Polystyrene Chain Growth Initiated from Dialkylzinc for Synthesis of Polyolefin-Polystyrene Block Copolymers
Abstract
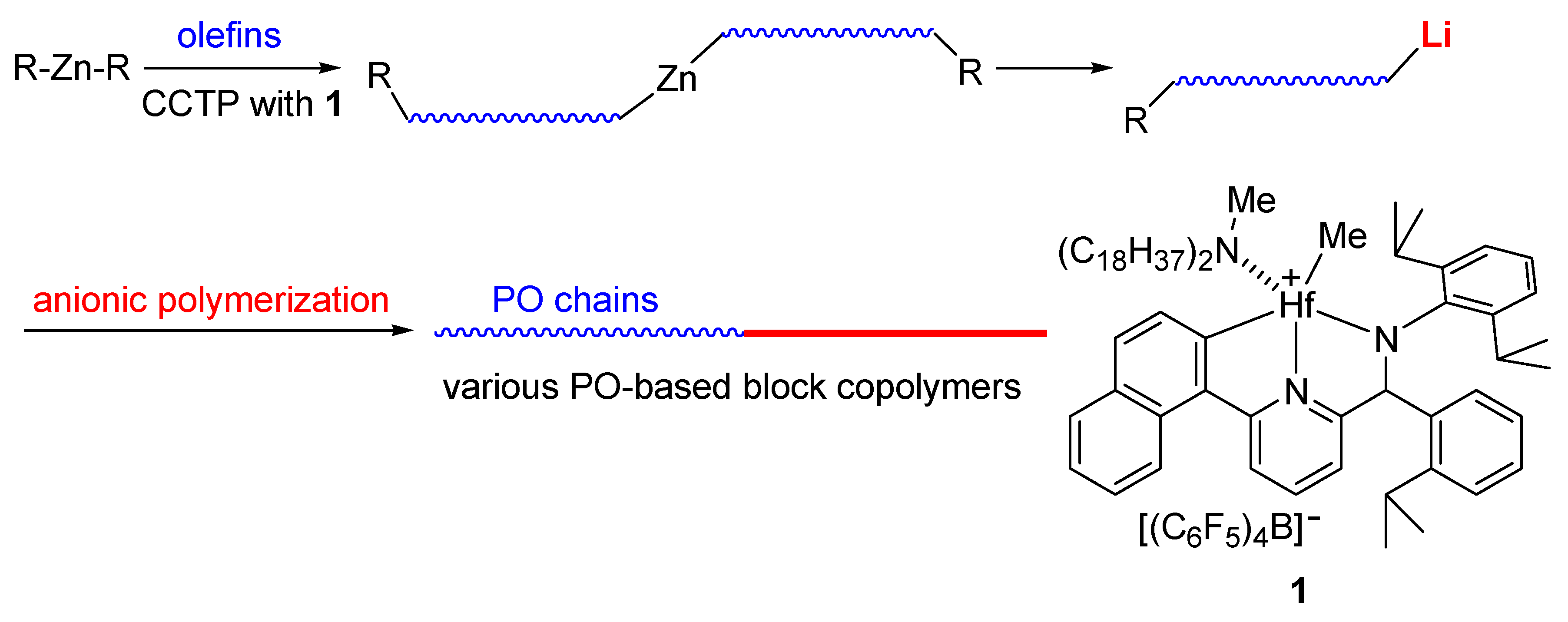
1. Introduction
2. Materials and Methods
3. Results and Discussion
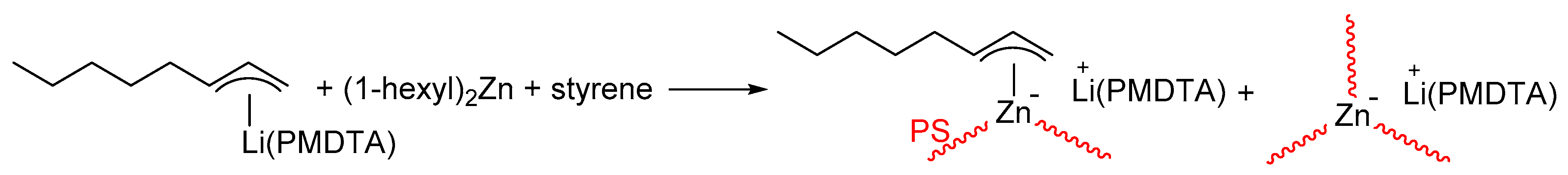
3.1. Converting Dialkylzinc to Alkyllithium
3.2. Attempts to Synthesize Block Copolymers
3.3. Initiators for PS Chain Growth from Dialkylzinc
3.4. Synthesis of PO-Block-PS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bates, C.M.; Bates, F.S. 50th Anniversary Perspective: Block Polymers—Pure Potential. Macromolecules 2017, 50, 3–22. [Google Scholar] [CrossRef]
- Guo, X.; Choi, B.; Feng, A.; Thang, S.H. Polymer Synthesis with More Than One Form of Living Polymerization Method. Macromol. Rapid Commun. 2018, 39. [Google Scholar] [CrossRef]
- Polymeropoulos, G.; Zapsas, G.; Ntetsikas, K.; Bilalis, P.; Gnanou, Y.; Hadjichristidis, N. 50th Anniversary Perspective: Polymers with Complex Architectures. Macromolecules 2017, 50, 1253–1290. [Google Scholar] [CrossRef]
- Kermagoret, A.; Debuigne, A.; Jérôme, C.; Detrembleur, C. Precision design of ethylene- and polar-monomer-based copolymers by organometallic-mediated radical polymerization. Nat. Chem. 2014, 6, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Dommanget, C.; D’Agosto, F.; Monteil, V. Polymerization of Ethylene through Reversible Addition–Fragmentation Chain Transfer (RAFT). Angew. Chem. Int. Ed. 2014, 53, 6683–6686. [Google Scholar] [CrossRef] [PubMed]
- Wolpers, A.; Bergerbit, C.; Ebeling, B.; D’Agosto, F.; Monteil, V. Aromatic Xanthates and Dithiocarbamates for the Polymerization of Ethylene through Reversible Addition–Fragmentation Chain Transfer (RAFT). Angew. Chem. Int. Ed. 2019, 58, 14295–14302. [Google Scholar] [CrossRef] [PubMed]
- Goring, P.D.; Morton, C.; Scott, P. End-functional polyolefins for block copolymer synthesis. Dalton Trans. 2019, 48, 3521–3530. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.Y.; Chung, T.C. Synthesis of Polyethylene Containing a Terminal p-Methylstyrene Group: Metallocene-Mediated Ethylene Polymerization with a Consecutive Chain Transfer Reaction to p-Methylstyrene and Hydrogen. Macromolecules 2002, 35, 1622–1631. [Google Scholar] [CrossRef]
- Chung, T.C.; Dong, J.Y. A Novel Consecutive Chain Transfer Reaction to p-Methylstyrene and Hydrogen during Metallocene-Mediated Olefin Polymerization. J. Am. Chem. Soc. 2001, 123, 4871–4876. [Google Scholar] [CrossRef]
- Yan, T.; Walsh, D.J.; Qiu, C.; Guironnet, D. One-Pot Synthesis of Block Copolymers Containing a Polyolefin Block. Macromolecules 2018, 51, 10167–10173. [Google Scholar] [CrossRef]
- Kay, C.J.; Goring, P.D.; Burnett, C.A.; Hornby, B.; Lewtas, K.; Morris, S.; Morton, C.; McNally, T.; Theaker, G.W.; Waterson, C.; et al. Polyolefin–Polar Block Copolymers from Versatile New Macromonomers. J. Am. Chem. Soc. 2018, 140, 13921–13934. [Google Scholar] [CrossRef] [PubMed]
- Jeon, C.; Kim, D.W.; Chang, S.; Kim, J.G.; Seo, M. Synthesis of Polypropylene via Catalytic Deoxygenation of Poly (methyl acrylate). ACS Macro Lett. 2019, 8, 1172–1178. [Google Scholar] [CrossRef]
- Kayser, F.; Fleury, G.; Thongkham, S.; Navarro, C.; Martin-Vaca, B.; Bourissou, D. Microphase Separation of Polybutyrolactone-Based Block Copolymers with Sub-20 nm Domains. Macromolecules 2018, 51, 6534–6541. [Google Scholar] [CrossRef]
- Walsh, D.J.; Su, E.; Guironnet, D. Catalytic synthesis of functionalized (polar and non-polar) polyolefin block copolymers. Chem. Sci. 2018, 9, 4703–4707. [Google Scholar] [CrossRef] [PubMed]
- Chapman, R.; Melodia, D.; Qu, J.-B.; Stenzel, M.H. Controlled poly (olefin)s via decarboxylation of poly(acrylic acid). Polym. Chem. 2017, 8, 6636–6643. [Google Scholar] [CrossRef]
- Higaki, Y.; Suzuki, K.; Kiyoshima, Y.; Toda, T.; Nishiura, M.; Ohta, N.; Masunaga, H.; Hou, Z.; Takahara, A. Molecular Aggregation States and Physical Properties of Syndiotactic Polystyrene/Hydrogenated Polyisoprene Multiblock Copolymers with Crystalline Hard Domain. Macromolecules 2017, 50, 6184–6191. [Google Scholar] [CrossRef]
- Hotta, A.; Cochran, E.; Ruokolainen, J.; Khanna, V.; Fredrickson, G.H.; Kramer, E.J.; Shin, Y.-W.; Shimizu, F.; Cherian, A.E.; Hustad, P.D.; et al. Semicrystalline thermoplastic elastomeric polyolefins: Advances through catalyst development and macromolecular design. Proc. Natl. Acad. Sci. USA 2006, 103, 15327–15332. [Google Scholar] [CrossRef]
- Eagan, J.M.; Xu, J.; Di Girolamo, R.; Thurber, C.M.; Macosko, C.W.; La Pointe, A.M.; Bates, F.S.; Coates, G.W. Combining polyethylene and polypropylene: Enhanced performance with PE/iPP multiblock polymers. Science 2017, 355, 814–816. [Google Scholar] [CrossRef]
- Song, X.; Cao, L.; Tanaka, R.; Shiono, T.; Cai, Z. Optically Transparent Functional Polyolefin Elastomer with Excellent Mechanical and Thermal Properties. ACS Macro Lett. 2019, 8, 299–303. [Google Scholar] [CrossRef]
- Valente, A.; Mortreux, A.; Visseaux, M.; Zinck, P. Coordinative chain transfer polymerization. Chem. Rev. 2013, 113, 3836–3857. [Google Scholar] [CrossRef]
- van Meurs, M.; Britovsek, G.J.P.; Gibson, V.C.; Cohen, S.A. Polyethylene Chain Growth on Zinc Catalyzed by Olefin Polymerization Catalysts: A Comparative Investigation of Highly Active Catalyst Systems across the Transition Series. J. Am. Chem. Soc. 2005, 127, 9913–9923. [Google Scholar] [CrossRef] [PubMed]
- Arriola, D.J.; Carnahan, E.M.; Hustad, P.D.; Kuhlman, R.L.; Wenzel, T.T. Catalytic production of olefin block copolymers via chain shuttling polymerization. Science 2006, 312, 714–719. [Google Scholar] [CrossRef] [PubMed]
- Hustad, P.O.; Kuhlman, R.L.; Arriola, D.J.; Carnahan, E.M.; Wenzel, T.T. Continuous production of ethylene-based diblock copolymers using coordinative chain transfer polymerization. Macromolecules 2007, 40, 7061–7064. [Google Scholar] [CrossRef]
- Saeb, M.R.; Mohammadi, Y.; Kermaniyan, T.S.; Zinck, P.; Stadler, F.J. Unspoken aspects of chain shuttling reactions: Patterning the molecular landscape of olefin multi-block copolymers. Polymer 2017, 116, 55–75. [Google Scholar] [CrossRef]
- Vittoria, A.; Busico, V.; Cannavacciuolo, F.D.; Cipullo, R. Molecular Kinetic Study of “Chain Shuttling” Olefin Copolymerization. ACS Catal. 2018, 8, 5051–5061. [Google Scholar] [CrossRef]
- Kim, S.D.; Kim, T.J.; Kwon, S.J.; Kim, T.H.; Baek, J.W.; Park, H.S.; Lee, H.J.; Lee, B.Y. Peroxide-Mediated Alkyl–Alkyl Coupling of Dialkylzinc: A Useful Tool for Synthesis of ABA-Type Olefin Triblock Copolymers. Macromolecules 2018, 51, 4821–4828. [Google Scholar] [CrossRef]
- Chenal, T.; Visseaux, M. Combining Polyethylene CCG and Stereoregular Isoprene Polymerization: First Synthesis of Poly (ethylene)-b-(trans-isoprene) by Neodymium Catalyzed Sequenced Copolymerization. Macromolecules 2012, 45, 5718–5727. [Google Scholar] [CrossRef]
- Rutkowski, S.; Zych, A.; Przybysz, M.; Bouyahyi, M.; Sowinski, P.; Koevoets, R.; Haponiuk, J.; Graf, R.; Hansen, M.R.; Jasinska-Walc, L.; et al. Toward Polyethylene–Polyester Block and Graft Copolymers with Tunable Polarity. Macromolecules 2017, 50, 107–122. [Google Scholar] [CrossRef]
- Li, T.; Wang, W.J.; Liu, R.; Liang, W.H.; Zhao, G.F.; Li, Z.; Wu, Q.; Zhu, F.M. Double-Crystalline Polyethylene-b-poly (ethylene oxide) with a Linear Polyethylene Block: Synthesis and Confined Crystallization in Self-Assembled Structure Formed from Aqueous Solution. Macromolecules 2009, 42, 3804–3810. [Google Scholar] [CrossRef]
- Thomas, T.S.; Hwang, W.; Sita, L.R. End-Group-Functionalized Poly (α-olefinates) as Non-Polar Building Blocks: Self-Assembly of Sugar-Polyolefin Hybrid Conjugates. Angew. Chem. Int. Ed. 2016, 55, 4683–4687. [Google Scholar] [CrossRef]
- Ota, Y.; Murayama, T.; Nozaki, K. One-step catalytic asymmetric synthesis of all-syn deoxypropionate motif from propylene: Total synthesis of (2R,4R,6R,8R)-2, 4, 6, 8-tetramethyldecanoic acid. Proc. Natl. Acad. Sci. USA 2016, 113, 2857–2861. [Google Scholar] [CrossRef]
- Jeon, J.Y.; Park, S.H.; Kim, D.H.; Park, S.S.; Park, G.H.; Lee, B.Y. Synthesis of polyolefin-block-polystyrene through sequential coordination and anionic polymerizations. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 3110–3118. [Google Scholar] [CrossRef]
- Park, S.S.; Kim, C.S.; Kim, S.D.; Kwon, S.J.; Lee, H.M.; Kim, T.H.; Jeon, J.Y.; Lee, B.Y. Biaxial Chain Growth of Polyolefin and Polystyrene from 1, 6-Hexanediylzinc Species for Triblock Copolymers. Macromolecules 2017, 50, 6606–6616. [Google Scholar] [CrossRef]
- Kim, C.S.; Park, S.S.; Kim, S.D.; Kwon, S.J.; Baek, J.W.; Lee, B.Y. Polystyrene chain growth from di-end-functional polyolefins for polystyrene-polyolefin-polystyrene block copolymers. Polymers 2017, 9, 481. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Park, S.S.; Park, S.H.; Jeon, J.Y.; Kim, H.B.; Lee, B.Y. Preparation of polystyrene-polyolefin multiblock copolymers by sequential coordination and anionic polymerization. RSC Adv. 2017, 7, 5948–5956. [Google Scholar] [CrossRef]
- Keyes, A.; Basbug Alhan, H.E.; Ordonez, E.; Ha, U.; Beezer, D.B.; Dau, H.; Liu, Y.-S.; Tsogtgerel, E.; Jones, G.R.; Harth, E. Olefins and Vinyl Polar Monomers: Bridging the Gap for Next Generation Materials. Angew. Chem. Int. Ed. 2019, 58, 12370–12391. [Google Scholar] [CrossRef]
- Tan, C.; Chen, C. Emerging Palladium and Nickel Catalysts for Copolymerization of Olefins with Polar Monomers. Angew. Chem. Int. Ed. 2019, 58, 7192–7200. [Google Scholar] [CrossRef]
- Zou, C.; Chen, C. Polar-Functionalized, Crosslinkable, Self-Healing, and Photoresponsive Polyolefins. Angew. Chem. Int. Ed. 2020, 59, 395–402. [Google Scholar] [CrossRef]
- Georges, S.; Hashmi, O.H.; Bria, M.; Zinck, P.; Champouret, Y.; Visseaux, M. Efficient One-Pot Synthesis of End-Functionalized trans-Stereoregular Polydiene Macromonomers. Macromolecules 2019, 52, 1210–1219. [Google Scholar] [CrossRef]
- Finnegan, R.A.; Kutta, H.W. Organometallic Chemistry. XII.1 The Thermal Decomposition of n-Butyllithium, a Kinetic Study2, 3. J. Org. Chem. 1965, 30, 4138–4144. [Google Scholar] [CrossRef]
- Boussie, T.R.; Diamond, G.M.; Goh, C.; Hall, K.A.; LaPointe, A.M.; Leclerc, M.K.; Murphy, V.; Shoemaker, J.A.W.; Turner, H.; Rosen, R.K.; et al. Nonconventional Catalysts for Isotactic Propene Polymerization in Solution Developed by Using High-Throughput-Screening Technologies. Angew. Chem. Int. Ed. 2006, 45, 3278–3283. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.J.; Baek, J.W.; Lee, H.J.; Kim, T.J.; Ryu, J.Y.; Lee, J.; Shin, E.J.; Lee, K.S.; Lee, B.Y. Preparation of Pincer Hafnium Complexes for Olefin Polymerization. Molecules 2019, 24, 1676. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.W.; Kwon, S.J.; Lee, H.J.; Kim, T.J.; Ryu, J.Y.; Lee, J.; Shin, E.J.; Lee, K.S.; Lee, B.Y. Preparation of half- and post-metallocene hafnium complexes with tetrahydroquinoline and tetrahydrophenanthroline frameworks for olefin polymerization. Polymers 2019, 11, 1093. [Google Scholar] [CrossRef]
- Rocchigiani, L.; Busico, V.; Pastore, A.; Macchioni, A. Comparative NMR Study on the Reactions of Hf(IV) Organometallic Complexes with Al/Zn Alkyls. Organometallics 2016, 35, 1241–1250. [Google Scholar] [CrossRef]
- De Rosa, C.; Di Girolamo, R.; Talarico, G. Expanding the Origin of Stereocontrol in Propene Polymerization Catalysis. ACS Catal. 2016, 6, 3767–3770. [Google Scholar] [CrossRef]
- Domski, G.J.; Eagan, J.M.; De Rosa, C.; Di Girolamo, R.; LaPointe, A.M.; Lobkovsky, E.B.; Talarico, G.; Coates, G.W. Combined Experimental and Theoretical Approach for Living and Isoselective Propylene Polymerization. ACS Catal. 2017, 7, 6930–6937. [Google Scholar] [CrossRef]
- Frazier, K.A.; Froese, R.D.; He, Y.; Klosin, J.; Theriault, C.N.; Vosejpka, P.C.; Zhou, Z.; Abboud, K.A. Pyridylamido hafnium and zirconium complexes: Synthesis, dynamic behavior, and ethylene/1-octene and propylene polymerization reactions. Organometallics 2011, 30, 3318–3329. [Google Scholar] [CrossRef]
- Cueny, E.S.; Landis, C.R. Zinc-Mediated Chain Transfer from Hafnium to Aluminum in the Hafnium-Pyridyl Amido-Catalyzed Polymerization of 1-Octene Revealed by Job Plot Analysis. Organometallics 2019, 38, 926–932. [Google Scholar] [CrossRef]
- Lee, H.J.; Baek, J.W.; Kim, T.J.; Park, H.S.; Moon, S.H.; Park, K.L.; Bae, S.M.; Park, J.; Lee, B.Y. Synthesis of Long-Chain Branched Polyolefins by Coordinative Chain Transfer Polymerization. Macromolecules 2019, 52, 9311–9320. [Google Scholar] [CrossRef]
- Strohmann, C.; Gessner, V.H. From the Alkyllithium Aggregate [{(nBuLi)2⋅PMDTA}2] to Lithiated PMDTA. Angew. Chem. Int. Ed. 2007, 46, 4566–4569. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, J.; Huang, L.; Zhu, R.; Huang, X.; Moir, R.; Huang, J. KOtBu-Catalyzed lithiation of PMDTA and the direct functionalization of bridged alkenes under mild conditions. Chem. Commun. 2017, 53, 4589–4592. [Google Scholar] [CrossRef] [PubMed]
- Luitjes, H.; Schakel, M.; Aarnts, M.P.; Schmitz, R.F.; de Kanter, F.J.J.; Klumpp, G.W. Reactions of the Butyllithiums with Tertiary Oligoethylenepolyamines. Tetrahedron 1997, 53, 9977–9988. [Google Scholar] [CrossRef]




| Entry | (1-hexyl)2Zn (μmol) | t-BuLi (μmol) | PO (g); FC3 (mol%) b | PS (g); Homo Fraction (%) | Mn (kDa); PDI before Styrene Polym c | Mn (kDa); PDI after Styrene Polym c |
|---|---|---|---|---|---|---|
| 1 d | 100 | 250 | 13.1; 23.4 | 5.0; - | 64.6 (2.10) | 61.3 (2.30) |
| 2 | 100 | 250 | 11.4; 20.5 | 5.0; 21 | 108 (1.48) | 121 (1.48) |
| 3 | 100 | 250 | 12.5; 22.6 | 10; 27 | 92 (1.62) | 111 (1.54) |
| 4 | 200 | 450 | 12.9; 22.4 | 5.0; 28 | 51 (1.66) | 75 (1.33) |
| 5 | 200 | 450 | 15.2; 22.8 | 10; 30 | 48 (1.74) | 89 (1.28) |
| Entry | Initiator | Li (μmol) | yield (g; %) | Mn (Da) b | Mw/Mn | # of PS chains (μmol) c |
|---|---|---|---|---|---|---|
| 1 | 1-octene + n-BuLi + PMDTA in MeCy | 50 | 4.69; 94 | 22,900 | 1.45 | 205 |
| 2 | 1-octene + n-BuLi + PMDTA in MeCy | 70 | 4.62; 92 | 22,800 | 1.39 | 203 |
| 3 | 1-octene + n-BuLi + PMDTA in MeCy | 100 | 4.82; 96 | 23,800 | 1.35 | 203 |
| 4 | Me2NCH2CH2N(Me)CH2CH2N(Me)CH2Li | 100 | 4.75; 95 | 19,700 | 1.25 | 240 |
| 5 | Me2NCH2CH2N(Me)Li | 100 | 1.14; 23 | 7400 | 2.10 | 154 |
| 6 | Me2NCH2CH2N(Me)Li·(PMDTA) | 50 | 4.56; 91 | 21,000 | 1.32 | 217 |
| 7 | Me2NCH2CH2N(Me)Li·(PMDTA) | 70 | 4.63; 93 | 22,300 | 1.33 | 208 |
| 8 | Me2NCH2CH2N(Me)Li·(PMDTA) | 100 | 4.67; 93 | 24,000 | 1.27 | 195 |
| 9 | pentylallyl-Li⋅(PMDTA) | 50 | 5.00; 100 | 21,500 | 1.28 | 233 |
| 10 | pentylallyl-Li⋅(PMDTA) | 70 | 5.00; 100 | 20,800 | 1.24 | 240 |
| 11 | pentylallyl-Li⋅(PMDTA) | 100 | 5.00; 100 | 19,400 | 1.30 | 258 |
| 12 | PhLi⋅(PMDTA) | 50 | 5.00;100 | 22,000 | 1.30 | 227 |
| 13 | PhLi⋅(PMDTA) | 70 | 4.98; 99 | 21,100 | 1.27 | 236 |
| 14 | PhLi⋅(PMDTA) | 100 | 4.98; 99 | 21,000 | 1.24 | 237 |
| 15 | n-BuLi⋅(PMDTA) | 100 | 4.96; 99 | 21,000 | 1.48 | 236 |
| 16 | Me3SiCH2Li⋅(PMDTA) | 100 | 5.00; 100 | 23,000 | 1.25 | 217 |
| Entry | (hexyl)2Zn (μmol) | Initiator | PO (g); FC3 (mol%) b | PS (g); Homo Fraction (%) | Homo-PS Mn (kDa); PDI | Mn (kDa); PDI before Styrene Polym c | Mn (kDa); PDI after Styrene Polymc |
|---|---|---|---|---|---|---|---|
| 1 | 150 | 1-octene + n-BuLi + PMDTA in MeCy | 15.6; 22 | 5.0; 29 | 24 (1.41) | 61 (1.75) | 66 (1.64) |
| 2 | 150 | Me2NCH2CH2N(Me)CH2CH2N(Me)CH2Li | 12.4; 21 | ~0 | 0 | 60 (1.65) | 59 (1.65) |
| 3 | 150 | Me2NCH2CH2N(Me)Li·(PMDTA) | 15.9; 23 | 3.5; 30 | 39 (2.77) | 60 (1.76) | 64 (1.70) |
| 4 | 150 | pentylallyl-Li⋅(PMDTA) | 13.1; 17 | 5.0; 29 | 16 (1.25) | 60 (1.61) | 82 (1.39) |
| 5 | 150 | pentylallyl-Li⋅(PMDTA) | 13.5; 21 | 10; 28 | 27 (1.24) | 62 (1.61) | 99 (1.30) |
| 6 | 300 | pentylallyl-Li⋅(PMDTA) | 14.2; 22 | 5.0; 27 | 11 (1.23) | 40 (1.50) | 51 (1.35) |
| 7 | 300 | pentylallyl-Li⋅(PMDTA) | 13.0; 19 | 10; 28 | 16 (1.24) | 35 (1.54) | 54 (1.26) |
| 8 | 150 | PhLi⋅(PMDTA) | 15.2; 24 | 5.0; 30 | 20 (1.52) | 64 (1.65) | 76 (1.49) |
| 9 | 150 | PhLi⋅(PMDTA) | 13.0; 17 | 10; 34 | 28 (1.39) | 67 (1.63) | 105 (1.29) |
| 10 | 300 | PhLi⋅(PMDTA) | 12.0; 22 | 5.0; 30 | 11 (1.40) | 33 (1.58) | 43 (1.41) |
| 11 | 300 | PhLi⋅(PMDTA) | 14.8; 23 | 10; 33 | 16 (1.38) | 38 (1.64) | 59 (1.34) |
| 12 | 150 | n-BuLi⋅(PMDTA) | 14.6; 24 | 5.0; 45 | 23 (1.33) | 63 (1.73) | 71 (1.65) |
| 13 | 150 | Me3SiCH2Li⋅(PMDTA) | 16.0; 21 | 5.0; 27 | 19 (1.35) | 71 (1.59) | 76 (1.49) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, T.J.; Baek, J.W.; Moon, S.H.; Lee, H.J.; Park, K.L.; Bae, S.M.; Lee, J.C.; Lee, P.C.; Lee, B.Y. Polystyrene Chain Growth Initiated from Dialkylzinc for Synthesis of Polyolefin-Polystyrene Block Copolymers. Polymers 2020, 12, 537. https://doi.org/10.3390/polym12030537
Kim TJ, Baek JW, Moon SH, Lee HJ, Park KL, Bae SM, Lee JC, Lee PC, Lee BY. Polystyrene Chain Growth Initiated from Dialkylzinc for Synthesis of Polyolefin-Polystyrene Block Copolymers. Polymers. 2020; 12(3):537. https://doi.org/10.3390/polym12030537
Chicago/Turabian StyleKim, Tae Jin, Jun Won Baek, Seung Hyun Moon, Hyun Ju Lee, Kyung Lee Park, Sung Moon Bae, Jong Chul Lee, Pyung Cheon Lee, and Bun Yeoul Lee. 2020. "Polystyrene Chain Growth Initiated from Dialkylzinc for Synthesis of Polyolefin-Polystyrene Block Copolymers" Polymers 12, no. 3: 537. https://doi.org/10.3390/polym12030537
APA StyleKim, T. J., Baek, J. W., Moon, S. H., Lee, H. J., Park, K. L., Bae, S. M., Lee, J. C., Lee, P. C., & Lee, B. Y. (2020). Polystyrene Chain Growth Initiated from Dialkylzinc for Synthesis of Polyolefin-Polystyrene Block Copolymers. Polymers, 12(3), 537. https://doi.org/10.3390/polym12030537







