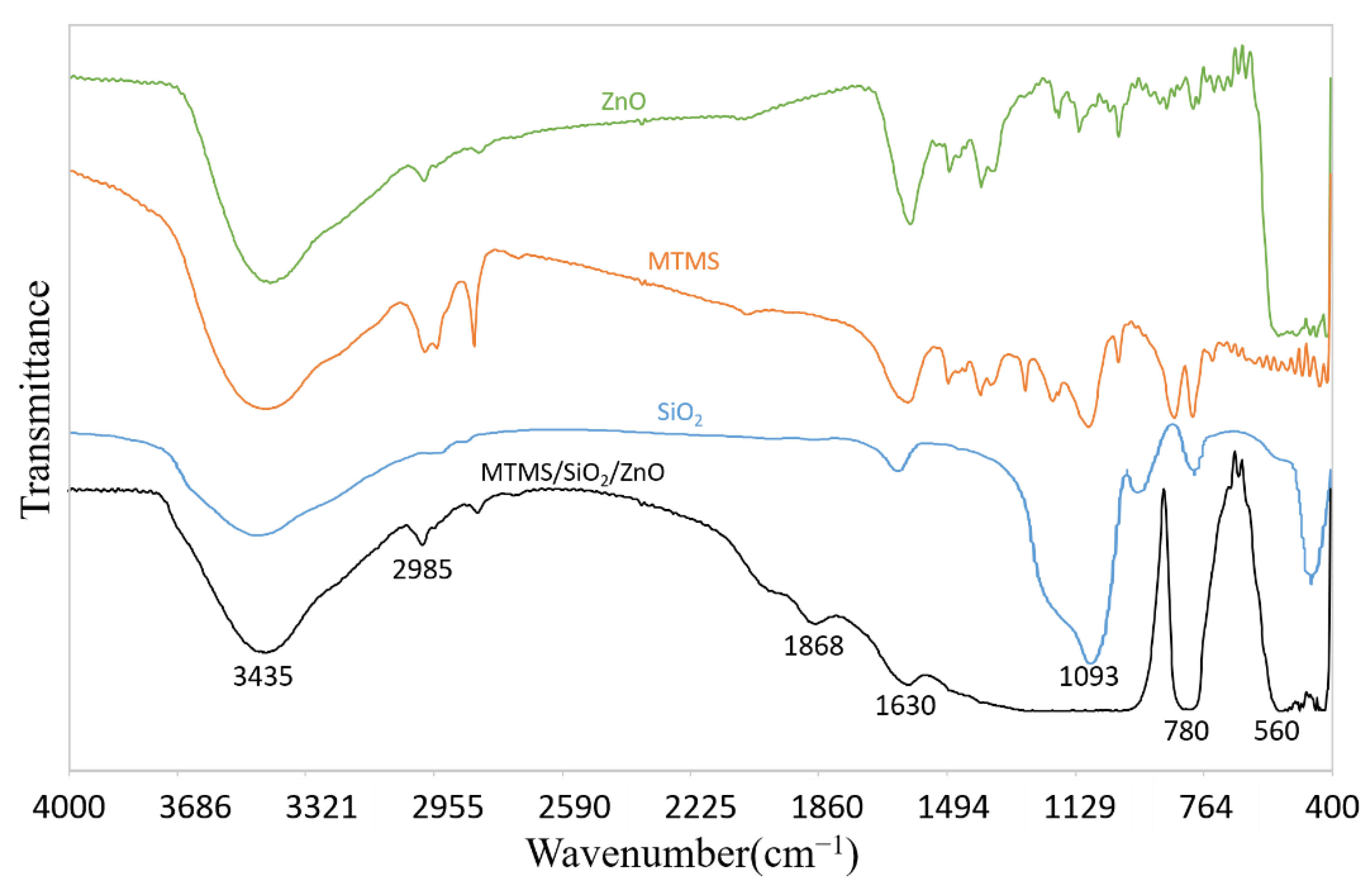
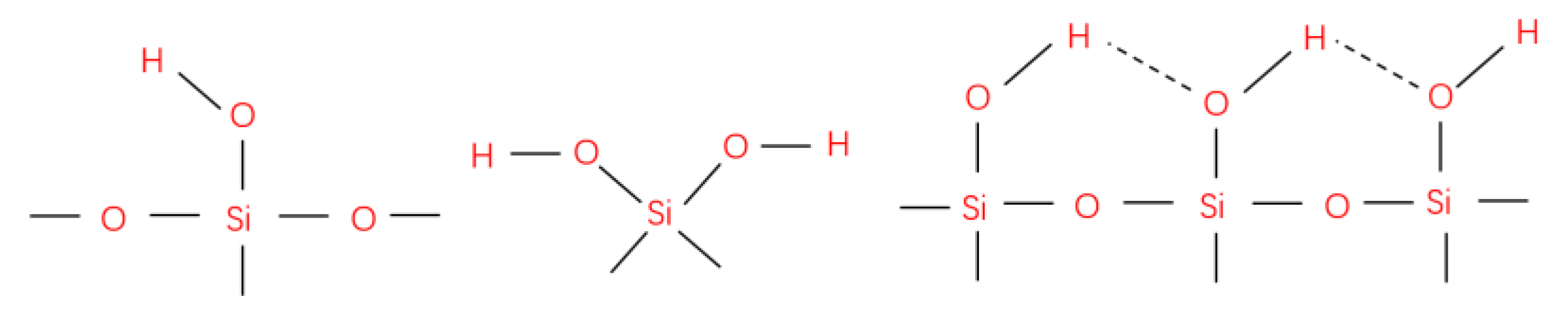
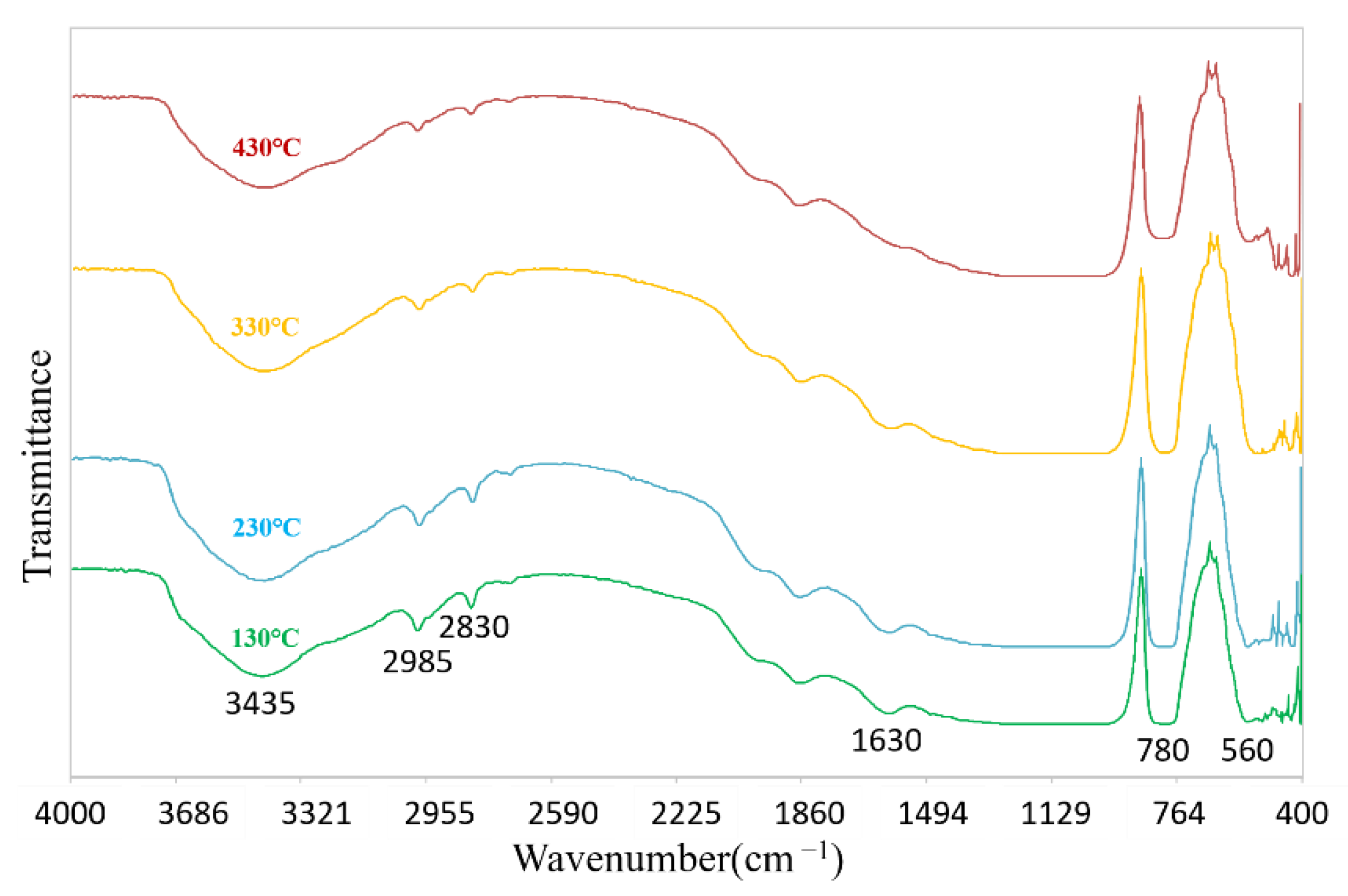
3.3. Chemical Composition of Superhydrophobic Coatings
- Hydrolysis:
- Water condensation:
- Alcohol condensation:
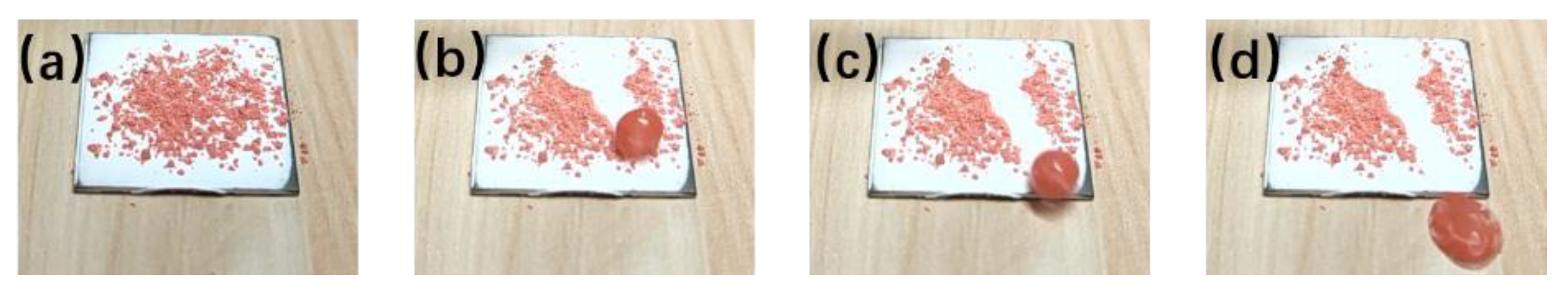
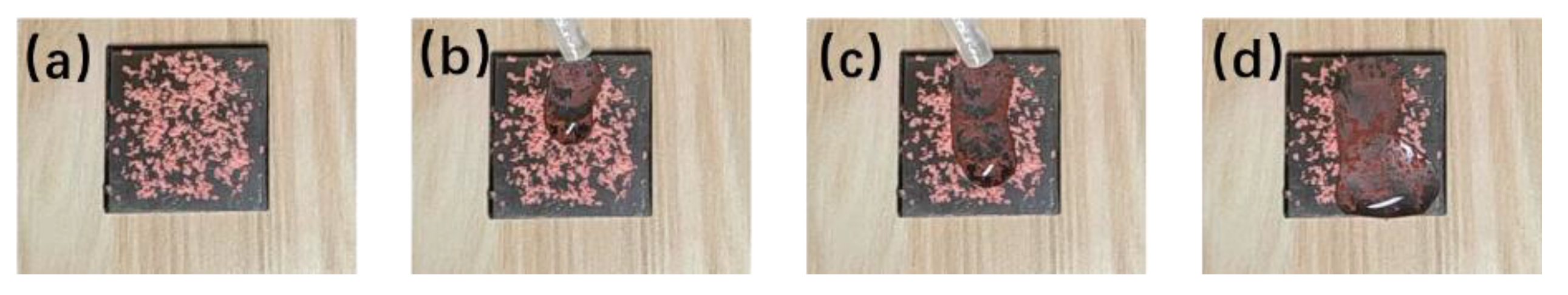
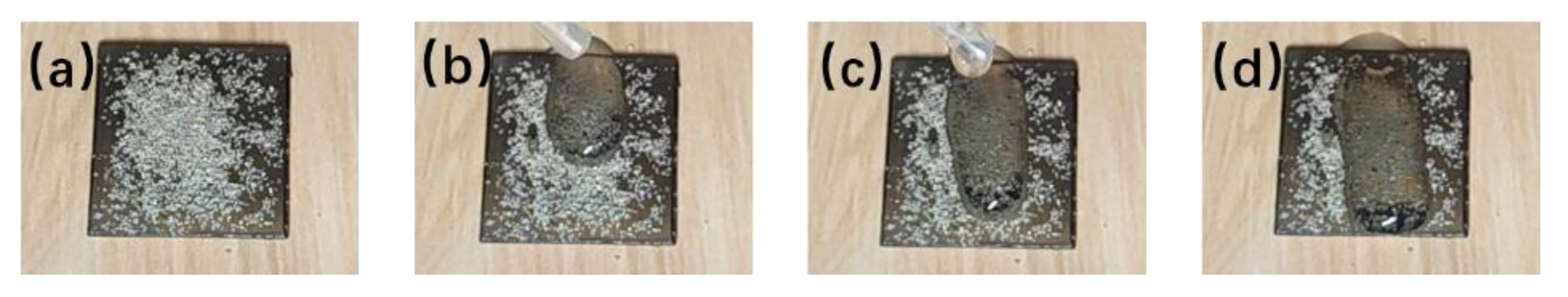
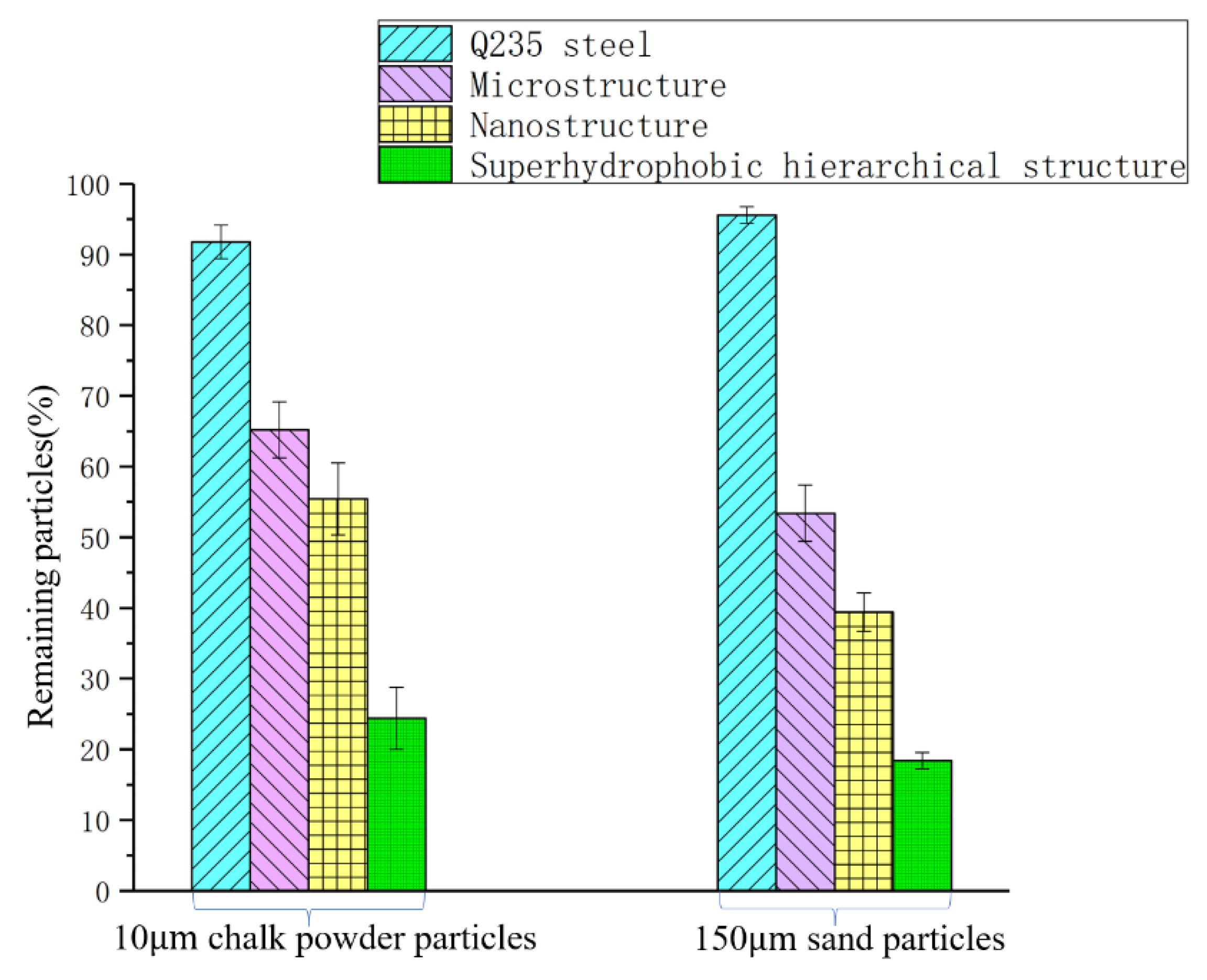
3.4. Self-Cleaning
3.5. Stability
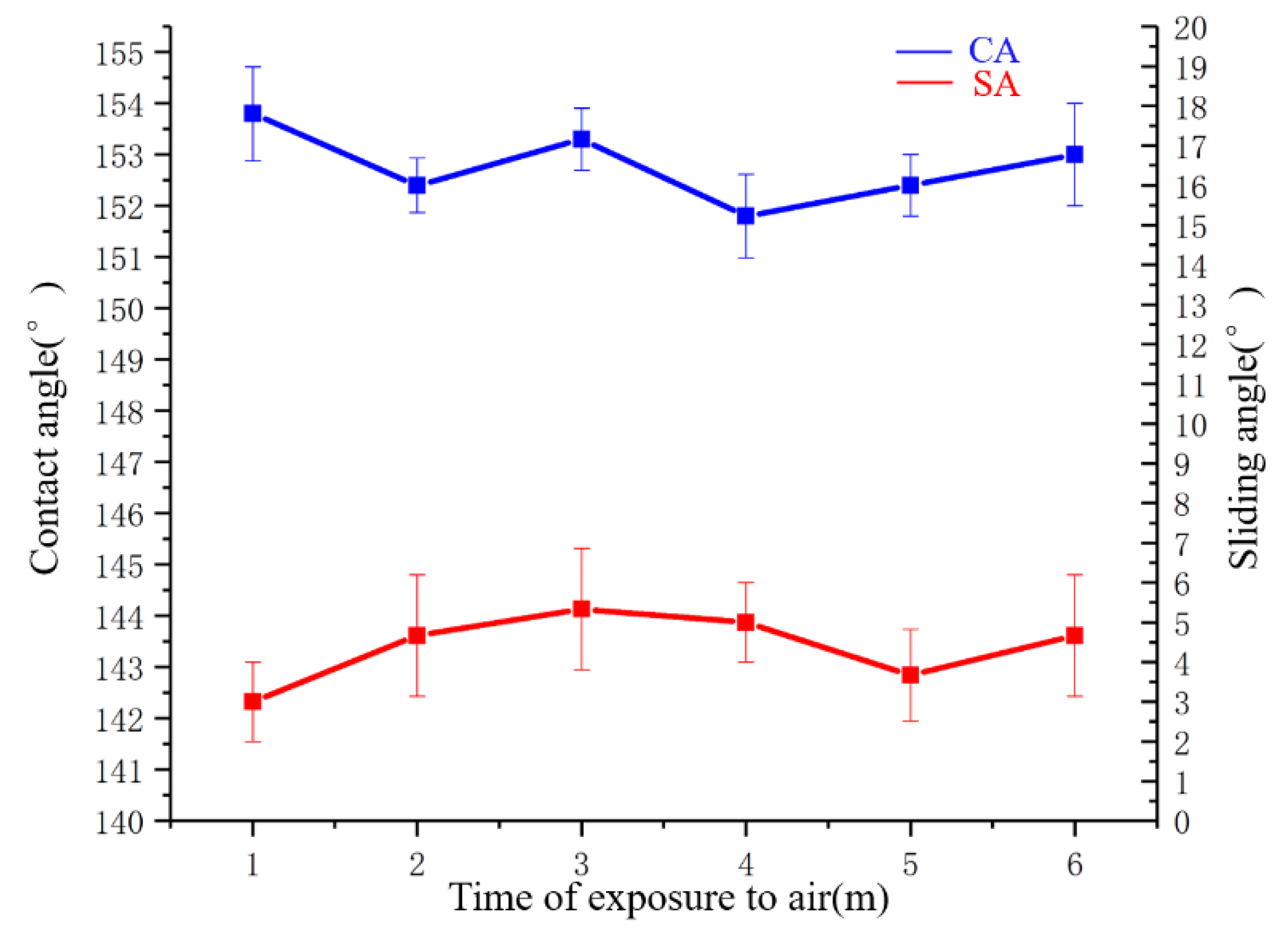
3.5.1. Atmospheric Exposure Test
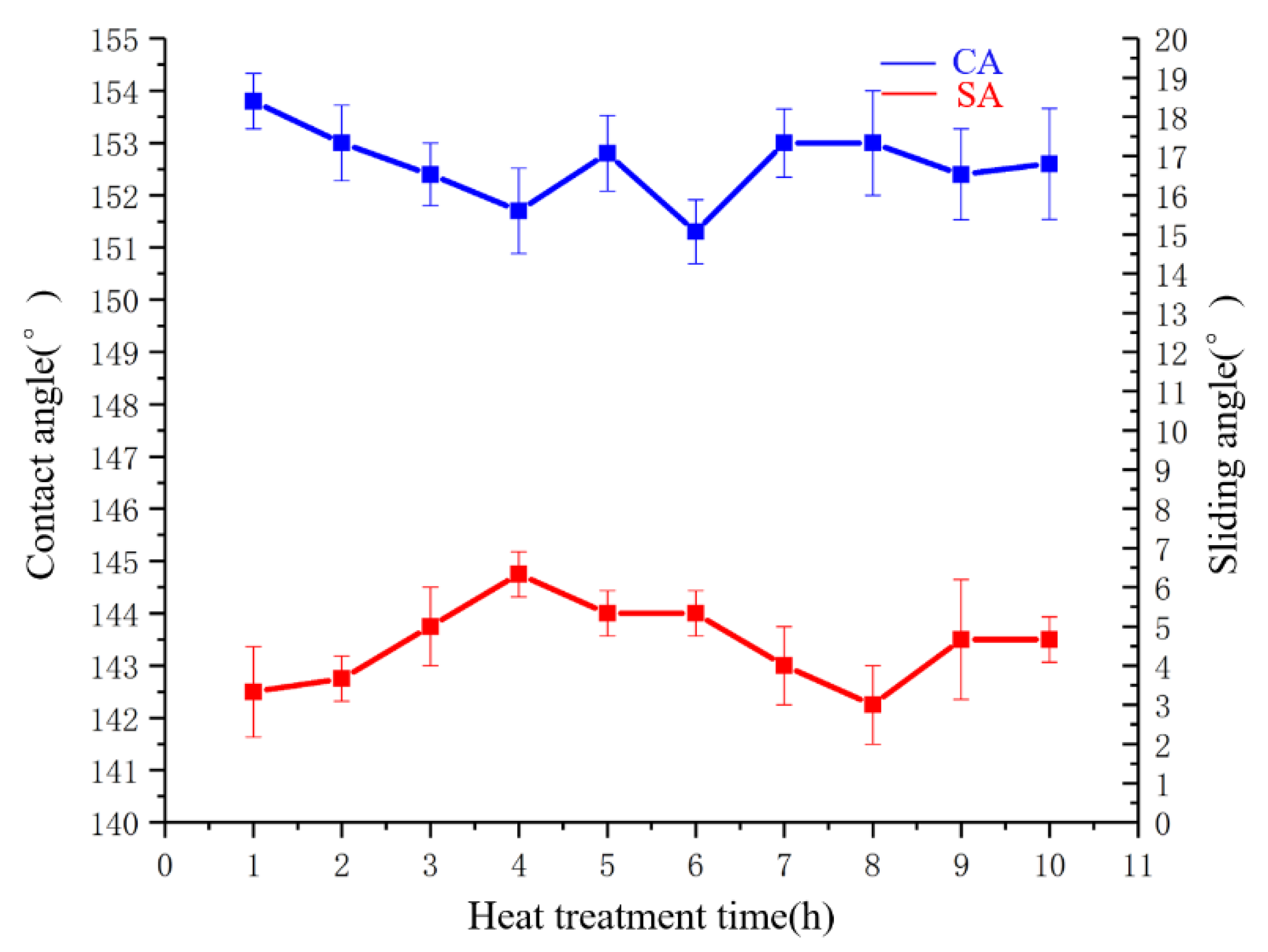
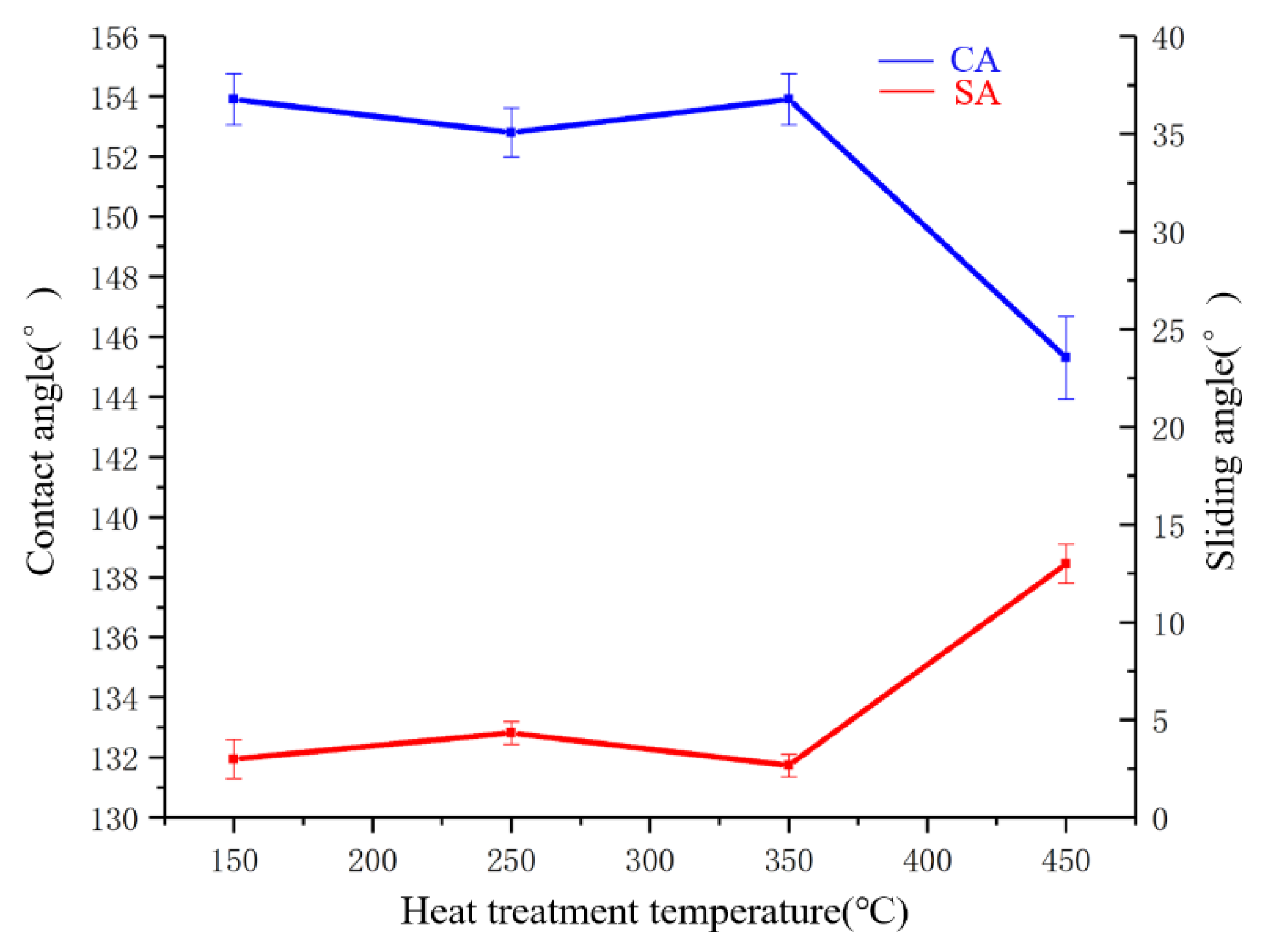
3.5.2. Heat Stability
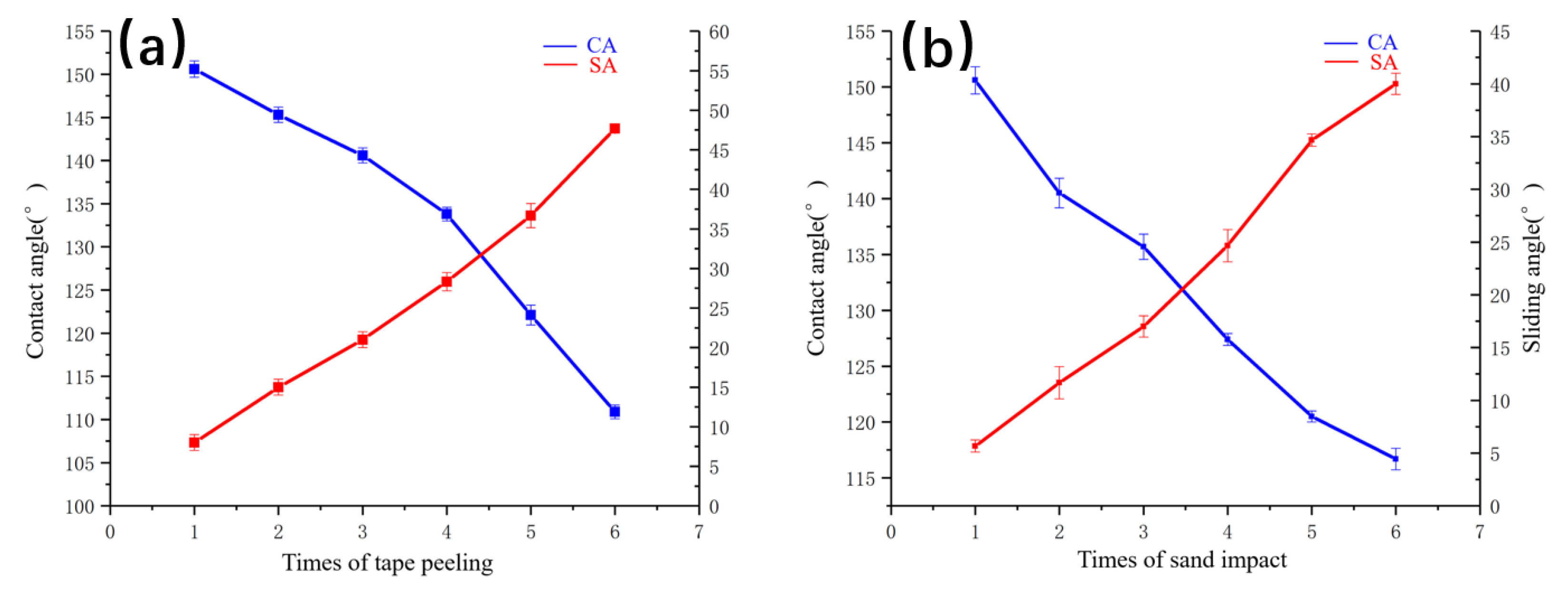
3.6. Binding and Impact Test
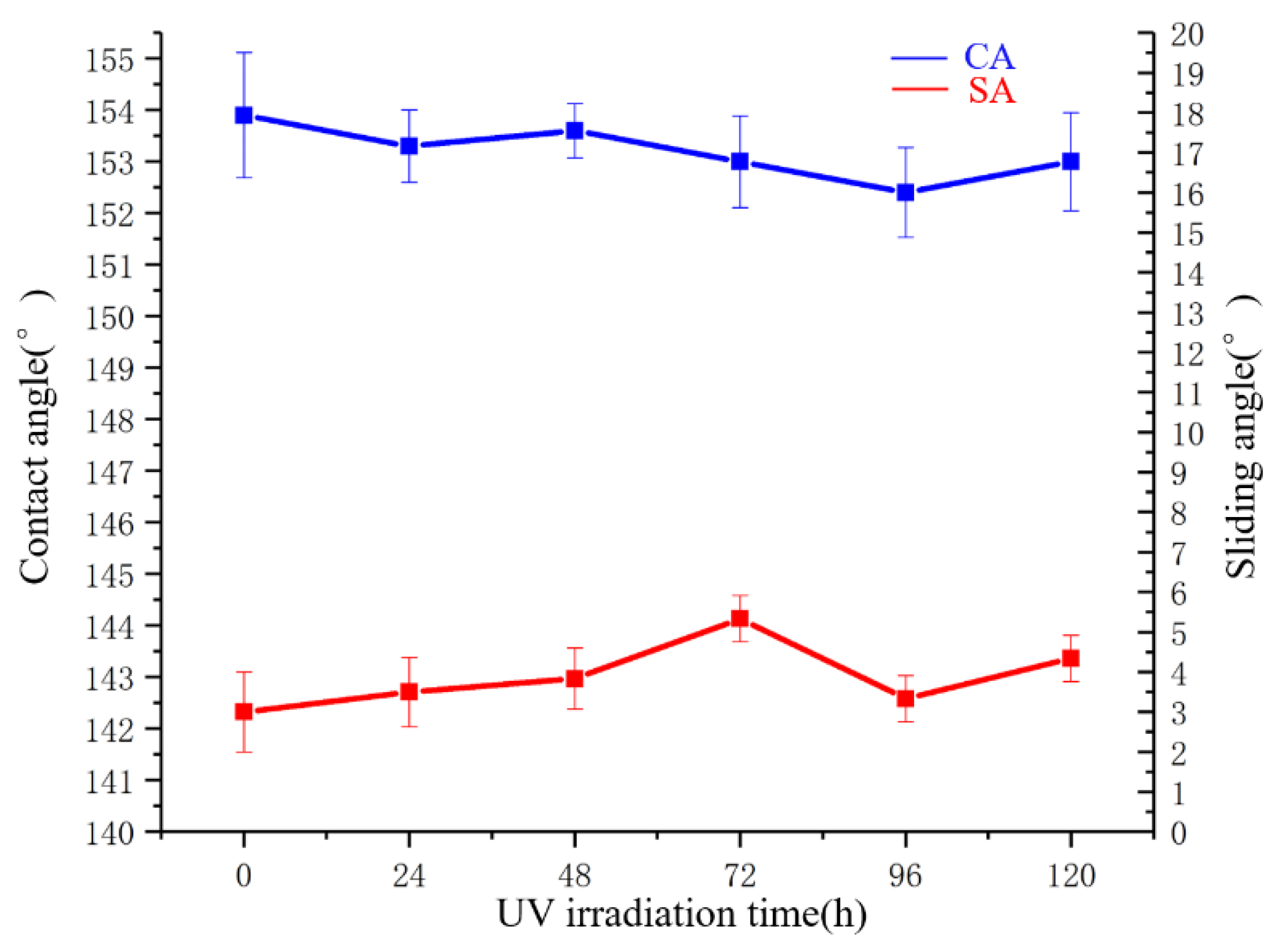
3.7. UV Irradiation Test
3.8. Icing Delay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Radulović, Ž.; Porter, L.M.; Kim, T.K.; Mulenga, A. Comparative bioinformatics, temporal and spatial expression analyses of ixodes scapularis organic anion transporting polypep-tides. Ticks Tick-Borne Dis. 2014, 5, 287–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boinovich, L.B.; Domantovskii, A.G.; Emelyanenko, A.M.; Miller, A.B.; Potapov, Y.F.; Khodan, A.N. Antiicing performance of superhydrophobic coatings on aluminum and stainless steel. Russ. Chem. Bull. 2013, 62, 380–387. [Google Scholar] [CrossRef]
- Antonini, C.; Innocenti, M.; Horn, T.; Marengo, M.; Amirfazli, A. Understanding the effect of superhydrophobic coatings on energy reduction in anti -icing systems. Cold Reg. Sci. Technol. 2011, 67, 58–67. [Google Scholar] [CrossRef]
- Barthlott, W.; Neinhuis, C. Purity of the sacred lotus, or es-cape from contamination in biological surfaces. Planta 1997, 202, 1–8. [Google Scholar] [CrossRef]
- Darmanin, T.; Givenchy, E.T.D.; Amigoni, S.; Guittard, F. Superhydrophobic surfaces by electrochemical processes. Adv. Mater. 2013, 25, 1378–1394. [Google Scholar] [CrossRef]
- Seo, D.; Shim, J.; Moon, B.; Lee, K.; Nam, Y. Passive anti-flooding superhydrophobic surfaces. ACS Appl. Mater. Interfaces 2020, 12, 4068–4080. [Google Scholar] [CrossRef]
- Cai, D.P.; Song, J.Y.; Xing, T.L.; Chen, G.Q. PDMS/MgO superhydrophobic finishing of silk fabric. Print. Dye. 2018, 18, 12–16. [Google Scholar]
- Du, M.Z.; Tian, M.W.; Qu, L.J. Construction of graphene/PDMS superhydrophobic cotton yarn and enhancement of water evaporation performance. J. Chengdu Text. Coll. 2017, 34, 1–5. [Google Scholar]
- Sebastian, D.; Yao, C.W.; Lian, L. Mechanical durability of engineered superhydrophobic surfaces for anti-corrosion. Coatings 2018, 8, 162. [Google Scholar] [CrossRef] [Green Version]
- Sebastian, D.; Yao, C.W.; Nipa, L.; Lian, L.; Twu, G. Corrosion behavior and mechanical properties of a nanocomposite superhydrophobic coating. Coatings 2021, 11, 652. [Google Scholar] [CrossRef]
- Lu, Y.Y.; Feng, Y.H.; Huang, Y.; Zhao, Y.Z.; Wang, Y.H. Study on preparation and properties of self-healing superhydrophobic antibacterial fabric. Funct. Mater. 2020, 7, 7110–7116. [Google Scholar]
- Zhang, M.; Du, X.L.; Shi, Y.H.; Shi, J.Y. Plasma pretreatment was used to prepare antibacterial and special wettability degreasing cotton fabrics. Funct. Mater. 2020, 3, 3157–3163. [Google Scholar]
- Yang, Y.S.; Shen, H.J.; Qin, L.; Qiu, J. Biomimetic construction of lotus leaf—Like self-cleaning superhydrophobic micro/nanostructures on wood surface. J. For. Eng. 2020, 5, 63–68. [Google Scholar]
- Yang, Y.S.; Shen, H.J.; Wang, X.; Qiu, J. Preparation and properties of self-cleaning superhydrophobic gel-free nanofibrillated composites. J. Northeast For. Univ. 2020, 48, 80–85. [Google Scholar]
- Gan, W.; Gao, L.; Sun, Q.; Jin, C.; Lu, Y.; Li, J. Multifunctional wood materials with magnetic, superhydrophobic and anti-ultraviolet properties. Appl. Surf. Sci. 2015, 332, 565–572. [Google Scholar] [CrossRef]
- Chan, H.M.; Yasmeen, S.; Park, K.; Gaiji, H.; Chung, C.; Kim, H.; Moon, H.S.; Choi, J.W.; Lee, H.B.R. Icephobic coating through a self-formed superhydrophobic surface using a polymer and microsized particles. ACS Appl. Mater. Interfaces 2022, 14, 3334–3343. [Google Scholar]
- Arumugam, G.K.; Veedu, V. Method for Preparing Icephobic/Superhydrophobic Surfaces on Metals, Ceramics, and Polymers. U.S. Patent No. 11,118,270, US11118270B1, 2021. [Google Scholar]
- Liu, G.H.; Xing, M.; Yu, T.; Lei, X.P. TSA and HCl chemically etched aluminum sheet to construct low adhesion superhydrophobic surface and its stability. Surf. Technol. 2019, 48, 140–149; 159. [Google Scholar]
- Park, B.; Hwang, W. A facile fabrication method for corrosion-resistant micro/nanostructures on stainless steel surfaces with tunable wettability. Scr. Mater. 2016, 113, 118–121. [Google Scholar] [CrossRef]
- Song, C.L.; Liu, J.B.; Weng, W.H.; Weng, W.J. Fluoride-doped TiO2 self-cleaning films were prepared by atmospheric chemical vapor deposition. J. Chin. Ceram. Soc. 2010, 38, 58–63. [Google Scholar]
- Akalak, H.; Ylmaz, K.; Gürsoy, M.; Karaman, M. Roll-to roll initiated chemical vapor deposition of super hydrophobic thin films on large-scale flexible substrates. Chem. Eng. Sci. 2020, 215, 115466. [Google Scholar] [CrossRef]
- Zhou, W.Y.; Zhang, Y.Y.; Shi, Y.D. Study on properties of TiO2 cotton fabric supported by dip-rolling hydrothermal method. Text. Aux. 2017, 34, 23–26. [Google Scholar]
- Zhang, B.; Wang, J.; Zhang, J. Bio-inspired one step hydrothermal fabricated superhydrophobic aluminum alloy with favorable corrosion resistance. Colloids Surf. A Physicochem. Eng. Asp. 2020, 589, 124469. [Google Scholar] [CrossRef]
- Yue, B.B. Study on Preparation of Thermal-Viscous Fiber Films Imitating Lotus Leaf Effect by Electrospinning. Master’s Thesis, University of Chinese Academy of Sciences, Shanghai, China, 27 April 2016. [Google Scholar]
- Pardo-Figuerez, M.; López-Córdoba, A.; Torres-Giner, S.; Lagaron, J.M. Superhydrophobic bio-coating made by co-continuous electrospinning and electrospraying on polyethylene terephthalate films proposed as easy emptying transparent food packaging. Coatings 2018, 8, 364. [Google Scholar] [CrossRef] [Green Version]
- Zheng, J.Y.; Feng, J.; Zhong, M.Q. Preparation of superhydrophilic/superhydrophobic polymer surfaces by CaCO3 particle template method. Acta Polym. Sin. 2010, 10, 1186–1192. [Google Scholar] [CrossRef]
- Wang, J.; Wu, Y.; Zhang, D.; Li, L.; Duan, S. Preparation of superhydrophobic flexible tubes with water and blood repellency based on template method. Colloids Surf. A Physicochem. Eng. Asp. 2019, 587, 124331. [Google Scholar] [CrossRef]
- Wu, X.; Fu, Q.; Kumar, D.; Ho, J.W.C.; Kanhere, P.; Zhou, H.; Zhong, C. Mechanically robust superhydrophobic and superoleophobic coatings derived by sol–gel method. Mater. Des. 2016, 89, 1302–1309. [Google Scholar] [CrossRef]
- Lee, J.W.; Hwang, W. Exploiting the silicon content of aluminum alloys to create a superhydrophobic surface using the sol–gel process. Mater. Lett. 2016, 168, 83–85. [Google Scholar] [CrossRef]
- Hou, K.K.; Chen, X.H.; Zhang, W.Q.; Zhai, T.T. Preparation of bionic superhydrophobic filter by electrodeposition method and its oil-water separation performance. Plat. Finish. 2020, 42, 1–6. [Google Scholar]
- Prado, L.H.; Virtanen, S. Cu–MoS2 superhydrophobic coating by composite electrodeposition. Coatings 2020, 10, 238. [Google Scholar] [CrossRef] [Green Version]
- Hao, L.F.; Yang, J.J.; Xu, W.; Zhao, G.H.; Liu, H.N.; Wang, X.C. Durable superhydrophobic coating on fabric surface prepared by layer self-assembly method and its properties. J. Shanxi Univ. Sci. Technol. 2019, 37, 68–73. [Google Scholar]
- Celik, N.; Torun, I.; Ruzi, M.; Esidir, A.; Onses, M.S. Fabrication of robust superhydrophobic surfaces by one-step spray coating: Evaporation driven self-assembly of wax and nanoparticles into hierarchical structures. Chem. Eng. J. 2020, 396, 125230. [Google Scholar] [CrossRef]
- Marchand, D.J.; Dilworth, Z.R.; Stauffer, R.J.; Hsiao, E.; Kim, J.H.; Kang, J.G.; Kim, S.H. Atmospheric rf plasma deposition of superhydrophobic coatings using tetramethylsilane precursor. Surf. Coat. Technol. 2013, 234, 14–20. [Google Scholar] [CrossRef]
- Satyaprasad, A.; Jain, V.; Nema, S.K. Deposition of superhydrophobic nanostructured teflon-like coating using expanding plasma arc. Appl. Surf. Sci. 2007, 253, 5462–5466. [Google Scholar] [CrossRef]
- Sebastian, D.; Yao, C.W. Effect of poly(dimethylsiloxane) binder in a silica-based superhydrophobic coating on mechanical properties, surface roughness, and wettability. MRS Commun. 2020, 10, 512–518. [Google Scholar] [CrossRef]
- Li, M.; Luo, W.; Sun, H.; Xu, J.; Ng, K.W.; Cheng, X. Micropatterned amorphous zr-based alloys coated with silica nanoparticles as superhydrophobic surfaces against abrasion. ACS Appl. Nano Mater. 2021, 4, 12300–12307. [Google Scholar] [CrossRef]
- Zhang, X.X.; Wang, Y.Y.; Gu, L.; Xu, Z.Y.; Peng, C.S. Superhydrophobic surface modified by sol-gel silica nanoparticle coating. Mater. Sci. Forum 2019, 960, 155–160. [Google Scholar] [CrossRef]
- Zhu, Z.T.; Tian, Y.; Liu, Y.B.; Fu, K.; Chen, Q.W.; Zhang, B.L.; Zhang, H.P.; Zhang, Q.Y. Facile synthesis of superhydrophobic coating with icing delay ability by the self-assembly of PVDF clusters. Colloids Surf. A Physicochem. Eng. Asp. 2022, 641, 128562. [Google Scholar] [CrossRef]
- Allahdini, A.; Jafari, R.; Momen, G. Transparent non-fluorinated superhydrophobic coating with enhanced anti-icing performance. Prog. Org. Coat. 2022, 165, 106758. [Google Scholar] [CrossRef]
- Hu, T.D.; Wu, Y.Z.; Zhao, N.; Liu, Y.L.; Zhou, P.C.; Xu, Y.C.; Xu, T.; Qu, W.S.; Wei, B.; Liao, Y.J. Biomimetic superhydrophobic films drop-coated with zinc oxide modified molecular sieves. Colloids Surf. A Physicochem. Eng. Asp. 2022, 642, 128669. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Bayat, A.; Ardekani, S.R.; Iranizad, E.S.; Moshfegh, A.Z. Sustainable superhydrophobic branched hierarchical ZnO nanowires: Stability and wettability phase diagram. Appl. Surf. Sci. 2021, 561, 150068. [Google Scholar] [CrossRef]
- Cui, X.J.; Li, G.J.; Ren, R.M. Process and mechanism of modification of industrial silica sols with methyltrimethoxysilane. J. Mater. Sci. Eng. 2012, 30, 528–532. [Google Scholar]
- Chantarachindawong, R.; Luangtip, W.; Chindaudom, P.; Osotchan, T.; Srikhirin, T. Development of the scratch resistance on acrylic sheet with basic colloidal silica (SiO2)—Methyltrimethoxysilane (MTMS) nanocomposite films by sol–gel technique. Can. J. Chem. Eng. 2012, 90, 888–896. [Google Scholar] [CrossRef]
- Liu, F.; Li, C.Y.; Li, G.J. Preparation and hydrophobicity analysis of methyl-modified silica sols. Electroplat. Finish. 2021, 40, 151–155. [Google Scholar]
- Fan, Y.; He, Y.; Luo, P.Y.; Chen, X.; Liu, B. A facile electrodeposition process to fabricate corrosion-resistant superhydrophobic surface on carbon steel. Appl. Surf. Sci. 2016, 368, 435–442. [Google Scholar] [CrossRef]
- Liu, F. Preparation and Study of Modified Silica Sol with Low Surface Energy. Master’s Thesis, Dalian Jiaotong University, Dalian, China, 13 June 2020. [Google Scholar]
- Wang, G.J.; Shi, Q.; Sha, H.X.; Liu, L.; Pan, J. Preparation of water-repellent membrane modified by methyl trimethoxysilane. J. Chem. Eng. 2009, 60, 2398–2403. [Google Scholar]
- Chen, Y.Y.; Hu, Z.J.; Yang, H.L.; Li, J.N.; Xu, Y.H. Research progress of organic group modified SiO2 aerogel. Aerosp. Mater. Technol. 2014, 2, 1–6. [Google Scholar]
- Yang, H.K.; Sun, Y.J. Surface modification of silica by gas phase method. Org. Silicon Mater. Its Appl. 1999, 13, 15–18. [Google Scholar]
- Rao, A.V.; Latthe, S.S.; Nadargi, Y.D.; Hirashima, H.; Ganesan, V. Preparation of MTMS based transparent superhydrophobic silica films by sol–gel method. J. Colloid Interface Sci. 2009, 332, 484–490. [Google Scholar]
- Bhushan, B.; Jung, Y.C.; Koch, K. Micro-, nano- and hierarchical structures for superhydrophobicity, self-cleaning and low adhesion. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2009, 367, 1631–1672. [Google Scholar] [CrossRef]
- Jesus, M.A.M.L.; Ferreira, A.M.; Lima, L.F.S.; Batista, G.F.; Mambrini, R.V.; Mohallem, N.D.S. Micro-mesoporous TiO2/SiO2 nanocomposites: Sol-gel synthesis, characterization, and enhanced photodegradation of quinoline. Ceram. Int. 2021, 47, 23844–23850. [Google Scholar] [CrossRef]
- Qi, Y.; Yang, Z.; Huang, W.; Zhang, J. Robust superhydrophobic surface for anti-icing and cooling performance: Application of fluorine-modified TiO2 and fumed SiO2. Appl. Surf. Sci. 2021, 538, 148131. [Google Scholar] [CrossRef]
- Yin, Z.; Yuan, F.; Zhou, D.; Xue, M.; Xie, C. Ultra dynamic water repellency and anti-icing performance of superhydrophobic zno surface on the printed circuit board (PCB). Chem. Phys. Lett. 2021, 771, 138558. [Google Scholar] [CrossRef]
- Ayres, J.; Simendinger, W.; Balik, C. Characterization of titanium alkoxide sol–gel systems designed for anti-icing coatings: I. Chemistry. J. Coat. Technol. Res. 2007, 4, 463–471. [Google Scholar] [CrossRef]
- Boinovich, L.B.; Emelyanenko, A.M. Anti-icing potential of superhydrophobic coatings. Mendeleev Commun. 2013, 23, 3–10. [Google Scholar] [CrossRef]
- Boinovich, L.B.; Emelyanenko, A.M.; Ivanov, V.K.; Pashinin, A.S. Durable icephobic coating for stainless steel. ACS Appl. Mater. Interfaces 2013, 5, 2549–2554. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shang, J.; Jiang, Y.; Wang, W. Heat Stability and Icing Delay on Superhydrophobic Coatings in Facile One Step. Polymers 2022, 14, 3124. https://doi.org/10.3390/polym14153124
Shang J, Jiang Y, Wang W. Heat Stability and Icing Delay on Superhydrophobic Coatings in Facile One Step. Polymers. 2022; 14(15):3124. https://doi.org/10.3390/polym14153124
Chicago/Turabian StyleShang, Jingyu, Yongfeng Jiang, and Wenhua Wang. 2022. "Heat Stability and Icing Delay on Superhydrophobic Coatings in Facile One Step" Polymers 14, no. 15: 3124. https://doi.org/10.3390/polym14153124
APA StyleShang, J., Jiang, Y., & Wang, W. (2022). Heat Stability and Icing Delay on Superhydrophobic Coatings in Facile One Step. Polymers, 14(15), 3124. https://doi.org/10.3390/polym14153124