Enhanced Biocompatibility of Multi-Layered, 3D Bio-Printed Artificial Vessels Composed of Autologous Mesenchymal Stem Cells
Abstract
1. Introduction
2. Materials and Methods
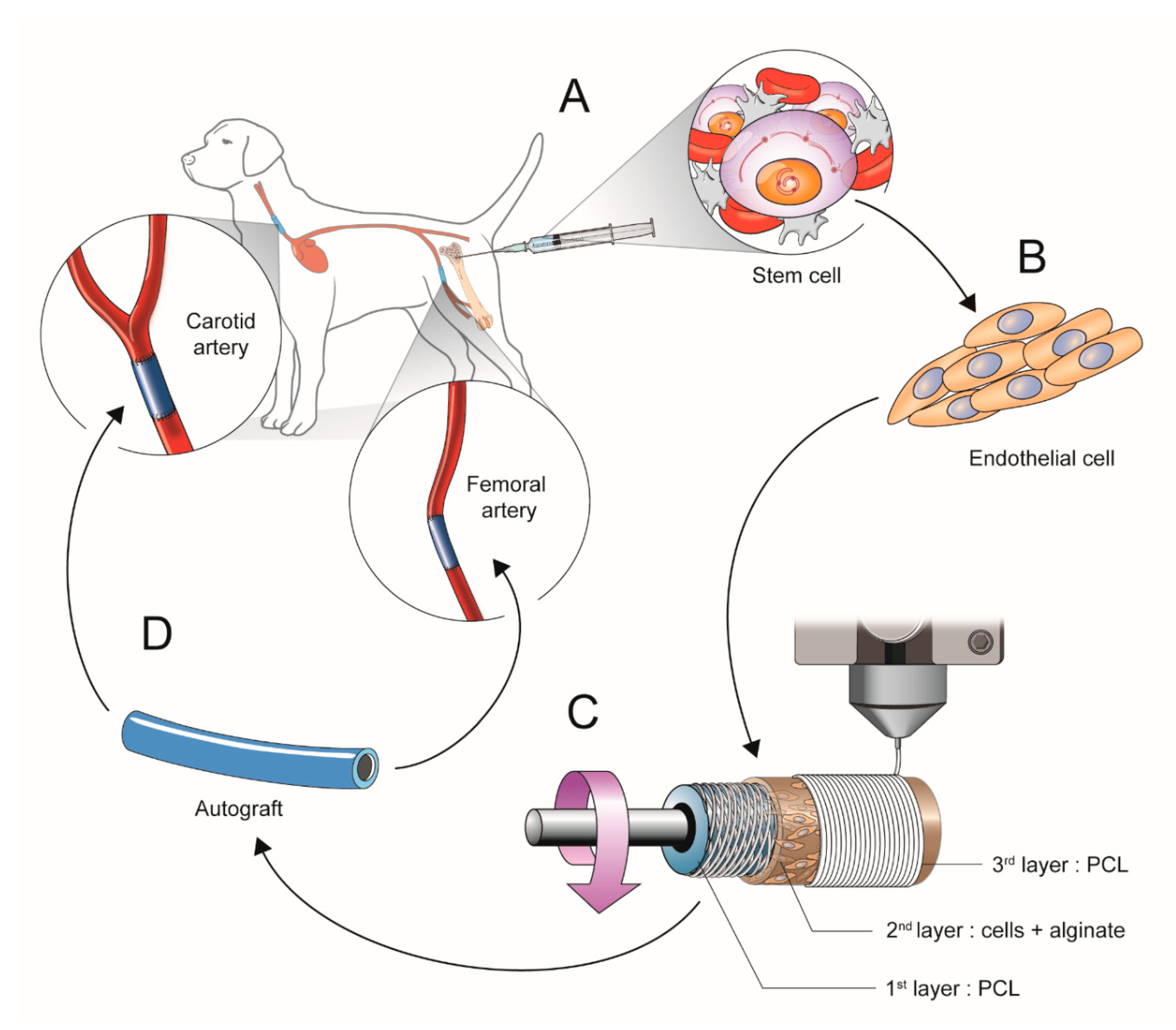
2.1. Scheme of Experimental Study Design
2.1.1. Isolation and Culture of Mesenchymal Stem Cells (b-MSCs) from Canine Bone Marrow
2.1.2. Cell Differentiation into Cell Resembling Endothelial Cells
2.1.3. Manufacturing the Artificial Vessels Using a 3D Bio-Printer
2.1.4. Surgical Implantation of the 3D Printed Vascular Grafts
2.2. Western Blot to Confirm Cell Differentiation
2.3. Scanning Electron Microscopy (SEM), Pre-Operation and Post-Operation.
2.4. Histological Assessment
2.5. Statistical Analysis
3. Results
3.1. Validation of the b-MSCs Differentiation and Artificial Vessels
3.2. In Vivo Studies of 3D Bio-Printed Artificial Vessels
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- McBane, J.; Sharifpoor, S.; Labow, R.S.; Ruel, M.; Suuronen, E.J.; Santerre, J.P. Tissue engineering a small diameter vessel substitute: Engineering constructs with select biomaterials and cells. Curr. Vasc. Pharmacol. 2012, 10, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, T.; Sellaro, T.; Badylak, S.F. Decellularization of tissues and organs. Biomaterials 2006, 27, 3675–3683. [Google Scholar] [CrossRef] [PubMed]
- Dar, A.; Shachar, M.; Leor, J.; Cohen, S. Optimization of cardiac cell seeding and distribution in 3D porous alginate scaffolds. Biotechnol. Bioeng. 2002, 80, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Liu, Z.; Wei, Z.; Liu, C.; Qiao, T.; Ran, F.; Bai, Y.; Jiang, X.; Ding, Y. Development and Validation of Small-diameter Vascular Tissue From a Decellularized Scaffold Coated With Heparin and Vascular Endothelial Growth Factor. Artif. Organs 2009, 33, 230–239. [Google Scholar] [CrossRef]
- Tabriz, A.G.; Hermida, M.A.; Leslie, N.R.; Shu, W. Three-dimensional bioprinting of complex cell laden alginate hydrogel structures. Biofabrication 2015, 7, 045012. [Google Scholar] [CrossRef]
- Hong, N.; Yang, G.-H.; Lee, J.; Kim, G. 3D bioprinting and its in vivo applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 444–459. [Google Scholar] [CrossRef]
- Gombotz, W. Protein release from alginate matrices. Adv. Drug Deliv. Rev. 1998, 31, 267–285. [Google Scholar] [CrossRef]
- Kong, H. Designing alginate hydrogels to maintain viability of immobilized cells. Biomaterials 2003, 24, 4023–4029. [Google Scholar] [CrossRef]
- Gu, F.; Amsden, B.; Neufeld, R.J. Sustained delivery of vascular endothelial growth factor with alginate beads. J. Control. Release 2004, 96, 463–472. [Google Scholar] [CrossRef]
- Naghieh, S.; Sarker, A.I.; Abelseth, E.; Chen, X. Indirect 3D bioprinting and characterization of alginate scaffolds for potential nerve tissue engineering applications. J. Mech. Behav. Biomed. Mater. 2019, 93, 183–193. [Google Scholar] [CrossRef]
- Park, J.-H.; Yoon, J.-K.; Lee, J.B.; Shin, Y.M.; Lee, K.-W.; Bae, S.-W.; Lee, J.; Yu, J.; Jung, C.-R.; Youn, Y.-N.; et al. Experimental Tracheal Replacement Using 3-dimensional Bioprinted Artificial Trachea with Autologous Epithelial Cells and Chondrocytes. Sci. Rep. 2019, 9, 2103. [Google Scholar] [CrossRef]
- Dreher, R.; Starly, B. Biofabrication of Multimaterial Three-Dimensional Constructs Embedded With Patterned Alginate Strands Encapsulating PC12 Neural Cell Lines. J. Nanotechnol. Eng. Med. 2015, 6, 021004. [Google Scholar] [CrossRef]
- Lee, K.; Kim, D.-H.; Lee, J.; Youn, Y.-N. The Effect of Pulsatile Flow on bMSC-Derived Endothelial-Like Cells in a Small-Sized Artificial Vessel Made by 3-Dimensional Bioprinting. Stem Cells Int. 2018, 2018, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Negoescu, A.; Lorimier, P.; Labat-Moleur, F.; Drouet, C.; Robert, C.; Guillermet, C.; Brambilla, C.; Brambilla, E. In situ apoptotic cell labeling by the TUNEL method: Improvement and evaluation on cell preparations. J. Histochem. Cytochem. 1996, 44, 959–968. [Google Scholar] [CrossRef] [PubMed]
- Stefanadis, C.; Toutouzas, K.; Stefanadi, E.; Lazaris, A.; Patsouris, E.; Kipshidze, N. Inhibition of plaque neovascularization and intimal hyperplasia by specific targeting vascular endothelial growth factor with bevacizumab-eluting stent: An experimental study. Atherosclerosis 2007, 195, 269–276. [Google Scholar] [CrossRef]
- Lim, S.Y.; Jeong, J.-O.; Hong, S.J.; Lim, D.-S.; Moon, J.Y.; Hong, Y.J.; Kim, J.H.; Ahn, Y.; Kang, J.C. Inflammation and delayed endothelization with overlapping drug-eluting stents in a porcine model of in-stent restenosis. Circ. J. 2008, 72, 463–468. [Google Scholar] [CrossRef][Green Version]
- Touchard, A.G.; Schwartz, R.S. Preclinical Restenosis Models: Challenges and Successes. Toxicol. Pathol. 2006, 34, 11–18. [Google Scholar] [CrossRef]
- Itoh, M.; Nakayama, K.; Noguchi, R.; Kamohara, K.; Furukawa, K.; Uchihashi, K.; Toda, S.; Oyama, J.I.; Node, K.; Morita, S. Scaffold-Free Tubular Tissues Created by a Bio-3D Printer Undergo Remodeling and Endothelialization when Implanted in Rat Aortae. PLoS ONE 2015, 10, e0136681. [Google Scholar]
- Haasters, F.; Prall, W.C.; Anz, D.; Bourquin, C.; Pautke, C.; Endres, S.; Mutschler, W.; Docheva, D.; Schieker, M. Morphological and immunocytochemical characteristics indicate the yield of early progenitors and represent a quality control for human mesenchymal stem cell culturing. J. Anat. 2009, 214, 759–767. [Google Scholar] [CrossRef]
- De Valence, S.; Tille, J.-C.; Chaâbane, C.; Gurny, R.; Bochaton-Piallat, M.-L.; Walpoth, B.; Möller, M. Plasma treatment for improving cell biocompatibility of a biodegradable polymer scaffold for vascular graft applications. Eur. J. Pharm. Biopharm. 2013, 85, 78–86. [Google Scholar] [CrossRef]
- Shi, J.; Chen, S.; Wang, L.; Zhang, X.; Gao, J.; Jiang, L.; Tang, D.; Zhang, L.; Midgley, A.C.; Kong, D.; et al. Rapid endothelialization and controlled smooth muscle regeneration by electrospun heparin-loaded polycaprolactone/gelatin hybrid vascular grafts. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 107, 2040–2049. [Google Scholar] [CrossRef] [PubMed]
- Tillman, B.W.; Yazdani, S.K.; Lee, S.J.; Geary, R.L.; Atala, A.; Yoo, J.J. The in vivo stability of electrospun polycaprolactone-collagen scaffolds in vascular reconstruction. Biomaterials 2009, 30, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Abelson, B.; Babbar, P.; Damaser, M.S. Harnessing the mesenchymal stem cell secretome for regenerative urology. Nat. Rev. Urol. 2019, 16, 363–375. [Google Scholar] [CrossRef] [PubMed]
- Kelm, J.M.; Sanchez-Bustamante, C.D.; Ehler, E.; Hoerstrup, S.P.; Djonov, V.; Ittner, L.M.; Fussenegger, M. VEGF profiling and angiogenesis in human microtissues. J. Biotechnol. 2005, 118, 213–229. [Google Scholar] [CrossRef] [PubMed]
- Kelm, J.M.; Ehler, E.; Nielsen, L.; Schlatter, S.; Perriard, J.-C.; Fussenegger, M. Design of Artificial Myocardial Microtissues. Tissue Eng. 2004, 10, 201–214. [Google Scholar] [CrossRef]
- Matsumura, G.; Hibino, N.; Ikada, Y.; Kurosawa, H.; Shin’Oka, T. Successful application of tissue engineered vascular autografts: Clinical experience. Biomaterials 2003, 24, 2303–2308. [Google Scholar] [CrossRef]



| Animal No. | Group | Position | Result |
|---|---|---|---|
| 1 | no cell (control) | left carotid artery (LCA) | damaged during dissection |
| right carotid artery (RCA) | occlusion | ||
| left femoral artery (LFA) | none implanted | ||
| right femoral artery (RFA) | none implanted | ||
| 2 | no cell (control) | left carotid artery (LCA) | occlusion |
| right carotid artery (RCA) | occlusion | ||
| left femoral artery (LFA) | none implanted | ||
| right femoral artery (RFA) | none implanted | ||
| 3 | no cell (control) | left carotid artery (LCA) | patent |
| right carotid artery (RCA) | patent | ||
| left femoral artery (LFA) | patent | ||
| right femoral artery (RFA) | patnet | ||
| 4 | no cell (control) | left carotid artery (LCA) | occlusion |
| right carotid artery (RCA) | patent | ||
| left femoral artery (LFA) | patent | ||
| right femoral artery (RFA) | occlusion | ||
| 5 | cell (cell-derived) | left carotid artery (LCA) | patent |
| right carotid artery (RCA) | patent | ||
| left femoral artery (LFA) | none implanted | ||
| right femoral artery (RFA) | none implanted | ||
| 6 | cell (cell-derived) | left carotid artery (LCA) | patent |
| right carotid artery (RCA) | patent | ||
| left femoral artery (LFA) | patent | ||
| right femoral artery (RFA) | patnet | ||
| 7 | cell (cell-derived) | left carotid artery (LCA) | occlusion |
| right carotid artery (RCA) | occlusion | ||
| left femoral artery (LFA) | patent | ||
| right femoral artery (RFA) | patnet | ||
| 8 | cell (cell-derived) | left carotid artery (LCA) | occlusion |
| right carotid artery (RCA) | occlusion | ||
| left femoral artery (LFA) | patent | ||
| right femoral artery (RFA) | occlusion |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, E.H.; Kim, J.-H.; Lee, J.H.; Kim, D.-H.; Youn, Y.-N. Enhanced Biocompatibility of Multi-Layered, 3D Bio-Printed Artificial Vessels Composed of Autologous Mesenchymal Stem Cells. Polymers 2020, 12, 538. https://doi.org/10.3390/polym12030538
Jang EH, Kim J-H, Lee JH, Kim D-H, Youn Y-N. Enhanced Biocompatibility of Multi-Layered, 3D Bio-Printed Artificial Vessels Composed of Autologous Mesenchymal Stem Cells. Polymers. 2020; 12(3):538. https://doi.org/10.3390/polym12030538
Chicago/Turabian StyleJang, Eui Hwa, Jung-Hwan Kim, Jun Hee Lee, Dae-Hyun Kim, and Young-Nam Youn. 2020. "Enhanced Biocompatibility of Multi-Layered, 3D Bio-Printed Artificial Vessels Composed of Autologous Mesenchymal Stem Cells" Polymers 12, no. 3: 538. https://doi.org/10.3390/polym12030538
APA StyleJang, E. H., Kim, J.-H., Lee, J. H., Kim, D.-H., & Youn, Y.-N. (2020). Enhanced Biocompatibility of Multi-Layered, 3D Bio-Printed Artificial Vessels Composed of Autologous Mesenchymal Stem Cells. Polymers, 12(3), 538. https://doi.org/10.3390/polym12030538





