Abstract
Many Zintl phases take up hydrogen and form hydrides. Hydrogen atoms occupy interstitial sites formed by alkali or alkaline earth metals and/or bind covalently to the polyanions. The latter is the case for polyanionic hydrides like SrTr2H2 (Tr = Al, Ga) with slightly puckered honeycomb-like polyanions decorated with hydrogen atoms. This study addresses the hydrogenation behavior of LnTr2, where the lanthanide metals Ln introduce one additional valence electron. Hydrogenation reactions were performed in autoclaves and followed by thermal analysis up to 5.0 MPa hydrogen gas pressure. Products were analyzed by powder X-ray and neutron diffraction, transmission electron microscopy, and NMR spectroscopy. Phases LnAl2 (Ln = La, Eu, Yb) decompose into binary hydrides and aluminium-rich intermetallics upon hydrogenation, while LaGa2 forms a ternary hydride LaGa2H0.71(2). Hydrogen atoms are statistically distributed over two kinds of trigonal-bipyramidal La3Ga2 interstitials with 67% and 4% occupancy, respectively. Ga-H distances (2.4992(2) Å) are considerably longer than in polyanionic hydrides and not indicative of covalent bonding. 2H solid-state NMR spectroscopy and theoretical calculations on Density Functional Theory (DFT) level confirm that LaGa2H0.7 is a typical interstitial metallic hydride.
1. Introduction
Zintl phases are polar intermetallic compounds formed by alkali or alkaline earth metals M and a main group element X of group 13–16. According to the Zintl–Klemm concept, M atoms formally transfer valence electrons to relatively electronegative X atoms, which form polyanions with the connectivity of an isoelectronic element [1,2,3,4,5,6]. K4Si4 is an archetypal example with (Si4)4− tetrahedra akin to P4 tetrahedra in white phosphorous [2]. Zintl phases and related compounds have recently been investigated for potential application, for example as thermoelectric materials (e.g., Mg2Si [7]), in magnetocaloric devices (e.g., Gd5Si4 [8]) or in thin-film solar cells (e.g., BaSi2 [9]).
The hydrogenation of Zintl phases has attracted considerable interest, since the incorporation of hydrogen can influence structures and physical properties (e.g., electrical conductivity, magnetic ordering) in a manifold way [10,11].
Hydrogen gas may oxidize the polyanion, e.g., from Xx− to X(x−1)−, thus decreasing the valence electron concentration (VEC) and—according to the Zintl–Klemm concept—increasing the connectivity of X atoms [12,13,14]. Hydrogen atoms are reduced to hydride ions, H−, during this reaction. They can be incorporated in voids of the crystal structure, e.g., M4 tetrahedra, or bound covalently to the polyanion. A plethora of structural motifs are observed, e.g., hydrogen-decorated polyanionic sheets (SrAl2H2 [13]), ribbons (SrGeH4/3−x [14]), chains (BaSiH2−x [15]), or small oligomers (Ba3Si4H1−2 [16]).
SrGa2 is a classical Zintl phase and crystallizes in the AlB2 structure type with honeycomb-like 63 nets of gallium atoms. According to the Zintl–Klemm concept, two electrons of the strontium atom are formally transferred to gallium atoms resulting in the limiting ionic formula Sr2+(Ga−)2. Ga− ions are iso-valence-electronic to carbon and form planar graphite-like layers. LaGa2 (AlB2 structure type [17], Figure 1) can be described by the limiting ionic formula La3+(Ga−)2e−. In contrast to the electron-precise SrGa2, the compound LaGa2 can be called a metallic or electronically imbalanced Zintl phase, since the excess electrons are mainly located in the conduction band and not transferred to the polyanion [18]. The concept of electronically imbalanced Zintl phases is well known and reported for several other compounds like A5Pn3 (A = Ca, Yb, Sm, Eu; Pn = Sb, Bi) [19,20,21]. A further Zintl phase with an excess electron exhibiting the AlB2 structure-type is SrAlSi (Sr2+(AlSi)−e−). Both SrGa2 and SrAlSi react with hydrogen to form polyanionic hydrides with covalent Tr-H (Tr = Al, Ga) bonds. SrGa2D2 is isostructural to SrAl2D2, where all atoms of the polyanion connect to one hydrogen atom, while in SrAlSiD only aluminium atoms bind to hydrogen [22,23]. Hydrogenation thus transforms the electronically imbalanced (metallic) Zintl phase SrAlSi to an electron-precise Zintl phase hydride SrAlSiH. Metallic Zintl phases LnTt (Ln = La, Nd; Tt = Si, Ge, Sn) react in the same manner and take up hydrogen to form electron-precise Zintl phase hydrides LnTtH [24]. The metallic Zintl phase LaSi2 adopts the ThSi2 structure type, which is characterized by a trigonal prismatic surrounding of silicon atoms by lanthanum atoms and a three-dimensional silicon network (Figure 1) [25]. The reactivity of LaSi2 towards hydrogen was found to be very low [26].
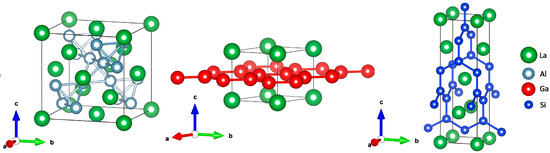
Figure 1.
Crystal structures of LaAl2 (MgCu2 type, left), LaGa2 (AlB2 type, middle), and LaSi2 (ThSi2 type, right). The polyanionic networks are emphasized.
So far, only interstitial hydrides were observed for Zintl phases containing lanthanide elements. The quest for polyanionic hydrides of lanthanide Zintl phases is still open. In this work, we (re)investigate the hydrogenation behavior of the metallic Zintl phases LaGa2 and LaSi2 and of the cubic Laves phases LnAl2 (Ln = La, Eu, Yb, MgCu2 type [27], Figure 1) in order to further elucidate crystal structure and bonding in their hydrides.
Thermal analysis under hydrogen atmosphere was used to characterize the reactivity towards hydrogen. Since hydrogenation reactions often do not yield single crystals, structural investigation was based on powder diffraction. Due to the low scattering power of hydrogen for X-rays, neutron diffraction was carried out for the structural analysis of a deuterated sample of LaGa2. 2H as a quadrupolar nucleus was also used for probing the local structure by NMR spectroscopy and DFT calculations gave insight into the electronic changes upon hydrogenation.
2. Materials and Methods
Synthesis: Due to air sensitivity, all handlings were carried out in an argon-filled glove box. Intermetallic compounds with lanthanum were synthesized from the elements in stoichiometric amounts (La: smart-elements (Vienna, Austria), ≥99.99%, surface cleaned mechanically, cut into small pieces; Si: abcr (Karlsruhe, Germany), 99.9999%; Al, Ga: Smart elements, 99.99%) by fusing the starting materials in a water-cooled copper crucible of an arc furnace under 80 kPa argon atmosphere (dried over P4O10, molecular sieve, silica gel and titanium sponge at 800 K). Ingots were turned over and remelted several times to ensure homogeneity. EuAl2 and YbAl2 were prepared from stoichiometric mixtures of the elements (Eu: Chempur (Karlsruhe, Germany), 99.9%; Yb: Kristallhandel Kelpin (Leimen, Germany), 99.9%) in sealed tantalum ampules, which were heated under dynamic vacuum in a silica-glass tube at 1423 K for 4 h and cooled by switching off the furnace. The hydrides (deuterides) were prepared from well-ground samples of the intermetallic precursors in crucibles made from hydrogen-resistant Böhler (Kapfenberg, Austria) L718-V alloy and reacted with hydrogen (deuterium) gas (hydrogen: Air Liquide (Paris, France), 99.9%; deuterium, Air Liquide (Paris, France), 99.8%) at 2.0 MPa in autoclaves made from the same alloy. The temperature was increased with a rate of 100 K/h to 573 K, held for 48 h, and cooled by switching off the furnace.
Elemental Analysis: The hydrogen contents of the hydrides were determined by elemental analysis with a VARIO EL (Elementar Analysensysteme GmbH, Hanau, Germany) microanalyzer using the carrier gas-hot extraction method in triplicate repetition.
Thermal Analysis: Hydrogenation experiments were carried out in a differential scanning calorimeter (DSC) Q1000 from TA Instruments (New Castle, Delaware, USA) equipped with a gas pressure cell. Powdered samples weighing 10–30 mg were loaded into aluminum pans, which were subsequently crimped. The experiments were performed under isochoric conditions with a heating rate of 10 K/min under 5.0 MPa hydrogen at 298 K, which increased to 6.7 MPa at the final temperature of 703 K.
Powder X-Ray Diffraction (PXRD) Powder X-ray diffraction data were collected on a Huber (Rimsting, Germany) G670 camera with image-plate detection system using Mo-Kα1 or Cu-Kα1 radiation. Flat transmission samples were prepared by grinding and mixing the moisture sensitive powders with Apiezon grease under an argon atmosphere and placing the sample between two sheets of Kapton foil.
Powder Neutron Diffraction (PND): Powder neutron diffraction was carried out at the E9 diffractometer (λ = 1.7982(1) Å) at Helmholtz-Zentrum Berlin für Materialien und Energie, Berlin, Germany [28]. Powdered samples (3 g) were held in gas-tight vanadium containers with 6 mm inner diameter and the measurement time was 8 h. Deuterides instead of hydrides were used in order to avoid the high incoherent scattering of 1H.
Rietveld Refinement: Rietveld refinements [29,30] were performed using TOPAS [31] and FULLPROF [32,33]. Deuterium atoms were located by difference Fourier analysis. Crystal structures were visualized with VESTA [34,35]. Further details of the crystal structure investigations may be obtained from FIZ Karlsruhe, 76344 Eggenstein-Leopoldshafen, Germany (fax: (+49)7247-808-666; e-mail: crysdata@fiz-karlsruhe.de, on quoting the deposition number CSD-1885556 (LaGa2D0.71(2)).
Density Functional Theory (DFT) Calculation: DFT calculations were performed with the Abinit software package [36,37,38,39,40] using generalized gradient approximation (GGA) and the Perdew–Burke–Ernzerhof (PBE) functional [41]. Projector augmented wave (PAW) [42] atomic data were taken from the JTH PAW atomic dataset table [43,44]. The convergence of calculations was tested regarding kinetical energy cutoff, k-point grid and smearing of metallic occupation levels with an accuracy of 0.1 mHartree. The kinetical energy cutoff (ecut) was set to 35 Hartree (pawecutdg = 70 Hartree). The compounds were treated metallic with 1 mHartree Gaussian smearing (occopt = 7, tsmear = 0.001). The smearing contribution of the total energy was lower than 0.1 mHartree. The hydrogen-containing structures were treated similarly. For structure relaxation a 5 × 5 × 4 (LaGa2) and 5 × 5 × 2 (LaGa2H) Monkhorst-Pack-grid was applied [45]. Lattice parameters reproduce experimental data with less than 1% deviation. The density of states was calculated using a 10 × 10 × 8 (LaGa2) and 10 × 10 × 4 (LaGa2H) k-grid and integration was performed using the tetrahedron method.
Transmission Electron Microscopy (TEM): A deuterated sample LaGa2Dx was investigated using a Philips (Amsterdam, Netherland) CM-200 STEM transmission electron microscope (acceleration voltage 200 kV, super twin objective lens, point resolution 0.23 nm) equipped with an EDAX (Mahwah, NJ, USA) energy-dispersive X-ray spectroscopy (EDX) system. A small amount of the sample (approx. 10 mg) was crushed under cyclohexane to a fine powder using an agate mortar. The suspension was allowed to sediment for a few seconds and subsequently one drop of the supernatant suspension was cast on a copper grid coated with holey carbon film (mesh 200; Plano, Wetzlar, Germany). The investigation was carried out using a double-tilt low-background sample holder (Gatan, Pleasanton, CA, USA). EDX analysis was conducted at several areas of the examined crystal (Figure S7 in Supplementary Materials). Kinematical selected-area electron diffraction (SAED) patterns (Figures S8–S10 in Supplementary Materials) were simulated using the JEMS software package [46,47].
Solid-state NMR:2H NMR measurements were performed with a Bruker Avance spectrometer (Bruker Corporation, Billerica, MA, USA) equipped with a B0 = 17.6 T superconducting magnet (frequency 114.92 MHz for 2H). For both static and MAS experiments (5 kHz spinning frequency) a 2.5 mm MAS probe was used. A one-pulse sequence with a 90° pulse length of 7 µs and a recycle delay of 10 s was used. About 1000 scans were recorded.
3. Results
3.1. Hydrogenation Reactions
The hydrogenation of LaGa2 at 2.0 MPa deuterium pressure and 573 K yields a new phase with a very similar X-ray powder diffraction pattern. A Rietveld refinement based on the structure model of LaGa2 shows a decrease of the c lattice parameter from 4.42521(7) Å to 4.33290(5) Å while the a lattice parameter slightly increases from 4.30975(5) Å to 4.32682(3) Å. This results in a negative volume change, which is known for lanthanide-containing hydrides undergoing a metal-semiconductor transition, e.g., LaH2 + ½ H2 → LaH3 [48,49]. The hydrogen content according to elemental analysis is 0.58(1) hydrogen atoms per formula unit, corresponding to a composition LaGa2H0.58.
The cubic Laves phases LnAl2 (Ln = La, Eu, Yb) decompose during the hydrogenation reaction at 5.0 MPa hydrogen pressure and 773 K to binary hydrides LnH2 and aluminium-rich phases (La3Al11, EuAl4, YbAl3; Figures S1–S5 in Supplementary Materials). Differential scanning calorimetry (DSC) of EuAl2 at 5.0 MPa shows a strong exothermic signal at 700 K in accordance with the above-mentioned reaction, while the hydrogenation of YbAl2 does not yield any thermal signal under the same conditions. Additionally, the hydrogenated sample still contains YbAl2 as a minor phase, suggesting a slow reaction.
The powder X-ray diffraction pattern of LaSi2 remains unchanged upon treatment at 773 K and 5.0 MPa hydrogen in an autoclave for 48 h (Figure S6 in Supplementary Materials). The lattice parameters before (a = 4.31565(3) Å, c = 13.8483(1) Å) and after (a = 4.31565(3) Å, c = 13.8407(1) Å) hydrogenation do not differ significantly, indicating little, if any reaction with hydrogen.
3.2. Crystal Structure of LaGa2D0.71(2)
In order to locate hydrogen in the crystal structure of hydrogenated LaGa2, neutron powder diffraction was carried out on a deuterated sample. The powder neutron diffraction pattern shows superstructure reflections, which were not observed in X-ray diffraction patterns (Figure 2), indicating a symmetry reduction with respect to hydrogen-free LaGa2 caused by deuterium atoms. A crystal structure model with a doubled c lattice parameter accounts for all reflections and was used for Rietveld refinements. Deuterium atoms were located in trigonal-bipyramidal La3Ga2 voids by difference Fourier analyses. Rietveld refinements based on this structure model (Table 1) and accounting for small amounts of the secondary phase LaGaDx show good agreement between measured and calculated powder neutron diffraction patterns (Figure 2). The formation of the superstructure can be explained by an ordering of deuterium atoms with alternating occupancies of 67(1)% and 4.5(8)% along [001] (Figure 3, Table 1). The gallium layers are closer to the deuterium position with lower occupancy (Figure 3). This results in two different Ga-D distances (2.099(3) Å and 2.247(3) Å). The crystal structure of LaGa2D0.71(2) is the first example of a Zintl phase hydride derived from the AlB2 type with a lanthanide. CeGa2D0.6 might be another example, but deuterium positions are not known yet [50]. LaGa2D0.71 and CeGa2D0.6 exhibit similar hydrogen contents and lattice parameter changes during hydrogenation. In contrast to the polyanionic hydrides SrAl2H2 and SrAlSiH with stoichiometric composition, LaGa2H0.71(2) is better described as an interstitial hydride with a variable composition. This is supported by the variable unit cell volumes for different samples of LaGa2Hx (V = 70.424(3) Å3 to 70.688(3) Å3).
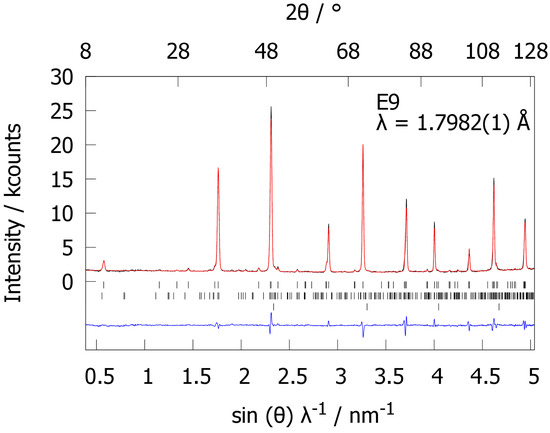
Figure 2.
Rietveld refinement of the crystal structure of LaGa2D0.71(2) based on powder neutron diffraction data (black, E9 at Helmholtz-Zentrum Berlin; red: calculated pattern), Rp = 3.96%, Rwp = 5.55%, Bragg makers from top to bottom: LaGa2D0.71(2) (97.2(8)%), LaGaDx (2.8(2)%), V (sample container); the difference plot is depicted in blue.

Table 1.
Structural parameters of LaGa2D0.71(2), space group P6/mmm, a = 4.3287(3) Å, c = 8.6910(5) Å.
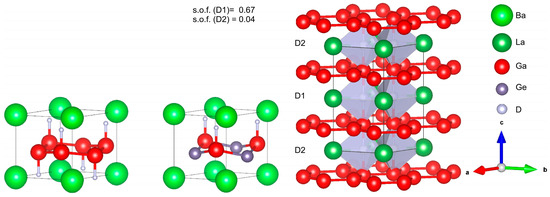
Figure 3.
Crystal structures of BaGa2D2 (left), BaGaGeD (middle) and LaGa2D0.71(2) (right). Covalent bonds of the polyanions are emphasized.
The good agreement between experimental and calculated SAED patterns of LaGa2D0.71(2) confirms the structure model derived by neutron diffraction (Figures S8–S10 in Supplementary Materials). Comparison of the simulated SAED patterns for both structure models and equivalent directions ([112] for LaGa2 and [111] for LaGa2Dx, respectively) of experimental data clearly indicates the presence of a two-fold superstructure (Figure S10 in Supplementary Materials). TEM-EDX analysis of the investigated crystal yields an atomic ratio of 33.5(6)% La and 66.5(6)% Ga (averaged over seven individual point measurements) are in good agreement with the crystal structure model (Table 1).
The comparison of LaGa2H0.71(2) with other Zintl phase hydrides derived from the AlB2 structure type reveals interesting structural differences. Both electron-precise AeGa2 and electronically imbalanced AeGaGe (Ae = Sr, Ba) take up hydrogen to form the polyanionic hydrides AeGa2H2 and AeGaGeH [12]. In these hydrides, bond lengths within the polyanion (Ga-Ga, Ga-Ge, Ge-Ge) are 1.7%–3.2% longer than in the hydrogen-free Zintl phases, as compared to only 0.4% for LaGa2H0.71 (all data for deuterides, Table 2). The chemical bonding within the polyanions can change tremendously upon hydrogenation: either π* states are depopulated and the Ga-Ga bonds contract or π states are depopulated and the bond expands [24]. The effect of the hydrogenation on the E-E bond length is comparably small for LaGa2H0.71(2) with respect to other Zintl phase hydrides (Table 2). The expansion of the Ga-Ga bond lengths in SrGa2D2 and BaGa2D2 can be explained by the depopulation of π states. In contrast, the oxidation of LaGa2 only depopulates La d states (vide infra, Section 3.4) and, hence, has little influence on Ga-Ga distances.

Table 2.
Interatomic distances, their relative changes upon deuteration and lattice parameter ratios of Zintl phases MGa2 (M = Sr, Ba, La), AeGaGe (Ae = Sr, Ba) and their deuterides.
The most significant structural difference between the hydrides is the coordination of hydrogen atoms. While hydrogen atoms in SrGa2H2, BaGa2H2 and BaGaGeH are tetrahedrally coordinated by metal atoms with one covalent Ga-H bond, hydrogen atoms in LaGa2H0.71 are located in trigonal bipyramidal La3Ga2 voids. This kind of fivefold coordination for hydrogen was also observed in ZrBe2H1.49 [51].
The Ga-D distances in SrGa2D2, BaGa2D2, SrGeGaD and BaGeGaD are in the typical range of covalent Ga-D bonds while those in LaGa2D0.71 are much longer (Table 2). The Sr-D and Ba-D bond lengths (Table 2) are comparable with bond lengths in mainly ionic compounds such as SrD2 and BaD2 [52,53]. The distances La-D (Table 2), however, are comparable to those in typical interstitial metallic hydrides, e.g., LaNi3D2.8 [54].
The c/a ratio is an important indicator for the anisotropy of chemical bonding in layered structures, which may be changed considerably upon hydrogenation. For the discussed examples it proves difficult to find a clear tendency. While c/a decreases upon hydrogenation for the compounds BaGa2 and LaGa2, it stays nearly the same for SrGa2 and even increases in the case of BaGeGa and SrGeGa (Table 2). It seems that, in addition to the VEC, the size of the cation plays an important role for the spatial demand of the layers.
3.3. Solid-State NMR
2H solid-state NMR spectroscopy was performed as a local probe for metal-deuterium interactions in LaGa2D0.71(2) (see Figure 4). As the 2H nucleus exhibits a spin quantum number of I = 1, the quadrupolar coupling can give evidence about covalent bonding. As shown for Zintl phase hydrides of silicon, germanium and tin, covalently bonded deuterium atoms show a distinct quadrupolar coupling due to the presence of strong electric field gradients (EFG), whereas investigations of deuterides with D atoms located in tetrahedral voids formed by alkaline earth atoms yield isotropic line spectra [14]. The static and 5 kHz 2H MAS spectra (Figure 4) of LaGa2D0.71(2) show no indication of covalent interactions that should be visible by a quadrupolar powder pattern (Pake doublet) in the static spectrum. The static linewidth is in the order of 6.5 kHz and can to a large extent be explained by dipolar coupling of deuterium nuclei with other NMR-active nuclei in the vicinity, i.e. 2H, 69Ga/71Ga, and 139La atoms. Calculating the total second moment [59] from all NMR-active spins yields a linewidth of 4.5 kHz. The difference to the experimentally observed one results from the residual linewidth present under MAS (2.2 kHz). The reason for this broad line is not fully understood. It could arise from chemical shift variations due to slight differences in the local atomic environment of deuterium atoms. However, the NMR results suggest that deuterium is not covalently bound, in agreement with the results of the crystal structure determination and crystal chemical considerations (Section 3.2).
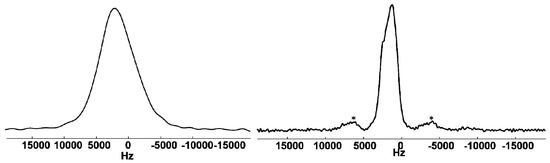
Figure 4.
Static (left) and MAS (right) solid-state 2H NMR spectra of LaGa2D0.71(2), asterisks indicating spinning sidebands.
3.4. Density Funtional Theory (DFT) Calcalations
Three models were used for quantum-mechanical modelling on DFT level, the hydrogen free Zintl phase, a hydride with one (LaGa2H) and a hydride with two hydrogen atoms per formula unit (LaGa2H2) (Tables S1–S3 in Supplementary Materials). The latter is a hypothetical model for evaluating the chances for higher hydrogen contents, e.g., at higher hydrogen pressures. For the calculation of LaGa2 and LaGa2H2, a simple AlB2 type model was used, while the calculation for LaGa2H was performed with only one of the two experimentally found H-atom positions (Table 1). The lattice parameters of LaGa2 calculated by structure optimization are in good agreement with experimental values (Figure S11 and Tables S1–S3 in Supplementary Materials). For LaGa2H (a = 4.351 Å, c = 4.320 Å), the lattice parameter c is decreased and for LaGa2H2 (a = 4.289 Å, c = 4.570 Å) it is expanded with respect to the parent compound LaGa2 (a = 4.3474 Å, c = 4.4085 Å). This observation is in agreement with the experimentally obtained structure model for LaGa2D0.71(2). The slight deviation of the lattice parameter c is attributed to the lower hydrogen content in the experimental structure (LaGa2H0.71 vs. LaGa2H). The Ga-H distance of 2.238 Å in the DFT-optimized LaGa2H matches the experimental value well.
The density of states at the Fermi level has a large d contribution from lanthanum for the Zintl phase, emphasizing the metallic character of LaGa2 (Figure 4), while electrons at the Fermi level in AeTr2 and AeTrTt (Ae = Ca, Sr, Ba; Tr =Al, Ga; Tt = Si, Ge) are rather located in the polyanion [56,58,60]. This is supported by the expansion of the bond lengths in the polyanion for alkaline-earth-containing compounds, while the Ga-Ga distance in LaGa2H0.71 is almost unaffected, since mostly d states are depopulated.
This finding supports the limiting ionic formula La3+(Ga−)2e− where the excess electron is in the conduction band. The density of states of LaGa2H shows a pseudo-gap at the Fermi level, which is quite common for hydrogenated Zintl phases [14,15,24]. The limitations of DFT methods in predicting bandgaps should be kept in mind, however. In comparison, the hydrides AeTr2H2 show pseudo-bandgaps as well, suggesting poor metallic conductivity [56], but AeTrTtH (Ae = Ca, Sr, Ba; Tr = Al, Ga; Tt = Si, Ge) are small band semiconductors [58,60]. Even though the structures of the different hydrides and the electron counts are similar, the consequences for the electronic properties are tremendous.
The contribution of gallium states at the Fermi level is very small (Figure 4). This finding explains the small expansion of the Ga-Ga bonds during hydrogenation, while the Ga-p contribution of SrGa2 is noticeably higher [55] and leads to a stronger expansion of Ga-Ga bonds.
The partial DOS for hydrogen atoms shows a large dispersion of the s band from −7 eV to 0 eV (Figure 5), which can be an indicator for a strong interaction of hydrogen atoms with all surrounding atoms as typical for metallic hydrides.
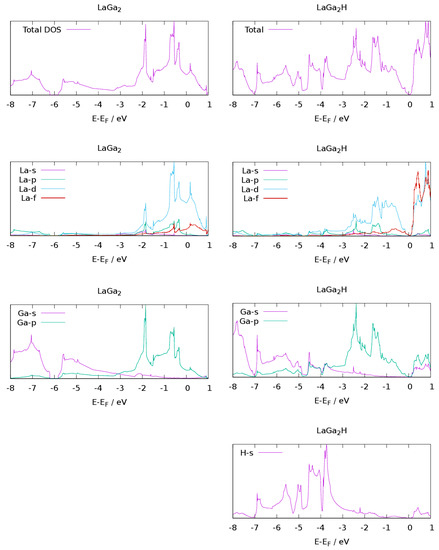
Figure 5.
Total (tDOS) and l-resolved partial (pDOS) density of states of LaGa2 and LaGa2H; energies are referenced to the Fermi level.
4. Discussion
The Laves phases LaAl2, EuAl2, and YbAl2 decompose into binary hydrides and aluminum-rich phases upon reaction with hydrogen. Despite the crystal-chemical similarity between europium and strontium, no analogous hydrides were formed. LaSi2 does not react with hydrogen up to 773 K and 5.0 MPa hydrogen even though it features trigonal-bipyramidal La3Si2 voids (in analogy to La3Ga2 in LaGa2).
The electronically imbalanced Zintl phase LaGa2 forms a metallic hydride with a nearly unchanged heavy atom structure. During the hydrogenation, d-states of lanthanum atoms are depopulated. Hydrogen atoms are incorporated in trigonal-bipyramidal La3Ga2 voids. The hydrides AeTr2H2, AeTrTtH and LaGa2H0.71 show pronounced differences with respect to the bonding character and electronic properties. Even though the VECs of LaGa2 and AeTrTt are equal, the incorporation of hydrogen atoms differs strongly. Compounds AeTrTtH exhibit covalent Tr-H bonds, comparable to those in AeTr2H2, while LaGa2H0.71 is a metallic hydride with hydrogen atoms in interstitial voids. The changes of the Tr-Tr and Tr-Tt bond lengths during hydrogenation suggest that the incorporation of hydrogen depopulates the π states of AeTr2 and AeTrTt, while the hydrogenation of LaGa2 mainly affects the d-states of lanthanum. The DOS of the pristine Zintl phases and their corresponding hydrides confirms this suggestion. The electronic properties of the corresponding hydrides are even more remarkable. While AeTrTtH and LaGa2H are narrow-bandgap semiconductors, AeTr2H2 remains metallic according to DFT calculations, although covalent Tr-H bonds are formed.
So far, covalent Tr-H or Tt-H (Tr = Al, Ga, In; Tt = Si, Ge, Sn) bonds in Zintl phase hydrides with lanthanide elements Ln are elusive. The strong Ln-H interaction seems to dominate chemical bonding, while covalent Tr-H or Tt-H bonds are competitive only with respect to the somewhat weaker Ae-H bonds. This leads to the occupation of interstitials with trigonal-bipyramidal (La3Ga2) or tetrahedral (Ln4) voids by hydrogen atoms for the lanthanide containing compounds and the exclusive formation of covalent Tr-H or Tt-H bonds for alkaline earth or alkali metal containing compounds. These examples emphasize the subtle influence of properties of Zintl phases and their hydrides due to small changes in element and/or electron count and show a potential way to tune electronic properties by an elegant combination of elements. Zintl phases with alkaline earth, lanthanide and Tr or Tt elements are an obvious target for further exploration of bonding situations in this class of compounds.
Supplementary Materials
Further data on thermal analyses, X-ray diffraction, selected-area electron diffraction and DFT calculations are available online at https://www.mdpi.com/2073-4352/9/4/193/s1.
Author Contributions
Conceptualization, H.K.; Methodology, H.K. and A.W.; Validation, A.W., M.B., A.F., and C.B.; Formal Analysis, A.W. and C.B.; Investigation, A.W., M.B., C.B., and A.F.; Resources, H.K. and O.O.; Data Curation, A.W.; Writing—Original Draft Preparation, A.W. and H.K.; Writing—Review and Editing, H.K., A.W., O.O., C.B., A.F., and M.B.; Visualization, A.W.; Supervision, H.K.; Project Administration, H.K.; Funding Acquisition, H.K.
Funding
This work was founded by the Deutsche Forschungsgemeinschaft (Ko 1803/8).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zintl, E.; Kaiser, H. Über die Fähigkeit der Elemente zur Bildung negativer Ionen. Z. Anorg. Allg. Chem. 1933, 211, 113–131. [Google Scholar] [CrossRef]
- Schäfer, H.; Eisenmann, B.; Müller, W. Zintl-Phasen: Übergangsformen zwischen Metall- und Ionenbindung. Angew. Chem. 1973, 85, 742–760. [Google Scholar] [CrossRef]
- Nesper, R. Chemische Bindungen—Intermetallische Verbindungen. Angew. Chem. 1991, 103, 805–834. [Google Scholar] [CrossRef]
- Kauzlarich, S.M. Chemistry, Structure and Bonding of Zintl Phases and Ions; Wiley-VCH: Weinheim, Germany, 1996; ISBN 978-0-471-18619-9. [Google Scholar]
- Nesper, R. The Zintl-Klemm Concept—A Historical Survey. Z. Anorg. Allg. Chem. 2014, 640, 2639–2648. [Google Scholar] [CrossRef]
- Corbett, J.D. Polyanionic Clusters and Networks of the Early p-Element Metals in the Solid State: Beyond the Zintl Boundary. Angew. Chem. Int. Ed. 2000, 39, 670–690. [Google Scholar] [CrossRef]
- Akasaka, M.; Iida, T.; Matsumoto, A.; Yamanaka, K.; Takanashi, Y.; Imai, T.; Hamada, N. The thermoelectric properties of bulk crystalline n- and p-type Mg2Si prepared by the vertical Bridgman method. J. Appl. Phys. 2008, 104, 013703. [Google Scholar] [CrossRef]
- Gschneidner, K.A., Jr.; Pecharsky, V.K. Magnetocaloric Materials. Annu. Rev. Mater. Sci. 2000, 30, 387–429. [Google Scholar] [CrossRef]
- Toh, K.; Saito, T.; Suemasu, T. Optical Absorption Properties of BaSi2 Epitaxial Films Grown on a Transparent Silicon-on-Insulator Substrate Using Molecular Beam Epitaxy. Jpn. J. Appl. Phys. 2011, 50, 068001. [Google Scholar] [CrossRef]
- Simon, A. Oxidation durch Wasserstoff in der Chemie und Physik der Seltenerdmetalle. Angew. Chem. 2012, 124, 4354–4361. [Google Scholar] [CrossRef]
- Ångstrom, J.; Johansson, R.; Sarkar, T.; Sørby, M.H.; Zlotea, C.; Andersson, M.S.; Nordblad, P.; Scheicher, R.H.; Häussermann, U.; Sahlberg, M. Hydrogenation-Induced Structure and Property Changes in the Rare- Earth Metal Gallide NdGa: Evolution of a [GaH]2− Polyanion Containing Peierls-like Ga−H Chains. Inorg. Chem. 2016, 55, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Häußermann, U. Coexistence of hydrogen and polyanions in multinary main group element hydrides. Z. Kristallogr. 2008, 223, 628–635. [Google Scholar] [CrossRef]
- Häussermann, U.; Kranak, V.F.; Puhakainen, K. Hydrogenous Zintl Phases: Interstitial Versus Polyanionic Hydrides. In Zintl Phases. Structure and Bonding; Fässler, T., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; Volume 139, ISBN 978-3-642-21150-8. [Google Scholar]
- Auer, H.; Guehne, R.; Bertmer, M.; Weber, S.; Wenderoth, P.; Hansen, T.C.; Haase, J.; Kohlmann, H. Hydrides of Alkaline Earth−Tetrel (AeTt) Zintl Phases: Covalent Tt−H Bonds from Silicon to Tin. Inorg. Chem. 2017, 56, 1061–1071. [Google Scholar] [CrossRef]
- Auer, H.; Schlegel, R.; Oeckler, O.; Kohlmann, H. Structural and Electronic Flexibility in Hydrides of Zintl Phases with Tetrel-Hydrogen and Tetrel-Tetrel Bonds. Angew. Chem. Int. Ed. 2017, 56, 12344–12347. [Google Scholar] [CrossRef] [PubMed]
- Kranak, V.F.; Benson, D.E.; Wollmann, L.; Mesgar, M.; Shafeie, S.; Grins, J.; Häussermann, U. Hydrogenous Zintl Phase Ba3Si4Hx (x = 1–2): Transforming Si4 “Butterfly” Anions into Tetrahedral Moieties. Inorg. Chem. 2014, 53, 756–764. [Google Scholar] [CrossRef] [PubMed]
- Laves, F. Die Kristallstrukturen von CaGa2, LaGa2 und CeGa2. Naturwissenschaften 1943, 11–13, 145. [Google Scholar] [CrossRef]
- Harima, H.; Yanase, Y. Electronic Structure and Fermi Surface of LaGa2. J. Phys. Soc. Jpn. 1991, 60, 2718–2723. [Google Scholar] [CrossRef]
- Leon-Escamilla, E.A.; Corbett, J.D. Hydrogen stabilization: Nine isotypic orthorhombic A5Pn3H phases (among A = Ca, Sr, Ba, Sm, Eu, Yb; Pn = Sb, Bi) formerly described as binary β-Yb5Sb3-type compounds. J. Alloys Compd. 1998, 265, 104–114. [Google Scholar] [CrossRef]
- Leon-Escamilla, E.A.; Corbett, J.D. Hydrogen in Polar Intermetallics. Binary Pnictides of Divalent Metals with Mn5Si3-type Structures and Their Isotypic Ternary Hydride Solutions. Chem. Mater. 2006, 18, 4782–4792. [Google Scholar] [CrossRef]
- Leon-Escamilla, E.A.; Stassi, P.D.C.; D, J. Corbett, Hydrogen in polar intermetallics: Syntheses and structures of the ternary Ca5Bi3D0.93, Yb5Bi3Hx, and Sm5Bi3H∼1 by powder neutron or single crystal X-ray diffraction. Solid State Chem. 2010, 183, 114–119. [Google Scholar] [CrossRef]
- Gingl, F.; Vogt, T.; Akiba, E. Trigonal SrAl2H2: The first Zintl phase hydride. J. Alloys Compd. 2000, 306, 127–132. [Google Scholar] [CrossRef]
- Björling, T.; Noréus, D.; Jansson, K.; Andersson, M.; Leonova, E.; Edén, M.; Hålenius, U.; Häussermann, U. SrAlSiH: A Polyanionic Semiconductor Hydride. Angew. Chem. Int. Ed. 2005, 44, 7269–7273. [Google Scholar] [CrossRef] [PubMed]
- Werwein, A.; Auer, H.; Kuske, L.; Kohlmann, H. From Metallic LnTt (Ln = La, Nd; Tt = Si, Ge, Sn) to Electron-precise Zintl Phase Hydrides LnTtH. Z. Anorg. Allg. Chem. 2018, 644, 1532–1539. [Google Scholar] [CrossRef]
- Brauer, G.; Haag, H. Über Darstellung und Kristallstruktur der Disilicide von einigen Metallen der Seltenen Erden. Z. Anorg. Allg. Chem. 1952, 267, 198–212. [Google Scholar] [CrossRef]
- Bohmhammel, K.; Henneberg, E. Hydriding and dehydriding behavior of lanthanum silicides. Solid State Ion. 2001, 141–142, 599–602. [Google Scholar] [CrossRef]
- Harris, I.R.; Mansey, R.C.; Raynor, G.V. Rare earth intermediate phases: III. The cubic laves phases formed with aluminium and cobalt. J. Less-Common Met. 1965, 9, 270–280. [Google Scholar] [CrossRef]
- Franz, A.; Hoser, A. E9: The Fine Resolution Powder Diffractometer (FIREPOD) at BER II. J. Large-Scale Res. Facil. 2017, 3, A103. [Google Scholar] [CrossRef]
- Rietveld, H.M. Line profiles of neutron powder-diffraction peaks for structure refinement. Acta Cryst. 1967, 22, 151–152. [Google Scholar] [CrossRef]
- Rietveld, H.M. A profile refinement method for nuclear and magnetic structures. J. Appl. Crystallogr. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Bruker AXS, TOPASc Version 5. Available online: www.bruker-axs.com (accessed on 28 March 2019).
- Rodrıguez-Carvajal, J. Recent advances in magnetic structure determination by neutron powder diffraction. Physica B 1993, 192, 55–69. [Google Scholar] [CrossRef]
- Rodrıguez-Carvajal, J. FullProf.2k, Version 5.30—Mar2012-ILL JRC. Institut Laue-Langevin: Grenoble, France, 2018. [Google Scholar]
- VESTA—Visualisation for Electronic and Structural Analysis; Version 3.3.1; Koichi Momma and Fujio Izumi: Tokyo, Japan, 2018.
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Abinit v. 8.8.2, GNU General Public License. Available online: http://www.abinit.org (accessed on 28 March 2019).
- Gonze, X.; Beuken, J.-M.; Caracas, R.; Detraux, F.; Fuchs, M.; Rignanese, G.-M.; Sindic, L.; Verstraete, M.; Zerah, G.; Jollet, F.; et al. First-principles computation of material properties: The ABINIT software project. Comput. Mater. Sci. 2002, 25, 478–492. [Google Scholar] [CrossRef]
- Gonze, X. A brief introduction to the ABINIT software package. Z. Kristallogr. Cryst. Mater. 2005, 220, 558–562. [Google Scholar] [CrossRef]
- Gonze, X.; Amadon, B.; Anglade, P.-M.; Beuken, J.-M.; Bottin, F.; Boulanger, P.; Bruneval, F.; Caliste, D.; Caracas, R.; Côté, M.; et al. ABINIT: First-principles approach to material and nanosystem properties. Comput. Phys. Commun. 2009, 180, 2582–2615. [Google Scholar] [CrossRef]
- Gonze, X.; Jollet, F.; Araujo, F.A.; Adams, D.; Amadon, B.; Applencourt, T.; Audouze, C.; Beuken, J.-M.; Bieder, J.; Bokhanchuk, A.; et al. Recent developments in the ABINIT software package. Comput. Phys. Commun. 2016, 205, 106–131. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef]
- JTH PAW Atomic Datasets, Version 1.0. Available online: https://www.abinit.org/downloads/PAW2 (accessed on 28 March 2019).
- Jollet, F.; Torrent, M.; Holzwarth, N. Generation of Projector Augmented-Wave atomic data: A 71 element validated table in the XML format. Comput. Phys. Commun. 2014, 185, 1246–1254. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Stadelmann, P.A. EMS—A software package for electron diffraction analysis and HREM image simulation in materials science. Ultramicroscopy 1987, 21, 131–146. [Google Scholar] [CrossRef]
- JEMS Version 4.4631U. JEMS-SAAS: Saas-Fee, Switzerland, 2016.
- Villars, P.; Calvert, L.D. Pearson’s Handbook of Crystallographic Data for Intermetallic Phases 1; American Society for Metals: Metals Park, OH, USA, 1985; ISBN 0-87170-217-7. [Google Scholar]
- Villars, P.; Calvert, L.D. Pearson’s Handbook of Crystallographic Data for Intermetallic Phases 2; American Society for Metals: Metals Park, OH, USA, 1985; ISBN 0-87170-217-7. [Google Scholar]
- Shashikalaa, K.; Sathyamoorthy, A.; Raj, P.; Dhar, S.K.; Malik, S.K. Structure and magnetic properties of CeGa2D0.6 system. J. Alloys Compd. 2007, 436, 19–22. [Google Scholar] [CrossRef]
- Andresen, A.F.; Otnes, K.; Maeland, A.J. Neutron scattering investigations of Be2ZrH1.5 and Be2ZrD1.5. J. Less-Common Met. 1983, 89, 201–204. [Google Scholar] [CrossRef]
- Bronger, W.; Chi-Chien, S.; Müller, P. Die Kristallstruktur von Bariumhydrid, ermittelt über Neutronenbeugungsexperimente an BaD2. Z. Anorg. Allg. Chem. 1987, 545, 69–74. [Google Scholar] [CrossRef]
- Brese, N.E.; O’Keeffe, M.; von Dreele, R.B. Synthesis and crystal structure of SrD2 and SrND and bond valence parameters for hydrides. J. Solid State Chem. 1990, 88, 571–576. [Google Scholar] [CrossRef]
- Denys, R.V.; Riabov, A.B.; Yartys, V.A.; Delaplane, R.G.; Sato, M. Hydrogen storage properties and structure of La1−xMgx(Ni1−yMny)3 intermetallics and their hydrides. J. Alloys Compd. 2007, 446–447, 166–172. [Google Scholar] [CrossRef]
- Harms, W.; Wendorff, M.; Röhr, C. Mixed Alkaline Earth Trielides AIIM1IIIxM2III2−x (AII = Ca, Sr, Ba; MIII = Al, Ga, In). A Structural and Theoretical Study. Z. Naturforsch. 2006, 62b, 177–194. [Google Scholar] [CrossRef]
- Björling, T.; Noréus, D.; Häussermannm, U. Polyanionic Hydrides from Polar Intermetallics AeE2 (Ae = Ca, Sr, Ba; E = Al, Ga, In). J. Am. Chem. Soc. 2006, 128, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.J.; Wu, Y.; Kranak, V.F.; Newman, N.; Garcia-Garcia, A.R.F.J.; Häussermann, U. Structural properties and superconductivity in the ternary intermetallic compounds MAB (M = Ca, Sr, Ba; A = Al, Ga, In; B = Si, Ge, Sn). Phys. Rev. B 2009, 80, 064514. [Google Scholar] [CrossRef]
- Evans, M.J.; Holland, G.P.; Garcia-Garcia, F.J.; Häussermann, U. Polyanionic gallium hydrides from AlB2-type precursors AeGaE (Ae = Ca, Sr, Ba; E = Si, Ge, Sn). J. Am. Chem. Soc. 2008, 130, 12139–12147. [Google Scholar] [CrossRef] [PubMed]
- Van Vleck, J.H. The Dipolar Broadening of Magnetic Resonance Lines in Crystals. Phys. Rev. 1948, 74, 1168–1183. [Google Scholar] [CrossRef]
- Lee, M.H.; Björling, T.; Hauback, B.C.; Utsumi, T.; Moser, D.; Bull, D.; Noréus, D.; Sankey, O.F.; Häussermann, U. Crystal structure, electronic structure, and vibrational properties of MAlSiH (M = Ca, Sr, Ba): Hydrogenation-induced semiconductors from the AlB2-type alloys MAlSi. Phys. Rev. B 2008, 78, 195209. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).