Abstract
Three 2D new coordination polymers Co2(L1)2(1,10-Phenanthroline)2(DMF)0.5(H2O) (1), (H2L1 = Pyridine-3,5-dicarboxylic acid) Co(L1)(2,2-bipyridine) (2), and Co(L2)(2,2-bipyridine) (DMF) (3) (H2L2 = Pyridine-3,4-dicarboxylic acid) were synthesized through a solvothermal reaction of cobalt nitrate and pyridine carboxylic acid ligand with the auxiliary ligand (1,10-Phenanthroline or 2,2-bipyridine). They were characterized by X-ray diffraction and elemental analysis, infrared spectroscopy, thermogravimetry analysis, and magnetism. Compounds 1–3 featured 2D hexagonal (6,3) networks which linked into 3D supramolecular architectures through π–π interaction. In addition, compounds 1 and 2 showed the antiferromagnetic exchange interactions, and the magnetic property of compound 3 exhibited ferromagnetic exchange interactions.
1. Introduction
The rational design and synthesis of coordination polymers have been under intensive investigation in the past two decades because of their potential applications in a number of fields, such as in the areas of storage, separation, catalysis, magnetism, and luminescence [1,2,3,4,5,6,7,8,9]. However, the reasonable synthesis and design of such target materials are still a great challenge in crystal engineering. There are many factors that can influence the final structures of coordination polymers, such as metal ions, ligands, metal-to-ligand ratio, solvent system, pH, etc., and among these factors, the selection of suitable organic ligands plays a crucial rule in the constructions of fascinating coordination polymers [10,11,12]. Just like the reported structures, it is a convenient strategy to construct coordination polymers with a single ligand containing N or O donor atoms [13,14]. Nevertheless, for the purpose of getting more coordination polymers with novel structures and excellent properties, single coordination atoms sometimes cannot meet the needs of researchers. Therefore, much attention has been paid to organic ligands containing various coordination atoms, of which pyridylcarboxylates are one variety. Accordingly, many works have been conducted to construct coordination polymers with different structures using pyridine carboxylic acid ligands, and various extended structures have been successfully constructed with different metal ions, for instance, six novel 3D coordination polymers with interesting topologies were synthesized by Zhu with pyridine-3,4-dicarboxylic acid [15], and a series of Ln coordination polymers with photoluminescent properties were constructed based on pyridine-3,5-dicarboxylic acid [16,17,18,19,20,21,22]. Taking into account the reported works, in this paper we chose the symmetrical pyridine-3,5-dicarboxylic acid and asymmetrical pyridine-3,4-dicarboxylic acid as the ligands because these two ligands can be deprotonated to form HL− and L2− anions which have the flexible and versatile coordination modes to bridge metal ions. Furthermore, the π-electric conjugated system of the pyridine dicarboxylic acid and auxiliary ligands (1,10-Phenanthroline and 2,2-bipyridine) are beneficial for the formation of stable supramolecular structures [15,21,22,23]. Meanwhile we chose cobalt nitrate as the metal source, and thus they could form both discrete and consecutive new Co compounds with magnetic behavior, having potential applications in magnetic materials [24,25].
In this contribution, three new 2D coordination polymers—Co2(L1)2(1,10-Phenanthroline)(DMF)0.5(H2O) (1), Co(L1)(2,2-bipyridine) (2), and Co(L2)(2,2-bipyridine)(DMF) (3)—were isolated under solvothermal conditions and characterized by single-crystal X-ray diffraction analysis, elemental analysis, infrared (IR) spectroscopy, and thermogravimetry analysis (TGA), and the magnetic susceptibilities were also investigated.
2. Materials and Methods
2.1. Materials and Physical Measurements
All reagents and solvents used in the experiment were obtained directly from a commercial source, and used without further purification. Inductively coupled plasma (ICP) analysis of Co and elemental analysis were performed on a Perkin-Elmer Optima 3300DV Spectrometer and a Perkin-Elmer 2400 element analyzer, respectively. Infrared (IR) spectra were recorded on a Nicolet Impact 410 FTIR spectrometer using a KBr pellet in the range of 4000–400 cm−1. Powder X-ray diffraction (PXRD) measurements were executed by using a Rigaku D/max 2550 X-Ray Powder Diffractometer. Thermogravimetric analysis was performed with a TGA Q500 V20.10 Build 36 instrument with a heating rate of 10 °C/min in a flowing N2 atmosphere. Magnetic susceptibility data were obtained by SQUID magnetometer (Quantum MPMS) in the range of 2–300 K using an applied field of 1000 Oe.
2.2. Methods
2.2.1. Synthesis of {Co2(L1)2(1,10-Phenanthroline)2}(DMF)0.5(H2O) (1)
H2L1 (0.05 mmol, 8.356 mg), Co(NO3)2·6H2O (0.1 mmol, 29.1 mg), and 1,10-Phenanthroline (0.1 mmol, 18 mg) were dissolved in DMF (5 mL), then the solution was transferred and sealed in a 15 mL Teflon-lined stainless steel autoclave, and heated at autogenous pressure at 120 °C for 3 days, followed by slow cooling (10 °C·h−1) to room temperature. The resulting mixture was washed by DMF, yielding purple block crystals and dried in the air. Yield for compound 1: 65%. IR (KBr disc, cm−1) 3449 (m), 3056 (w), 2359 (w), 1617 (s), 1571 (s), 1514 (m), 1359 (s), 1275 (m), 1120 (w), 1093 (m), 842 (s), 765 (s), 720 (s), 680 (s), 565 (w), 442 (w). Elemental analysis (%): Calcd for: C39.5H27.5Co2N6.5O9.5: Co 13.65 C 54.94 H 3.21 N 10.54; found: Co 13.49 C 55.03 H 3.26 N 10.52.
2.2.2. Synthesis of Co(L1)(2,2-bipyridine) (2)
H2L1 (0.05 mmol, 8.356 mg), Co(NO3)2·6H2O (0.1 mmol, 29.1 mg), and 2,2-bipyridine (0.1 mmol, 18 mg) were dissolved in DMF (5 mL), then the solution was transferred to a 15 mL Teflon-lined stainless autoclave, which was heated to 120 °C for 3 days, followed by slow cooling (10 °C·h−1) to room temperature. The resulting mixture was washed by DMF, then the purple block crystals were collected and air-dried. Yield for compound 2: 65%. IR (KBr disc, cm−1) 3449 (m), 3056 (w), 2359 (w), 1617 (s), 1571 (s), 1514 (m), 1359 (s), 1275 (m), 1120 (w), 1093 (m), 842 (s), 765 (s), 720 (s), 680 (s), 565 (w), 442 (w). Elemental analysis (%): Calcd for: Co 15.5 C38H22Co2N6O8: C 56.4 H 2.72 N 10.4; found: Co 15.45 C 56.61 H 2.83 N 10.36.
2.2.3. Synthesis of Co(L2)(2,2-bipyridine)(DMF) (3)
The mixture of Co(NO3)2·6H2O (0.1 mmol, 29.1 mg), H2L2 (0.05 mmol, 8.356 mg), 2,2-bipyridine (0.2 mmol, 31.2 mg), DMF(3 mL), CH3CH2OH (2 mL), and H2O (1 mL) were transferred to a 15 mL Teflon-lined stainless steel autoclave, and heated at 100 °C for 3 days, followed by slow cooling (10 °C·h−1) to room temperature. The resulting mixture was washed by DMF, and purple columnar crystals were collected and air-dried (yield 42%). IR (KBr disc, cm−1) 3449 (m), 3095 (w), 3056 (w), 1972 (w), 1623 (s), 1559 (s), 1436 (m), 1404 (m), 1378 (s), 1294 (w), 1165 (w), 1062 (w), 875 (m), 842 (m), 765 (s), 733 (m), 675 (m), 604 (w), 572 (w), 456 (w). Elemental analysis (%): Calcd for: C20H18CoN4O5: Co 13.0 C 53.0 H 4.0 N 12.36; found: Co 13.16 C 53.06 H 3.98 N 12.47.
2.3. Crystal Structural Determination
Single crystal X-ray diffraction data for compounds 1–3 were obtained using a Rigaku RAXIS-RAPID equipped with graphite-monochromated Mo Kα radiation (λ = 0.71073 Å) at 293 K. The data processing was accomplished with the PROCESS-AUTO processing program. The structures were solved with the direct methods of the SHELXL crystallographic software package and refined on F2 by full-matrix least square techniques. All non-hydrogen atoms of the three compounds were refined with anisotropic thermal parameters. All hydrogen atoms of the organic molecule were geometrically placed and added to the structure factor calculation. Crystal data and structure refinement details of compounds 1–3 are summarized in Table 1. Crystallographic data for the structures reported in this paper have been deposited in the Cambridge Crystallographic Data Centre (CCDC), and the CCDC numbers of the three compounds are CCDC-1876151 (1), CCDC-1876152 (2), and CCDC-1876153 (3). As for the molecular formula of compounds 1 and 3, it is difficult to obtain the exact solvent molecules in the structure, so we further determined these using Elemental analysis, TGA and Platon program, and the solvent composition (compound 1: 0.5DMF + H2O; compound 2: DMF) was calculated from the TGA and elemental analysis that were in agreement with the data from Platon program [26].

Table 1.
Crystallographic data for compounds 1–3.
3. Results and Discussion
3.1. Crystal Structures of Co2(L1)2(1,10-Phenanthroline)2(DMF)0.5(H2O) (1)
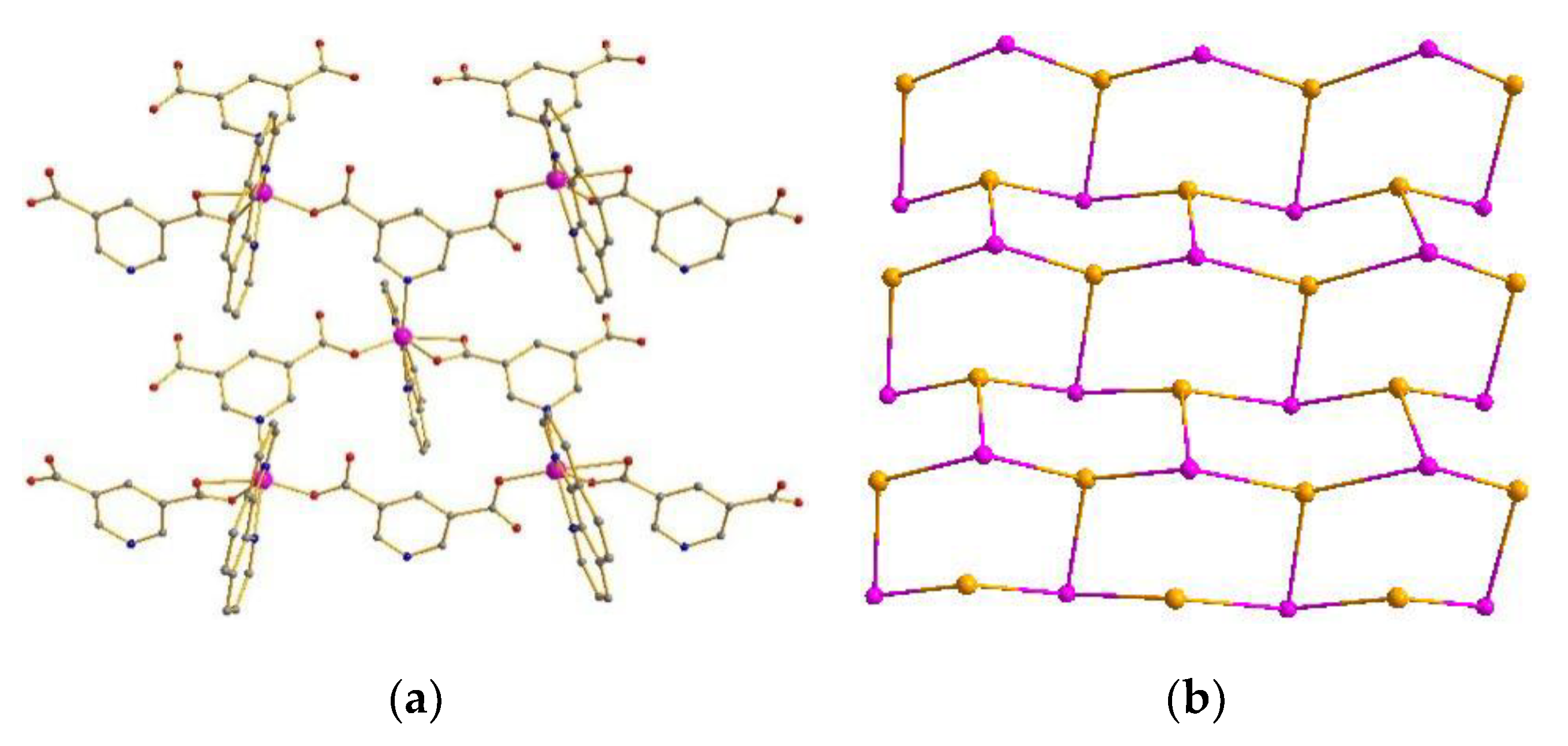
Compound 1 crystallizes in the monoclinic space group P21/n. The asymmetric unit of 1 contains two Co(II) atoms, two L1 ligands, and two 1,10-Phenanthroline molecules. 1,10-Phenanthroline molecule (Figure S1 in the supplementary materials). The Co atom is hexacoordinated, adopting [CoN3O3] distorted octahedron geometry coordination environments. Each Co atom is coordinated with one O atom from one L1 ligand, two O atoms from one L1′ ligand in bidentate coordinated mode, one N atom from one L1 ligand, and two N atoms from one 1,10-Phenanthroline molecule (Figures S1 and S2). Accordingly, the Co–O and Co–N bond lengths are 2.000 Å−2.417 Å and 2.097−2.165 Å, respectively. The Co–O and Co–N distances are quite similar to the normal Co–O and Co–N distances [27,28,29,30,31]. In compound 1, the L1 ligand shows two types of coordination modes. In addition, L1a adopts a μ2–η1:η1 bridging coordination mode to connect three Co ions with the Co···Co separations of 0.7402 nm, 0.9902 nm, and 1.031 nm. Meanwhile, L1b adopts the μ4–η1:η1:η1:η1 coordination mode to connect three Co ions with the Co···Co separations of 0.9639 nm, 0.9874 nm, and 1.031 nm (Figure S2). Compound 1 features a 2D hexagonal (6,3) layer with hcb topology, where both L1 and L1′ act as tri-connected nodes, and the auxiliary ligand occupies two reaction sites of the distorted octahedron. As a result, the hexacoordinated Co atom provides a tri-connected node (Figure 1a,b) [32,33,34,35]. Furthermore, the 2D layers are extended into a 3D supramolecular architecture by π–π interactions between the 1,10-Phenanthroline molecules with center-to-center distances of 3.34−3.45 Å (Figure S7) [36,37,38,39,40].
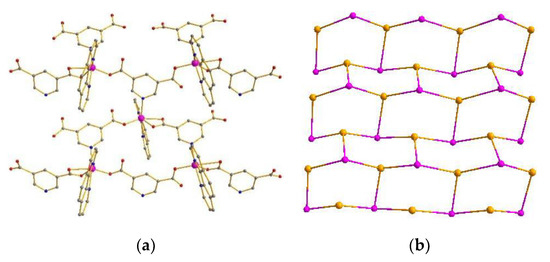
Figure 1.
(a) 2D network of compound 1. (b) Topology of compound 1.
3.2. Crystal Structures of Co(L1)(2,2-bipyridine) (2)
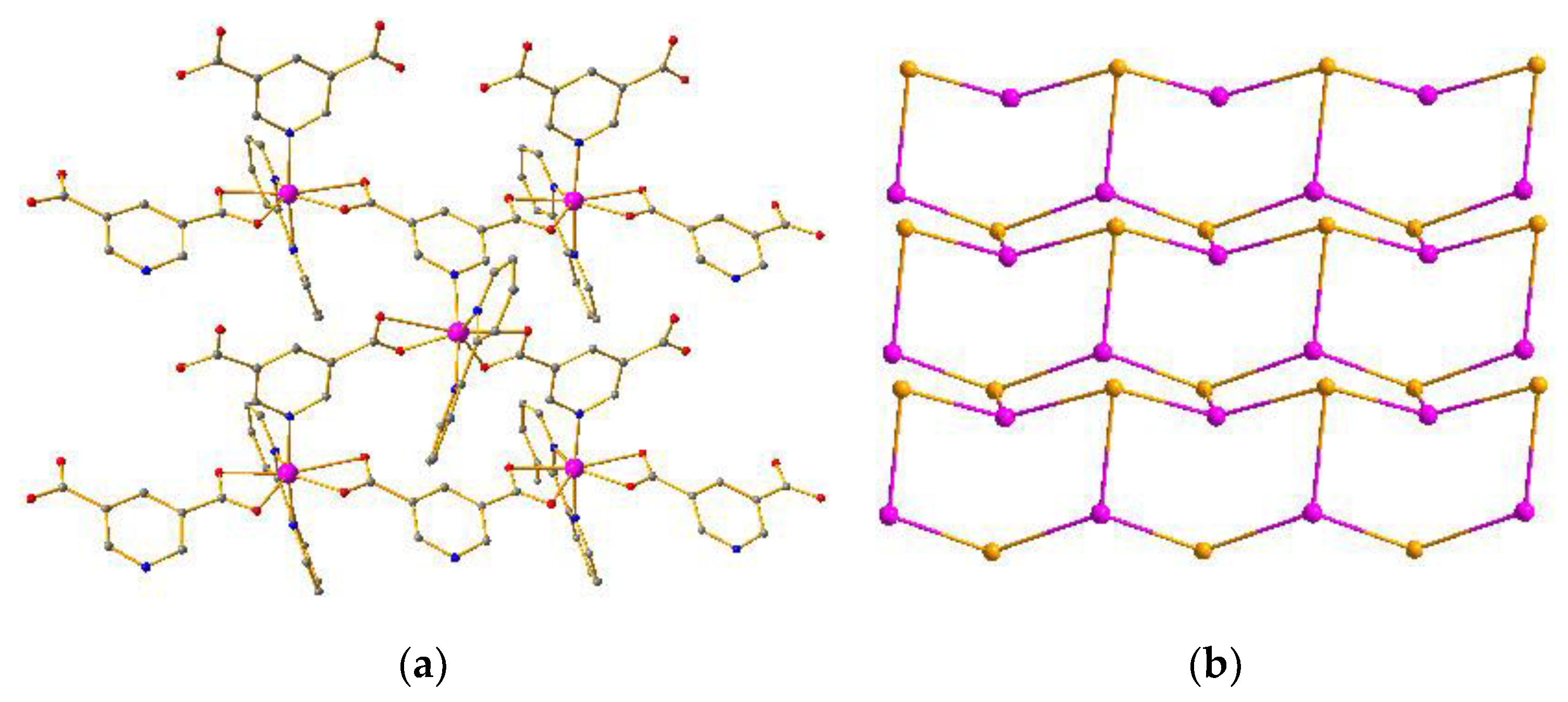
Compound 2 crystallizes in the orthorhombic system with the P212121 space group. The asymmetric unit of crystal 2 consists of one Co atom, one L1 ligand, and one 2,2-bypyridine. As depicted in Figure 2a, the Co center adopts a distorted heptacoordinated pentagonal bipyramid configuration through the coordination of three nitrogen atoms from one 2,2-bypyridine and one L1 ligand, and four oxygen atoms from two individual L1 ligands in a chelating mode (Figures S3 and S4). The Co–O (Co–O = 2.0642 Å−2.7512 Å) and Co–N (Co–N = 2.1052 Å−2.1340 Å) distances are quite similar to the normal ranges [27,28,29,30,31]. The L1 ligand adopts a bidentate coordination mode and utilizes one nitrogen atom to connect three Co atoms with the Co···Co separations of 0.7395 nm, 0.7714 nm, and 0.9977 nm. The neighboring three Co atoms are bridged by six L1 ligands into a 2D hexagonal (6,3) network (Figure 2a) with hcb topology (Figure 2b) [32,33,34,35]. Furthermore, the adjacent layers are further linked into a 3D supramolecular architecture by π–π interactions between the 2,2-bipyridine molecules with center-to-center distances of 3.66−3.80 Å (Figure S8) [36,37,38,39,40].
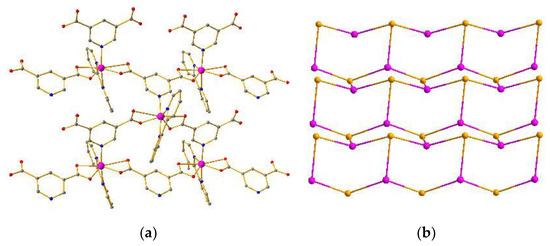
Figure 2.
(a) 2D network of compound 2. (b) Topology of compound 2.
3.3. Crystal Structures of Co(L2)(2,2-bipyridine)(DMF) (3)
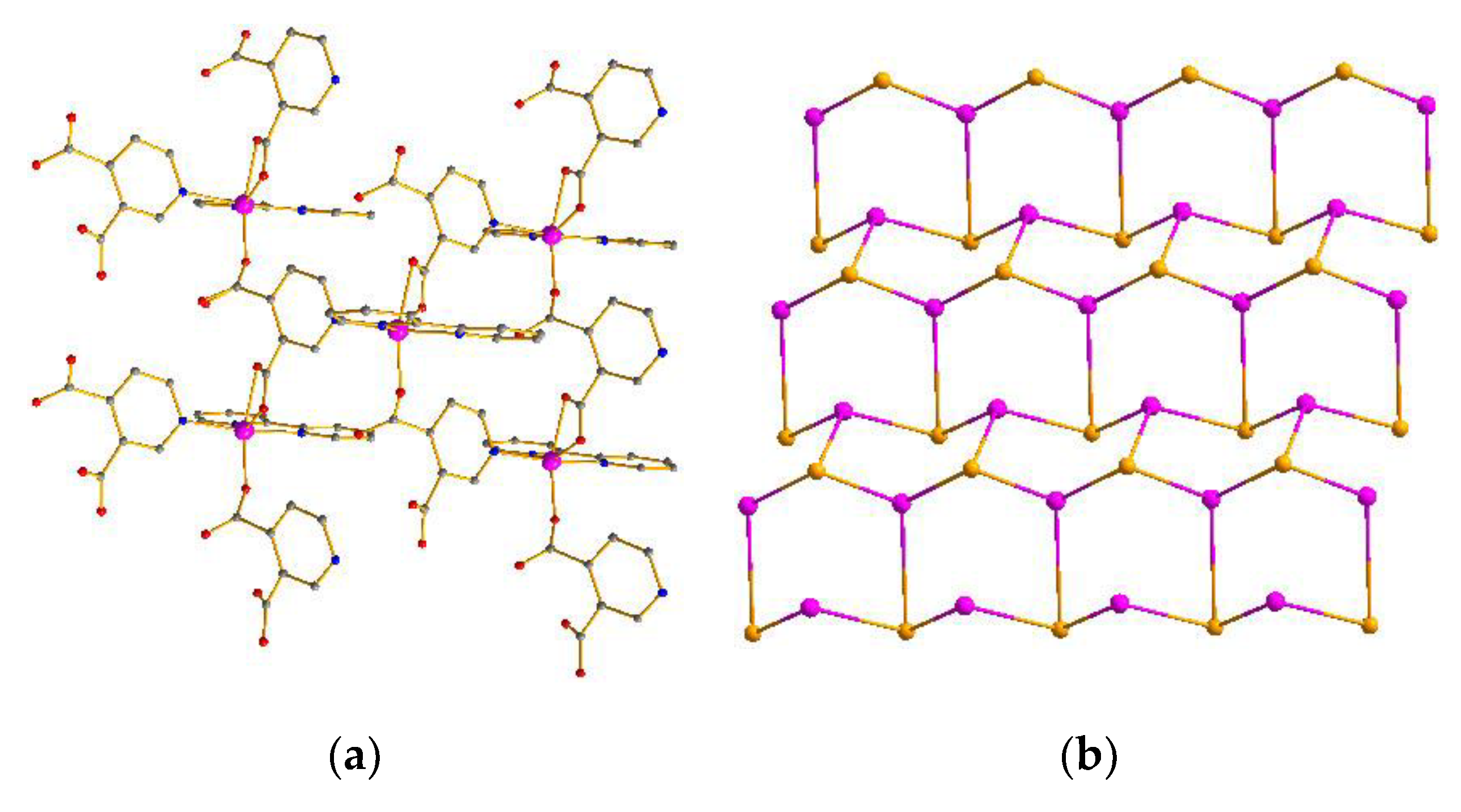
Compound 3 crystallizes in the monoclinic system with the P1n1 space group. The asymmetric unit of compound 3 contains a crystallographically independent Co atom, a L1 ligand, and a 2,2-bipyridine molecule. As depicted in Figure 3a, the Co atom is hexacoordinated, adopting [CoN3O3] distorted octahedron geometry coordination environments. Each Co atom is coordinated with three oxygen atoms of two different L2 ligands, three nitrogen atoms from one 2,2-bipyridine, and one L2 ligand (Figure S5). Accordingly, Co−O and Co−N bond lengths are 2.000 Å−2.2275 Å and 2.088−2.172 Å, respectively, which are acceptable bond lengths [27,28,29,30,31]. The L2 ligand adopts a chelate-monodentate coordination and utilizes one nitrogen atom to connect three Co atoms with the Co···Co separations of 0.7642 nm, 0.7662 nm, and 0.8146 nm (Figure S6). The neighboring three Co atoms are bridged by six other L2 ligands into a 2D hexagonal (6,3) network (Figure 3a) with hcb topology (Figure 3b) [32,33,34,35]. Furthermore, the adjacent layers are further linked into a 3D supramolecular architecture by π–π interactions between the 2,2-bipyridine molecules with center-to-center distances of 3.56–3.92 Å (Figure S9) [36,37,38,39,40].
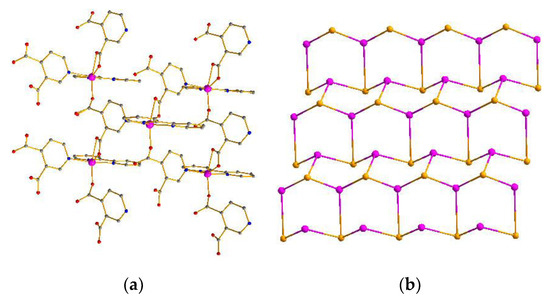
Figure 3.
(a) 2D network of compound 3. (b) Topology of compound 3.
3.4. X-Ray Power Diffraction Analysis and Thermal Analysis
The diffraction peaks of compounds 1–3 were confirmed with good agreement between the experimental (black line) and simulated PXRD patterns (red line) (Figures S10–S12), and the phenomenon indicates that the synthesized compounds 1–3 have good phase purity.
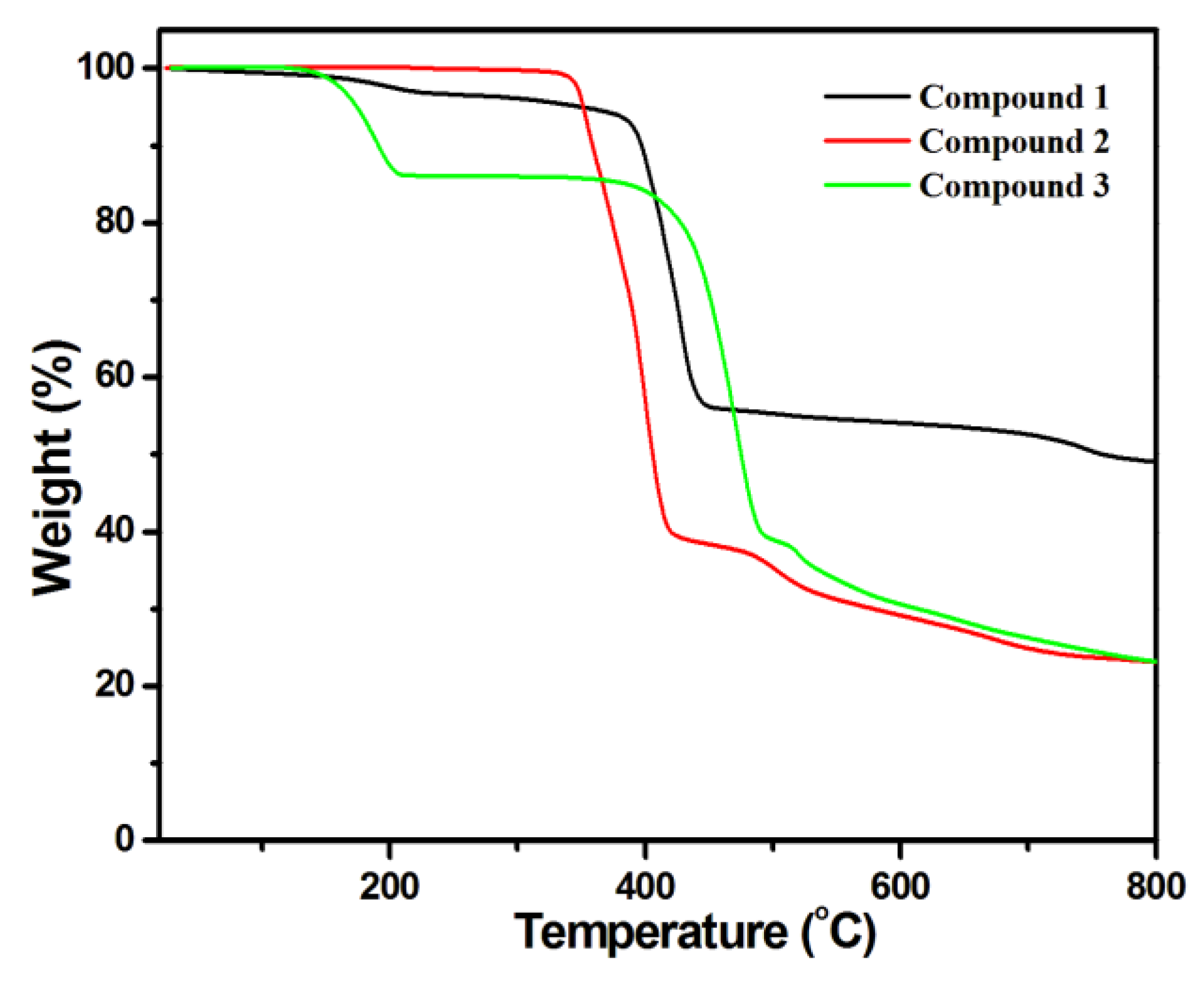
The thermal stability of compounds 1–3 were examined by TG analysis in the range of 25−800 °C, and the results are given in Figure 4. For 1, this led to weight loss from 135 to 378 °C as uncoordinated water and DMF molecules were separated from the framework (Obsd. 6.26%, Calcd. 6.3%), and further loss resulted from the decomposition of organic components. Finally, a residue of Co−O was left. For 2, the framework started to decompose from 338 to 800 °C, and Co−O was the final residue. In terms of 3, weight loss occurred at 141−400 °C because of the removal of uncoordinated DMF molecules in the framework (Obsd. 16.3%, Calcd. 16.14%), and the decomposition of the framework occurred at 220 °C, so a residue of CoO remained.
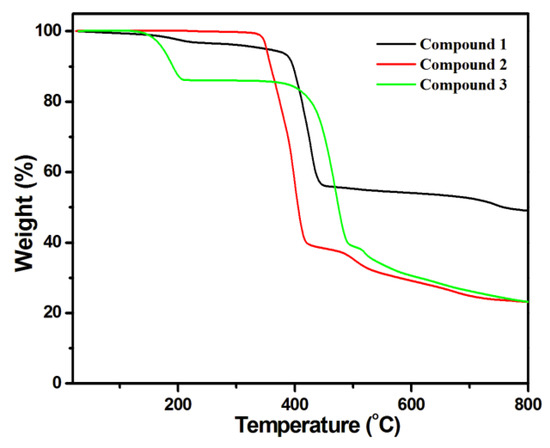
Figure 4.
Thermogravimetric analysis for compounds 1–3.
3.5. Magnetic Properties
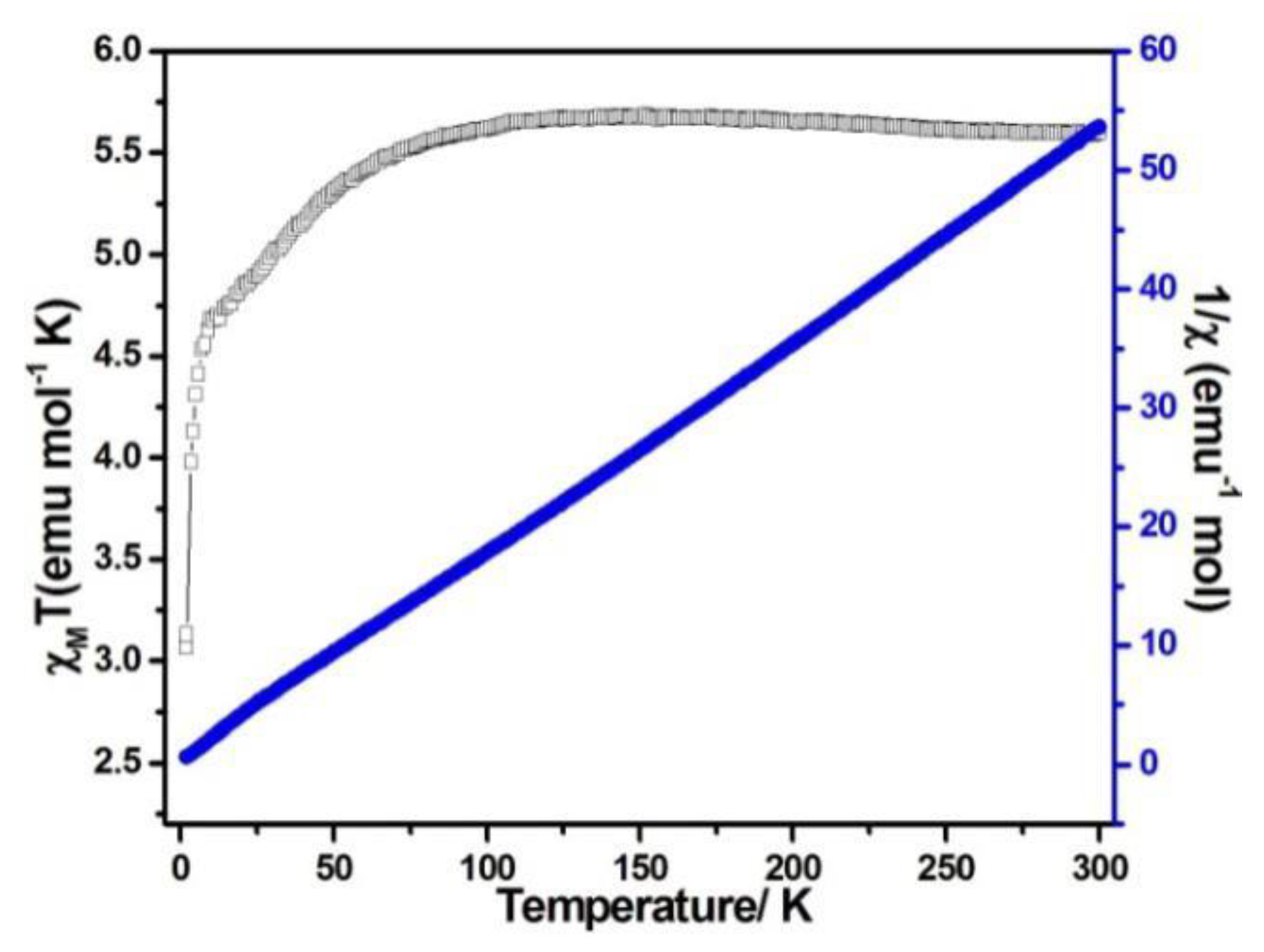
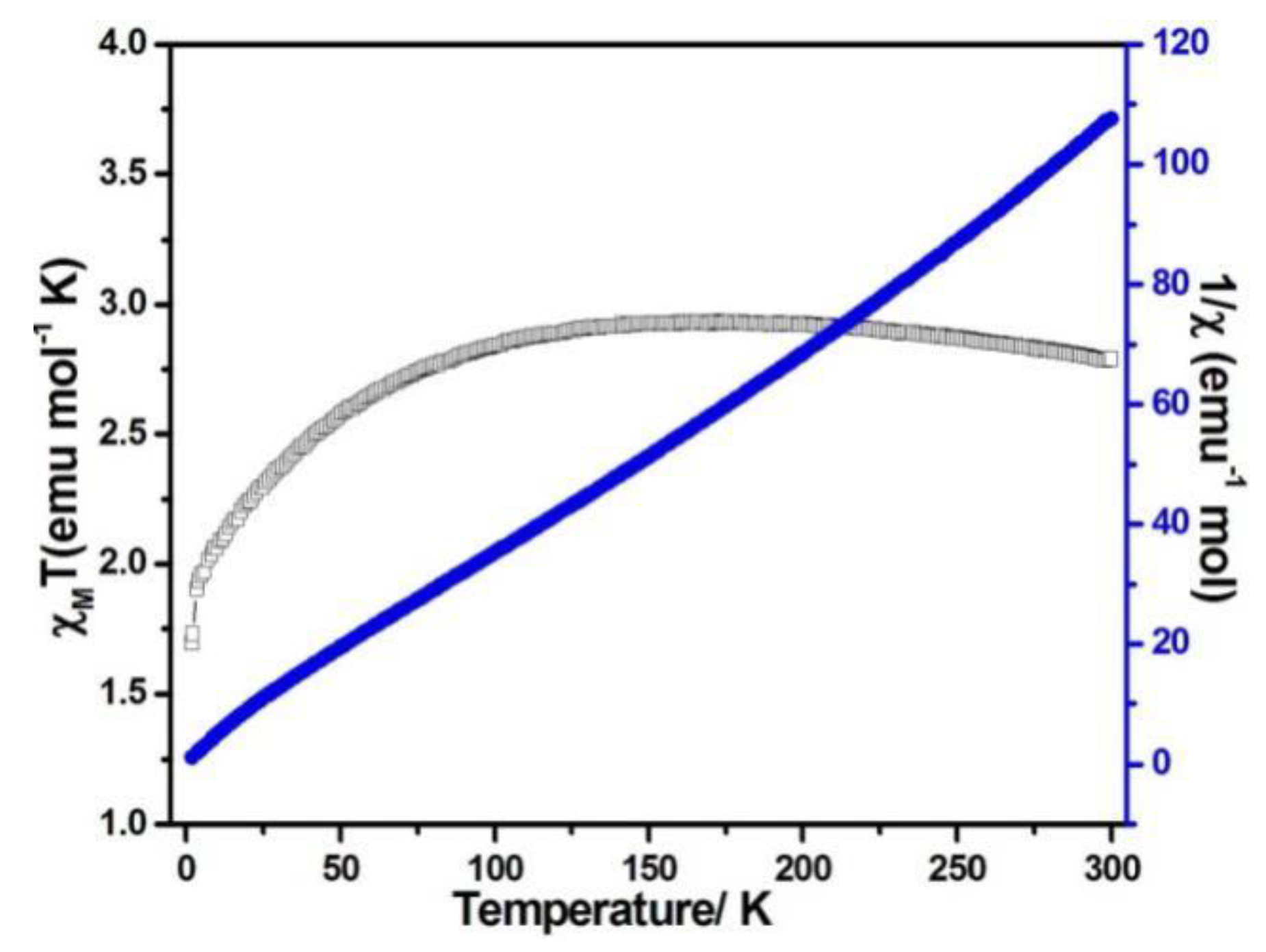
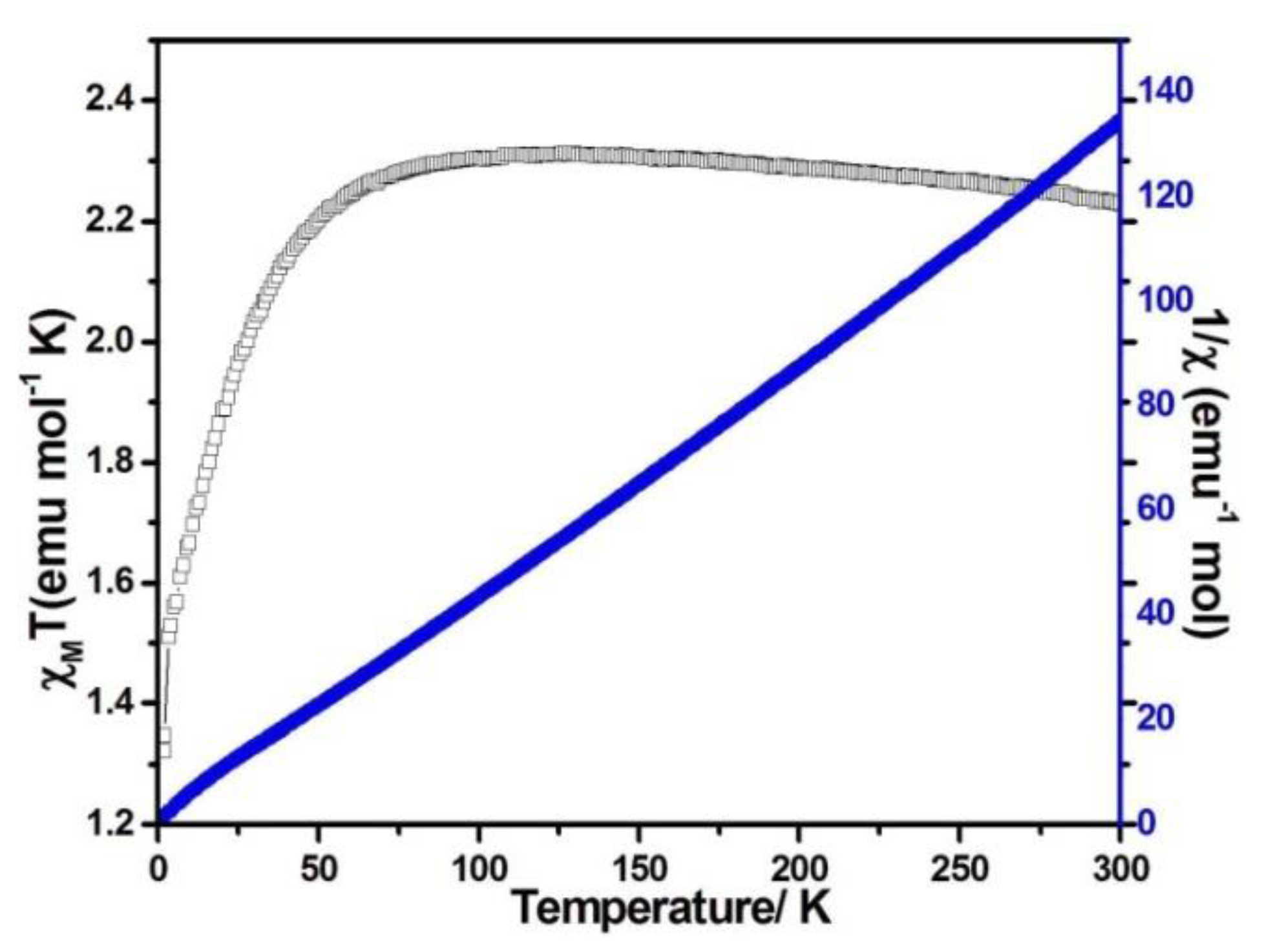
When the magnetic field was 1000 Oe and the temperature was in the range of 2–300 K, the variable temperature magnetic susceptibility of compounds 1–3 was measured. The curves of χMT and χM−1 vs. T of compounds 1–3 are presented in Figure 5, Figure 6, and Figure 7, respectively. The χMT value of compound 1 at 300 K was 5.60 emu·K mol−1 for a Co2 unit, where the value was much higher than the theoretical χMT value of the spin-only one for two isolated Co2+ ions (3.75 emu·K mol−1 and S = 3/2), which is mainly due to the spin-orbit coupling of the high-spin Co2+ ions. Upon the temperature cooling to 50 K, the χMT value was kept roughly constant. Nevertheless, it decreased suddenly and gave the χMT value 3.07 emu·K mol−1 at 2 K. The sudden decrease below 50 K was due to the antiferromagnetic coupling between the paramagnetic centers and the zero-field splitting (Figure 5). The χMT value of compound 2 at 300 K was 2.79 emu·K mol−1 for a Co ion, where the value was much higher than the theoretical χMT value of the spin-only one for one isolated Co2+ ion (1.875 emu·K mol−1 and S = 3/2), but it still fell within the usual range for octahedral Co2+ ions in the 4T2g state. Upon the temperature cooling to 50 K, the χMT value was kept roughly constant. The phenomenon is reminiscent of antiferromagnetic behavior (Figure 6). The χMT value of compound 3 at 300 K was 2.23 emu·K mol−1 for a Co ion, where the value was much higher than the theoretical χMT value for one isolated Co2+ ion (1.875 emu·K mol−1 and S = 3/2), though it fell within the usual range for octahedral Co2+ ions in the 4T2g state. The χMT value was kept roughly constant until the temperature decreased to 50 K, suggesting a ferromagnetic interaction between Co ions (Figure 7). Curie−Weiss law was used to fit the χM−1 data from 2–300 K, acquiring θ = −1.77 K, C = 5.67 emu·K mol−1 for compound 1. The negative θ value and χMT trend with temperature fully demonstrated an antiferromagnetic effect of compound 1. In compound 2, the parameters C = 1.0 emu·K mol−1 and θ = 0 K indicated an antiferromagnetic effect of compound 2, while in compound 3, giving C = 2.2 emu·K mol−1 and θ = 6.5 K, a ferromagnetic effect was suggested between the Co2+ ions (Table 2) [24,25].
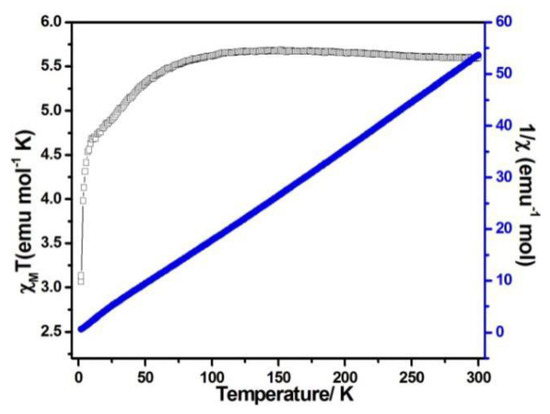
Figure 5.
χMT vs. T (filled triangles) and χM−1 (filled squares) plots for compound 1 at 0.1 T.
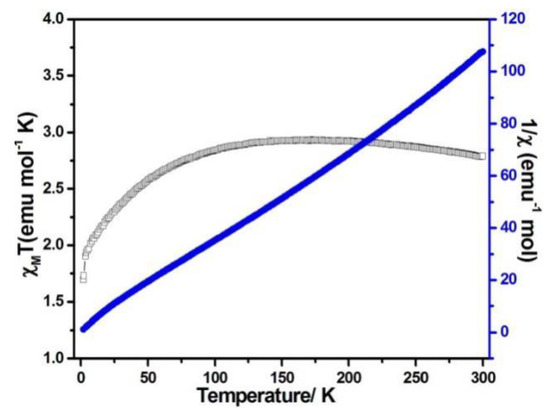
Figure 6.
χMT vs. T (filled triangles) and χM−1 (filled squares) plots for compound 2 at 0.1 T.
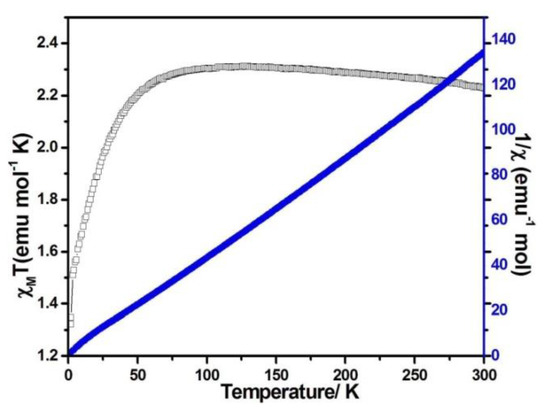
Figure 7.
χMT vs. T (filled triangles) and χM−1 (filled squares) plots for compound 3 at 0.1 T.

Table 2.
Magnetic susceptibility of compounds 1–3.
4. Conclusions
Three 2D coordination polymers were prepared based on cobalt nitrate and H2L1, H2L2 ligands. Compounds 1–3 all featured 2D hexagonal (6,3) layers with hcb topology. Further stacking of these layers on the basis of π–π interactions resulted in 3D supramolecular architectures. Additionally, the magnetic measurements of compounds 1 and 2 showed antiferromagnetic exchange interactions, while compound 3 exhibited ferromagnetic exchange interactions. This investigation provides some useful information to design and yield new coordination polymers with pyridine-type ligands and further enriches the magnetism of Co coordination polymers.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4352/9/3/166/s1. Tables S1–S3: Selected bond distances and angles, Figures S1–S6: Structural information of compounds 1–3, Figure S7–S9: 3D supramolecular architecture of compounds 1–3, Figures S10–S13: PXRD data of compounds 1–3, the ligands of H2L1 and H2L2, Figures S14–S16: IR of compounds 1–3, Figure S17: Photographs of compounds 1–3, Figure S18: SEM images of compounds 1–3.
Author Contributions
X.Z. and X.G. conceived and designed the experiments; X.Z. performed the experiments; L.L. and Y.P. analyzed the data; Z.S. collected the data; X.T. supervised the work. All the authors contributed to the manuscript revision.
Funding
This work was supported by the Open Project of State Key Laboratory of Inorganic Synthesis and Preparation of Jilin University 2017−35, the Research Fund for the Doctoral Program of Weifang University (2016BS06), the Natural Science Foundation of Shandong Province of China (ZR2017MB056), and the National Natural Science Foundation Youth Fund (201802104).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Espallargas, G.M.; Coronado, E. Magnetic functionalities in MOFs: From the framework to the pore. Chem. Soc. Rev. 2018, 47, 533–557. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.X.; Jiao, X.L.; Li, C.; Chen, D.R. Flexible self-supported metal–organic framework mats with exceptionally high porosity for enhanced separation and catalysis. J. Mater. Chem. A 2018, 6, 334–341. [Google Scholar] [CrossRef]
- Chughtai, A.H.; Ahmad, N.; Younus, H.A.; Laypkov, A.; Verpoort, F. Metal–organic frameworks: Versatile heterogeneous catalysts for efficient catalytic organic transformations. Chem. Rev. 2015, 44, 6804–6849. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.W.; Chen, L.F.; Cui, H.; Zhang, J.Y.; Zhang, L.; Su, C.Y. Applications of metal–organic frameworks in heterogeneous supramolecular catalysis. Chem. Soc. Rev. 2014, 43, 6011–6061. [Google Scholar] [CrossRef]
- Hu, Z.C.; Deibert, B.G.; Li, J. Luminescent metal–organic frameworks for chemical sensing and explosive detection. Chem. Soc. Rev. 2014, 43, 5815–5840. [Google Scholar] [CrossRef] [PubMed]
- Getman, R.B.; Bae, Y.S.; Wilmer, C.E.; Snurr, R.Q. Review and Analysis of Molecular Simulations of Methane, Hydrogen, and Acetylene Storage in Metal–Organic Frameworks. Chem. Rev. 2012, 112, 703–723. [Google Scholar] [CrossRef] [PubMed]
- Li, J.R.; Sculley, J.; Zhou, H.C. Metal–Organic Frameworks for Separations. Chem. Rev. 2012, 112, 869–932. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.J.; Yue, Y.F.; Qian, G.D.; Chen, B.L. Luminescent Functional Metal–Organic Frameworks. Chem. Rev. 2012, 112, 1126–1162. [Google Scholar] [CrossRef]
- Corma, A.; García, H.; Llabrés i Xamena, F.X. Engineering Metal Organic Frameworks for Heterogeneous Catalysis. Chem. Rev. 2010, 110, 4606–4655. [Google Scholar] [CrossRef]
- Li, B.Y.; Zhou, X.J.; Zhou, Q.; Li, G.H.; Hua, J.; Bi, Y.; Li, Y.J.; Shi, Z.; Feng, S.H. Design and construction of coordinationpolymers based on 2,2′-dinitro-4,4′-biphenyldicarboxylate and imidazole-basedligands: The effect of ligand length and metal ions. CrystEngComm 2011, 13, 4592–4598. [Google Scholar] [CrossRef]
- Zhang, J.P.; Lin, Y.Y.; Zhang, W.X.; Chen, X.M. Temperature or Guest-Induced Drastic Single-Crystal-to-Single-Crystal Transformations of a Nanoporous Coordination Polymer. J. Am. Chem. Soc. 2005, 127, 14162–14163. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.P.; Li, D.S.; Fu, F.; Dong, W.W.; Zhao, J.; Zou, K.; Wang, Y.Y. Stoichiometry of N-Donor Ligand Mediated Assembly in the ZnII-Hfipbb System: From a 2-Fold Interpenetrating Pillared-Network to Unique (3,4)-Connected Isomeric Nets. Cryst. Growth Des. 2011, 11, 3850–3857. [Google Scholar] [CrossRef]
- Li, B.Y.; Zhang, Z.J.; Li, Y.; Yao, K.X.; Zhu, Y.H.; Deng, Z.Y.; Yang, F.; Zhou, X.J.; Li, G.H.; Wu, H.H.; et al. Enhanced Binding Affinity, Remarkable Selectivity, and High Capacity of CO2 by Dual Functionalization of a rht-Type Metal-Organic Framework. Angew. Chem. Int. Ed. 2012, 1, 1412–1415. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, X.M.; Ma, J.P.; Liu, Q.K.; Wang, P.; Dong, Y.B. Cu(I)-MOF: Naked-eye colorimetric sensor for humidity and formaldehyde in single-crystal-to-single-crystal fashion. Chem. Commun. 2014, 50, 1444–1446. [Google Scholar]
- Xue, M.; Zhu, G.S.; Ding, H.; Wu, L.; Zhao, X.J.; Jin, Z.; Qiu, S.L. Six Three-Dimensional Metal–Organic Frameworks with (3,4)-, (4,5)-, and (3,4,5)-Connected Nets Based on Mixed Ligands: Synthesis, Structures, and Adsorption Properties. Cryst. Growth Des. 2009, 9, 1481–1488. [Google Scholar] [CrossRef]
- Zhou, X.J.; Zhang, Z.J.; Li, B.Y.; Yang, F.; Peng, Y.; Li, G.H.; Shi, Z.; Feng, S.H.; Li, J. Two three-dimensional metal–organic frameworks constructed by thiazole-spaced pyridinecarboxylates exhibiting selective gassorption or antiferromagnetic coupling. New J. Chem. 2013, 37, 425–430. [Google Scholar] [CrossRef]
- Zhou, X.J.; Li, B.Y.; Li, G.H.; Zhou, Q.; Shi, Z.; Feng, S.H. Synthesis, structures and luminescent properties of cadmium(II) metal organic frameworks based on 3-pyrid-4-ylbenzoic acid, 4-pyrid-4-ylbenzoic acid ligands. CrystEngComm 2012, 14, 4664–4669. [Google Scholar] [CrossRef]
- Gao, Q.; Jiang, F.L.; Wu, M.Y.; Huang, Y.G.; Yuan, D.Q.; Wei, W.; Hong, M.C. Indium(III)-2,5-pyridine dicarboxylate complexes with mononuclear, 1D chain, 2D layer and 3D chiral frameworks. CrystEngComm 2009, 11, 918–926. [Google Scholar] [CrossRef]
- Das, M.C.; Ghosh, S.K.; Sanudo, E.C.; Bharadwaj, P.K. Coordination polymers with pyridine-2,4,6-tricarboxylic acid and alkaline-earth/lanthanide/transition metals: Synthesis and X-ray structures. Dalton Trans. 2009, 1644–1658. [Google Scholar] [CrossRef]
- Sun, F.X.; Zhu, G.S. Solvent-directed synthesis of chiral and non-centrosymmetric metal-organic frameworks based on pyridine-3,5-dicarboxylate. Inorg. Chem. Commun. 2013, 38, 115–118. [Google Scholar] [CrossRef]
- Liu, Y.T.; Du, Y.Q.; Wu, X.; Zheng, Z.P.; Lin, X.M.; Zhua, L.C.; Cai, Y.P. A series of lanthanide complexes based on pyridine-3,5-dicarboxylate and succinate ligands: Syntheses, structures and properties. CrystEngComm 2014, 16, 6797–6802. [Google Scholar] [CrossRef]
- Wang, X.L.; Qin, C.; Wang, E.B.; Xu, L.; Su, Z.M. Synthesis, structure and luminescent properties of two three-dimensional d10 metal complexes constructed from pyridine-3,4-dicarboxylic acid with new network topologies. J. Mol. Struct. 2006, 796, 172–178. [Google Scholar] [CrossRef]
- Lin, X.M.; Niu, J.L.; Wen, P.X.; Lu, Y.N.; Hu, L.; Zhang, D.L.; Cai, Y.P. From 1D to 3D lanthanide coordination polymers constructed with pyridine-3,5-dicarboxylic acid: Synthesis, crystal structures, and catalytic properties. RSC Adv. 2016, 6, 63425–63432. [Google Scholar] [CrossRef]
- Yang, F.; Zhou, Q.; Zeng, G.; Li, G.H.; Gao, L.; Shi, Z.; Feng, S.H. Anion effects on the structures and magnetic properties of binuclear lanthanide single-molecule magnets. Dalton Trans. 2014, 43, 1238–1245. [Google Scholar] [CrossRef] [PubMed]
- Li, S.D.; Lu, L.P.; Feng, S.S.; Zhu, M.L. Syntheses, structures, magnetic properties and luminescence of four coordination polymers based on an asymmetric semirigid tricarboxylate ligand. J. Solid State Chem. 2019, 269, 56–64. [Google Scholar] [CrossRef]
- He, Y.P.; Tan, Y.X.; Wang, F.; Zhang, J. Microporous Zinc Tris [(4-carboxyl) phenylduryl] amine Framework with an Unusual Topological Net for Gas Storage and Separation. Inorg. Chem. 2012, 51, 1995–1997. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Liu, B.; Wu, Y.P.; Bai, L.; Zhang, J.; Li, D.S. [Co(H2O)6]2+ and H3O+ encapsulated in a unique 3D anionic Co(II) framework with hydrophilic hexagonal and circular channels. CrystEngComm 2015, 17, 7034–7037. [Google Scholar] [CrossRef]
- Wang, J.C.; Ding, F.W.; Ma, J.P.; Liu, Q.K.; Cheng, J.Y.; Dong, Y.B. Co(II)-MOF: A Highly Efficient Organic Oxidation Catalyst with Open Metal Sites. Inorg. Chem. 2015, 54, 10865–10872. [Google Scholar] [CrossRef]
- Chae, S.H.; Kim, H.C.; Lee, Y.S.; Huh, S.; Kim, S.J.; Kim, Y.; Lee, S.J. Thermally Robust 3-D Co-DpyDtolP-MOF with Hexagonally Oriented Micropores: Formation of Polyiodine Chains in a MOF Single Crystal. Cryst. Growth Des. 2015, 15, 268–277. [Google Scholar] [CrossRef]
- Jin, J.; Gong, Y.Y.; Li, L.; Han, X.; Meng, Q.; Liu, Y.H.; Niu, S.Y. Spectroscopic properties of a series of Co(II) coordination polymers and the influence of Co(II) coordination environment on photoelectric property. Spectrochim. Acta Part A 2015, 137, 856–863. [Google Scholar] [CrossRef]
- Murinzi, T.W.; Hosten, E.; Watkins, G.M. Synthesis and characterization of a cobalt-2,6-pyridinedicarboxylate MOF with potential application in electrochemical sensing. Polyhedron 2017, 137, 188–196. [Google Scholar] [CrossRef]
- Gu, J.Z.; Liang, X.X.; Cai, Y.; Wu, J.; Shi, Z.F.; Kirillov, A.M. Hydrothermal assembly, structures, topologies, luminescence, and magnetism of a novel series of coordination polymers driven by a trifunctional nicotinicacid building block. Dalton Trans. 2017, 46, 10908–10925. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.B.; Xiao, Z.Y.; Huang, A.; Wang, W.; Zhang, L.L.; Wang, R.M.; Sun, D.F. Crystal structures, topological analysis and luminescence properties of three coordination polymers based on a semi-rigid ligand and N-donor ligand linkers. New J. Chem. 2016, 40, 5957–5965. [Google Scholar] [CrossRef]
- Bo, Y.X.; Na, S.Y.; Yang, W.; Juan, Z.L.; Wei, L.Q. Synthesis, structure and luminescence properties of metal-organic frameworks based on benzo-bis(imidazole). Sci. China Chem. 2014, 57, 135–140. [Google Scholar]
- O’Keeffe, M.; Peskov, M.A.; Ramsden, S.J.; Yaghi, O.M. The Reticular Chemistry Structure Resource (RCSR) Database of, and Symbols for, Crystal Nets. Acc. Chem. Res. 2008, 41, 1782–1789. [Google Scholar] [CrossRef]
- Noh, T.H.; Lee, H.; Jang, J.; Jung, O.S. Organization and Energy Transfer of Fused Aromatic Hydrocarbon Guests within Anion-Confining Nanochannel MOFs. Angew. Chem. Int. Ed. 2015, 54, 9284–9288. [Google Scholar] [CrossRef]
- Wang, C.C.; Yang, C.C.; Chung, W.C.; Lee, G.H.; Ho, M.L.; Yu, Y.C.; Chung, M.W.; Sheu, H.S.; Shih, C.H.; Cheng, K.Y.; et al. A New Coordination Polymer Exhibiting Unique 2D Hydrogen-Bonded (H2O)16 Ring Formation and Water-Dependent Luminescence Properties. Chem. A Eur. J. 2011, 17, 9232–9241. [Google Scholar] [CrossRef]
- Lupton, J.M.; Samuel, I.D.W.; Burn, P.L. Origin of spectral broadening in π-conjugated amorphous semiconductors. Phys. Rev. B 2002, 66, 155206. [Google Scholar] [CrossRef]
- Akpinar, I.; Drout, R.J.; Islamoglu, J.; Kato, S.; Lyu, J.F.; Farha, O.K. Exploiting π–π Interactions to Design an Efficient Sorbent for Atrazine Removal from Water. ACS Appl. Mater. Interfaces 2019, 11, 6097–6103. [Google Scholar] [CrossRef]
- Yao, R.X.; Cui, X.; Jia, X.X.; Zhang, F.Q.; Zhang, X.M. A Luminescent Zinc(II) Metal–Organic Framework (MOF) with Conjugated π-Electron Ligand for High Iodine Capture and Nitro-Explosive Detection. Inorg. Chem. 2016, 55, 9270–9275. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).